Adsorption of Calcium Ions on Calcite Surface and Its Influence on Flotation Separation of Scheelite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
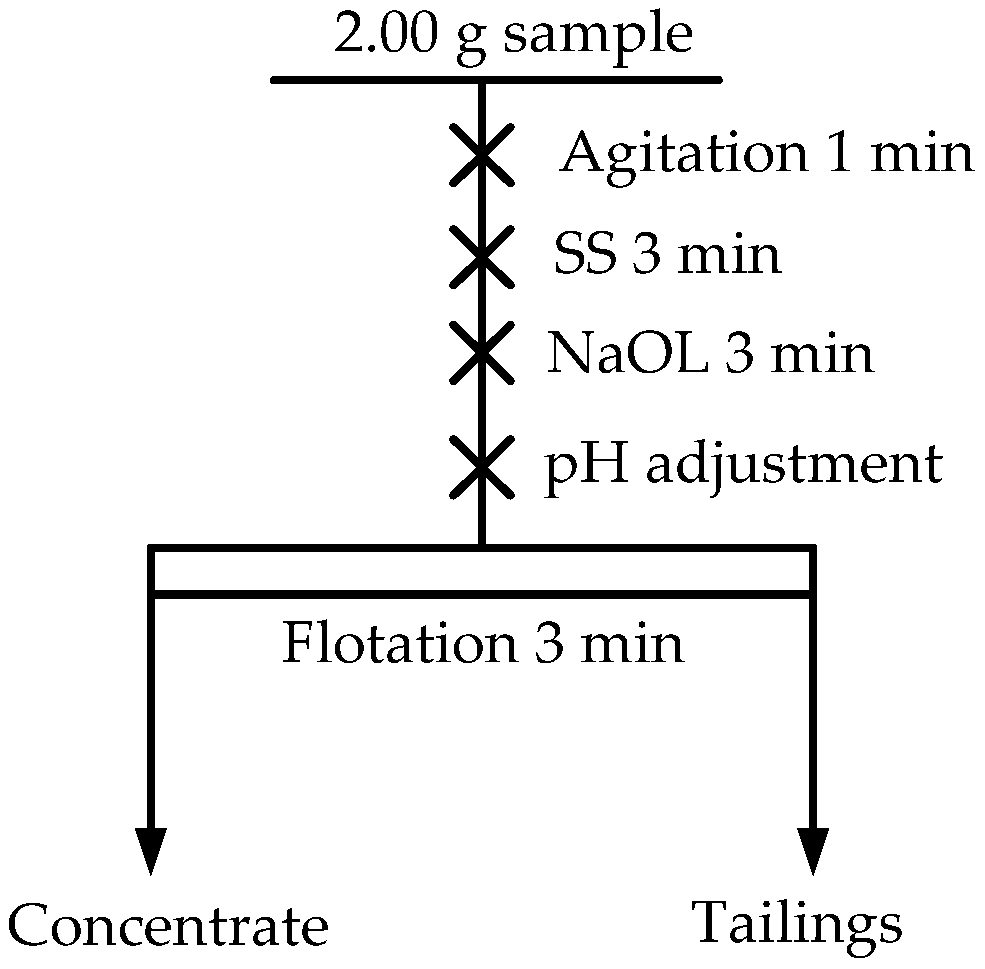
2.2. Flotation Tests
2.3. Contact Angles Measurements
2.4. Zeta Potential Measurements
2.5. X-Ray Photoelectron Spectroscopy (XPS) Analysis
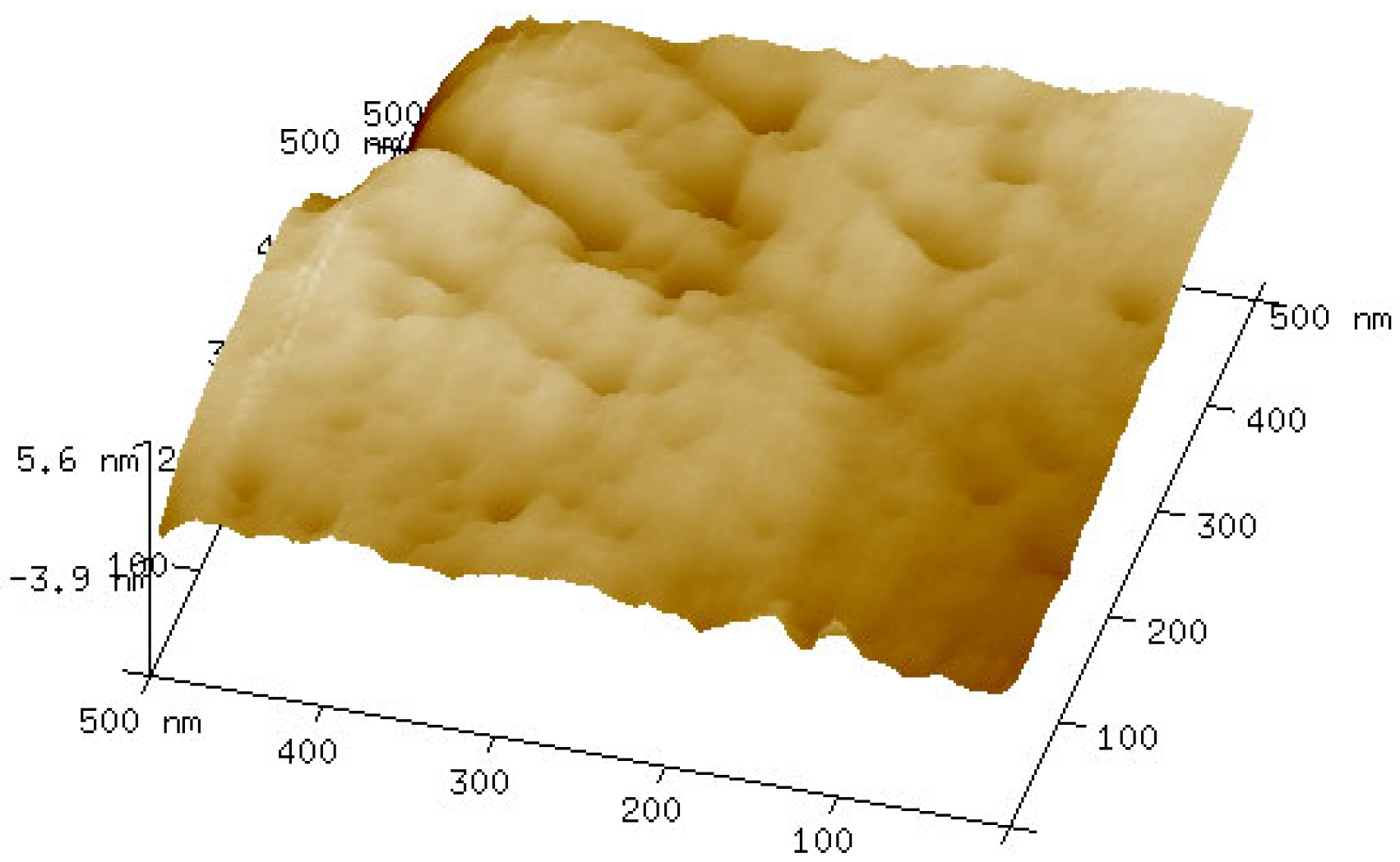
2.6. Atomic Force Microscope (AFM) Measurements
3. Results and Discussion
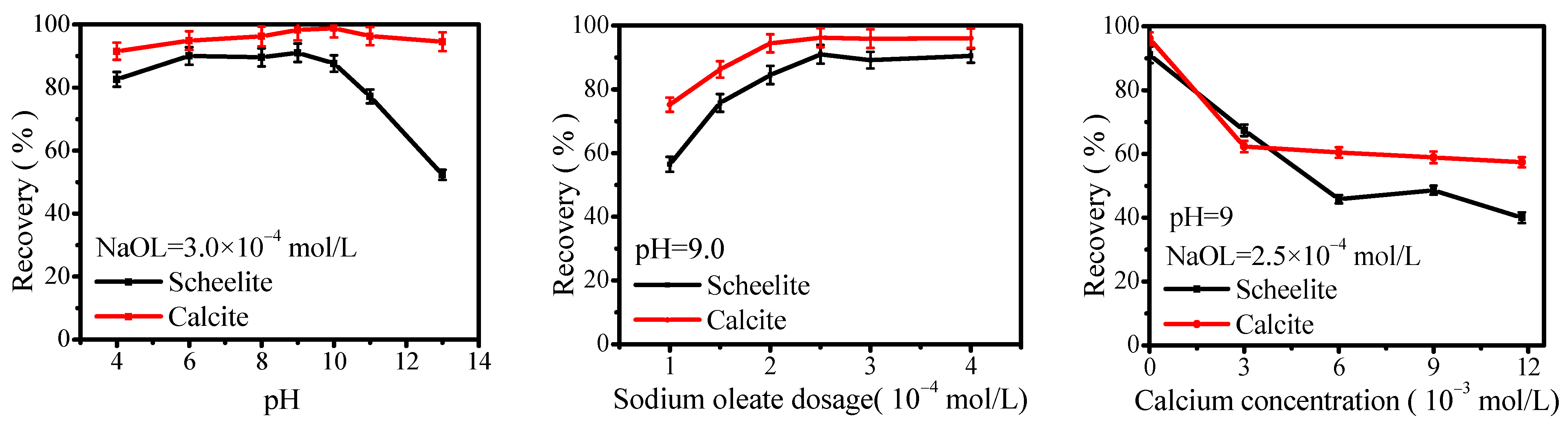
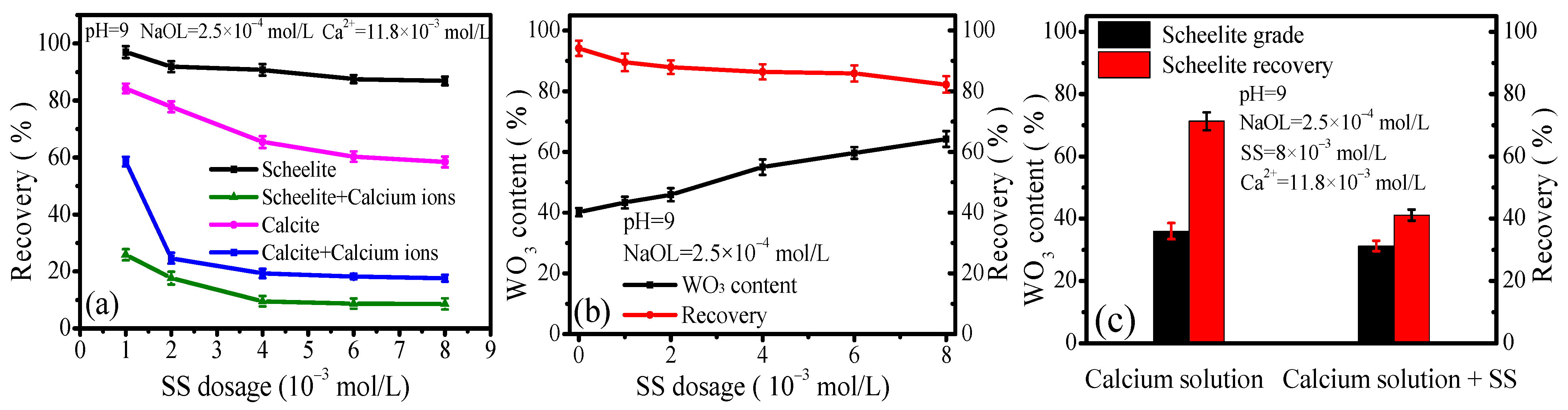
3.1. Flotation Behavior of Scheelite and Calcite
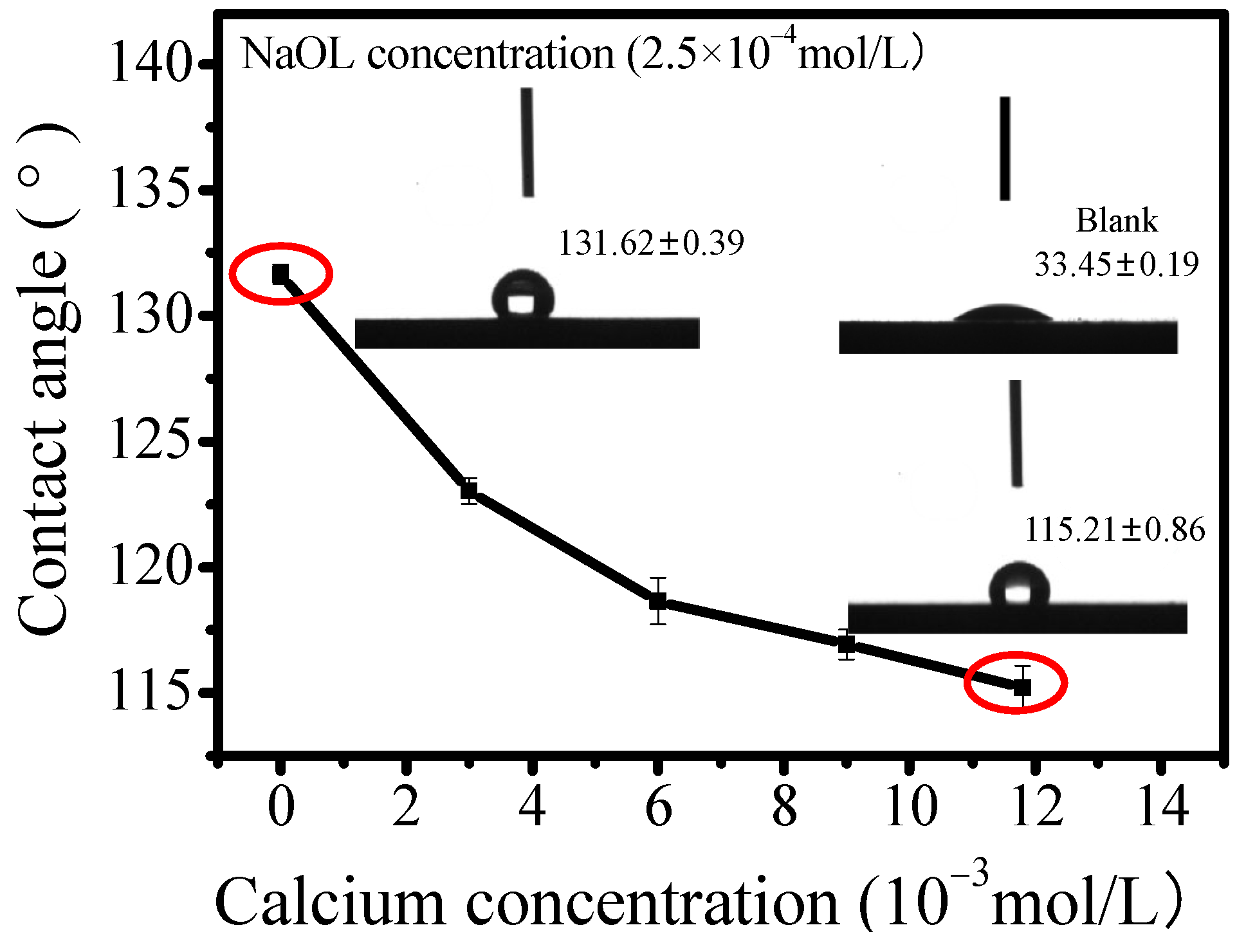
3.2. Influence of Calcium Ions Adsorption on Calcite Hydrophobicity
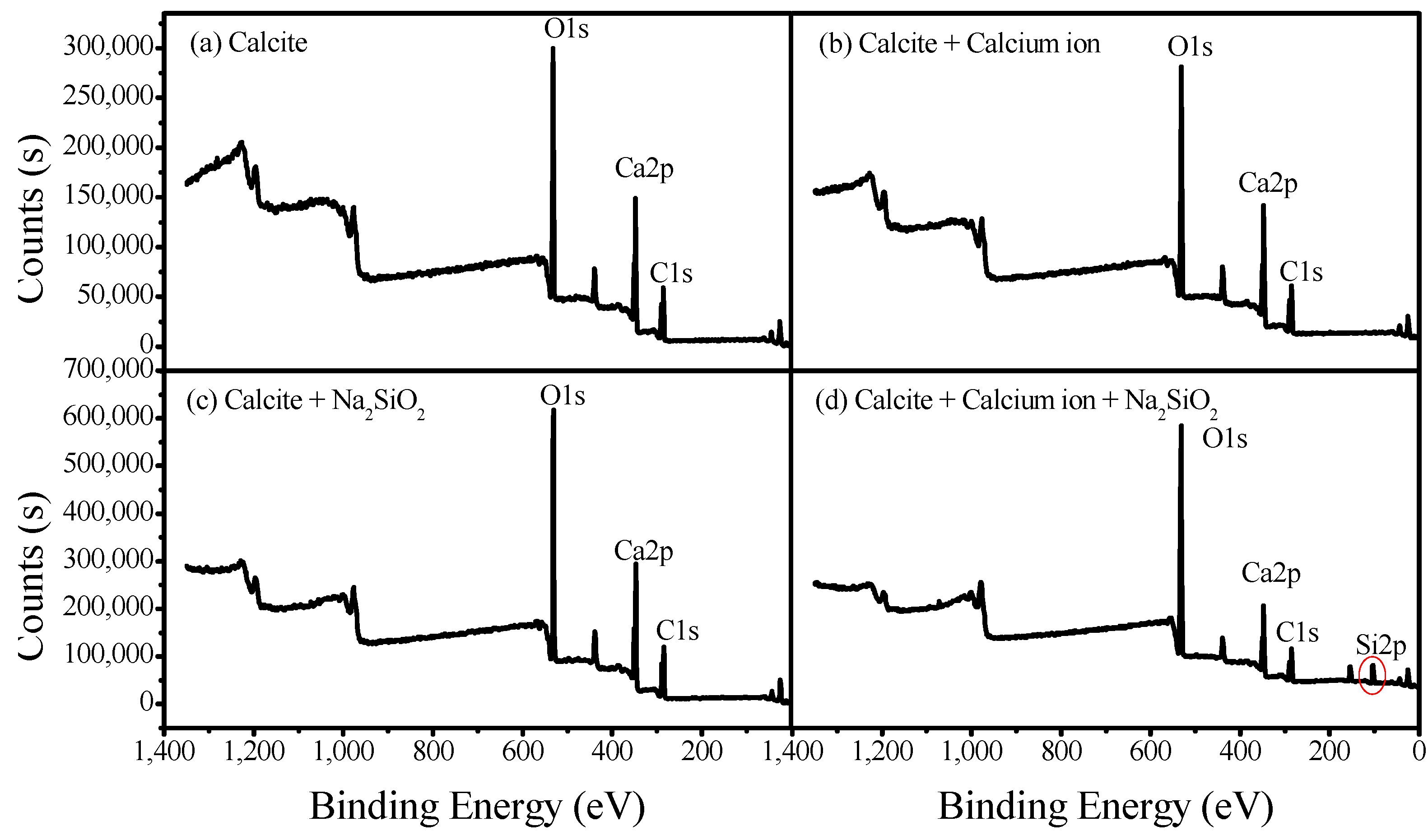
3.3. XPS Analysis of Adsorption Mechanisms
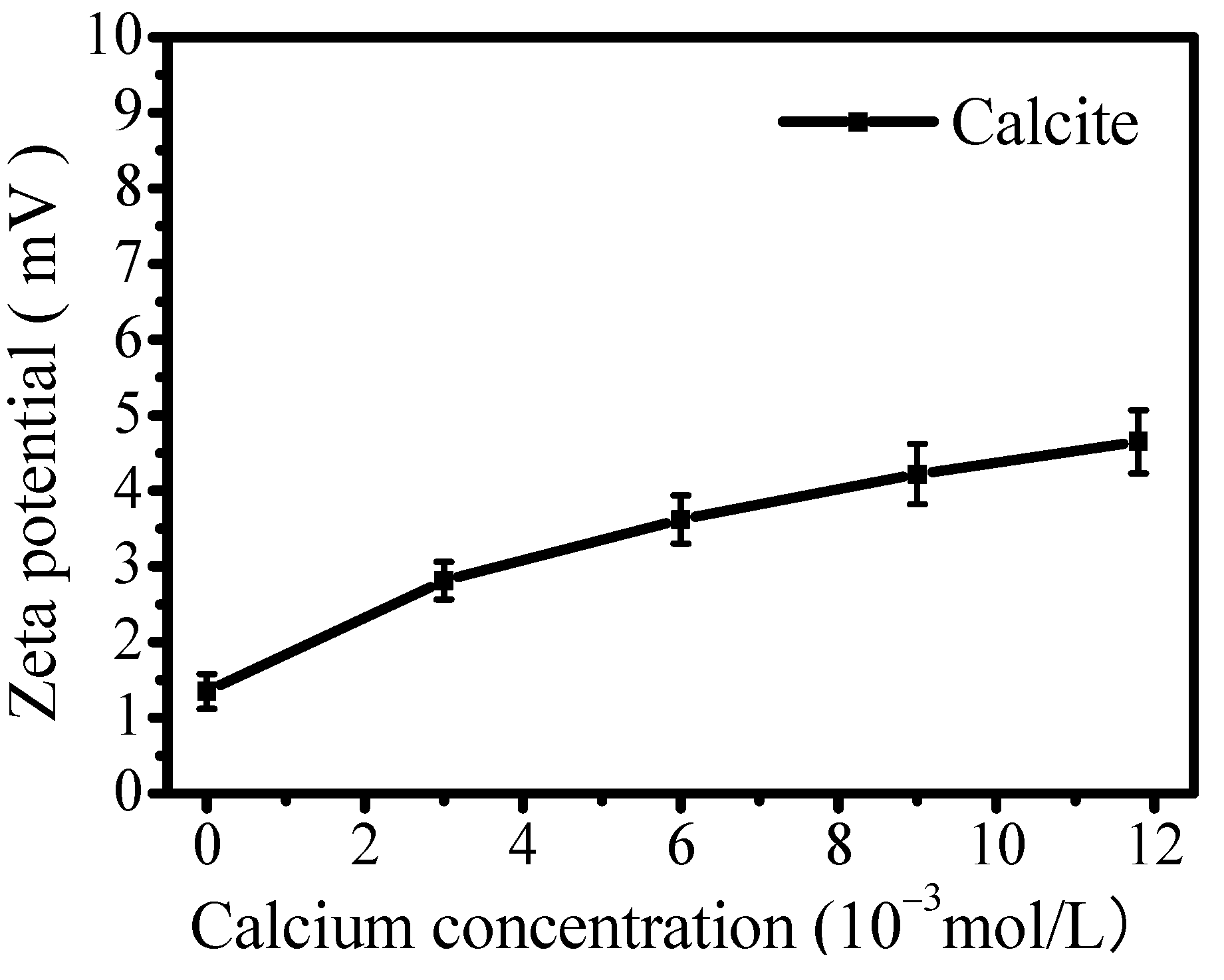
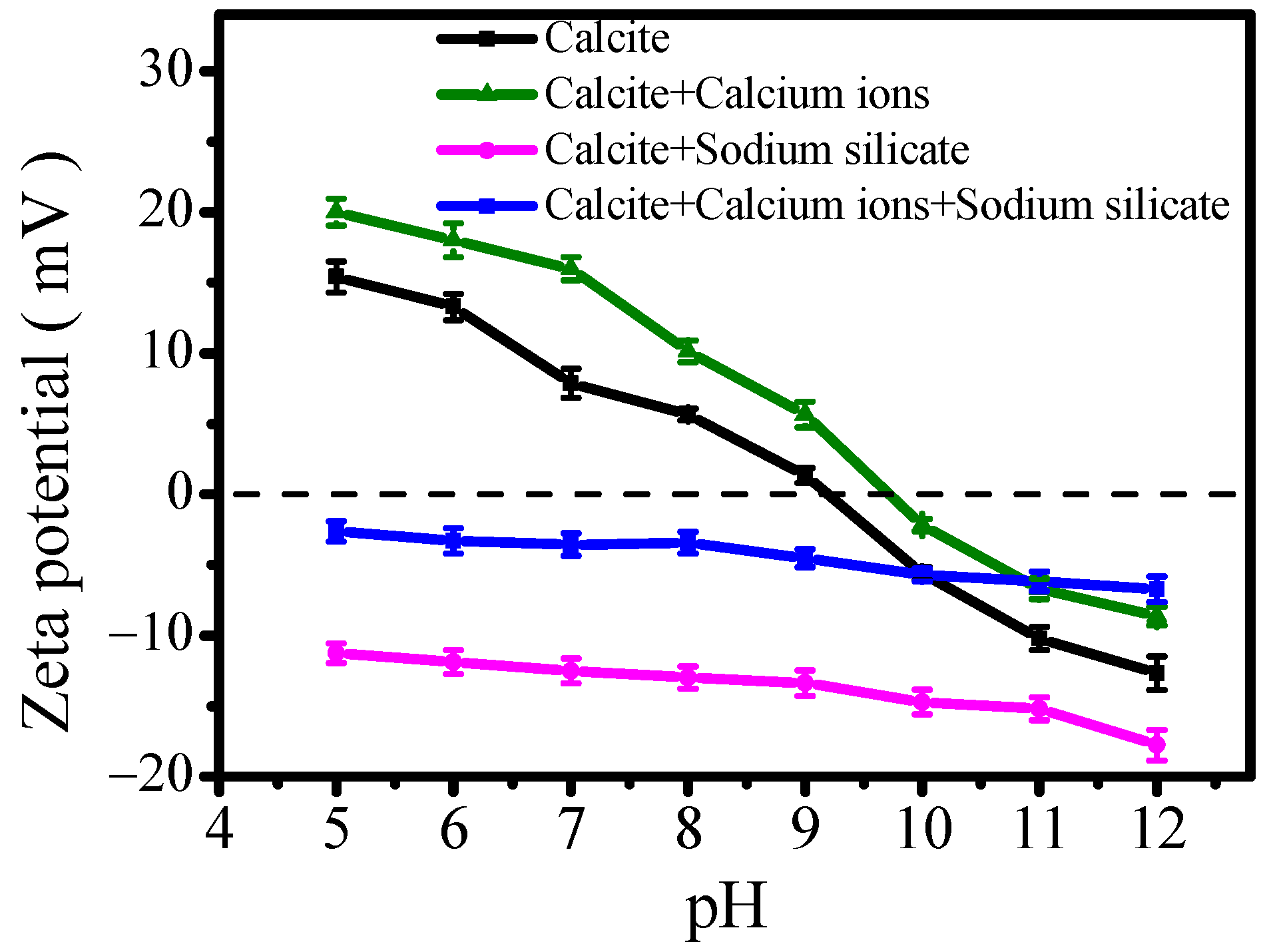
3.4. Effect of Calcium Ions on Surface Zeta Potential
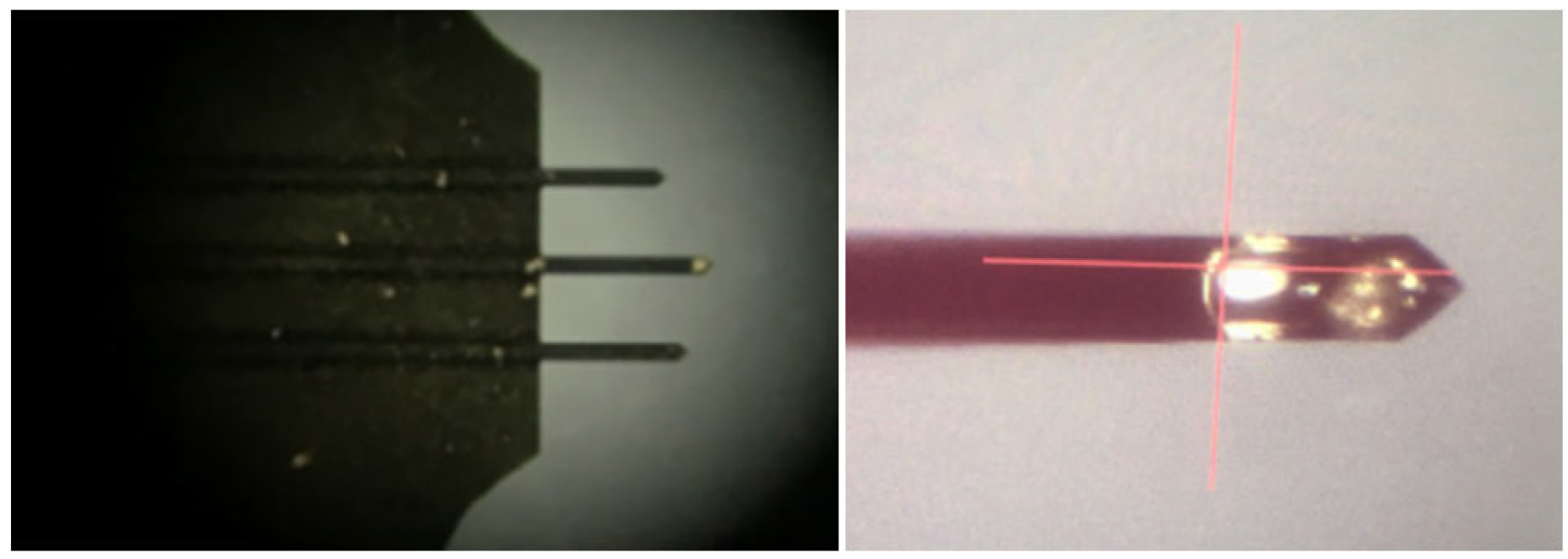
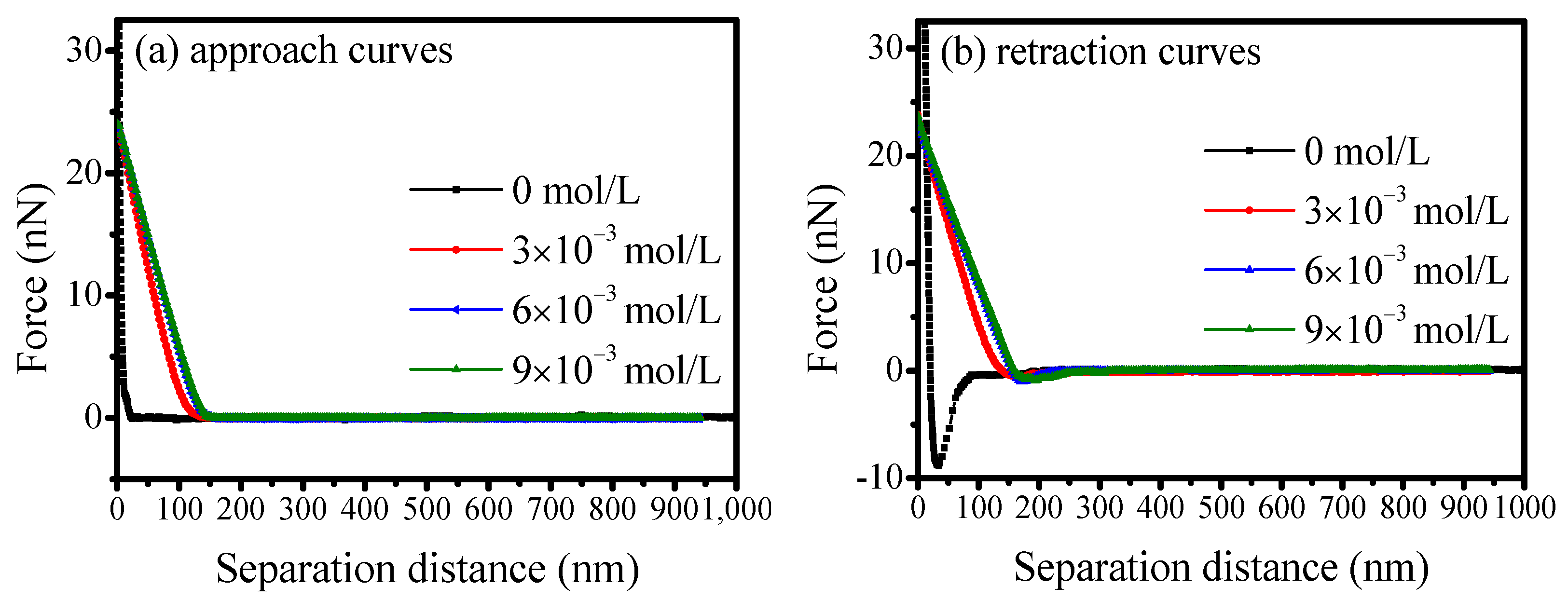
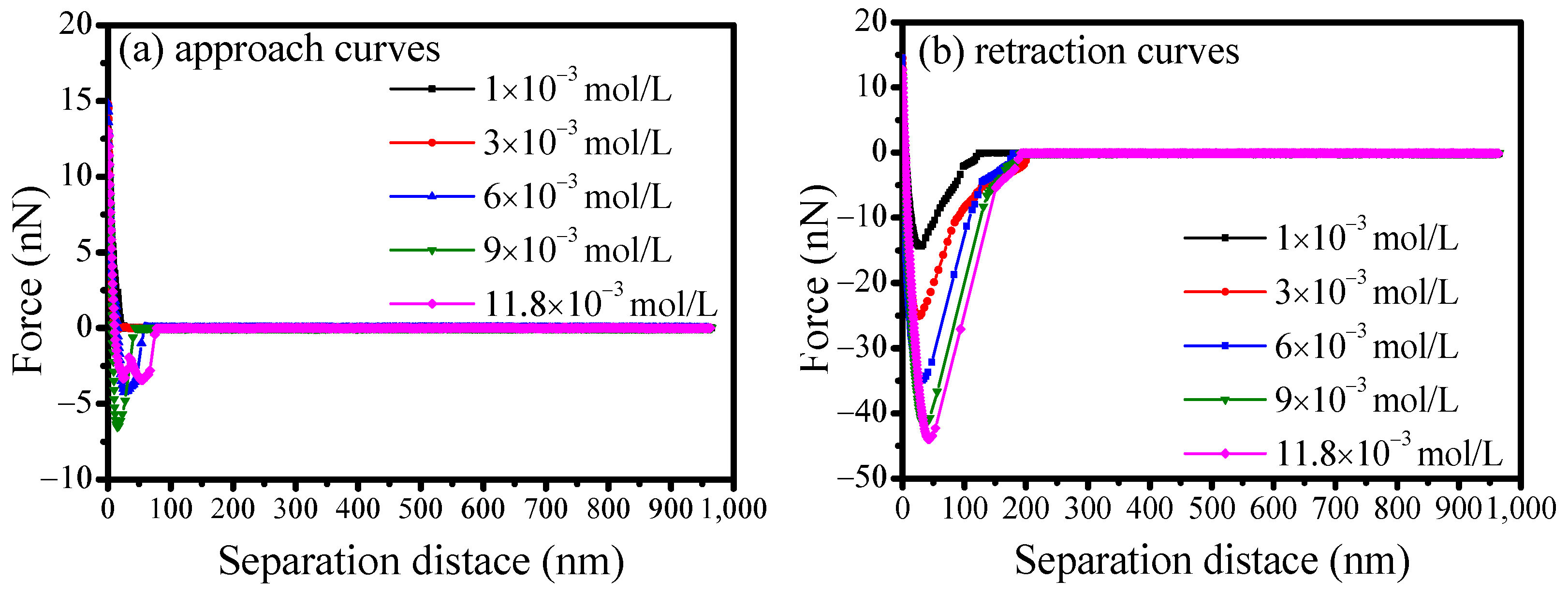
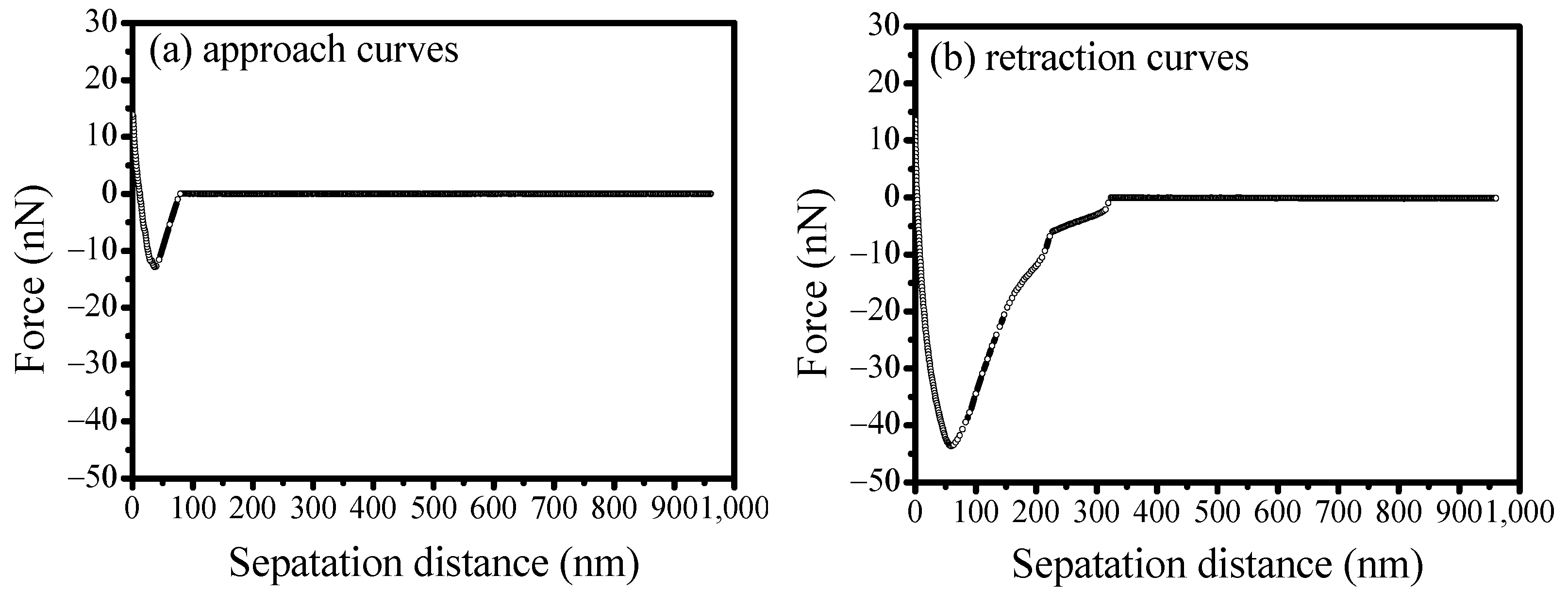
3.5. AFM Force Measurements Between Scheelite and Calcite
4. Conclusions
- (1)
- Calcium ions dissolved from calcium-bearing minerals would adsorb on calcite’s surface, leading to increased surface positive charge. This behavior is consistent with previous findings on scheelite and fluorite surface adsorption. Furthermore, competitive adsorption between calcium ions and oleate at surface the Ca site would reduce oleate adsorption capacity, thereby increasing the hydrophilicity of both minerals.
- (2)
- Results from both mixed binary mineral flotation and adhesion force measurements suggest that calcium ions would enhance SS adsorption on scheelite and calcite surfaces, thereby eliminating its selectivity for calcite. The adhesion forces would increase with the adsorption of calcium ions on mineral surfaces, hindering the effective separation of scheelite from calcite. Therefore, to achieve efficient separation of scheelite from calcite, the pulp environment and reagent regime should be carefully optimized to mitigate the detrimental effects of unavoidable calcium ion accumulation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gu, X.; Zhu, Y.; Li, Y.; Han, Y. Selective flotation of siderite and quartz from a carbonate-containing refractory iron ore using a novel amino-acid-based collector. Physicochem. Probl. Mineral Process. 2018, 54, 803–813. [Google Scholar] [CrossRef]
- Foucaud, Y.; Filippov, L.; Filippova, I.; Badawi, M. The challenge of tungsten skarn processing by froth flotation: A review. Front. Chem. 2020, 8, 230. [Google Scholar] [CrossRef]
- Wang, J.; Liu, R.; Jing, M.; Han, S. Research progress on mineral interaction in flotation system composed of calcite and its associated minerals. Nonferrous Met. Eng. 2023, 13, 78–87. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Y.; Wen, S.; Ma, M.; Sun, C.; Yin, W.; Ma, Y. Effect of carbonate minerals on quartz flotation behavior under conditions of reverse anionic flotation of iron ores. Int. J. Miner. Process. 2016, 152, 1–6. [Google Scholar] [CrossRef]
- Lu, Y.; Ding, R.; Li, G.; Song, X.; Cao, Y.; Jia, K. Research Progress with Scheelite Flotation Reagents: A Review. Minerals 2023, 13, 1257. [Google Scholar] [CrossRef]
- Filippova, I.V.; Filippov, L.O.; Duverger, A.; Severov, V.V. Synergetic effect of a mixture of anionic and nonionic reagents: Ca mineral contrast separation by flotation at neutral pH. Miner. Eng. 2014, 66–68, 135–144. [Google Scholar] [CrossRef]
- Xu, L.; Liu, D.; Sun, R.; Wang, Y.; Liu, Z.; Shao, P.; Wang, C.; Wen, S. Flotation separation of smithsonite and calcite in sodium oleate system using soluble starch as depressant. Miner. Eng. 2024, 205, 108490. [Google Scholar] [CrossRef]
- Kupka, N.; Kaden, P.; Jantschke, A.; Schach, E.; Rudolph, M. Acidified water glass in the selective flotation of scheelite from calcite, part II: Species in solution and related mechanism of the depressant. Physicochem. Probl. Mineral Pro. 2020, 56, 797–817. [Google Scholar] [CrossRef]
- Kupka, N.; Mockel, R.; Rudolph, M. Acidified water glass in the selective flotation of scheelite from calcite, Part I: Performance and impact of the acid type. Physicochem. Probl. Mineral Process. 2020, 56, 238–251. [Google Scholar]
- Pan, Z.; Zhang, Y.; Hu, J.; Jiao, F.; Qin, W. Camph or leaf extract as neoteric and environmentally friendly depressant in flotation separation of scheelite and calcite. Trans. Nonferrous Met. Soc. China 2023, 33, 275–284. [Google Scholar] [CrossRef]
- Dong, L.; Qin, W.; Jiao, F.; Zhu, H. Flotation separation of scheelite and calcite using mixed cationic-anionic collectors. Min. Metall. Eng. 2018, 38, 61–64. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, W.; Hu, Y. New insights into the dodecylamine adsorption on scheelite and calcite: An adsorption model. Miner. Eng. 2015, 79, 54–61. [Google Scholar] [CrossRef]
- Kupka, N.; Rudolph, M. Froth flotation of scheelite—A review. Int. J. Min. Sci. Technol. 2018, 28, 373–384. [Google Scholar] [CrossRef]
- Wei, Z.; Sun, W.; Han, H.; Gui, X.; Xing, Y. Flotation chemistry of scheelite and its practice: A comprehensive review. Miner. Eng. 2023, 204, 108404. [Google Scholar] [CrossRef]
- Dong, L.; Cui, Y.; Qiao, L.; Lan, S.; Zheng, Q.; Shen, P.; Liu, D. A critical review on the flotation of calcium-containing minerals. Sep. Purif. Technol. 2025, 360, 131082. [Google Scholar] [CrossRef]
- Sun, W.; Wei, Z.; Han, H.; Gao, Z.; Wang, J.; Wang, R. Flotation chemistry of tungsten ore and its practice. Met. Mine 2021, 535, 24–41. [Google Scholar] [CrossRef]
- Gao, Z.; Fan, R.; Ralston, J.; Sun, W.; Hu, Y. Surface broken bonds: An efficient way to assess the surface behaviour of fluorite. Miner. Eng. 2019, 130, 15–23. [Google Scholar] [CrossRef]
- Gao, Z.; Li, C.; Sun, W.; Hu, Y. Anisotropic surface properties of calcite: A consideration of surface broken bonds. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 53–61. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, C.; Sun, W.; Gao, Y.; Kowalczuk, P.B. Froth flotation of fluorite: A review. Adv. Colloid Interfac. 2021, 290, 102382. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, B.; Chen, Y. Flotation separation depressants for scheelite and calcium-bearing minerals: A review. Int. J. Miner. Metall. Mater. 2023, 30, 1621–1632. [Google Scholar] [CrossRef]
- Wang, R.; Han, H.; Sun, W.; Sun, W.; Zhang, H.; Cheng, Y. Selective inhibition behavior and mechanism of Al-starch complex on ultrafine calcite in scheelite flotation. Conserv. Util. Miner. Resour. 2023, 43, 1–10. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Q.; Wang, S.; Wang, C.; Chen, M.; Li, H. Efficient selective flotation separation of fluorite from calcite using ferrous and ferric species as combined depressant. Miner. Eng. 2024, 205, 108451. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Fu, J.; Zhang, Z.; Li, W.; Han, H.; Wei, Z.; Chen, W.; Liu, W. Recycle of Pb-BHA collectors based on the adsorption and desorption mechanism on scheelite surface. J. Clean Prod. 2024, 435, 140555. [Google Scholar] [CrossRef]
- Wei, Z.; Sun, W.; Wang, P.; Liu, D.; Han, H. A novel metal-organic complex surfactant for high-efficiency mineral flotation. Chem. Eng. J. 2021, 426, 130853. [Google Scholar] [CrossRef]
- Gao, Z.; Jiang, Z.; Sun, W.; Gao, Y. Typical roles of metal ions in mineral flotation: A review. Trans. Nonferrous Met. Soc. China 2021, 31, 2081–2101. [Google Scholar] [CrossRef]
- Corin, K.C.; Charamba, A.; Manono, M.S. Water quality impact on flotation response: A focus on specific ions and temperature. Miner. Eng. 2024, 207, 108549. [Google Scholar] [CrossRef]
- Chen, J. The interaction of flotation reagents with metal ions in mineral surfaces: A perspective from coordination chemistry. Miner. Eng. 2021, 171, 107067. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, H.; Xu, Y.; Shu, K.; Fang, S.; Xu, L. The effect of dissolved calcite species on the flotation of bastnaesite using sodium oleate. Miner. Eng. 2020, 145, 106095. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, Y.; Ma, Z.; Liao, Y. Impact of calcium and gypsum on separation of scheelite from fluorite using sodium silicate as depressant. Sep. Purif. Technol. 2019, 215, 249–258. [Google Scholar] [CrossRef]
- Guo, W.; Chang, J.; Wang, S.; Liu, Q.; Zhang, H. Probing the interaction of calcium and magnesium ions on scheelite surface by atomic force microscopy. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131200. [Google Scholar] [CrossRef]
- Sun, W.; Han, H.; Zou, Q.; Chen, W. Study on the effect of calcium ion on the flotation behaviors of scheelite and its regulation. Met. Mine 2023, 567, 90–97. [Google Scholar] [CrossRef]
- Sun, W.; Han, H.; Sun, W.; Wang, R.; Wei, Z. Novel insights into the role of colloidal calcium dioleate in the flotation of calcium minerals. Miner. Eng. 2022, 175, 107274. [Google Scholar] [CrossRef]
- Sun, W.; Han, H.; Sun, W.; Wang, R. Novel insights into the mechanism of lime method based on calcium dioleate and mineral surface transformation. J. Cent. South Univ. 2023, 30, 2983–2992. [Google Scholar] [CrossRef]
- Filippov, L.O.; Duverger, A.; Filippova, I.V.; Kasaini, H.; Thiry, J. Selective flotation of silicates and Ca-bearing minerals: The role of non-ionic reagent on cationic flotation. Miner. Eng. 2012, 36–38, 314–323. [Google Scholar] [CrossRef]
- Ducker, W.A.; Senden, T.J.; Pashley, R.M. Direct measurement of colloidal forces using an atomic force microscope. Nature 1991, 353, 239–241. [Google Scholar] [CrossRef]
- Ducker, W.A.; Senden, T.J.; Pashley, R.M. Measurement of forces in liquids using a force microscope. Langmuir 1992, 8, 1831–1836. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, M.; Gui, X.; Cao, Y.; Babel, B.; Rudolph, M.; Weber, S.; Kappl, M.; Butt, H. The application of atomic force microscopy in mineral flotation. Adv. Colloid Interfac. 2018, 256, 373–392. [Google Scholar] [CrossRef]
- Cao, Z.; Cheng, Z.; Wang, J.; Cao, Y. Synergistic depression mechanism of Ca2+ ions and sodium silicate on bastnaesite flotation. J. Rare Earth 2022, 40, 988–995. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, Y.; Liao, Y.; Ma, Z. Impact of gypsum on flotation of scheelite and fluorite using sodium oleate as collector. Sep. Sci. Technol. 2020, 55, 2528–2537. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Q.; Zhang, G.; Chen, W.; Chen, Y. Effect of depressants in the selective flotation of scheelite and calcite using oxidized paraffin soap as collector. Int. J. Miner. Process. 2016, 157, 210–215. [Google Scholar] [CrossRef]
- Feng, B.; Guo, W.; Xu, H.; Peng, J.; Luo, X.; Zhu, X. The combined effect of lead ion and sodium silicate in the flotation separation of scheelite from calcite. Sep. Sci. Technol. 2017, 52, 567–573. [Google Scholar] [CrossRef]
- Yao, W.; Li, M.; Zhang, M.; Cui, R.; Shi, J.; Ning, J. Effects of Pb2+ ions on the flotation behavior of scheelite, calcite, and fluorite in the presence of water glass. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127826. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Liao, Y.; Jin, C.; Ma, Z. Effect of unavoidable ion (Ca2+) in pulp on the dispersion behavior of fine smithsonite. Molecules 2022, 27, 9026. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xu, Z. Interactions of amphoteric amino phosphoric acids with calcium-containing minerals and selective flotation. Int. J. Miner. Process. 2003, 72, 87–94. [Google Scholar] [CrossRef]
- Gao, Z.; Bai, D.; Sun, W.; Cao, X.; Hu, Y. Selective flotation of scheelite from calcite and fluorite using a collector mixture. Miner. Eng. 2015, 72, 23–26. [Google Scholar] [CrossRef]
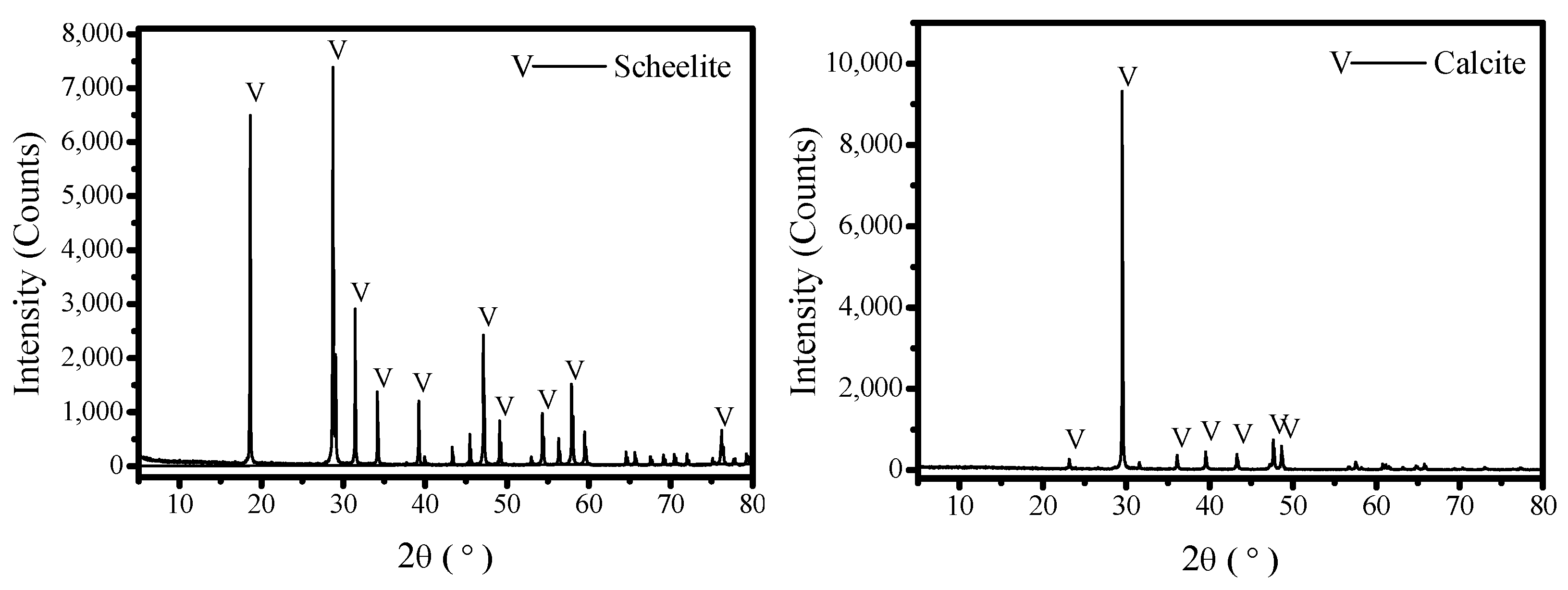
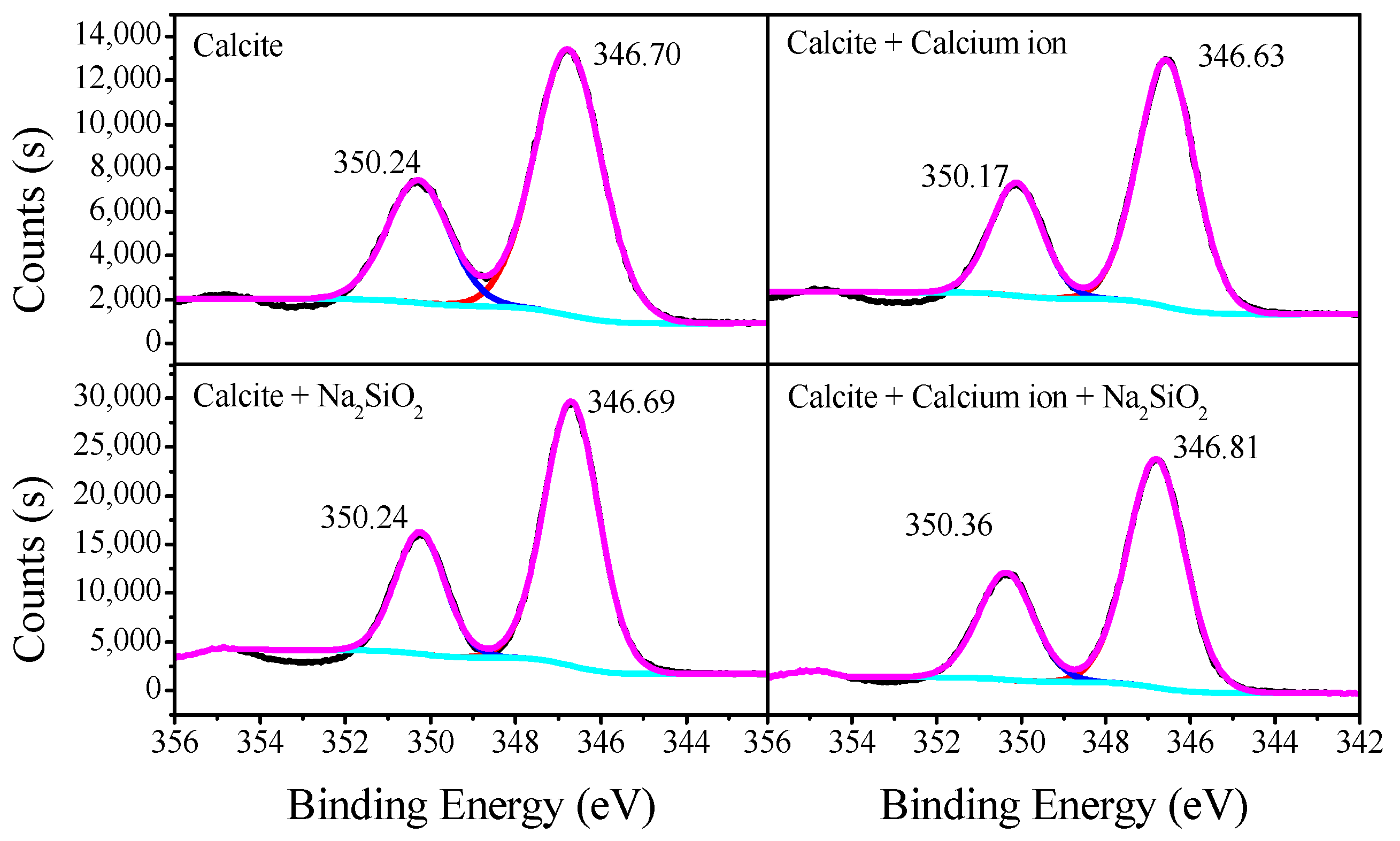
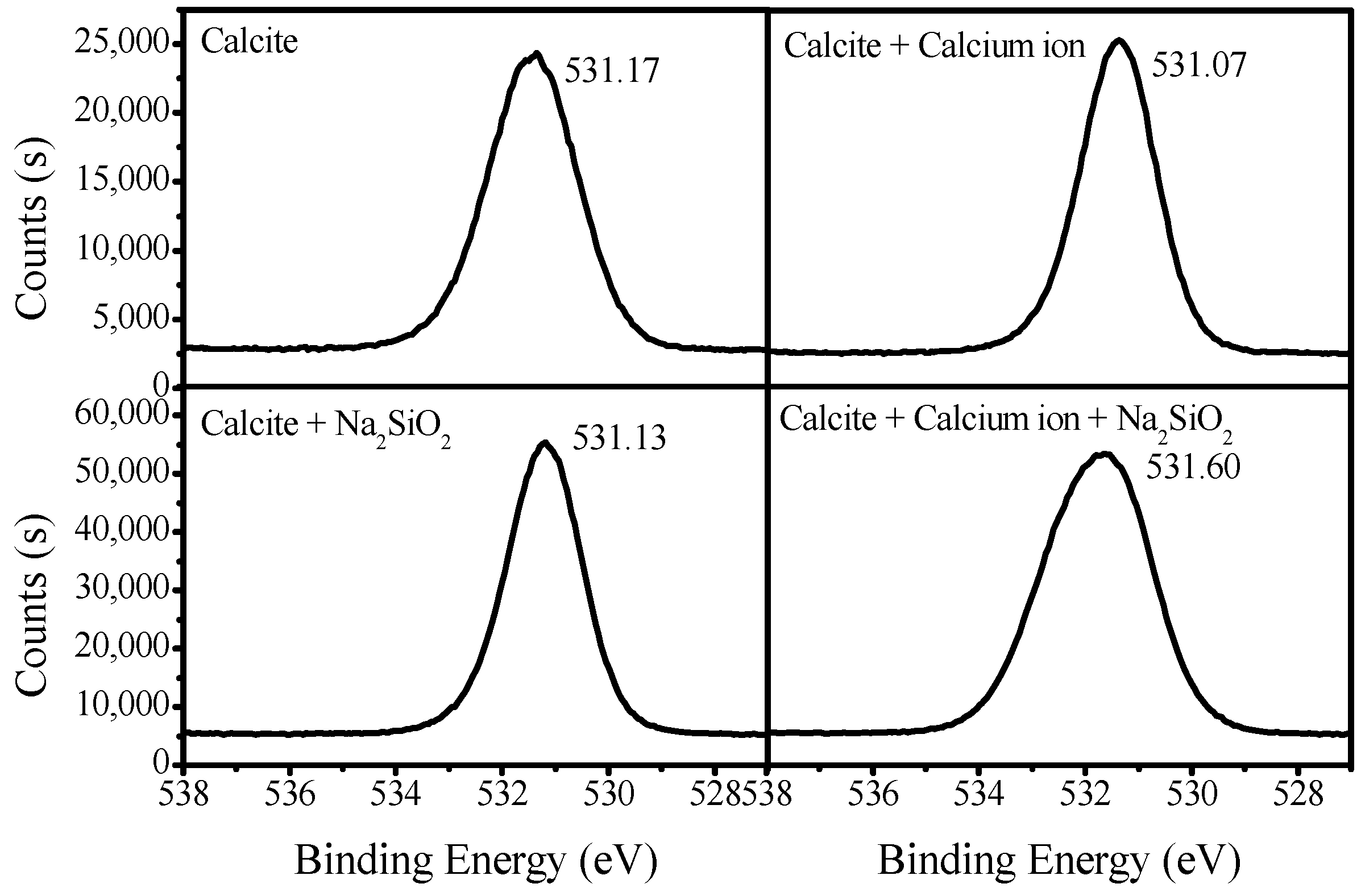
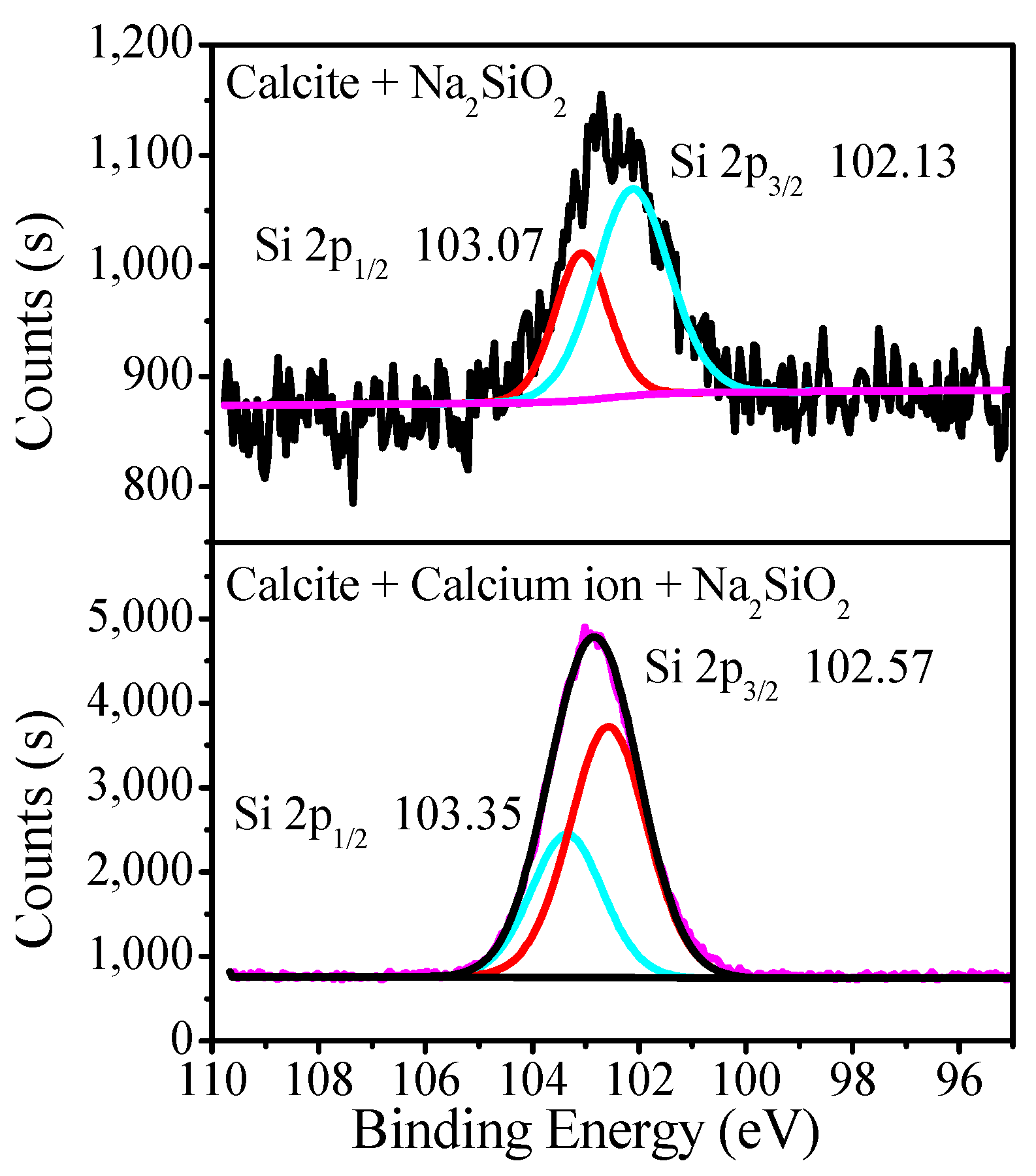
| Sample | WO3 | CaO | CO3 | SiO2 | Fe2O3 | Purity |
|---|---|---|---|---|---|---|
| Scheelite | 80.48 | 19.35 | / | 0.71 | 0.045 | 97.97 |
| Calcite | / | 57.03 | 42.40 | 0.11 | 0.036 | 96.70 |
| Tests | Atomic Concentration of the Main Elements (%) | |||
|---|---|---|---|---|
| Ca | O | Si | C | |
| Calcite | 13.33 | 48.13 | - | 38.54 |
| Calcite + CaCl2 | 13.38 | 48.52 | - | 38.1 |
| Calcite + Na2SiO3 | 12.34 | 48.27 | 2.08 | 37.31 |
| Calcite + CaCl2 + Na2SiO3 | 8.53 | 54.1 | 10.79 | 26.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhang, X.; Deng, X.; Shi, C.; Xing, B. Adsorption of Calcium Ions on Calcite Surface and Its Influence on Flotation Separation of Scheelite. Minerals 2025, 15, 1225. https://doi.org/10.3390/min15111225
Zhang Z, Zhang X, Deng X, Shi C, Xing B. Adsorption of Calcium Ions on Calcite Surface and Its Influence on Flotation Separation of Scheelite. Minerals. 2025; 15(11):1225. https://doi.org/10.3390/min15111225
Chicago/Turabian StyleZhang, Zhiguo, Xiaolong Zhang, Xiaowei Deng, Changliang Shi, and Baolin Xing. 2025. "Adsorption of Calcium Ions on Calcite Surface and Its Influence on Flotation Separation of Scheelite" Minerals 15, no. 11: 1225. https://doi.org/10.3390/min15111225
APA StyleZhang, Z., Zhang, X., Deng, X., Shi, C., & Xing, B. (2025). Adsorption of Calcium Ions on Calcite Surface and Its Influence on Flotation Separation of Scheelite. Minerals, 15(11), 1225. https://doi.org/10.3390/min15111225







