Evolutionary Model of the Sepid-Sarve Manto-Type Copper Mineralization, Doruneh Fault Volcanic-Plutonic Belt (Central Iran Domain, NE Iran): An Integrated Geological, Geochemical, Fluid-Inclusion and Stable O–S Isotope Study
Abstract
1. Introduction
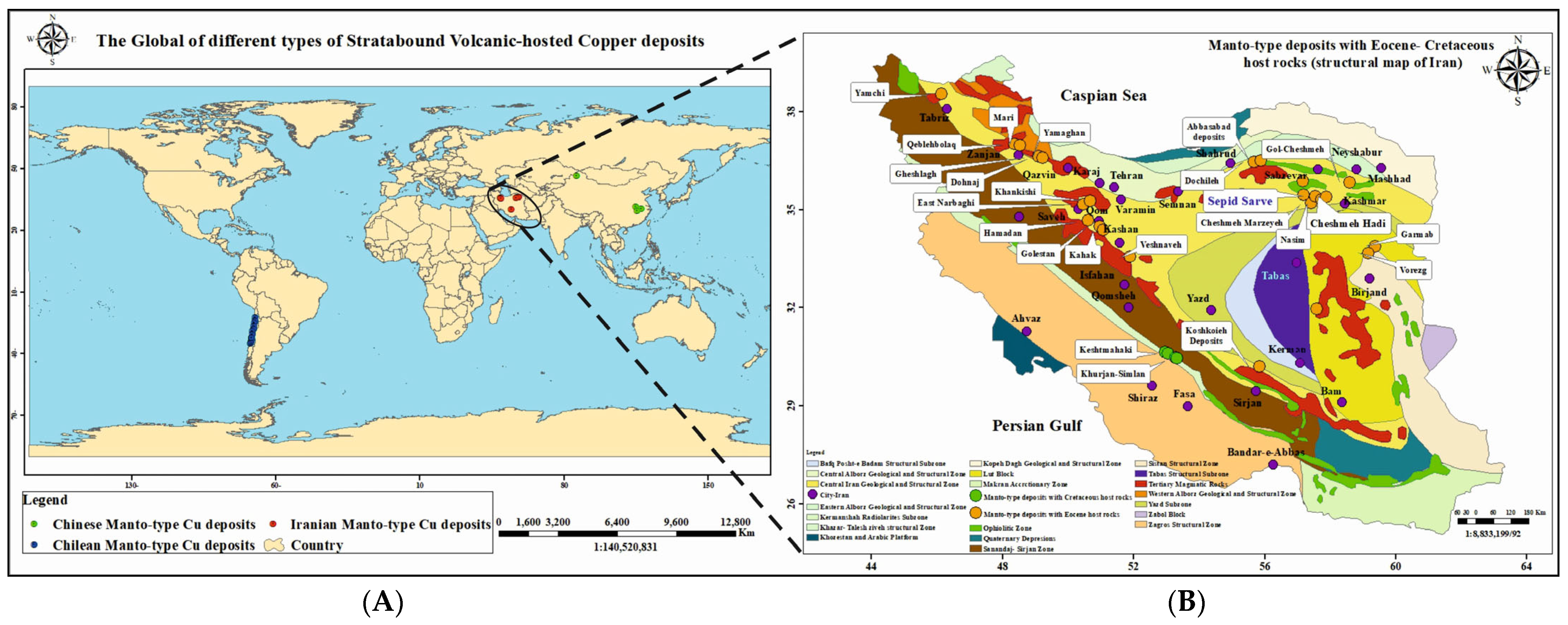
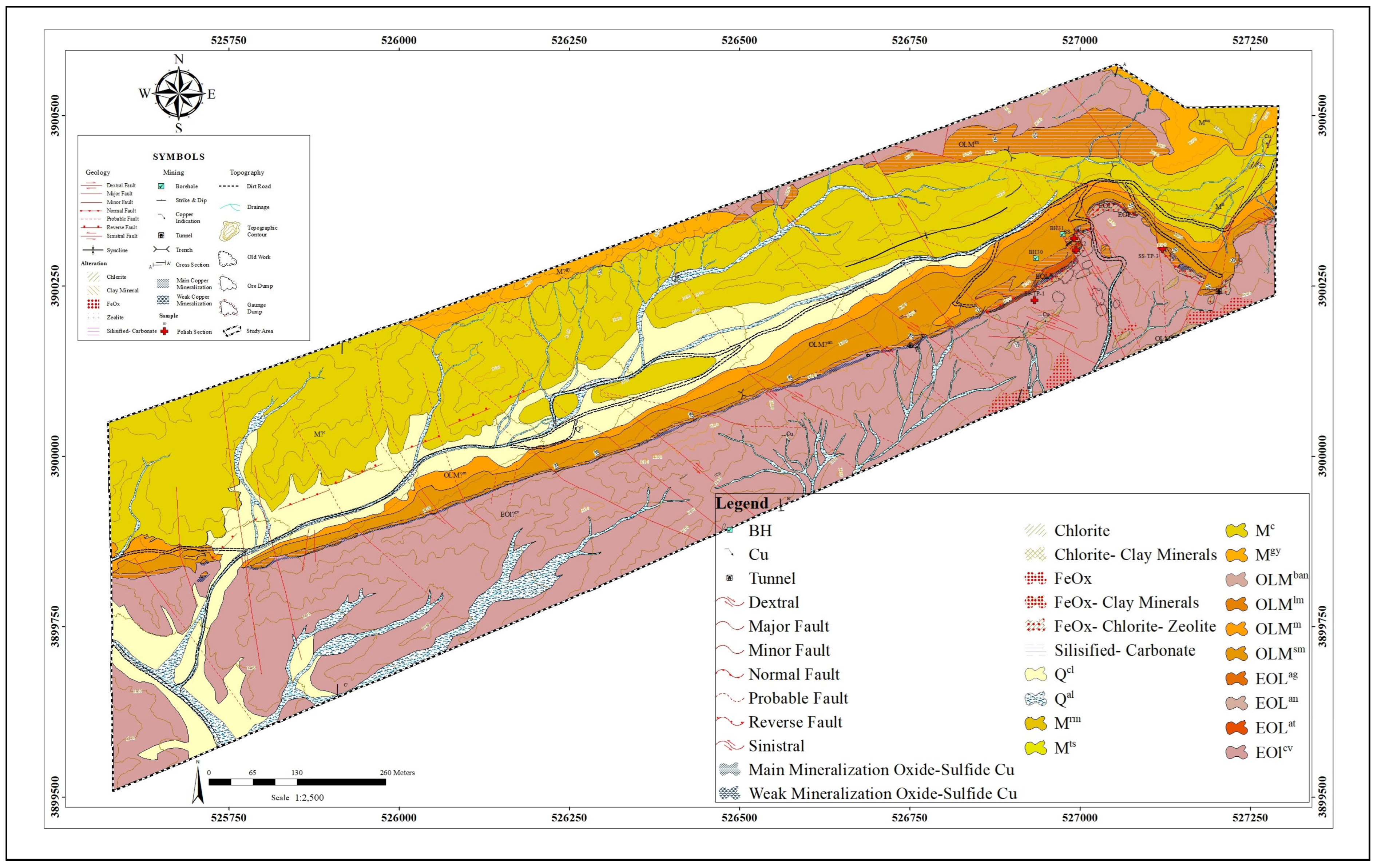
2. Geologic Setting
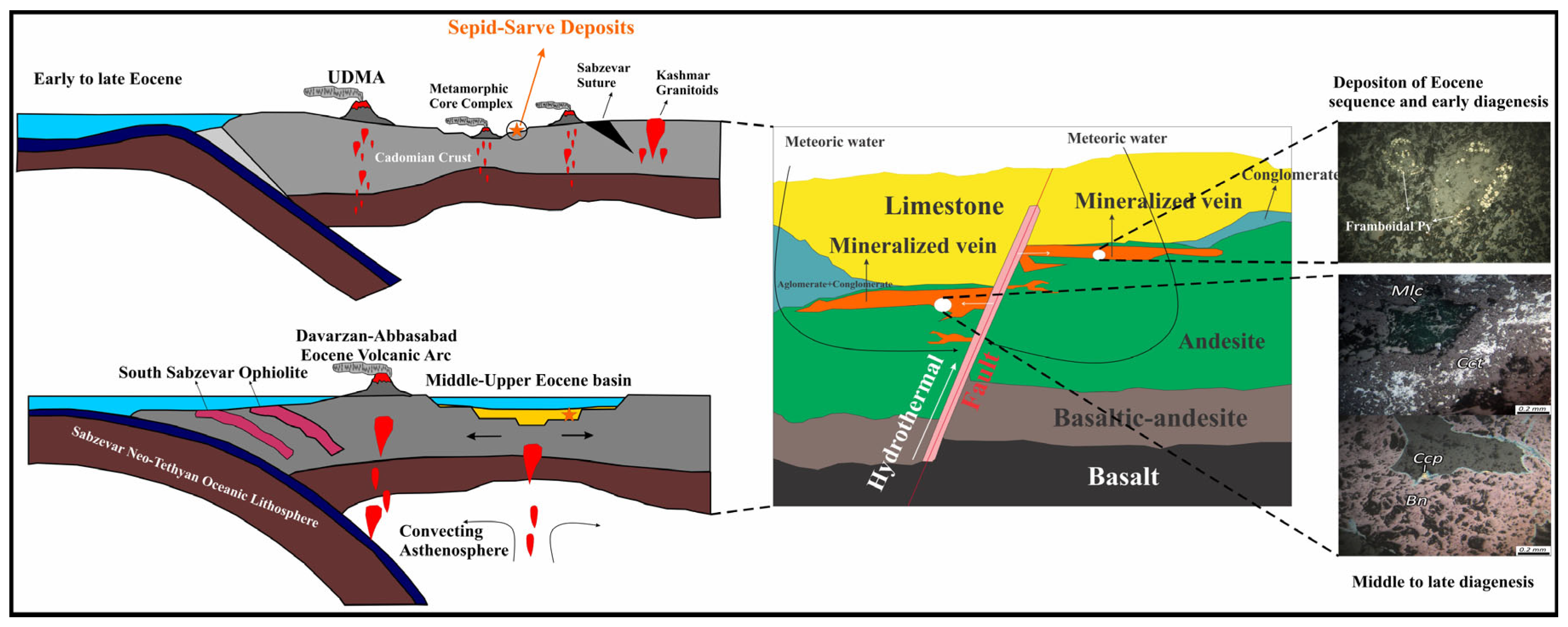
2.1. Regional Geology and Tectonic Setting
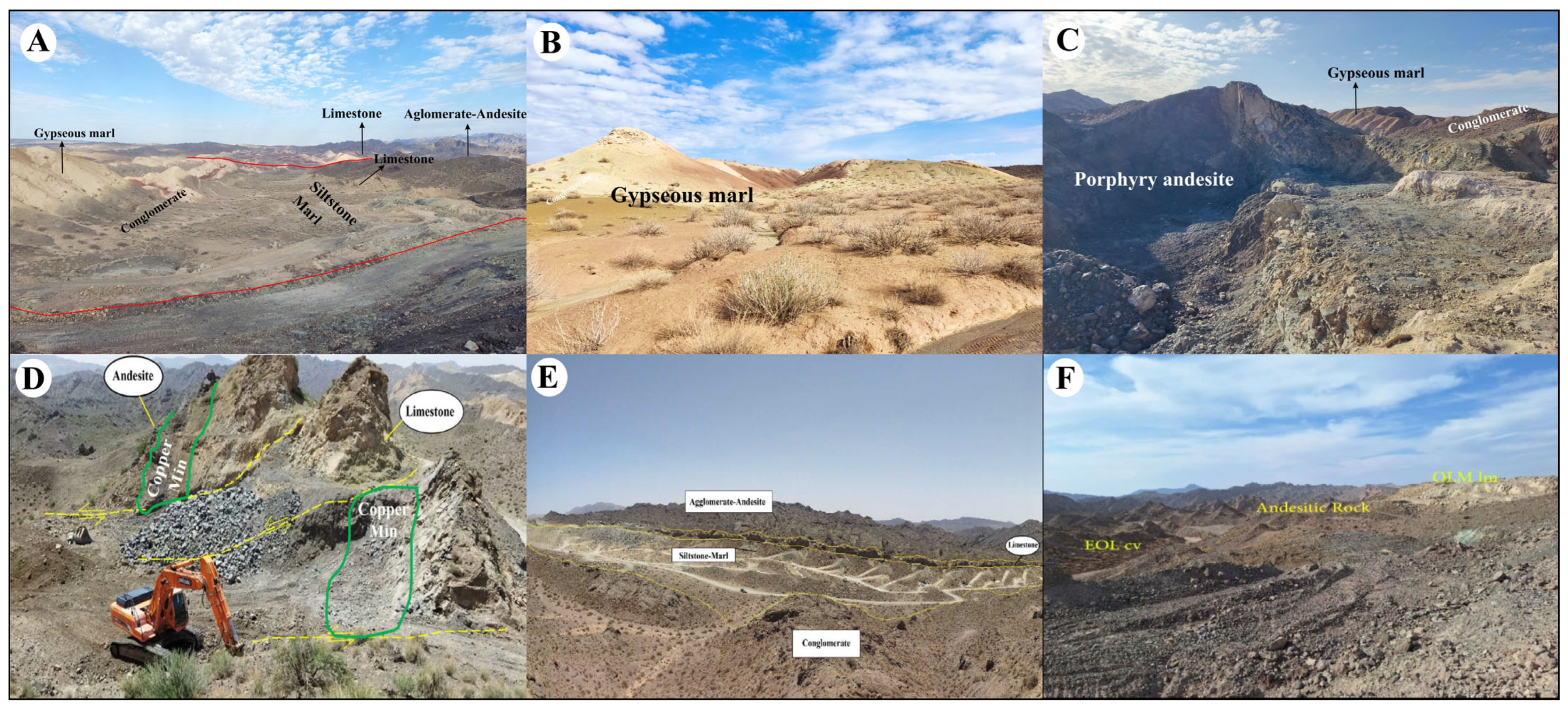
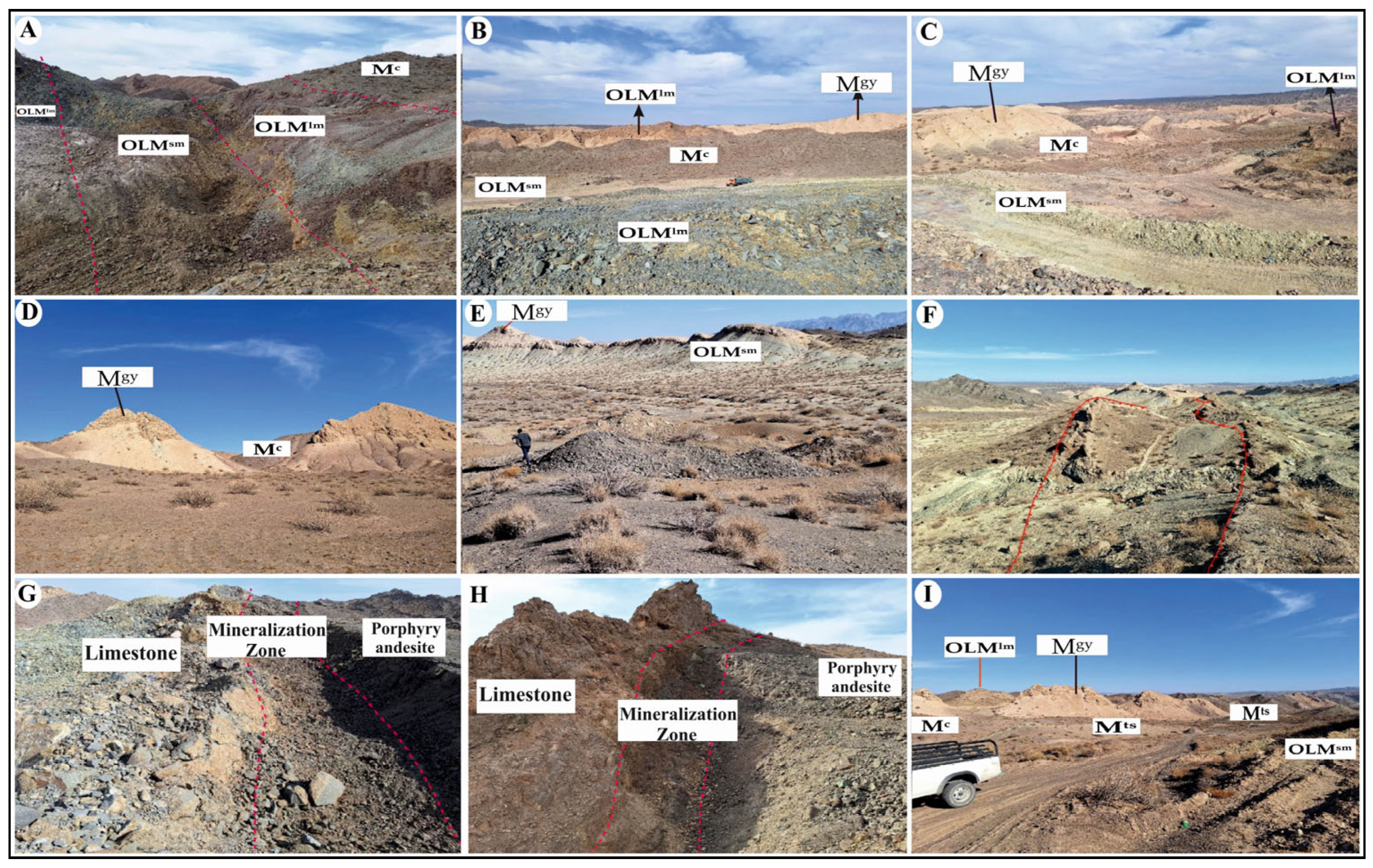
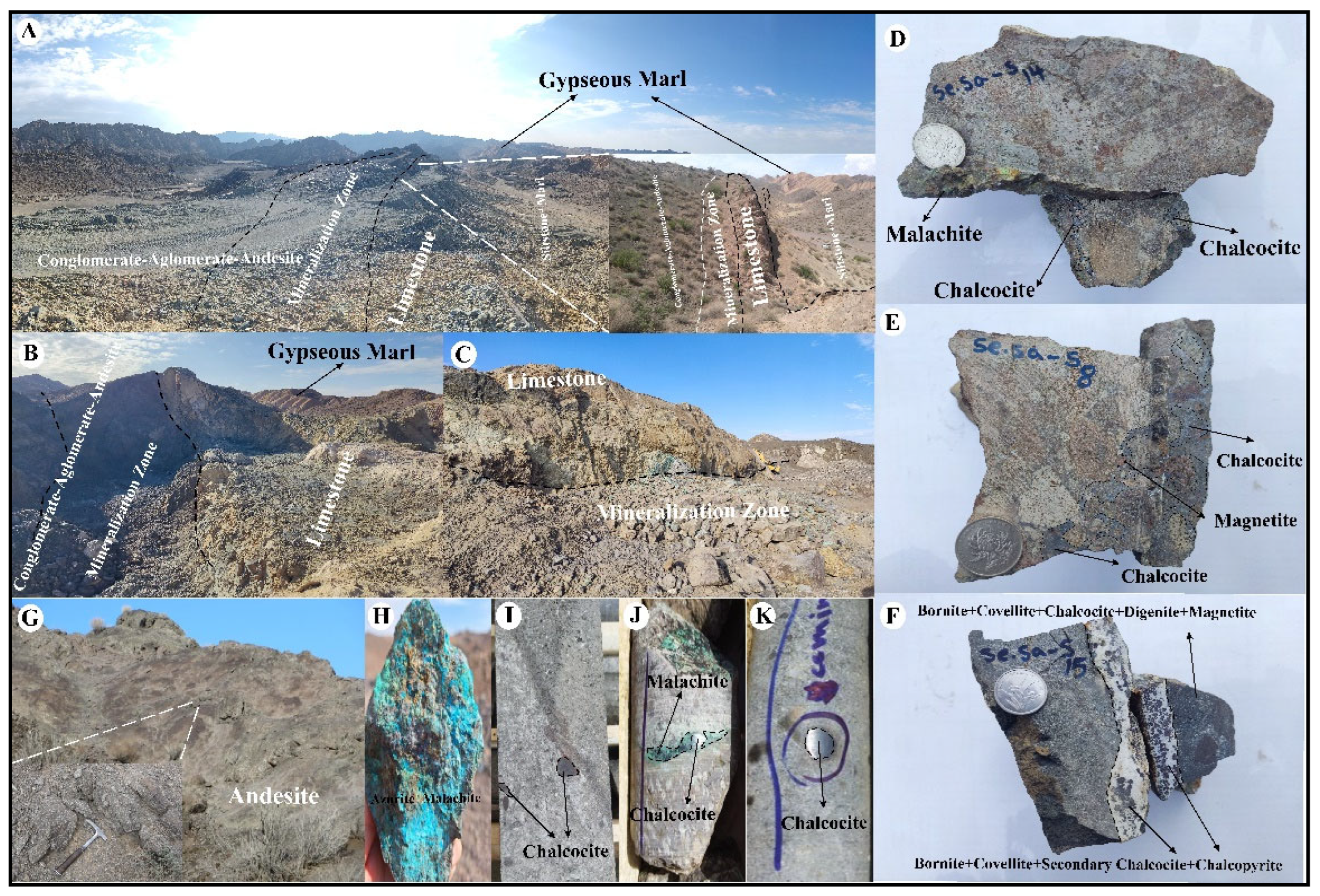
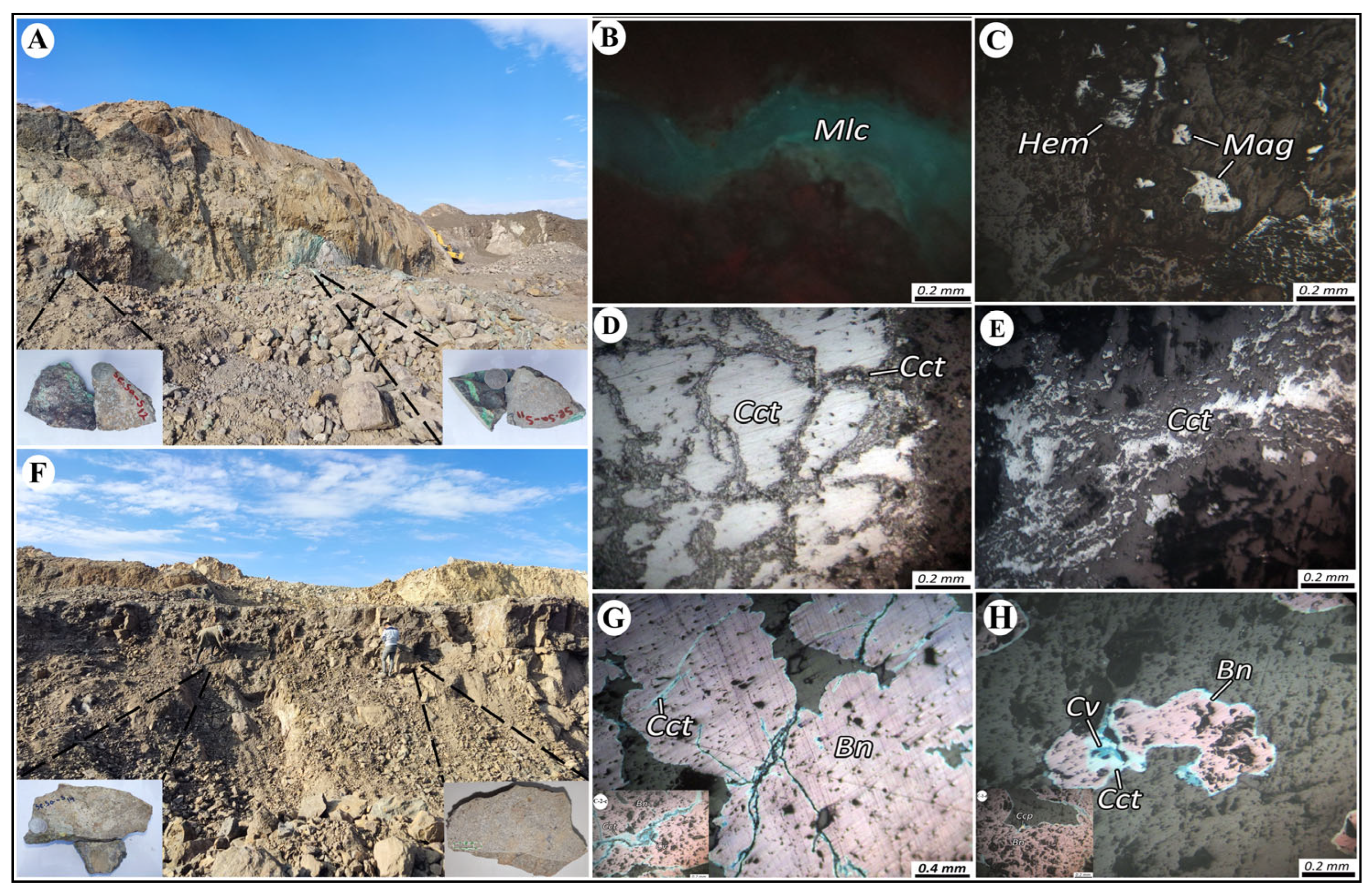
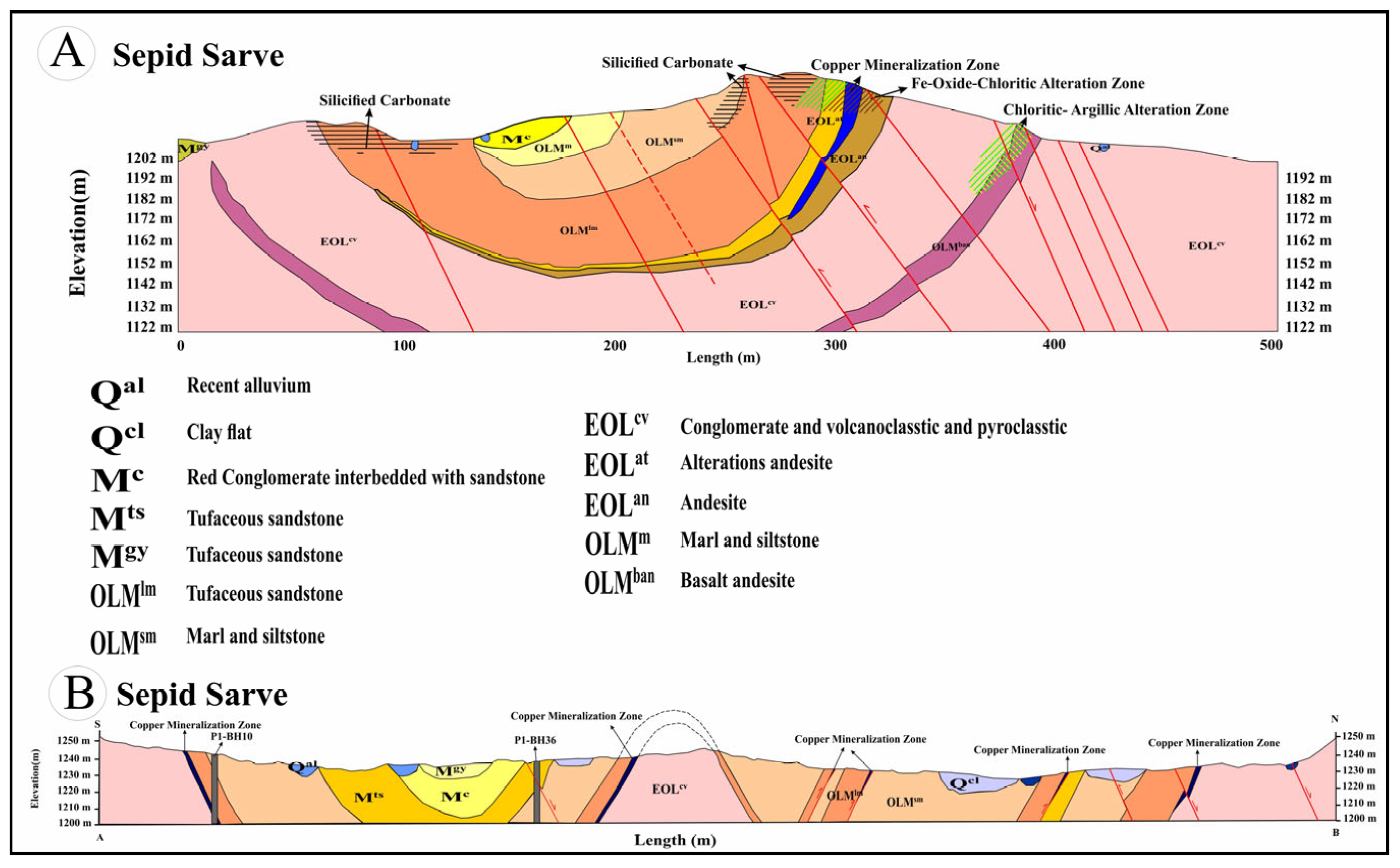
2.2. Ore Deposit Geological Characteristics
2.2.1. Conglomerate
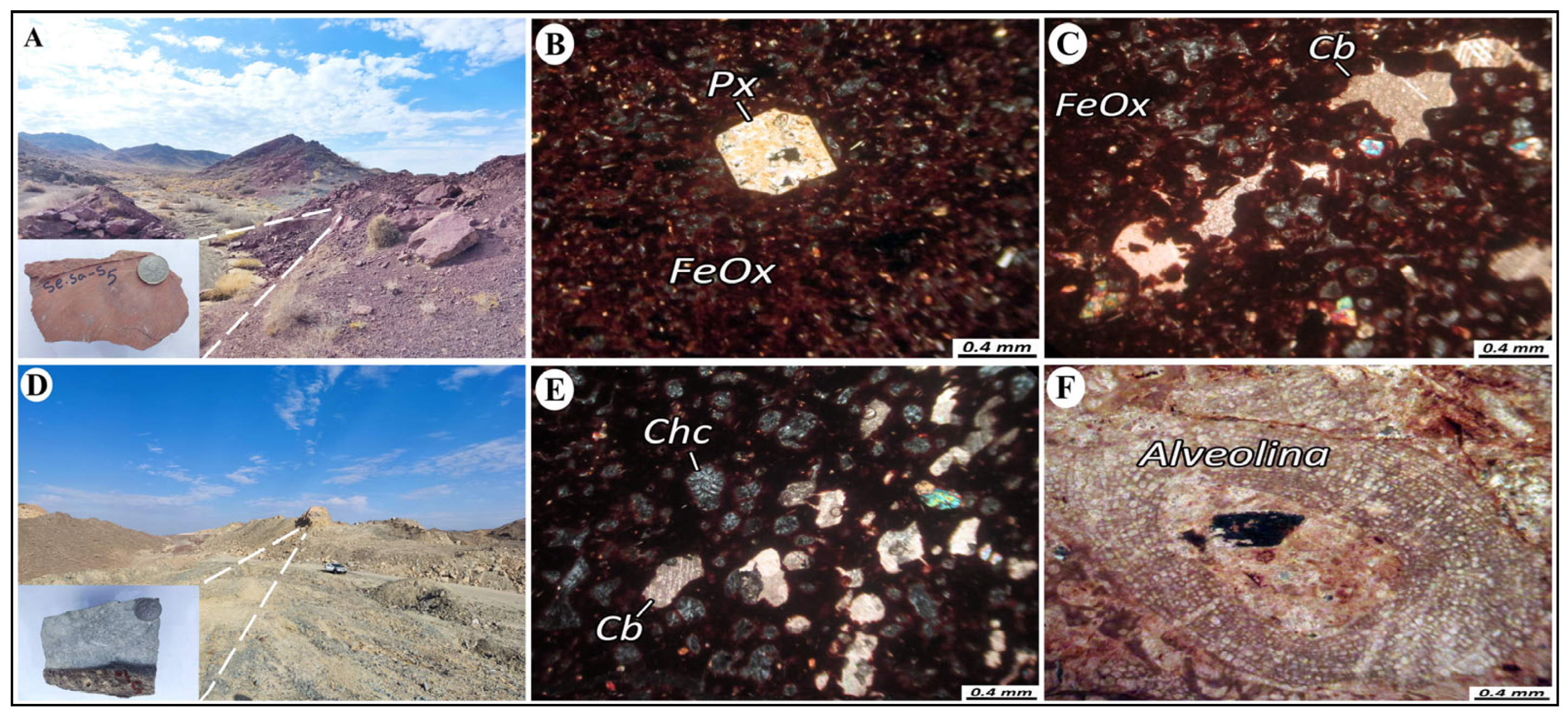
2.2.2. Andesitic and Basaltic Andesite
2.2.3. Pyroxene Andesite
2.2.4. Hornblende-Pyroxene Andesite
2.2.5. Basalt, Olivine Basalt, and Microgabbro
2.2.6. Agglomerate
2.2.7. Limestone
2.2.8. Marl and Siltstone
3. Materials and Methods
3.1. Petrographic and Geochemical Analyses
3.2. Fluid Inclusions Measurements
3.3. Stable Isotope Measurements
3.3.1. Oxygen Isotope Analysis
3.3.2. Sulfur Isotope Analysis
4. Results and Discussion
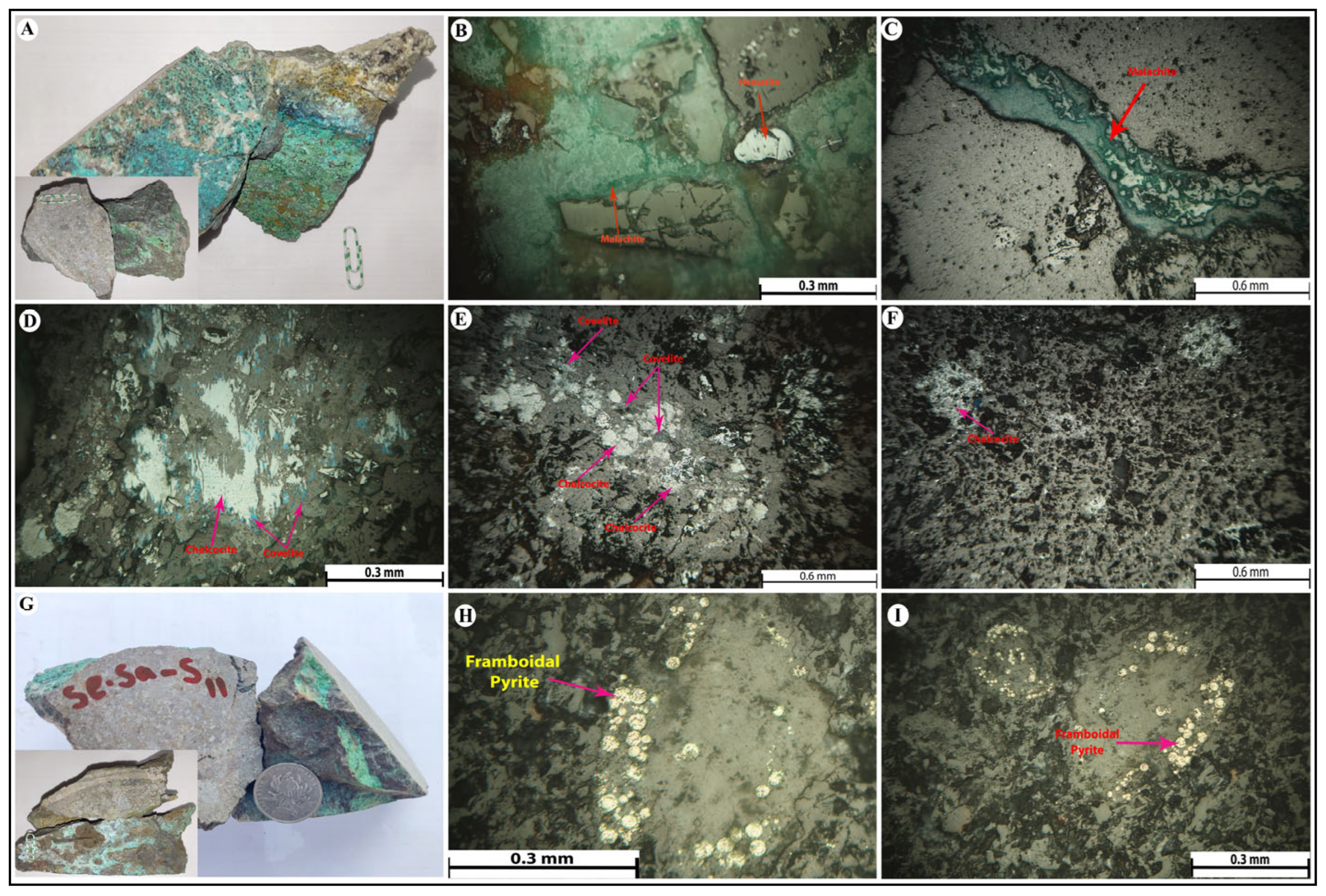
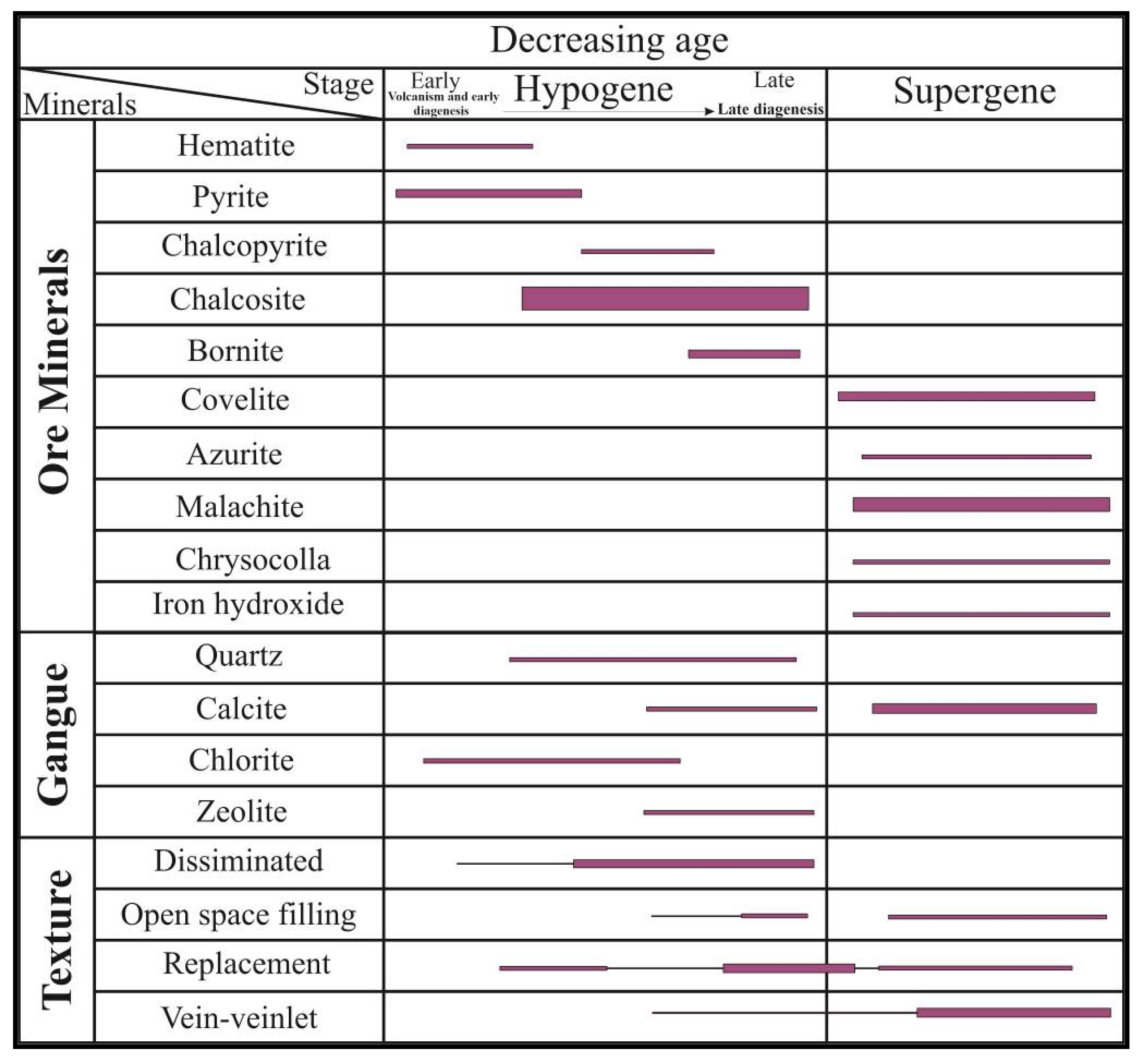
4.1. Ore Mineralogy and Paragenetic Sequence
4.2. Alteration
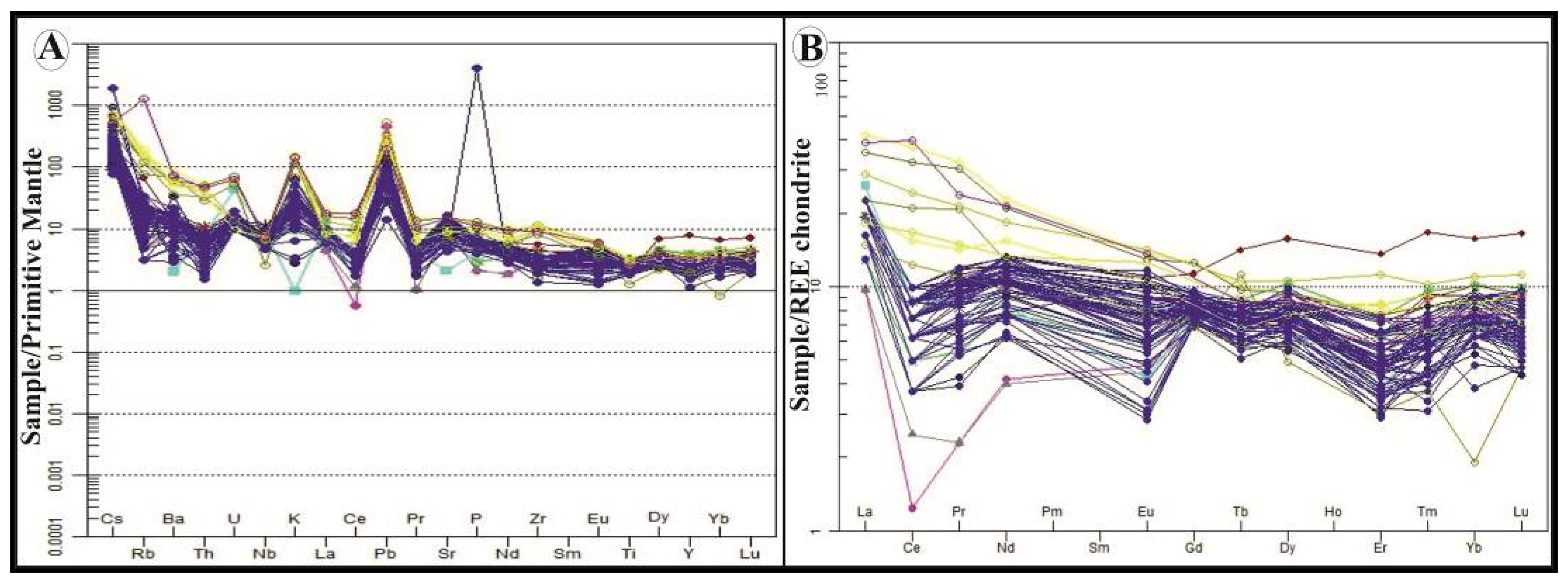
4.3. Geochemistry of Major and Trace Elements
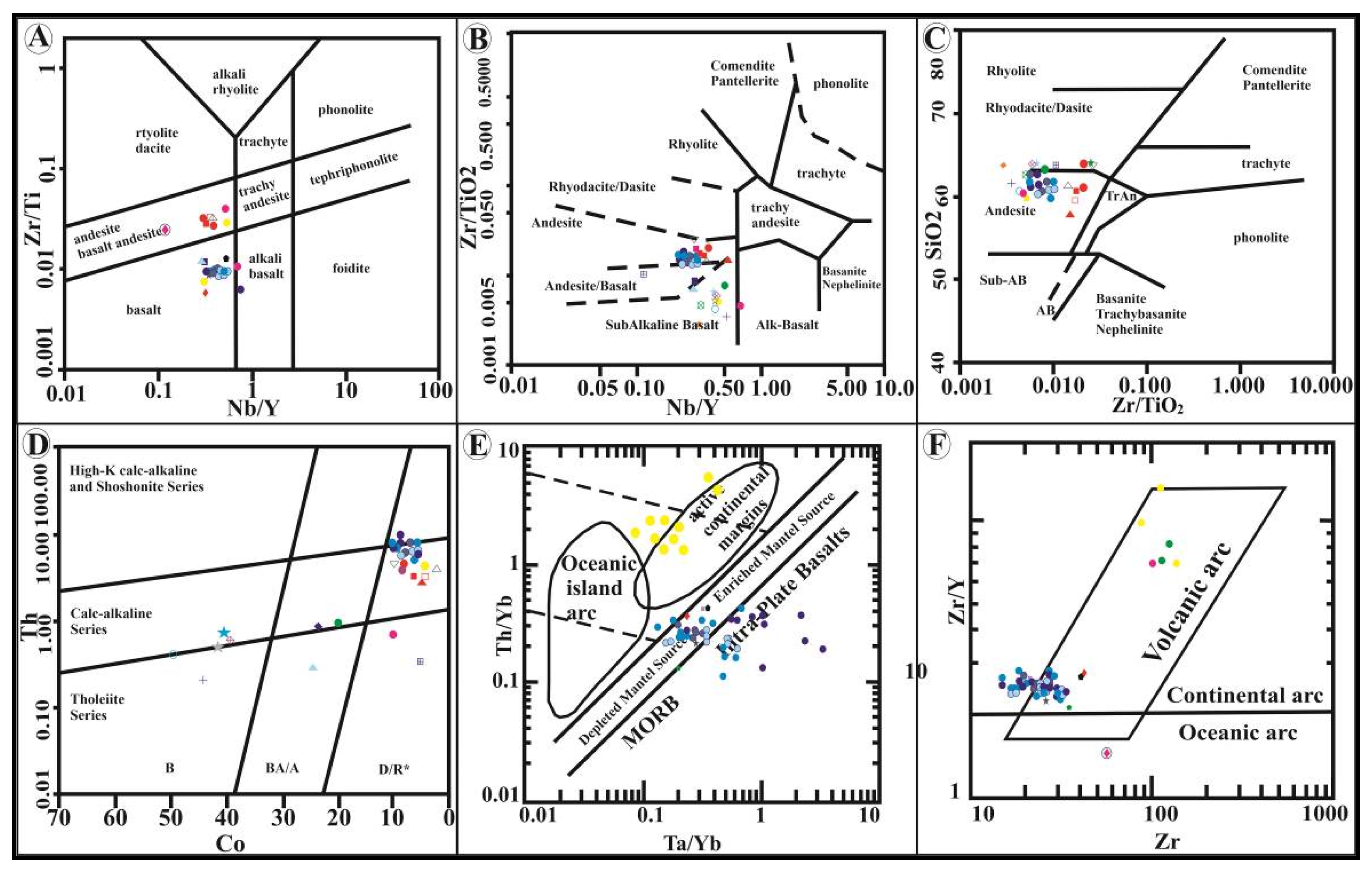
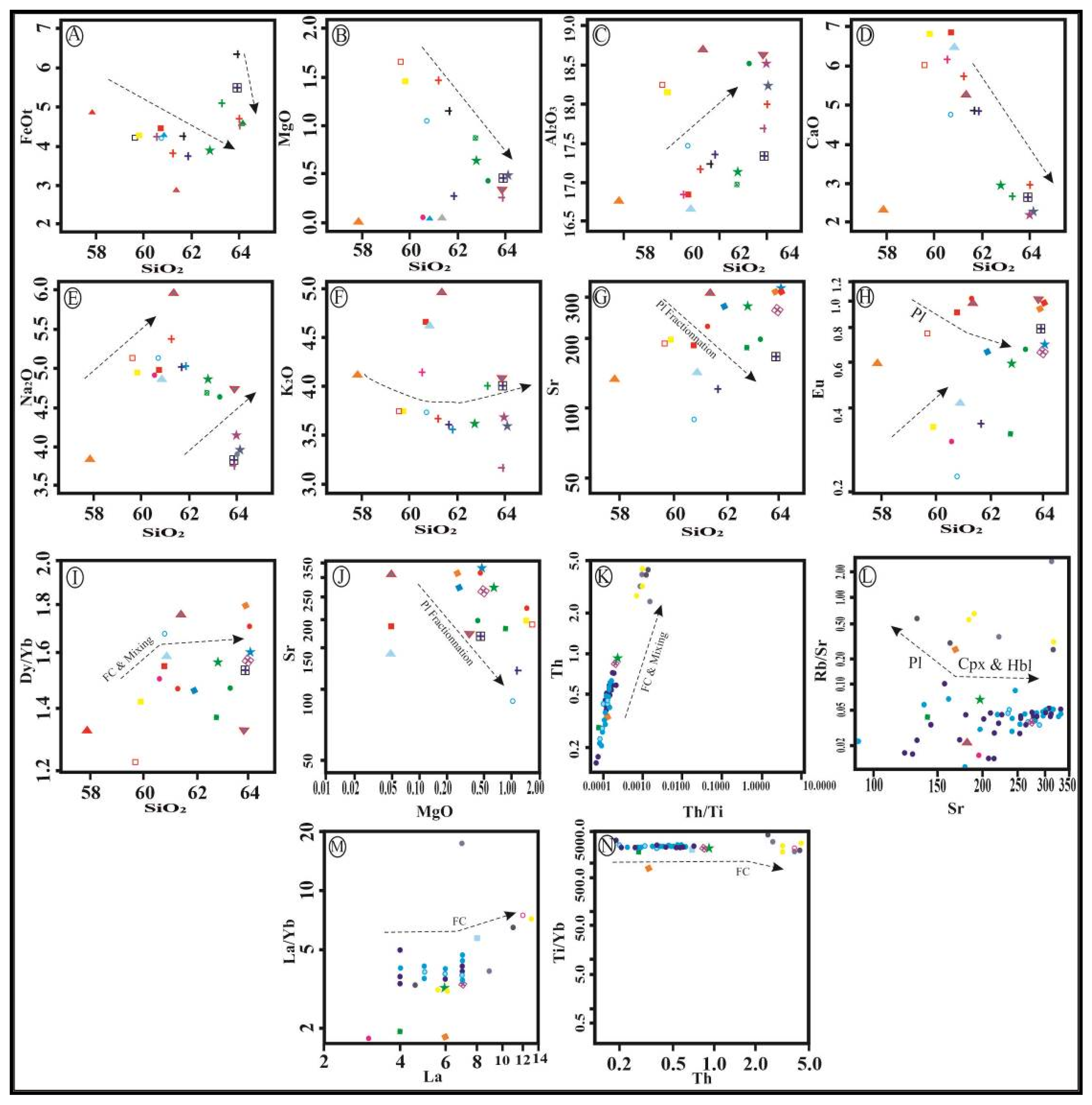
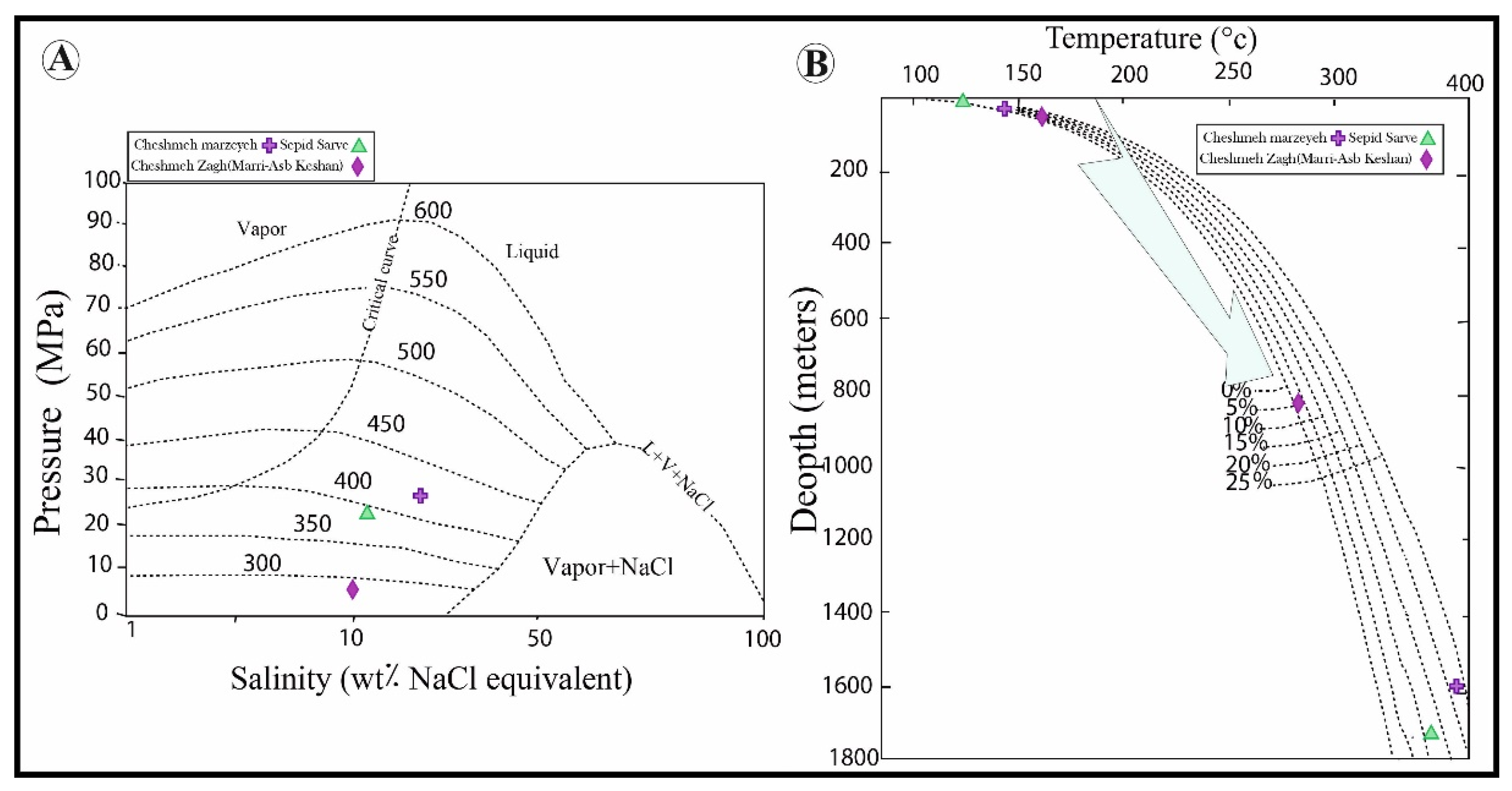
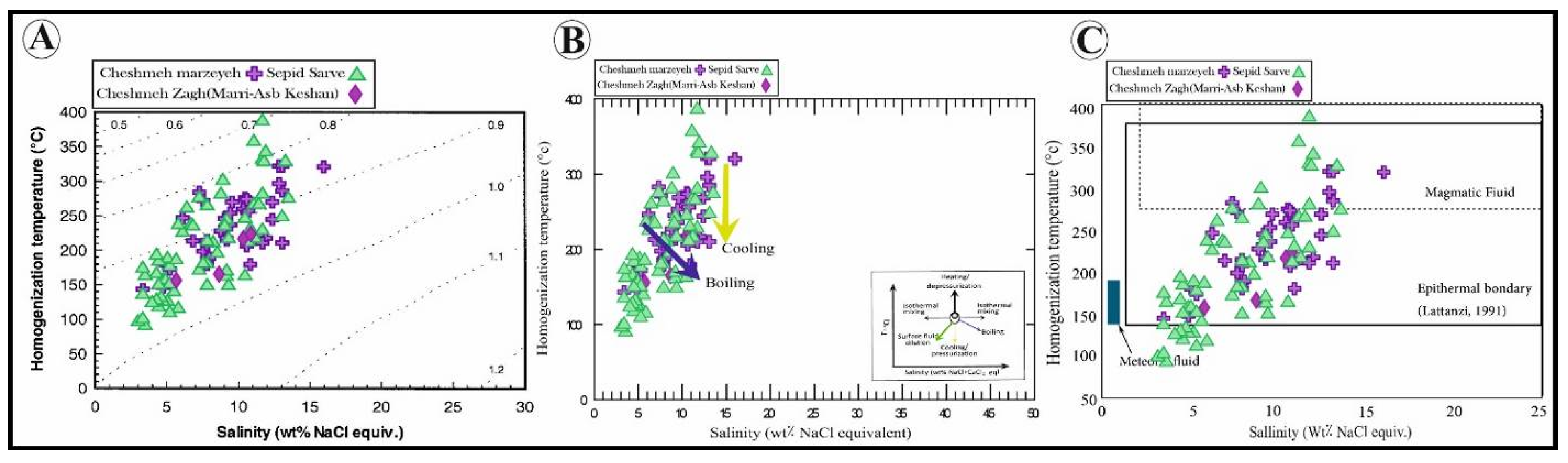
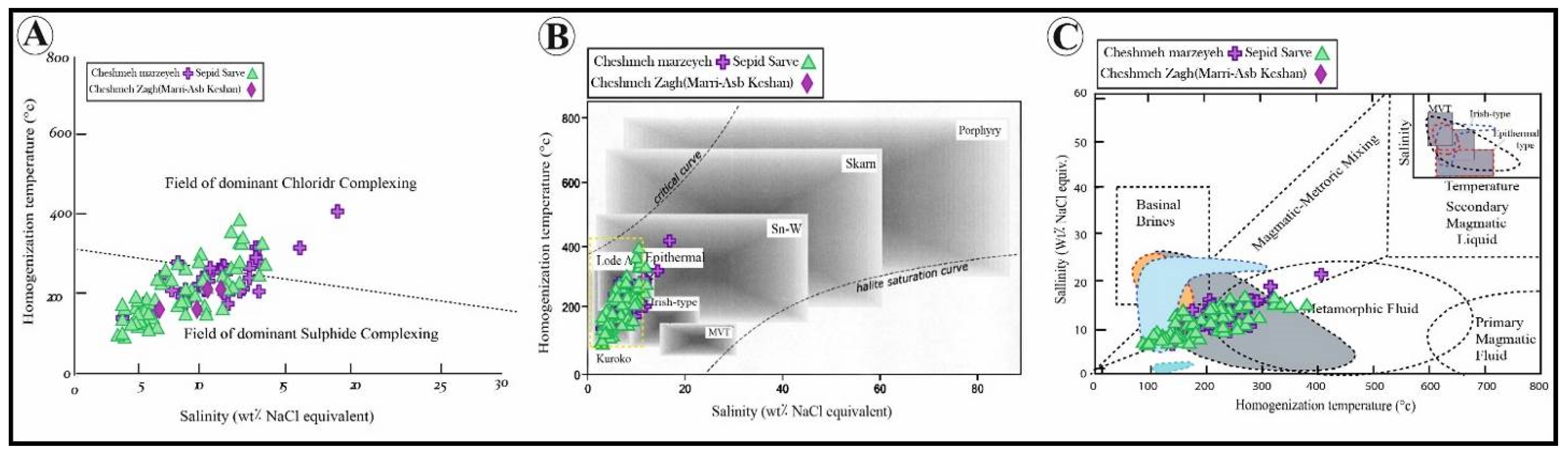
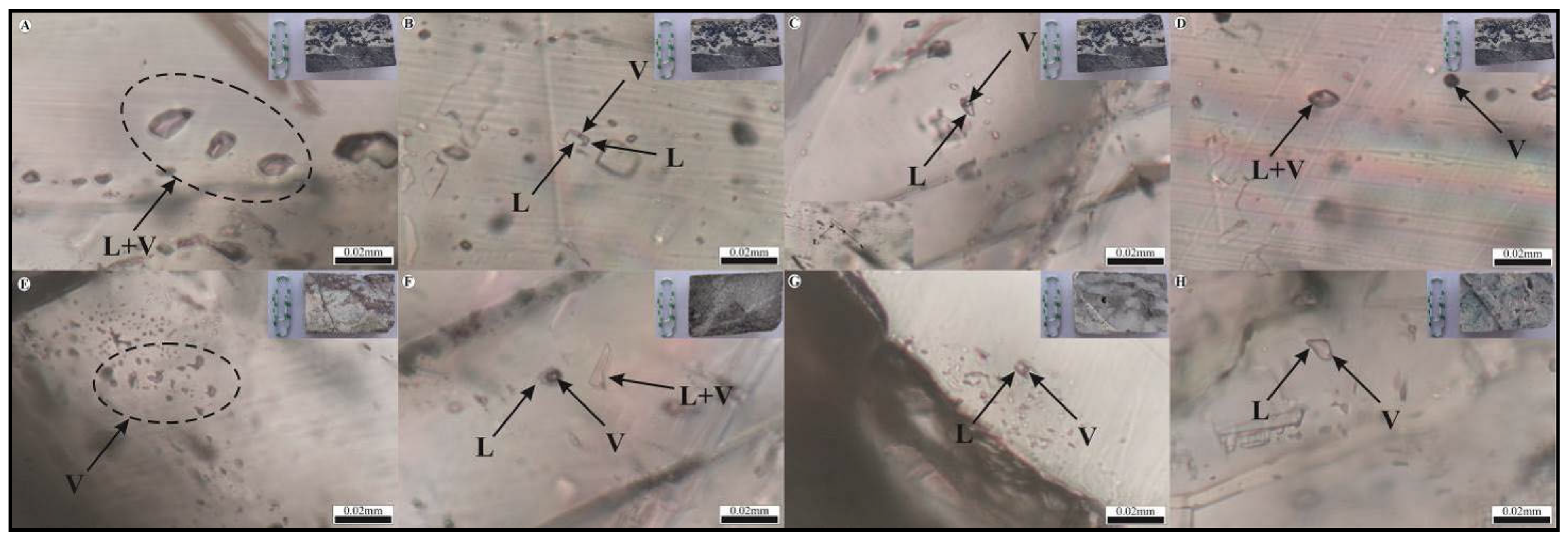
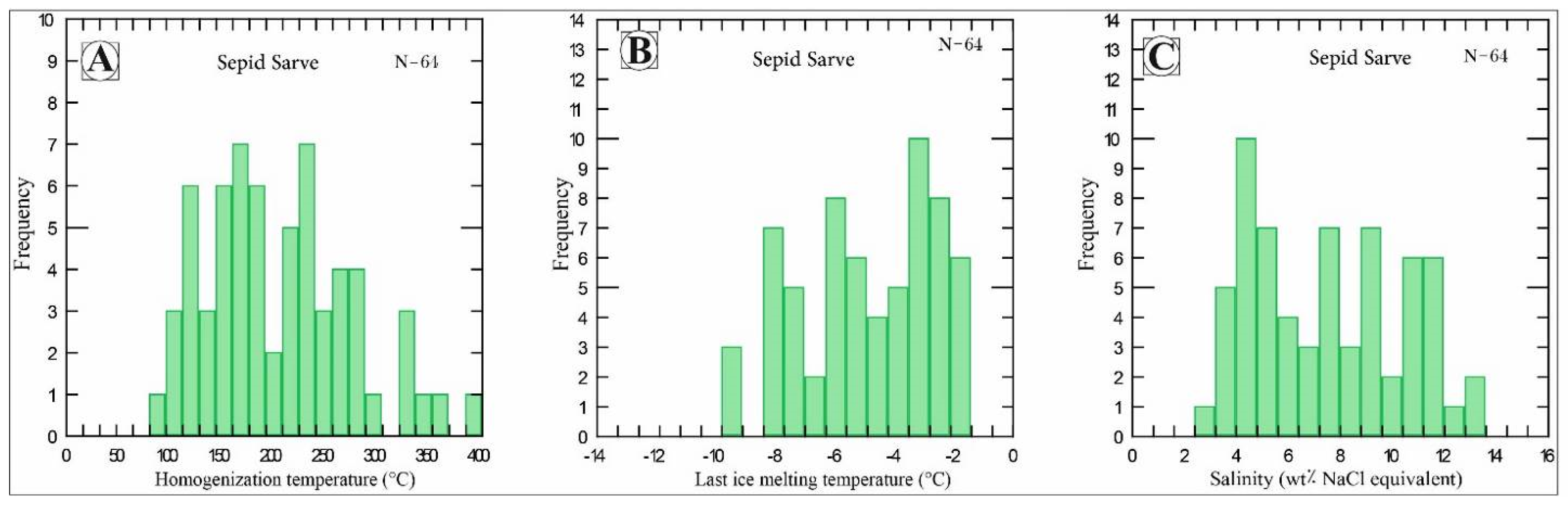
4.4. Fluid Inclusions
4.5. Stable Isotopes
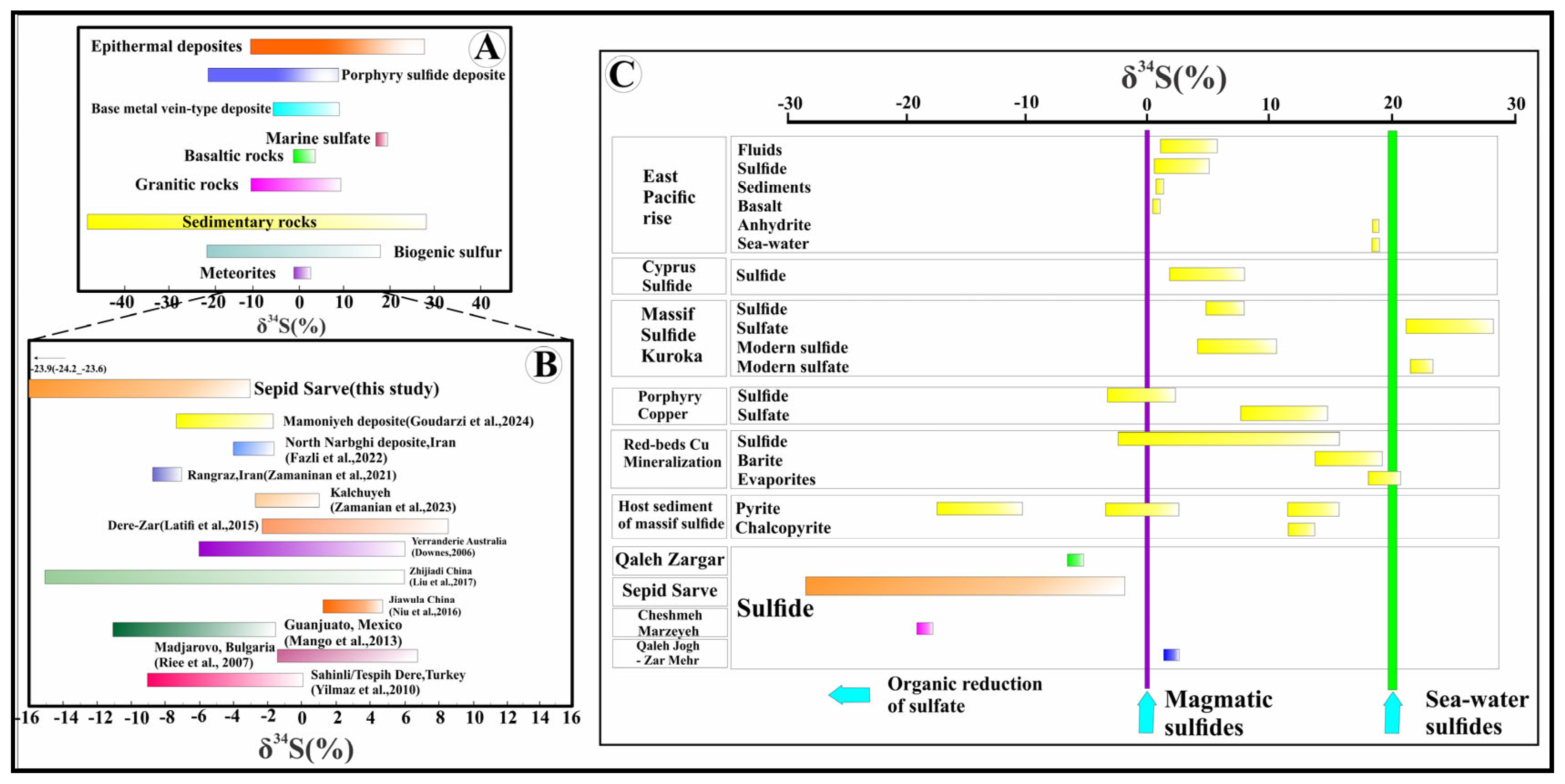
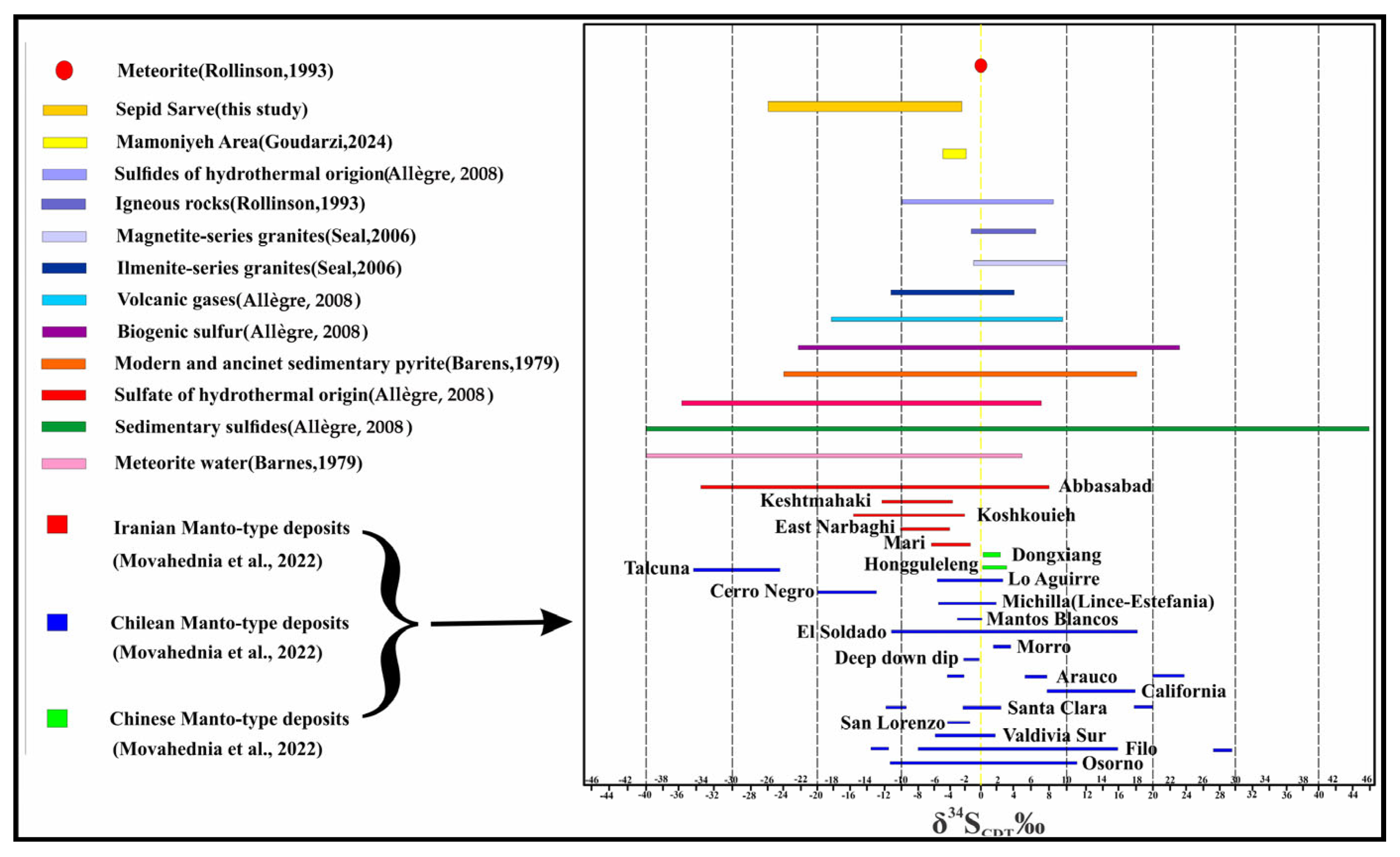
4.5.1. Sulfur Isotopes
- During sulfide mineralization, the hydrothermal fluid expelled and dispersed the sulfate phase away from the vein system. The scarcity of sulfate minerals alongside sulfides may support this removal of sulfate-bearing fluids during or after mineralization. This scenario is consistent with acidic and oxidizing conditions that facilitate sulfide precipitation from sulfur complexes and promote sulfate phase separation.
- Prior to the current stage of sulfide deposition, the sulfate phase may have precipitated at greater depths, allowing the upward migration of a sulfide-dominant hydrothermal fluid into the vein system, where it precipitated 34S-depleted sulfide minerals.
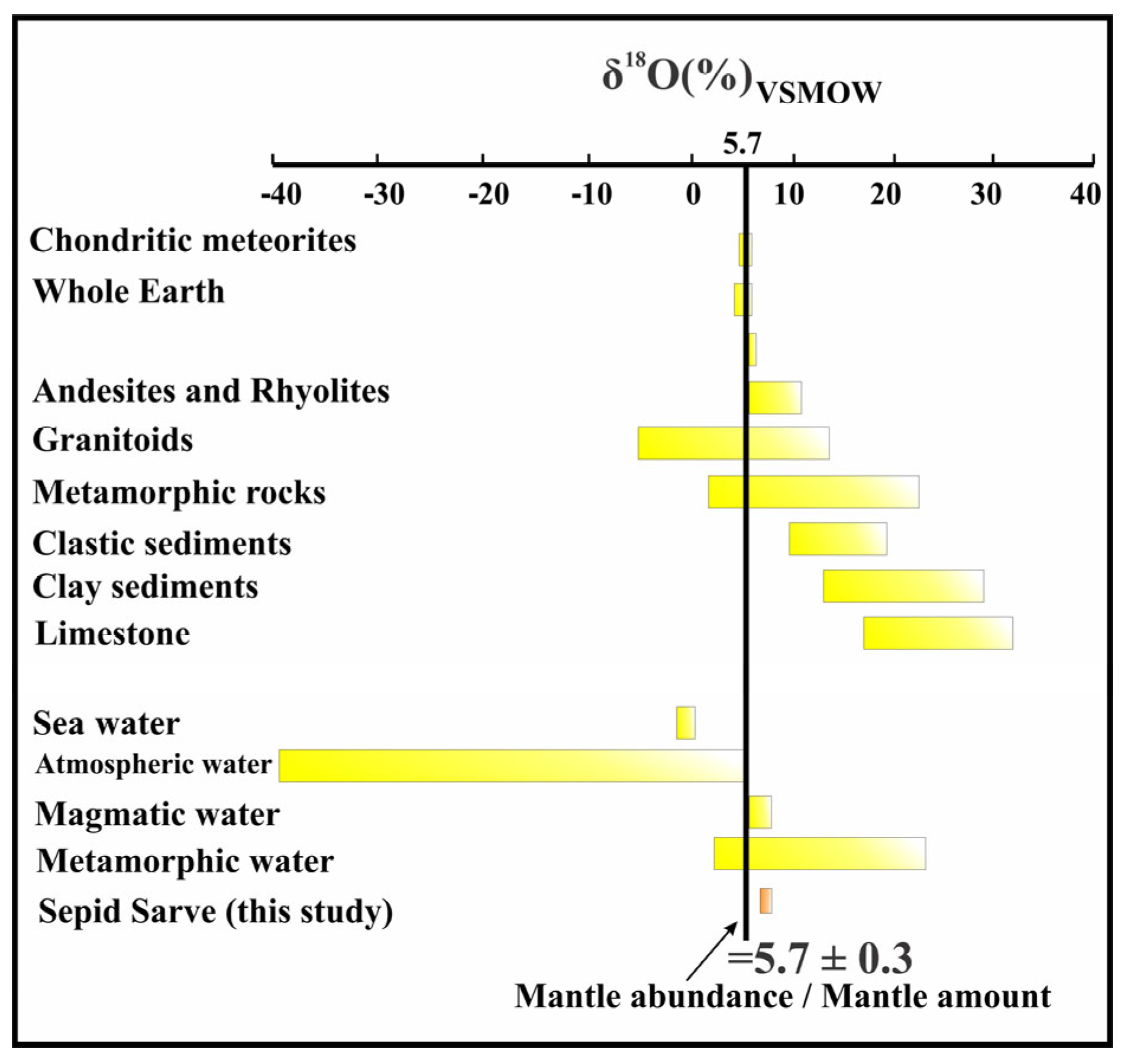
4.5.2. Oxygen Isotopes
4.6. Tectono-Magmatic Model
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Campus, F. Distroto Minero Punta del cobre, modelo interpretative. Rev. Geol. Chile 1980, 11, 51–76. [Google Scholar]
- Wilson, N.S.F.; Zentilli, M. The role of organic matter in the genesis of the El Soldado volcanic-hosted Manto-type Cu deposit, Chile. Econ. Geol. 1999, 94, 1115–1136. [Google Scholar] [CrossRef]
- Ruiz, C.; Aguirre, L.; Corvalan, J.; Klohn, C.; Klohn, E.; Levi, B. Geología y Yacimientos Metalíferos de Chile; Instituto de Investigaciones Geol´ ogicas: Santiago, Chile, 1965; p. 302. [Google Scholar]
- Ruiz, C.; Peebles, F. Geología, Distribuci´on y g´ Enesis de los Yacimientos Metalíferos Chilenos; Editorial Universitaria: Santiago, Chile, 1988; p. 305. [Google Scholar]
- Palacios, C. Subvolcanic copper deposits in the Coastal Range of northern Chile. Zeitbl. Geol. Palaontol 1986, 1, 1605–1615. [Google Scholar] [CrossRef]
- Vivallo, W.; Henríquez, F. Genesis común de los dep´ositos estratoligados y vetiformes de cobre del Jur´ asico Medio a Superior en la Cordillera de la Costa, Regi´ on de Antofagasta, Chile. Rev. Geol. Chile 1998, 25, 199–228. [Google Scholar] [CrossRef]
- Losert, J. Genesis of Copper Mineralizations and Associated Alterations in the Jurassic Volcanic Rocks of the Buena Esperanza Mining Area; Departamento de Geología, Universidad. de Chile: Santiago, Chile, 1973; Volume 40, p. 64. [Google Scholar]
- Sato, T. Manto type copper deposits in Chile: A review. Bull. Geol. Surv. Jpn. 1984, 35, 565–582. [Google Scholar]
- Tosdal, R.M.; Munizaga, F. Lead sources in Mesozoic and Cenozoic Andean ore deposits, north-central Chile (30–34 S). Mineral. Depos. 2003, 38, 234–250. [Google Scholar] [CrossRef]
- Merinero, R.; Ortega, L.; Lunar, R.; Pina, R.; Cardenes, V. Framboidal chalcopyrite and bornite constrain redox conditions during formation of their host rocks in the copper stratabound mineralization of Picachos, north-central Chile. Ore Geol. Rev. 2019, 112, 103037. [Google Scholar] [CrossRef]
- Kojima, S.; Trist´a-Aguilera, D.; Hayashi, K. Genetic aspects of the Manto-type copper deposits based on geochemical studies of north Chilean deposits. Resour. Geol. 2009, 59, 87–98. [Google Scholar] [CrossRef]
- Samani, B. Metallogeny of Manto-Type Deposits in Iran; 6th Symposium on Geosciences; Geological Survey of Iran: Tehran, Iran, 2001; (In Persian with English abstract). [Google Scholar]
- Nezafati, N.; Stöllner, T. Economic Geology, Mining Archaeological and Archaeometric Investigations at the Veshnaveh Ancient Copper Mine, Central Iran. Metalla 2017, 23, 67–90. [Google Scholar] [CrossRef]
- Fazeli, A. Type of the Copper Mineralization at Veshnaveh Deposit, South of Qom. Unpublished Master’s Thesis, Kharazmi university, Tehran, Iran, 2002; p. 157. [Google Scholar]
- Boveiri Konari, M.; Rastad, E.; Rashidnejad Omran, N. Volcanic redbed-type copper mineralization in the Keshtmahaki, Southern Sanandaj-Sirjan Zone, southeastern Iran. In Proceedings of the 11th SGA Biennial Meeting Let’s Talk Ore Deposits, Antofagasta, Chile, 26–29 September 2011. [Google Scholar]
- Abolipour, M.; Rastad, E.; Rashidnejad Omran, N. Manto-type copper mineralization in pyrobitumen-bearing porphyritic andesite, Koshkoiye district of Rafsanjan, Dehaj-Sardoiye subzone. Sci. Q. J. Geosci. 2015, 24, 123–144. [Google Scholar]
- Alizadeh, V.; Momenzadeh, M.; Emami, M.H. Petrography, geochemistry, mineralogy, fluid inclusions and mineralization study of Vorezg-Qayen copper deposit. Sci. Q. J. Geosci. 2013, 22, 47–58. [Google Scholar]
- Maghfouri, S.; Movahednia, M. Investigation of geology and mineralization of Abbas Abad copper deposit and comparison with Manto-type deposit. In Proceedings of the 18th Symposium of the Geological Society of Iran, Tehran, Iran, 24 December 2014; Tarbiat Modares University: Tehran, Iran (In Persian with English abstract). [Google Scholar]
- Maghfouri, S.; Hosseinzadeh, M.R.; Moayyed, M.; Movahednia, M.; Choulet, F. Geology, mineralization and sulfur isotopes geochemistry of the Mari Cu (Ag) Manto-type deposit, northern Zanjan, Iran. Ore Geol. Rev. 2017, 81, 10–22. [Google Scholar] [CrossRef]
- Kaboodi, Z.; Ghaderi, M.; Rastad, E. Mineralogy, texture and structure and genetic model of Kahak Manto-type copper deposit in the Eocene volcano-sedimentary sequence, south Qom. Sci. Q. J. Geosci. 2019, 29, 145–154. [Google Scholar]
- Fazli, N.; Ghaderi, M.; Movahednia, M.; Maghfouri, S. Manto-type copper mineralization in the central part of the Urumieh-Dokhtar magmatic arc (Qom-Saveh region) with emphasis on the East Narbaghi deposit, northeast Saveh. Iranian J. Geol. 2021, 15, 69–90. [Google Scholar]
- Jiba, Z.; Ghaderi, M.; Maghfouri, S. Geology, mineralogy, and fluid inclusion studies of the Yamaghan Manto-type Cu (Ag) deposit, southeast Zanjan, NW Iran. Adv. Appl. Geol. 2021, 11, 594–615. [Google Scholar]
- Salehi, L.; Rasa, I. Sulfur isotopic characteristics of the chalcocite in Madan Bozorg Cu deposits, Abbas Abad, NE Iran. In Proceedings of the 34th Geosciences Congress, Tehran, Iran, 22–24 February 2016. [Google Scholar]
- Glennie, K.W. Cretaceous Tectonic Evolution of Arabia’s Eastern Plate Margin: A Tale of Two Oceans, in Middle East Models of Jurassic/Cretaceous Carbonate Systems; SEPM (Society for Sedimentary Geology) Special Publication: Claremore, OK, USA, 2000; Volume 69, pp. 9–20. [Google Scholar] [CrossRef]
- Rossetti, F.; Nasrabady, M.; Vignaroli, G.; Theye, T.; Gerdes, A.; Razavi, M.; Moin Vaziri, H. Early Cretaceous migmatitic mafic granulites from the Sabzevar range (NE Iran): Implications for the closure of the Mesozoic peri-Tethyan oceans in central Iran. Terra Nova 2010, 22, 26–34. [Google Scholar] [CrossRef]
- Maghfouri, S. Geology, Mineralogy, Geochemistry, and Genesis of Cu Mineralization within Late Cretaceous Volcano-Sedimentary Sequence in Southwest of Sabzevar, with Emphasis on the Nudeh Deposit. Unpublished. Master’s Thesis, Tarbiat Modares University, Tehran, Iran, 2012; 312p. (In Persian with English abstract). [Google Scholar]
- Soltani, A. Mineralogy, geochemistry and genesis of the Abri, Rahbari, and Cheshmeh Marziyeh cu deposit, NW Darooneh. Master’s Thesis, Shahrood University of Technology, Shahrud, Iran, 2016; 191p. [Google Scholar]
- Monazzami Bagherzadeh, R.; Karimpour, M.H.; Farmer, J.L.; Stern, C.; Santos, J.F.; Ribeiro, S.; Rahimi, B.; Haidarian Shahri, M.R. Geochronology, petrology and geochemistry of intermediate and mafic Rocks of the Bornaward plutonic complex (northwest of Bardeskan, Iran). J. Econ. Geol. 2019, 10, 425–448. [Google Scholar]
- Rezaeihamid, R.; Tale Fazel, A. Mineralogy, minerals-chemistry and sulfur isotope geochemistry of Baharieh copper deposit (NE Kashmar): Implications for ore genesis. J. Petrol. 2019, 10, 53–78. [Google Scholar]
- Jabari, A.; Malekzadeh Shafaroudi, A.; Karimpoor, M.H. The stratabound (mantle-type) copper deposit of Kal Abri in the Eocene volcano-sedimentary complex, NW Bardaskan, NE Iran. J. Adv. Appl. Geol. 2016, 23, 142. [Google Scholar]
- Ramezaniabbakhsh, T.; Karimpour, M.H.; Azizi, H.; Rahimi, B.; Saadat, S. Metallogeny of mantle-copper deposits, special view in Nasim copper deposit, northwest of Bardaskan, Khorasan Razavi, Iran. J. Econ. Geol. 2023, 15, 143–174. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Arab-Amiri, A.; Ghanbari, H. Mineralogy, alteration, fluid inclusion and stable isotopes studies of the Sharifabad-Bardeskan copper deposit, NE Iran. Sci. Q. J. Geosci. 2020, 30, 135–146. [Google Scholar]
- Heidari, M. Study of Geochemistry, Sulfur Stable isotopes, and Genesis of Madan Bozorg Copper Deposit (Abbasabad, East of Shahroud). Unpublished Master’s Thesis, Bu-Ali Sina University, Hamadan, Iran, 2012; 169p. (In Persian with English abstract). [Google Scholar]
- Taefi, N.; Mousivand, F.; Sadeghian, M. Mineralogy, geochemistry, and genesis of copper mineralization in the Grik and Gurkhan Southeast of Shahrood. In Proceedings of the 6th Symposium of Iranian Society of Economic Geology, Zahedan, Iran, 13 September 2014; University of Birjand: Birjand, Iran (In Persian with English abstract). [Google Scholar]
- Entezari Harsini, A. Geology, Geochemistry, Mineralization, Genesis and Isotope studies of Golcheshmeh deposit, South Neyshabour, Northeastern Iran. Unpublished Ph.D. Thesis, Ferdowsi University of Mashhad, Mashhad, Iran, 2017; 310p. (In Persian with English abstract). [Google Scholar]
- Rezapanah Khour, H. Investigation of Geology, Alteration and the Cu Mineralization in Cheshmeh-Hadi Area, Bardaskan (Khorasan Razavi Province, Northeast Iran). Unpublished Master’s Thesis, Shahid Bahonar University of Kerman, Kerman, Iran, 2016; 148p. (In Persian with English abstract). [Google Scholar]
- Aghanabati, A. Geology of Iran; Geological Survey of Iran: Tehran, Iran, 2004; 586p. [Google Scholar]
- Alavi, M. Sedimentary and structural characteristics of the Paleo Tethys remnants in northeastern Iran. Geol. Soc. Am. Bull. 1991, 103, 983–992. [Google Scholar] [CrossRef]
- Steele-MacInnis, M.; Lecumberri-Sanchez, P.; Bodnar, R.J. HokieFlincs_H2O-NaCl: A Microsoft Excel spreadsheet for interpreting microthermometric data from fluid inclusions based on the PVTX properties of H2O-NaCl. Comput. Geosci. 2012, 49, 334–337. [Google Scholar] [CrossRef]
- Eftekharnejad, J. Tectonic division of Iran with respect to sedimentary basins. J. Iran. Petrol. Soc. 1981, 82, 19–28. (In Persian) [Google Scholar]
- Shahrabi, M.; Hosseini, M.; Shabani, K. Geological Survey and Mineral Exploration of Iran; 1:250,000 Bardeskan quadrangle; Geological Survey & Mineral Exploration of Iran (GSI): Tehran, Iran, 2007. [Google Scholar]
- Ghaeni, F.; Moussavi Harami, R. Geological Survey and Mineral Exploration of Iran; 1:100,000 Doruneh geological map; Ferdowsi university of Mashhad: Mashhad, Iran, 2007. [Google Scholar]
- VSMOW2/SLAP2; Reference Sheet for VSMOW2 and SLAP2—International Measurement Standards. Vienna International Centre: Vienna, Austria, 2017.
- IAEA-S-4; Soufre de Lacq, Elemental Sulfur—Sulfur Stable Isotope Reference Material. Vienna International Centre: Vienna, Austria, 2022.
- Zentilli, M.; Munizaga, F.; Graves, M.C.; Boric, R.; Wilson, N.S.F.; Mukhopadhyay, P.K.; Snowdon, L.R. Hydrocarbon involvement in the genesis of ore deposits: An example in Cretaceous strata-bound (Manto-type) copper deposits of central Chile. Int. Geol. Rev. 1997, 39, 1–21. [Google Scholar] [CrossRef]
- Wilson, N.S.F. Organic petrology, chemical composition, and reflectance of pyrobitumen from the El Soldado Cu deposit, Chile. Int. J. Coal Geol. 2000, 43, 53–82. [Google Scholar] [CrossRef]
- Herazo, A.; Reich, M.; Barra, F.; Morata, D.; Real, I.D.; Pagès, A. Assessing the role of bitumen in the formation of stratabound Cu-(Ag) deposits: Insights from the Lorena deposit, Las Luces district, northern Chile. Ore Geol. Rev. 2020, 124, 103639. [Google Scholar] [CrossRef]
- Dissanayake, C.B. Gold and other metals in graphite. In Bitumen in Ore Deposits. Society for Geology Applied to Mineral Deposits; Parnell, J., Kucha, H., Landais, P., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; Volume 9, pp. 138–152. [Google Scholar] [CrossRef]
- Winchester, J.A.; Floyd, P.A. Geochemical discrimination of different magma series and their differentiation products using immobile elements. Chem. Geol. 1977, 20, 325–343. [Google Scholar] [CrossRef]
- Hasite, A.R.; Kerr, A.C.; Pearce, J.A.; Mitchell, S.F. Classification of altered volcanic island arc rocks using immobile trace elements: Development of the Th-Co discrimination diagram. J. Petrol. 2007, 48, 2341–2357. [Google Scholar] [CrossRef]
- Pearce, J.A. Role of the Sub-Continental Lithosphere in Magma Genesis at Active Continental Margins. In Continental Basalts and Mantle Xenoliths; Hawkesworth, C.J., Norry, M.J., Eds.; Shiva: Cheshire, UK, 1983; pp. 230–249. [Google Scholar]
- Harker, A. The Natural History of Igneous Rocks; Cambridge University Press: Cambridge, UK, 1909. [Google Scholar] [CrossRef]
- Wilson, M. Igneous Petrogenesis; Unwin Hyman: London, UK, 2007; 461p. [Google Scholar]
- Feely, T.C.; Cosca, M.A.; Lindsay, C.R. Petrogenesis and implications of calc-alkaline cryptic hybrid magmas from Washburn volcano, Absaroka volcanic province, U.S.A. J. Petrol. 2002, 43, 663–703. [Google Scholar] [CrossRef]
- Davidson, J.; Turner, S.; Handley, H.; Macpherson, C.; Dosseto, A. Amphibole “sponge” in arc crust? Geology 2007, 35, 787–790. [Google Scholar] [CrossRef]
- Schiano, P.; Monzier, M.; Eissen, J.P.; Martin, H.; Koga, K.T. Simple mixing as the major control of the evolution of volcanic suites in the Ecuadorian Andes. Contrib. Mineral. Petrol. 2010, 160, 297–312. [Google Scholar] [CrossRef]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Pearce, J.A. A user’s guide to basalt discrimination diagrams. In Trace Element Geochemistry of Volcanic Rocks: Applications for Massive Sulfide Exploration; Wyman, D.A., Ed.; Geological Association of Canada: St. John’s, NL, Canada, 1996; Volume 12, pp. 79–113. [Google Scholar]
- Stepanov, A.; Mavrogenes, J.A.; Meffre, S.; Davidson, P. The key role of mica during igneous concentration of tantalum. Contrib. Miner. Petrol. 2014, 167, 1009. [Google Scholar] [CrossRef]
- Klimm, K.; Holtz, F.; King, P.L. Fractionation vs. magma mixing in the Wangrah suite A-type granites, Lachlan Fold Belt, Australia, experimental constraints. Lithos 2008, 102, 415–434. [Google Scholar] [CrossRef]
- Boynton, W.V. Cosmochemistry of the rare earth elements, Meteorite studies. In Rare Earth Element Geochemistry; Developments in Geochemistry; Elsevier: Amsterdam, The Netherlands, 1984; pp. 115–1522. [Google Scholar] [CrossRef]
- Sheppherd, T.J.; Rankin, A.H.; Alderton, D.H.M. A Practical Guide to Fluid Inclusion Studies; Blackie and Son: Glasgow, Scotland, 1985; 239p. [Google Scholar]
- Hedenquist, J.W.; Henley, R.W. The important of CO2 on freezing point measurements of fluid inclusion: Evidence from active geothermal system and implication for epithermal ore deposition. Econ. Geol. 1985, 80, 1379–1406. [Google Scholar] [CrossRef]
- Kant, W.; Warmada, W.; Idrus, A.; Setijadji, L.D.; Watanabe, K. Fluid inclusion study of the polymetalic epithermal quartz veins at Soripesa Prospect Area, Sumbawa island, Indonesia. J. Appl. Geol. 2012, 4, 77–89. [Google Scholar] [CrossRef]
- Wilkinson, J.J. Fluid Inclusions in Hydrothermal Ore Deposits. Lithos 2001, 55, 229–272. [Google Scholar] [CrossRef]
- Haas, J.L. The effect of salinity on the maximum thermal gradient of a hydrothermal system at hydrostatic pressure. Econ. Geol. 1971, 66, 940–946. [Google Scholar] [CrossRef]
- Pirajno, F. Hydrothermal Mineral Deposits, Principle and Fundamental Concept for the Exploration Geologist; Springer: Berlin/Heidelberg, Germany, 2009; 706p. [Google Scholar]
- Kesler, S.E. Ore-forming fluids. Elements 2005, 1, 13–18. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Frantz, J.D. Determination of the homogenisation temperatures and densities of superficial fluids in the system NaCl-KCl-CaCl2-H2O using synthetic f luids inclusions. Chem. Geol. 1987, 64, 335–345. [Google Scholar] [CrossRef]
- Bodnar, R.J. A method of calculating fluid inclusion volumes based on vapor bubble diameters and P-V-T-X properties on inclusion fluids. Econ. Geol. 1983, 78, 534–542. [Google Scholar] [CrossRef]
- Lattanzi, P. Applications of fluid inclusions in the study and exploration of mineral deposits. Eur. J. Miner. 1991, 3, 689–697. [Google Scholar] [CrossRef]
- Beane, R.E. The magmatic-meteoric transition. Geotherm. Resour. Counc. Spec. Rep. 1983, 13, 245–253. [Google Scholar]
- Hoefs, J. Stable Isotope Geochemistry; Springer: Heidelberg, Germany, 2004; 287p. [Google Scholar] [CrossRef]
- Méheut, M.; Lazzeri, M.; Balan, E.; Mauri, F. Equilibrium isotopic fractionation in the kaolinite, quartz, water system: Prediction from first-principles density-functional theory. Geochim. Cosmochim. Acta 2007, 71, 3170–3181. [Google Scholar] [CrossRef]
- Li, Y.B.; Liu, J.M. Calculation of sulfur isotope fractionation in sulfides. Geochim. Cosmochim. Acta 2006, 70, 1789–1795. [Google Scholar] [CrossRef]
- Ohmoto, H.; Goldhaber, M.B. Sulfur and Carbon Isotope. In Geochemistry of Hydrothermal Ore Deposits, 3rd ed.; Barnes, H.L., Ed.; John Wiley & Sons: New York, NY, USA, 1997; pp. 517–611. [Google Scholar]
- Marini, L.; Moretti, R.; Accornero, M. Sulfur Isotopes in Magmatic-Hydrothermal Systems, Melts, and Magmas. In Sulfur in Magmas and Melts: Its Importance for Natural and Technical Processes; Behrens, H., Webster, J., Eds.; De Gruyter: Berlin, Germany, 2011; pp. 423–492. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry, 8th ed.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Fazli, N.; Ghaderi, M.; Movahednia, M.; Li, J.W.; Lentz, D.R.; Yan, S. Geology and genesis of the North Narbaghi Cu-Ag deposit in the Urumieh-Dokhtar magmatic arc, Iran: Fluid inclusion and stable isotope constraints. Ore Geol. Rev. 2022, 144, 104801. [Google Scholar] [CrossRef]
- Zamanian, H.; Dolatshahi, S.; Yang, X.; Somarin, A.K.; Meshkani, S.A. Geochemical, fluid inclusion and O-H-S isotope constraints on the origin of the Rangraz copper deposit, Central Iran. Ore Geol. Rev. 2021, 128, 103877. [Google Scholar] [CrossRef]
- Zamanian, H.; Tale Fazel, E.; Sameti, M.; Asadi Haroni, H.; Yang, X. The petrogenesis and metallogenesis of the Kalchuyeh epithermal gold deposit, central Iran: Constraints from geochemistry, fluid inclusion, and H–O–S isotopes. J. Asian Earth Sci. 2023, 242, 105505. [Google Scholar] [CrossRef]
- Latifi Saii, F.; Mirnejad, H.; Alipour Asl, M.; Niroomand, S. Evaluating the Au mineralization in the Darrehzar vein system (Pariz area—Kerman Province), with emphasis on fluid inclusion and sulfur isotope studies. Adv. Appl. Geol. 2014, 4, 65–75. [Google Scholar]
- Downes, P.M. Yerranderie, a Late Devonian silver–gold–lead intermediate sulfidation epithermal district, eastern Lachlan Orogen, New South Wales, Australia. Resour. Geol. 2007, 57, 1–23. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Li, Y. Genesis of the Zhijiadi Ag–Pb–Zn Deposit, Central North China Craton: Constraints from Fluid Inclusions and Stable Isotope Data. Geofluids 2017, 2017, 4153618. [Google Scholar] [CrossRef]
- Niu, S.-D.; Li, S.-R.; Santosh, M.; Zhang, D.-H.; Li, Z.-D.; Shan, M.-J.; Lan, Y.-X.; Gao, D.-R. Mineralogical and isotopic studies of base metal sulfides from the Jiawula Ag–Pb–Zn deposit, Inner Mongolia, NE China. J. Asian Earth Sci. 2016, 115, 480–494. [Google Scholar] [CrossRef]
- Mango, H.; Oreskes, N.; Zantop, H. Origin of epithermal Ag–Au–Cu–Pb–Zn mineralization in Guanajuato, Mexico. Miner. Depos. 2013, 49, 119–143. [Google Scholar] [CrossRef]
- Rice, C.M.; McCoyd, R.J.; Boyce, A.J.; Marchev, P. Stable isotope study of the mineralization and alteration in the Madjarovo Pb–Zn district, south-east Bulgaria. Miner. Depos. 2007, 42, 691–713. [Google Scholar] [CrossRef]
- Yilmaz, H.; Oyman, T.; Nuran Sonmez, F.; Arehart, G.; Zeki, B. Intermediate sulfidation epithermal gold-base metal deposits in Tertiary subaerial volcanic rocks, Sahinli/Tespih Dere (Lapseki/Western Turkey). Ore Geol. Rev. 2010, 37, 236–258. [Google Scholar] [CrossRef]
- Goudarzi, M.; Zamanian, H.; Klötzli, U.; Lentz, D.; Ullah, M. Genesis of the Mamuniyeh Copper Deposit in the Central Urumieh-Dokhtar Magmatic Arc, Iran: Constraints from Geology, Geochemistry, Fluid Inclusions, and H–O–S Isotopes. Ore Geol. Rev. 2024, 175, 106279. [Google Scholar] [CrossRef]
- Rollinson, H. Rising Geochemical Data: Evaluation, Presentation, Interpretation; Longman John Wiley and Sons: New York, NY, USA, 1993; 352p. [Google Scholar]
- Allègre, C.J. Isotope Geology; Sutcliffe, C., Translator; Cambridge University Press: Cambridge, UK, 2008; ISBN 978-0-521-86228-8. [Google Scholar] [CrossRef]
- Seal, R.R. Sulfur Isotope Geochemistry of Sulfide Minerals. Rev. Mineral. Geochem. 2006, 61, 633–677. [Google Scholar] [CrossRef]
- Barnes, H.L. Geochemistry of Hydrothermal Ore Deposite; Sulfur and Carbon Isotope, Ohmoto and Goldbar; John Wiley & Sons: New York, NY, USA, 1979; Chapter 11. [Google Scholar]
- Movahednia, M.; Maghfouri, S.; Fazli, N.; Rastad, E.; Ghaderi, M.; González, F.J. Metallogeny of Manto-type stratabound Cu-(Ag) mineralization in Iran: Relationship with NeoTethyan evolution and implications for future exploration. Ore Geol. Rev. 2022, 149, 105064. [Google Scholar] [CrossRef]
- Sasaki, A.; Ulriksen, C.E.; Sato, K.; Ishihara, S. Sulfur isotope reconnaissance of porphyry copper and Manto-type deposits in Chile and the Philippines. Bull. Geol. Surv. Jpn. 1984, 35, 615–622. [Google Scholar]
- Puig, A.; Spiro, B. The source of sulphur in polymetallic deposits in the Cretaceous magmatic arc, Chilean Andes. J. South Am. Earth Sci. 1988, 1, 261–266. [Google Scholar] [CrossRef]
- Munizaga, F.; Reyes, J.C.; Nystrom, J.O. Razones isotópicas de los sulfuros del distrito minero de Cerro Negro: Un posible indicador de los dep´ositos estratoligados de Cu hospedados en rocas sedimentarias lacustres. Andean Geol. 1994, 21, 189–195. [Google Scholar]
- Munizaga, F.; Zentilli, M. Sulphur Isotope Characterization of Stratabound Copper Deposits in Chile; Comucicaciones, Universidad de Chile: Santiago, Chile, 1994; Volume 45, pp. 127–134. [Google Scholar]
- Saric, N.; Kreft, C.; Huete, C. Geología del yacimiento Lo Aguirre, Chile. Rev. Geol. Chile 2003, 30, 317–331. [Google Scholar] [CrossRef]
- Wilson, N.S.F.; Zentilli, M.; Reynolds, P.H. Age of mineralization by basinal f luids at the El Soldado Manto-type Cu deposit, Chile: 40 feldspar. Chem. Geol. 2003, 197, 161–176. [Google Scholar] [CrossRef]
- Wilson, N.S.F.; Zentilli, M. Association of pyrobitumen with copper mineralization from the Uchumi and Talcuna districts, central Chile. Int. J. Coal Geol. 2006, 65, 158–169. [Google Scholar] [CrossRef]
- Cai, Y.T.; Ni, P.; Wang, G.G.; Pan, J.Y.; Zhu, X.T.; Chen, H.; Ding, J.Y. Fluid inclusion and H-O–S–Pb isotopic evidence for the Dongxiang Manto-type copper deposit, South China. J. Geochem. Explor. 2016, 171, 71–82. [Google Scholar] [CrossRef]
- Shen, P.; Pan, H.; Li, Z.; Sun, J.; Shen, Y.; Li, C.; Feng, H.; Cao, C. A Manto-type Cu deposit in the Central Asian Orogenic Belt: The Hongguleleng example (Xinjiang, China). Ore Geol. Rev. 2020, 124, 103656. [Google Scholar] [CrossRef]
- Zheng, Y.F. Calculation of oxygen isotope fractionation in hydroxyl-bearing silicates. Earth Planet. Sci. Lett. 1993, 120, 247–263. [Google Scholar] [CrossRef]
- Rollinson, H.R. Using Geochemical Data: Evaluation, Presentation, Interpretation; Mor, F.; Madadri, S., Translators; Routledge: Oxfordshire, UK, 2005. [Google Scholar]
- Shafaii Moghadam, H.; Hua Li, X.; Ling, X.X.; Santos, J.F.; Stern, R.J.; Li, Q.L.; Ghorbani, G. Eocene Kashmar granitoids (NE Iran): Petrogenetic constraints from U-Pb zircon geochronology and isotope geochemistry. Lithos 2015, 216, 118–135. [Google Scholar] [CrossRef]
- Ghasemi, H.; Rezaei-Kahkhaei, M. Petrochemistry and tectonic setting of the Davarzan-Abbasabad Eocene Volcanic (DAEV) rocks, NE Iran. Mineral. Petrol. 2015, 109, 235–252. [Google Scholar] [CrossRef]
- Berberian, M.; King, G.C.P. Towards a paleogeography and tectonic evolution of Iran. Can. J. Earth Sci 1981, 18, 210–265. [Google Scholar] [CrossRef]
- Ghasemi, A.; Talbot, C.J. A new tectonic scenario for the Sanandaj-Sirjan Zone (Iran). J. Asian Earth Sci. 2006, 26, 683–693. [Google Scholar] [CrossRef]
- Asiabanha, A.; Bardintzeff, J.M.; Kananian, A.; Rahimi, G. Post-Eocene volcanics of the Abazar district, Qazvin, Iran: Mineralogical and geochemical evidence for a complex magmatic evolution. J. Asian Earth Sci. 2012, 45, 79–94. [Google Scholar] [CrossRef]
- Asiabanha, A.; Foden, J. Post Collisional transition from an extensional volcano sedimentary basin to a continental arc in the Alborz Ranges, N Iran. Lithos 2012, 148, 98–111. [Google Scholar] [CrossRef]


| Sample | SiO2 | Al2O3 | Na2O | MgO | K2O | TiO2 | CaO | Fe2O3 | Ag | Pb | Cu | Mo | Bi | Fe | Co | Ni | Ti | Sb | Tl | Li | Be | Na | K | Ca | Mg | Al | Ga | Cs | Rb | Ba | Sr | Th | U | Y | Zr | Hf | Nb | Ta | P | La | Ce | Pr | Nd | Sm | Eu | Er | Gd | Tb | Dy | Ho | Tm | Yb | Lu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Se.Sa-12A | 64/05 | 18 | 3/9 | 0/46 | 3/66 | 0/58 | 2/95 | 5/17 | 0/6 | 15 | 1068 | 2/2 | 0/05 | 35,794 | 8/2 | 29 | 4456 | 1/1 | 0/55 | 11 | 4/9 | 31,348 | 34,626 | 24,966 | 2871 | 7465 | 15/2 | 4/8 | 94 | 613 | 316 | 4/35 | 1/2 | 14/4 | 119 | 3/08 | 5/5 | 0/4 | 1090 | 13 | 30 | 3/93 | 13/7 | 2/98 | 1 | 1/66 | 3/26 | 0/47 | 3/07 | 0/39 | 0/27 | 1/8 | 0/29 |
| Se.Sa-13A | 63/9 | 17/69 | 3/76 | 0/26 | 3/17 | 0/63 | 2/68 | 7/07 | 31/9 | 23 | >5% | 3/8 | 0/05 | 35,658 | 4/2 | 18 | 3139 | 2/2 | 0/41 | 10 | 3/9 | 27,389 | 28,248 | 18,024 | 1856 | 6497 | 15/2 | 3/9 | 79 | 420 | 315 | 4/29 | 1/5 | 14/5 | 18 | 3/12 | 4/6 | 0/31 | 1237 | 11 | 26 | 3/7 | 12/6 | 2/89 | 0/95 | 1/61 | 3/26 | 0/46 | 3/05 | 0/39 | 0/27 | 1/7 | 0/26 |
| Se.Sa-15A | 57/85 | 16/75 | 3/84 | 0/01 | 4/12 | 0/74 | 2/31 | 5/38 | >10 | 38 | >1000 | 6/82 | 0/46 | 41,000 | 4/9 | 13/8 | 3800 | 1/33 | 0/6 | 15/8 | 1/1 | 32,600 | 35,200 | 13,500 | 2400 | 2/66 | 15/2 | 5/39 | 74/3 | 254 | 132 | 2/7 | 0/3 | 8/7 | 109 | 3/78 | 4/7 | 0/46 | 1000 | 4/6 | 9/91 | 1/35 | 5/8 | 1/5 | 0/6 | 1/3 | 0 | 0/27 | 1/85 | 0/39 | 0/21 | 1/4 | 0/23 |
| Se.Sa-16A | 61/25 | 17/16 | 5/38 | 1/46 | 3/67 | 0/63 | 5/71 | 4/2 | 0/42 | 22 | 541 | 3/01 | 0/16 | 37,900 | 8/4 | 19/6 | 4000 | 0/58 | 0/51 | 22/6 | 1/4 | 36,600 | 36,200 | 47,700 | 3900 | 4/17 | 18/6 | 5/67 | 78/2 | 368 | 223 | 3/9 | 0/3 | 18 | 128 | 3/65 | 5/4 | 0/43 | 1000 | 8/9 | 19/5 | 2/6 | 11 | 3/02 | 1/04 | 2/34 | 0 | 0/5 | 3/37 | 0/68 | 0/33 | 2/3 | 0/36 |
| Se.Sa-17A | 60/75 | 16/85 | 4/99 | 0/05 | 4/66 | 0/63 | 6/81 | 4/92 | 0/43 | 20 | 575 | 3/75 | 0/12 | 34,700 | 6/2 | 16/2 | 3900 | 0/84 | 0/58 | 18/5 | 1/2 | 35,700 | 38,100 | 34,500 | 1400 | 2/56 | 17/3 | 6/69 | 101 | 400 | 184 | 3/2 | 0/2 | 15 | 110 | 3/15 | 4/9 | 0/37 | 800 | 5/6 | 13/5 | 1/82 | 8/02 | 2/35 | 0/92 | 1/77 | 0 | 0/42 | 2/78 | 0/57 | 0/3 | 1/8 | 0/3 |
| Se.Sa-17B | 59/64 | 18/25 | 5/15 | 1/66 | 3/75 | 0/66 | 6/01 | 4/69 | 0/44 | 22 | 458 | 3/25 | 0/14 | 32,000 | 4/2 | 17/3 | 3400 | 0/84 | 0/56 | 17/3 | 1/5 | 35,723 | 37,512 | 35,000 | 1290 | 2/36 | 16/2 | 6/78 | 122 | 359 | 190 | 3/2 | 0/2 | 15 | 110 | 3/14 | 5/3 | 0/25 | 900 | 6/1 | 12/5 | 1/73 | 9/26 | 2/36 | 0/77 | 1/8 | 0 | 0/32 | 2/45 | 0/58 | 0/21 | 2 | 0/23 |
| Se.Sa-18A | 61/35 | 18/7 | 5/97 | 0/05 | 4/97 | 0/71 | 5/23 | 3/18 | 0/5 | 14 | 1068 | 2/1 | 0/05 | 3421 | 2/3 | 29 | 3215 | 1/5 | 0/39 | 10 | 3/9 | 30,221 | 36,620 | 23,230 | 2781 | 7365 | 15 | 4 | 802 | 512 | 312 | 3/9 | 1/3 | 14 | 100 | 3/04 | 5/2 | 0/26 | 1032 | 12 | 32 | 2/9 | 12/8 | 2/7 | 1 | 1/56 | 0 | 0/41 | 2/8 | 0/34 | 0/24 | 1/6 | 0/29 |
| Se.Sa-18B | 63/85 | 18/63 | 4/75 | 0/35 | 4/08 | 0/55 | 2/68 | 6/07 | 0/14 | 17 | 397 | 2/79 | 0/11 | 32,200 | 9/9 | 18/7 | 0/44 | 1/84 | 0/56 | 20/6 | 1/3 | 29,000 | 42,100 | 32,220 | 4100 | 3450 | 17/8 | 5/51 | 86/1 | 398 | 173 | 4/5 | 0/2 | 20/6 | 142 | 4/15 | 6 | 0/48 | 1000 | 7/4 | 18 | 2/31 | 10/8 | 3/24 | 1/02 | 2/5 | 3/39 | 0/52 | 3/3 | 0/74 | 0/32 | 2/5 | 0/41 |
| Se.Sa-01 | 63/3 | 18/51 | 4/65 | 0/43 | 4/01 | 0/61 | 2/64 | 5/64 | 0/1 | 2 | 54 | 2 | 0/1 | 38,519 | 20 | 31 | 3776 | <0.5 | <0.1 | 14 | 0/2 | 22,946 | 7106 | 47,451 | >2% | 75,884 | 15/1 | 0/7 | 13 | 126 | 197 | 0/91 | 0/3 | 15/5 | 48 | 1/75 | 8/1 | 0/83 | 407 | 6 | 7 | 1 | 6/9 | 0/22 | 0/67 | 1/34 | 2/16 | 0/39 | 2/79 | 0/39 | 0/3 | 1/9 | 0/31 |
| Se.Sa-02 | 61/9 | 17/36 | 5/05 | 0/27 | 3/56 | 0/58 | 4/85 | 4/15 | 0/1 | 4 | 40 | 0/1 | 0/1 | 47,492 | 23/5 | 37 | 4245 | <0.5 | <0.1 | 12 | 0/2 | 23,126 | 6382 | 53,087 | >2% | 81,673 | 15/4 | 1 | 10 | 105 | 272 | 0/84 | 0/4 | 15/5 | 49 | 1/81 | 4/6 | 0/57 | 523 | 7 | 7 | 1/33 | 7/4 | 0/21 | 0/66 | 1/29 | 2/23 | 0/39 | 3/07 | 0/39 | 0/29 | 2/1 | 0/3 |
| Se.Sa-03 | 60/85 | 16/65 | 4/86 | 0/05 | 4/61 | 0/6 | 6/45 | 4/73 | 0/1 | 4 | 63 | 0/1 | 0/1 | 48,663 | 24/6 | 38 | 3605 | 1 | 0/16 | 3 | 0/2 | 19,532 | 2373 | 55,661 | >2% | 82,422 | 15/8 | 1/7 | 6 | 61 | 141 | 0/28 | 0/2 | 17/2 | 42 | 1/64 | 4/9 | 0/6 | 278 | 4 | 4 | 0/66 | 4/7 | 0/02 | 0/43 | 1/57 | 2/1 | 0/42 | 3/33 | 0/65 | 0/31 | 2/1 | 0/32 |
| Se.Sa-04 | 60/55 | 16/85 | 4/91 | 0/06 | 4/15 | 0/62 | 6/15 | 4/68 | 0/1 | 9 | 6186 | 0/1 | 0/1 | 37,322 | 10 | 46 | 2650 | <0.5 | <0.1 | <1 | 0/2 | 1761 | 244 | >10% | 7241 | 89,852 | 15/7 | <0/5 | <1 | 14 | 43/7 | 0/68 | 0/9 | 9/3 | 28 | 1/29 | 6/4 | 0/58 | 397 | 8 | 7 | 1/1 | 5 | 0/02 | 0/31 | 0/81 | 1/97 | 0/31 | 2/1 | 0/58 | 0/2 | 1/4 | 0/18 |
| Se.Sa-05 | 59/85 | 18/15 | 4/94 | 1/45 | 3/74 | 0/65 | 6/82 | 4/72 | 0/7 | 32 | 284 | 0/1 | 0/1 | 45,855 | 25/4 | 52 | 3746 | <0.5 | 0/15 | 6 | 0/2 | 25,505 | 3146 | 48,663 | >2% | 86,485 | 16 | 0/9 | 3 | 60 | 196 | <0.1 | 0/2 | 11/8 | 33 | 1/32 | 5/4 | 0/53 | 195 | 3 | 1 | 0/28 | 2/5 | 0/02 | 0/35 | 1/19 | 1/79 | 0/32 | 2/41 | 0/57 | 0/22 | 1/7 | 0/21 |
| Se.Sa-06 | 62/75 | 16/95 | 4/7 | 0/85 | 3/64 | 0/66 | 2/95 | 4/33 | 0/1 | 16 | 153 | 0/1 | 0/1 | 45,710 | 26 | 54 | 3712 | <0.5 | <0.1 | 7 | 0/2 | 24,429 | 3515 | 54,628 | >2% | 88,529 | 16/1 | 0/9 | 4 | 61 | 182 | <0.1 | 0/2 | 12/9 | 33 | 1/36 | 4/5 | 0/5 | 205 | 3 | 2 | 0/28 | 2/4 | 0/02 | 0/33 | 1/13 | 1/78 | 0/32 | 2/46 | 0/58 | 0/21 | 1/8 | 0/17 |
| Se.Sa-07 | 63/9 | 17/35 | 3/84 | 0/46 | 4/01 | 0/6 | 2/63 | 6/1 | 0/1 | 3 | 40 | 0/1 | 0/1 | 27,859 | 5/1 | 5 | 2572 | <0.5 | 0/1 | 18 | 0/2 | 26,564 | 16,425 | 17,194 | 9009 | 70,541 | 15/9 | 1/8 | 42 | 92 | 169 | 0/33 | 0/4 | 35/7 | 62 | 2/16 | 4/1 | 0/39 | 590 | 6 | 8 | 1/46 | 7/8 | 0/48 | 0/8 | 2/86 | 2/93 | 0/67 | 5/06 | 0/64 | 0/54 | 3/3 | 0/53 |
| Se.Sa-08 | 64/1 | 18/24 | 3/95 | 0/48 | 3/59 | 0/58 | 2/24 | 5/08 | 0/1 | 4 | 138 | 0/1 | 0/1 | 54,893 | 40/6 | 299 | 3802 | <0.5 | <0.1 | 17 | 0/2 | 15,347 | 8660 | 66,205 | >2% | 68,084 | 15/6 | 2/5 | 17 | 110 | 330 | 0/71 | 0/3 | 13/1 | 37 | 1/54 | 5/6 | 0/62 | 656 | 7 | 8 | 1/43 | 7/2 | 0/26 | 0/7 | 1 | 2/36 | 0/39 | 2/88 | 0/43 | 0/2 | 1/8 | 0/23 |
| Se.Sa-09 | 64 | 18/52 | 4/15 | 0/49 | 3/68 | 0/57 | 2/18 | 5/04 | 0/1 | 7 | 29 | 1 | 0/1 | 54,821 | 39/5 | 282 | 3446 | <0.5 | <0.1 | 32 | 0/2 | 12,006 | 7428 | 48,237 | >2% | 62,404 | 16/3 | 1/1 | 9 | 105 | 264 | 0/58 | 0/34 | 11/6 | 34 | 1/34 | 5/2 | 0/66 | 619 | 7 | 8 | 1/44 | 7/4 | 0/12 | 0/66 | 1/13 | 2/13 | 0/36 | 2/51 | 0/42 | 0/23 | 1/6 | 0/17 |
| Se.Sa-10 | 62/8 | 17/15 | 4/86 | 0/64 | 3/62 | 0/63 | 2/9 | 4/3 | 0/1 | 6 | 19 | 0/1 | 0/1 | 53,398 | 41/8 | 329 | 3458 | <0.5 | <0.1 | 33 | 0/2 | 12,023 | 7004 | 64,240 | >2% | 59,380 | 16/7 | 1/4 | 13 | 89 | 274 | 0/47 | 0/2 | 11/3 | 33 | 1/33 | 4/9 | 0/49 | 594 | 7 | 7 | 1/43 | 7/9 | 0/02 | 0/6 | 1 | 2/29 | 0/36 | 2/5 | 0/71 | 0/17 | 1/6 | 0/21 |
| Se.Sa-11 | 61/65 | 17/25 | 5/03 | 1/15 | 3/61 | 0/61 | 4/86 | 4/72 | 0/1 | 5 | 25 | 0/1 | 0/1 | 48,511 | 44/4 | 467 | 2298 | <0.5 | <0.1 | 39 | 0/2 | 2871 | 3508 | 42,747 | >2% | 34,053 | 16/6 | 0/7 | <1 | 55 | 121 | 0/2 | 0/3 | 6/9 | 21 | 1/02 | 3/7 | 0/34 | 373 | 4 | 3 | 0/66 | 3/7 | 0/02 | 0/36 | 0/67 | 1/76 | 0/24 | 1/97 | 0/67 | 0/1 | 1 | 0/15 |
| Se.Sa-12 | 60/75 | 17/45 | 5/14 | 1/04 | 3/74 | 0/59 | 4/74 | 4/66 | 0/1 | 3 | 16 | 0/1 | 0/1 | 52,377 | 49/6 | 506 | 2671 | <0.5 | 0/1 | 40 | 0/2 | 4524 | 1614 | 40,212 | >2% | 42,514 | 16/3 | 4/7 | 2 | 20 | 89/4 | 0/39 | 0/3 | 8/7 | 25 | 1/09 | 3/8 | 0/36 | 432 | 5 | 5 | 0/86 | 4/3 | 0/02 | 0/23 | 0/76 | 1/92 | 0/32 | 2/18 | 0/65 | 0/12 | 1/3 | 0/24 |
| Sample N. | Mineralization Type | Mineral | Phase | Tice | TICE+ | Salinity | Th V-L | Th L-V | Tutec | THT |
|---|---|---|---|---|---|---|---|---|---|---|
| S4 | Mlc + Az + Cal | Calcite | L + V | (−2)–(−5/5) | 2/0–5/5 | 3/38–9/09 | 135–210 | −36/3 | 135–210 | |
| S7 | Mlc + Az + Cal | Calcite | L + V | (−2/7)–(9/2) | 2/7–9/2 | 4/48–12/63 | 176–330 | −43 | 176–330 | |
| S7 | Mlc + Az + Cal | Calcite | V + L | (−7/8)–(−8/20) | 7/8–8/2 | 11/19–11/6 | 265–341 | 265–341 | ||
| S6 | Cct + Cal | Calcite | L + V | (−1/8)–(−9/4) | 1/8–9/4 | 3/08–13/17 | 90–386 | −49/6 | 90–386 | |
| S9 | Cct + Cal | Calcite | L + V | (−2)–(−5) | 2/0–5/0 | 3/39–7/86 | 149–275 | −37/2 | 149–275 | |
| S9 | Cct + Cal | Calcite | V + L | (−5/7)–(−5/8) | 5/7–5/8 | 8/76–8/98 | 280–300 | 280–300 | ||
| S15 | Cct + Bn + Ccp + Mag + Qtz | Quartz | L + V | (−2)–(−9/6) | 2/0–9/6 | 3/39–13/38 | 100–234 | −39 | 100–234 | |
| S15 | Cct + Bn + Ccp + Mag + Qtz | Quartz | L + V + S | −8 | 8 | 11/55 | 280 | 280 |
| Chalcopyrite <=> H2S [75] | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample Point | Mineral | δ34Smin (%) | T (°C)-Min | T (°C)-Ave | T (°C)-Max | 1000 Ln α-T(min) | 1000 Ln α-T(Ave) | 1000 Ln α-T(max) | δ34SH2Sfluid (‰) |
| Se-Sa-S8 | Chalcocite | −2/9 ± 0/2 | |||||||
| Se-Sa-S14 | Chalcocite-Malachite-Azurite | −7/1 ± 0/0 | |||||||
| Se-Sa-S15-2 | Chalcocite-Bornite-Chalcopyrite-Covellite-Silica(Quartz) | −23/9 ± 0/3 | 100 | 193/69 | 280 | 0/4 | 0/2 | 0/2 | −24/1 ± 0/3 |
| Galena <=> H2S [75] | |||||||||
| Sample Point | Mineral | δ34Smin(%) | T (°C)-Min | T (°C)-Ave | T (°C)-Max | 1000 Ln α-T(min) | 1000 Ln α-T(Ave) | 1000 Ln α-T(max) | δ34SH2Sfluid (‰) |
| Se-Sa-S8 | Chalcocite | −2/9 ± 0/2 | |||||||
| Se-Sa-S14 | Chalcocite-Malachite-Azurite | −7/1 ± 0/0 | |||||||
| Se-Sa-S15-2 | Chalcocite-Bornite-Chalcopyrite-Covellite-Silica(Quartz) | −23/9 ± 0/3 | 100 | 193/69 | 280 | −4/6 | −2/9 | −2/1 | −21/0 ± 0/3 |
| Galena <=> Chalcopyrite [75] | |||||||||
| Sample Point | Mineral | δ34Smin(%) | T (°C)-Min | T (°C)-Ave | T (°C)-Max | 1000 Ln α-T(min) | 1000 Ln α-T(Ave) | 1000 Ln α-T(max) | δ34SH2Sfluid (‰) |
| Se-Sa-S8 | Chalcocite | −2/9 ± 0/2 | |||||||
| Se-Sa-S14 | Chalcocite-Malachite-Azurite | −7/1 ± 0/0 | |||||||
| Se-Sa-S15-2 | Chalcocite-Bornite-Chalcopyrite-Covellite-Silica(Quartz) | −23/9 ± 0/3 | 100 | 193/69 | 280 | −3/3 | −1/5 | −0/6 | −22/4 ± 0/3 |
| Muscovite <=> H2O [104] | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample Point | Mineral | δ18Omin(%) | T (°C)-Min | T (°C)-Ave | T (°C)-Max | 1000 Ln α-T(min) | 1000 Ln α-T(Ave) | 1000 Ln α-T(max) | δ18OH2Ofluid (%) |
| Se.Sa-S6 | Silica(Quartz) | +7/5 ± 0/0 | 90 | 177/5 | 386 | 12/4 | 5/6 | 0/1 | +1/9 ± 0/0 |
| Se.Sa-S15-1 | Chalcocite-Bornite-Chalcopyrite- Covellite-Silica(Quartz) | +7/6 ± 0/3 | 100 | 193/69 | 280 | 11/3 | 4/8 | 1/9 | +2/8 ± 0/3 |
| Quartz <=> H2O [74] | |||||||||
| Sample Point | Mineral | δ18Omin(%) | T (°C)-Min | T (°C)-Ave | T (°C)-Max | 1000 Ln α-T(min) | 1000 Ln α-T(Ave) | 1000 Ln α-T(max) | δ18OH2Ofluid (%) |
| Se.Sa-S6 | Silica(Quartz) | +7/5 ± 0/0 | 90 | 177/5 | 386 | 24/4 | 13/5 | 2/8 | −6/0 ± 0/0 |
| Se.Sa-S15-1 | Chalcocite-Bornite-Chalcopyrite- Covellite-Silica(Quartz) | +7/6 ± 0/3 | 100 | 193/69 | 280 | 22/9 | 12/1 | 6/5 | −4/5 ± 0/3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esform, M.; Zamanian, H.; Klötzli, U.; Zarasvandi, A.; Almasi, A.; Goudarzi, M. Evolutionary Model of the Sepid-Sarve Manto-Type Copper Mineralization, Doruneh Fault Volcanic-Plutonic Belt (Central Iran Domain, NE Iran): An Integrated Geological, Geochemical, Fluid-Inclusion and Stable O–S Isotope Study. Minerals 2025, 15, 1246. https://doi.org/10.3390/min15121246
Esform M, Zamanian H, Klötzli U, Zarasvandi A, Almasi A, Goudarzi M. Evolutionary Model of the Sepid-Sarve Manto-Type Copper Mineralization, Doruneh Fault Volcanic-Plutonic Belt (Central Iran Domain, NE Iran): An Integrated Geological, Geochemical, Fluid-Inclusion and Stable O–S Isotope Study. Minerals. 2025; 15(12):1246. https://doi.org/10.3390/min15121246
Chicago/Turabian StyleEsform, Morteza, Hasan Zamanian, Urs Klötzli, Alireza Zarasvandi, Alireza Almasi, and Mohammad Goudarzi. 2025. "Evolutionary Model of the Sepid-Sarve Manto-Type Copper Mineralization, Doruneh Fault Volcanic-Plutonic Belt (Central Iran Domain, NE Iran): An Integrated Geological, Geochemical, Fluid-Inclusion and Stable O–S Isotope Study" Minerals 15, no. 12: 1246. https://doi.org/10.3390/min15121246
APA StyleEsform, M., Zamanian, H., Klötzli, U., Zarasvandi, A., Almasi, A., & Goudarzi, M. (2025). Evolutionary Model of the Sepid-Sarve Manto-Type Copper Mineralization, Doruneh Fault Volcanic-Plutonic Belt (Central Iran Domain, NE Iran): An Integrated Geological, Geochemical, Fluid-Inclusion and Stable O–S Isotope Study. Minerals, 15(12), 1246. https://doi.org/10.3390/min15121246