Improving the Processing of Copper–Arsenic-Bearing Ores: Enhancing Separation and Extraction Methods Through Mediator Insights—A Brief Review
Abstract
1. Introduction
1.1. Selective Separation of Enargite via Froth Flotation
1.2. Selective Extraction of Arsenic via Leaching
1.2.1. Leaching of Arsenic in Acidic Conditions
1.2.2. Leaching of Arsenic in Alkaline Conditions
2. The Role of Mediators
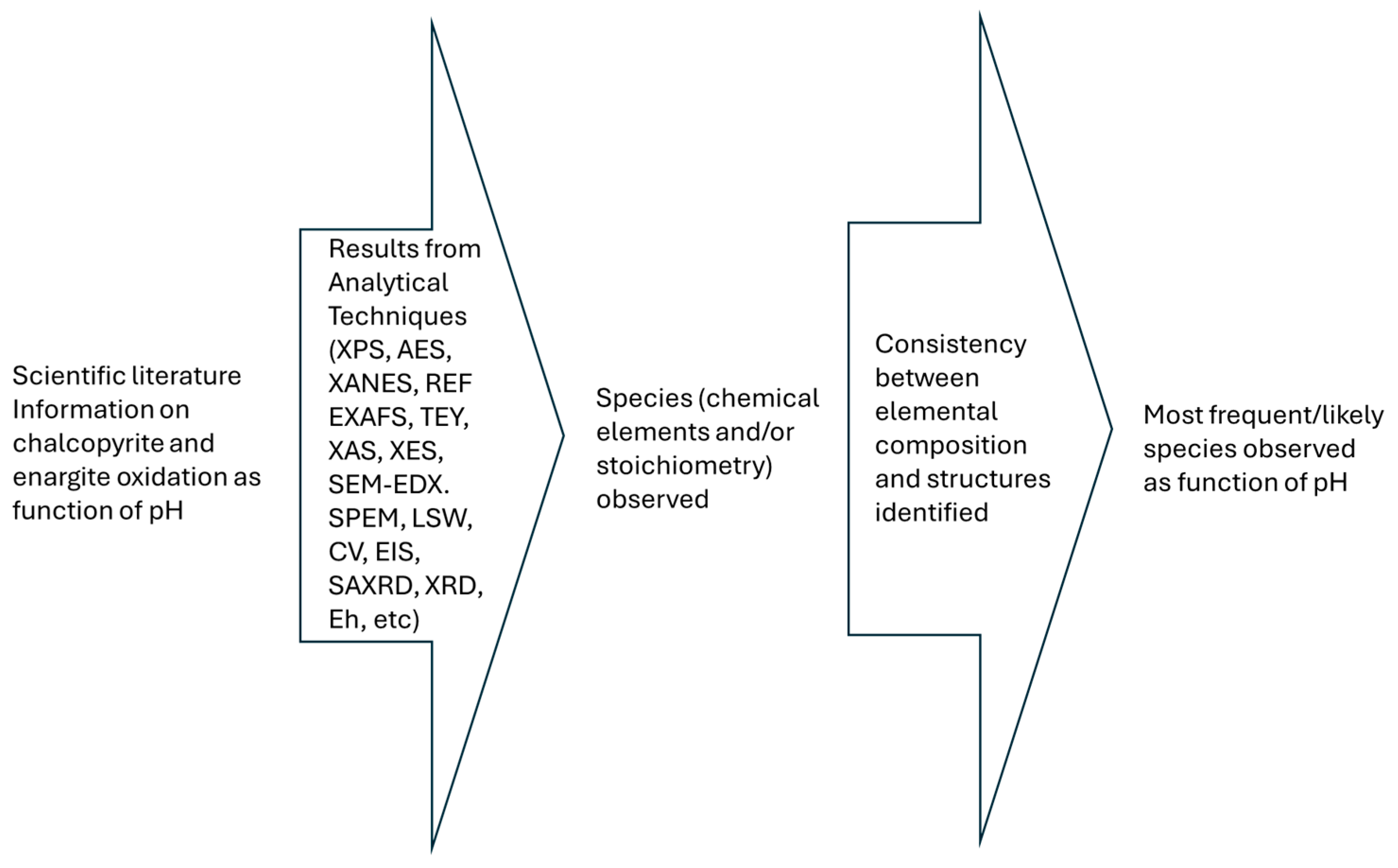
3. Methodology
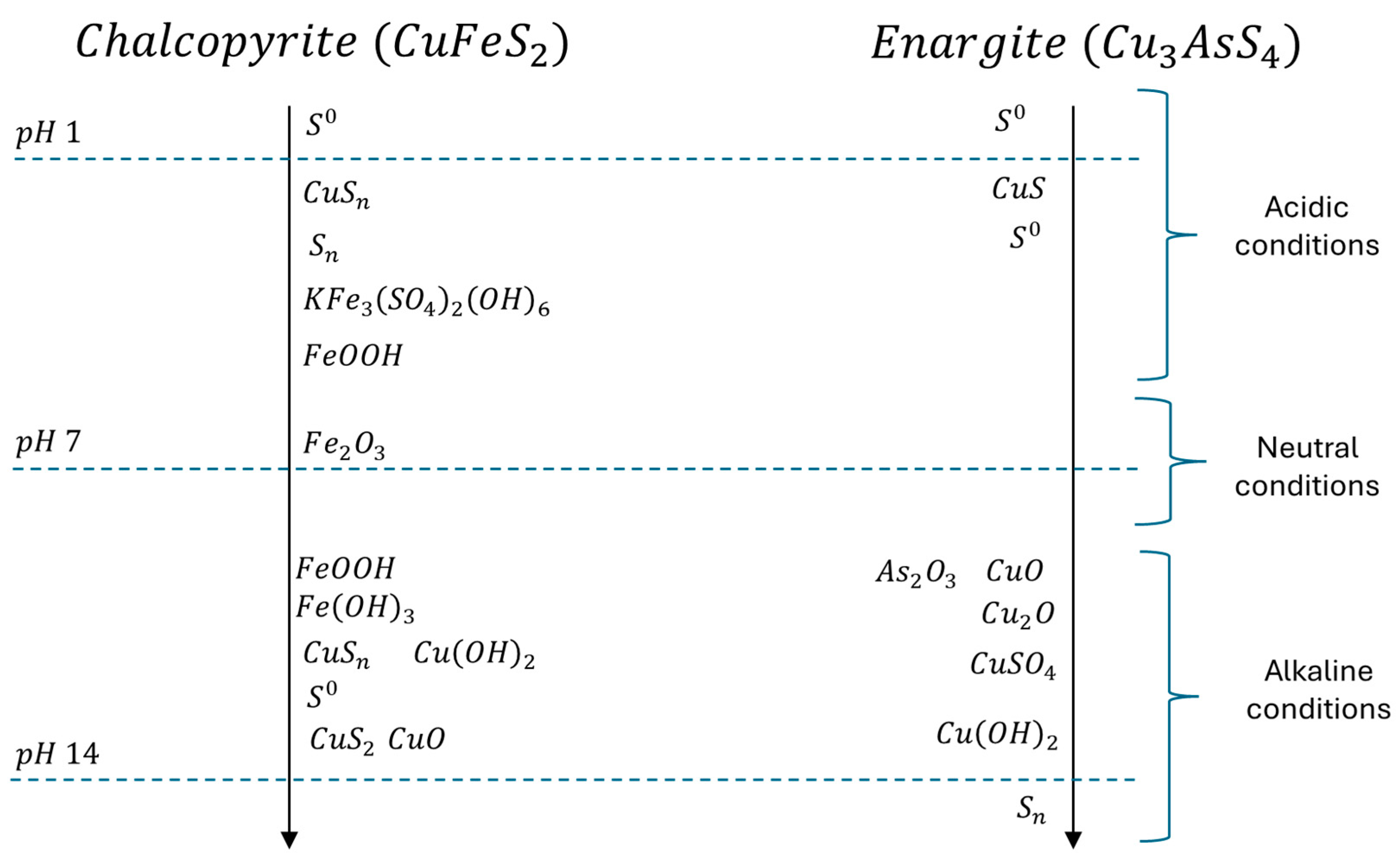
4. Specific Mediators Observed in Copper–Arsenic–Sulphide Minerals
4.1. Chalcopyrite
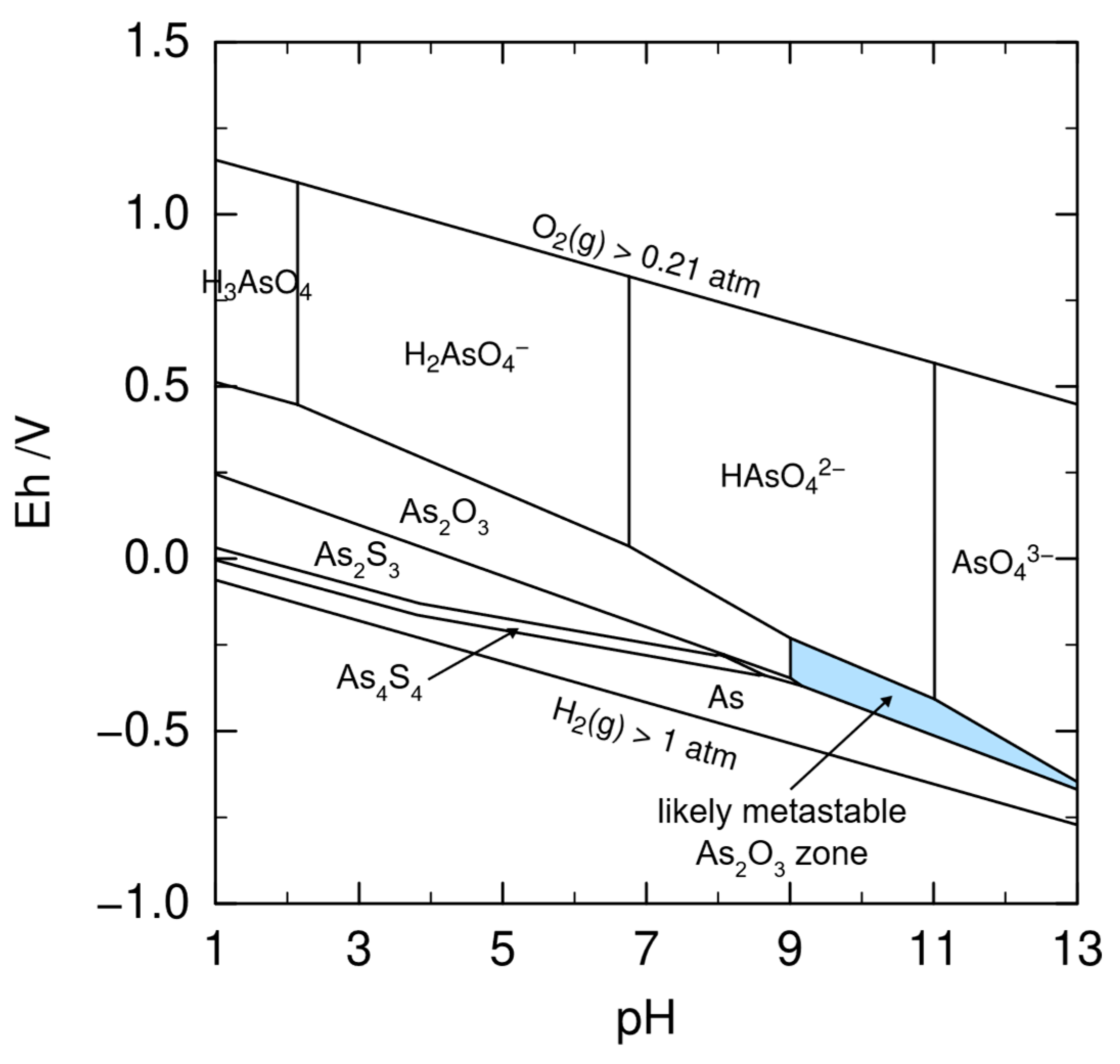
4.2. Enargite
5. Most Probable Mediators Across Different pH Conditions
6. Discussion
6.1. Selective Arsenic Leaching
6.2. Selective Enargite Separation from Copper Concentrates (Governed by Chalcopyrite)
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- West, J. Decreasing Metal Ore Grades. J. Ind. Ecol. 2011, 15, 165–168. [Google Scholar] [CrossRef]
- Voigt, P.; Hourn, M.; Lawson, V.; Anderson, G.; Mallah, D. Economic Recovery and Upgrade of Metals from Middling and Tailing Streams. In Proceedings of the 49th Annual Canadian Mineral Processors Operators Conference, Ottawa, ON, Canada, 17–19 January 2017; pp. 254–263. [Google Scholar]
- Safarzadeh, M.S.; Moats, M.S.; Miller, J.D. An Update to “Recent Trends in the Processing of Enargite Concentrates. ” Miner. Process. Extr. Metall. Rev. 2014, 35, 390–422. [Google Scholar] [CrossRef]
- Chung, K.W.; Yoon, H.-S.; Kim, C.-J.; Jeon, H.-S. Selective Leaching of Molybdenum from Bulk Concentrate by Electro-Oxidation. Metals 2021, 11, 1904. [Google Scholar] [CrossRef]
- Fuerstenau, M.C.; Jameson, G.J.; Yoon, R.-H. Froth Flotation: A Century of Innovation; SME: Littleton, CO, USA, 2007; ISBN 0-87335-252-1. [Google Scholar]
- Tayebi-Khorami, M.; Manlapig, E.; Forbes, E.; Bradshaw, D.; Edraki, M. Selective Flotation of Enargite from Copper Sulphides in Tampakan Deposit. Miner. Eng. 2017, 112, 1–10. [Google Scholar] [CrossRef]
- Guo, H.; Yen, W.-T. Selective Flotation of Enargite from Chalcopyrite by Electrochemical Control. Miner. Eng. 2005, 18, 605–612. [Google Scholar] [CrossRef]
- Woods, R. Electrochemical Potential Controlling Flotation. Int. J. Miner. Process. 2003, 72, 151–162. [Google Scholar] [CrossRef]
- Gao, X. Discussion on Arsenic Removal in Arsenic-Containing Copper Concentrate. Discov. Miner. 2024, 1, 3. [Google Scholar] [CrossRef]
- Yang, W.; Qian, L.; Jin, B.; Feng, Q.; Li, L.; He, K.; Yang, J. Leaching Behaviors of Copper and Arsenic from High-Arsenic Copper Sulfide Concentrates by Oxygen-Rich Sulfuric Acid Leaching at Atmospheric Pressure. J. Environ. Chem. Eng. 2022, 10, 107358. [Google Scholar] [CrossRef]
- Fredes Muñoz, S.A. Diseño de Mejores Prácticas Operacionales en el Procesamiento de Concentrados de Molibdeno Para Minera los Pelambres. Bachelor’s Thesis, Universidad de Chile, Santiago, Chile, 2015. [Google Scholar]
- Tongamp, W.; Takasaki, Y.; Shibayama, A. Arsenic Removal from Copper Ores and Concentrates through Alkaline Leaching in NaHS Media. Hydrometallurgy 2009, 98, 213–218. [Google Scholar] [CrossRef]
- Boyraz, T.; Türk, T.; Alp, I. Investigation of Arsenic Removal from Copper Concentrates by Alkaline Sulphur Leaching. Physicochem. Probl. Miner. Process. 2025, 61, 204050. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Bello, R.; Padilla, R. Removal of Arsenic from Enargite Rich Copper Concentrates. In Materials Processing Fundamentals; Zhang, L., Allanore, A., Wang, C., Yurko, J.A., Crapps, J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 249–256. ISBN 978-3-319-48197-5. [Google Scholar]
- Montes-Atenas, G.; de Guevara, R.L.; Lizama-Allende, K.; Valenzuela, F. Can Hydrogen Sulphide Gas Be Produced during Alkaline Leach of Enargitic Copper Concentrates? Hydrometallurgy 2019, 184, 109–115. [Google Scholar] [CrossRef]
- Wills, B.; Napier-Munn, T. Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 7th ed.; Butterworth-Heinemann: Oxford, UK, 2006; ISBN 978-0-7506-4450-1. [Google Scholar]
- Habashi, F. Textbook of Hydrometallurgy, 2nd ed.; Metallurgie Extractive Quebec: Sainte-Foy, QC, Canada, 1999; ISBN 2-9803247-7-9. [Google Scholar]
- Butt, H.-J.; Graf, K.; Kappl, M. Physics and Chemistry of Interfaces, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2013; ISBN 978-3-527-41216-7. [Google Scholar]
- Fichter, F. Organische elektrochemie. In Die Chemische Reaktion, 1st ed.; Steinkopff, T., Ed.; Wiley: Hoboken, NJ, USA, 1942; Volume 6, ISBN 0-598-84008-7. [Google Scholar]
- Steckhan, E. Indirect Electroorganic Syntheses—A Modern Chapter of Organic Electrochemistry. Angew. Chem. Int. Ed. Engl. 1986, 25, 683–701. [Google Scholar] [CrossRef]
- Watling, H.R. The Bioleaching of Sulphide Minerals with Emphasis on Copper Sulphides—A Review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, C.; Kou, J.; Zhao, H.; Wei, D.; Xing, Y. Enhancing the Leaching of Chalcopyrite Using Acidithiobacillus Ferrooxidans under the Induction of Surfactant Triton X-100. Minerals 2019, 9, 11. [Google Scholar] [CrossRef]
- Pullin, H.; Springell, R.; Parry, S.; Scott, T. The Effect of Aqueous Corrosion on the Structure and Reactivity of Zero-Valent Iron Nanoparticles. Chem. Eng. J. 2017, 308, 568–577. [Google Scholar] [CrossRef]
- Gonzalo, M.-A. Rôle de l’état de Surface du fer Métal sur le Mécanisme et la Cinétique de Décomposition de Colorants Azoïques. Ph.D. Thesis, Institut National Polytechnique de Lorraine, Nancy, France, 2004. [Google Scholar]
- Zeng, W.; Wang, Y.; Peng, C.; Qiu, Y. Organo-Mediator Enabled Electrochemical Transformations. Chem. Soc. Rev. 2025, 54, 4468–4501. [Google Scholar] [CrossRef]
- Krishna, D.N.G.; Philip, J. Review on Surface-Characterization Applications of X-Ray Photoelectron Spectroscopy (XPS): Recent Developments and Challenges. Appl. Surf. Sci. Adv. 2022, 12, 100332. [Google Scholar] [CrossRef]
- Mamiya, K.; Sanada, N. Auger Electron Spectroscopy. J. Jpn. Soc. Colour Mater. 2013, 86, 175–178. [Google Scholar] [CrossRef]
- Henderson, G.S.; de Groot, F.M.F.; Moulton, B.J.A. X-Ray Absorption Near-Edge Structure (XANES) Spectroscopy. Rev. Mineral. Geochem. 2014, 78, 75–138. [Google Scholar] [CrossRef]
- Vaughan, D.J.; Pattrick, R.A.D.; Wogelius, R.A. Minerals, Metals and Molecules: Ore and Environmental Mineralogy in the New Millennium. Mineral. Mag. 2002, 66, 653–676. [Google Scholar] [CrossRef]
- Harmer, S.L.; Thomas, J.E.; Fornasiero, D.; Gerson, A.R. The Evolution of Surface Layers Formed during Chalcopyrite Leaching. Geochim. Et Cosmochim. Acta 2006, 70, 4392–4402. [Google Scholar] [CrossRef]
- Biegler, T.; Horne, M.D. The Electrochemistry of Surface Oxidation of Chalcopyrite. J. Electrochem. Soc. 1985, 132, 1363. [Google Scholar] [CrossRef]
- Yin, Q.; Kelsall, G.H.; Vaughan, D.J.; England, K.E.R. Atmospheric and Electrochemical Oxidation of the Surface of Chalcopyrite (CuFeS2). Geochim. Et Cosmochim. Acta 1995, 59, 1091–1100. [Google Scholar] [CrossRef]
- Holliday, R.I.; Richmond, W.R. An Electrochemical Study of the Oxidation of Chalcopyrite in Acidic Solution. J. Electroanal. Chem. Interfacial Electrochem. 1990, 288, 83–98. [Google Scholar] [CrossRef]
- Wang, S. Copper Leaching from Chalcopyrite Concentrates. JOM 2005, 57, 48–51. [Google Scholar] [CrossRef]
- Klauber, C. A Critical Review of the Surface Chemistry of Acidic Ferric Sulphate Dissolution of Chalcopyrite with Regards to Hindered Dissolution. Int. J. Miner. Process. 2008, 86, 1–17. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Muñoz, J.A.; Blázquez, M.L.; González, F.; Ballester, A. Leaching of Chalcopyrite with Ferric Ion. Part I: General Aspects. Hydrometallurgy 2008, 93, 81–87. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.P.; Gerson, A.R. A Review of the Structure, and Fundamental Mechanisms and Kinetics of the Leaching of Chalcopyrite. Adv. Colloid Interface Sci. 2013, 197–198, 1–32. [Google Scholar] [CrossRef]
- Hernández, P.C.; Taboada, M.E.; Herreros, O.O.; Graber, T.A.; Ghorbani, Y. Leaching of Chalcopyrite in Acidified Nitrate Using Seawater-Based Media. Minerals 2018, 8, 238. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.; Zhang, X.; Qian, L.; Sun, M.; Yang, Y.; Zhang, Y.; Wang, J.; Kim, H.; Qiu, G. The Dissolution and Passivation Mechanism of Chalcopyrite in Bioleaching: An Overview. Miner. Eng. 2019, 136, 140–154. [Google Scholar] [CrossRef]
- Xiong, X.; Hua, X.; Zheng, Y.; Lu, X.; Li, S.; Cheng, H.; Xu, Q. Oxidation Mechanism of Chalcopyrite Revealed by X-Ray Photoelectron Spectroscopy and First Principles Studies. Appl. Surf. Sci. 2018, 427, 233–241. [Google Scholar] [CrossRef]
- Buckley, A.; Woods, R. An X-Ray Photoelectron Spectroscopic Study of the Oxidation of Chalcopyrite. Aust. J. Chem. 1984, 37, 2403–2413. [Google Scholar] [CrossRef]
- Todd, E.C.; Sherman, D.M.; Purton, J.A. Surface Oxidation of Chalcopyrite (CuFeS2) under Ambient Atmospheric and Aqueous (pH 2-10) Conditions: Cu, Fe L- and O K-Edge X-Ray Spectroscopy. Geochim. Et Cosmochim. Acta 2003, 67, 2137–2146. [Google Scholar] [CrossRef]
- Ghahremaninezhad, A.; Asselin, E.; Dixon, D.G. Electrochemical Evaluation of the Surface of Chalcopyrite during Dissolution in Sulfuric Acid Solution. Electrochim. Acta 2010, 55, 5041–5056. [Google Scholar] [CrossRef]
- Dutrizac, J.E. Elemental Sulphur Formation during the Ferric Sulphate Leaching of Chalcopyrite. Can. Metall. Q. 1989, 28, 337–344. [Google Scholar] [CrossRef]
- Parker, G.K.; Woods, R.; Hope, G.A. Raman Investigation of Chalcopyrite Oxidation. Colloids Surf. A Physicochem. Eng. Asp. 2008, 318, 160–168. [Google Scholar] [CrossRef]
- Majuste, D.; Ciminelli, V.S.T.; Osseo-Asare, K.; Dantas, M.S.S.; Magalhães-Paniago, R. Electrochemical Dissolution of Chalcopyrite: Detection of Bornite by Synchrotron Small Angle X-Ray Diffraction and Its Correlation with the Hindered Dissolution Process. Hydrometallurgy 2012, 111–112, 114–123. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Miki, H.; Hirajima, T.; Tsunekawa, M. A Model for Ferrous-Promoted Chalcopyrite Leaching. Hydrometallurgy 2000, 57, 31–38. [Google Scholar] [CrossRef]
- Li, Y.; Qian, G.; Brown, P.L.; Gerson, A.R. Chalcopyrite Dissolution: Scanning Photoelectron Microscopy Examination of the Evolution of Sulfur Species with and without Added Iron or Pyrite. Geochim. Et Cosmochim. Acta 2017, 212, 33–47. [Google Scholar] [CrossRef]
- Sandström, Å.; Shchukarev, A.; Paul, J. XPS Characterisation of Chalcopyrite Chemically and Bio-Leached at High and Low Redox Potential. Miner. Eng. 2005, 18, 505–515. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, W.; Chen, M. A Copper and Iron K-Edge XANES Study on Chalcopyrite Leached by Mesophiles and Moderate Thermophiles. Miner. Eng. 2013, 48, 31–35. [Google Scholar] [CrossRef]
- Yang, Y.; Harmer, S.; Chen, M. Synchrotron-Based XPS and NEXAFS Study of Surface Chemical Species during Electrochemical Oxidation of Chalcopyrite. Hydrometallurgy 2015, 156, 89–98. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Muñoz, J.A.; Blázquez, M.L.; González, F.; Ballester, A. Leaching of Chalcopyrite with Ferric Ion. Part II: Effect of Redox Potential. Hydrometallurgy 2008, 93, 88–96. [Google Scholar] [CrossRef]
- Vizcarra, T.G.; Harmer, S.L.; Wightman, E.M.; Johnson, N.W.; Manlapig, E.V. The Influence of Particle Shape Properties and Associated Surface Chemistry on the Flotation Kinetics of Chalcopyrite. Miner. Eng. 2011, 24, 807–816. [Google Scholar] [CrossRef]
- England, K.E.R.; Charnock, J.M.; Pattrick, R.A.D.; Vaughan, D.J. Surface Oxidation Studies of Chalcopyrite and Pyrite by Glancing-Angle x-Ray Absorption Spectroscopy (REFLEXAFS). Mineral. Mag. 1999, 63, 559–566. [Google Scholar] [CrossRef]
- Yin, Q.; Vaughan, D.J.; England, K.E.R.; Kelsall, G.H.; Brandon, N.P. Surface Oxidation of Chalcopyrite (CuFeS2) in Alkaline Solutions. J. Electrochem. Soc. 2000, 147, 2945. [Google Scholar] [CrossRef]
- Mielczarski, J.A.; Cases, J.M.; Alnot, M.; Ehrhardt, J.J. XPS Characterization of Chalcopyrite, Tetrahedrite, and Tennantite Surface Products after Different Conditioning. 1. Aqueous Solution at pH 10. Langmuir 1996, 12, 2519–2530. [Google Scholar] [CrossRef]
- Parker, A.; Paul, R.; Power, G. Electrochemical Aspects of Leaching Copper from Chalcopyrite in Ferric and Cupric Salt Solutions. Aust. J. Chem. 1981, 34, 13–34. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Gan, X.; Hu, M.; Tao, L.; Qin, W.; Qiu, G. Role of Pyrite in Sulfuric Acid Leaching of Chalcopyrite: An Elimination of Polysulfide by Controlling Redox Potential. Hydrometallurgy 2016, 164, 159–165. [Google Scholar] [CrossRef]
- Linge, H.G. A Study of Chalcopyrite Dissolution in Acidic Ferric Nitrate by Potentiometric Titration. Hydrometallurgy 1976, 2, 51–64. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Z.; Qian, G.; Li, J.; Gerson, A.R. Kinetics and Mechanisms of Chalcopyrite Dissolution at Controlled Redox Potential of 750 mV in Sulfuric Acid Solution. Minerals 2016, 6, 83. [Google Scholar] [CrossRef]
- Dutrizac, J.E. The Dissolution of Chalcopyrite in Ferric Sulfate and Ferric Chloride Media. Metall. Trans. B 1981, 12, 371–378. [Google Scholar] [CrossRef]
- Salinas, K.E.; Herreros, O.; Torres, C.M. Leaching of Primary Copper Sulfide Ore in Chloride-Ferrous Media. Minerals 2018, 8, 312. [Google Scholar] [CrossRef]
- Fisher, W.W.; Flores, F.A.; Henderson, J.A. Comparison of Chalcocite Dissolution in the Oxygenated, Aqueous Sulfate and Chloride Systems. Miner. Eng. 1992, 5, 817–834. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Lawson, F. The Kinetics of Leaching Chalcocite in Acidic Oxygenated Sulphate-Chloride Solutions. Hydrometallurgy 1991, 27, 249–268. [Google Scholar] [CrossRef]
- Biegler, T. Reduction Kinetics of a Chalcopyrite Electrode Surface. J. Electroanal. Chem. Interfacial Electrochem. 1977, 85, 101–106. [Google Scholar] [CrossRef]
- Warren, G.W.; Sohn, H.-J.; Wadsworth, M.E.; Wang, T.G. The Effect of Electrolyte Composition on the Cathodic Reduction of CuFeS2. Hydrometallurgy 1985, 14, 133–149. [Google Scholar] [CrossRef]
- Dixon, D.G.; Mayne, D.D.; Baxter, K.G. GalvanoxTM—A Novel Galvanically-Assisted Atmospheric Leaching Technology for Copper Concentrates. Can. Metall. Q. 2008, 47, 327–336. [Google Scholar] [CrossRef]
- Wang, J.; Tao, L.; Zhao, H.; Hu, M.; Zheng, X.; Peng, H.; Gan, X.; Xiao, W.; Cao, P.; Qin, W.; et al. Cooperative Effect of Chalcopyrite and Bornite Interactions during Bioleaching by Mixed Moderately Thermophilic Culture. Miner. Eng. 2016, 95, 116–123. [Google Scholar] [CrossRef]
- Welham, N.J. Mechanochemical Processing of Enargite (Cu3AsS4). Hydrometallurgy 2001, 62, 165–173. [Google Scholar] [CrossRef]
- Berkh, K.; Majzlan, J.; Meima, J.A.; Plášil, J.; Rammlmair, D. The Effect of Chemical Variability and Weathering on Raman Spectra of Enargite and Fahlore. Eur. J. Mineral. 2023, 35, 737–754. [Google Scholar] [CrossRef]
- Da Pelo, S. Environmental Geochemistry and Mineralogy of Active and Abandoned Mine Sites. Ph.D. Thesis, University of Cagliari, Cagliari, Italy, 1998. [Google Scholar]
- Rossi, A.; Atzei, D.; Da Pelo, S.; Frau, F.; Lattanzi, P.; England, K.E.R.; Vaughan, D.J. Quantitative X-Ray Photoelectron Spectroscopy Study of Enargite (Cu3AsS4) Surface. Surf. Interface Anal. 2001, 31, 465–470. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; MacDonald, R.J.C. The Kinetics of Dissolution of Enargite in Acidified Ferric Sulphate Solutions. Can. Metall. Q. 1972, 11, 469–476. [Google Scholar] [CrossRef]
- Herreros, O.; Quiroz, R.; Hernández, M.C.; Viñals, J. Dissolution Kinetics of Enargite in Dilute Cl2/Cl− Media. Hydrometallurgy 2002, 64, 153–160. [Google Scholar] [CrossRef]
- Ásbjörnsson, J.; Kelsall, G.H.; Pattrick, R.A.D.; Vaughan, D.J.; Wincott, P.L.; Hope, G.A. Electrochemical and Surface Analytical Studies of Enargite in Acid Solution. J. Electrochem. Soc. 2004, 151, E250. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, Y.; Skinner, W.; Chen, M. Electrochemical and Spectroscopic Analysis of Enargite (Cu3AsS4) Dissolution Mechanism in Sulfuric Acid Solution. Hydrometallurgy 2020, 194, 105346. [Google Scholar] [CrossRef]
- Chen, M.; Ma, Y.L.; Bruckard, W. An Electrochemical and Spectroscopic Study of the Leaching Mechanisms of Enargite and Chalcopyrite in Sulfuric Acid. In Proceedings of the International Mining Processing Congress (IMPC) Asia-Pacific Conference 2022, Melbourne, Australia, 22–24 August 2022; pp. 512–520. [Google Scholar]
- Corkhill, C.L.; Wincott, P.L.; Lloyd, J.R.; Vaughan, D.J. The Oxidative Dissolution of Arsenopyrite (FeAsS) and Enargite (Cu3AsS4) by Leptospirillum Ferrooxidans. Geochim. Et Cosmochim. Acta 2008, 72, 5616–5633. [Google Scholar] [CrossRef]
- Plackowski, C.; Hampton, M.A.; Nguyen, A.V.; Bruckard, W.J. The Effects of X-Ray Irradiation and Temperature on the Formation and Stability of Chemical Species on Enargite Surfaces during XPS. Miner. Eng. 2013, 45, 59–66. [Google Scholar] [CrossRef]
- Plackowski, C.; Hampton, M.A.; Nguyen, A.V.; Bruckard, W.J. Fundamental Studies of Electrochemically Controlled Surface Oxidation and Hydrophobicity of Natural Enargite. Langmuir 2013, 29, 2371–2386. [Google Scholar] [CrossRef]
- Ishihara, S.; Shinoda, K.; Kano, J. Mechanochemical Treatment to Remove Arsenic from Copper Ore. Minerals 2019, 9, 349. [Google Scholar] [CrossRef]
- Elsener, B.; Atzei, D.; Fantauzzi, M.; Rossi, A. Electrochemical and XPS Surface Analytical Studies on the Reactivity of Enargite. Eur. J. Mineral. 2007, 19, 353–361. [Google Scholar] [CrossRef]
- Córdova, R.; Gómez, H.; Real, S.G.; Schrebler, R.; Vilche, J.R. Characterization of Natural Enargite/Aqueous Solution Systems by Electrochemical Techniques. J. Electrochem. Soc. 1997, 144, 2628. [Google Scholar] [CrossRef]
- Kantar, C. Solution and Flotation Chemistry of Enargite. Colloids Surf. A Physicochem. Eng. Asp. 2002, 210, 23–31. [Google Scholar] [CrossRef]
- Lattanzi, P.; Da Pelo, S.; Musu, E.; Atzei, D.; Elsener, B.; Fantauzzi, M.; Rossi, A. Enargite Oxidation: A Review. Earth-Sci. Rev. 2008, 86, 62–88. [Google Scholar] [CrossRef]
- Sasaki, K.; Takatsugi, K.; Ishikura, K.; Hirajima, T. Spectroscopic Study on Oxidative Dissolution of Chalcopyrite, Enargite and Tennantite at Different pH Values. Hydrometallurgy 2010, 100, 144–151. [Google Scholar] [CrossRef]
- Fullston, D.; Fornasiero, D.; Ralston, J. Oxidation of Synthetic and Natural Samples of Enargite and Tennantite: 2. X-Ray Photoelectron Spectroscopic Study. Langmuir 1999, 15, 4530–4536. [Google Scholar] [CrossRef]
- Viñals, J.; Roca, A.; Hernández, M.C.; Benavente, O. Topochemical Transformation of Enargite into Copper Oxide by Hypochlorite Leaching. Hydrometallurgy 2003, 68, 183–193. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovičová, M.; Bastl, Z.; Ohtani, T.; Sánchez, M. Influence of Mechanical Activation on the Alkaline Leaching of Enargite Concentrate. Hydrometallurgy 2000, 54, 205–216. [Google Scholar] [CrossRef]
- Gow, R.N.; Young, C.; Huang, H.; Hope, G.; Takasaki, Y. Electrochemistry of Enargite: Reactivity in Alkaline Solutions. In Electrometallurgy 2012; Wiley: Hoboken, NJ, USA, 2012; pp. 217–225. ISBN 978-1-118-37135-0. [Google Scholar]
- Tongamp, W.; Takasaki, Y.; Shibayama, A. Selective Leaching of Arsenic from Enargite in NaHS–NaOH Media. Hydrometallurgy 2010, 101, 64–68. [Google Scholar] [CrossRef]
- Di Benedetto, F.; Pelo, S.D.; Caneschi, A.; Lattanzi, P. Chemical State of Arsenic and Copper in Enargite: Evidences from EPR and X-Ray Absorption Spectroscopies, and SQUID Magnetometry. Neues Jahrb. Für Mineral. Abh. 2011, 188, 11–19. [Google Scholar] [CrossRef]
- Davis, A.; Ruby, M.V.; Bergstrom, P.D. Bioavailability of Arsenic and Lead in Soils from the Butte, Montana, Mining District. Environ. Sci. Technol. 1992, 26, 461–468. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; National Association of Corrosion Engineers: Brussels, Belgium, 1974; ISBN 978-0-915567-98-0. [Google Scholar]
- Kinniburgh, D.; Cooper, D. PhreePlot: Creating Graphical Output with PHREEQC; Natural Environment Research Council: Swindon, UK, 2011; p. 574.
- Ball, J.W.; Nordstrom, D.K. User’s Manual for WATEQ4F, with Revised Thermodynamic Data Base and Text Cases for Calculating Speciation of Major, Trace, and Redox Elements in Natural Waters; US Geological Survey: Reston, VA, USA, 1991.
- Gow, R.; Huang, H.; Young, C. Utility of Mass-Balanced EH-pH Diagrams I—Applications of Gibbs’ Phase Rule. Miner. Metall. Process. 2016, 33, 58–67. [Google Scholar] [CrossRef]
- Malki, B.; Guillotte, I.; Baroux, B. Deriving Metastable Pourbaix Diagrams of Stainless Steels Using Density Functional Theory Calculations. J. Electrochem. Soc. 2023, 170, 091501. [Google Scholar] [CrossRef]
- Drissi, S.H.; Refait, P.; Abdelmoula, M.; Génin, J.M.R. The Preparation and Thermodynamic Properties of Fe(II) Fe(III) Hydroxide-Carbonate (Green Rust 1); Pourbaix Diagram of Iron in Carbonate-Containing Aqueous Media. Corros. Sci. 1995, 37, 2025–2041. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Zhang, B.; Jiao, F.; Qin, W. Flotation Separation of Enargite from Complex Copper Concentrates by Selective Surface Oxidation. Physicochem. Probl. Miner. Process. 2019, 55, 852–864. [Google Scholar] [CrossRef]
- Voigt, D.E.; Brantley, S.L.; Hennet, R.J.-C. Chemical Fixation of Arsenic in Contaminated Soils. Appl. Geochem. 1996, 11, 633–643. [Google Scholar] [CrossRef]
- Tang, Z.; Tang, X.; Xiao, Z.; Liu, H. Mechanism and Kinetics of Scorodite Formation in Arsenic-Bearing Solutions Using Fe(OH)3 as a Solid Iron Source. Process Saf. Environ. Prot. 2024, 191, 1218–1238. [Google Scholar] [CrossRef]
- Kossoff, D.; Welch, M.D.; Hudson-Edwards, K.A. Scorodite Precipitation in the Presence of Antimony. Chem. Geol. 2015, 406, 1–9. [Google Scholar] [CrossRef]

| Mediator | pH | Temp. [°C] | Experimental Conditions | Technique | Ref. |
|---|---|---|---|---|---|
| Atmospheric oxidation | |||||
| FeOOH | - | 90 | Water vapor at 500 kPa | XPS | [40] |
| Fe2O3·xH2O CuS2 metastable CuSO4 | - | 22 | Air, 2–120 days | XPS | [41] |
| Fe(OH)3 FeOOH Fe2O3 CuS2 metastable CuS | - | N/R | Air | LSV | [32] |
| FeOOH CuO Cu2O/Cu2S Cu/Fe sulphate | - | 20 | Air, 7 days | TEY-XAS, XES | [42] |
| Acid leaching | |||||
| Cu0.75S2 CuS S0 | ~0 | 25 | N2-purged solution 1 M H2SO4, 5·10−4 M CuSO4 −0.45–0.55 V vs. SCE | LSV, CV | [31] |
| CuS2 metastable S0 | 0 | 25 | N2-purged solution 0.5 V vs. SCE 1 M HClO4 | XPS | [32] |
| Cu1−xFe1−yS2 Cu1−x−zS2 CuS Fe2(SO4)3 Jarosite | ~0.3 | 25 | 0.5 M H2SO4 0.1–0.75 V vs. MSE | EIS | [43] |
| S0 | ~0.3–0.5 | 95 | 0.1–2 M Fe2(SO4)3 0.3–0.5 M H2SO4 | SEM-EDS | [44] |
| S0 Polymeric sulphur | ~1.0 | 25 | N2-purged solution 0.1 M HCl 0.57–0.69 V vs. Ag/AgCl | Raman | [45] |
| Cu5FeS4 (bornite) S0 Sulphide CuS | ~1.0 | 25 | N2-purged solution 0.1 M H2SO4 | LSV, SAXRD µ-Raman | [46] |
| Cu2S S0 | ~1.0 | 30 | 0.1 or 0.5 M FeSO4 0.01 or 0.001 M CuSO4 Fe2(SO4)3 0.1 M H2SO4 | Thermodynamic model | [47] |
| S22− (disulphide) Sn2− (polysulphide) | 1.0 | 75 | 4 mM FeCl2 HClO4 0.65 V vs. SHE 5–10 days | SPEM, XPS | [48] |
| Sn2− (polysulphide) S0 | 1.0 | 85 | 0.1 M HClO4 | SEM, XPS | [18] |
| S22− S0 | 1.5 | 65 | culture medium 0.2 M KMnO4 50 mM FeSO4·7H2O 50 mM CuSO4·5H2O H2SO4 | Redox titration XPS, XRD | [49] |
| S22− S0 Jarosite | 1.5 | 65 | culture medium Sulfolobus metallicus 0.2 M KMnO4 50 mM FeSO4·7H2O 50 mM CuSO4·5H2O H2SO4 | Redox titration XPS, XRD | [49] |
| CuSn-like phase | 1.8 | 30 | 9K basic salt medium mesophilic consortium H2SO4 | XANES | [50] |
| CuSn-like phase Jarosite | 1.8 | 45 | 9 K basic salt medium thermophilic consortium H2SO4 | XANES | [50] |
| Sn2−/S0 S22−/CuS Jarosite/FeOOH | 1.8 | N/R | 9 K basic salt medium 0.1–1.2 V vs. Ag/AgCl H2SO4 | CV, XPS TEY-XANES Raman | [51] |
| S0 CuS FeOOH K-Jarosite | 1.8 | 35 | 0 K basic salt medium with different ratios Fe2(SO4)3/FeSO4 0.4–0.6 V vs. Ag/AgCl H2SO4 | XRD SEM-EDS | [52] |
| S0 FeOOH K-Jarosite | 1.8 | 65 | Norris nutrient medium with different ratios Fe2(SO4)3/FeSO4: 0.4–0.6 V vs. Ag/AgCl H2SO4 | XRD SEM-EDS | [52] |
| Jarosite | 1.83 | N/R | Air-saturated 0.1 M NaNO3 HNO3—7 days | TEY-XAS, XES | [42] |
| Cu0.8S2 | ~2.88 | N/R | Air-saturated 0.1 M CH3COOH 40 days | XPS | [41] |
| Fe2O3 | 6.53 | N/R | Air-saturated 0.1 M NaNO3 HNO3—7 days | TEY-XAS, XES | [42] |
| Alkaline leaching | |||||
| S22− Sn2− | 9.0 | N/R | KOH 100 ppm PAX | XPS | [53] |
| FeOOH Cu(OH)2 | 9.2 | N/R | 0.1 M Na2B4O7 1.5 V vs. SCE—7 min | REFLEXAFS | [54] |
| Fe(OH)3 Fe2O3 CuS2 metastable CuO S0 | 9.2 | 25 | 0.1 M Na2B4O7 | CV, XPS, AES | [55] |
| Fe(OH)3 CuFe1−xS2 | 10.0 | N/R | Aerated water KOH 25 min | XPS | [56] |
| FeOOH | 10.67 | N/R | Air-saturated 0.1 M NaNO3 NaOH—7 days | TEY-XAS, XES | [42] |
| Fe(OH)3 Fe2O3 CuS2 metastable CuO S0 | 12.7 | 25 | 0.05 M NaOH | CV, XPS, AES | [55] |
| Mediator | pH | Temp. [°C] | Experimental Conditions | Technique | Ref. |
|---|---|---|---|---|---|
| Atmospheric oxidation | |||||
| As2O3 | - | 25/100 | Enargite concentrate, after milling with oxygen | XRD | [69] |
| CuSO4·5H2O | - | 25/100 | XRD | ||
| Tennantite as an intermediate phase | - | Enargite weathering (air) | Laser Raman microprobe, EPMA, XRD | [70] | |
| Cu3(SO4)(OH)4 | - | 80 | Enargite massive sample, after 28 days, in air at 85 °C and 80%, antlerite-like structures observed in cracks | XRD | [71] |
| S2−, S0, and S6+ | - | Ambient temperature | Oxidized enargite, the surface is enriched also with arsenic in a thin layer of 0.5 nm with a inner layer of cooper depleted in sulphur. Cu appeared as Cu(I) and Cu(II) bonding oxygen atoms. As is mainly associated to oxygen. | XPS | [72] |
| Acid leaching | |||||
| CuS | 0 | 25/100 | Enargite concentrate, in acid (0.5 M HCl, pH ~0) after 1 h | XRD | [69] |
| S0 | 60–95 | Enargite oxidized with Fe3+ ions | Soxhlet extraction | [73] | |
| S0 amorphous | 1 | 25 | Enargite specimen, acid pH (close to 1), after 30 min contact with a NaClO/HCl mixture | SEM-EDS XRD | [74] |
| AsIII/AsV, with As(III)-Oxygen and CuII sulphate and chloride | 1 | Room temperature | After cyclic voltammetry tests, at potential higher than 0.2 V vs. SCE | XPS | [75] |
| S0 | 1 | Room Temperature | In oxidation potentials of 0.3 V vs. SCE | In-situ Raman | [75] |
| S0 | 1 | 60–95 | Synthetic enargite in presence of acid ferric sulphate solution after 80 h contact | Soxhlet extraction | [73] |
| and while increasing redox potential sulphide and polysulphide to sulphur | 1 | Room Temperature | Enargite electrode, dissolution in 0.1 M sulphuric acid, 450 to 750 mV Ag/AgClsat and then 750 to 900 mV. | EIS, CV, XPS, XANES, Raman, | [76] |
| 1 | 20 | Electrochemical oxidation of enargite demonstrated an obvious passivation region from 500 to 750 mV (Ag/AgCl). The formed passivation film was found a n-type semiconductor behavior, which is different from the original enargite with a p-type behavior. | XPS, XANES, CV, Raman | [77] | |
| As(III)-O increases in time, As(III)-S reduces in time, forming thiosulphate | 1.8 | Not reported (close to 30) | Enargite submitted to oxidation in presence and absence of the acidophilic microorganism Leptospirillum ferrooxidans. | XPS, SEM-EDS | [78] |
| S0 | 4 | 25 | Crushed natural enargite, 30 min, | XPS, AFM, CA | [79,80] |
| Around pH 7 | Atmospheric temperature | Mechanochemical treatment using planetary ball milling in dry conditions speeds up oxidation in air. Arsenic bonds with oxygen and it is easily dissolved in water or alkaline solution | XRD, XAS | [81] | |
| Three-layer structure: layer 1. thin metal deficient layer (0.7 nm) Cu, layer 2. 5–10 nm of As depleted and below a layer depleted in Cu and enriched in Sulphur–polysulphide structure | pH 2–4 (and 7.0 approx. for distilled water) | Room Temperature | Enargite dissolution distilled water, sulphuric acid at pH 4, and pH 2 ferric chloride/ferric sulphate mixture a mixed with 0.025 M Fe(III) | SEM-EDS, XPS with sputtering, OCP measurements | [82] |
| Alkaline leaching | |||||
| 9.2 | 25 | Natural enargite, electrochemical oxidation | CV | [83] | |
| CuO, Cu(SO)4 Cu(OH)2 | 10 | 25 | Crushed natural enargite, 30 min | XPS, AFM, CA | [79,80] |
| Cu2O CuO | 10 | 35 | Natural enargite, longer times of X-ray exposure (184 min) | XPS | [79] |
| CuO | 10.5 | Ambient temperature | Enargite microflotation tests controlled by hydrogen peroxide and sodium sulphide, conditioning times 2–5 min. | Thermodynamic studies | [84] |
(alkaline conditions) (acidic conditions) | pH 1–11 | Various | If iron is present in the system (for instance, in natural environments coming from pyrite) In presence of strong oxidation conditions copper oxide is predominant At redox potentials close to 0.56 vs. SHE | Various techniques | [85] |
| S0 at pH 2 f Cu3(AsO4)2 close to neutral pH Cu(OH)2 at pH 11 | pH 2, 5 and 11 | 25 | Copper ore containing Enargite oxidation with 0.013% H2O2 and O2 and microflotation tests conducted with prior oxidation for 1 h. | XPS | [86] |
| 25 | Natural enargite, electrochemical oxidation | CV, EIS and XPS | [83] | ||
| As2O3 As4S4 As2S3 Cu(OH)2 CuO Cu2O Sulphur-rich layer Sn (polysulphide structure) | 11 | 20–22 | Synthetic and natural enargite, nitrogen bubbling for 20 min and oxygen bubbling for 60 min, thin layer of oxidized species | XPS | [87] |
| CuO Cu(OH)2 Structure depleted in S in the form of polysulphide, As2O3 | 11.5–12.5 | 25–60 | Natural enargite, particle sizes in three ranges 20–25 um, 40–53 um and 90–110 um, after leaching experiments with NaClO 0.07 M–0.47 M | XPS | [88] |
| 60, 80, 90 | 120 min alkaline leaching after 15,30- and 60-min activation stirring ball millwith, with sodium sulphide, of enriched enargite concentrate | XRD an XPS | [89] | ||
| Close to 13 | Atmosphec temperature | Mechanochemical treatment using planetary ball milling in wet alkaline conditions speeds up oxidation in air. Arsenic bonds with oxygen and it is easily dissolved in water or alkaline solution for up to 50 h. Residue with higher crystallinity than dry conditions. | XRD, XAS | [81] | |
| CuS-like structures S0 (pH9 up to 12) and longer conditioning times | pH8–13 | Not reported (should be close to 25) | Enargite samples were used as electrodes, above −200 mV vs. SHE suggesting As-leaching. | In-situ Raman, CV, and thermodynamic computations | [90] |
| Cu2S | 13.7 | 25/80 | After 120 min leaching in presence of NaSH (0.68–1.35 M) | XRD | [91] |
| CuS | 13.7 | 60/80/90 | Enargite concentrate, activated with stirring mill using steel balls after 60 min maximum, and leached after 120 min leaching in presence of 100 g/L Na2S | XRD | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes-Atenas, G.; Alsina, M.A.; Valenzuela, F.; Yarmuch, J.L.; Basualto, C. Improving the Processing of Copper–Arsenic-Bearing Ores: Enhancing Separation and Extraction Methods Through Mediator Insights—A Brief Review. Minerals 2025, 15, 1157. https://doi.org/10.3390/min15111157
Montes-Atenas G, Alsina MA, Valenzuela F, Yarmuch JL, Basualto C. Improving the Processing of Copper–Arsenic-Bearing Ores: Enhancing Separation and Extraction Methods Through Mediator Insights—A Brief Review. Minerals. 2025; 15(11):1157. https://doi.org/10.3390/min15111157
Chicago/Turabian StyleMontes-Atenas, Gonzalo, Marco A. Alsina, Fernando Valenzuela, Juan L. Yarmuch, and Carlos Basualto. 2025. "Improving the Processing of Copper–Arsenic-Bearing Ores: Enhancing Separation and Extraction Methods Through Mediator Insights—A Brief Review" Minerals 15, no. 11: 1157. https://doi.org/10.3390/min15111157
APA StyleMontes-Atenas, G., Alsina, M. A., Valenzuela, F., Yarmuch, J. L., & Basualto, C. (2025). Improving the Processing of Copper–Arsenic-Bearing Ores: Enhancing Separation and Extraction Methods Through Mediator Insights—A Brief Review. Minerals, 15(11), 1157. https://doi.org/10.3390/min15111157








