1. Introduction
Copper is one of most the important non-ferrous metal raw materials in promoting national economic construction. Due to it is excellent ductility, electrical conductivity and thermal conductivity, it is widely used in electrical, mechanical, military, new energy, aerospace, and other fields [
1,
2]. Most domestic copper resources exist in the form of copper sulfide; chalcopyrite is a typical representative, and constitutes 70% of the world’s copper resources [
3]. With the deep exploitation of copper sulfide resources, it is necessary to enrich copper bearing minerals by flotation to obtain standard copper concentrate to meet metallurgical requirements [
4]. However, in actual mining industry production, pyrite and pyrrhotite, as high-sulfide ores, have physical and chemical properties similar to those of chalcopyrite. This leads to difficulties in the flotation separation of copper and sulfur, seriously affecting the efficient recovery and utilization of copper resources, and has adverse effects on subsequent copper smelting [
1,
5].
It is well known that in process mineralogy, the physical, chemical, mineralogical, and structural properties of mineral raw materials and their performance, product quality, and utilization in the processing process are studied, addressing problems and challenges in the mineral processing and helping to increase the value of the concentrate [
6,
7]. At present, there are few studies on the potential valuable mineral components in chalcopyrite, and the structural properties of ore samples in different regions, have not been fully understood. Therefore, conducting process mineralogical research on sulfide ores to identify their mineral composition, distribution characteristics, elemental occurrence states, and monomer dissociation of useful components is of crucial importance for understanding the mineral factors in the grinding process, separation, and metallurgical processes of useful minerals [
8]. However, mineralogical research alone is insufficient to provide adequate theoretical support for improving flotation indicators. Combining and adjusting process mineralogical research with the optimization of flotation processes will provide sufficient theoretical support for the optimization of industrial production processes [
7].
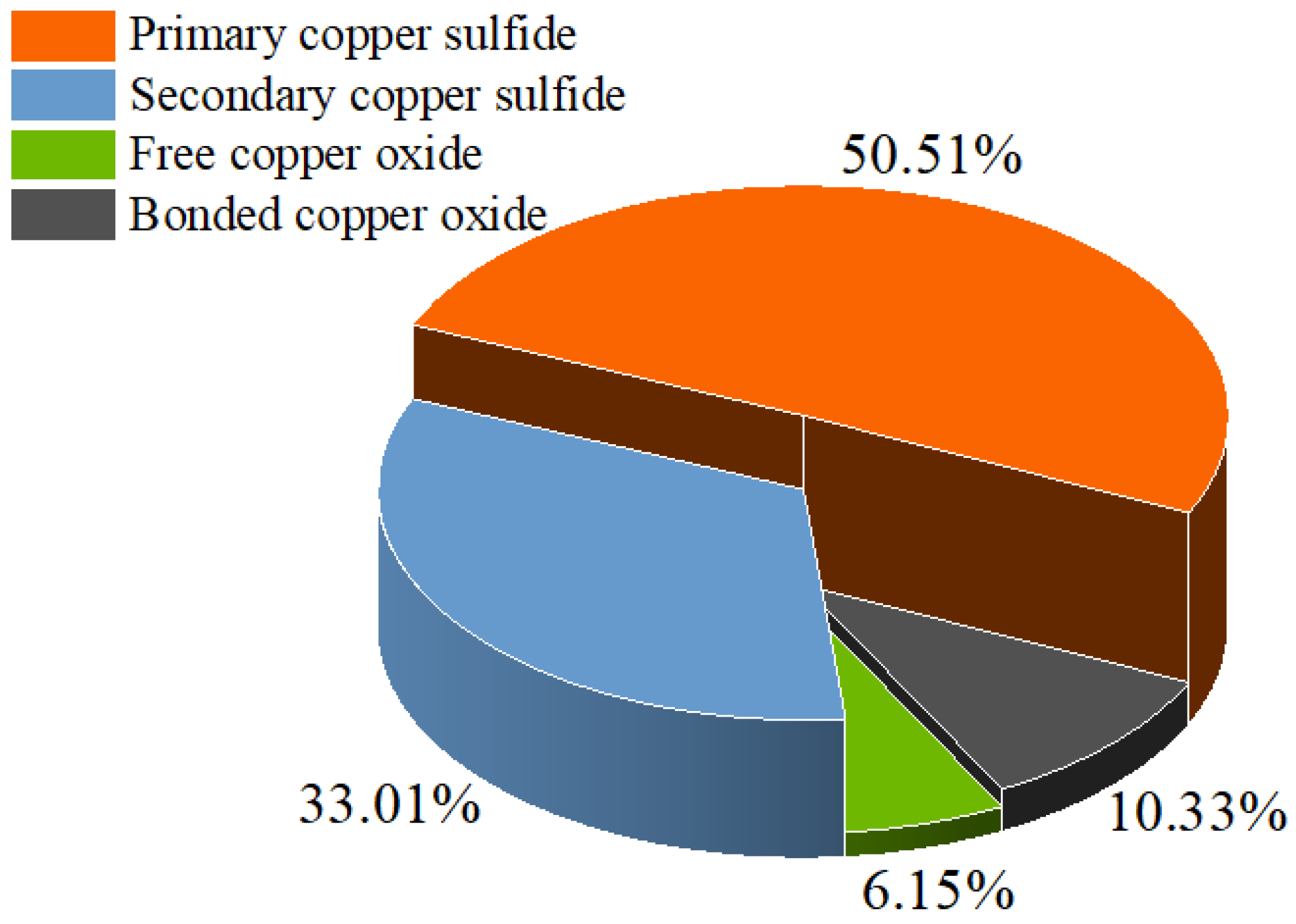
The main copper sulfide minerals include chalcopyrite, bornite and chalcocite [
7]. During the flotation process of copper sulfide, iron sulfide minerals are the main obstacle affecting the flotation of copper sulfide [
9]. For a long time, there have been mainly two methods used for the flotation separation of copper sulfide minerals and iron sulfide minerals: the selective inhibition of iron sulfide minerals, and the selective capture of copper sulfide minerals. In industry, the method of selectively inhibiting iron sulfide minerals is often employed to achieve the separation of copper and sulfur. The inhibitors for iron sulfide minerals mainly include inorganic inhibitors and organic inhibitors. Organic inhibitors alter the hydrophilicity of minerals by bonding their functional groups with metal atoms on the surface of iron sulfide through chemical bonds and electrostatic adsorption [
10]. Common organic inhibitors mainly include carboxymethyl cellulose (CMC), tannic acid, lactic acid, guar gum, pyrogallic acid, sodium humate, sodium lignosulfonate, dextrin, starch, polyacrylic acid, and other macromolecular organic substances [
8,
9,
10,
11,
12,
13,
14,
15]. These organic inhibitors usually contain multiple functional groups that can adsorb onto the surface of pyrite, generating hydrophilic substances that further hinder the flotation of pyrite. In recent years, it has received extensive attention due to its environmental friendliness, low cost, and strong selectivity [
16]. But, at present, there are relatively few studies on the industrial applications of these organic inhibitors, whether they can be applied in actual industrial production still requires further research. The inorganic inhibitors mainly include lime [
17], cyanide [
18], and sodium sulfide and sulfites [
19]. Lime is often used in production to adjust the high pH of the pulp, and research shows that a high pH value is conducive to the surface oxidation of pyrite; in addition, the depression effect of lime on pyrite is mainly due to the formation of the hydrophilic film composed of CaCO
3, CaSO
4, and Fe(OH)
3 on the pyrite surface, which hinders the contact between the collector and pyrite [
20,
21]. However, the extensive use of lime as an inhibitor for copper–sulfur separation can cause pipe blockage, high flotation froth viscosity, and the severe loss of rare metals [
3,
22,
23]. Although cyanide can prioritize the inhibition of pyrite, it is highly toxic and can cause harm to the environment and human health [
24].
As a strong oxidant, Ca(ClO)
2 is usually used as a disinfectant and bleaching agent. In recent years, due to it is low toxicity and high stability, it has gradually been used as an inhibitor of iron sulfide minerals [
25]. Research indicates that the main inhibitory mechanism of Ca(ClO)
2 on pyrite is as follows: Ca(ClO)
2 has strong oxidizing properties, and when dissolved in water, it decomposes to release oxygen and Ca
2+, which is less prone to hydrolysis. The surface of pyrite is oxidized, generating hydrophilic substances such as CaSO
4 [
26]. In addition, when the pulp is aerated and stirred, it absorbs a small amount of carbon dioxide, generating CO
32−, and CO
32−,combines with Ca
2+ dissolved from calcium hypochlorite and eventually covers the surface of pyrite with a layer of highly hydrophilic CaCO
3 precipitate, which prevents the adsorption of pyrite by the collector [
27,
28,
29,
30,
31].
This research was based on the process mineralogy study of the raw ore, the analysis of different processing products, and the determination of the optimal flotation process. As a result, the flotation indicators were significantly improved, and this provided a reference for industrial trials.
2. Materials and Discussion
2.1. Materials
The ore samples in this test were taken from Honghe Prefecture, Yunnan Province. Samples were taken from different mines according to the site mining conditions, and representative ore blocks were mixed and selected for process mineralogy research. The remaining ore samples were crushed to −2 mm in a closed circuit by a jaw crusher and a high-pressure roll crusher, and then reduced and mixed as test ore samples for chemical multi-element analysis, copper chemical image analysis, and flotation experimental research.
2.2. Research on Process Mineralogy
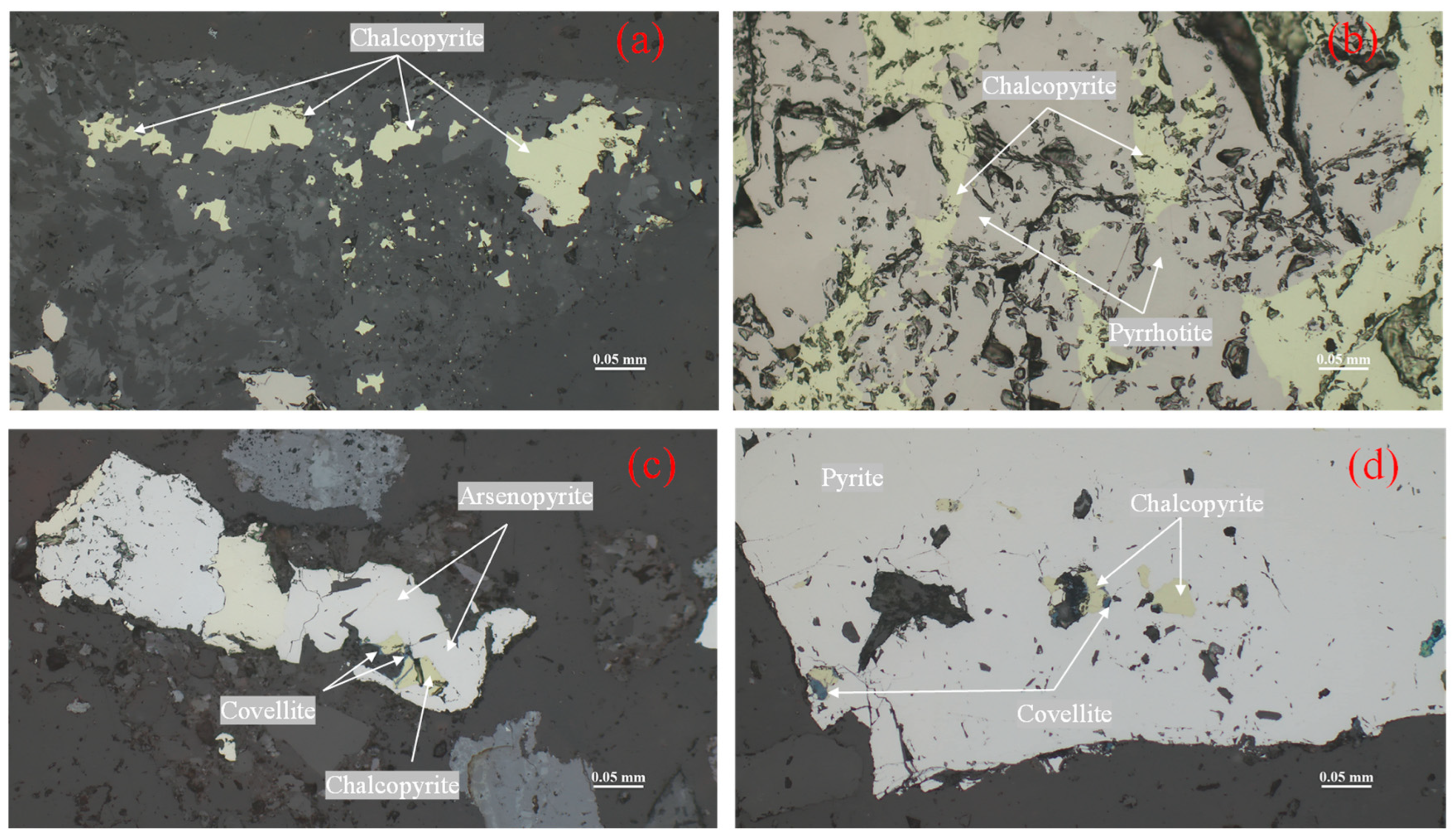
In this study, the ore fragments were selected and polished, and thin flakes were used for rock and ore identification. After the ore sample was mixed and divided, 200 g of ore sample were ground to −74 μm, which was used for X-ray fluorescence spectrum analysis, chemical multi-element analysis, and X-ray diffraction analysis. The 100 g ore sample was ground to −270 μm and then inlaid with resin for MLA analysis, SEM analysis, and X-ray energy spectrum analysis. The structural structure, elemental composition, mineral composition, distribution characteristics of the target minerals, and the occurrence state of copper were identified, and the possible influence of mineral mineralogy factors on the later beneficiation process was analyzed to understand the difficulty of flotation of the ore.
2.3. Flotation Experiments
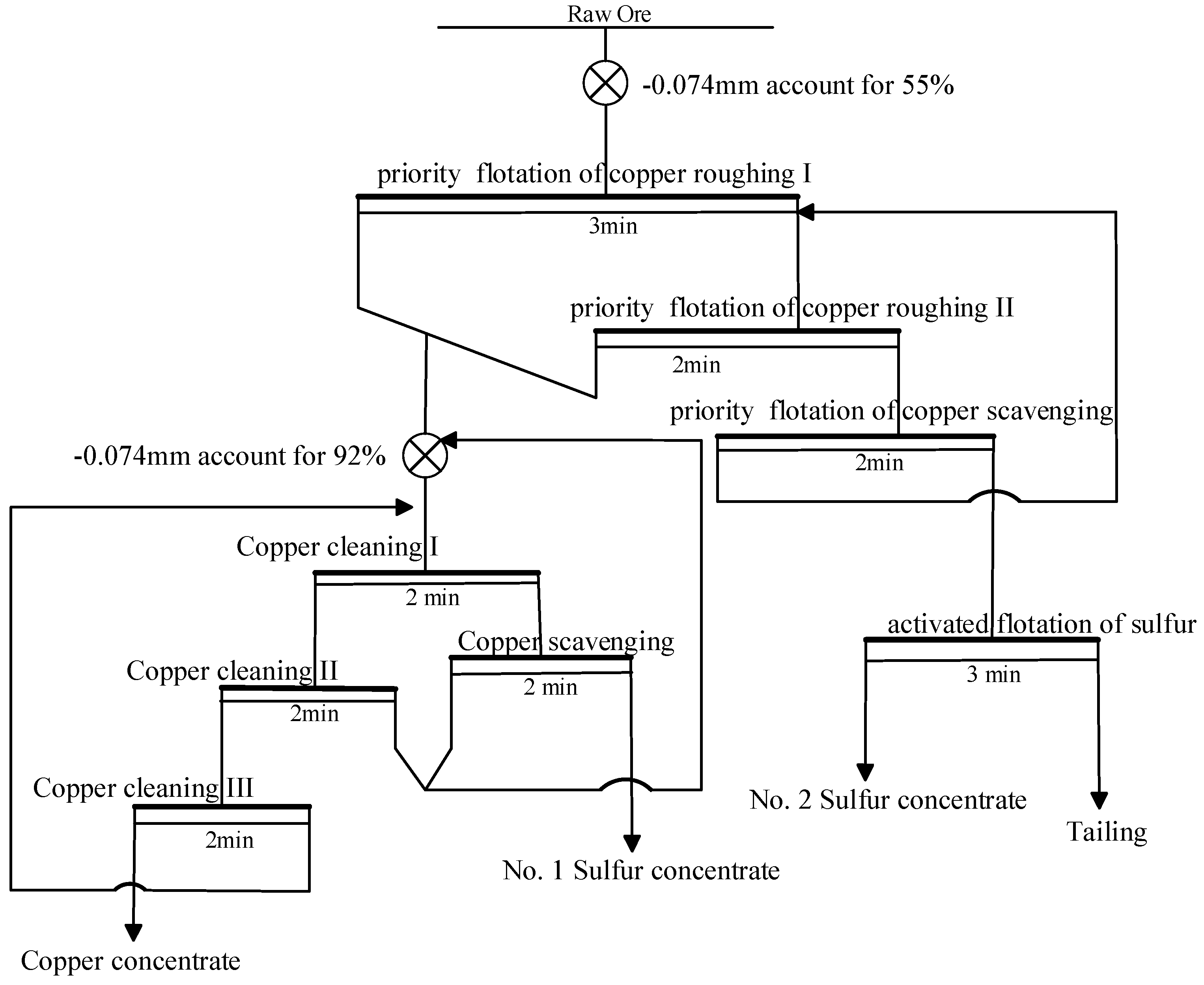
Before each flotation test, 500 g ore samples were taken and ground using a wet test ball mill (manufactured by the Wuhan Exploration Machinery Factory, China). The grinding concentration was 65%, ensuring that 55% of the ore could pass through the 74 μm sieve. The flotation test used a self-inflating flotation machine (XFD-1.5 L, Jilin Exploration Factory, China, with specifications of 1.5 L, 0.75 L, 0.5 L and 0.2 L). After grinding, the ground ore slurry was added to a 1.5 L flotation tank, and the liquid level of the flotation tank was adjusted by adding tap water to keep it constant. The agitator speed of flotation machine was fixed at 2110 r/min, and the pH value was set within the range of 9.5 to 10. Finally, the flotation tailings were sent to the shaking table for reprocessing to recover cassiterite. The flotation froth products were collected, and the yield and recovery of each product were calculated by filtration, drying, weighing, and testing. The flotation recovery was calculated based on the grade of copper and sulfur and the weight of flotation froth and tailings according to Equation (1).
The specific experiments were as follows:
Rough selection conditions test, including tests on the types and dosages of inhibitors;
Selection conditions test, including copper sulfur separation inhibitor dosage and types test, copper sulfur separation collector dosage test;
Whole process of open and closed circuit experiment
where
ε refers to the recovery (%);
α1 and
α2 represent the grade (%) of the target element in concentrate and tailings, respectively.
m1 and
m2 are the mass (g) of concentrate and tailings, respectively.
2.4. Reagent
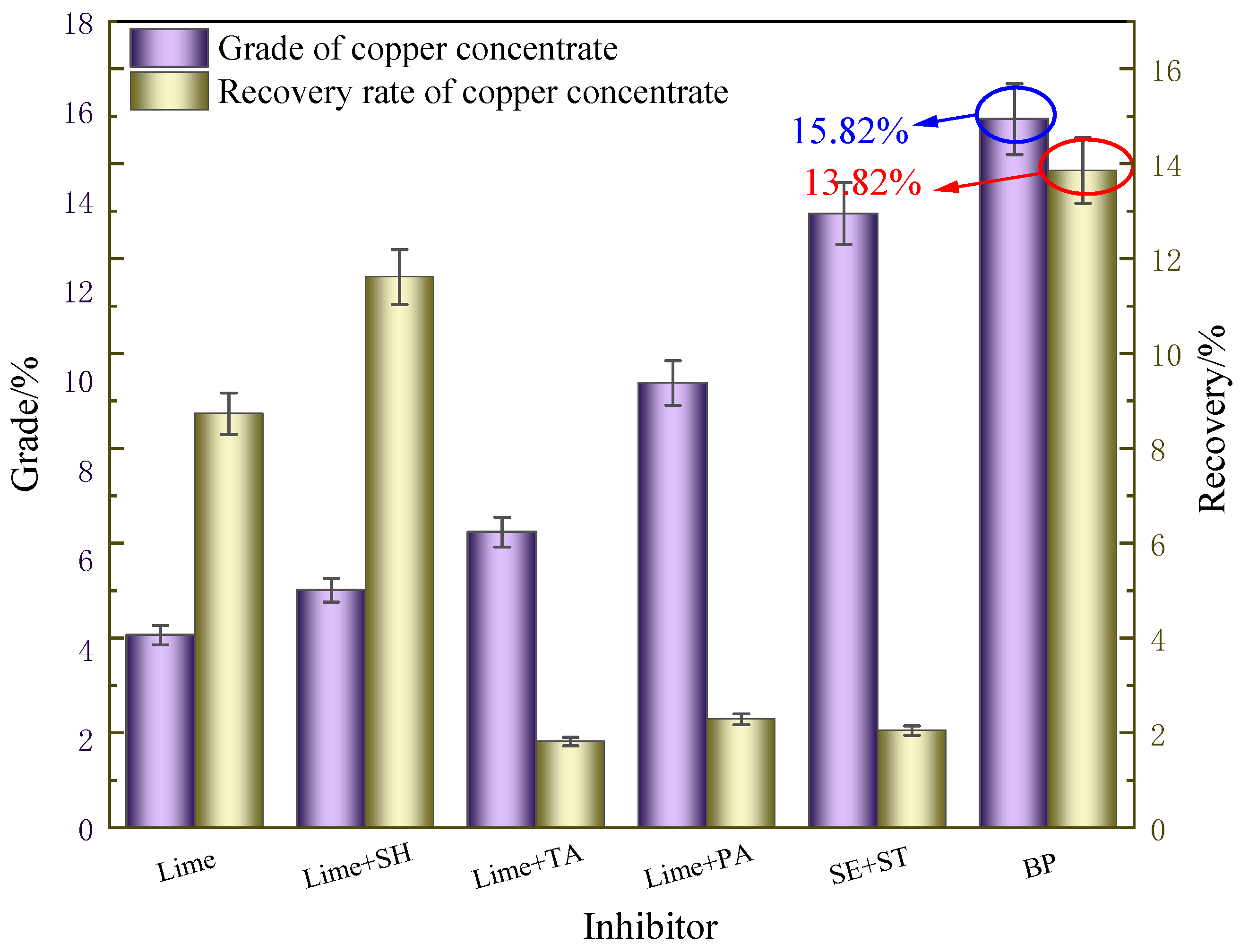
Sodium humate (SH), pyrogallic acid (PA), tannic acid (TA), sodium hypochlorite (SE), starch (ST), and calcium hypochlorite (BP) were used as Cu-S separation inhibitors. Sodium butyl xanthate (SBX) and ammonium dibutyl dithiophosphate (ADD) were used as copper flotation collectors, and methyl isobutyl methanol (MIBC) was used as a frother in the experiment. All these reagents were of analytical-purity grade and were purchased from Shanghai Alding Biochemical Technology Co., LTD. The copper flotation collector ML-8 was independently developed, and its purity was of industrial grade.