3.1. Pentlandite (Fe5Ni4S8) Bulk and Surface Models
The bulk Fe-rich pentlandite (Fe
5Ni
4S
8) was reported as the most stable compound based on cluster expansion [
31]. As shown in
Figure 1, the structure of Fe
5Ni
4S
8 was tetragonal, possessing a P4_2/nmc space group. The structural lattice properties were a = b = 7.020 Å and c = 9.930 Å. It was noted that a and b lattice takes the value of the primitive structure of Fe
5Ni
4S
8 [
31]. It was reported that in the conversion of the primitive structure to conventional structure, the lattice is multiplied by
, which gave
. This suggested that the generated structure could be cubic. The lattice parameters of 9.930 Å agreed with the previously reported values of 9.991 Å [
32] and 9.977 Å [
29]. The electronic density of states for pentlandite (Fe
5Ni
4S
8) were previously reported [
31].
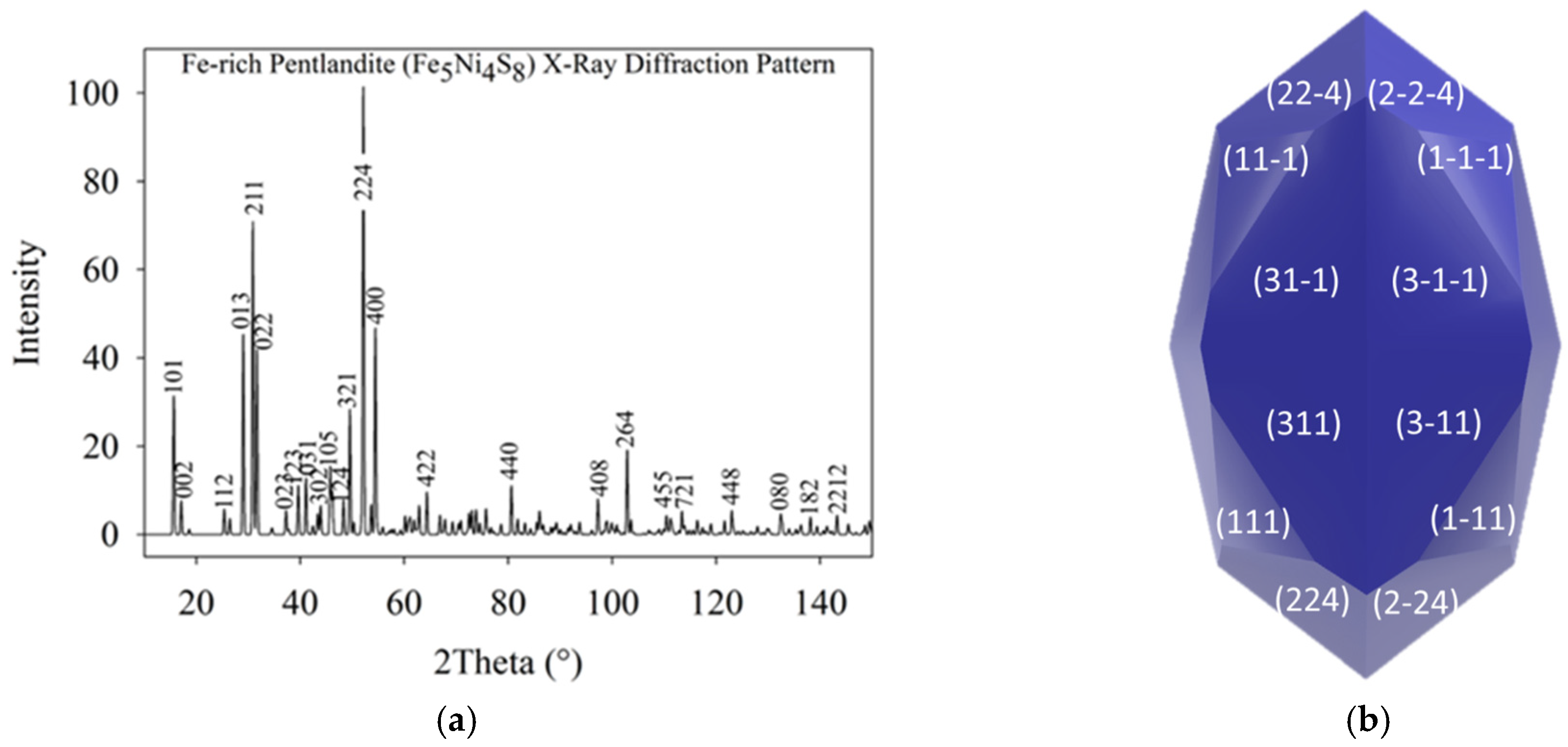
The Fe
5Ni
4S
8 structure was analysed using computational X-ray diffraction (XRD) to determine its structural composition, as illustrated in
Figure 2a. The XRD pattern was simulated using MedeA software, version 3.9 with copper as the radiation source, revealing that the (224) and (211) planes exhibited higher intensities. This computational XRD data was then compared to experimental XRD results of a similar compound [
33]. Experimentally, the (111), (311), (511), and (440) planes of Fe
5Ni
4S
8 were reported as having the highest peak intensities, with the (311) and (440) planes reaching 100% intensity [
33].
Due to the tetragonal nature of the generated Fe
5Ni
4S
8 structure, the computational XRD results displayed different plane indices compared to experimental findings. This information will be utilised in subsequent surface studies, where the plane surfaces (111), (311), (224), and (211) will be selected for further investigation to determine the most stable surface of the tetragonal Fe
5Ni
4S
8. These surfaces were cleaved from the relaxed bulk structure, and to prevent the adsorbates from interacting with the upper repeating slab model, the vacuum elevation was set to 30.0 Å for all surface models. Their surface energies were calculated as 1.62 J.m
−2 (plane surface of 111), 1.84 J.m
−2 (plane surface of 211), 1.84 J.m
−2 (plane surface of 224), and 1.48 J.m
−2 (plane surface of 311). These were used to construct the surface crystal morphology as shown in
Figure 2b, which clearly showed that the plane surface of (311) appears in a large plane on the morphology. The plane surface (211) energy ratio to the plane surface of (311) was E
Surf-(211)/E
Surf-(311) = 1.242 <
. Although the ratio is less than
, the plane surface of (211) was not expressed in the morphology due to competition with the plane surface of (311) (see
Figure 2b).
It has been reported that the plane surface of (111) for (Fe,Ni)
9S
8 was the most stable [
34]. However, in this study it has been demonstrated that the plane surface of (311) was found to be the most stable surface and dominant on the surface morphology since it had the lowest surface energy of 1.48 J.m
−2, followed by the plane surface of (111) and then the plane surfaces of (211) and (224), as shown in
Table 1. This was in good agreement with the reported experimental XRD [
33], which showed that the plane surface of the (311) plane had the highest intensity; therefore, it was the most dominant surface.
As shown in
Figure 3, the plane surface of (2 × 1) (311) displayed a drastic relaxation where the Fe and Ni atoms relaxed inwards, resulting in a sulphur-terminated surface.
Table 2 shows the displacement of the metal atoms relaxation. Clearly the top Fe1, Fe2, Fe3, Ni1, and Ni2 atoms on the surface of the (311) plane relaxed inwards by −0.0410 Å, −0.0851 Å, −0.0535 Å, −0.0133 Å, and −0.0417 Å, respectively (
Table 2). The S1 and S2 were noted to relax inward by −0.0448 and −0.0328, whereas S3 relaxed upward by 0.0049. It was observed that the Fe1 atoms relaxed and formed a new bond with the S atom within the surface, while the Fe2 atoms formed new bonds with three S atoms within the surface after relaxation. Furthermore, Ni2 atoms formed new bonds with four S atoms after relaxation. At the bottom of the surface, it was noted that there was also dramatic relaxation, where some atoms relaxed downwards and some inwards to the surface.
Density of States of Fe5Ni4S8 of the Plane Surface of (311)
The computed density of states (DOS) for the Fe
5Ni
4S
8 plane surface of (311) is presented in
Figure 4. The total density of states (TDOS) analysis indicated that the Fe
5Ni
4S
8 plane surface of (311) exhibited metallic behaviour, as no band gap was observed at the Fermi level (E
F). Additionally, the E
F was positioned deep within the pseudo-gap, suggesting structural stability. A broad peak spanning from −8 eV in the VB to 2 eV in the conduction band (CB) was observed on the TDOS, which consisted of states from S, Ni, and Fe atoms.
The projected partial density of states (PDOS) revealed that the Fe atoms were the most active at EF, with dominant 3d-orbitals. The S atoms displayed minimal states at the EF, indicating low surface activity. The Ni atoms displayed a 3d-orbital broad sharp peak just below the EF. In the valence band (VB) region, contributions from the S atom s-orbital were observed between −15 eV and −11 eV. The S1 atom’s p-orbital contributed within the range of −8 eV to −3 eV, while the Ni1 atom’s 3d-orbital contributed between −3 eV and −1 eV. Additionally, the Fe atom’s 3d-orbitals were active from −1 eV to 2 eV.
3.2. Isolated Dithiocarbamate, Thionocarbamate, Mercaptobenzothiazole, and S-Triazine Molecular Geometries
The VASP (models) and DMol
3 (HOMO and LUMO) optimised collector geometries are illustrated in
Figure 5, while
Table 3 presents the bonds and torsion parameters for their polar head groups. The selected bond lengths and bond angles for these functional groups contain polar sulphur atoms, which serve as the active sites for reactions.
The reactivity of these collectors before adsorption was characterised by their HOMO and LUMO energies.
Figure 5 shows the isosurfaces for the HOMO and LUMO orbitals. It was observed that, for all collectors, the HOMO isosurface was concentrated on the sulphur (S) and nitrogen (N) atoms, indicating that electron donation would likely occur from these atoms. The LUMO isosurface was predominantly located on the sodium (Na), nitrogen (N), oxygen (O), and sulphur (S) atoms for all collectors, suggesting that these atoms can accept electrons. This implied that sulphur atoms have the ability to both donate and accept electrons. The high-density regions of the HOMO orbital can be associated with electrophilic attacks [
35]. Previous research suggested that the donor and acceptor behaviour of these collectors on the mineral surface can be predicted by identifying the molecule with the highest HOMO energy and the lowest LUMO energy.
A higher HOMO energy level indicates strong ability to donate electrons, while a lower LUMO energy level indicates strong ability to accept electrons. According to this theory, the electron-donating ability (HOMO energies) decreased in the following order, SDTBAT > SMBT > ADEDTC > IPDETC, while the electron-accepting ability (LUMO energies) followed the order: SMBT > SDTBAT> ADEDTC > IPDETC. This pattern clearly showed that SDTBAT had greater capacity for donating electrons, while SMBT had the greater capacity for accepting electrons. The band gaps reflect the HOMO–LUMO gap which predicted the reactivity of the collector molecules. In this regard a molecule with a small gap would be more reactive compared to a molecule with a larger gap [
36]. This was due to the easy mobility of electrons from the valence band to the conduction for a molecule with a small gap. Based on this, as shown in
Table 3, SDTBAT had the smallest band gap (1.93 eV), suggesting strong reactivity, and the reactivity order decreased as follows: SDTBAT > ADEDTC > SMBT > IPDETC. Although the reactivity may be predicted from the H-L gap, adsorption of the collector molecules on the surface depends highly on the orientation and matching of the orbital for reactivity. As such, the predicted reactivity may differ from the surface adsorption energies. The calculated bond lengths and torsion angles for the ADEDTC, IPDETC, SMBT, and SDTBAT collectors are summarised in
Table 4. It was observed that the double bond in ADEDTC, IPDETC, and SMBT was located at the S1 atom (C=S1). In the case of SDTBAT, the S1 and S2 atoms were single-bonded and carried a negative charge. A sodium ion (Na
+) was included to neutralise this charge of the charged S atoms, in particular for SMBT and SDTBAT molecules. Among the collectors, the C=S1 bond length in IPDETC (1.658 Å) was the shortest, followed by ADEDTC (1.665 Å), indicating greater stability and lower reactivity.
In contrast, the C–S bond lengths in SDTBAT (1.725 Å and 1.723 Å) and SMBT (C–S2 = 1.770 Å) were longer, suggesting reduced stability and higher reactivity. The C–O and C–N bonds were approximately 1.35 Å in length. The torsion angles for ((S1–C=N1)–N) and ((S2–C–N1)=N) were nearly 120°, while ((S1–C–N)=S), ((N–C–O)=S1), and ((S1=C–S2)–N/=C) in ADEDTC were close to 115°. Similarly, the torsion angles for ((S1=C–N)–O) and ((S1=C–S2)–N/=C) in SMBT were around 125°.
3.3. Fe5Ni4S8 Plane Surface of (311) Collector Adsorptions
This section analyses the adsorption behaviour of ADEDTC, IPDETC, SMBT, and SDTBAT collectors on the pentlandite (Fe
5Ni
4S
8) plane surface of (311). Note that the naming of the metal sites on the adsorbed surface figures was independent of the naming of the clean surface figures. It is important to note that the SMBT collector was adsorbed in both vertical and horizontal orientations, and the strongest orientation adsorption site was reported. The adsorption geometries, reactivity, and bonding modes of these collectors on the Fe
5Ni
4S
8 plane surface of (311), both before and after relaxation, are illustrated in the
Supporting Information, Section SI 1. The most exothermic of the various tested adsorption configurations of ADEDTC, IPDETC, SMBT, and SDTBAT on the Fe
5Ni
4S
8 plane surface of (311) at Ni and Fe sites, after relaxation, are illustrated in
Figure 6 and
Figure 7. The theoretical Ni–S and Fe–S bond lengths, measured as 2.350 Å and 2.400 Å, respectively, were derived from the sum of the empirically determined covalent radii of Ni (1.350 Å), Fe (1.400 Å), and S (1.000 Å), as established by Slater [
37]. These values were used as a reference for comparison with the bond lengths obtained from the adsorption analysis.
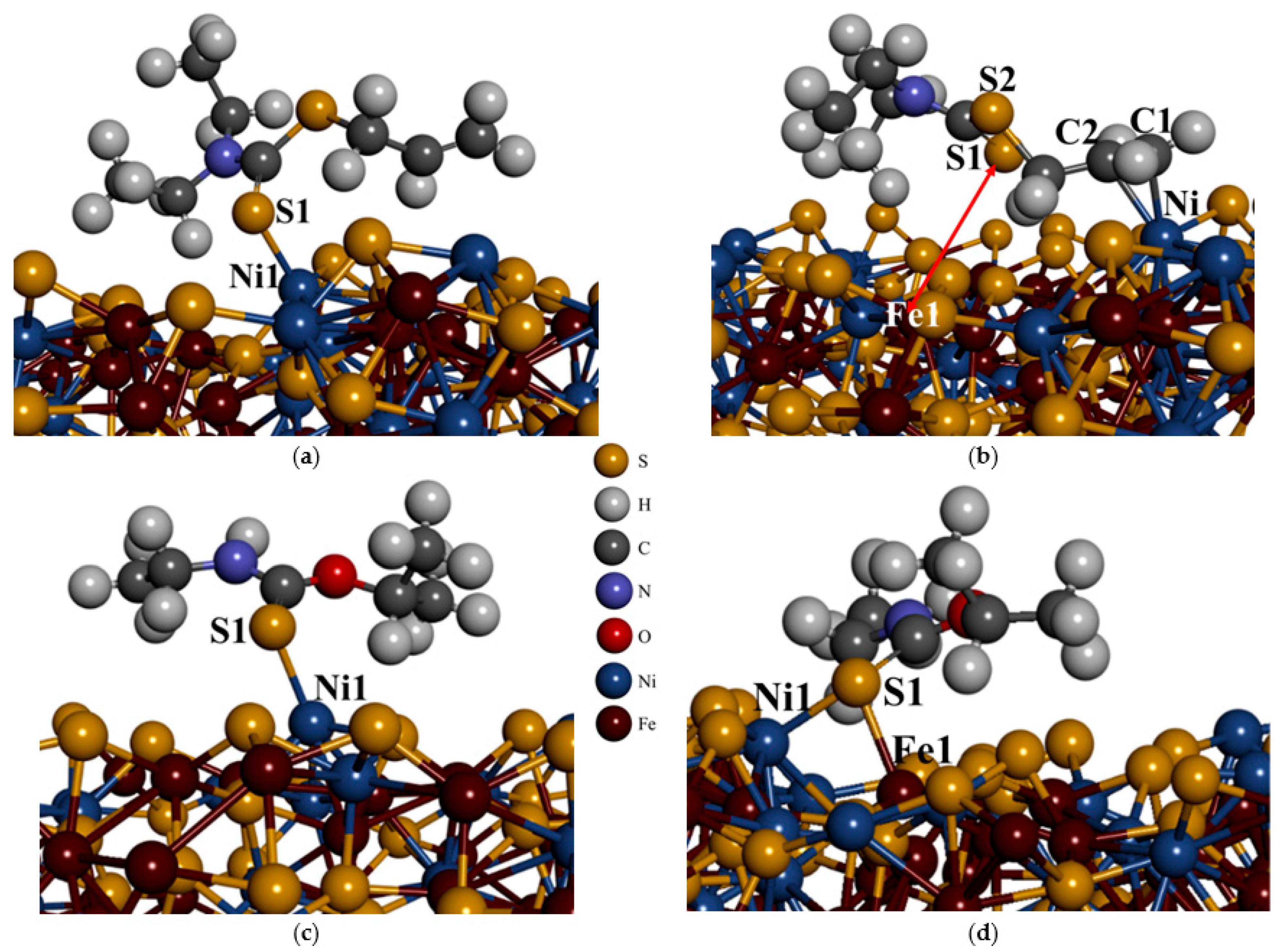
In
Figure 6a,b, the most exothermic adsorption of the ADEDTC collector on both Ni and Fe sites is displayed. It was observed that for Ni site adsorption (see
Figure 6a), a Ni1–S1 bond length of 2.250 Å was obtained. In the case of Fe site adsorption (
Figure 6b), the ADEDTC S1 atom underwent desorption from the Fe1 atom as shown by the red arrow, resulting in an Fe1–S1 distance of 3.788 Å. Notably, the allyl chain interacted with the surface Ni atom, forming C2–Ni and C1–Ni bond lengths of 2.111 Å and 2.139 Å, respectively. In comparison with the empirically measured covalent radii (Ni–S = 2.350 Å) determined by Slater [
37], the S1–Ni bond length of 2.250 Å was shorter, indicating a stronger bond. This shorter bond length suggested a strong interaction between the pentlandite mineral surface and ADEDTC collector. The adsorption and dispersion energies for the most exothermic adsorption models on Ni and Fe sites are presented in
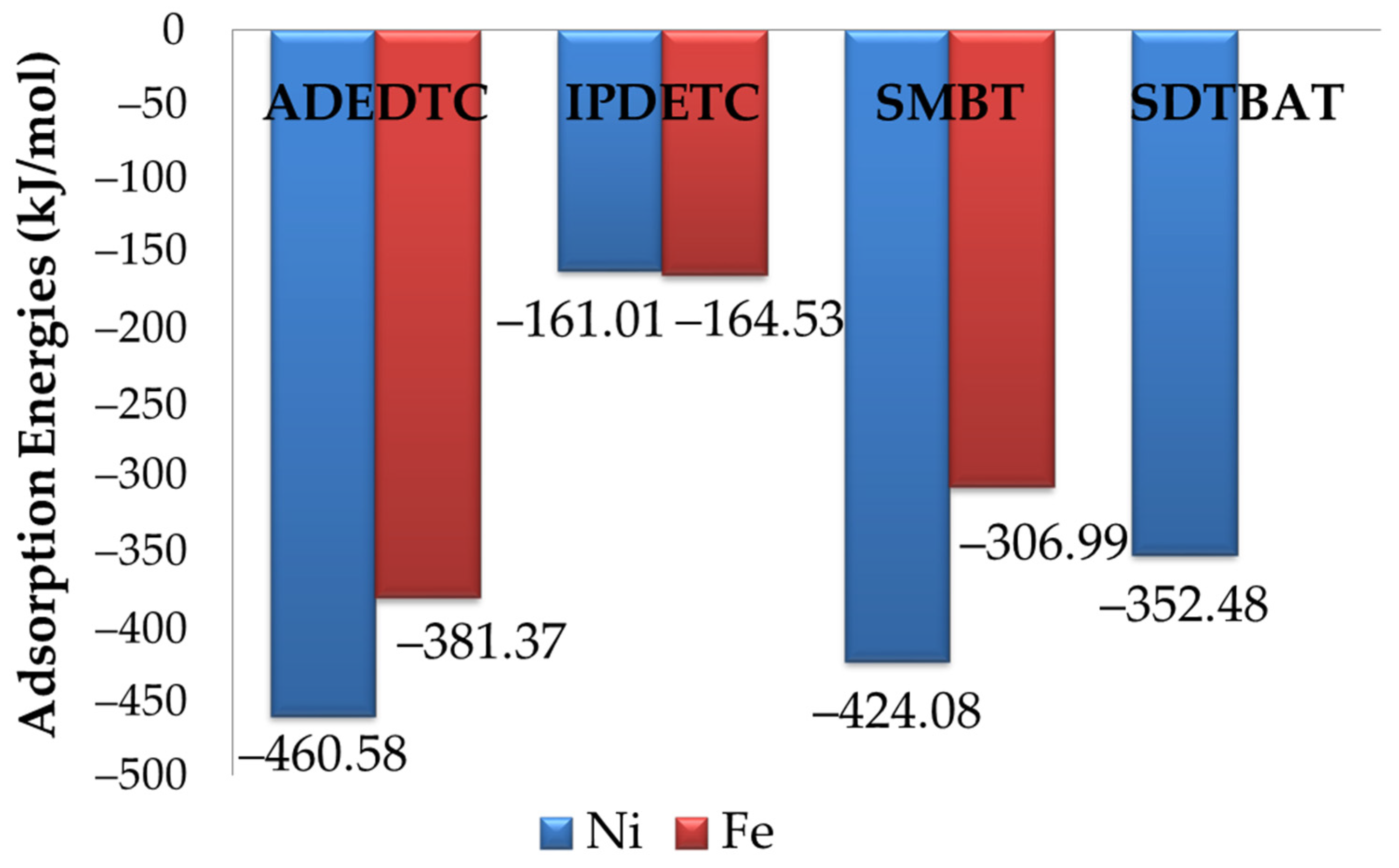
Table 5. The adsorption of ADEDTC on Ni sites resulted in adsorption and dispersion energies of E
ads = −460.58 kJ.mol
−1 and E
disp = −97.15 kJ.mol
−1, whereas adsorption on Fe sites yielded E
ads = −381.37 kJ.mol
−1 and E
disp = −108.05 kJ.mol
−1. The adsorption energy results clearly indicated that ADEDTC exhibited stronger exothermic adsorption on Ni sites than on Fe sites, suggesting a stronger interaction with Ni atoms on pentlandite mineral surface. This finding implies that ADEDTC collector, which has been found to be selective towards Cu on chalcopyrite [
11], could be effectively used for the selective flotation of pentlandite minerals. A previous experimental study has also shown that allyl collectors such as NAOITC collector had great potential to enhance the recovery rate of pentlandite and nickel in industrial beneficiation [
21]. This suggested that the ADEDTC collector’s strong adsorption predicts its potential application in the improvement of pentlandite recovery. Interestingly, the dispersion energies showed that adsorption on Fe sites were more exothermic than on Ni sites. This was attributed to the long-range interactions of the allyl-hydrocarbon chain and its bonding with the surface.
In the case of IPDETC adsorption, as shown in
Figure 6c,d, the most exothermic adsorption on the Ni site for the IPDETC collector was the S1–Ni1 monodentate (see
Figure 6c), which gave a bond length of 2.244 Å. The adsorption of the IPDETC collector on the Fe site, as shown in
Figure 6d, resulted in mono-bridge bonding on the Fe and Ni atoms, forming bond lengths of Fe1–S1 = 2.355 Å and Ni1–S1 = 2.285 Å. Since the initial adsorption site was on Fe, the shift towards Ni to bridge indicated that the collector preferred to also bond with Ni atoms on the pentlandite surface. For both Ni and Fe site adsorption, IPDETC preferred to orientate at an angle on the surface. The IPDETC adsorption bond lengths on Ni and Fe sites were smaller than the sum of the empirically measured covalent radii (Fe–S = 2.400 Å and Ni–S = 2.350 Å) determined by Slater [
37], which indicated a stronger bond. Clearly the bond length of Ni1–S1 = 2.285 Å was much stronger compared to Fe1–S1 = 2.355 Å and predicted that the IPDETC collector preferred the Ni site. These smaller bonds of the collector adsorption predicted the strong interaction of the pentlandite mineral with IPDETC collectors.
The obtained adsorption energies and dispersion energies for the most exothermic IPDETC adsorption on Ni and Fe sites are displayed in
Table 5. The IPDETC adsorption on Ni sites gave adsorption and dispersion energies of E
ads = −161.01 kJ.mol
−1, including E
disp = −87.80 kJ.mol
−1, while on Fe sites it gave E
ads = −164.53 kJ.mol
−1, including E
disp = −56.27 kJ.mol
−1. The adsorption energies clearly showed that the IPDETC on the Fe site was more exothermic than on the Ni site, which was attributed to the bridging on the Fe and Ni atoms. This still indicated the preferential IPDETC collector interaction with Ni atoms on Fe
5Ni
4S
8. This showed that the chalcopyrite collector, such as IPDETC [
11], was predicted to be used for selective flotation of pentlandite minerals. This was also supported by the previous experimental study which showed that high cumulative nickel recoveries were obtained with IPETC and its mixtures with PAX and SIBX [
19]. This suggested that the IPDETC collector with a diethyl chain is predicted to have potential ability to improve the recovery of pentlandite based on its strong adsorption on the pentlandite surface towards Ni sites. It was noted that the dispersion energies depicted that the Ni site adsorption was more exothermic than the Fe site adsorption. This was ascribed to the hydrocarbon chain’s long-range interaction with the surface, which also indicated the preferential adsorption on the Ni site.
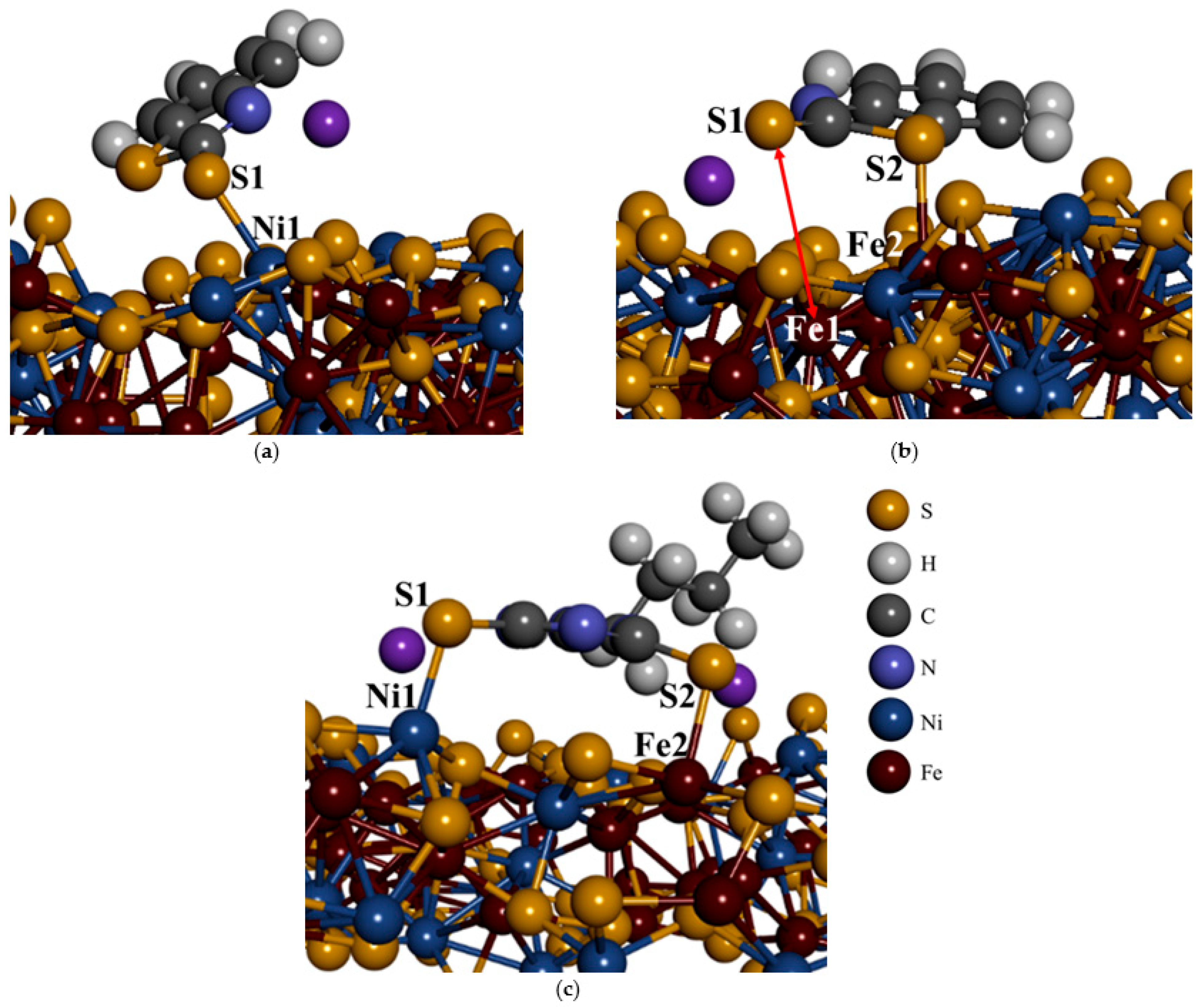
Figure 7a,b illustrates the most exothermic horizontal adsorption of SMBT on the Fe
5Ni
4S
8 plane surface of (311). The less exothermic vertical adsorption is shown in the
Supporting Information, Section SI 1. Among the configurations, the most exothermic adsorption occurred at the Ni site through a monodentate interaction between the S1 atom and Ni1 (
Figure 7a), with a bond length of 2.339 Å. In contrast, when SMBT was adsorbed on the Fe site (see
Figure 7b), the exocyclic S1 atom detached from the surface, resulting in an Fe1–S1 distance of 4.863 Å as reflected by the red arrow. However, the endocyclic S2 atom remained bonded, forming a monodentate bond (Fe2–S2 = 2.438 Å). In both Ni and Fe site adsorptions, SMBT adopted a flat orientation on the surface, ascribed to the horizontal orientation bonding. The bond formed with the Ni site was shorter than the empirical Ni–S covalent radius (2.350 Å), while the Fe–S bond was longer than the Fe–S covalent radius (2.400 Å), as reported by Slater [
37]. This suggested a stronger interaction between SMBT and Ni atoms and a weaker interaction with Fe atoms. The shorter bond at the Ni site further indicated a more favourable interaction with the Fe
5Ni
4S
8 surface through Ni sites.
Table 5 presents the calculated adsorption and dispersion energies for the most exothermic horizontal configurations of SMBT on Ni and Fe sites. The SMBT adsorption on the Ni site yielded an adsorption energy of E
ads = −424.08 kJ.mol
−1, including a dispersion component of E
disp = −109.29 kJ.mol
−1. In comparison, with the Fe site, adsorption of SMBT gave lower energies: E
ads = −306.99 kJ.mol
−1 and E
disp = −104.69 kJ.mol
−1. These results confirm that SMBT adsorption was more energetically favourable on Ni than on Fe sites, indicating a preferential Ni adsorption on pentlandite. Additionally, the greater dispersion energy at the Ni site was attributed to enhanced long-range interactions between SMBT hydrocarbon chain and the surface. Similar flat preferential adsorption of the SMBT was observed on the pyrite surface [
13].
Figure 7c shows that SDTBAT adsorption onto the Fe
5Ni
4S
8 plane surface of (311) was through bridging on both Ni and Fe atoms. The molecule adopted a flat orientation with its hydrocarbon chain positioned vertically relative to the surface. The adsorption occurred in a bidentate manner, forming bond lengths of Ni1–S1 = 2.261 Å and Fe1–S2 = 2.380 Å. These bond lengths were shorter than the empirical covalent radii for Ni–S (2.350 Å) and Fe–S (2.400 Å), as reported by Slater [
37], indicating a strong interaction between SDTBAT and the Fe and Ni atoms. Notably, the Ni–S1 bond was significantly shorter, suggesting a stronger bond on the Ni site. This shorter bond also implied a strong interaction between SDTBAT and the pentlandite mineral surface. The adsorption and dispersion energies for SDTBAT on Ni and Fe sites are summarised in
Table 5. The adsorption on Ni yielded adsorption energy of E
ads = −352.48 kJ.mol
−1, including a dispersion energy of E
disp = −183.85 kJ.mol
−1. Among all tested collectors, SDTBAT showed the most negative dispersion energy, attributed to its flat adsorption orientation and the strong long-range interaction between the triazine group and hydrocarbon chain with the mineral surface. This emphasised the importance of including dispersion corrections when evaluating collector adsorption.
When comparing all collector adsorptions (see Figure 13), it was evident that all collectors showed a preference for Ni over Fe sites on the Fe
5Ni
4S
8 plane surface of (311). The order of adsorption strength, based on adsorption energy was as follows: ADEDTC (−460.58 kJ.mol
−1) > SMBT (−424.08 kJ.mol
−1) > SDTBAT (−352.48 kJ.mol
−1) > IPDETC (−164.53 kJ.mol
−1). ADEDTC exhibited the most exothermic adsorption energy, clearly highlighting its strong affinity for Ni atoms and its high selectivity for pentlandite (see Figure 13). These predicted their potential as highly selective collectors for the separation of pentlandite from other sulphide minerals. The dispersion energies in descending order were as follows: SDTBAT > SMBT > ADEDTC > IPDETC. Similar trends have been observed in previous studies, where ADEDTC and IPDETC displayed a preference for Cu atoms during the separation of chalcopyrite from pyrite [
11].
3.4. Electronic Properties of the Adsorbed Plane Surface of (311)
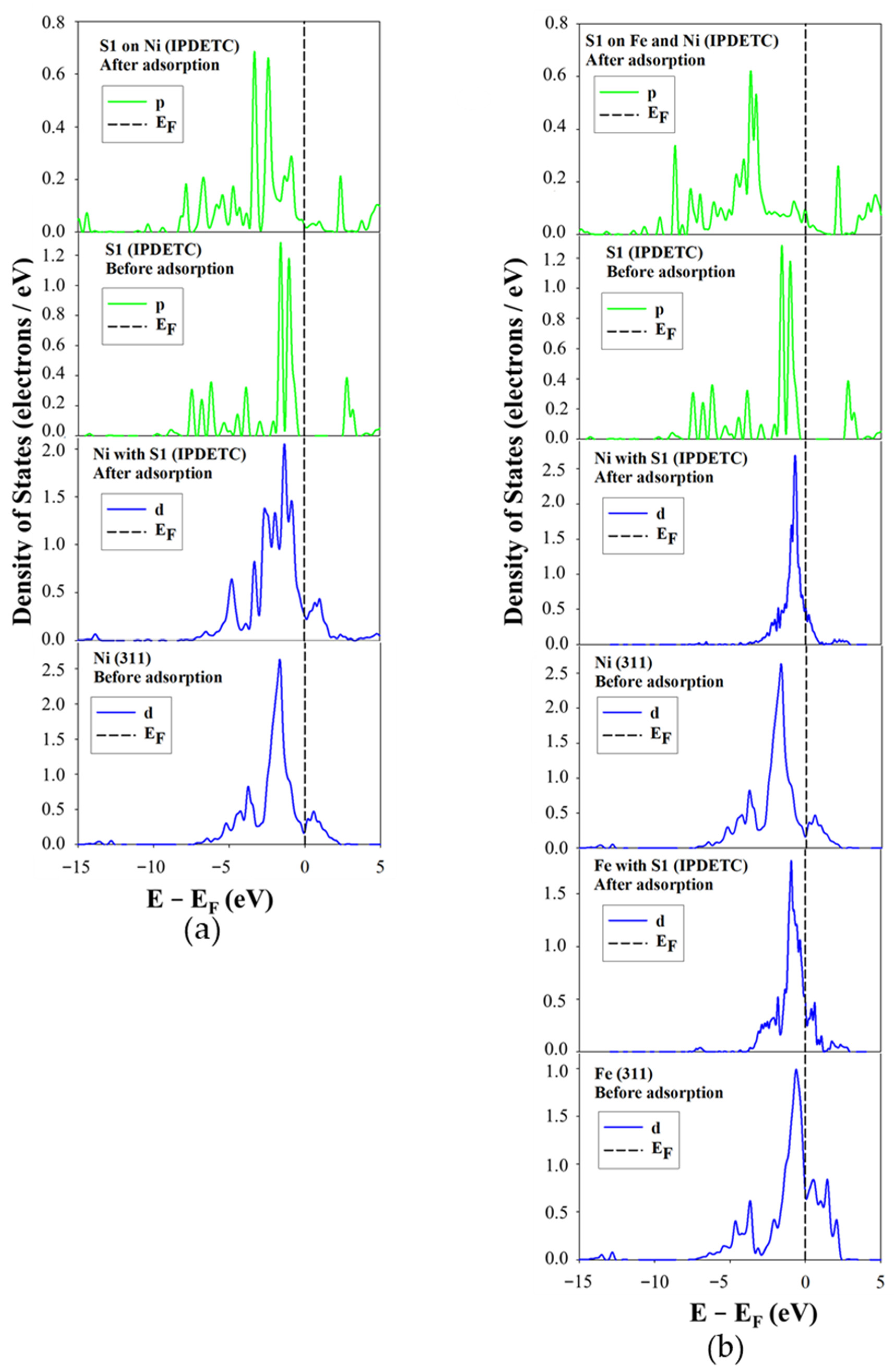
The projected density of states (PDOS) for the adsorption of ADEDTC, IPDETC, SMBT, and SDTBAT collectors on the Fe5Ni4S8 plane surface of (311) was analysed to assess changes in chemical reactivity before and after adsorption on Ni and Fe atoms. Variations in the PDOS of the collectors’ sulphur atoms and the surface Ni and Fe atoms were attributed to the hybridisation between sulphur 3p-orbitals and metal 3d-orbitals.
Figure 8 illustrates the PDOS for ADEDTC adsorption on Ni and Fe atoms. In
Figure 8a, the Ni site interaction is shown, which depicts that after adsorption, the Ni 3d-orbital profile just below the Fermi level (E
F) changed significantly, where a sharp peak split into two peaks, indicating orbital hybridisation with the S1 3p-orbitals. Although the sharpest peak remained unchanged, a new peak formed on the right of the sharp peak, suggesting electron transfer from S1 to Ni1. The S1 3p-orbital peaks just below E
F decreased in intensity (from above 1.0 to below 0.5 electrons/eV), confirming electron donation to Ni1 3d-orbitals and indicating that ADEDTC binds to Ni via distinctive covalent bonding.
Figure 9b shows the PDOS for ADEDTC adsorbed on Fe atoms. It was noted that the Fe 3d-orbitals exhibited changes at E
F, with a previously split peak below E
F merging into a broader splitting peak. A rise in the peak above E
F indicated electron loss, and the peak below E
F decreased in intensity (from just below 2.0 to slightly above 1.5 electrons/eV), demonstrating electron depletion from Fe1 3d-orbitals. Meanwhile, the S1 3p-orbitals showed a merged peak pattern with minor changes as the S1 atom desorbed from the Fe atom. Overall, the changes suggested that Fe1 atoms transferred electrons to neighbouring surface sulphur atoms following the adsorption behaviour of desorption of the collector.
The PDOS for IPDETC adsorption is shown in
Figure 9. In
Figure 9a, the Ni site adsorption was examined, which indicated that after adsorption, the Ni 3d-orbitals showed a peak split into four component peaks just below E
F. This was caused by interaction with S1 3p-orbitals, accompanied by a general reduction in state density. The S1 3p-orbital peaks also decreased evidently (from above 1.0 to below 0.5 electrons/eV), indicating electron transfer to the Ni atoms, again suggesting covalent bonding.
Figure 9b illustrates the PDOS for IPDETC on Fe atoms. The Fe 3d-orbitals featured a sharper peak just below E
F with minor side splitting. The peak above E
F shifted closer to E
F and decreased in intensity, suggesting electron gain by the Fe1 atom. The Ni1 atom 3d-orbital sharp peak was observed to move closer to the E
F, suggesting hybridisation with the S1 3p-orbitals. At the same time, the S1 3p-orbital peaks just below E
F nearly vanished, indicating electron loss. These changes imply that the Fe1 atom accepted electrons from the S1 atom of the collector, confirming a covalent interaction.
The projected density of states (PDOS) for horizontal adsorption of the SMBT collector on Ni and Fe atoms of the Fe
5Ni
4S
8 plane surface of (311) is shown in
Figure 10. It was observed that the PDOS for SMBT adsorption on the Ni site exhibited a minor splitting of the 3d-orbital peaks just below the Fermi level (E
F), which led to an increase in the density of states (see
Figure 10a). Notably, at the E
F, the conduction band (CB) peak shifted toward the valence band (VB), with a smaller peak fully moving into the VB, suggesting electron transfer from the sulphur atoms to the Ni1 atoms. Moreover, a small peak emerged at the E
F, suggesting movement of the peaks toward the VB. Additionally, the two highest peaks of the S1 3p-orbital just below E
F significantly decreased in intensity after adsorption. It was noted that some states near the E
F shifted toward the CB. This behaviour indicated electron donation from S1 to the Ni1 atom, pointing to covalent bonding between SMBT and the Ni1 atom.
In
Figure 10b, the PDOS for horizontal adsorption of SMBT on Fe atoms is presented. The Fe1 3d-orbitals showed a reduction in state density just below E
F (dropping from about 2.0 to slightly over 1.0 electrons/eV). Meanwhile, the peak just above E
F increased, implying electron transfer from Fe1 to the sulphur atom. The S2 3p-orbitals also showed significant changes, with the two main peaks below E
F decreasing in intensity and splitting into several smaller peaks. In addition, the conduction band peaks shifted closer to E
F and showed a slight increase in state density at E
F. These changes suggested that the S2 atom accepted electrons from Fe1 atom, forming a back-donation covalent bond.
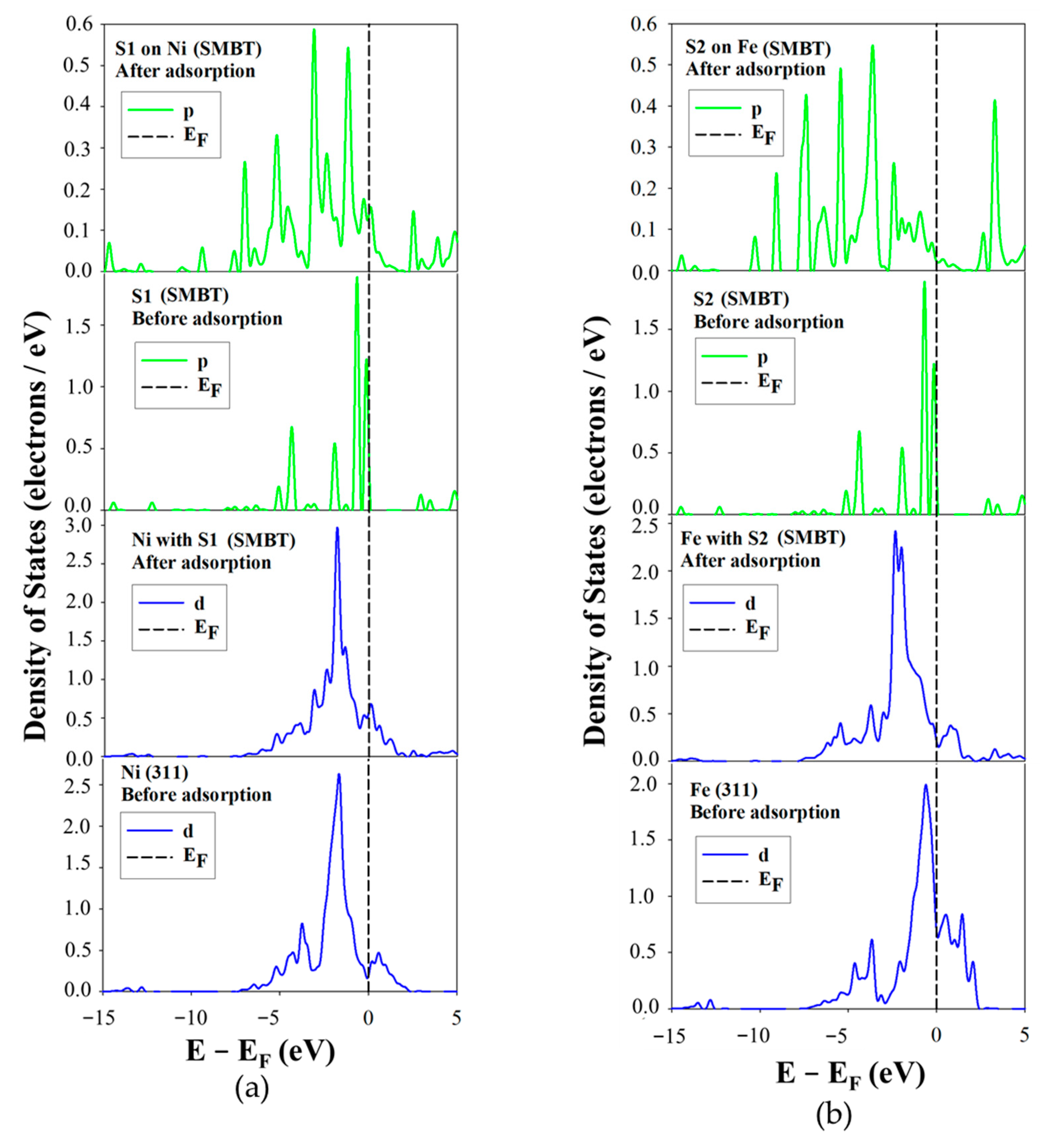
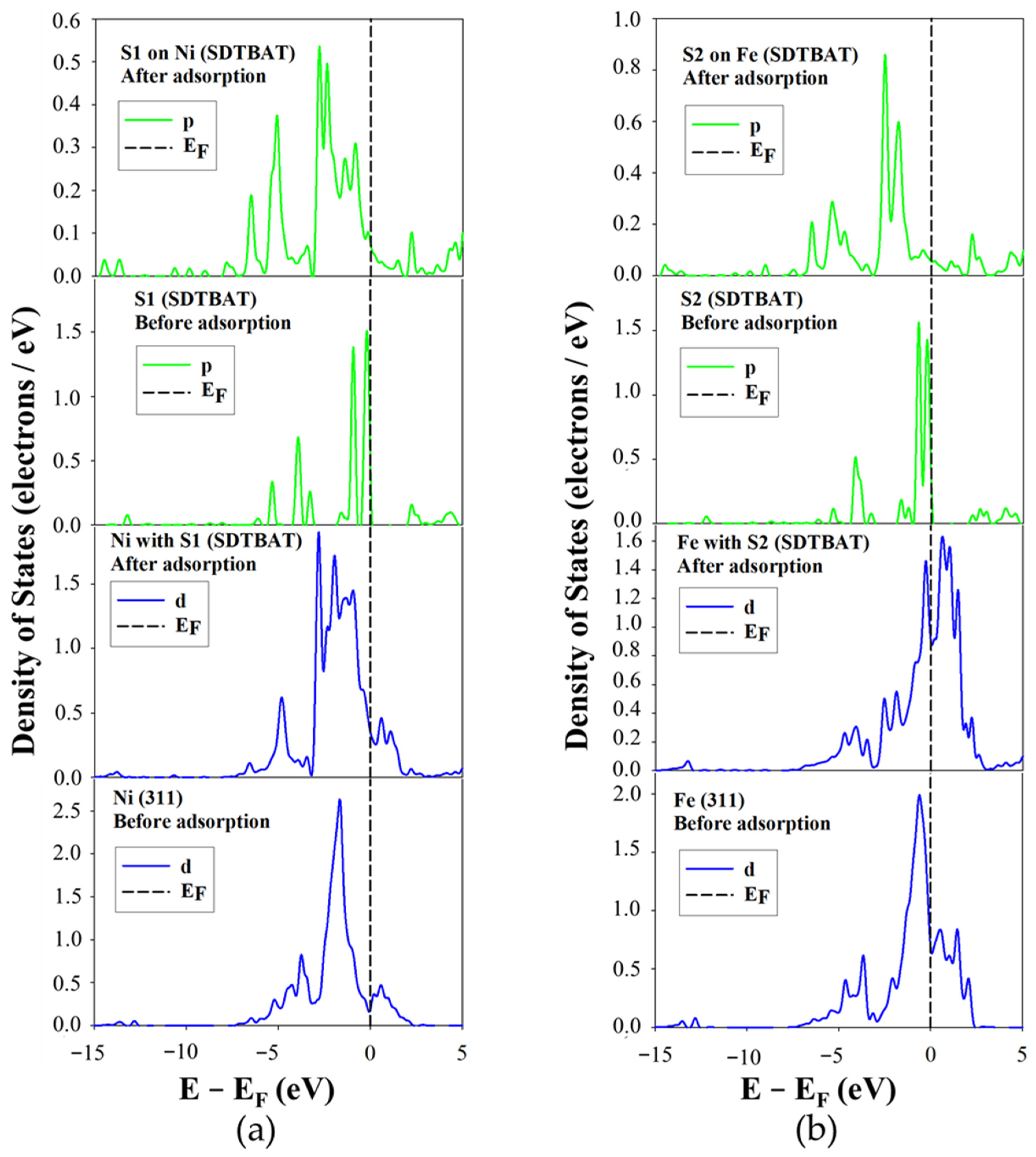
The projected density of states (PDOS) for SDTBAT collector adsorption on Ni and Fe atoms of the Fe
5Ni
4S
8 pentlandite (311) surface is illustrated in
Figure 11. Clearly the PDOS corresponding to SDTBAT adsorption on the Ni site also exhibited a broad 3d-orbital splitting peak just below the E
F, resulting in a reduction in the density of states. Additionally, at E
F, the states shifted toward the conduction band (CB), indicating that Ni atoms lost electrons to the sulphur atoms.
Meanwhile, the two highest peaks of the S1 3p-orbital just below E
F significantly decreased in intensity, while some new states appeared at E
F, suggesting that the S1 atom gained electrons from Ni atoms. This pattern indicated that SDTBAT binds to Ni atoms through a back-donation covalent mechanism.
Figure 11b presents the PDOS for SDTBAT adsorption on Fe atoms. After adsorption, the sharp Fe 3d-orbital peak just below E
F decreased in intensity, dropping from approximately 2.0 to below 1.5 electrons/eV. Notably, the peak just above E
F increased significantly, surpassing the peak at the conduction band. This implied an electron transfer from Fe1 to the sulphur atoms. The S1 3p-orbital peaks just below E
F also decreased considerably and shifted toward lower energy levels, with a noticeable increase in states at the E
F. This indicated that the S1 atom accepted electrons from the Fe 3d-orbitals, confirming the formation of a back-donation covalent bond.
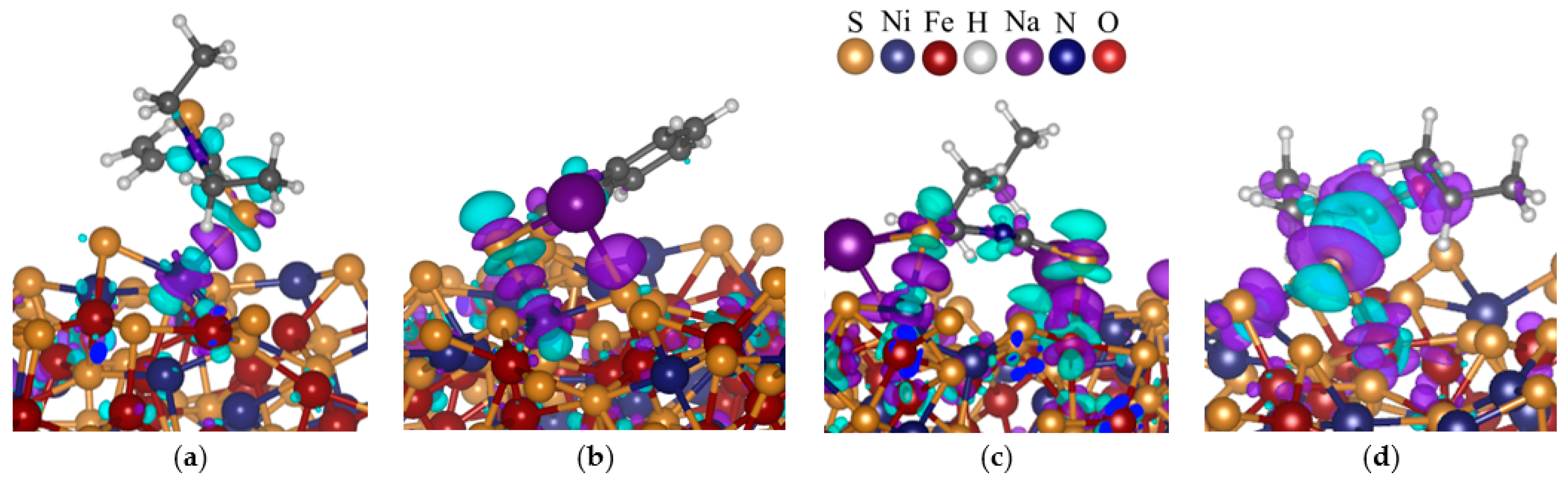
The charge density differences were computed to complement the density of states and clearly depicted which atoms gained or lost charges, as shown in
Figure 12. It was noted that both metals (Ni and/or Fe) and the S atoms had cyan cloud colour above and beneath the atom, with purple cloud colour around the atom. The purple colour was also observed on the bond between the metal and S atoms. These indicated that the electrons were largely localised on the bond, suggesting a covalent bond formation.