Bacteria-like Ferruginous Structures in Carboniferous Limestones as Remains of Post-Variscan Hydrothermal Activity in Southern Poland
Abstract
1. Introduction
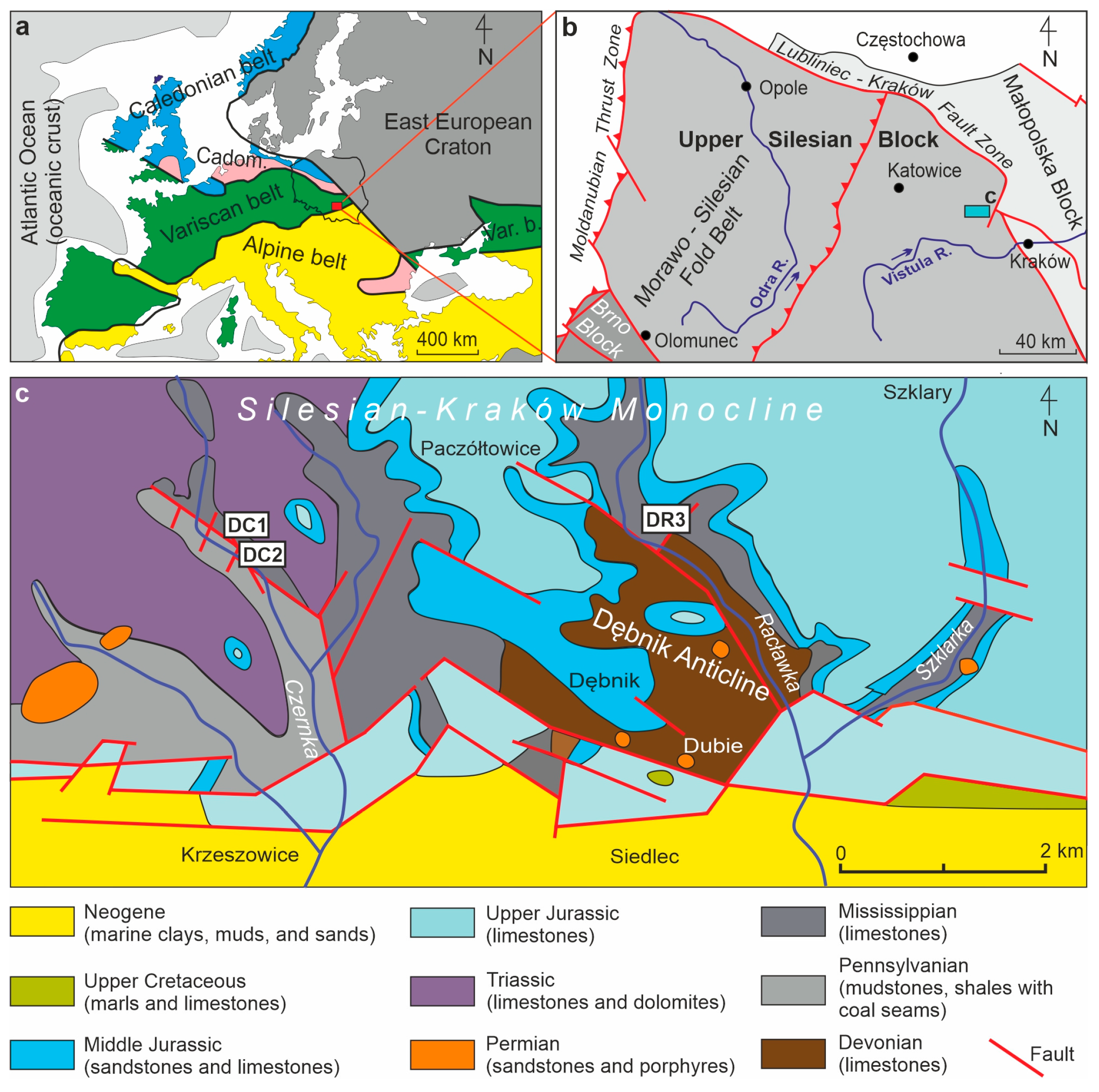
2. Geological Setting
3. Material and Methods
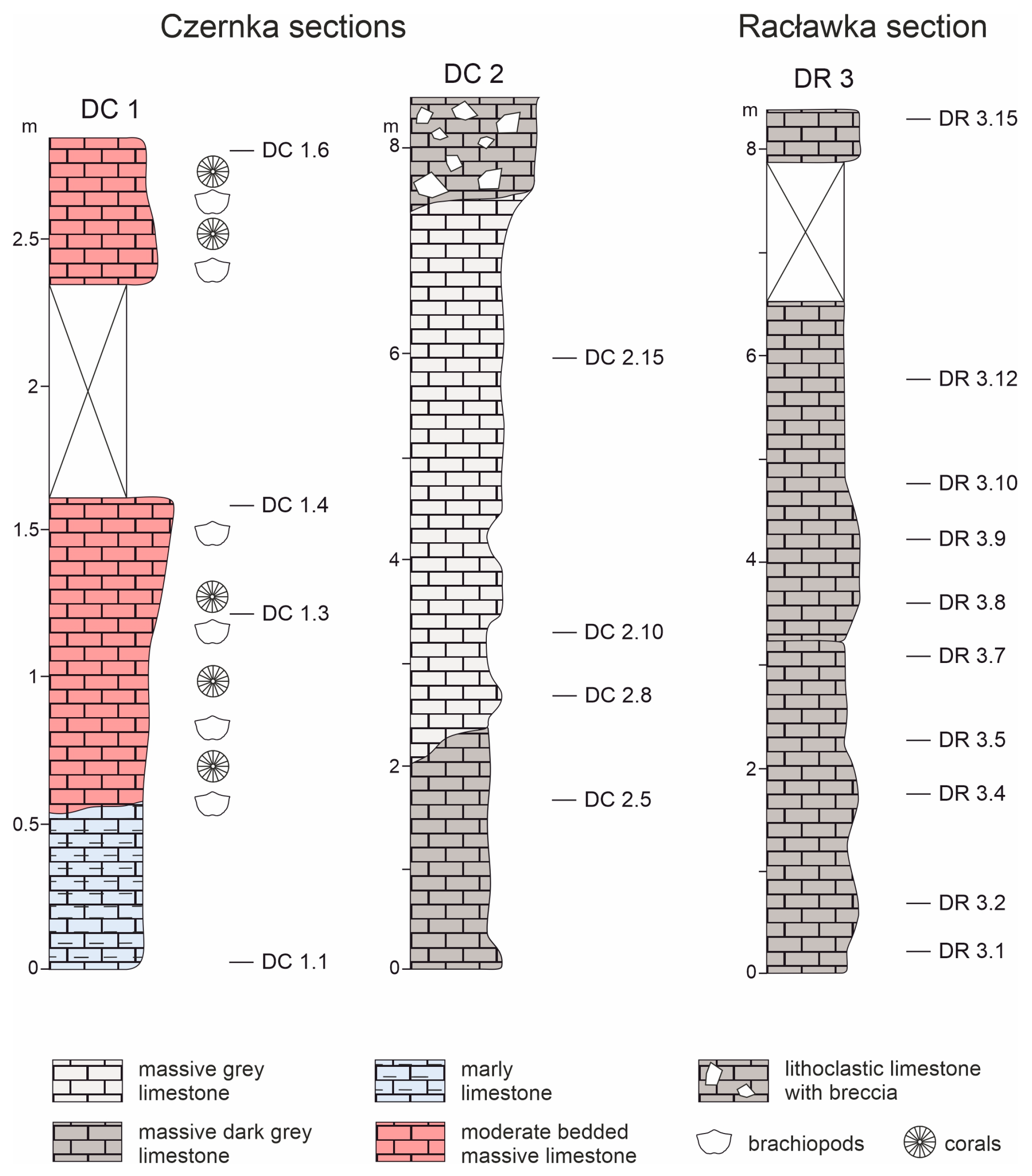
3.1. Sampling
3.2. Petrology and Geochemistry Studies
4. Results
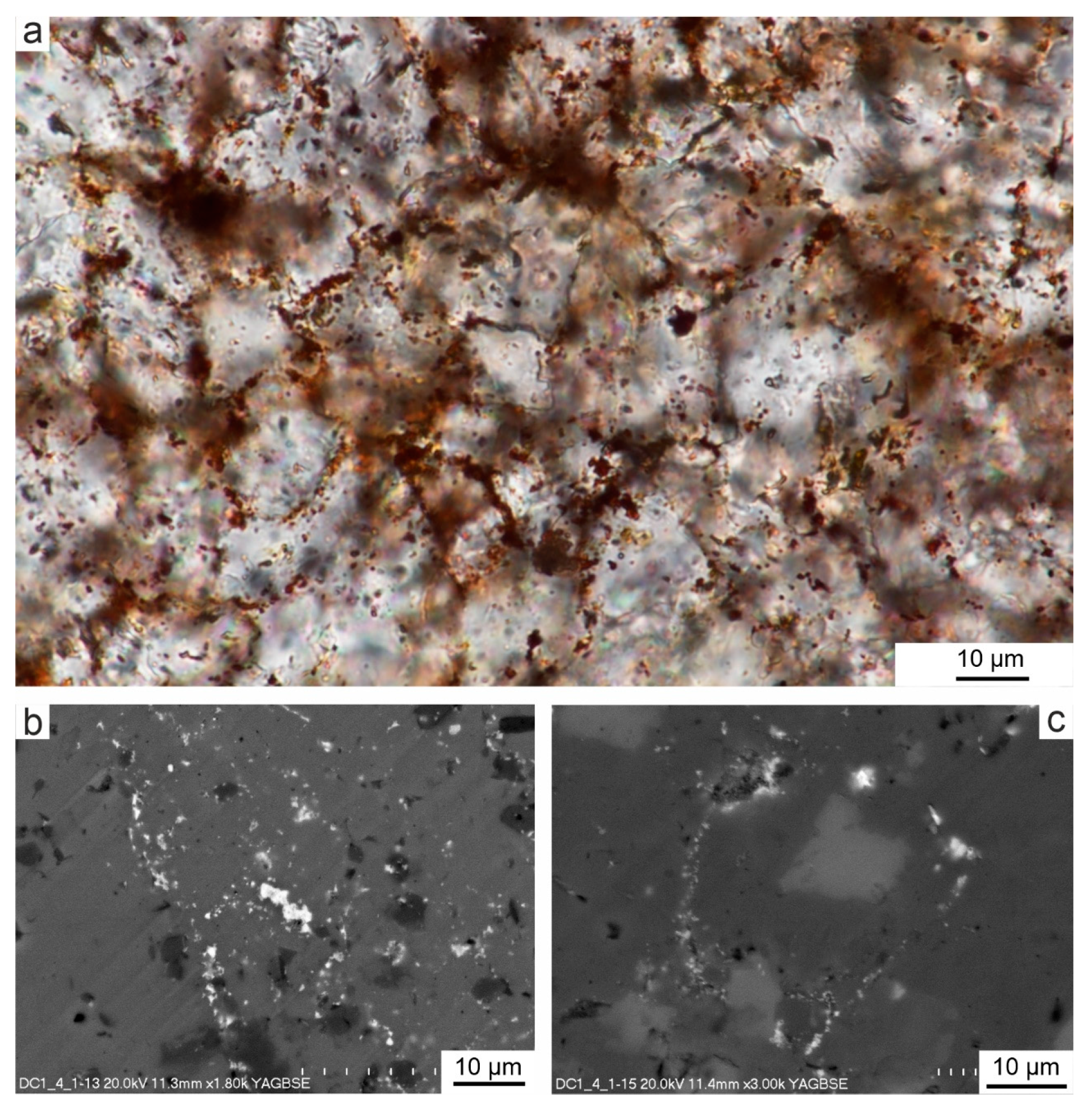
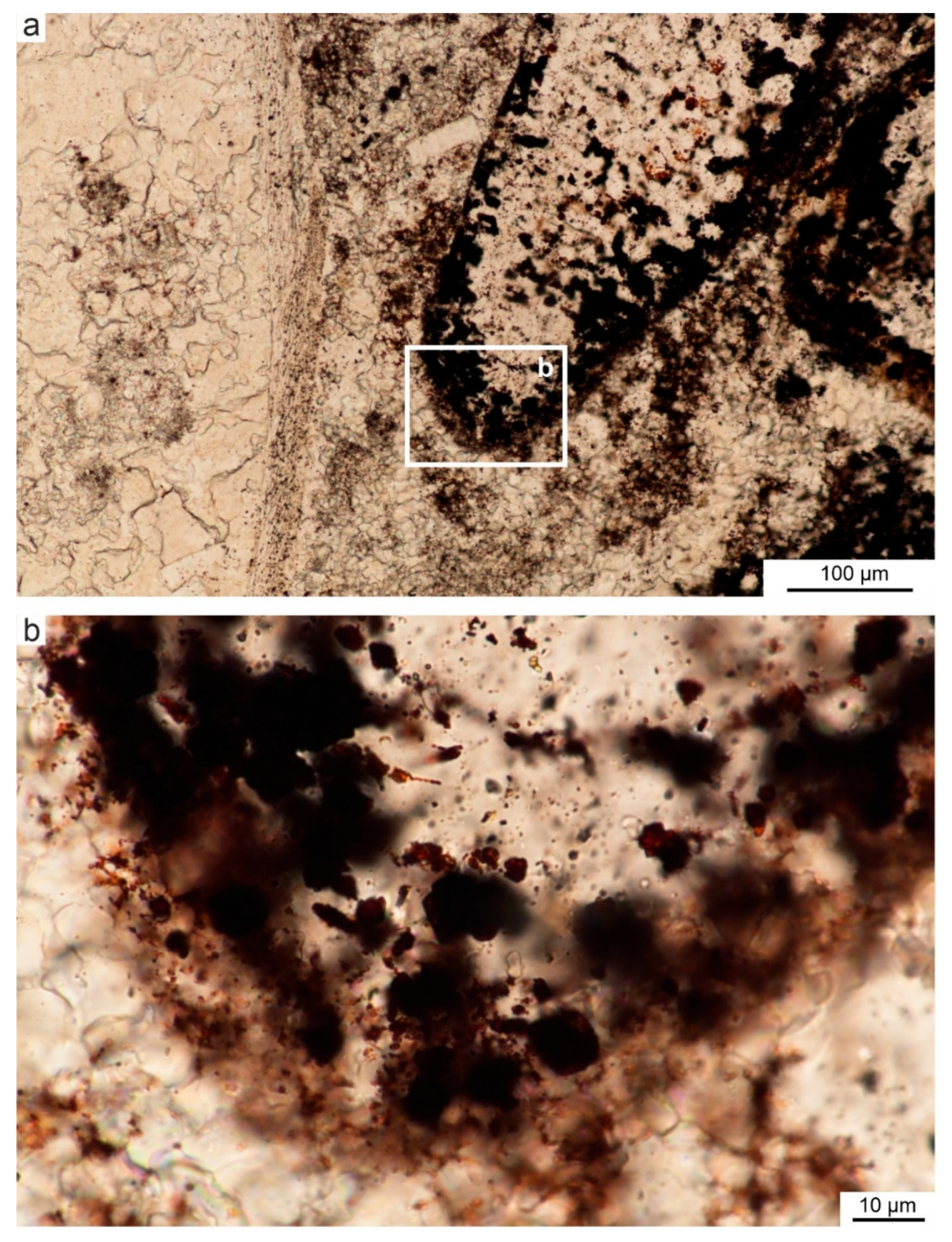
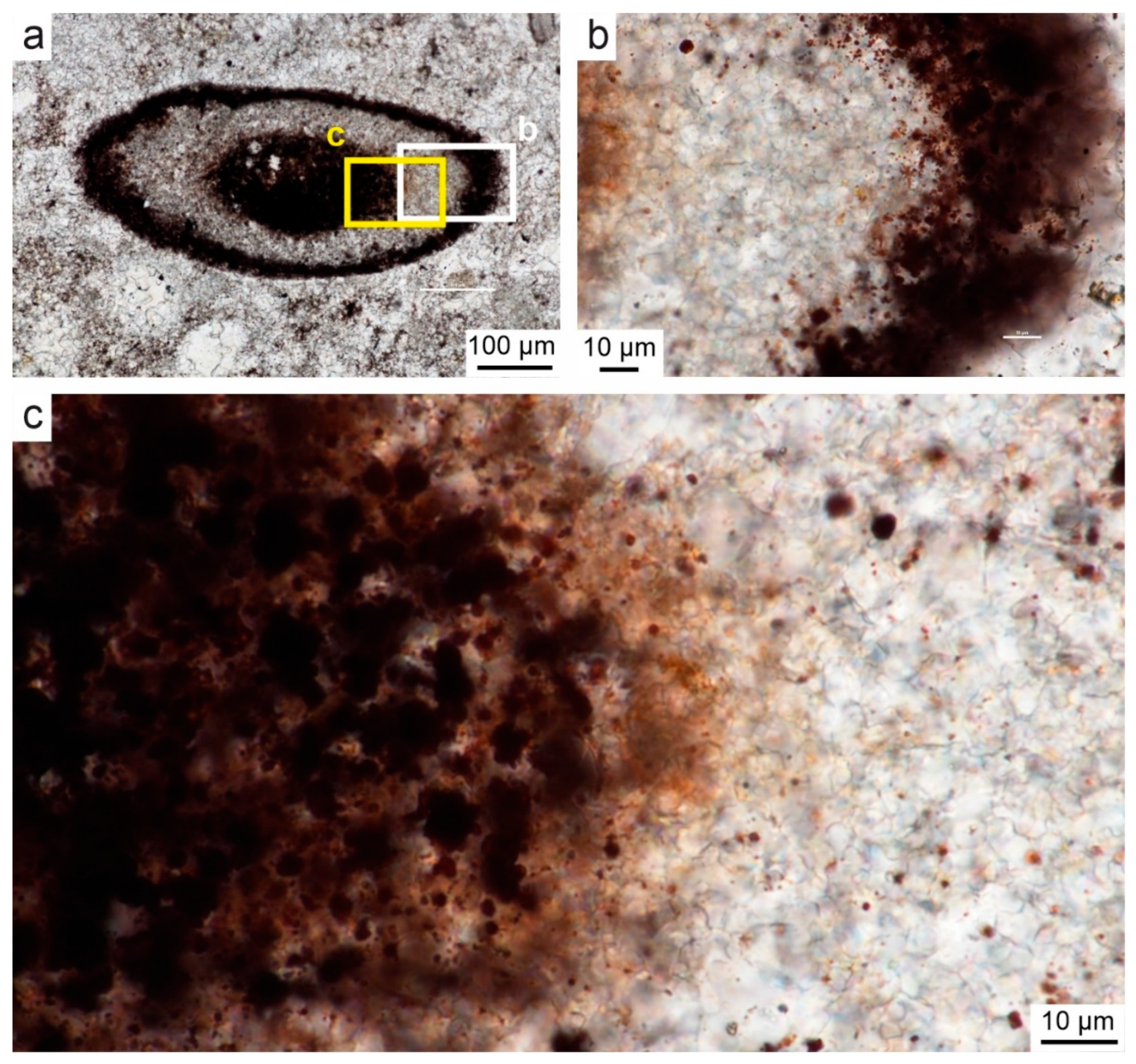
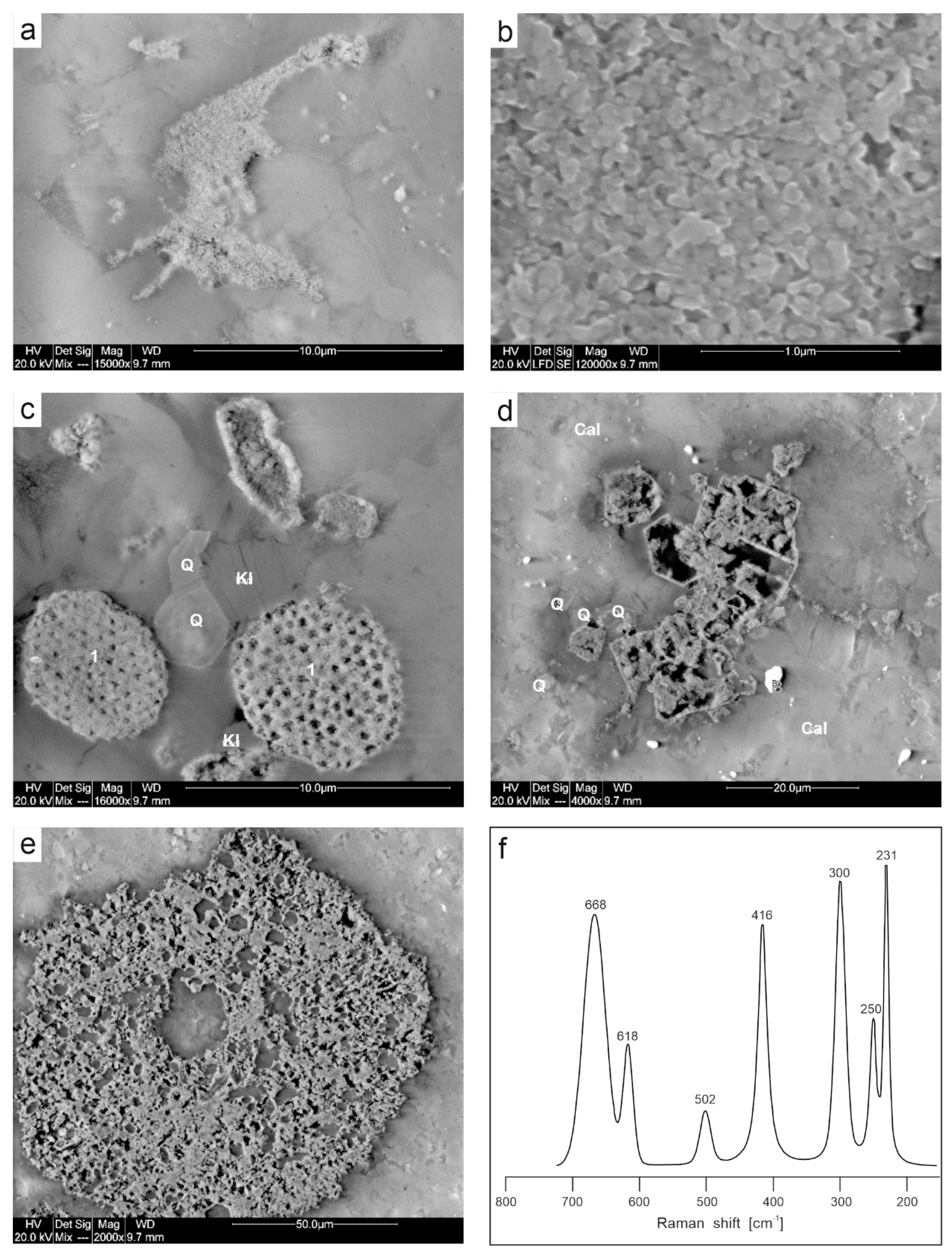
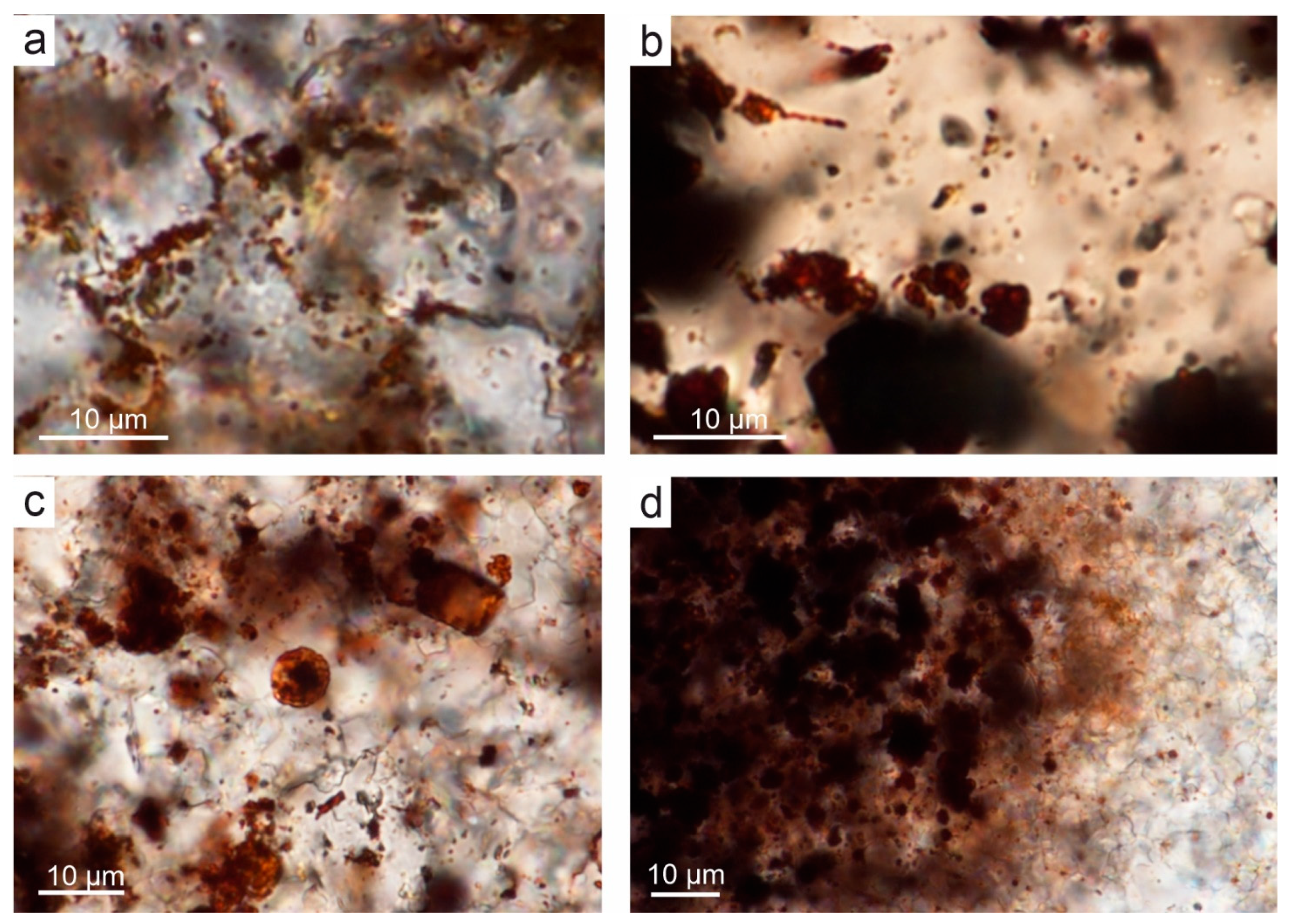
4.1. Microstructure of Limestones with Ferruginous Pigments
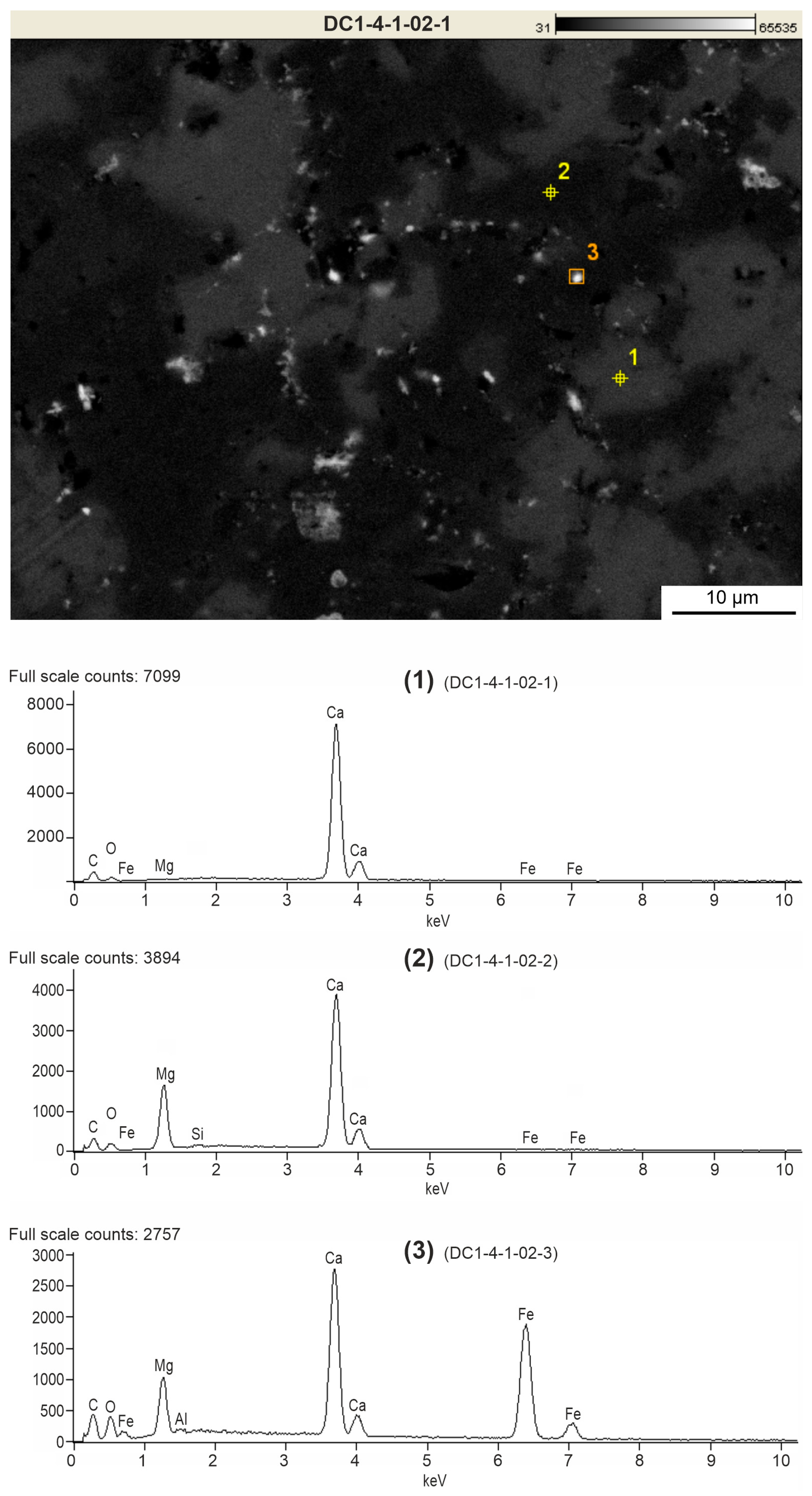
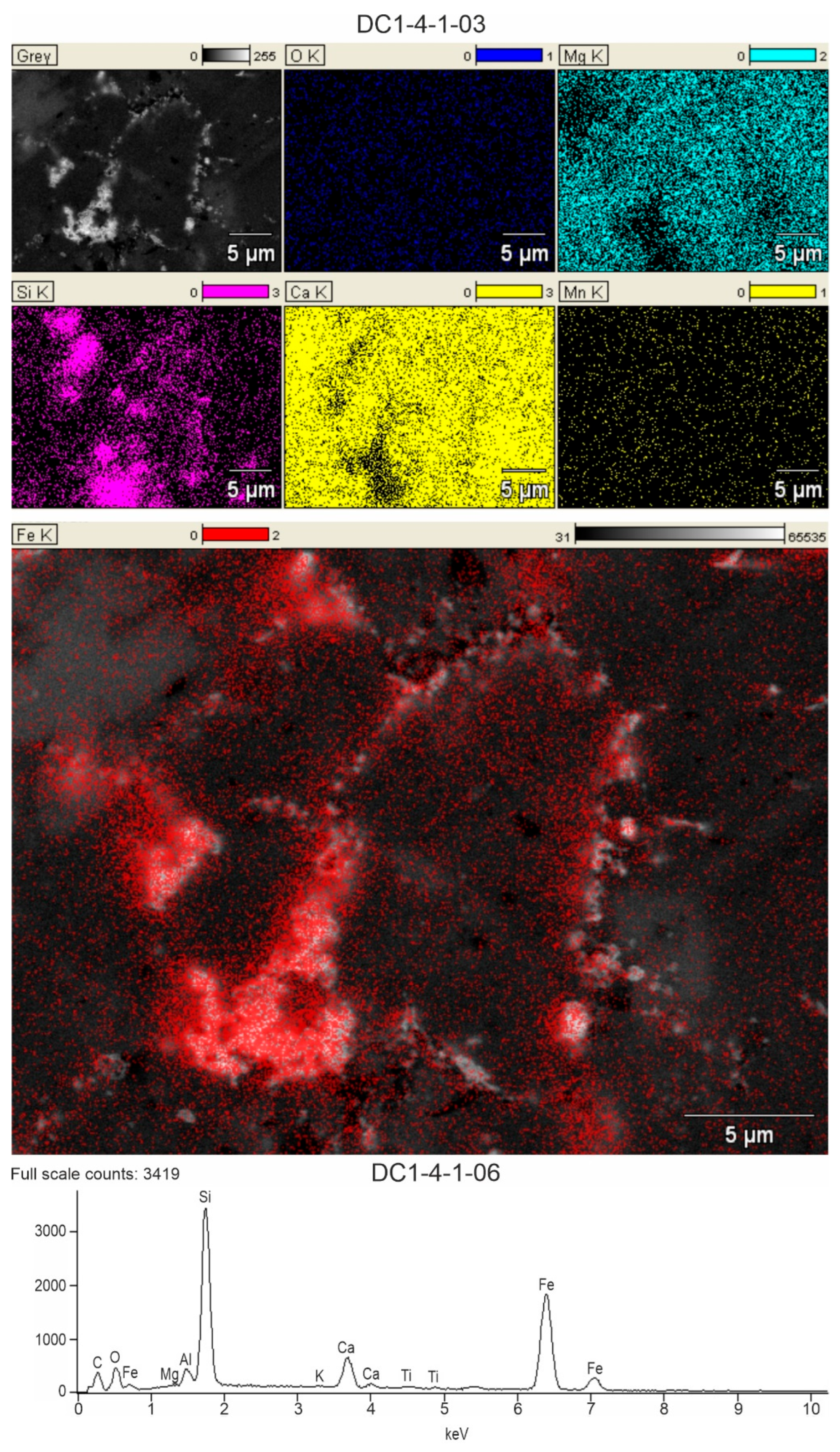
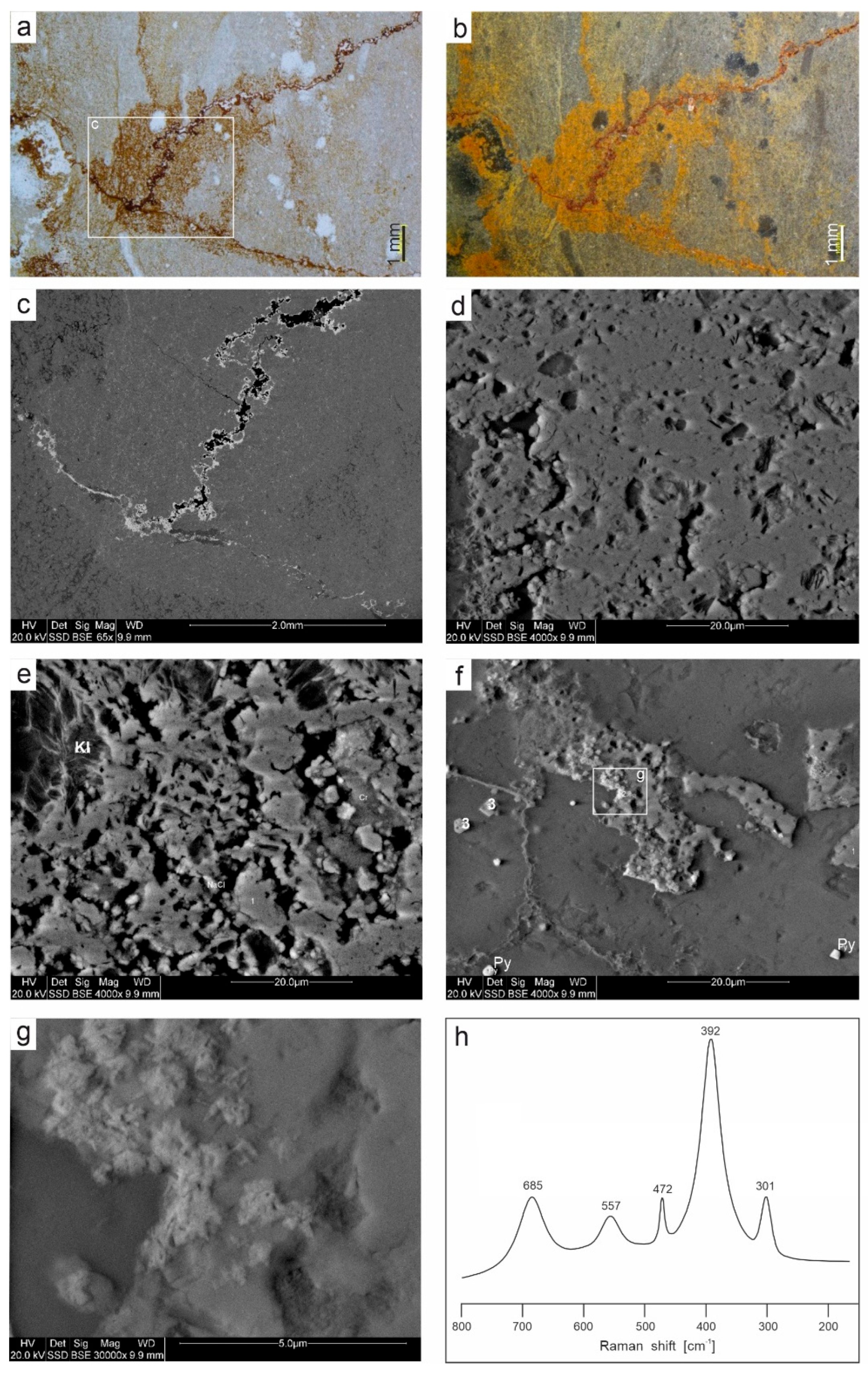
4.2. Mineralogy of Ferruginous Discolourations in Limestones
4.3. Geochemistry of Major Oxides and Trace Elements
4.4. Correlation Between Main Elements and SEM-EDS Mapping
4.5. Trace Elements
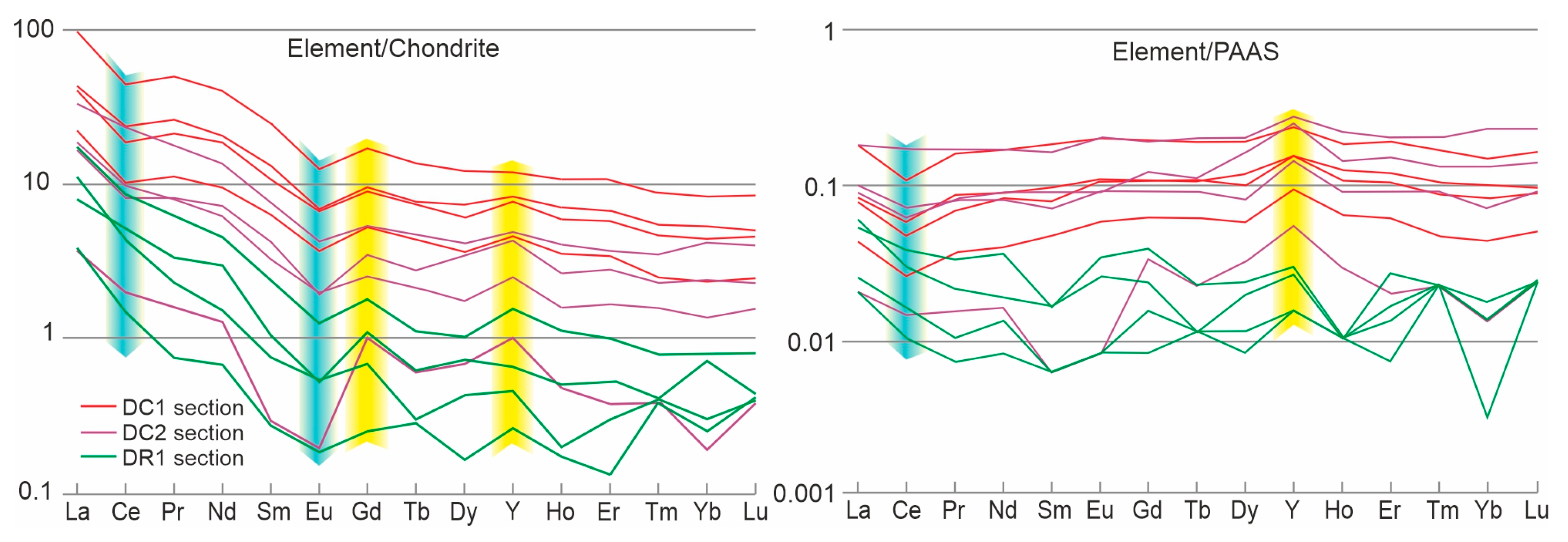
4.6. REE Signatures
5. Discussion
5.1. Bacterial Origin of Rust-Coloured and Brown Pigments
5.2. Timing for IRB Growth
5.3. Potential Sources of Hydrothermal Fluids
6. Conclusions
- In the Viséan part of the thick limestone succession located along the Upper Silesian Block (northern part of the Brunovistulicum), there are local zones of red-coloured rocks, which, based on morphological and chemical evidence, have been classified here as bacterial structures. These are spherical or oval forms, 1–2 µm in diameter, that occur as individual capsules or tubes arranged linearly between the walls of calcite crystals. The single capsule is hollow inside and has a wall that is approximately 0.1 µm thick, which contains a large amount of iron oxides and oxyhydroxides; this may be a remnant of the original bacterial sheath. Some oval, dark brown pigment clusters, surrounded by translucent halos with a floccular structure, resemble the bacterial holdfasts. We present here the morphology and chemistry of the microstructures showing their similarities to IRB from the present-day Sphaerotilus-Leptothrix group, the Galionella group, and the Mariprofundus ferrooxydans species.
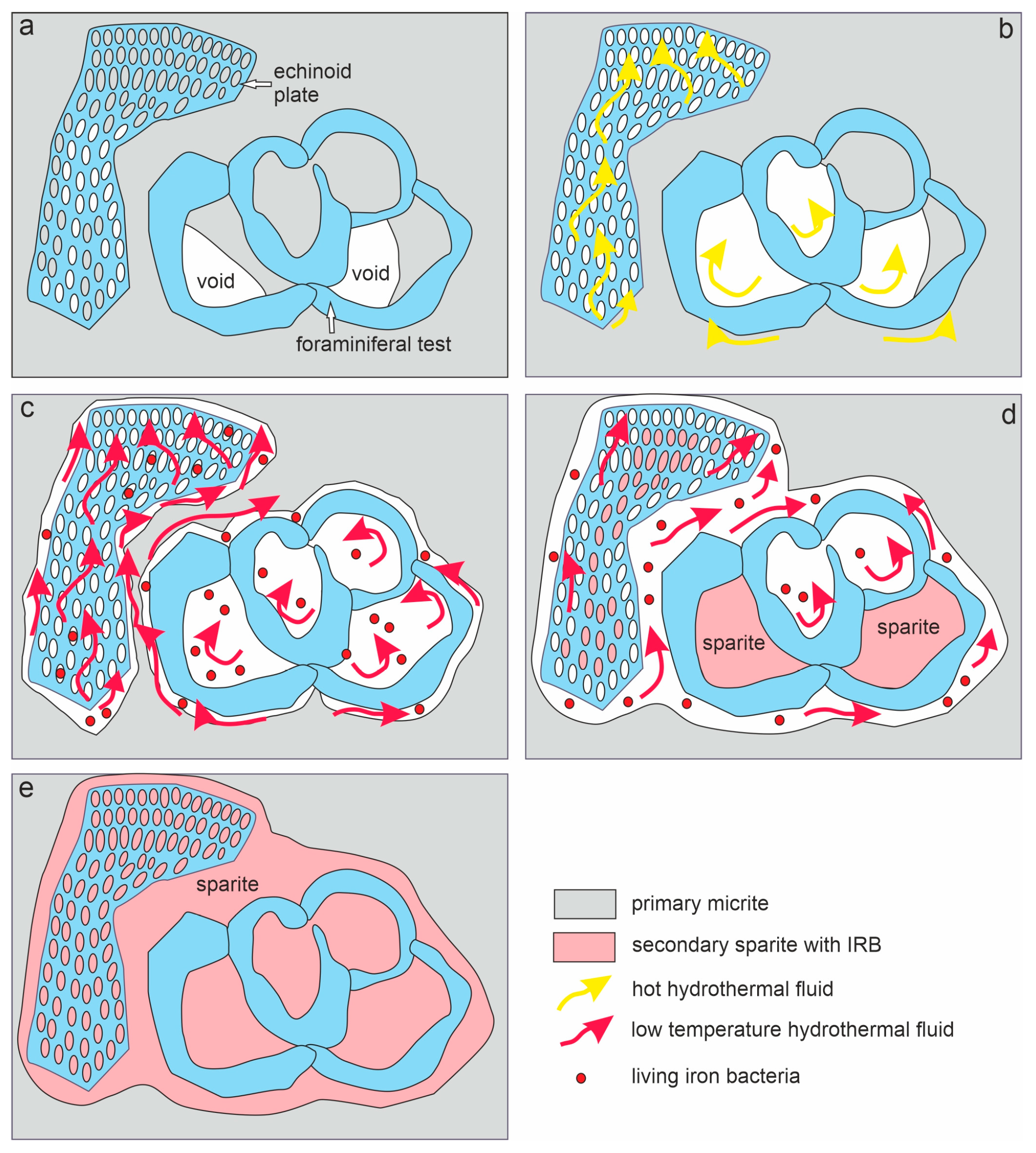
- The taphonomic analysis shows that bacteria-like remnants are not associated with the sedimentation of shallow marine carbonate mud. They are present only in narrow zones that are a few millimetres to several centimetres wide, unrelated to bedding, and they also occur across various microfossils. Based on microfacies analysis, we indicate that bacterial growth occurred in the originally empty micropores of microfossil skeletons and shells, between bioclasts, or in secondary voids formed during the selective dissolution of micrite or smaller sparite crystals. The dissolution of primary micrite and the subsequent filling of spaces with iron oxides/xyhydroxideswere associated with repeated hydrothermal pulses. The solutions must have been relatively cold, which is evidenced by the increased content of Ba, Sr, As, and Sb.
- Taking into account the types of mineralization associated with post-Variscan plutonism and volcanism, well documented in the immediate vicinity of the studied Mississippian sedimentary succession, we suggest that the hydrothermal pulses that associate with the formation of bacterial structures are probably related to felsite magmatism (Pennsylvanian), which was characterized, among other, by Cu-Mo mineralization, typical of many neighbouring porphyry deposits and skarn metasomatic deposits. However, this issue requires further geochemical studies on these sediments, in conjunction with analogous studies on carbonate rocks around the Zn-Pb mineralization zones, but outside the ore bodies and their transition zones.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volesky, B. Biosorption and Biosorbents. In Biosorption of Heavy Metals; CRC Press: Boca Raton, FL, USA, 1990; pp. 3–5. [Google Scholar]
- Cygnarowska, K. The use of algae to remove copper and lead from industrial wastewater. Geol. Geophys. Environ. 2023, 7, 85–93. [Google Scholar] [CrossRef]
- Gyollai, I.; Polgari, M.; Fintor, K.; Pal-Molnar, E.; Popp, F.; Koeberl, C. Microbial activity records in Marinoan Snowball Earth postglacial transition layers connecting diamictite WITH cap carbonate (Otavi Group, NW-Namibia). Austrian J. Earth Sci. 2017, 110, 4–20. [Google Scholar] [CrossRef]
- Ciurej, A.; Bąk, M.; Szczerba, M. Biostratinomy and diagenetic impact on exceptional preservation of coccospheres from Lower Oligocene coccolith limestones. Minerals 2020, 10, 616. [Google Scholar] [CrossRef]
- Mulder, E.G.; Deinema, M.H. The sheathed bacteria. In The Prokaryotes, A Handbook on the Biology of Bacteria: Ecophysiology, Isolation, Identification, Applications; Balows, A., Ed.; Springer-Verlag: Berlin/ Heidelberg, Germany, 1992; p. 4126. [Google Scholar]
- Pan, J.; Zhao, H.; Tucker, M.E.; Zhou, J.; Jiang, M.; Wang, Y.; Zhao, Y.; Sun, B.; Han, Z.; Yan, H. Biomineralization of monohydrocalcite induced by the halophile Halomonas smyrnensis WMS-3. Minerals 2019, 9, 632. [Google Scholar] [CrossRef]
- Bąk, M.; Bąk, K.; Górny, Z.; Stożek, B. Evidence of bacteriogenic iron and manganese oxyhydroxides in Albian–Cenomanian marine sediments of the Carpathian realm (Poland). Ann. Soc. Geol. Pol. 2015, 85, 371–385. Available online: http://www.asgp.pl/85_2_371_385 (accessed on 17 May 2025). [CrossRef]
- Noike, T.N.; Nakamura, K.; Matsumoto, J. Oxidation of ferrous iron by acidophilic iron-oxidizing bacteria from a stream receiving acid mine drainage. Water Res. 1983, 17, 21–27. [Google Scholar] [CrossRef]
- Schrenk, M.O.; Edwards, K.J.; Goodman, R.M.; Hamers, R.J.; Banfield, J.F. Distribution of Thiobacillus ferrooxidans and Leptospirillum ferrooxidans: Implications for generation of acid mine drainage. Science 1998, 279, 1519–1522. [Google Scholar] [CrossRef] [PubMed]
- Kirby, C.S.; Thomas, H.M.; Southam, G.; Donald, R. Relative contributions of abiotic and biological factors in Fe(II) oxidation in mine drainage. Appl. Geochem. 1999, 14, 511–530. [Google Scholar] [CrossRef]
- Francis, C.A.; Tebo, B.M. Marine Bacillus spores as catalysts for the oxidative precipitation and sorption of metals. J. Mol. Microbiol. Biotechnol. 1999, 1, 71–78. Available online: https://www.caister.com/backlist/jmmb/v/v1/v1n1/11.pdf (accessed on 15 October 2020).
- Konhauser, K.O.; Riding, R. Bacterial biomineralization. In Fundamentals in Geobiology; Knoll, A.H., Canfield, D.E., Konhauser, K.O., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2012; pp. 105–130. Available online: https://robertriding.com/pdf/konhauser_riding2012.pdf (accessed on 15 October 2020).
- Schopf, J.W.; Fairchild, T.R. Late Precambrian microfossils: A new stromatolitic biota from Boorthanna, south Australia. Nature 1973, 242, 537–538. Available online: https://www.nature.com/articles/242537a0 (accessed on 15 October 2020). [CrossRef]
- Schelble, R.T.; Westall, F.; Allen, C.C. 1.8 Ga iron-mineralized microbiota from the Gunflint Iron Formation, Ontario, Canada: Implications for Mars. Adv. Space Res. 2004, 33, 1268–1273. [Google Scholar] [CrossRef]
- Awramik, S.M.; Schopf, J.W.; Walter, M.R. Filamentous fossil bacteria from the Archean of Western Australia. Precamb. Res. 1983, 20, 357–374. [Google Scholar] [CrossRef]
- Sommaruga, R.; Psenner, R. Trophic interactions within the microbial food web in Piburger See (Austria). Archiv Hydrobiol. 1995, 132, 257–278. [Google Scholar] [CrossRef]
- Préat, A.; Loreau, J.P.; Durlet, C.; Mamet, B. Petrography and biosedimentology of the Rosso Ammonitico Veronese (Middle–Upper Jurassic, north-eastern Italy). Facies 2006, 52, 265–278. Available online: https://link.springer.com/article/10.1007/s10347-005-0032-2 (accessed on 8 May 2021). [CrossRef]
- Mamet, B.; Préat, A. Iron-bacterial mediation in Phanerozoic red limestones: State of the art. Sedim. Geol. 2006, 185, 147–157. [Google Scholar] [CrossRef]
- Bąk, M.; Natkaniec-Nowak, L.; Naglik, B.; Bąk, K.; Dulemba, P. Organic-walled microfossils from the early Middle Cambrian sediments of the Holy Cross Mountains, Poland: Possible implications for sedimentary environment in the SE margin of the Baltica. Acta Geol. Sin.—Engl. Ed. 2017, 91, 39–50. [Google Scholar] [CrossRef]
- Hanert, H.H. Bacterial and chemical iron oxide deposition in a shallow bay on Palaea Kameni, Santorini, Greece: Microscopy, electron probe microanalysis, and photometry of in situ experiments. Geomicrobiol. J. 2002, 19, 317–342. [Google Scholar] [CrossRef]
- Hallam, A.; Bradshaw, M.G. Bituminous shales and oolitic ironstones as indicators of transgressions and regressions. J. Geol. Soc. 1979, 136, 157–164. [Google Scholar] [CrossRef]
- Rudmin, R.; Reva, I.; Sokol, E.; Abdullayev, E.; Ruban, A.; Kudryavtsev, A.; Tolkachev, O.; Mazurov, A. Minerals of rare earth elements in high-phosphorus ooidal ironstones of the Western Siberia and Turgai Depression. Minerals 2020, 10, 11. [Google Scholar] [CrossRef]
- Krajewski, K. Pelagic stromatolites from the High-Tatric Albian limestones in the Tatra Mts. Kwart. Geol. 1981, 25, 731–759. Available online: https://gq.pgi.gov.pl/article/view/9044 (accessed on 16 November 2024).
- Bąk, M.; Górny, Z.; Bąk, K. Sponge growth on the Cenomanian carbonate shelves of the Carpathian Basin: A record from spicule-rich turbidites. Bull. Geosci. 2015, 90, 651–666. Available online: http://www.geology.cz/bulletin/contents/art1544# (accessed on 16 November 2024). [CrossRef]
- Boyd, T.D.; Scott, S.D. Microbial and hydrothermal aspects of ferric oxyhydroxides and ferrosic hydroxides: The example of Franklin Seamount, Western Woodlark Basin, Papua New Guinea. Geochem. Transact. 2001, 7, 45. [Google Scholar] [CrossRef]
- Mazur, S.; Aleksandrowski, P.; Gągała, Ł.; Krzywiec, P.; Żaba, J.; Gaidzik, K. Late Palaeozoic strike—Slip tectonics versus oroclinal bending at the SW outskirts of Baltica: Case of the Variscan belt’s eastern end in Poland. Int. J. Earth Sci. 2020, 109, 1133–1160. Available online: https://link.springer.com/article/10.1007/s00531-019-01814-7 (accessed on 10 December 2020). [CrossRef]
- Dvorák, J.; Galle, A.; Herbig, H.G.; Krejèí, Z.; Malec, J.; Paszkowski, M.; Racki, G.; Skompski, S.; Szulczewski, M.; Żakowa, H. Evolution of the Polish-Moravian carbonate platform in the Late Devonian and Early Carboniferous: Holy Cross Mts., Krakow Upland, Moravian Karst. In XIII International Congress on Carboniferous-Permian; Guide to Excursion B4; Małecka, J., Ed.; Państwowy Instytut Geologiczny: Warszawa, Poland, 1995; pp. 1–35. [Google Scholar]
- Alexandrowicz, S.W.; Siedlecka, A. Lithological profile of Dinantian limestones of Czerna near Krzeszowice. Ann. Soc. Geol. Pol. 1964, 34, 395–423. Available online: http://www.asgp.pl/sites/default/files/volumes/34_3_395_424.pdf (accessed on 15 December 2020).
- Timmerman, M.J. Timing, geodynamic setting and character of Permo-Carboniferous magmatism in the foreland of the Variscan Orogen, NW Europe. Geol. Soc. Lond. Spec. Publ. 2004, 223, 41–74. [Google Scholar] [CrossRef]
- Mikulski, S.Z.; Williams, I.S.; Markowski, M. Carboniferous–Permian magmatism and Mo–Cu (W) mineralization in the contact zone between the Małopolska and Upper Silesia Blocks (south Poland): An echo of the Baltica–Gondwana collision. Int. J. Earth Sci. 2019, 108, 1467–1492. Available online: https://link.springer.com/article/10.1007/s00531-019-01715-9 (accessed on 17 October 2020). [CrossRef]
- Sass-Gustkiewicz, M.; Dżułyński, S.; Ridge, J.D. The emplacement of zinc-lead sulfide ores in the Upper Silesian district—A contribution to the understanding of Mississippi Valley-type deposits. Econ. Geol. 1982, 77, 392–412. [Google Scholar] [CrossRef]
- Żelaźniewicz, A.; Aleksandrowski, P.; Buła, Z.; Karnkowski, P.H.; Konon, A.; Oszczypko, N.; Ślączka, A.; Żaba, J.; Żytko, K. Tectonic Subdivision of Poland; Komitet Nauk Geologicznych PAN: Wrocław, Poland, 2011; p. 60. Available online: https://www.kngpan.agh.edu.pl/wp-content/uploads/Regionalizacja_Tektoniczna_Polski_20111.pdf (accessed on 17 October 2024).
- Gradziński, R. Przewodnik Geologiczny po Okolicach Krakowa; Wydawnictwa Geologiczne: Warszawa, Poland, 1972; p. 3. [Google Scholar]
- Żaba, J.; Buła, A.; Jachowicz, M. Principal characteristics of the Upper Silesian Block and Małopolska Block border zone (southern Poland). Geol. Mag. 1997, 5, 669–677. [Google Scholar] [CrossRef]
- Kalvoda, J.; Leichmann, J.; Bábek, O.; Melichar, R. Brunovistulian terrane (Central Europe) and Istanbul Zone (NW Turkey): Late Proterozoic and Paleozoic tectonostratigraphic development and paleogeography. Geol. Carp. 2003, 54, 139–152. Available online: https://geologicacarpathica.com/browse/archive/?journal_article_no=2661 (accessed on 20 October 2020).
- Buła, Z.; Habryn, R.; Jachowicz-Zdanowska, M.; Żaba, J. The Precambrian and Lower Paleozoic of the Brunovistulicum (eastern part of the Upper Silesian Block, southern Poland)—The state of the art. Geol. Quart. 2015, 59, 123–134. [Google Scholar] [CrossRef]
- Belka, Z. The development and decline of a Dinantian carbonate platform: An example from the Moravia-Silesia Basin, Poland. In European Dinantian Environments; Miller, J., Wright, P.V., Adams, A.E., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1987; pp. 177–188. [Google Scholar]
- Hladil, J. Structure and microfacies of Middle and Upper Devonian Carbonate Buildups in Moravia, Czechoslovakia. Mem. Can. Soc. Petrol. Geol. 1988, 14, 607–618. Available online: https://archives.datapages.com/data/dgs/014/014002/607_cspgsp014b0607.htm (accessed on 15 January 2021).
- Szulczewski, M.; Belka, Z.; Skompski, S. The drowning of a carbonate platform: An example from the Devonian–Carboniferous of the south western Holy Cross Mountains, Poland. Sedim. Geol. 1996, 106, 21–49. [Google Scholar] [CrossRef]
- Bábek, O. Thinning and wining upward megasequence in Middle Devonian carbonate slope deposits, Moravia, Czech Republic. Neues Jahrb. Geol. Paläontologie Abh. 1996, 202, 409–432. [Google Scholar] [CrossRef]
- Dvořák, J. The Famennian of Moravia (CSSR); the relation between tectonics and sedimentary facies. Ann. Soc. Geol. Belg. 1986, 109, 131–136. Available online: https://popups.uliege.be/0037-9395/index.php?id=2426 (accessed on 20 October 2020).
- Szulczewski, M. Depositional evolution of the Holy Cross Mts. (Poland) in the Devonian and Carboniferous—A review. Geol. Quart. 1995, 39, 471–488. Available online: https://gq.pgi.gov.pl/article/view/8261 (accessed on 25 October 2020).
- Bábek, O.; Plikryl, T.; Hladil, J. Progressive drowning of carbonate platform in the Moravo-Silesian Basin (Czech Republic) before the Frasnian/Famennian event: Facies, compositional variations and gamma-ray spectrometry. Facies 2007, 53, 293–316. [Google Scholar] [CrossRef]
- Franke, W.; Cocks, R.M.; Torsvik, T.H. The Palaeozoic Variscan oceans revisited. Gondwana Res. 2017, 48, 257–284. [Google Scholar] [CrossRef]
- Słaby, E.; Breitkreuz, C.; Żaba, J.; Domańska-Siuda, J.; Gajdzik, K.; Falenty, K.; Falenty, A. Magma generation in an alternating transtensional–transpressional regime, the Kraków–Lubliniec Fault Zone, Poland. Lithos 2010, 119, 251–268. [Google Scholar] [CrossRef]
- Harańczyk, C. Mineral parageneses of Cracovides and its cover (Southern Poland). Ann. Soc. Geol. Pol. 1985, 53, 91–126. Available online: http://www.asgp.pl/sites/default/files/volumes/53_1-4_091_126.pdf (accessed on 14 October 2020).
- Czerny, J.; Muszyński, M. Co-magmatism of the Permian volcanites of the Krzeszowice area in the light of petrochemical data. Miner. Pol. 1997, 28, 3–25. Available online: http://www.mineralogia.pl/dokumenty/282.pdf (accessed on 14 October 2020).
- Lewandowska, A.; Rospondek, M.J.; Nawrocki, J. Stephanian–Early Permian intermediate volcanic rocks from the Nieporaz-Brodla and Sławków grabens near Kraków, Southern Poland. Ann. Soc. Geol. Pol. 2010, 80, 227–251. Available online: http://www.asgp.pl/sites/default/files/volumes/80_3_227_251.pdf (accessed on 14 October 2020).
- Lewandowska, A.; Rospondek, M.J.; Kobuszewski, Ł. Alleged Carboniferous (Viséan) volcanism at the eastern margin of the Moravo-Silesian Basin, Kraków region, southern Poland. Ann. Soc. Geol. Pol. 2018, 88, 59–69. [Google Scholar] [CrossRef]
- Nawrocki, J.; Lewandowska, A.; Fanning, M. Isotope and paleomagnetic ages of the Zalas rhyodacites (S Poland). Przegl. Geol. 2007, 55, 476–478. Available online: https://geojournals.pgi.gov.pl/pg/article/view/31129 (accessed on 14 October 2020).
- Nawrocki, J.; Fanning, M.; Lewandowska, A.; Polechońska, O.; Werner, T. Palaeomagnetism and the age of the Cracow volcanic rocks (S Poland). Geoph. J. Intern. 2008, 174, 475–488. [Google Scholar] [CrossRef]
- Nawrocki, J.; Krzemiński, L.; Pańczyk, M. 40Ar-39Ar ages of selected rocks and minerals from the Kraków–Lubliniec Fault Zone, and their relation to the Paleozoic structural evolution of the Malopolska and Brunovistulian terranes (S Poland). Geol. Quart. 2010, 54, 289–300. Available online: https://gq.pgi.gov.pl/article/view/7589 (accessed on 14 October 2020).
- Szulc, J. Anisian–Carnian evolution of the Germanic basin and its eustatic, tectonic and climatic controls. Zentralblatt Geol. Paläeontologie 1999, 1, 813–852. Available online: https://www.schweizerbart.de/publications/detail/isbn/9783510660117/Epicontinental_Triassic_Volume_1 (accessed on 14 October 2020).
- Leach, D.L.; Viets, J.G.; Kozłowski, A.; Kibitlewski, S. Geology, geochemistry, and genesis of the Silesia-Kraków zinc-lead district, southern Poland. Soc. Econ. Geol. Spec. Publ. 1996, 4, 171–181. [Google Scholar]
- Szuwarzyński, M. Ore bodies in the Silesia-Cracow Zn-Pb ore district, Poland. Prace Państw. Inst. Geol. 1996, 154, 9–24. [Google Scholar]
- Mikulski, S.Z.; Oszczepalski, S.; Sadłowska, K.; Chmielewski, A.; Małek, R. The occurrence of associated and critical elements in selected documented Zn-Pb, Cu-Ag, Fe-Ti-V, Mo-Cu-W, Sn, Au-As and Ni deposits in Poland. Biul. Państw. Inst. Geol. 2018, 472, 21–52. [Google Scholar] [CrossRef]
- Szczurek, S. Late Devonian–Early Carboniferous Foraminifera of the Upper Silesian Block (Kraków region, southern Poland). Geol. Quart. 2023, 67, 36. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Kamber, B.S. The behaviour of the rare earth elements during estuarine mixing—Revisited. Mar. Chem. 2006, 100, 147–161. [Google Scholar] [CrossRef]
- Morse, J.W.; Mackenzie, F.T. Geochemistry of sedimentary carbonates. In Developments in Sedimentology; Elsevier: Amsterdam, The Netherlands, 1990; Volume 48, p. 706. [Google Scholar]
- Shields, G.A.; Webb, G.R. Has the REE Composition of Seawater Changed over Geological Time? Chem. Geol. 2004, 1–2, 103–107. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.S. Composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution: An Examination of the Geochemical Record Preserved in Sedimentary Rocks; Carlton Blackwell Scientific Publication: Oxford, UK, 1985; p. 312. Available online: https://core.ac.uk/download/621678419.pdf (accessed on 20 October 2020).
- McLennan, S.M. Relationships between the trace element composition of sedimentary rocks and upper continental crust. Geochem. Geophys. Geosyst. 2001, 2, 1–24. [Google Scholar] [CrossRef]
- Chan, C.S.; Fakra, S.C.; Emerson, D.; Fleming, E.J.; Edwards, K.J. Lithotrophic iron-oxidizing bacteria produce organic stalks to control iron mineral growth: Implications for biosignature formation. ISME J. 2011, 5, 717–727. Available online: https://www.nature.com/articles/ismej2010173 (accessed on 10 March 2020). [CrossRef]
- Comolli, L.R.; Luef, B.; Chan, C.S. High-resolution 2D and 3D cryo-TEM reveals structural adaptations of two stalk-forming bacteria to an Fe-oxidizing lifestyle. Environ. Microbiol. 2011, 13, 2915–2929. [Google Scholar] [CrossRef]
- Emerson, D.; Moyer, C.L. Microbiology of Seamounts: Common Patterns Observed in Community Structure. Oceanography 2002, 23, 148–163. [Google Scholar] [CrossRef]
- Van Veen, W.L.; Mulder, E.G.; Deinema, M.H. The Sphaerotilus-Leptothrix group of bacteria. Microbiol. Rev. 1978, 42, 329–356. [Google Scholar] [CrossRef] [PubMed]
- Kalvoda, J.; Bábek, O.; Leichmann, J.; Fatka, O.; Laichmann, J.; Melichar, R.; Nechyba, S.; Spacek, P. Brunovistulian terrane (Bohemian Massif, Central Europe) from late Proterozoic to late Paleozoic: A review. Int. J. Earth Sci. 2007, 97, 497–518. [Google Scholar] [CrossRef]
- Belka, Z.; Valverde-Vaquero, P.; Dörr, W.; Ahrendt, H.; Wemmer, K.; Franke, W.; Schafer, J. Accretion of first Gondwana-derived terranes at the margin of Baltica. Geol. Soc. Lond. Spec. Publ. 2002, 201, 19–36. [Google Scholar] [CrossRef]
- Madondo, J.; Canet, C.; Núñez-Useche, F.; González-Partida, E. Geology and geochemistry of jasperoids from the ‘Montaña de Manganeso’ district, San Luis Potosí, north-central Mexico. Rev. Mex. Cienc. Geol. 2021, 38, 193–209. [Google Scholar] [CrossRef]
- Piepgras, D.J.; Gacobsen, S.B. The behavior of rare earth elements in seawater: Precise determination of variations in the North Pacific water column. Geochim. Cosmochim. Acta 1992, 56, 1851–1862. [Google Scholar] [CrossRef]
- Piper, D.Z.; Bau, M. Normalized rare earth elements in water, sediments, and wine: Identifying sources and environmental redox conditions. Am. J. Analyt. Chem. 2013, 4, 69–83. [Google Scholar] [CrossRef]
- Siedlecka, A.; Krysowska, M. Studies of origin and distribution of the Karniowice sandstones north of the Krzeszowice graben. Ann. Soc. Geol. Pol. 1962, 32, 371–398. Available online: http://www.asgp.pl/sites/default/files/volumes/32_3_371_398.pdf (accessed on 12 January 2020).
- Wolska, A. Petrology and geochemistry of granitoids and their mafic microgranular enclaves (MME) in marginal part of the Małopolska Block (S Poland). Mineralogia 2012, 43, 3–127. [Google Scholar] [CrossRef]
- Truszel, M.; Karwowski, Ł.; Lasoń, K.; Markiewicz, J.; Żaba, J. Magmatism and metamorphism of the Kraków–Lubliniec tectonic zone as a clue to the occurrence of polymetallic deposits. Biul. Państw. Inst. Geol. 2006, 418, 55–103. Available online: https://geojournals.pgi.gov.pl/bp/article/view/29624 (accessed on 22 October 2020).
- Piekarski, D. Metallogenic and prognostic analysis of the Paleozoic sequence of the NE margin of the Upper Silesian Coal Basin. Ann. Soc. Geol. Pol. 1985, 53, 207–234. Available online: http://www.asgp.pl/sites/default/files/volumes/53_1-4_207_234.pdf (accessed on 22 October 2020).
- Harańczyk, C.; Lankosz, A.; Wolska, A. Jerzmanowice granodioryte, porphyres and Co-Mo ores. Rudy Met. Nieżelaz. 1995, 40, 334–341. [Google Scholar]
- Koszowska, E.; Wolska, A. Mineralogical and geochemical study of thermally altered country rocks of granodioritic intrusion in the Będkowska Valley near Kraków (S Poland). Ann. Soc. Geol. Pol. 2000, 70, 261–280. Available online: http://www.asgp.pl/sites/default/files/volumes/70_3-4_261_281.pdf (accessed on 22 October 2020).
- Truszel, M.; Karwowski, Ł. Skarns and skarn mineralization in the Cracow-Lubliniec region (Southern Poland). Mineral. Soc. Pol. Spec. Pap. 2003, 23, 175–178. [Google Scholar]
- Oszczepalski, S.; Markowiak, M.; Mikulski, S.Z.; Lasoń, K.; Buła, Z.; Habryn, R. Porphyry Mo–Cu–W mineralization within Precambrian-Paleozoic rocks—Prospectivity analysis of the border zone of the Upper Silesia and Małopolska Blocks. Biul. Państw. Inst. Geol. 2010, 439, 339–354. [Google Scholar]
- Mikulski, S.Z.; Oszczepalski, S.; Markowiak, M. The occurrence and prospective resources of molybdenum and tungsten ores in Poland. Biul. Państw. Inst. Geol. 2012, 465, 297–314. [Google Scholar]
- Markowiak, M. Description of ore mineralization against the background of thermal-metasomatic alterations of rocks in the Żarki-Kotowice area. Prace Państw. Inst. Geol. 2015, 203, 74. Available online: https://www.pgi.gov.pl/oferta-inst/wydawnictwa/serie-wydawnicze/prace-pig/6853-prace-pig-tom-203-2015.html (accessed on 25 October 2020).
- Mikulski, S.Z.; Markowiak, M.; Sadłowska, K.; Chmielewski, A.; Zieliński, G. Pilot studies of rare earths (REE) in the contact zone of the Upper Silesia Block with the Małopolska Block. Biul. Państw. Inst. Geol. 2015, 465, 77–98. [Google Scholar] [CrossRef]
- Małek, R. Porphyry-type Cu-Mo mineralization in the Będkowska Valley region of the Kraków-Częstochowa Upland. Prz. Geol. 2018, 66, 252–258. Available online: https://geojournals.pgi.gov.pl/pg/article/view/26933/18651 (accessed on 25 October 2020).
- Sass-Gustkiewicz, M.; Dżułyński, S. On the origin of strata-bound Zn-Pb ores in the Upper Silesia, Poland. Ann. Geol. Soc. Pol. 1998, 68, 267–278. Available online: http://www.asgp.pl/sites/default/files/volumes/68_4_267_278.pdf (accessed on 22 October 2020).
- Heijlen, W.; Muchez, P.; Banks, D.A.; Schneider, J.; Kucha, H. Carbonate-hosted Zn-Pb deposits in Upper Silesia, Poland: Origin and evolution of mineralizing fluids and constraints on genetic models. Econ. Geol. 2003, 9, 911–932. [Google Scholar] [CrossRef]
- Mikulski, S.Z.; Oszczepalski, S.; Sadłowska, K.; Chmielowski, A.; Małek, R. Trace Element Distributions in the Zn-Pb (Mississippi Valley-Type) and Cu-Ag (Kupferschiefer) Sediment-Hosted Deposits in Poland. Minerals 2020, 10, 75. [Google Scholar] [CrossRef]



| DC1 (n = 4) | DC2 (n = 4) | DR3 (n = 10) | |
|---|---|---|---|
| CaO | 51.88–44.70 (av. 48.98) | 55.51–49.72 (av. 53.85) | 55.34–54.16 (av. 54.79) |
| Σ CaO + LOI | 94.38–82.00 (av. 89.40) | 98.82–90.22 (av. 95.57) | 99.03–98.02 (av. 98.59) |
| Σ other elements | 17.81–5.56 (av. 10.49) | 9.83–1.19 (av. 3.44) | 2.02–0.97 (av. 1.40) |
| SiO2 | 12.14–3.28 (av. 6.80) | 8.04–0.59 (av. 2.58) | 1.06–0.41 (av. 0.66) |
| MgO | 1.26–0.30 (av. 0.68) | 0.33–0.24 (av. 0.27) | 0.66–0.40 (av. 0.58) |
| Fe2O3 | 1.69–0.44 (av. 0.92) | 0.43–0.07 (av. 0.17) | 0.13–0.05 (av. 0.07) |
| Al2O3 | 3.34–0.46 (av. 1.84) | 0.88–0.02 (av. 0.33) | 0.19–0.06 (av. 0.09) |
| K2O | 0.14–0.03 (av. 0.09) | 0.07–0.01 (av. 0.03) | 0.06–0.01 (av. 0.02) |
| TiO2 | 0.10–0.02 (av. 0.06) | 0.03–0.01 (av. 0.02) | 0.01 |
| MnO | 0.07–0.02 (av. 0.05) | 0.03–0.01 (av. 0.02) | 0.01 |
| P2O5 | 0.06–0.01 (av. 0.04) | 0.01 | 0.01 |
| Na2O | 0.03–0.01 (av. (0.02) | 0.01 | 0.01 |
| SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | TiO2 | P2O5 | MnO | |
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 0.93352 | 0.93112 | −0.08947 | −0.98424 | 0.25506 | 0.8845 | 0.92725 | 0.78691 | 0.7502 | |
| Al2O3 | 0.93352 | 0.96874 | −0.10711 | −0.95836 | 0.34937 | 0.9138 | 0.99673 | 0.93623 | 0.76123 | |
| Fe2O3 | 0.93112 | 0.96874 | −0.012 | −0.9598 | 0.35121 | 0.89023 | 0.96939 | 0.86091 | 0.70297 | |
| MgO | −0.08947 | −0.10711 | −0.012 | −0.02716 | 0.1545 | −0.01154 | −0.09549 | −0.09094 | 0.30166 | |
| CaO | −0.98424 | −0.95836 | −0.9598 | −0.02716 | −0.33029 | −0.91739 | −0.95411 | −0.83796 | −0.79583 | |
| Na2O | 0.25506 | 0.34937 | 0.35121 | 0.1545 | −0.33029 | 0.44729 | 0.39502 | 0.53825 | 0.32657 | |
| K2O | 0.8845 | 0.9138 | 0.89023 | −0.01154 | −0.91739 | 0.44729 | 0.9092 | 0.85725 | 0.6789 | |
| TiO2 | 0.92725 | 0.99673 | 0.96939 | −0.09549 | −0.95411 | 0.39502 | 0.9092 | 0.94817 | 0.75895 | |
| P2O5 | 0.78691 | 0.93623 | 0.86091 | −0.09094 | −0.83796 | 0.53825 | 0.85725 | 0.94817 | 0.75491 | |
| MnO | 0.7502 | 0.76123 | 0.70297 | 0.30166 | −0.79583 | 0.32657 | 0.6789 | 0.75895 | 0.75491 |
| DC1 (n = 4) | DC2 (n = 4) | DR3 (n = 10) | |
|---|---|---|---|
| Sr | 758.3–354.4 (av. 607.4) | 155.0–109.2 (av. 140.7) | 329.5–184.8 (av. 187.7) |
| Rb | 5.4–3.0 (av. 3.7) | 1.7–0.1 (av. 0.6) | 2.6–0.4 (av. 1.1) |
| Ba | 30–18 (av. 26) | 30–4 (av. 11) | 6–2 (av. 4) |
| Mo | 1.1–0.4 (av. 0.6) | 0.2–0.1 (av. 0.2) | 0.3–0.1 (av. 0.17) |
| Cu | 4.6–2.0 (av. 3.5) | 2.8–1.1 (av. 1.8) | 2.1.7–0.2 (av. 0.84) |
| Pb | 191.0–8.8 (av. 83.5) | 33.2–3.5 (av. 14.8) | 5.0–0.7 (av. 2.3) |
| Zn | 298–15 (av. 151) | 86–17 (av. 36) | 19–15 (av. 10.7) |
| Zr | 35.2–5.0 (av. 19.2) | 35.9–1.7 (av. 11.8) | 7.8–2.8 (av. 2.3) |
| U | 3.7–1.3 (av. 2.5) | 2.6–0.7 (av. 1.6) | 4.1–1.1 (av. 2.5) |
| Th | 5.7–0.7 (av. 2.9) | 3.3–1.0 (av. 1.8) | 0.2 |
| Hf | 1.0–0.1 (av. 0.6) | 0.9–0.1 (av. 0.3) | 0.3–0.1 (av. 0.1) |
| ΣREE | 86.6–20.9 (av. 47.8) | 36.1–3.5 (av. 18.3) | 13.1–2.5 (av. 6.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bąk, M.; Bąk, K.; Wolska, A.; Rzepa, G.; Szczurek, S.; Strzeboński, P.; Bębenek, S.; Dolnicki, P. Bacteria-like Ferruginous Structures in Carboniferous Limestones as Remains of Post-Variscan Hydrothermal Activity in Southern Poland. Minerals 2025, 15, 1158. https://doi.org/10.3390/min15111158
Bąk M, Bąk K, Wolska A, Rzepa G, Szczurek S, Strzeboński P, Bębenek S, Dolnicki P. Bacteria-like Ferruginous Structures in Carboniferous Limestones as Remains of Post-Variscan Hydrothermal Activity in Southern Poland. Minerals. 2025; 15(11):1158. https://doi.org/10.3390/min15111158
Chicago/Turabian StyleBąk, Marta, Krzysztof Bąk, Anna Wolska, Grzegorz Rzepa, Stanisław Szczurek, Piotr Strzeboński, Sławomir Bębenek, and Piotr Dolnicki. 2025. "Bacteria-like Ferruginous Structures in Carboniferous Limestones as Remains of Post-Variscan Hydrothermal Activity in Southern Poland" Minerals 15, no. 11: 1158. https://doi.org/10.3390/min15111158
APA StyleBąk, M., Bąk, K., Wolska, A., Rzepa, G., Szczurek, S., Strzeboński, P., Bębenek, S., & Dolnicki, P. (2025). Bacteria-like Ferruginous Structures in Carboniferous Limestones as Remains of Post-Variscan Hydrothermal Activity in Southern Poland. Minerals, 15(11), 1158. https://doi.org/10.3390/min15111158








