Study on the Inhibition and Activation of Pyrite Under Low Alkalinity Conditions Created by Hydrogen Peroxide and Lime
Abstract
1. Introduction
2. Materials and Methods
2.1. Ore Sample
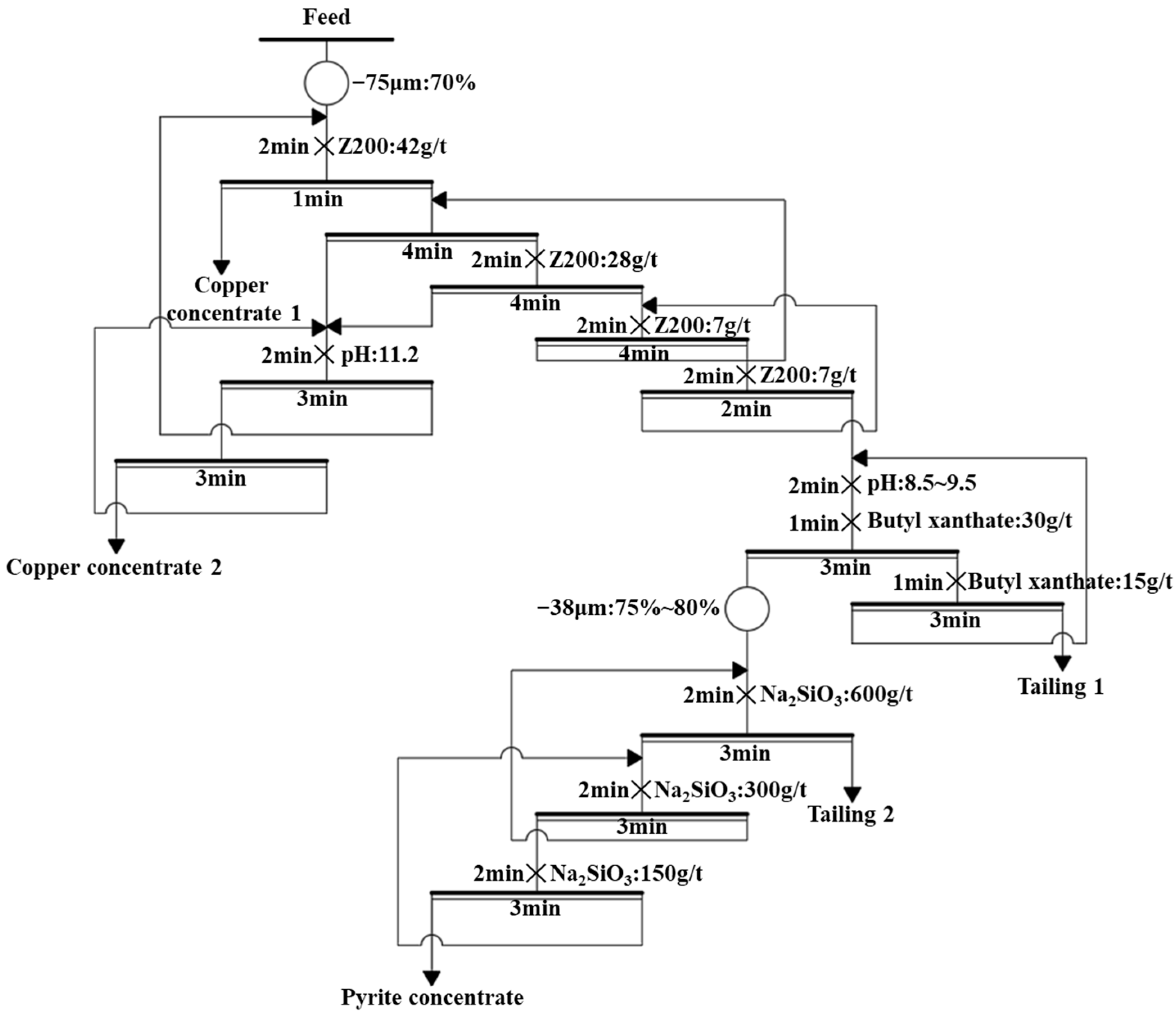
2.2. Flotation Process of the Mine
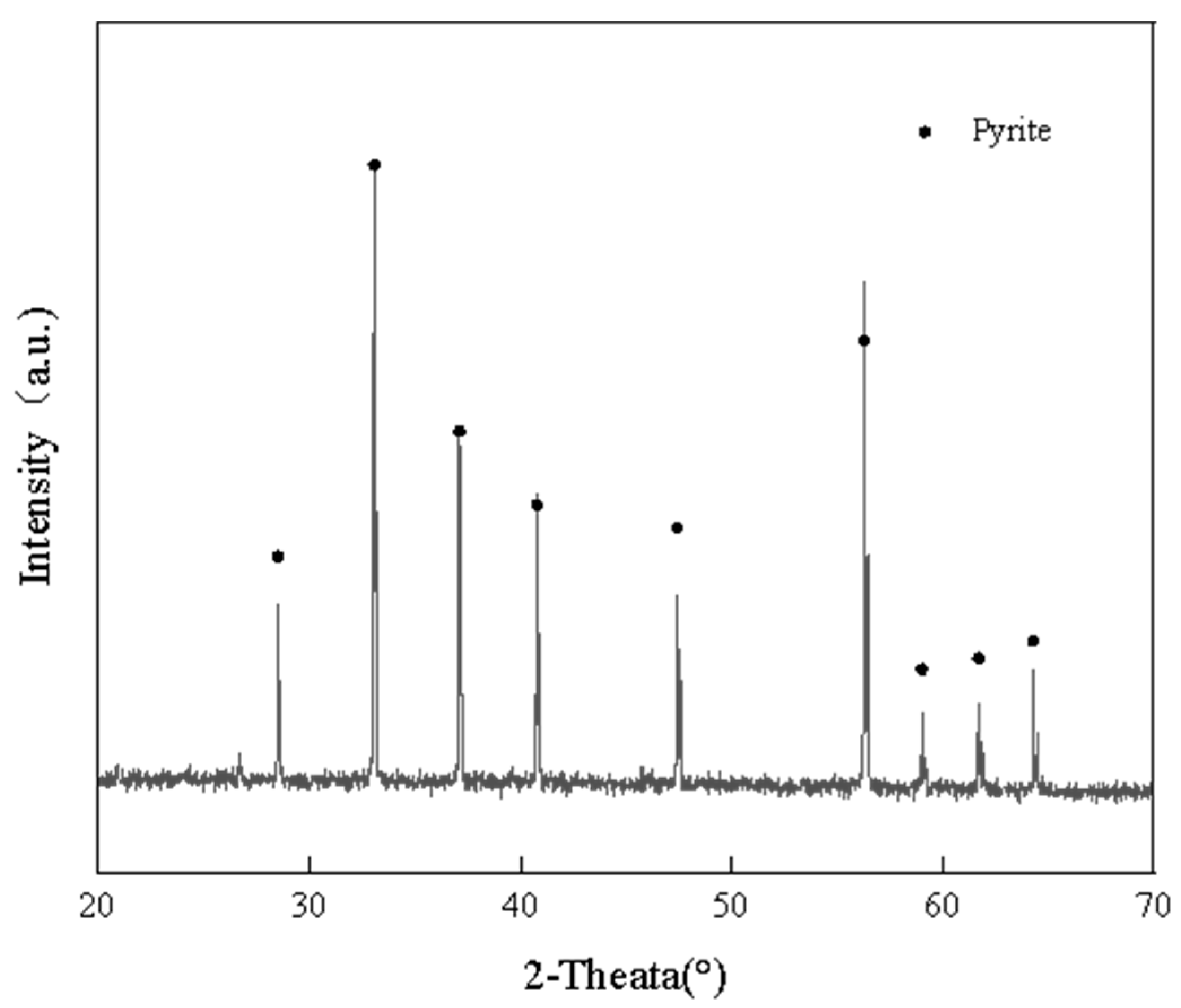
2.3. Pure Mineral
2.4. Reagents
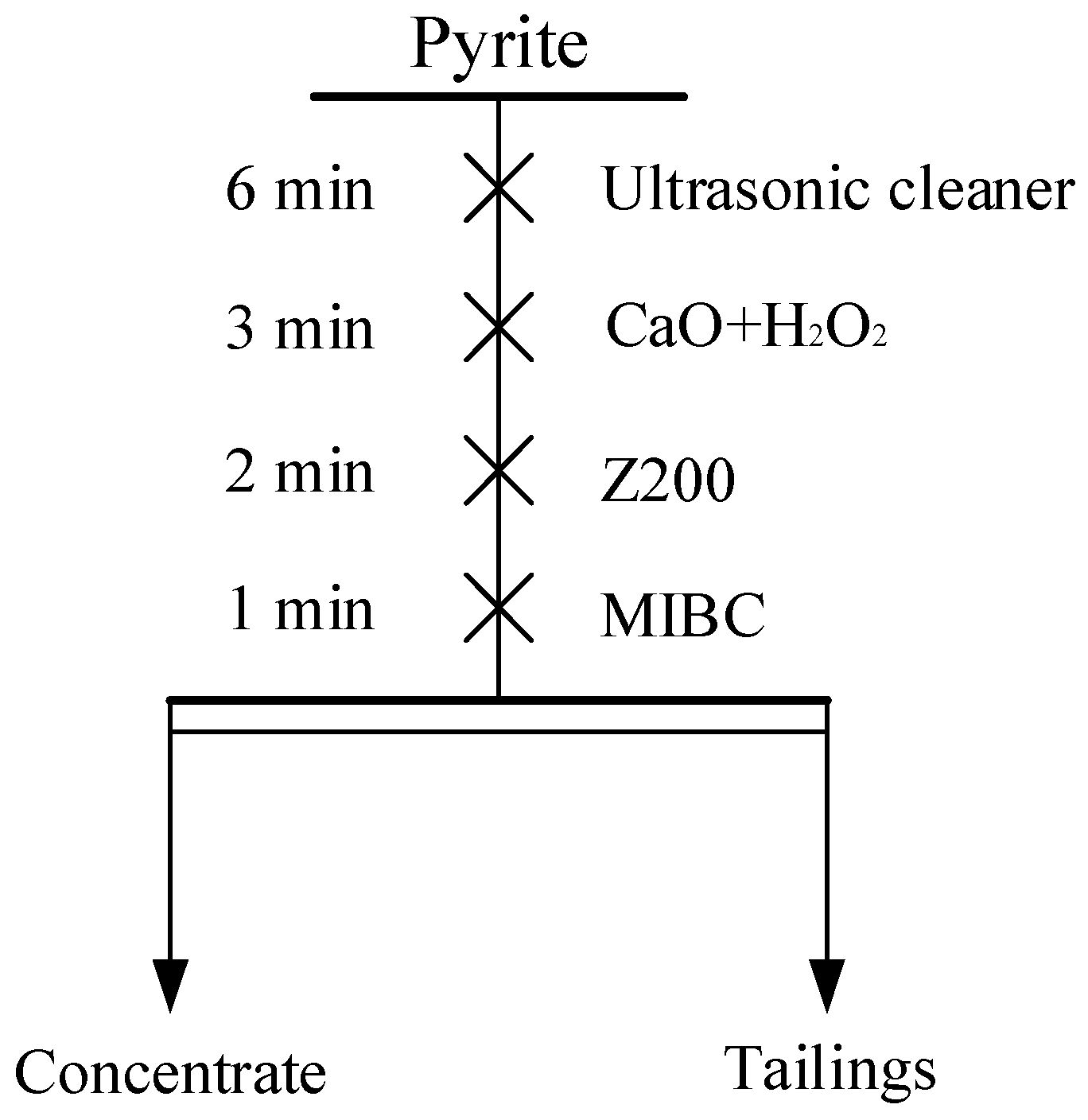
2.5. Methods and Processes
2.6. Testing and Analysis
2.6.1. Scanning Electron Microscopy (SEM)
2.6.2. Contact Angle Measurement
2.6.3. Process Mineralogy Parameter Testing Using a Mineral Liberation Analyzer (MLA)
2.6.4. XPS Measurement
3. Results and Discussion
3.1. Effects of Different Inhibition Systems on Pyrite Flotation
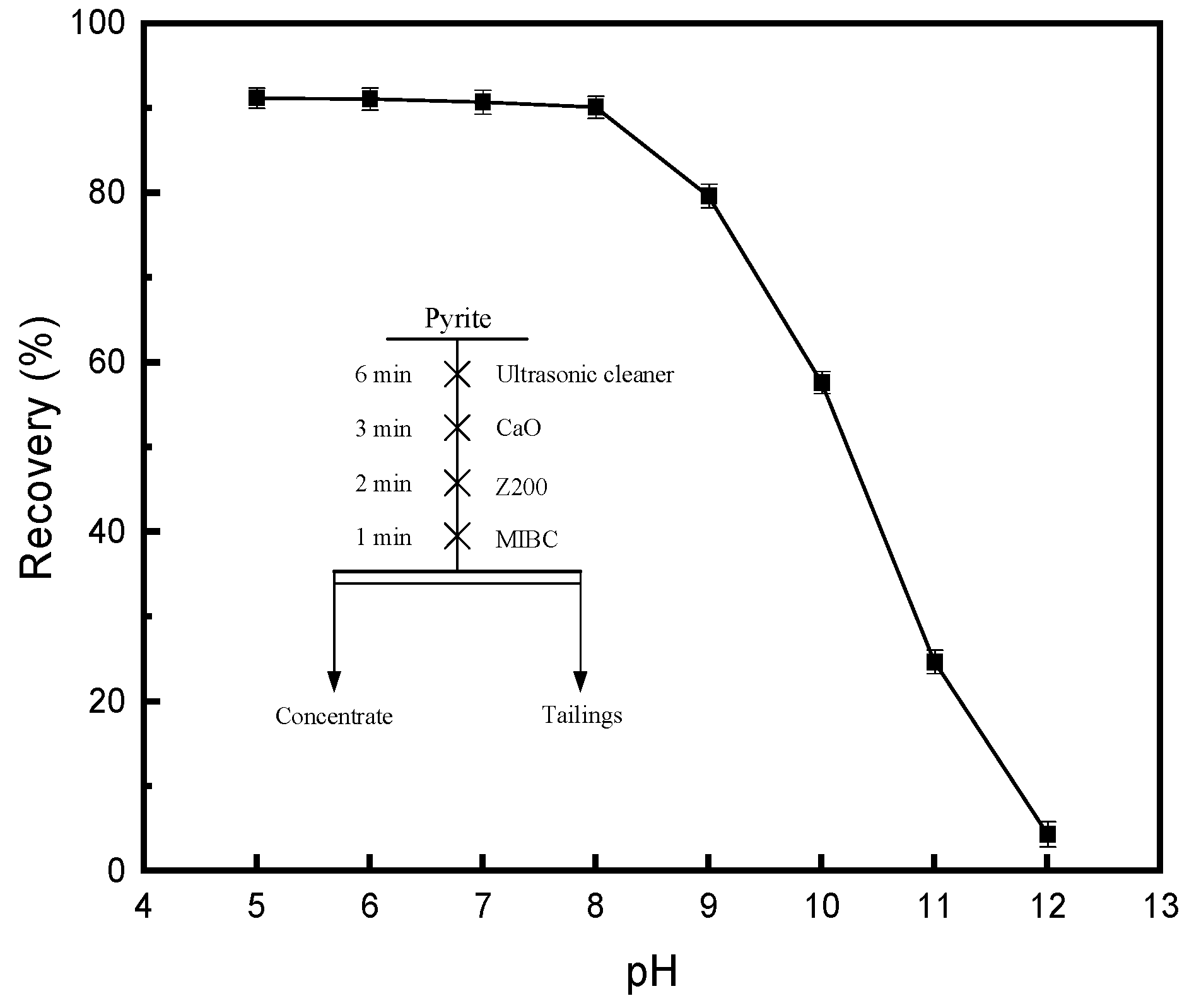
3.1.1. Effect of Lime on the Floatability of Pyrite in the Z200 System
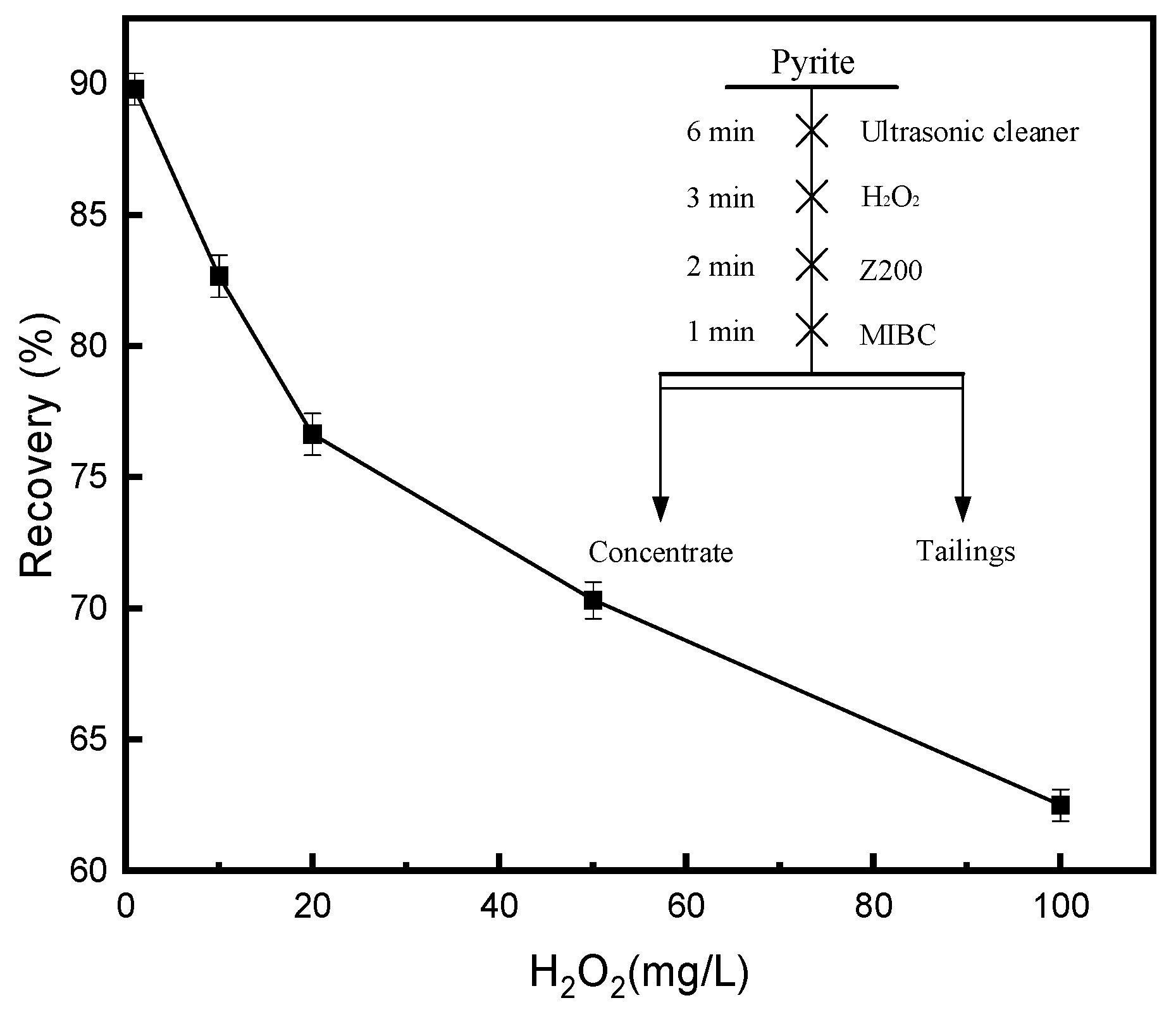
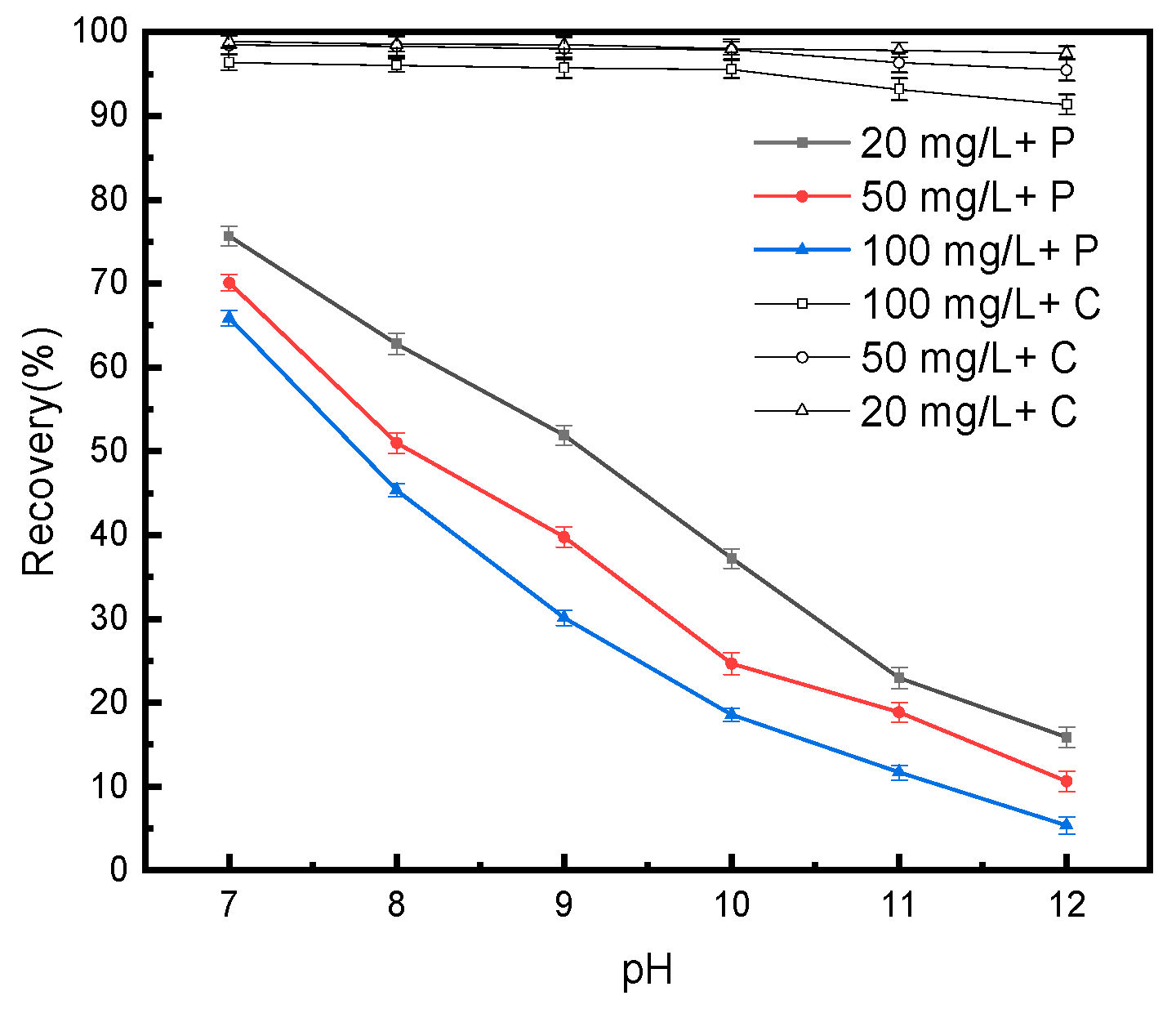
3.1.2. Effect of H2O2 on Pyrite in the Z200 System
3.1.3. Effect of Combined Lime-H2O2 Inhibition on Pyrite in the Z200 System
3.2. Activation of Depressed Pyrite
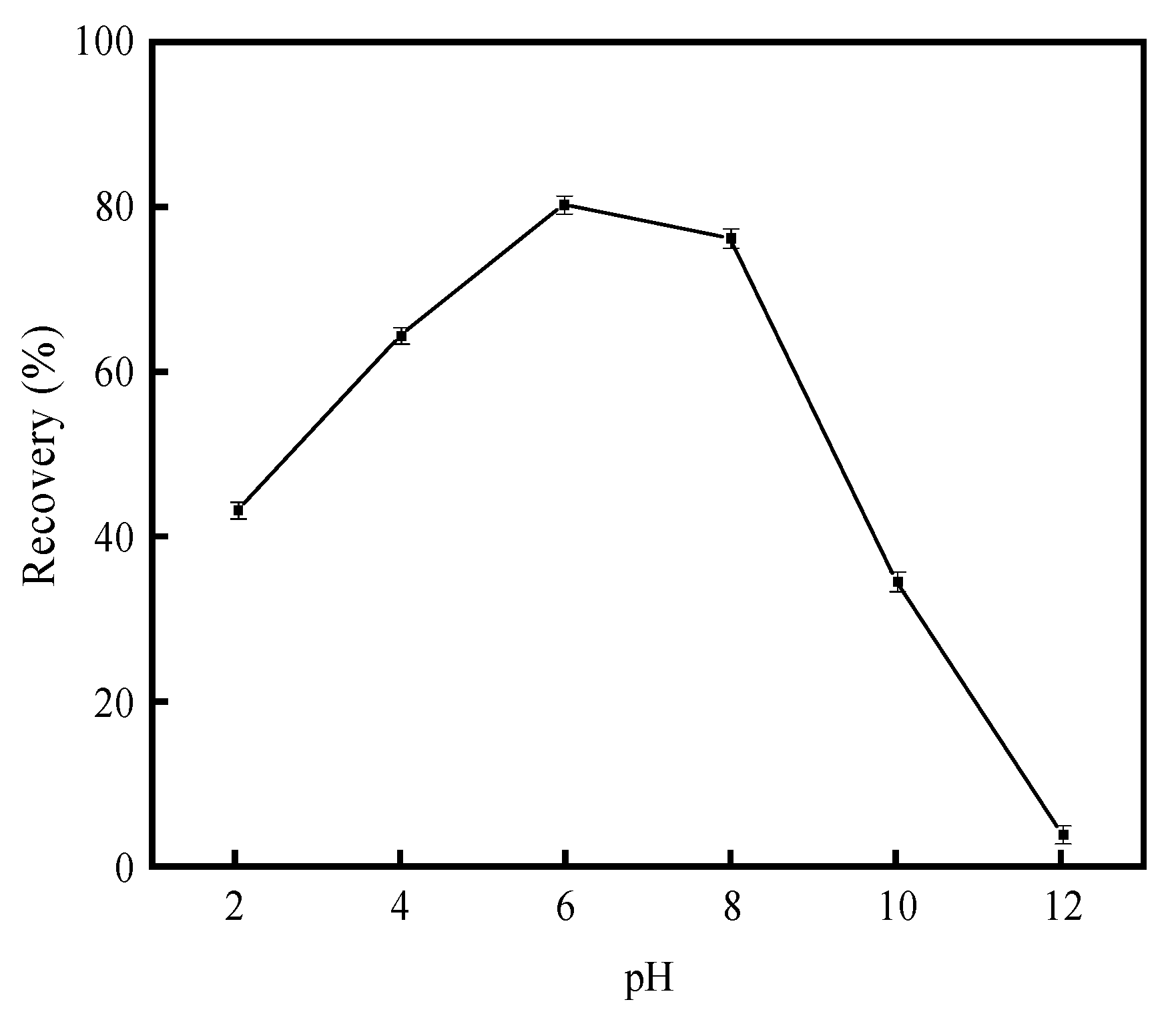
3.2.1. Sulfuric Acid Activation Test
3.2.2. Copper Sulfate Activation Test
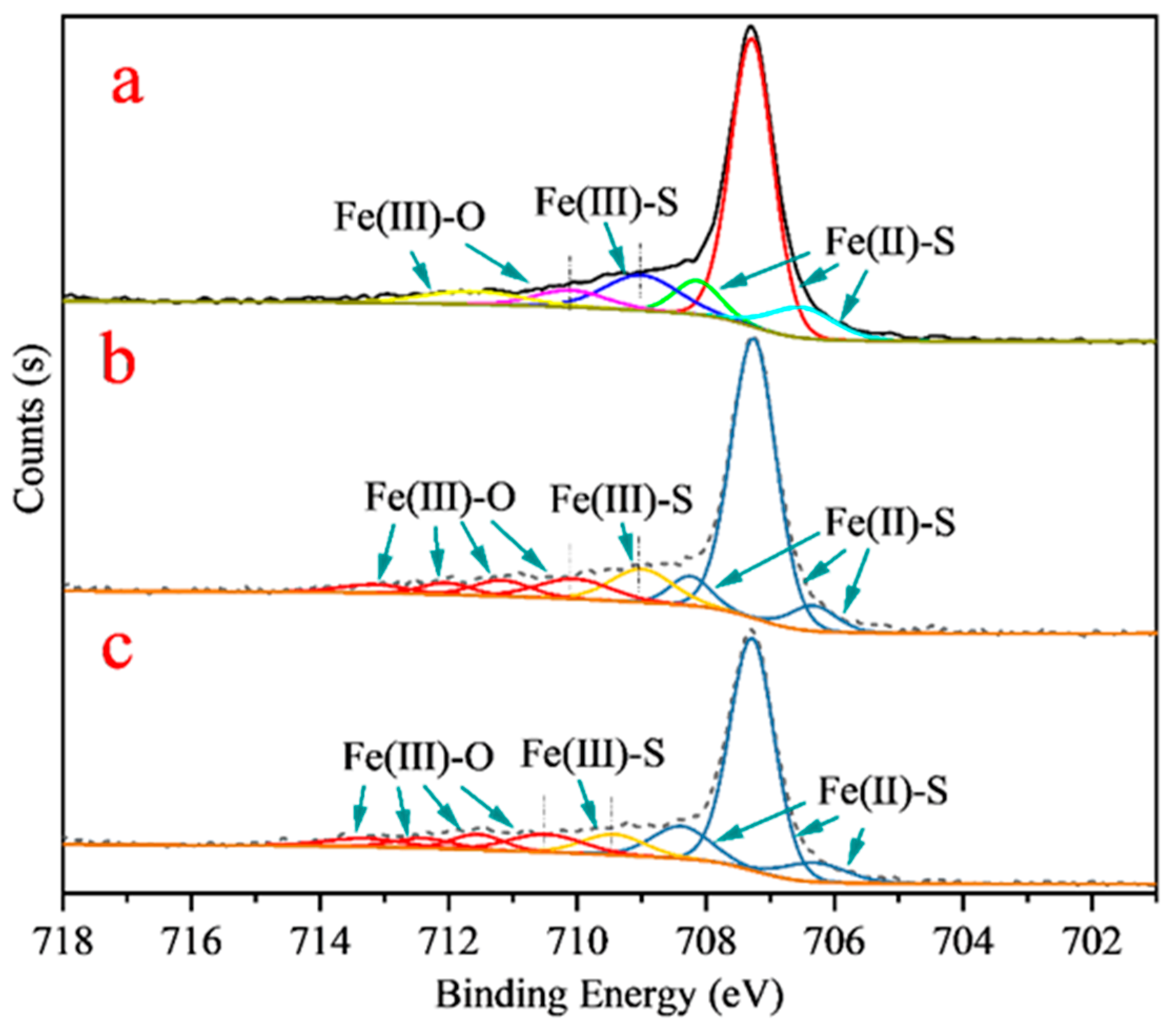
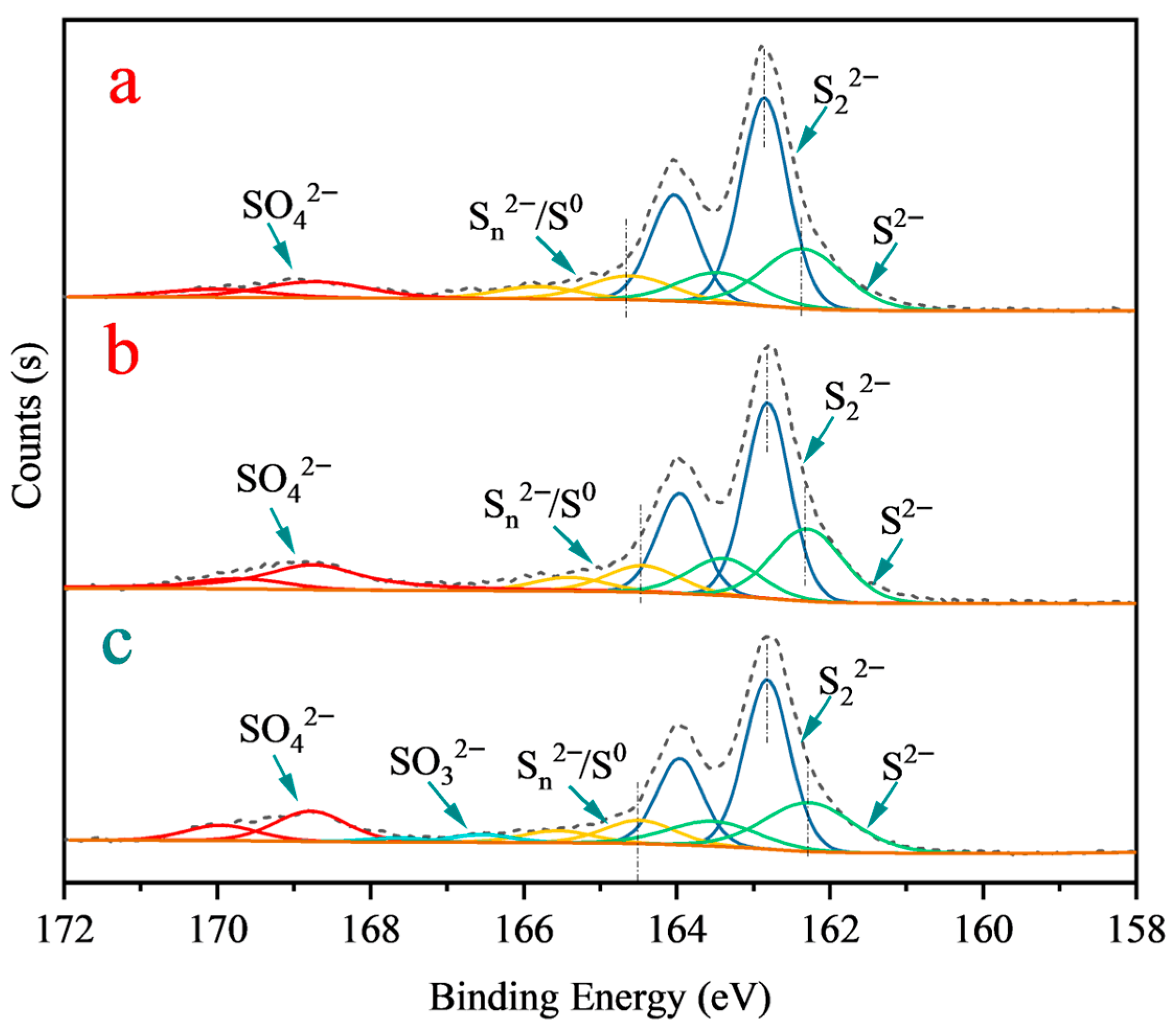
3.3. XPS Analysis
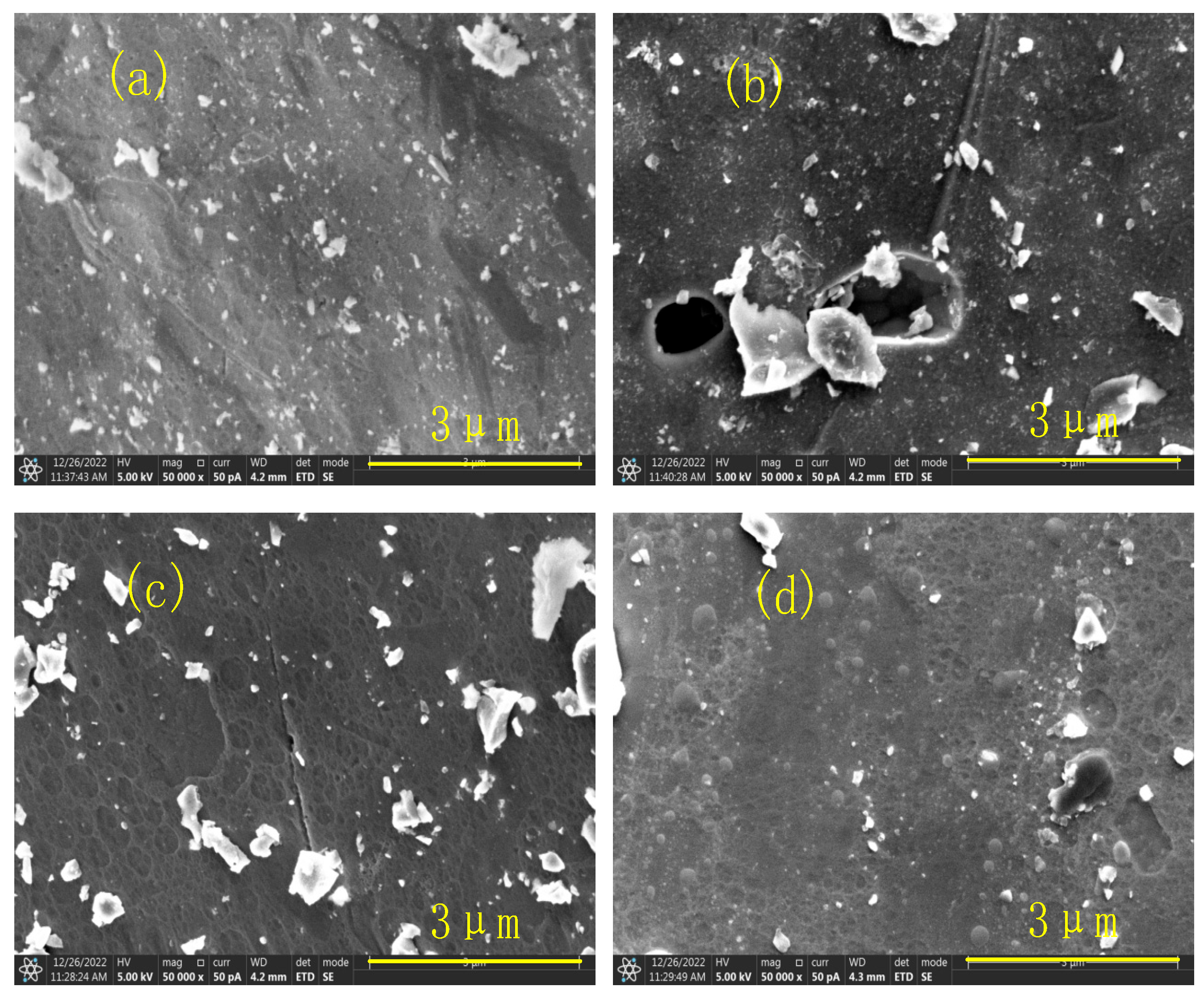
3.4. SEM Analysis
3.5. Contact Angle Analysis
3.6. Validation Through Actual Ore Flotation Testing
4. Conclusions
- Micro-flotation test results indicate that the addition of H2O2 reduces the pH required for pyrite suppression from 12 to 10. The combined use of lime and H2O2 as an inhibitor system enables efficient separation of covellite from pyrite.
- An investigation into the mechanisms revealed that the added H2O2 oxidized and corroded the pyrite surface, leading to increased oxide and hydroxide generation. This contributed to enhanced surface hydrophilicity and thereby to effective pyrite inhibition.
- Pyrite that has been inhibited can partially regain its floatability upon the addition of sulfuric acid or copper sulfate, with sulfuric acid demonstrating greater effectiveness.
- The Serbian copper ore exhibits a high sulfur content. When lime is used alone for pyrite inhibition, pyrite and gold recovery rates remain low. However, employing lime and H2O2 as a combined inhibitor reduces lime consumption in industrial operations, maintains copper flotation performance, and increases pyrite recovery by 2.3% and gold recovery by nearly 4%.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MLA | Mineral liberation analyzer |
| SEM | Scanning electron microscopy |
| XPS | X-ray photoelectron spectroscopy |
References
- Luo, S.X.; Chen, H.; Mou, Q.S.; Wu, Y.H. Research situation and progress of adsorption properties of pyrite. Multipurp. Util. Miner. Resour. 2020, 5, 27–33. [Google Scholar]
- Chen, W.; Chen, T.; Bu, X.; Chen, F.; Ding, Y.; Zhang, C.; Deng, S.; Song, Y. Theselective flotation of chalcopyrite against galena using a lginatesas depressant. Miner. Eng. 2019, 141, 105848. [Google Scholar] [CrossRef]
- Wang, F.L. Basic Theoretical Research on Flotation of Main Sulfide Oxide Minerals of Copper, Lead, Zinc and Iro. Ph.D. Thesis, Northeastern University, Shenyang, China, 2008. [Google Scholar]
- Chimonyo, W.; Fletcher, B.; Peng, Y. The differential depression of anoxidized starch on the flotation of chalcopyrite and graphite. Miner. Eng. 2020, 146, 106114. [Google Scholar] [CrossRef]
- Schlesinger, M.E.; Sole, K.C.; Davenport, W.G.; King, M.J. Chapter 1—Overview. In Extractive Metallurgy of Copper, 5th ed.; Schlesinger, M.E., King, M.J., Sole, K.C., Davenport, W.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–12. [Google Scholar]
- Wang, J.; Zheng, S.; Liu, W.; Chen, L.; Wen, Z.; Li, X. Prediction, evaluation and optimization of China’s copper resource supply system under carbon constraints. Sustain. Prod. Consum. 2023, 39, 285–300. [Google Scholar] [CrossRef]
- Li, W.Y.; Bai, X.; Ge, F.; Quan, X.; Chen, M.J.; Hao, J.M. Development Status of Copper-sulfur Separation Influence Factors and Depressants. Multipurp. Util. Miner. Resour. 2025, 1–18. [Google Scholar]
- Long, Y.Y.; Yin, W.Z.; Long, K.Y. Synergistic oxidation of NaClO and KMnO4 on flotation separation of chalcopyrite and pyrite and mechanism therein. Min. Metall. Eng. 2024, 44, 51–56. [Google Scholar]
- Tang, X.; Chen, Y. A review of flotation and selective separation of pyrrhotite: A perspective from crystal structures international. J. Min. Sci. Technol. 2022, 32, 847–863. [Google Scholar] [CrossRef]
- Shan, Z.Q.; Xia, Y.; Zhang, X.H. Copper selection experimental on a copper-gold sulphide Ore in Balai County, Lao People’s Democratic republic. Met. Mine 2016, 9, 96–99. [Google Scholar]
- Chen, J.H.; Li, Y.Q.; Long, Q.R. Molecular structures and activity of organic depressants for marmatite, jamesonite and pyrite flotation. Trans. Nonferrous Met. Soc. China 2010, 20, 1993–1999. [Google Scholar] [CrossRef]
- Bi, Y.X.; Yu, P.; Ding, Z.; Bai, S.J.; Wen, S.M. The Development of research on the pyrite flotation depressants. Conserv. Util. Miner. Resour. 2020, 40, 157–166. [Google Scholar]
- Cao, Q.; Zhang, H.; Yan, Y.; Li, Y.; Liu, D. Flotation separation of pyrite and chalcopyrite with potassium permanganate as a depressant. Chem. Pap. 2024, 78, 1761–1773. [Google Scholar] [CrossRef]
- Mu, Y.; Peng, Y.; Lauten, R.A. The depression of pyrite in selective flotation by different reagent systems—A Literature review. Miner. Eng. 2016, 96–97, 143–156. [Google Scholar] [CrossRef]
- Li, S.H.; Ma, Q.; Wang, L.; Yang, X. Research Progress on Activation Mechanism of Pyrite Flotation. Multipurp. Util. Miner. Resour. 2023, 2, 124–130. [Google Scholar]
- Qi, X.; Li, X.; Liang, Y.; Wang, H.; Guo, W.; Cong, X.; Lv, F.; Zhang, H. Surface structure-dependent hydrophobicity/oleophilicity of pyrite and its influence on coal flotation. J. Ind. Eng. Chem. 2020, 87, 136–144. [Google Scholar] [CrossRef]
- Bi, Y. Research on Selective Flotation Separation of Chalcopyrite and Pyrite Under Low Alkalinity. Ph.D. Thesis, Kunming University of Science and Technology, Kunming, China, 2023. [Google Scholar]
- Khoso, S.A.; Fei, L.Ü.; Ya, G.A.O.; Wei, S.U.N. Xanthate interaction and flotation separation of H2O2-treated chalcopyrite and pyrite. Trans. Nonferrous Met. Soc. China 2019, 29, 2604–2614. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, D.; Wen, S.; Lin, Q.; Song, Z.; Luo, X. Enhanced pyrite depression through nitrogen-aerated flotation under low alkalinity conditions. Adv. Powder Technol. 2024, 35, 104424. [Google Scholar] [CrossRef]
- Ozun, S.; Hassas, B.V.; Miller, J.D. Collectorless flotation of oxidized pyrite. Colloids Surf. A Physicochem. Eng. Asp. 2019, 561, 349–356. [Google Scholar] [CrossRef]
- Luo, X.; Yang, W.; Wen, S.; Lin, Q.; Wei, D.; Li, C.; Zhang, Y.; Song, Z.; Wang, Y. Effect of regulating dissolved oxygen concentration in pulp with aerated gas on pyrite flotation. Miner. Eng. 2023, 202, 108290. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, W.; Guo, Y.; Wang, T.; Luo, G.; Wang, H.; He, G. The flotation separation of galena and pyrite using serpentine as depressant. Powder Technol. 2019, 342, 486–490. [Google Scholar] [CrossRef]
- Lee, R.L.J.; Chen, X.; Peng, Y. Flotation performance of chalcopyrite in the presence of an elevated pyrite proportion. Miner. Eng. 2022, 177, 107387. [Google Scholar] [CrossRef]
- Hodgkinson, G.; Sandenberght, R.F.; Hunter, C.J.; De Wet, J.R. Pyrite flotation from gold leach residues. Miner. Eng. 1994, 7, 691–698. [Google Scholar] [CrossRef]
- Yuan, J.; Li, Y.; Ding, Z.; Zhang, Y.; Chen, L.; Wen, S.; Bai, S. Cleaner flotation separation of chalcopyrite from pyrite at low alkalinity: Based on calcium hypochlorite oxidative modification in acid mine drainage system. Sep. Purif. Technol. 2024, 344, 127325. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Fan, R.; Duan, W.; Huang, L.; Xiao, Q. Separation mechanism of chalcopyrite and pyrite due to H2O2 treatment in low-alkaline seawater flotation system. Miner. Eng. 2022, 176, 107356. [Google Scholar] [CrossRef]
- Duan, W.; Wang, Y.; Rao, F.; Liu, W.; Yang, Y.; Deng, R. Correlation between the mineralogical properties and oxidation rate of different minerogenetic pyrites: A Reflection on Floatability. Min. Metall. Explor. 2023, 40, 1369–1381. [Google Scholar] [CrossRef]
- Schippers, A.; Jozsa, P.; Sand, W. Sulfur chemistry in bacterial leaching of pyrite. Appl. Environ. Microbiol. 1996, 62, 3424–3431. [Google Scholar] [CrossRef] [PubMed]
- Holmes, P.R.; Crundwell, F.K. The kinetics of the oxidation of pyrite by ferric ions and dissolved oxygen: An electrochemical study. Geochim. Cosmochim. Acta 2000, 64, 263–274. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Nguyen, A.V. Characterisation of sphalerite and pyrite surfaces activated by copper sulphate. Miner. Eng. 2017, 100, 223–232. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Bancroft, G.M.; Pratt, A.R.; Scaini, M.J. Sulfur and iron surface states on fractured pyrite surfaces. Am. Mineral. Gruyter 1998, 83, 1067–1076. [Google Scholar] [CrossRef]
- Niu, X.; Chen, J.; Li, Y.; Xia, L.; Li, L.; Sun, H.; Ruan, R. Correlation of surface oxidation with xanthate adsorption and pyrite flotation. Appl. Surf. Sci. 2019, 495, 143411. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Muir, I.J. X-ray photoelectron spectroscopic study of a pristine pyrite surface reacted with water vapour and air. Geochim. Cosmochim. Acta 1994, 58, 4667–4679. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Muir, I.J.; Prarr, A.R. Oxidation of arsenopyrite by air and air-saturated, distilled water, and implications for mechanism of oxidation. Geochim. Cosmochim. Acta 1995, 59, 1773–1786. [Google Scholar] [CrossRef]
- Bonnissel-Gissinger, P.; Alnot, M.; Ehrhardt, J.J.; Behra, P. Surface oxidation of pyrite as a function of pH. Environ. Sci. Technol. 1998, 32, 2839–2845. [Google Scholar] [CrossRef]
- Chiriță, P.; Schlegel, M.L. Pyrite oxidation in air-equilibrated solutions: An electrochemical study. Chem. Geol. 2017, 470, 67–74. [Google Scholar] [CrossRef]
- Cai, Y.; Pan, Y.; Xue, J.; Sun, Q.; Su, G.; Li, X. Comparative XPS study between experimentally and naturally weathered pyrites. Appl. Surf. Sci. 2009, 255, 8750–8760. [Google Scholar] [CrossRef]


| Elements | Cu | Au * | S | As | Fe | Pb | SO3 | Zn | SiO2 |
|---|---|---|---|---|---|---|---|---|---|
| Contents | 2.8 | 1.64 | 20.39 | 0.035 | 18.87 | 0.01 | 3.74 | 0.02 | 3.74 |
| Copper–Sulfur Mineral Intergrowth Type | Yield (%) | Intergrowth Association | Copper Sulfide Particle Size Distribution (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| With Py | With Others | −10 µm | −20 + 10 µm | −38 + 20 µm | −74 + 38 µm | −150 + 74 µm | +150 µm | ||
| X = 100% | 85.88 | -- | -- | 0.62 | 14.7 | 26.15 | 30.03 | 14.12 | 0.26 |
| 80% ≤ X < 100% | 6.22 | 4.34 | 1.88 | -- | 0.12 | 0.77 | 2.26 | 2.81 | 0.26 |
| 50% ≤ X < 80% | 3.86 | 3.29 | 0.57 | -- | 0.24 | 1.47 | 1.74 | 0.41 | -- |
| X < 50% | 4.04 | 3.07 | 0.97 | 0.6 | 1.04 | 1.69 | 0.71 | -- | -- |
| Sum | 100.00 | 10.7 | 3.42 | 1.22 | 16.1 | 30.08 | 34.74 | 17.34 | 0.52 |
| Type of Pyrite Intergrowth | Yield (%) | Intergrowth Association | Pyrite Particle Size Distribution (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| With Cu | With Others | −10 µm | −20 + 10 µm | −38 + 20 µm | −74 + 38 µm | −150 + 74 µm | +150 µm | ||
| X = 100% | 83.77 | -- | -- | 0.23 | 18.47 | 30.35 | 27.41 | 7.22 | 0.09 |
| 80% ≤ X < 100% | 6.12 | 2.08 | 4.04 | 0.01 | 0.24 | 1.43 | 3.06 | 1.38 | -- |
| 50% ≤ X < 80% | 3.37 | 0.58 | 2.79 | 0.03 | 0.49 | 0.96 | 1.73 | 0.16 | -- |
| X < 50% | 6.74 | 0.63 | 6.11 | 0.90 | 2.22 | 2.51 | 1.10 | 0.01 | -- |
| Sum | 100.00 | 3.29 | 12.94 | 1.17 | 21.42 | 35.25 | 33.30 | 8.77 | 0.09 |
| Mineral Species | Mineral Content/% |
|---|---|
| Covellite (CuS) | 11.49 |
| Tennantite (Cu3AsS4) | 0.89 |
| Pyrite (FeS2) | 39.31 |
| Quartz (SiO2) | 27.74 |
| Product | Yield (%) | Grade (%) | Recovery (%) | ||||
|---|---|---|---|---|---|---|---|
| Cu | Au (g·t−1) | S | Cu | Au | S | ||
| Copper Concentrate 1 | 7.57 | 30.35 | 4.07 | 34.70 | 80.42 | 18.81 | 12.89 |
| Copper Concentrate 2 | 4.76 | 8.39 | 4.62 | 36.80 | 13.97 | 13.42 | 8.58 |
| Pyrite Concentrate | 23.41 | 0.52 | 3.77 | 49.40 | 4.26 | 53.87 | 56.70 |
| Tailing 2 | 14.53 | 0.096 | 0.78 | 7.61 | 0.49 | 6.92 | 5.42 |
| Tailing 1 | 49.73 | 0.050 | 0.23 | 6.73 | 0.87 | 6.98 | 16.41 |
| Feed | 100.00 | 2.86 | 1.64 | 20.39 | 100.00 | 100.00 | 100.00 |
| Copper Concentrate 1 + 2 | 12.33 | 21.88 | 4.28 | 35.51 | 94.38 | 32.23 | 21.47 |
| Tailing 1 + 2 | 64.26 | 0.06 | 0.35 | 6.93 | 1.36 | 13.90 | 21.83 |
| Chemical Formula | Content (wt.%) | Supplier | Function |
|---|---|---|---|
| CaO | 98 | Macklin Biochemical (Shanghai), Shanghai, China | Depressant |
| H2SO4 | 98.08 | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China | pH regulator |
| NaBX | — | Zhuzhou Flotation Reagents Co., Ltd., Zhuzhou, China | collector |
| MIBC | 99 | Shanghai EON Chemical Technology Co., Shanghai, China | frother |
| Z200(C3H7CH(CH3)C(S)NHC2H5) | — | Zhuzhou Flotation Reagents Co., Ltd., Zhuzhou, China | collector |
| H2O2 | 30 | Macklin Biochemical (Shanghai), Shanghai, China | depressant |
| Species | B.E. (eV) | Atomic Concentration (at. %) | |||
|---|---|---|---|---|---|
| Blank | CaO | H2O2 + CaO | |||
| Fe 2p3/2 | Fe(II)-S | 707.2 ± 0.1 | 30.2 | 27.1 | 26.5 |
| Fe(III)-S | 709.2 ± 0.3 | 5.7 | 4.3 | 4.1 | |
| Fe(III)-O | 710.3 ± 0.2 | 4.5 | 7.23 | 8.27 | |
| S 2p3/2 | S22− | 162.9 ± 0.1 | 21.3 | 20.8 | 19.4 |
| S2− | 162.5 ± 0.2 | 10.5 | 9.2 | 9.6 | |
| Sn2−/S0 | 164.3 ± 0.2 | 3.1 | 4.6 | 4.1 | |
| SO42− | 168.7 ± 0.1 | 1.2 | 1.8 | 2.6 | |
| SO32− | 166.5 ± 0.1 | - | - | 1.13 | |
| O 1s | O-H | 532.8 ± 0.2 | 8.0 | 24.5 | 25.3 |
| Me-O | 530.1 ± 0.2 | 2.3 | 12.8 | 13.6 | |
| Ca 2p | Ca-O | 348.1 ± 0.2 | - | 0.1 | 0.1 |
| Test Condition | Pyrite | |
|---|---|---|
| Appearance of Contact Angle | Contact Angle (°) | |
| Fresh Surface | 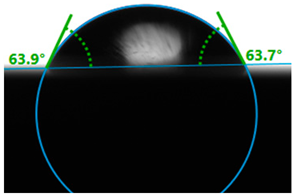 | 63.8 |
| Lime | 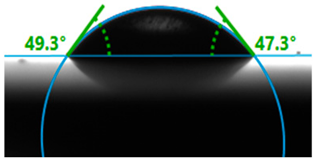 | 48.3 |
| Lime + Hydrogen Peroxide | 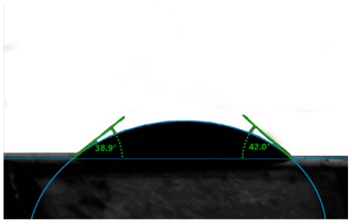 | 40.5 |
| Product | Yield (%) | Grade (%) | Recovery (%) | ||||
|---|---|---|---|---|---|---|---|
| Cu | Au (g·t−1) | S | Cu | Au | S | ||
| Copper Concentrate 1 | 7.42 | 31.12 | 4.13 | 34.6 | 80.74 | 18.69 | 12.59 |
| Copper Concentrate 2 | 4.68 | 8.51 | 4.58 | 36.8 | 13.93 | 13.07 | 8.45 |
| Pyrite Concentrate | 24.35 | 0.47 | 3.92 | 49.4 | 4.00 | 58.20 | 58.99 |
| Tailing 2 | 14.53 | 0.084 | 0.5 | 7.61 | 0.43 | 4.43 | 5.42 |
| Tailing 1 | 49.02 | 0.05 | 0.19 | 6.05 | 0.91 | 5.61 | 14.55 |
| Feed | 100 | 2.86 | 1.64 | 20.39 | 100 | 100 | 100 |
| Copper Concentrate 1 + 2 | 12.33 | 22.37 | 4.30 | / | 94.66 | 31.76 | 21.04 |
| Tailing 1 + 2 | 63.55 | 0.06 | 0.26 | 6.41 | 1.34 | 10.04 | 19.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Kostović, M.; Deng, R.; Liao, Y. Study on the Inhibition and Activation of Pyrite Under Low Alkalinity Conditions Created by Hydrogen Peroxide and Lime. Minerals 2025, 15, 1177. https://doi.org/10.3390/min15111177
Yang Y, Kostović M, Deng R, Liao Y. Study on the Inhibition and Activation of Pyrite Under Low Alkalinity Conditions Created by Hydrogen Peroxide and Lime. Minerals. 2025; 15(11):1177. https://doi.org/10.3390/min15111177
Chicago/Turabian StyleYang, Yuankun, Milena Kostović, Rongdong Deng, and Yinying Liao. 2025. "Study on the Inhibition and Activation of Pyrite Under Low Alkalinity Conditions Created by Hydrogen Peroxide and Lime" Minerals 15, no. 11: 1177. https://doi.org/10.3390/min15111177
APA StyleYang, Y., Kostović, M., Deng, R., & Liao, Y. (2025). Study on the Inhibition and Activation of Pyrite Under Low Alkalinity Conditions Created by Hydrogen Peroxide and Lime. Minerals, 15(11), 1177. https://doi.org/10.3390/min15111177





