Abstract
This study investigated the solvent extraction of a high-nickel-content metal solution using nickel-preloaded extractants. A synthetic high-nickel lithium-ion battery (LIB) black mass leachate was prepared to extract Cu, Al, and Mn using Ni-preloaded D2EHPA (Ni-D2EHPA). Then, Co was extracted from the raffinate using Ni-preloaded PC88A (Ni-PC88A). The results showed that Ni-preloaded D2EHPA extracted more than 99% of the Al, Cu, and Mn. Co was also co-extracted at a rate of 53%, but 99% of the Co was scrubbed with 0.2 M H2SO4. Co was extracted from the raffinate using Ni-PC88A at a rate of 99% with 1.0 O/A. Finally, 99% of the Co in the organic phase was stripped using 2.0 M sulfuric acid. After Co extraction using Ni-PC88A, 80 g/L Ni and 1.38 g/L Li remained in the raffinate. Crude nickel sulfate was produced from the raffinate after precipitation of Li as lithium carbonate.
Keywords:
preloading; solvent extraction; lithium-ion battery; recycling; PC88A; D2EHPA; critical minerals 1. Introduction
The global market for electric vehicles (EVs) has grown dramatically owing to environmental regulations on conventional vehicles with internal combustion engines. This has resulted in global market growth of lithium-ion batteries (LIBs) for electric vehicles, as well as the generation of end-of-life (EOL) LIBs. The annual growth rate of the global LIB market for EVs is approximately 32%, and the LIB supply in 2030 is expected to increase to approximately 3670 GWh. In addition, the generation of EOL LIBs for EVs is expected to grow and is estimated to reach approximately 1322 GWh by 2040 [1,2].
EOL LIBs contain lithium (Li), nickel (Ni), and cobalt (Co), which are their main cathodic materials. These materials are regarded as critical minerals because they are maldistributed, which means that mining and production occur in different countries. This can threaten the global supply of materials through international trade disputes [3,4]. Therefore, recycling EOL LIBs can be an alternative for reducing the supply chain risk of critical minerals. LIBs also contain impurities such as iron (Fe), aluminum (Al), copper (Cu), and manganese (Mn). Therefore, an appropriate recycling process for EOL LIBs that can separate and purify Li, Ni, and Co is essential [5,6].
Hydrometallurgical processes can also be used to recycle spent LIBs. To recover metals via hydrometallurgy, spent LIBs should be pretreated. Various pretreatment processes can be applied, depending on the battery type and technology. Generally, battery packs are deactivated and dismantled. The dismantled cells are then pyrolyzed to remove organic binders and carbon black for comminution processes, such as crushing and grinding. Black mass, metal-concentrated oxide materials, can then be produced as the final product of the pretreatment [7,8].
The black mass can be dissolved to recover metals as the solution phase during leaching. The metal ions must be purified and concentrated to produce metal products, such as nickel sulfate (NiSO4), cobalt sulfate (CoSO4), and lithium carbonate (Li2CO3), for the reproduction of cathodic materials [9,10,11]. Processes for removing impurities and refining target metals include precipitation, solvent extraction, adsorption, and ion exchange. Among these, solvent extraction is essential for selectively purifying and concentrating target metal ions from a mixture of metal ions using an organic extractant [12,13,14,15].
Solvent extraction can be used to separate, purify, and concentrate target metals. The principle of solvent extraction is the exchange of protons between the organic and target metal ions in the aqueous phase, as shown in Equation (1):
where M2+ is the metal ion and RH is the organic extractant.
[M2+]aq. + 2[RH]org. = [R2M]org. + 2[H+]aq.
Once metal ions are extracted from the organic phase, protons are released into the aqueous phase. This leads to a decrease in pH, which decreases extraction efficiency. As proton concentration is the driving force of solvent extraction, the equilibrium pH should be maintained to achieve efficient metal extraction. Saponification can be applied to maintain the equilibrium pH during the solvent extraction phase.
Saponification is the process in which protons are replaced with sodium ions (Na+). Once Na+ ions are loaded after saponification, metal ions exchange with them, as shown in Equation (2).
[M2+]aq. + 2[RNa]org. = [MR2]org. + 2[Na+]aq.
Saponification can maintain extraction efficiency because the equilibrium pH can be maintained during the extraction reaction. However, Na+ may remain in the aqueous phase after solvent extraction. They then accumulate and inevitably contaminate the final metal products in the raffinate. In addition, excessive Na+ in the aqueous phase increases the wastewater sodium sulfate concentration, which can be a potential pollutant once released into the environment [16]. Therefore, preloading can be used in place of saponification [17,18].
Preloading is an alternative to saponification. The principles of the two processes are similar, as they prevent a pH drop during extraction. The difference lies in the loading of the metals in the organic phase. During the preloading process, metal ions other than Na+ react with the organic phase and replace the protons in the organic phase, as shown in Equation (3).
where N denotes the preloaded metal.
[Nx+]aq. + x[RH]org. = [RxN]org. + x[H+]aq.
Once the preloaded organic ([RxN]org) reacts with the aqueous phase containing the target metals, an ion exchange reaction between Nx+ and Mx+ occurs without a decrease in pH, as shown in Equation (4). This prevents a decrease in solvent extraction efficiency.
[Mx+]aq. + [RxN]org. = [RxM]org. + [Nx+]aq.
In addition, it is possible to collect concentrated metal solutions in the raffinate, which can be used as the final product for crystallization or electrowinning after additional purification [19].
Preloading has been studied using various extractants for recovering Ni and Co from impurities. Oliver et al. studied the Ni-preloading of Cyanex 272 to recover Ni and Co from the sulfuric acid-based leachate of a platinum group metals (PGMs) converter matte. The authors used Ni-preloaded Cyanex 272 (Ni-Cyanex 272) (Cyanex 272, Syensqo, Brussels, Belgium) to recover 99% of the cobalt in the organic phase and successfully recovered 99% Co with 0.5 M sulfuric acid. They reported the production of refined NiSO4 in the raffinate of Co and Fe solvent extraction using Ni-Cyanex 272 [16]. Chen et al. used Ni-loaded Mextral®272P (Ni-Mextral®272P) (Mextral®272P, KopperChem, Chongqing, China) to extract Co from the pregnant leach solution (PLS) of spent NCM LIBs with Cu, Mn, and Fe removed. Cobalt % was extracted at a rate of 99 when 20% volume of Ni-Mextral®272P was used with A/O = 2.0 at 25 °C. Hydroxide precipitation was then carried out on the raffinate to collect Ni in the form of Ni(OH)2 [17]. Chen et al. also suggested the preloading of D2EHPA (Shandong Lanhai Industry, Jinan, China) with Co (Co-D2EHPA) to extract Mn from Ni-, Co-, and Mn-containing PLS in waste cathode materials from spent LIBs. They first precipitated Ni using dimethylglyoxime and extracted 99% of the Mn using Co-D2EHPA. Finally, they recovered Co and Li in the form of CoC2O4·2H2O (cobalt oxalate) and Li2CO3, respectively [18].
Some solvent extraction studies using preloading have been conducted for the recovery of metals from the leachate of low-Ni nickel–cobalt–manganese (NCM) black mass or PGM converter mattes. Meanwhile, the demand for high-nickel NCM batteries containing over 60% Ni in cathodic materials is expected to increase owing to their high energy density, but decrease production cost owing to the smaller cobalt portion [20,21]. Therefore, the hydrometallurgical recycling of spent high-nickel LIBs also needs to be investigated. However, only a few studies have focused on solvent extraction using preloading for metal recovery from high-Ni NCM LIBs.
This study focuses on the recovery of Co and Ni from Ni-preloaded D2EHPA (Ni-D2EHPA) to extract Al, Cu, and Mn from high-Ni-content PLS. Subsequently, the aqueous phases of the Ni and Co solutions were recovered to separate Co and Ni using Ni-preloaded PC88A (Ni-PC88A) (PC88A, DAIHACHI Chemical Industry, Osaka, Japan). This concept is believed to shorten the solvent extraction time required to separate Al, Cu, Mn, Co, and Ni from the leachate of NCM (nickel, cobalt, and manganese) black mass by excluding the Ni solvent extraction circuit.
2. Materials and Methods
2.1. Solvent Extraction Chemicals
In this study, a synthetic leachate of high-Ni black mass was prepared for solvent extraction tests. The solution was produced using reagent-grade metal sulfates (Al2(SO4)3·14–18H2O, CuSO4·5H2O, MnSO4·5H2O, CoSO4·7H2O, NiSO4·6H2O, and Li2SO4·H2O) dissolved in deionized water (DI water). Two types of solutions were prepared. Solution 1 was prepared to simulate the solution after the precipitation of Fe and Al from the leachate of high-nickel black mass. The solution contains Ni 33.0 g/L, Co 15.0 g/L, Mn 17.0 g/L, and Li 1.5 g/L. The composition of the solution seems similar to previous research with high-nickel black mass. Koo et al. (2024) conducted solvent extraction tests with leachate from black mass containing Ni 36.3 g/L, Co 12.4 g/L, Mn 10.96 g/L, and Cu 2.86 g/L [22]. In addition, Zou et al. (2024) studied the leaching of high-nickel black mass combined with LFP black mass, and the composition of the leachate is Ni 13.8 g/L, Co 8.8 g/L, Mn 7.6 g/L, and Li 6.7 g/L [23]. Based on the results, the range of elemental composition in this study is within the range of the literature reviews, and the composition of the solution seems appropriate for solvent extraction studies.
Solution 2 was prepared to mimic the aqueous phase after the extraction of Mn, Cu, and Al. The compositions of solutions 1 and 2 are listed in Table 1 and Table 2, respectively.

Table 1.
Composition of the synthetic solution used in Al, Cu, and Mn solvent extraction.

Table 2.
Composition of the synthetic solution used in the Co solvent extraction.
Two extractants, di-(2-ethylhexyl)phosphoric acid (D2EHPA) and 2-ethylhexyl 2-ethylhexyphosphonic acid (PC88A), were used, and the solvents were diluted using Exxsol D-80 (Exxon Mobil Chem. Exxon Mobil, Baytown, TX, USA). Finally, NiSO4 solution was prepared for the preloading reagent produced by dissolving reagent-grade NiSO4·6H2O in DI water.
2.2. Experimental and Analytical Methods
In this study, organic extractants of D2EHPA and PC88A were used for the study. D2EHPA is a well-known organophosphorus acid extractant to extract Al, Cu and Mn at lower solution pH. The extractant can efficiently extract the elements relatively inexpensive and regarded as impurities in advance [17,18]. Then, Co extraction was conducted using PC88A. PC88A is an acidic phosphonate, widely used to extract cobalt. Another extractant, Cyanex 272, can be an alternative to PC88A due to the higher performance of Co separation from Ni-containing solution [22]. However, Cyanex 272 is about 2 to 4 times more expensive than PC88A, and this makes PC88A more encouraging to apply to cobalt extraction [24]. In terms of economic operation, PC88A was used for preloading studies.
Preloading, Ni ions were loaded on the extractants before the solvent extraction tests. First, 40% NaOH solution was used to saponify the PC88A and D2EHPA extractants without pH control. The saponification rate was set between 60% and 100% based on the extractant concentration in the organic phase. Following saponification, a NiSO4 solution was prepared for preloading. The saponified organic phase was then preloaded with the NiSO4 solution. Preloading was tested to investigate the effects of extractant concentration and saponification rate.
Subsequently, solvent extraction was performed to extract Al, Cu, and Mn from solution 1 using Ni-preloaded D2EHPA and Co from solution 2 using Ni-preloaded PC88A. Solvent extraction was performed using three-stage batch simulation tests to investigate the extraction behavior of each element. Subsequently, the extracted organic phases were scrubbed and stripped using sulfuric acid. The detailed methodology of the batch simulation tests is shown in Figure 1. After completing preloading and solvent extractions, the metal solutions in the aqueous phase were analyzed using inductively coupled plasma-atomic emission spectrometry (ICP-AES) (PerkinElmer/Optima-4300DV, Shelton, CT, USA). The percentages of extraction, scrubbing, and stripping were calculated using Equations (5)–(7).
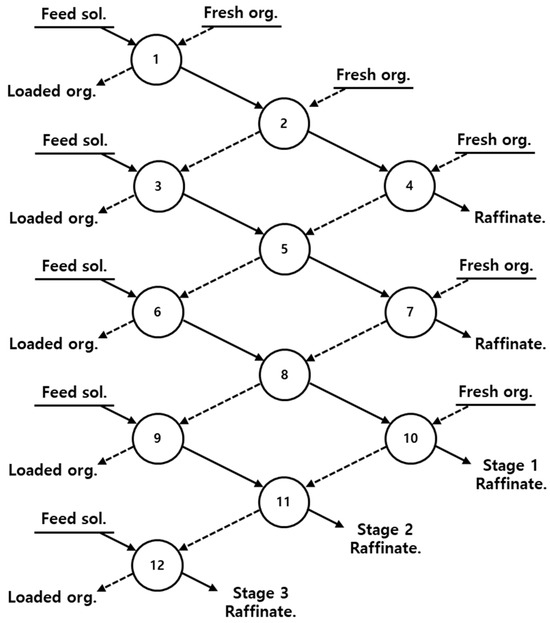
Figure 1.
Batch simulation of three-stage counter-current extraction.
3. Results
3.1. Extraction of Al, Cu, and Mn with Ni-Preloaded D2EHPA
3.1.1. Preloading Behavior of Co and Ni in D2EHPA
The preloading behaviors of Co and Ni were investigated using saponified D2EHPA. The degree of saponification was maintained between 60% and 100% in 1.2 M D2EHPA. The metal solutions were prepared using Ni or Co at a concentration of 0.42 mol/L. This was approximately 70% of the extraction capacity of the prepared saponified D2EHPA for preloading Ni or Co. Preloading was conducted at an O/A ratio of 1.0 at 25 °C. As shown in Figure 2, higher Co and Ni extraction occurred at higher degrees of saponification. At a saponification rate of 80%, the Co and Ni preloading efficiencies were 95% and 84%, respectively. By increasing the saponification rate to 100%, the Ni preloading efficiency increased to 93%. Therefore, preloading of Ni and Co in D2EHPA is feasible. However, we observed an increase in viscosity in the organic phase, which can be explained by oligomerization of the D2EHPA extractant in the presence of cobalt. This led to an increase in the phase separation time between the organic and aqueous phases [25,26,27]. Therefore, Ni preloading is appropriate when D2EHPA is used as the extractant. The solvent extraction reaction depends on the formation of monomers and dimers, as follows:
where M2+ is the metal ion (Ni or Co) and RH is an organic extractant (D2EHPA).
Dimer: [M2+]aq. + 2[R2H2]org. = [M·(HR2)2]org. + 2[H+]aq.
Monomer: [M2+]aq. + 2[RH]org. = [MR2]org. + 2[H+]aq.
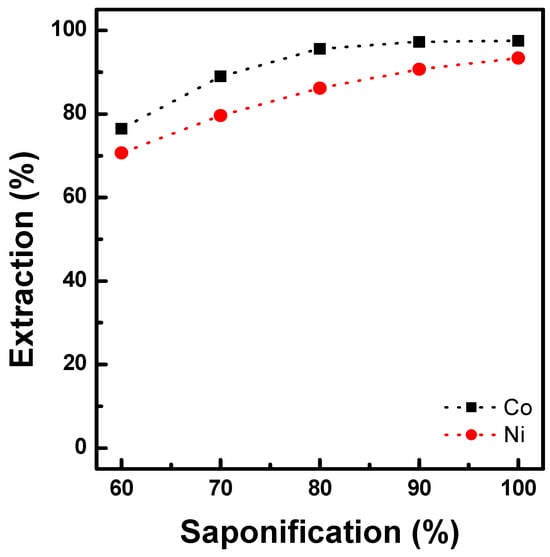
Figure 2.
Effect of saponification on the extraction of Co & Ni with 1.2 M D2EHPA at O/A 1.0 and 25 °C.
The saponification reaction with the extractant (D2EHPA or PC88A) and metal extraction with saponified organic compounds can be expressed as follows: After saponification, the dimers are converted into monomers, which can then be extracted.
2[Na+]aq. + [R2H2]org. = 2[Na·R]org. + 2[H+]aq.
[M2+]aq. + 2[Na·R]org. = [MR2]org. + 2[Na+]aq.
3.1.2. Extraction Behavior of Al, Cu, and Mn with Ni-D2EHPA
The extraction of Al, Mn, and Cu was tested with Ni-preloaded D2EHPA (Ni-D2EHPA), with 80%–100% saponification followed by Ni preloading at an O/A ratio of 1 and 25 °C. Synthetic solutions containing 3.0 g/L Al, 1.5 g/L Cu, and 17.0 g/L Mn were prepared for the extraction tests, and the results are shown in Figure 3. Al extraction was above 95%, regardless of the saponification rate. Meanwhile, the extraction of Cu and Mn increased to 84% and 83%, respectively, when the saponification rate increased from 80% to 100%. This can be explained by the increase in the equilibrium pH in the aqueous phase, which enhances metal extraction in the organic phase.
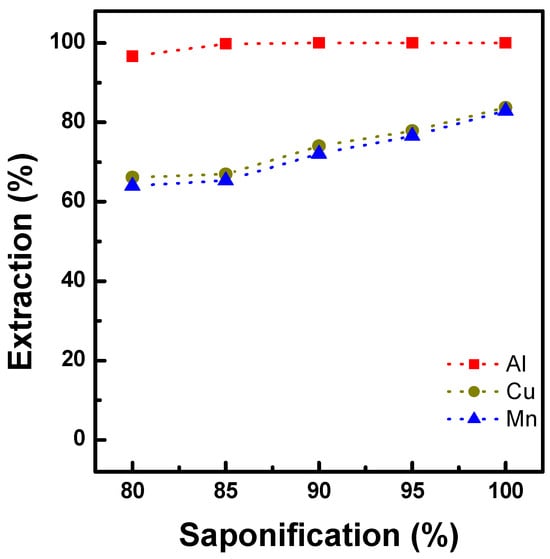
Figure 3.
Effect of saponification (%) on the extraction of Al, Cu, and Mn with 1.2M D2EHPA at O/A 1.0 and 25 °C.
Figure 4 shows metal extraction from the synthetic solution in Table 1 using Ni-D2EHPA at various concentrations of the organic phase under 80% saponification conditions. When the concentration of Ni-D2EHPA increased, the extraction of Al, Cu, Mn, and Co improved. In addition, more than 99% Al, 84% Cu, and 83% Mn were observed. Co was also extracted at 45%; however, the extraction rate of Li was approximately 7%, and most of the Li remained in the raffinate.
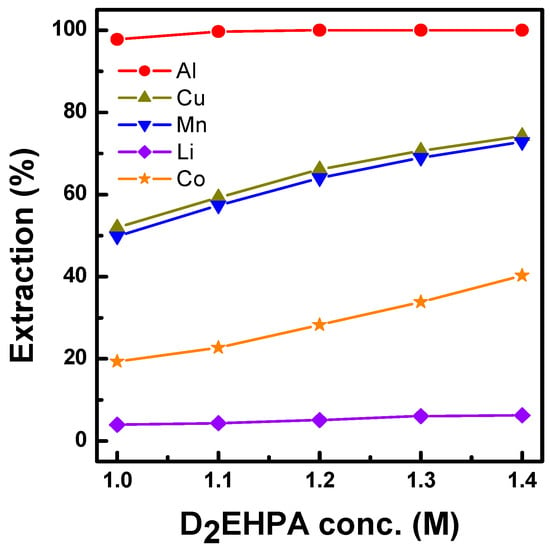
Figure 4.
Effect of 80% saponified Ni-D2EHPA concentration. on the extraction of Al, Cu, and Mn at O/A 1.0 and 25 °C.
3.1.3. Three-Stage Batch Simulation of Solvent Extraction by Ni-D2EHPA
A three-stage batch simulation of solvent extraction was conducted using Ni-preloaded (32.77 g/L) and 80%-saponified 1.2 M D2EHPA to investigate the Al, Cu, and Mn extraction behaviors. The extraction tests were conducted at equilibrium pH between 3.5 and 4.4 with an O/A ratio of 1.0 at 25 °C. Figure 5 shows the mass balance of the metals during each extraction phase using Ni-loaded D2EHPA. After three sequential extraction phases, the final organic phase contained 3.000 g/L of Al, 1.495 g/L of Cu, and 16.85 g/L of Mn, all of which had extraction percentages above 99%. Therefore, it is feasible to extract Al, Cu, and Mn impurities using Ni-loaded D2EHPA. Meanwhile, the Ni concentration in the raffinate after the extraction phase increased from 33 to 65.77 g/L as 99% of the Ni in the Ni-preloaded D2EHPA was distributed to the aqueous phase. Co and Li remained in the final raffinate at concentrations of 7.05 and 1.27 g/L, respectively; thus, purified Li, Co, and Ni solutions could be produced. Meanwhile, 53% of Co was extracted, with 7.95 g/L in the organic phase. Li (0.23 g/L) was then loaded into the organic phase. Because the co-extracted Li and Co were present in the organic phase, scrubbing tests were conducted to redistribute the metals to the aqueous phase.
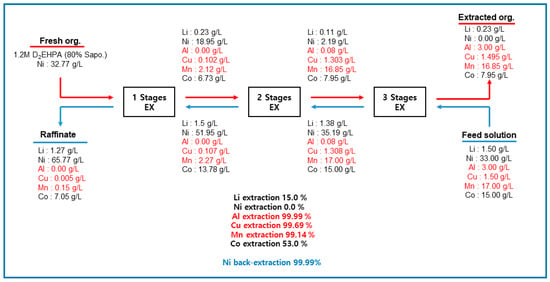
Figure 5.
Mass balance of metal ions after 3-stage simulation tests using 80% Saponified 1.2M D2EHPA, O/A 1.0 at 25 °C (Red color: Target materials in the circuit, red underline; organic phase, blue underline: aqueous phase).
3.1.4. Scrubbing and Stripping Behavior After Extraction with Ni-D2EHPA
The scrubbing of Co and Li was tested using the organic phase after three sequential extractions of acidic Al, Cu, Mn, Co, Ni, and Li solutions. The compositions of the metals in the organic phase are listed in Table 3. First, sulfuric acid (a scrubbing agent) concentrations from 0.02 to 0.3 M were tested to investigate their effect. As shown in Figure 6, the scrubbing efficiency increased for all metals, except Al, with increasing sulfuric acid concentration, and 99% of Li was scrubbed from the organic phase. At a sulfuric acid concentration of 0.1 M, 86% of Co and 19% of Mn were scrubbed, and the efficiency improved to >99% and 55% with 0.2 M sulfuric acid, respectively. The effects of the O/A ratio were also tested by varying it from 1.0 to 5.0. Figure 7 shows that the Co scrubbing efficiency decreased from 99% to 40% as the O/A ratio increased from 1.0 to 5.0. Based on the results, a McCabe–Thiele diagram was constructed, as shown in Figure 8, to calculate the theoretical Co scrubbing stage. The results data set of cobalt scrubbing from Figure 7 was collected. Then, the cobalt concentration in both the organic and aqueous phases was plotted at 25 °C as an isotherm graph. Based on the McCabe–Thiele diagram, 99% Co could be scrubbed in two stages from the organic phase containing 7.85 g/L Co using 0.2 M H2SO4 at an O/A ratio of 0.9.

Table 3.
Composition of organic phase used in scrubbing test.
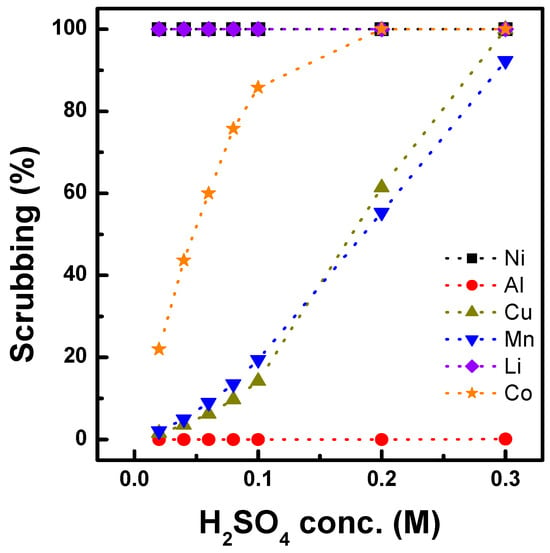
Figure 6.
Effect of H2SO4 concentration on scrubbing process with O/A 1.0 at 25 °C.
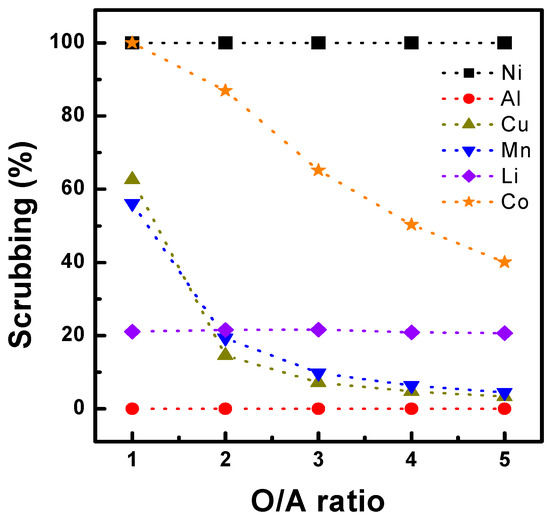
Figure 7.
Effect O/A ratio on scrubbing using 0.2M H2SO4, at 25 °C.
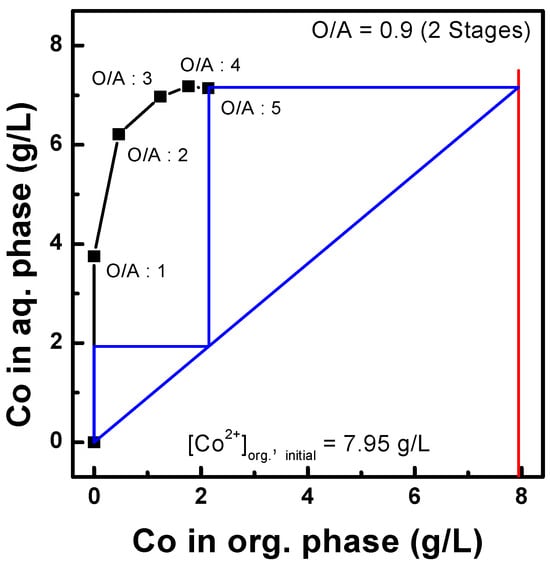
Figure 8.
McCabe–Thiele diagram on Co scrubbing using 0.2M H2SO4, at 25 °C.
Stripping of Al-, Cu-, and Mn-loaded organics was conducted after the scrubbing of Co in the organic phase at O/A 1.0. Stripping tests were conducted with the organic phase after solvent extraction of the synthetic solution (Table 1) with Ni-D2EHPA, followed by Co scrubbing with 0.2 M H2SO4. Sulfuric acid was used as the stripping agent at concentrations between 0.5 and 5.0 M, and the temperature varied between 25 °C and 60 °C. Figure 9 shows that 99% of Cu and Mn were stripped at 25 °C, regardless of sulfuric acid concentration. However, in the case of Al, a low sulfuric acid concentration was unsuitable for stripping in the organic phase. By increasing the sulfuric acid to 5.0 M concentration, 55% of the Al was stripped. Additionally, Al stripping was enhanced to 99% by increasing the temperature to 30 °C, as shown in Figure 10, with 4.0 M sulfuric acid. Furthermore, lower sulfuric acid concentrations of 2.0 M achieved 99% Al stripping efficiency at a higher temperature of 40 °C. The stripping efficiency increased with increasing temperature. Therefore, over 99% of Al, Cu, and Mn could be stripped by increasing both the sulfuric acid concentration and the temperature.
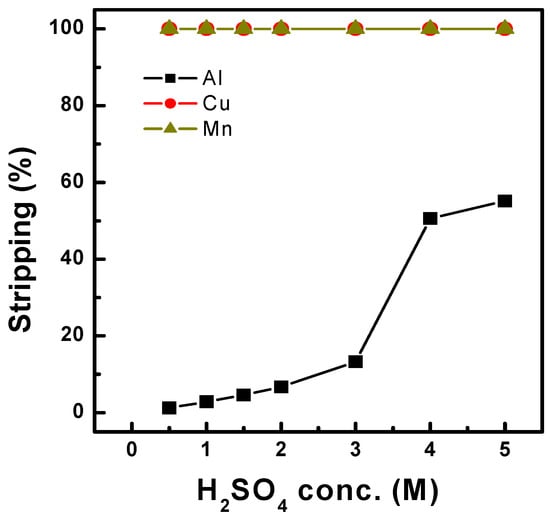
Figure 9.
Effect of H2SO4 conc. on the stripping of Cu, Mn, and Al at O/A 1.0 and 25 °C.
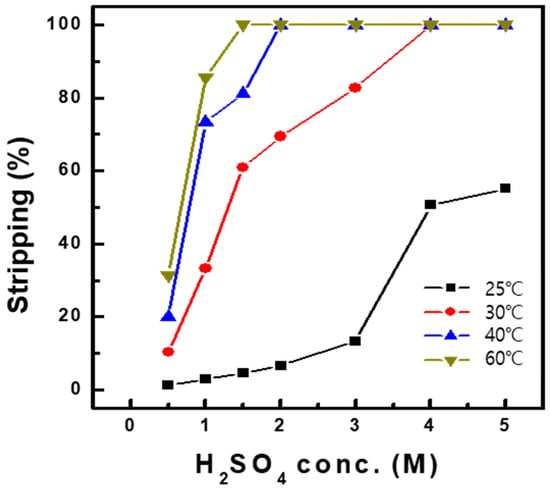
Figure 10.
Effect of H2SO4 concentration and temperature on the stripping of Al at O/A 1.0.
3.2. Extraction of Co with Ni Preloaded PC88A
3.2.1. Preloading of Ni in PC88A
Co solvent extraction tests using Ni-preloaded PC88A (Ni-PC88A) were conducted using the raffinate obtained from the solvent extraction of Al, Cu, and Mn from a synthetic black mass leaching solution. First, Ni was preloaded at 0.8 M PC88A reacting with a NiSO4 solution containing 14.97 g/L Ni at an O/A ratio of 1.0 at 25 °C. The degree of saponification of the organic phase varied from 60% to 85%. As shown in Figure 11, the nickel preloading efficiency increased with increasing saponification. Ni (85%) was preloaded with 60% saponification, which loaded 14.24 g/L Ni in the organic phase. It then increased to 97% when saponification increased to 80%, resulting in a Ni concentration of 16.29 g/L in the organic phase. When Ni was preloaded, the Na loaded in the organic phase was distributed to the aqueous phase by ionic exchange with Ni ions from the aqueous phase.
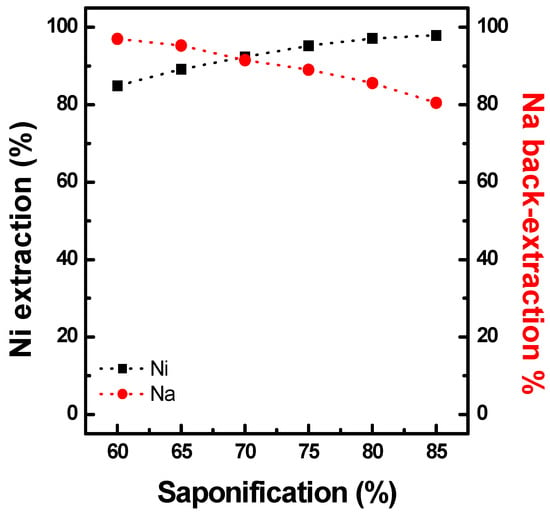
Figure 11.
Effect of saponification on Ni preloading using 0.8M PC88A at O/A 1.0 and 25 °C.
3.2.2. Extraction of Co with Ni-PC88A
After preloading PC-88A with Ni, Co was extracted from a synthetic solution using Ni-PC88A, the composition of which is shown in Table 2. The Co extraction increased from 80% to 95% by increasing the degree of saponification from 60% to 80%, as shown in Figure 12. Simultaneously, the preloaded nickel in the organic phase was back-extracted to the raffinate. In addition, an increase in Co extraction and a decrease in Ni back-extraction were observed at higher degrees of saponification. This can be explained by the Co ions in the aqueous phase exchanging with priority to the remaining Na+ rather than the preloaded Ni ions exchanged in the organic phase. A higher degree of saponification indicates that more Na+ remained in the organic phase; therefore, the remaining Na was initially exchanged with Co. This was followed by ion exchange between Ni in the organic phase and Co ions in the solution. Therefore, the concentration of the extractant and degree of saponification should be controlled to ensure 100% back-extraction of Na+ in the organic phase before cobalt extraction with Ni-preloaded PC88A. The Li-extraction efficiency of Ni-PC88A was less than 1% for all saponification conditions.
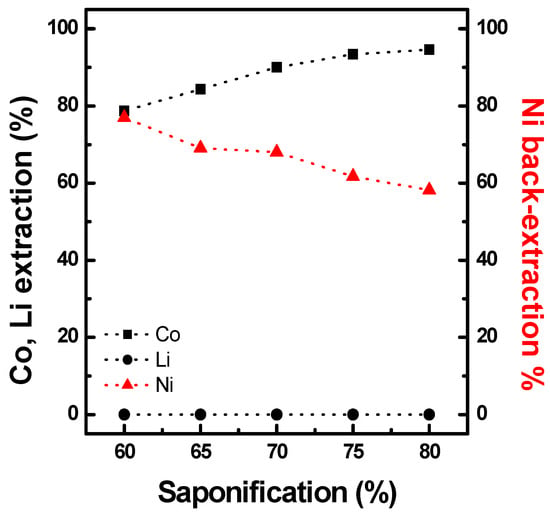
Figure 12.
Effect of saponification on the extraction of Co, Ni, and Li using 0.8M PC88A at O/A 1.0 and 25 °C.
3.2.3. Three-Stage Batch Simulation of Solvent Extraction by Ni-PC88A
Co was separated from Li-, Co-, and Ni-containing solutions using PC88A with 65% saponification followed by 15.41 g/L Ni preloading. Three-stage batch simulation tests were conducted, and the mass balance of the metal ions is shown in Figure 13. The equilibrium pH for extraction was initially set at 4.3, and the final pH increased to 6.3 with an O/A ratio of 1.0 at 25 °C. The initial Co ion concentration was 15.0 g/L in the aqueous phase, and 14.91 g/L was extracted to the organic phase, equivalent to a Co extraction efficiency of 99%. Of Li, 7.8% was also co-extracted, with a concentration of 0.12 g/L in the organic phase. Meanwhile, about 97% of the preloaded Ni ions were redistributed from the organic phase to the aqueous phase. The final Ni concentration in the aqueous phase was 80 g/L. Also, the concentrations of Li and Co in the aqueous phase were 1.38 and 0.09 g/L, respectively, so NiSO4 can be produced from the raffinate.
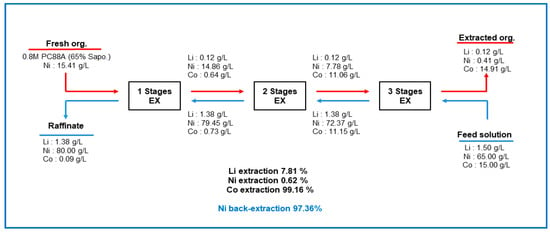
Figure 13.
Mass balance of metal ions after 3 stages simulation test by using Ni preloaded 0.8M PC88A at O/A 1.0 and 25 °C (Red underline; organic phase, blue underline: aqueous phase).
3.2.4. Stripping of Co After Extraction with Ni-PC88A
Co stripping was conducted using an organic phase containing 14.91 g/L of Co ions after the three-stage batch simulation tests. Figure 14 shows the stripping efficiency under different concentrations of sulfuric acid as a stripping agent with an O/A ratio of 1.0 at 25 °C. Co stripping above 99% was observed between 1.0 and 5.0 M sulfuric acid. In addition, the effects of the O/A ratio were investigated by varying the ratio between 1.0 and 10.0, as shown in Figure 15. An O/A ratio of 1.0 achieved more than 99% Co stripping, but it started to decrease at an O/A ratio of 5.0, and 74% stripping was observed at 10.0. Therefore, a higher O/A ratio resulted in lower stripping efficiency. Finally, a McCabe–Thiele diagram was constructed, as shown in Figure 16. The results data set of cobalt stripping from Figure 15 was collected. Then, the cobalt concentration in both the organic and aqueous phases was plotted at 25 °C as an isotherm graph. Finally, the target concentration of cobalt was set to 14.91 g/L and slope of operation line was set to 7.0 based on the optimization condition of O/A from Figure 15. The McCabe–Thiele diagram showed that 104.37 g/L Co could be stripped from the organic phase (initial Co concentration of 14.91 g/L) within three stages using 2.0 M sulfuric acid with an O/A ratio of 7.0. This means that cobalt of 14.91 g/L in the organic phase can be redistributed and concentrated to 105 g/L as a lower volume of sulfuric acid is used as a stripping agent. The extracted cobalt was stripped at 7 times lower aqueous phase, and a cobalt concentration of 105 g/L in the aqueous phase could be achieved.
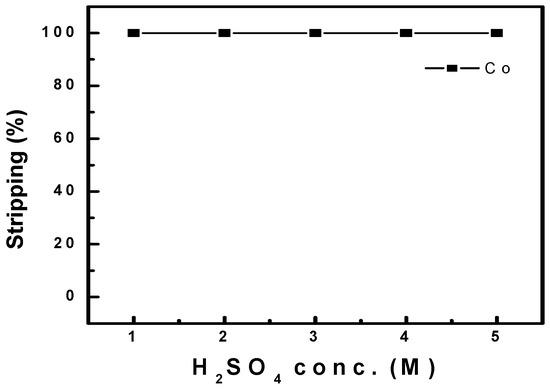
Figure 14.
Effect of H2SO4 conc. on the stripping of Co at O/A 1.0 and 25 °C.
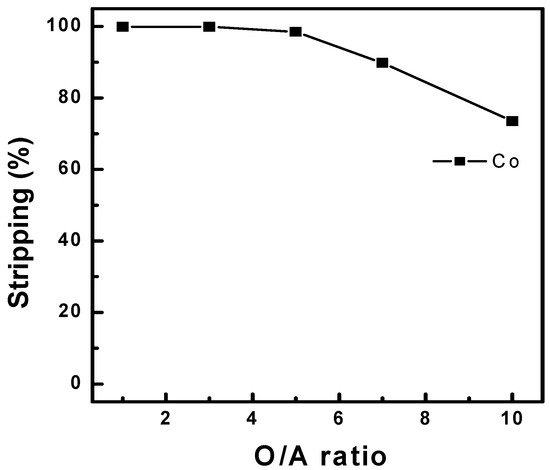
Figure 15.
Effect of O/A ratio on the stripping of Co using 2.0 M H2SO4 at 25 °C.
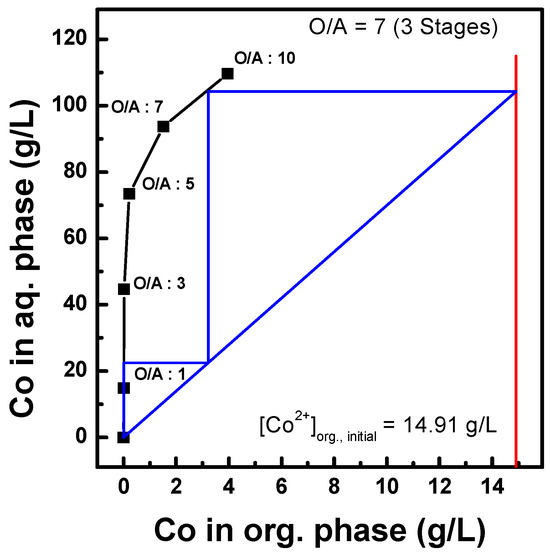
Figure 16.
McCabe–Thiele diagram of Co stripping using 2.0 M H2SO4, at 25 °C.
3.3. Overall Process Flowsheet
Figure 17 shows a novel solvent extraction process with mass balance using Ni-preloaded D2EHPA for the removal of Al, Cu, and Mn, followed by Co solvent extraction with Ni-preloaded PC88A. Al, Cu, and Mn could be extracted (99%) with 80%-saponified 1.2 M D2EHPA, followed by Ni preloading of 32.77 g/L. During the extraction, 99% of the Ni was redistributed to the raffinate; thus, the concentration of Ni in the aqueous solution increased from 33 to 65.77 g/L. Cobalt (53%) was co-extracted, but 99% of the Co from the organic could be scrubbed using 0.2 M sulfuric acid at an O/A ratio of 0.9 within two theoretical scrubbing stages. Cu and Mn in D2EHPA could be stripped at a rate above 99% using 0.5 M sulfuric acid at 25 °C. Al could be stripped efficiently when the temperature increased to 60 °C with 1.5 M sulfuric acid. The stripped D2EHPA does not contain any metal ions due to achieving 99% metal stripping efficiency, so it can be recycled to Ni preloading followed by Al, Cu, and Mn extraction circuit, again. After extracting Al, Cu, and Mn using Ni-D2EHPA, Co was separated from the raffinate containing Co, Ni, and Li using 65% saponified 0.8 M PC88A, followed by the preloading of 15.41 g/L Ni. More than 99% of Co was extracted, and 97% of Ni was redistributed to the aqueous phase. The extracted Co was then stripped using sulfuric acid and collected as a purified cobalt sulfate (CoSO4) solution. As the stripped PC88A does not contain metal ions in the organic phase, it can be recycled by preloading it again with nickel and extracting cobalt again. The Final raffinate contained 80 g/L Ni, 1.38 g/L Li, and 0.09 g/L Co, and the solution was processed for the production of NiSO4 after the additional precipitation of Li as Li2CO3. Both the cobalt sulfate solution from stripping and the nickel sulfate solution after Li removal can be crystallized. The final product from CoSO4 and NiSO4 solutions is crystallized metal sulfate salts such as CoSO4·6H2O and NiSO4·7H2O, respectively.
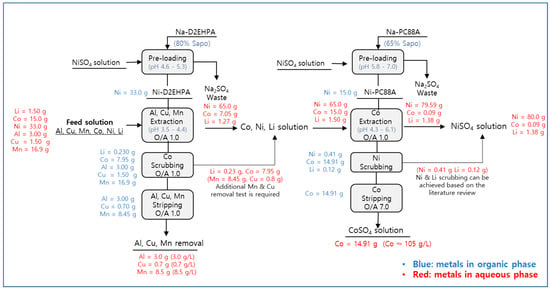
Figure 17.
Overall mass balance for the recovery of Li, Co, and Ni from the leachate of the high-nickel LIBs black mass.
Still, there are additional studies conducted to make this process available. First of all, Cu and Mn can be co-scrubbed during the scrubbing process, based on this study. When Co scrubbing tests were conducted, 60% Cu (0.8 g/L) and 50% Mn (8.45 g/L) were also scrubbed and distributed to the Co scrubbed aqueous solution. This means that an additional process is required to recover cobalt from the Cu- and Mn-contaminated solution. In order to enhance cobalt scrubbing efficiency and decrease Cu and Mn contents in the solution, scrubbing with Mn and Cu sulfate solution can be suggested. It has been reported that Mn and Cu ions in the scrubbing solution can exchange the cobalt ions in the organic phase. The scrubbing efficiency is higher than in the case when sulfuric acid was only used as a scrubbing agent [28,29]. Therefore, additional scrubbing tests using acidified Cu and Mn sulfate solution can be suggested to increase the purity of Co in the scrubbed solution.
In addition, Ni and Li scrubbing from the organic phase should also be considered after the extraction of Co using Ni-PC88A. Co extraction of 99% was achieved in this study, but Li 0.12 g and Ni 0.41 g were also co-extracted. In order to recover additional Li and Ni from the organic phase, scrubbing of Li and Ni from PC88A is essential. Nguyen et al. (2014) scrubbed 99.9% Li and Ni from PC88A using CoSO4 solution at pH 4.75 and O/A 2.0 within 2 stages. The key driving force of Ni and Li is the ionic exchange of Ni, Li in the organic and Co in the aqueous phase. Co has a higher affinity than Ni and Li at pH 4.75 of PC88A [30]. This means that cobalt has priority to be extracted in the organic phase. Then, extracted Co has ionic exchange with Li and Ni, and this makes Li and Ni distribute to the aqueous phase. Therefore, Li and Ni can be technically scrubbed to the aqueous phase and finally recovered without loss in the overall process.
4. Conclusions
Solvent extraction tests were conducted using a preloaded extractant to remove Al, Cu, and Mn and separate Co from the pregnant leaching solution of black mass from spent LIBs. The Ni-preloaded D2EHPA extracted 99% of the Al, Cu, and Mn. Subsequently, 99% of the Co was separated from the Ni-containing solution using Ni-preloaded PC88A. The raffinate was obtained as a high-purity NiSO4 solution without additional solvent extraction. In addition, Ni preloading can prevent Na contamination in both the organic and aqueous phases; therefore, it can be used to produce higher-purity metal sulfate solutions compared with conventional saponified solvent extraction. Therefore, this study suggests that solvent extraction with a preloaded extractant can be advantageous for metal production.
Author Contributions
Conceptualization, G.-G.L. and J.A. (Jaewoo Ahn); methodology, Y.-C.C.; validation, K.-H.K. and Y.-C.C.; formal analysis, K.-H.K.; investigation, K.-H.K. and J.A. (Junmo Ahn); resources, J.A. (Junmo Ahn); data curation, B.K. and Y.H.; writing—original draft preparation, K.-H.K.; writing—review and editing, J.A. (Junmo Ahn); visualization, J.A. (Junmo Ahn); supervision, J.A. (Jaewoo Ahn); project administration, G.-G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Authors appreciate efforts of grammar and wording corrections from Editage, and financial support of the article correction cost from Jeonbuk National University.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bibra, E.M.; Connelly, E.; Dhir, S.; Drtil, M.; Henriot, P.; Hwang, I.; Le Marois, J.-B.; McBain, S.; Paoli, L.; Teter, J. Global EV Outlook 2022: Securing Supplies for an Electric Future; National Academy of Sciences: Washington, DC, USA, 2022. [Google Scholar]
- Zhao, Y.; Pohl, O.; Bhatt, A.I.; Collis, G.E.; Mahon, P.J.; Rüther, T.; Hollenkamp, A.F. A review on battery market trends, second-life reuse, and recycling. Sustain. Chem. 2021, 2, 167–205. [Google Scholar] [CrossRef]
- Lehtimäki, H.; Karhu, M.; Kotilainen, J.M.; Sairinen, R.; Jokilaakso, A.; Lassi, U.; Huttunen-Saarivirta, E. Sustainability of the use of critical raw materials in electric vehicle batteries: A transdisciplinary review. Environ. Chall. 2024, 16, 100966. [Google Scholar] [CrossRef]
- Miao, Y.; Liu, L.; Zhang, Y.; Tan, Q.; Li, J. An overview of global power lithium-ion batteries and associated critical metal recycling. J. Hazard. Mater. 2022, 425, 127900. [Google Scholar] [CrossRef]
- Khatibi, H.; Hassan, E.; Frisone, D.; Amiriyan, M.; Farahati, R.; Farhad, S. Recycling and Reusing Copper and Aluminum Current-Collectors from Spent Lithium-Ion Batteries. Energies 2022, 15, 9069. [Google Scholar] [CrossRef]
- Ferreira, D.A.; Prados, L.M.Z.; Majuste, D.; Mansur, M.B. Hydrometallurgical separation of aluminium, cobalt, copper and lithium from spent Li-ion batteries. J. Power Sources 2009, 187, 238–246. [Google Scholar] [CrossRef]
- Yoo, K.; Heo, W.; Kim, B. The Enhancement of Recycling Processes Efficiency of Lithium Ion Batteries; A Review. Resour. Recycl. 2024, 33, 24–36. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, Z.; Ma, W.; Zhao, Q. Pretreatment options for the recycling of spent lithium-ion batteries: A comprehensive review. J. Energy Storage 2023, 72, 108691. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A mini-review on metal recycling from spent lithium ion batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Virolainen, S.; Wesselborg, T.; Kaukinen, A.; Sainio, T. Removal of iron, aluminium, manganese and copper from leach solutions of lithium-ion battery waste using ion exchange. Hydrometallurgy 2021, 202, 105602. [Google Scholar] [CrossRef]
- Brückner, L.; Frank, J.; Elwert, T. Industrial recycling of lithium-ion batteries—A critical review of metallurgical process routes. Metals 2020, 10, 1107. [Google Scholar] [CrossRef]
- Lee, M.-S.; Kim, S.-B.; Chae, J.-G. Adsorption of Ni (II), Co (II), and Mg (II) from Sulfuric Acid Solution by Diphonix Resin for the Utilization of Laterite Ore. J. Miner. Soc. Korea 2010, 23, 183–189. [Google Scholar]
- Agatzini-Leonardou, S.; Dimaki, D. Recovery of Nickel and Cobalt from Low-Grade Nickel Oxide Ores by the Technique of Extraction in Heaps Using Dilute Sulphuric Acid at Ambient Temperature. Greek Patent GR1001555, 22 March 1994. [Google Scholar]
- Whittington, B.; Muir*, D. Pressure acid leaching of nickel laterites: A review. Miner. Process. Extr. Metullargy Rev. 2000, 21, 527–599. [Google Scholar] [CrossRef]
- Jung, Y.J.; Son, S.H.; Park, S.C.; Kim, Y.H.; Yoo, B.Y.; Lee, M.S. Study on selective lithium leaching effect on roasting conditions of the waste electric vehicle cell powder. Resour. Recycl. 2019, 28, 79–86. [Google Scholar] [CrossRef]
- Olivier, M.; Dorfling, C.; Eksteen, J. Evaluating a solvent extraction process route incorporating nickel preloading of Cyanex 272 for the removal of cobalt and iron from nickel sulphate solutions. Miner. Eng. 2012, 27, 37–51. [Google Scholar] [CrossRef]
- Chen, X.; Xu, B.; Zhou, T.; Liu, D.; Hu, H.; Fan, S. Separation and recovery of metal values from leaching liquor of mixed-type of spent lithium-ion batteries. Sep. Purif. Technol. 2015, 144, 197–205. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Zhou, T.; Liu, D.; Hu, H.; Fan, S. Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries. Waste Manag. 2015, 38, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Sole, K.; Siwela, L.; Khuzwayo, N. Separation of manganese from cobalt by solvent extraction: Application to the recycling of spent cathode material from lithium-ion batteries. In Proceedings of the Battery Materials Conference 2022, Johannesburg, South Africa, 24–25 August 2022; p. 93. [Google Scholar]
- Dong, T.; Zhang, S.; Ren, Z.; Huang, L.; Xu, G.; Liu, T.; Wang, S.; Cui, G. Electrolyte Engineering Toward High Performance High Nickel (Ni ≥ 80%) Lithium—Ion Batteries. Adv. Sci. 2024, 11, 2305753. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Seong, W.M.; Manthiram, A. Cobalt-free, high-nickel layered oxide cathodes for lithium-ion batteries: Progress, challenges, and perspectives. Energy Storage Mater. 2021, 34, 250–259. [Google Scholar] [CrossRef]
- Koo, N.; Kim, B.; Kim, H.-I.; Kwon, K. Extraction strategies from black alloy leachate: A comparative study of solvent extractants. Batteries 2024, 10, 221. [Google Scholar] [CrossRef]
- Zou, Y.; Chernyaev, A.; Ossama, M.; Seisko, S.; Lundström, M. Leaching of NMC industrial black mass in the presence of LFP. Sci. Rep. 2024, 14, 10818. [Google Scholar] [CrossRef]
- Alibaba.com. Available online: http://www.alibaba.com (accessed on 28 October 2025).
- Carson, I.; Tasker, P.A.; Love, J.B.; Moser, M.; Fischmann, A.J.; Jakovljevic, B.; Soderstrom, M.D.; Morrison, C.A. The supramolecular and coordination chemistry of cobalt (II) extraction by phosphinic acids. Eur. J. Inorg. Chem. 2018, 2018, 1511–1521. [Google Scholar] [CrossRef]
- Moon, H.S.; Song, S.J.; Tran, T.T.; Lee, M.S. Separation of Co (II), Ni (II), and Cu (II) from Sulfuric Acid Solution by Solvent Extraction. Resour. Recycl. 2022, 31, 21–28. [Google Scholar] [CrossRef]
- Ohnuma, T.; Kobayashi, T. X-ray absorption near edge structure simulation of LiNi0.5Co 0.2Mn0.3O2 via first-principles calculation. RSC Adv. 2019, 9, 35655–35661. [Google Scholar] [CrossRef] [PubMed]
- Vieceli, N.; Vonderstein, C.; Swiontekc, T.; Stopić, S.; Dertmann, C.; Sojka, R.; Reinhardt, N.; Ekberg, C.; Friedrich, B.; Petranikova, M. Recycling of Li-ion batteries from industrial processing: Upscaled hydrometallurgical treatment and recovery of high purity manganese by solvent extraction. Solvent Extr. Ion Exch. 2023, 41, 205–220. [Google Scholar] [CrossRef]
- Chu Cheng, M.H. Solvent Extraction Process for Recovering Nickel and Cobalt from Each Solutions. US20040050212A1, 18 March 2004. [Google Scholar]
- Nguyen, V.T.; Lee, J.-c.; Jeong, J.; Kim, B.-S.; Pandey, B. Selective recovery of cobalt, nickel and lithium from sulfate leachate of cathode scrap of Li-ion batteries using liquid-liquid extraction. Met. Mater. Int. 2014, 20, 357–365. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).