A Preliminary Study on the Solvent Extraction of Molybdenum and Rhenium from an Industrial Pregnant Leach Solution Using Alamine336 as the Extractant and the Ionic Liquid 1-Octyl-3-Methylimidazolium Bis(trifluoromethylsufonyl)imide as the Diluent
Abstract
1. Introduction
2. Methodology
2.1. Materials and Chemicals
2.2. Experimental Procedure
3. Result and Discussion
3.1. Extraction and Losses of Ionic Solvent
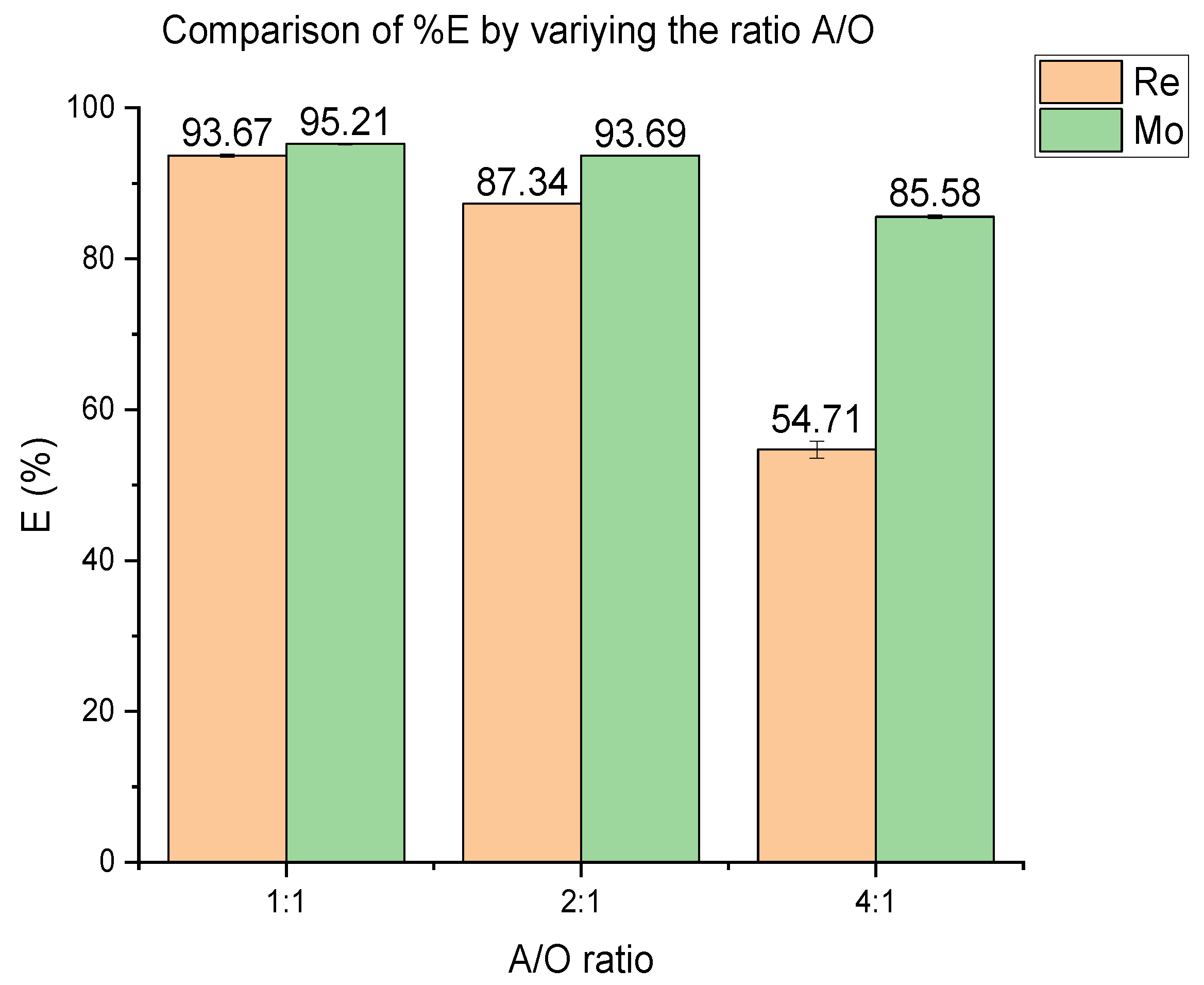
3.2. Effect of % Extractant and Aqueous/Organic Ratio
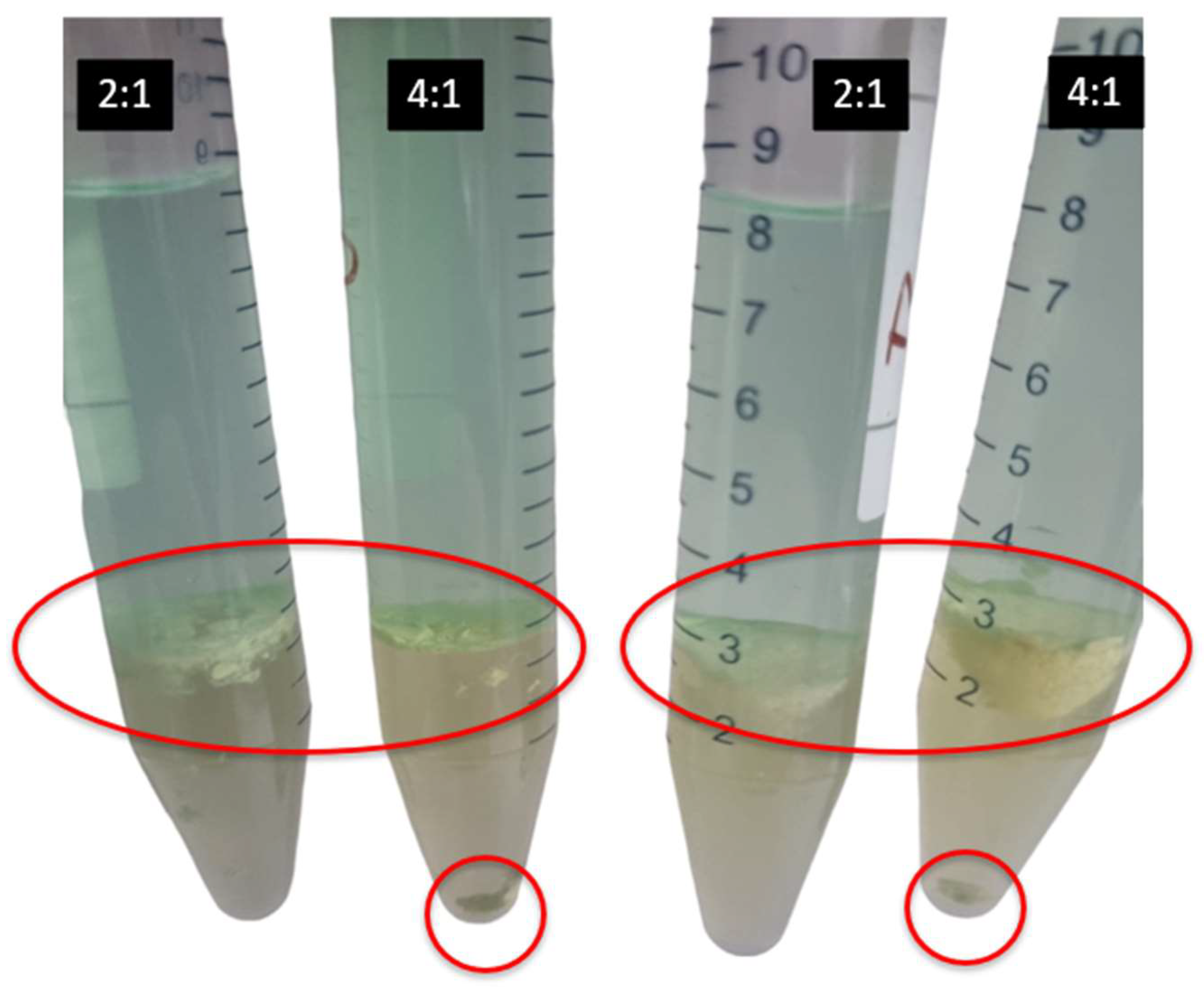
3.3. Third Phase Formation
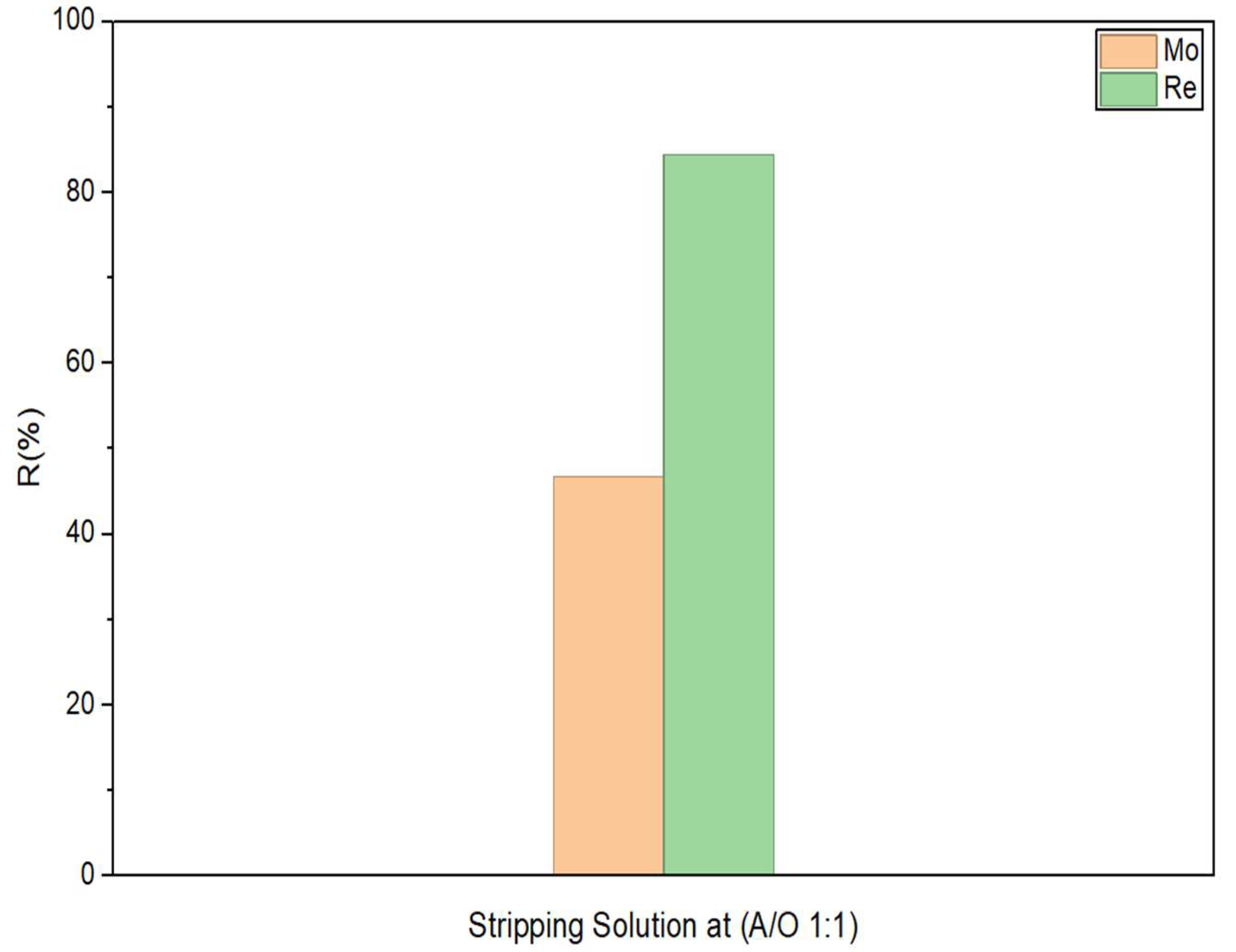
3.4. Stripping
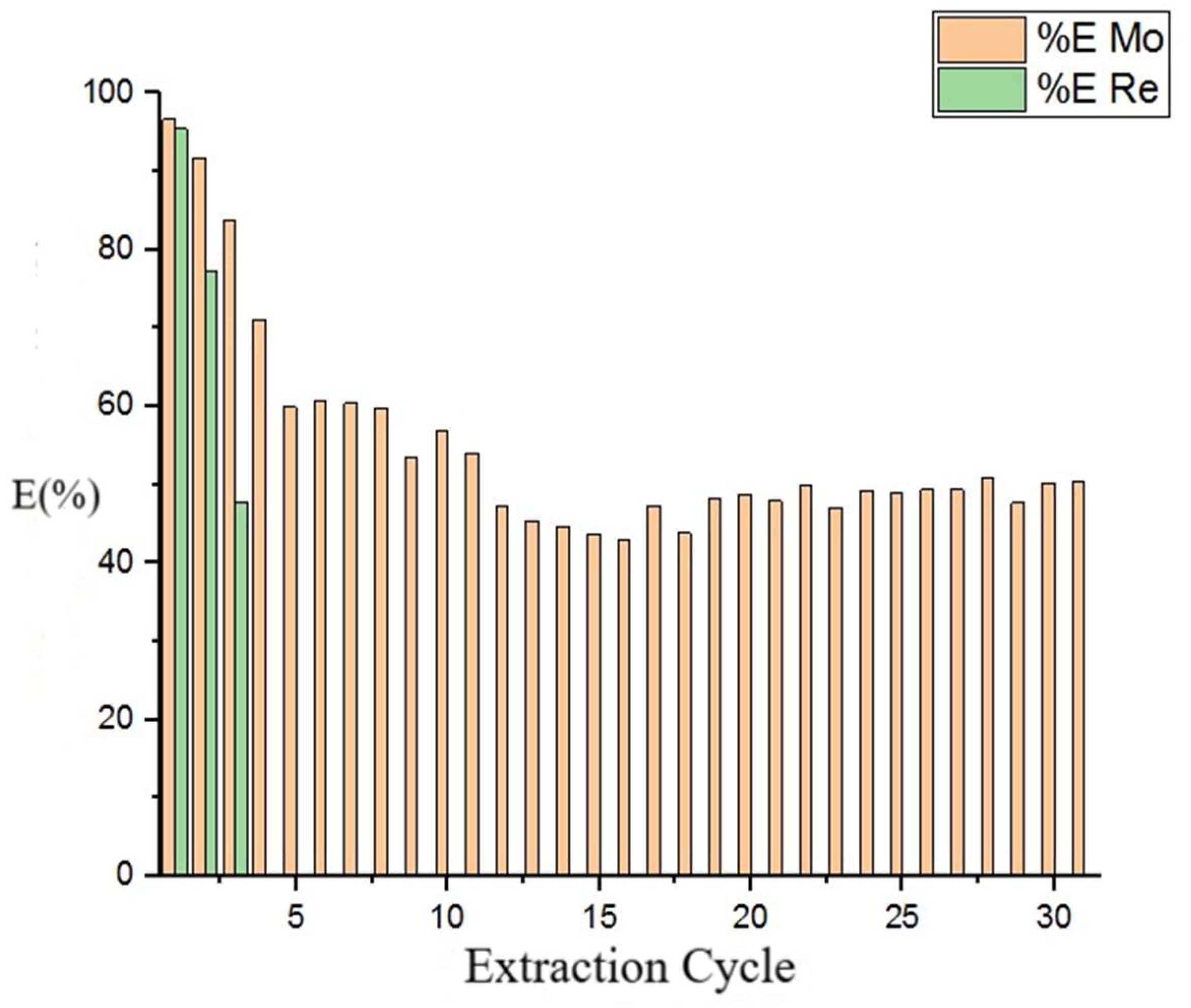
3.5. Stability of Ionic Solvent Across Cycles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Habashi, F. Handbook of Extractive Metallurgy; Wiley-VCH: Weinheim, Germany, 1997. [Google Scholar]
- Zhao, Z.; Zhang, G.; Huo, G.; Li, H. Kinetics of Atmospheric Leaching Molybdenum from Metalliferous Black Shales by Air Oxidation in Alkali Solution. Hydrometallurgy 2009, 97, 233–236. [Google Scholar] [CrossRef]
- Zhan-Fang, C.; Hong, Z.; Zhao-Hui, Q. Solvent Extraction of Rhenium from Molybdenum in Alkaline Solution. Hydrometallurgy 2009, 97, 153–157. [Google Scholar] [CrossRef]
- Gaur, R.P.S.; Wolfe, T.A.; Braymiller, S.A. Recycling of Rhenium-Containing Wire Scrap. Int. J. Refract. Met. Hard Mater. 2015, 50, 79–85. [Google Scholar] [CrossRef]
- Dilworth, J.R. Rhenium Chemistry–Then and Now. Coord. Chem. Rev. 2021, 436, 213822. [Google Scholar] [CrossRef]
- Kessler, V.G.; Seisenbaeva, G.A. Rhenium Nanochemistry for Catalyst Preparation. Minerals 2012, 2, 244–257. [Google Scholar] [CrossRef]
- Lunk, H.-J.; Drobot, D.V.; Hartl, H. Discovery, Properties and Applications of Rhenium and Its Compounds. ChemTexts 2021, 7, 6. [Google Scholar] [CrossRef]
- da Silva, T.P.; Figueiredo, M.-O.; de Oliveira, D.; Veiga, J.P.; Batista, M.J. Molybdenite as a Rhenium Carrier: First Results of a Spectroscopic Approach Using Synchrotron Radiation. J. Miner. Mater. Charact. Eng. 2013, 2013, 207–211. [Google Scholar] [CrossRef]
- Anderson, C.D.; Taylor, P.R.; Anderson, C.G. Extractive Metallurgy of Rhenium: A Review. Min. Met. Explor. 2013, 30, 59–73. [Google Scholar] [CrossRef]
- Guo, X.; Ma, Z.; Li, D.; Tian, Q.; Xu, Z. Recovery of Re (VII) from Aqueous Solutions with Coated Impregnated Resins Containing Ionic Liquid Aliquat 336. Hydrometallurgy 2019, 190, 105149. [Google Scholar] [CrossRef]
- Khoshnevisan, A.; Yoozbashizadeh, H.; Mohammadi, M.; Abazarpoor, A.; Maarefvand, M. Separation of Rhenium and Molybdenum from Molybdenite Leach Liquor by the Solvent Extraction Method. Min. Met. Explor. 2013, 30, 53–58. [Google Scholar] [CrossRef]
- Valenzuela, F.R.; Andrade, J.P.; Sapag, J.; Tapia, C.; Basualto, C. The Solvent Extraction Separation of Molybdenum and Copper from Acid Leach Residual Solution of Chilean Molybdenite Concentrate. Min. Eng. 1995, 8, 893–904. [Google Scholar] [CrossRef]
- Khoshnevisan, A.; Yoozbashizadeh, H.; Mozammel, M.; Sadrnezhaad, S.K. Kinetics of Pressure Oxidative Leaching of Molybdenite Concentrate by Nitric Acid. Hydrometallurgy 2012, 111–112, 52–57. [Google Scholar] [CrossRef]
- Mirvaliev, R.; Inoue, K. Pressure Oxidative Leaching of Molybdenite in Alkaline Media. J. Min. Mater. Process. Inst. Jpn 2001, 117, 72–76. [Google Scholar] [CrossRef][Green Version]
- Shen, L.; Tesfaye, F.; Li, X.; Lindberg, D.; Taskinen, P. Review of Rhenium Extraction and Recycling Technologies from Primary and Secondary Resources. Min. Eng. 2021, 161, 106719. [Google Scholar] [CrossRef]
- Nabardi, S.; Aghazadeh, V.; Esrafili, M.D.; Kiyani, N. Separation of Rhenium and Molybdenum from Autoclave Acid-Leach Liquor of Molybdenite Concentrate Using Bifunctional Ionic Liquid [A336][Cy272] in Xylene. Hydrometallurgy 2025, 235, 106472. [Google Scholar] [CrossRef]
- Quijada-Maldonado, E.; Sánchez, F.; Pérez, B.; Tapia, R.; Romero, J. Task-Specific Ionic Liquids as Extractants for the Solvent Extraction of Molybdenum (VI) from Aqueous Solution Using Different Commercial Ionic Liquids as Diluents. Ind. Eng. Chem. Res. 2018, 57, 1621–1629. [Google Scholar] [CrossRef]
- Quijada-Maldonado, E.; Román, R.; Merlet, G.; Pérez, B.; Cabezas, R.; Tapia, R.; Olea, F.; Villarroel, E.; Araya-López, C.; Romero, J. Rhenium (VII) Extraction from Sulfuric Aqueous Solutions Using Ionic Liquids as Diluent and Extractant: Insights on the Extraction Stoichiometry and Process Parameters. J. Chem. Technol. Biotechnol. 2022, 97, 1224–1233. [Google Scholar] [CrossRef]
- Srivastava, R.R.; Kim, M.; Lee, J.; Ilyas, S. Liquid–Liquid Extraction of Rhenium (VII) from an Acidic Chloride Solution Using Cyanex 923. Hydrometallurgy 2015, 157, 33–38. [Google Scholar] [CrossRef]
- Mitchell, P.C.H. Oxo-Species of Molybdenum-(V) and-(VI). Q. Rev. Chem. Soc. 1966, 20, 103–118. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Jiang, C.-J.; Wang, X.-W.; Xian, P.-F.; Wang, H.-G.; Yang, Y. Existing Form of Mo (VI) in Acidic Sulfate Solution. Rare Met. 2017, 36, 612–616. [Google Scholar] [CrossRef]
- Olea, F.; Valenzuela, M.; Zurob, E.; Parraguez, B.; Abejón, R.; Cabezas, R.; Merlet, G.; Tapia, R.; Romero, J.; Quijada-Maldonado, E. Hydrophobic Eutectic Solvents for the Selective Solvent Extraction of Molybdenum (VI) and Rhenium (VII) from a Synthetic Pregnant Leach Solution. J. Mol. Liq. 2023, 385, 122415. [Google Scholar] [CrossRef]
- Nakashima, K.; Kubota, F.; Maruyama, T.; Goto, M. Feasibility of Ionic Liquids as Alternative Separation Media for Industrial Solvent Extraction Processes. Ind. Eng. Chem. Res. 2005, 44, 4368–4372. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Keim, W. Ionic Liquids—New “Solutions” for Transition Metal Catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Sivaramakrishna, M.; Raut, D.R.; Nayak, S.; Nayak, S.K.; Mohapatra, P.K. Unusual Selective Extraction of Pu4+ by Some Novel Diamide Ligands in a Room Temperature Ionic Liquid. Sep. Purif. Technol. 2017, 181, 69–75. [Google Scholar] [CrossRef]
- Joo, S.-H.; Kim, Y.-U.; Kang, J.-G.; Yoon, H.-S.; Kim, D.-S.; Shin, S.M. Recovery of Molybdenum and Rhenium Using Selective Precipitation Method from Molybdenite Roasting Dust in Alkali Leaching Solution. Mater. Trans. 2012, 53, 2038–2042. [Google Scholar] [CrossRef]
- Dietz, M.L. Ionic Liquids as Extraction Solvents: Where Do We Stand? Sep. Sci. Technol. 2006, 41, 2047–2063. [Google Scholar] [CrossRef]
- Quijada-Maldonado, E.; Allain, A.; Pérez, B.; Merlet, G.; Cabezas, R.; Tapia, R.; Romero, J. Selective Liquid-Liquid Extraction of Molybdenum (VI) and Rhenium (VII) from a Synthetic Pregnant Leach Solution: Comparison between Extractants and Diluents. Min. Eng. 2020, 145, 106060. [Google Scholar] [CrossRef]
- Cheema, H.A.; Ilyas, S.; Masud, S.; Muhsan, M.A.; Mahmood, I.; Lee, J. Selective Recovery of Rhenium from Molybdenite Flue-Dust Leach Liquor Using Solvent Extraction with TBP. Sep. Purif. Technol. 2018, 191, 116–121. [Google Scholar] [CrossRef]
- Chapeaux, A.; Simoni, L.D.; Stadtherr, M.A.; Brennecke, J.F. Liquid Phase Behavior of Ionic Liquids with Water and 1-Octanol and Modeling of 1-Octanol/Water Partition Coefficients. J. Chem. Eng. Data 2007, 52, 2462–2467. [Google Scholar] [CrossRef]
- Mehdi, H.; Binnemans, K.; Van Hecke, K.; Van Meervelt, L.; Nockemann, P. Hydrophobic Ionic Liquids with Strongly Coordinating Anions. Chem. Commun. 2010, 46, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Papović, S.; Bešter-Rogač, M.; Vraneš, M.; Gadžurić, S. The Effect of the Alkyl Chain Length on Physicochemical Features of (Ionic Liquids+ γ-Butyrolactone) Binary Mixtures. J. Chem. Thermodyn. 2016, 99, 1–10. [Google Scholar] [CrossRef]
- Mesbah, M.; Soroush, E.; Kakroudi, M.R. Predicting Physical Properties (Viscosity, Density, and Refractive Index) of Ternary Systems Containing 1-Octyl-3-Methyl-Imidazolium Bis (Trifluoromethylsulfonyl) Imide, Esters and Alcohols at 298.15 K and Atmospheric Pressure, Using Rigorous Classification Techniques. J. Mol. Liq. 2017, 225, 778–787. [Google Scholar]
- Olazabal, M.A.; Orive, M.M.; Fernandez, L.A.; Madariaga, J.M. Selective Extraction of Vanadium (V) from Solutions Containing Molybdenum (VI) by Ammonium Salts Dissolved in Toluene. Solvent Extr. Ion Exch. 1992, 10, 623–635. [Google Scholar] [CrossRef]
- Quijada-Maldonado, E.; Torres, M.J.; Romero, J. Solvent Extraction of Molybdenum (VI) from Aqueous Solution Using Ionic Liquids as Diluents. Sep. Purif. Technol. 2017, 177, 200–206. [Google Scholar] [CrossRef]
- Mohammadi, M.; Forsberg, K.; Kloo, L.; Martinez De La Cruz, J.; Rasmuson, Å. Separation of ND(III), DY(III) and Y(III) by Solvent Extraction Using D2EHPA and EHEHPA. Hydrometallurgy 2015, 156, 215–224. [Google Scholar] [CrossRef]
- Wang, L.Y.; Lee, M.S. Development of a Separation Process for the Selective Extraction of Hafnium(IV) over Zirconium(IV) from Sulfuric Acid Solutions by Using D2EHPA. Hydrometallurgy 2016, 160, 12–17. [Google Scholar] [CrossRef]
- Rao, K.S.; Devi, N.B.; Reddy, B.R. Solvent Extraction of Copper from Sulphate Medium Using MOC 45 as Extractant. Hydrometallurgy 2000, 57, 269–275. [Google Scholar] [CrossRef]
- Aminian, H.; Bazin, C. Solvent Extraction Equilibria in Copper (II)-Iron (III)-LIX984 System. Min. Eng. 2000, 13, 667–672. [Google Scholar] [CrossRef]
- Tereshatov, E.E.; Boltoeva, M.Y.; Folden, C.M. First Evidence of Metal Transfer into Hydrophobic Deep Eutectic and Low-Transition-Temperature Mixtures: Indium Extraction from Hydrochloric and Oxalic Acids. Green Chem. 2016, 18, 4616–4622. [Google Scholar] [CrossRef]
- Tan, R.Y.; Lee, C.S.; Pichika, M.R.; Cheng, S.F.; Lam, K.Y. PH Responsive Polyurethane for the Advancement of Biomedical and Drug Delivery. Polymers 2022, 14, 1672. [Google Scholar] [CrossRef]
- Freire, M.G.; Carvalho, P.J.; Gardas, R.L.; Marrucho, I.M.; Santos, L.M.; Coutinho, J.A.P. Mutual Solubilities of Water and the [C n Mim][Tf2N] Hydrophobic Ionic Liquids. J. Phys. Chem. B 2008, 112, 1604–1610. [Google Scholar] [CrossRef]
- Hidayah, N.N.; Abidin, S.Z. Extraction of Light, Medium and Heavy Rare-Earth Elements Using Synergist Extractants Developed from Ionic Liquid and Conventional Extractants. Comptes Rendus Chim. 2019, 22, 728–744. [Google Scholar] [CrossRef]
- Adavodi, R.; Zuffranieri, A.; Romano, P.; Rahmati, S.; Vegliò, F. Selective Recovery of Molybdenum over Nickel and Cobalt from Simulated Secondary Sources Using Bifunctional Ionic Liquid [TOA][Cy272]. Materials 2025, 18, 3826. [Google Scholar] [CrossRef]
- Mudring, A.; Babai, A.; Arenz, S.; Giernoth, R. The “Noncoordinating” Anion Tf2N− Coordinates to Yb2+: A Structurally Characterized Tf2N− Complex from the Ionic Liquid [Mppyr][Tf2N]. Angew. Chem. Int. Ed. 2005, 44, 5485–5488. [Google Scholar] [CrossRef]
- Emam, S.S.; Elgoud, E.M.A.; Abd-Elhamid, A.I.; Aly, H.F. Selective Extraction of Molybdenum (VI) with Novel Ionic Liquid from Nitric Acid Solution. Sep. Sci. Technol. 2023, 58, 2123–2137. [Google Scholar] [CrossRef]
- Schaeffer, N.; Martins, M.A.R.; Neves, C.M.S.S.; Pinho, S.P.; Coutinho, J.A.P. Sustainable Hydrophobic Terpene-Based Eutectic Solvents for the Extraction and Separation of Metals. Chem. Commun. 2018, 54, 8104–8107. [Google Scholar] [CrossRef]
- Ola, P.D.; Matsumoto, M. Use of Deep Eutectic Solvent as Extractant for Separation of Fe (III) and Mn (II) from Aqueous Solution. Sep. Sci. Technol. 2019, 54, 759–765. [Google Scholar] [CrossRef]
- Coca, J.; Díez, F.V.; Morís, M.A. Solvent Extraction of Molybdenum and Tungsten by Alamine 336 and DEHPA. Hydrometallurgy 1990, 25, 125–135. [Google Scholar] [CrossRef]
- Basualto, C.; Marchese, J.; Valenzuela, F.; Acosta, A. Extraction of Molybdenum by a Supported Liquid Membrane Method. Talanta 2003, 59, 999–1007. [Google Scholar] [CrossRef]
- Marchese, J.; Valenzuela, F.; Basualto, C.; Acosta, A. Transport of Molybdenum with Alamine 336 Using Supported Liquid Membrane. Hydrometallurgy 2004, 72, 309–317. [Google Scholar] [CrossRef]
- Osseo-Asare, K. Third Phase Formation in Solvent Extraction: A Microemulsion Model. In Proceedings of the Metal Separation Technology Beyond 2000: Integrating Novel Chemistry with Processing, Oahu, HI, USA, 13–18 June 1999. [Google Scholar]
- Zhang, M.; Song, H.; Zheng, C.; Liu, S.; Lin, Z.; Liu, Y.; Wu, W.; Gao, X. Highly Efficient Selective Extraction of Mo with Novel Hydrophobic Deep Eutectic Solvents. J. Air Waste Manag. Assoc. 2021, 71, 1492–1501. [Google Scholar] [CrossRef]
- Lasheen, T.A.; El-Ahmady, M.E.; Hassib, H.B.; Helal, A.S. Molybdenum Metallurgy Review: Hydrometallurgical Routes to Recovery of Molybdenum from Ores and Mineral Raw Materials. Miner. Process. Extr. Metall. Rev. 2015, 36, 145–173. [Google Scholar] [CrossRef]
- Singh, R.K.; Dhadke, P.M. Extraction and Separation Studies of Zinc (II) and Copper (II) with D2EHPA and PC-88A from Perchlorate Media. J. Serbian Chem. Soc. 2002, 67, 41–51. [Google Scholar] [CrossRef]
- Fan, R.; Liu, R.; Zhao, Z.; Li, Y.; Liu, D.; Wang, D.; Jia, S. Hydrometallurgical Separation of Mo and Re from Rhenium-Containing Molybdenum Calcine for Efficient Rhenium Recovery. Sep. Purif. Technol. 2025, 363, 132135. [Google Scholar] [CrossRef]

| Concentrations (mg/L) | ||
|---|---|---|
| Metal Ion | PLS-Mo | PLS-Re |
| Mo (VI) | 4.216 | 1.775 |
| Re (VII) | 0.02 | 0.308 |
| Cu (II) | 2.679 | 0.008 |
| Fe (III) | 3.479 | 0.002 |
| pH | 0.21 | 0.94 |
| Extractant | PLS | % v/v | Form 3rd Phase | Ionic s. Losses (ppm) | pH eq. |
|---|---|---|---|---|---|
| Alamine 336 | Mo | 5 | No | 103.3 | 0.28 |
| Mo | 10 | No | 666.6 | 0.35 | |
| Re | 5 | No | 404.9 | 1.12 | |
| Re | 10 | No | 1118.8 | 1.28 | |
| Re | 5 | No | 319.7 | 1.09 | |
| Re | 10 | No | 1272.5 | 1.26 |
| PLS | % v/v | Extraction Percentage (%) | Selectivity | |||||
|---|---|---|---|---|---|---|---|---|
| Re | Cu | Fe | Mo | Mo-Re | Mo-Cu | Mo-Fe | ||
| Mo | 5 | 95.5 | 2.9 | 0 | 96.6 | 1.4 | 976.2 | Very selective |
| Mo | 10 | 95.5 | 6.4 | 0 | 97.9 | 2.2 | 691.1 | Very selective |
| Re | 5 | 93.5 | 0 | 0 | 95.5 | 1.5 | Very selective | Very selective |
| Re | 10 | 86.7 | 0 | 0 | 95.0 | 2.9 | Very selective | Very selective |
| Re | 5 | 93.7 | 0 | 0 | 95.2 | 1.3 | Very selective | Very selective |
| Re | 10 | 86.0 | 0 | 0 | 94.7 | 2.9 | Very selective | Very selective |
| A/O | Mo—Re | Mo—Cu | Mo—Fe |
|---|---|---|---|
| 1:1 | 1.34 | Very selective | Very selective |
| 2:1 | 2.15 | Very selective | Very selective |
| 4:1 | 4.91 | Very selective | Very selective |
| PLS | A/O | % v/v Ext | 3rd Phase Form? |
|---|---|---|---|
| PLS-Mo | 2:1 | 5 | Yes |
| 4:1 | 5 | Yes | |
| 2:1 | 10 | Yes | |
| 4:1 | 10 | Yes | |
| 2:1 | 5 | Yes | |
| 4:1 | 5 | Yes | |
| 2:1 | 5 | Yes | |
| 4:1 | 5 | Yes | |
| 2:1 | 5 | Yes | |
| 2:1 | 5 | Yes | |
| 2:1 | 5 | Yes | |
| 2:1 | 5 | Yes | |
| 1.5:1 | 5 | Yes | |
| 1.5:1 | 5 | Yes | |
| 2:1 | 10 | Yes | |
| 1.5:1 | 10 | Yes | |
| 2:1 | 10 | Yes | |
| 2:1 | 15 | Yes | |
| 1.5:1 | 15 | Yes | |
| PLS-Re | 2:1 | 5 | No |
| 4:1 | 5 | No |
| Selectivity | |||
|---|---|---|---|
| A/O | Mo—Re | Mo—Cu | Mo—Fe |
| 1:1 | 1.37 | 976.24 | >1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayat, M.; Allendes, C.; Araya, A.; Cabezas, R.; Urzúa-Ahumada, J.; Quijada-Maldonado, E. A Preliminary Study on the Solvent Extraction of Molybdenum and Rhenium from an Industrial Pregnant Leach Solution Using Alamine336 as the Extractant and the Ionic Liquid 1-Octyl-3-Methylimidazolium Bis(trifluoromethylsufonyl)imide as the Diluent. Minerals 2025, 15, 1204. https://doi.org/10.3390/min15111204
Hayat M, Allendes C, Araya A, Cabezas R, Urzúa-Ahumada J, Quijada-Maldonado E. A Preliminary Study on the Solvent Extraction of Molybdenum and Rhenium from an Industrial Pregnant Leach Solution Using Alamine336 as the Extractant and the Ionic Liquid 1-Octyl-3-Methylimidazolium Bis(trifluoromethylsufonyl)imide as the Diluent. Minerals. 2025; 15(11):1204. https://doi.org/10.3390/min15111204
Chicago/Turabian StyleHayat, Muhammad, Cristian Allendes, Alejandro Araya, Rene Cabezas, Julio Urzúa-Ahumada, and Esteban Quijada-Maldonado. 2025. "A Preliminary Study on the Solvent Extraction of Molybdenum and Rhenium from an Industrial Pregnant Leach Solution Using Alamine336 as the Extractant and the Ionic Liquid 1-Octyl-3-Methylimidazolium Bis(trifluoromethylsufonyl)imide as the Diluent" Minerals 15, no. 11: 1204. https://doi.org/10.3390/min15111204
APA StyleHayat, M., Allendes, C., Araya, A., Cabezas, R., Urzúa-Ahumada, J., & Quijada-Maldonado, E. (2025). A Preliminary Study on the Solvent Extraction of Molybdenum and Rhenium from an Industrial Pregnant Leach Solution Using Alamine336 as the Extractant and the Ionic Liquid 1-Octyl-3-Methylimidazolium Bis(trifluoromethylsufonyl)imide as the Diluent. Minerals, 15(11), 1204. https://doi.org/10.3390/min15111204









