1. Introduction
According to [
1], geometallurgy, a subfield of applied mineralogy, is defined as the metallurgical study of economically viable portions of the Earth’s crust with exploration potential. Geometallurgy integrates knowledge from geology, metallurgy, and geostatistics to determine the optimal configuration of a processing plant, guided by a central question: How do specific sample types behave during metallurgical processing stages? Addressing this question requires comprehensive understanding of the mineralogical characteristics of both ore and gangue minerals.
The efficiency of gold extraction processes is directly correlated with the mineralogical properties of the ore body being processed, which dictate the chemical and physical attributes employed in metallurgical operations. Historically, the definition of such mineralogical properties was time-consuming, costly, and primarily qualitative. However, advancements in mineralogical analysis technologies, particularly scanning electron microscopy (SEM), have enabled the large-scale optimization of sample characterization. When combined with traditional geological methods used to correlate lithologies and their spatial variations, these technologies have transformed mineral characterization into a powerful semi-automated quantitative approach.
Geometallurgy has become an essential approach for gold deposits in the Iron Quadrangle, as it integrates mineralogical, geological, and metallurgical data to improve the predictability of processing performance. Recent studies in the region ([
2,
3,
4,
5,
6,
7,
8]) have shown that variations in lithology, mineral associations, and texture strongly influence comminution behavior, flotation selectivity, gravity recovery, and even the potential recovery of residual gold in tailings. Despite these advances, there is still a lack of comprehensive frameworks that combine modal mineralogy with detailed metallurgical testing in small and medium-sized deposits, which are increasingly relevant in current production scenarios. Addressing this gap is critical for optimizing recovery strategies and tailoring processing flowsheets to lithotype-specific characteristics in complex systems such as the Lamego gold deposit.
The widespread adoption of sublevel open stoping, enabled by advancements in long-hole drilling, brings to the fore the critical challenge of dilution. This phenomenon, defined as the mixture of ore with waste rock, is a pivotal factor for economic viability and is categorized as planned (within the ore block) or unplanned (from external factors like wall instability). Consequently, the final dilution is not solely a function of the designed ore geometry but is also dictated by the interplay of geotechnical conditions and mining practices, making its accurate prediction and control essential for operational efficiency [
9,
10,
11,
12,
13,
14].
Since 2004, the price of gold per ounce has reached multiple peaks, rendering previously uneconomic mining areas financially viable. Nevertheless, due to their distinct characteristics when compared to larger deposits, these smaller-scale areas require customized studies and techniques to define their geological, mining, and metallurgical parameters [
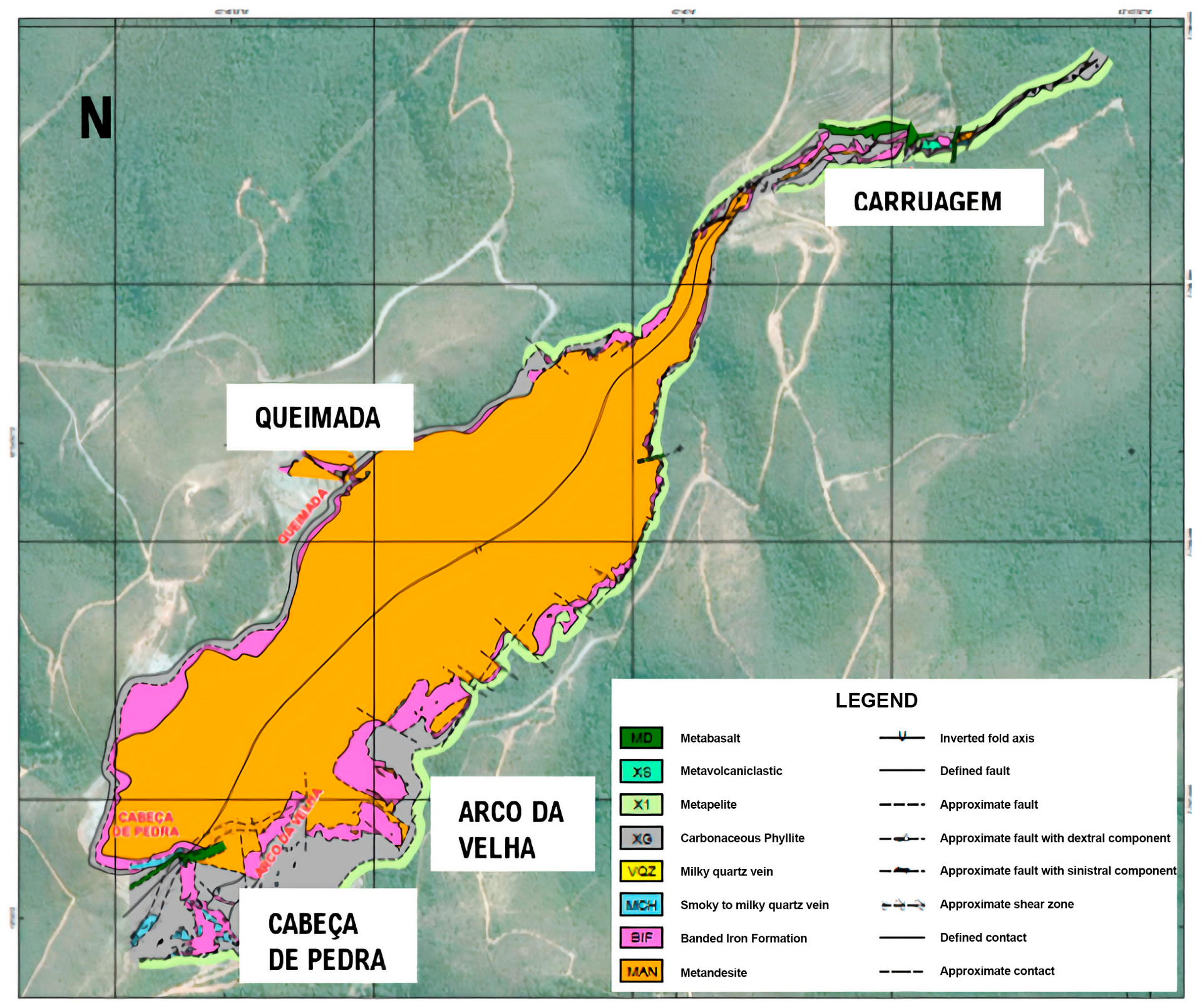
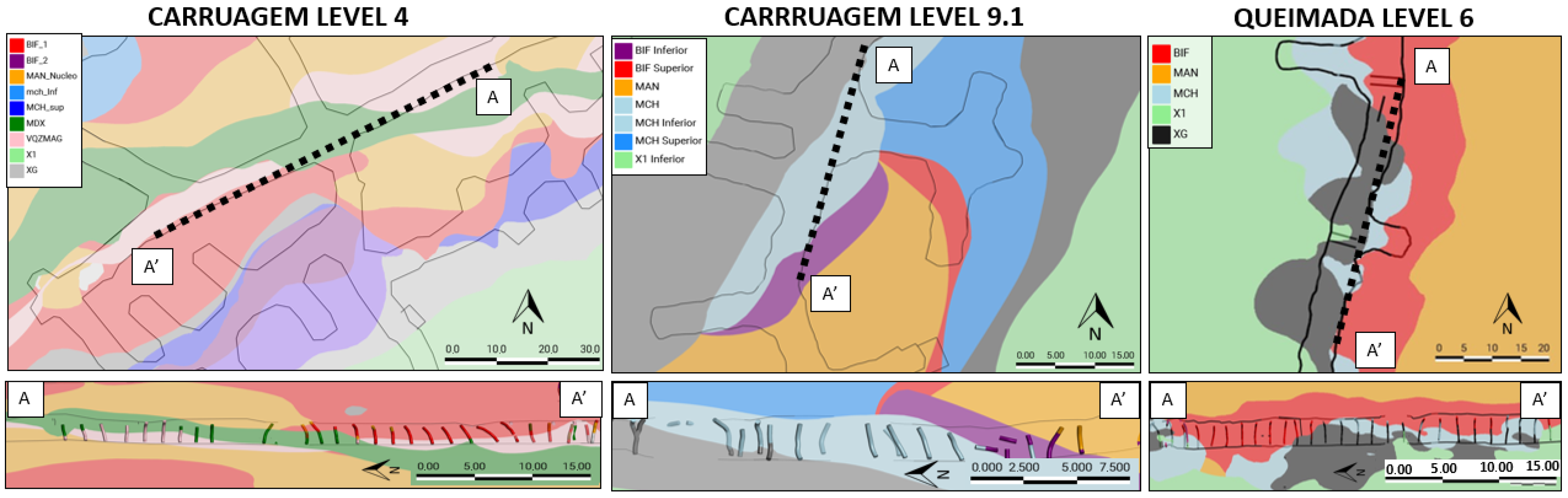
15]. As a result, numerous small-scale mining projects have become feasible. One such example is the Lamego deposit, which is integrated into production at the Cuiabá mine, which is an area of recognized importance for gold production in Brazil. The Lamego mine is composed of four ore bodies: (1) Cabeça de Pedra: Located in the southwest hinge of the local main folded structure, this ore body is associated with sulfide-bearing Banded Iron Formation (BIF). Mining operations were halted at level 4; (2) Arco da Velha: This body lies on the normal limb of the fold and hosts ore in both main lithotypes—BIF and smoky quartz. It was mined up to level 3 and is currently outside the production planning for upcoming years; (3) Queimada: Corresponding to the overturned limb, this body hosts its primary ore zone in sulfide-rich BIF and is currently at level 7; and (4) Carruagem: The most important ore body in the mine, located at the fold closure, with mineralization predominantly associated with smoky quartz. Mining is currently taking place at level 11. In this context, geometallurgical research demonstrates significant potential to correlate ore properties with the environmental and economic aspects of extraction, integrating data from mine planning to mineral behavior during processing.
The aim of this paper adopts such a perspective by presenting the mineralogical characterization and its main metallurgical implications for the two operational ore bodies at the Lamego mine, Carruagem (CAR) and Queimada (QMD) in the levels 6, 4.1, and 9. The study focuses on the characterization of both mineralized and non-mineralized lithotypes during gravity separation and flotation processes, emphasizing modal mineralogy and its associations with other minerals within the two main production bodies of the mine.
Geological Context
The Lamego orogenic gold deposit, currently owned by AngloGold Ashanti Córrego do Sítio Mineração, is in the central-northern region of the Iron Quadrangle [
16]. It lies between the municipalities of Sabará and Caeté, Minas Gerais, approximately 20 km from the state capital, Belo Horizonte. The deposit is mined and operated via underground methods at depths reaching up to 600 m, with estimated mineral resources of 1.05 million ounces (Moz) and reserves of 120 thousand ounces (Koz) at an average grade of 2.90 g/t [
17].
Regionally, the deposit is part of the greenstone belt of the Rio das Velhas Super-group, specifically within the Mestre Caetano unit of the Nova Lima Group, adjacent to the Córrego do Sítio and Mindá units [
18]. It is recognized as part of the Lamego Fold [
19]. The lithological sequence at Lamego (
Figure 1) comprises mafic volcanic rocks, chert, BIF, silicification zones (smoky quartz), carbonaceous pelitic metamorphic rocks, and micaceous turbidites within the greenschist facies [
20]. The mineralization is associated with quartz veins and Algoma-type BIF altered by hydrothermal silicification [
21].
It is possible to describe five geological units in the Lamego deposit: metandesite, BIF, silicification zone, carbonaceous phyllite, and pelite.
The metandesite (MAN) is hydrothermally altered and metamorphosed basic volcanic rocks that displays a mineral assemblage characteristic of lower greenschist facies. These rocks consist of schistose basalt (with possible andesitic variations), composed of chlorite, carbonate, quartz, sericite, and plagioclase [
20]. The author of [
20] identified five distinct hydrothermal parageneses within the volcanic unit, defined by variable proportions of actinolite, epidote, albite, quartz, carbonates, sericite, and localized sulfides.
The banded iron formation was described [
21] as alternating dark and light bands of carbonate–quartz. Mineralogically, it consists of quartz, carbonate (predominantly siderite), chlorite, sericite, pyrite, arsenopyrite, pyrrhotite, and accessory minerals (e.g., rutile, carbonaceous matter). Transitions to chert occur both laterally and vertically, with gold mineralization hosted in sulfides (mainly pyrite, pyrrhotite, and arsenopyrite).
The silicification zone (MCH) comprises large volumes of smoky quartz (ranging from centimeters to decameters in thickness) strongly associated with carbonates and sulfides. This zone occurs near BIF and carbonaceous phyllite, with gold predominantly occurring in free form.
The carbonaceous phyllite (XG) overlies the BIF/chert units and consists of quartz, sericite, and carbonaceous material [
20,
21].
The pelite is the youngest lithotype, displaying preserved gradational compositional layering and crenulation cleavage. It is composed of quartz, carbonate, sericite/muscovite, pyrite, chalcopyrite, and sphalerite [
22].
Figure 1.
Geological map (B) of the Lamego mine, highlighting lithology, structures, and orebody positions [
23].
Figure 1.
Geological map (B) of the Lamego mine, highlighting lithology, structures, and orebody positions [
23].
2. Materials and Methods
2.1. Materials and Sampling
The sampling campaign was conducted at the Lamego underground mine, focusing on the operational levels of the Carruagem (CAR) and Queimada (QMD) ore bodies. The methodology was designed to capture the full variability of lithotypes that constitute the mine’s ore and the potential dilution (incorporation of waste rock) sent for metallurgical processing.
Channel samples were collected from gallery walls at a consistent spacing of 3 m. Each sample had a minimum weight of 2 kg to ensure sufficient mass for subsequent chemical and metallurgical analyses. Lithological contacts and textural variations were carefully respected during sampling to prevent cross-contamination and preserve the integrity of each lithotype.
This strategy ensured the representation of all mineralized lithotypes (e.g., banded iron formation—BIF, smoky quartz) and their host rocks (e.g., metandesite, carbonaceous phyllite). Based on geographical location and lithological similarity, individual samples were composited into 11 representative composite samples for metallurgical testing. The sampling areas are illustrated in
Figure 2.
The collected samples were sent to AngloGold Ashanti’s (AGA) internal laboratory for initial chemical characterization. Gold content was determined by fire assay at AGA internal laboratory, and total sulfur was analyzed using a Leco analyzer (LECO Corporation, St. Joseph, MI, USA). These results were used as a basis for defining the final composite samples.
To ensure representativeness of lithotypes in each orebody, lithological contacts and potential textural variations were respected, preventing contamination and preserving the integrity of studied lithotypes.
This sampling strategy captured all mineralized lithotypes and their host rocks. Samples of the same lithology were composited into a single representative sample, also considering their geographical location.
The collected samples were sent to AngloGold Ashanti’s (AGA) internal laboratory for gold (fire assay) and sulfur (Leco) analyses. Gold and sulfur results formed the basis for sample compositing.
2.2. Mineralogical Analysis
Mineralogical characterization was performed using a QUANTA FEI 650F scanning electron microscope equipped with the QEMSCAN (Quantitative Evaluation of Minerals by Scanning Electron Microscopy—FEI Company, now part of Thermo Fisher Scientific, Hillsboro, OR, USA) system. The analyses were conducted on feed samples, gravity concentrates, and flotation products (concentrates and tailings), as indicated in the processing flowchart (
Figure 3).
The system operated at an acceleration voltage of 25 kV and a beam current of 10 nA. The acquisition was performed in Particle Mineral Analysis (PMA) mode using the SIP CuS1 measurement protocol, adapted for the samples. A field size of 1.5 cm and a point spacing of 10 µm were used, ensuring adequate resolution for quantitative mineralogical characterization.
The data generated provided modal mineralogy and mineral association analysis. In the results, minerals present in amounts below 1% were grouped into an “others” category.
Chemical analysis for gold (fire assay) and sulfur (Leco) was conducted on solid samples digested with aqua regia. The gold content was determined using atomic absorption spectrometry, and sulfur was measured by infrared detection after combustion.
2.3. Metallurgical Testing
Metallurgical tests were conducted at AGA’s laboratory in the Queiroz plant, Nova Lima, Minas Gerais. The composites were prepared according to lithology, ore body, and spatial proximity, and they met the minimum mass requirement for testing (13 kg). A total of 11 composite samples were generated, covering all study areas. The processing route is illustrated in
Figure 3.
Gravity separation tests were focused on samples with higher gold grades in host rocks (BIF and smoky quartz), as this process is generally ineffective for gold in the other type of samples due to the limited density variation between associated minerals.
The tests followed AGA’s internal protocols, with aliquots collected at each stage for internal analyses of Au, S, and C.
The tests began with the crushing process, in which the entire sample mass was crushed as a preparatory step for splitting, in case the mass exceeded the required amount. The crushed material was then sent for grinding to reach a particle size of 74 µm, which is the necessary fraction to ensure optimal mineral liberation for the separation processes.
In the gravity separation stage, selected composites were fed into the Knelson concentrator and separated into underflow and overflow streams. The overflow represents the higher-density material and is considered the final product, while the underflow, which still contains unrecovered gold, was sent to the flotation cell.
At this stage, the material underwent two processes: the first, called cleaner flotation, recovered particles with lower water affinity using collectors. The tailings from this first step were subjected to rougher flotation, in which another reagent was used to recover any remaining gold. The test conditions for each stage are shown in the
Table 1.
3. Results
The results are presented below, organized by the four main lithotypes: metandesite, banded iron formation (BIF), smoky quartz, and carbonaceous phyllite.
3.1. Metandesite
The metandesite exhibited a grinding time of 14 min to achieve a particle size passing 74 µm, with an energy consumption of 5.94 kW. The variable K, representing the particle breakage rate per unit of energy, was 0.18.
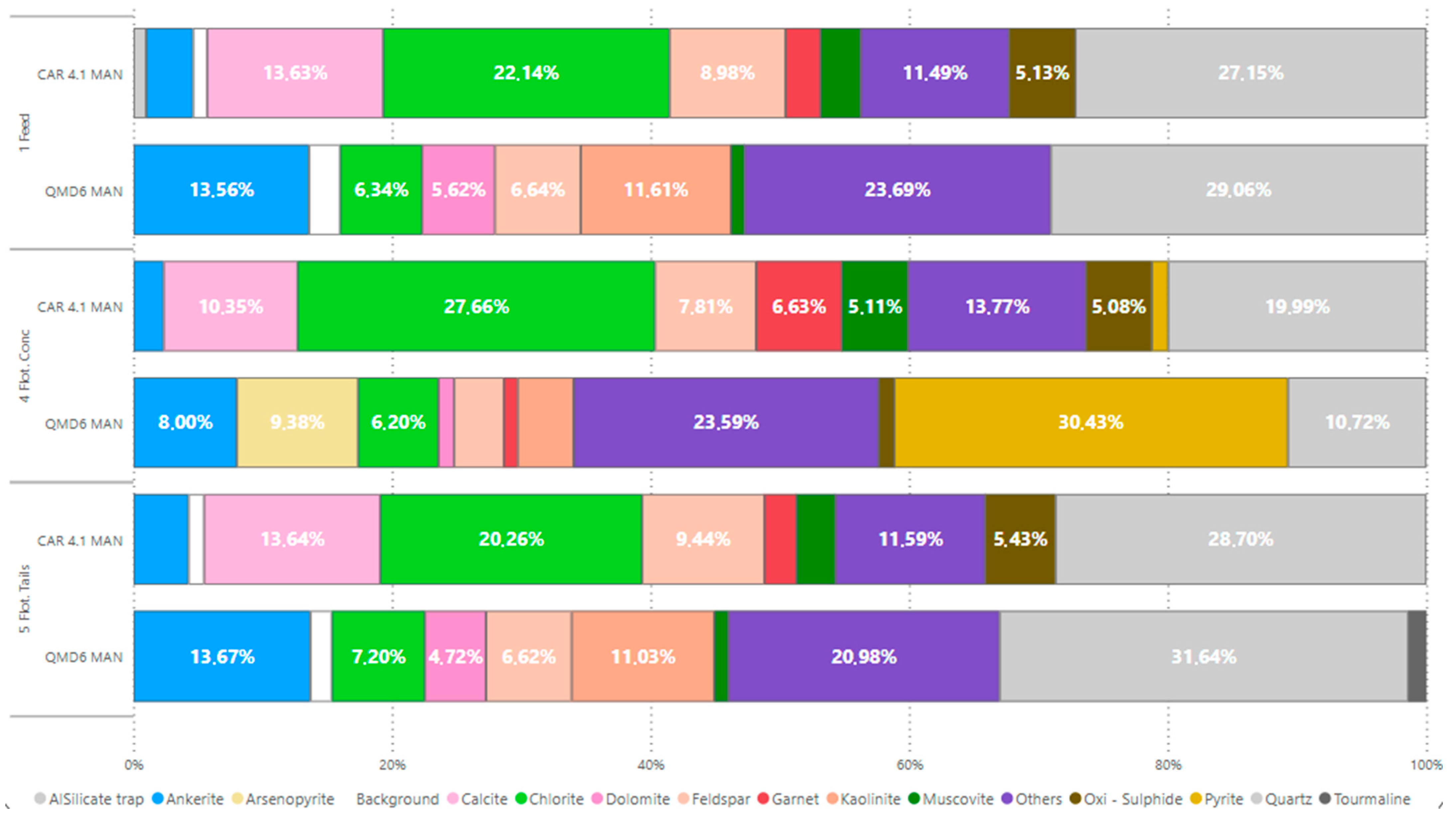
In general, the mineralogical composition of the feed samples is similar, dominated by silicates and carbonates. However, notable differences exist in chlorite content among the samples: 22.39% in CAR4.1 MAN versus 6.5% in QMD6 MAN. Carbonate composition also varies: QMD6 MAN contains ankerite and dolomite (13.9% and 3.76%, respectively), while CAR4.1 MAN consists solely of calcite (13.78%).
The lithologies showed an average mass recovery of 3.42%, with metallic recovery of 19% for CAR4.1 MAN and 74% for QMD6 MAN. The latter’s higher recovery correlates with its greater sulfide content (29.48% pyrite and 9.44% arsenopyrite).
CAR4.1 MAN concentrate was primarily composed of chlorite (27.76%), quartz (20.06%), and calcite (10.42%), with iron oxides and muscovite as minor constituents (12% and 5.13%, respectively). Tails composition closely resembled the feed, with only a reduction in mass quantity (
Figure 4).
The mineral association analyses will be presented below (
Table 2), highlighting the main minerals identified in the modal mineralogy and their key associated minerals in the feed samples:
The chemical analyses detected peaks in Au along with a slight reduction in C during flotation for both samples. In the QMD6 MAN sample, the feed grades of Au (0.37 g/t) and S (0.63%) showed concentration increases of 17.9 times (reaching 6.62 g/t) and 30.8 times (reaching 19.40%), respectively. Conversely, in the CAR4.1 MAN sample, the Au (0.06 g/t) and S (0.05%) feed grades exhibited concentration factors of 7x and 31.6x, respectively. Additionally, the carbon content in the concentrate decreased from 4.37% to 2.51% for QMD6 MAN and from 3.12% to 2.5% for CAR4.1 MAN.
Regarding metallurgical performance, the QMD6 MAN sample achieved 4.14% mass recovery and 74.09% metal recovery in flotation, whereas the CAR4.1 MAN sample yielded lower recoveries of 2.70% (mass) and 18.92% (metallic).
3.2. Banded Iron Formation
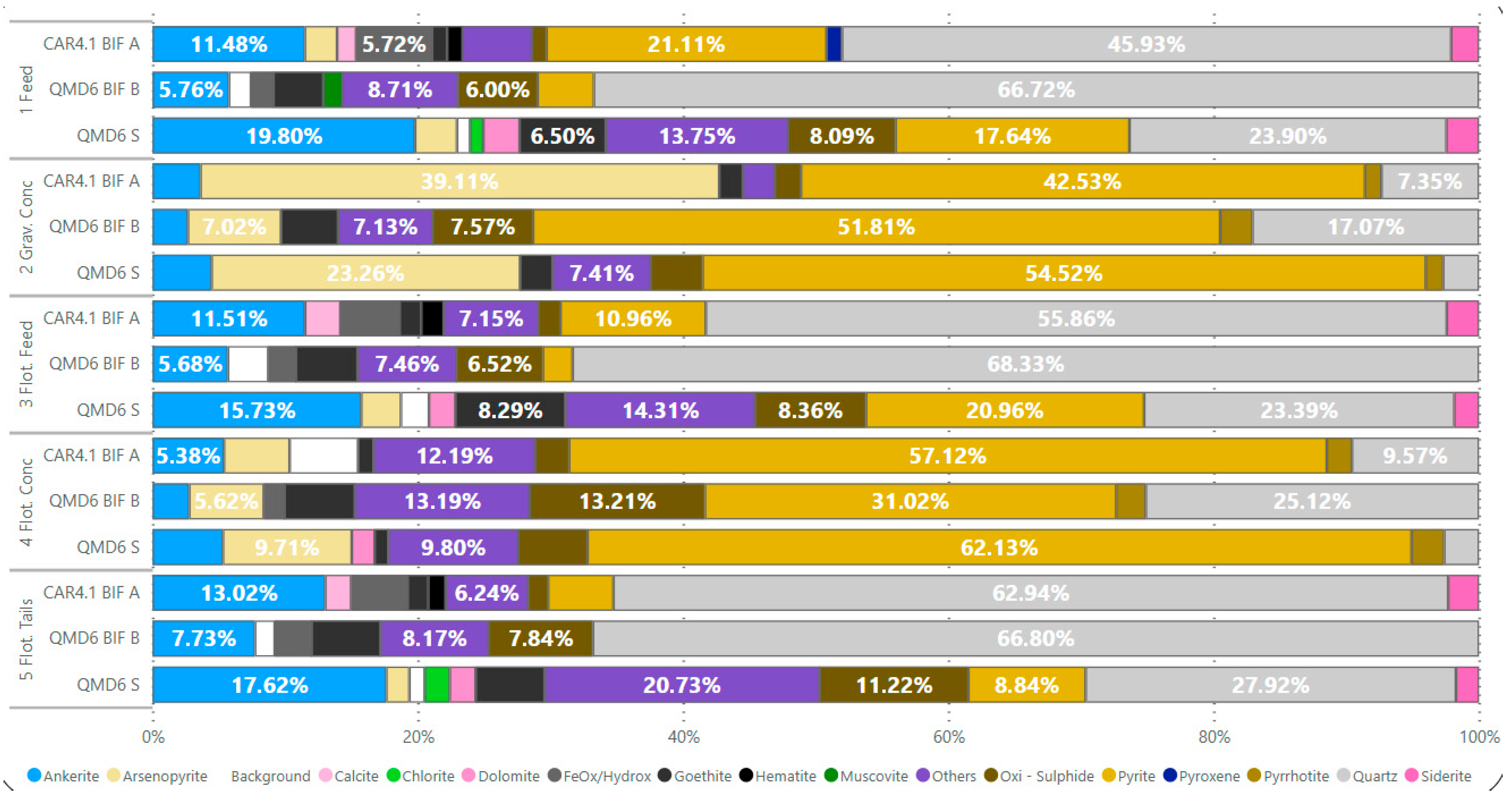
The BIF samples exhibited grinding times to achieve 80% passing 74 µm of 15.49 min for sample CAR4.1 BIF A with energy consumption of 6.7 kW. The variable K, representing the particle breakage rate per unit of energy, showed a value of 0.19. The QMD6 S sample presented a grinding time of 20.22 min, energy consumption of 8.63 kW, and K value of 0.15.
The feed mineralogy of the BIF is represented by silicates (predominantly quartz), sulfides (mainly pyrite), and carbonates (mostly ankerite). The QMD6 S sample contains arsenopyrite (3.19%) in addition to pyrite (17.80%) in its sulfide composition and ankerite (19.98%), dolomite (2.75%), and siderite (2.47%) as carbonates. The QMD6 BIF B sample has quartz as the predominant mineral (67.85%), along with oxidized sulfides (6.10%), ankerite (5.86%), and pyrite (4.32%). The CAR4.1 BIF A sample composition includes quartz (43.99%), pyrite (20.22%), arsenopyrite (2.33%), ankerite (11%), and siderite (2%).
The gravity concentrates of the samples are primarily composed of pyrite and arsenopyrite. For QMD6 S, the proportions were 52.12% of pyrite and 21.34% of arsenopyrite; QMD6 BIF B contained 48.93% of pyrite, 6.93% of arsenopyrite, and 16.86% of quartz; CAR4.1 BIF A showed 51.05% of pyrite, 44.78% of arsenopyrite, and 8.83% of quartz. Ankerite was concentrated in the samples at percentages of 4.35%, 2.63%, and 4.34%, respectively. The mineralogy of gravity tailings consisted of 53.02% of quartz, 10.92% of ankerite, and 10.40% of pyrite.
The composition of flotation concentrates from the QMD6 S included 58.76% of pyrite, 9.75% of arsenopyrite, 2.47% of pyrrhotite, and 5.33% of ankerite. QMD6 BIF B presented 28.96% of pyrite, 5.64% of arsenopyrite, 5.32% of goethite, 9.06% of oxidized sulfides, and 25.22% of quartz. CAR4.1 BIF A consisted of 54.83% of pyrite, 9.55% of quartz, and 5.37% of ankerite. The final tailings of all samples showed mineralogy like their respective feed compositions. QMD6 S tailings contained 28.23% of quartz, 17.82% of ankerite, 7.85% of pyrite, 6.69% of oxidized sulfides, and 5.23% of siderite. QMD6 BIF B tailings comprised 67.78% of quartz, 7.96% of oxidized sulfides, 5.27% of goethite, and 7.84% of ankerite. CAR4.1 BIF A tailings showed 60.69% of quartz, 12.56% of ankerite, and 4.75% of pyrite (
Figure 5).
The predominant minerals in the modal mineralogy and their principal associated minerals within the feed samples are shown in
Table 3.
The metallurgical results for sample QMD6 S showed 0.65% of mass recovery and 2.76% of metal recovery in gravity separation, while flotation achieved 21.78% of mass recovery and 41.09% of metal recovery. For QMD6 BIF B, gravity separation yielded 0.65% of mass recovery and 7.86% of metal recovery, with flotation reaching 9.69% of mass recovery and 89.97% of metal recovery. The CAR4.1 sample demonstrated 0.97% of mass recovery and 10.62% of metal recovery in gravity separation, while flotation produced 18.56% of mass recovery and 89.96% of metal recovery.
3.3. Smoke Quartz
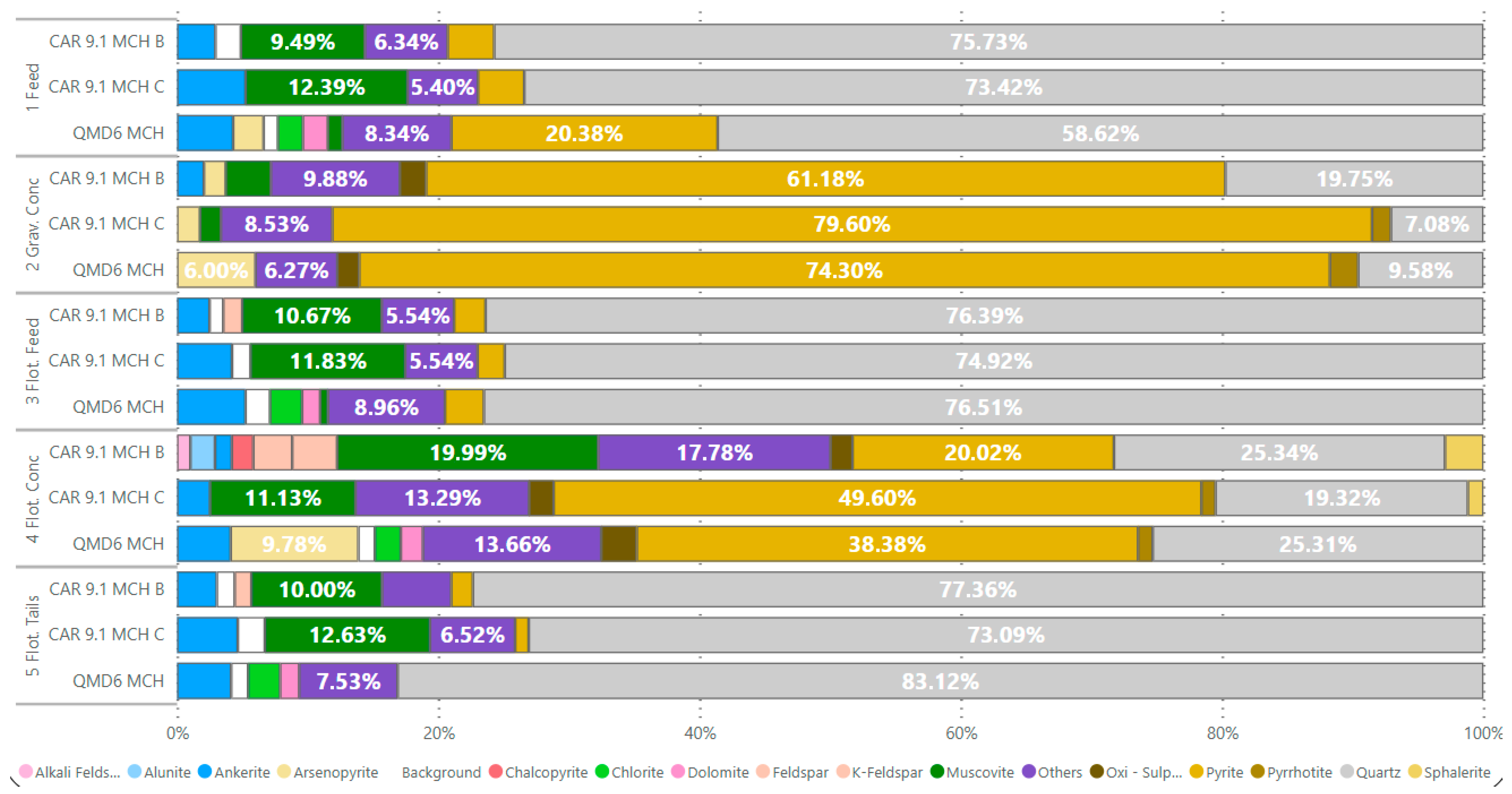
The smoke quartz showed a grinding time of 21 min to achieve 80% passing 74 µm particle size with energy consumption between 10.32 kW. The variable K, representing the particle breakage rate per unit of energy, had a value of 0.120. The feed of sample QMD6 MCH is composed of quartz (59.24%), pyrite (20.60%), ankerite (4.33%), and arsenopyrite (2.36%). The gravity concentrate contains arsenopyrite (6.01%), pyrite (72.32%), and quartz (9.60%), while the gravity tailings contain quartz (77.64%) and ankerite (10.66%). The flotation concentrate contains 37.25% of pyrite, 25.63% of quartz, and 8.77% of arsenopyrite. In the flotation tailings, quartz had the highest mineralogical proportion at 84.19%.
Sample CAR9.1 MCH C has a feed mineralogical composition consisting mainly of quartz and muscovite (75.32%), pyrite (12.41%), and ankerite (3.55% and 5.25%, respectively). The gravity concentrate contains pyrite (78.21%) and quartz (7.12%). The gravity tailings are represented by quartz (75.97%) and muscovite (12%). In the flotation concentrate, the sample showed 44.77% of pyrite, 18.19% of quartz, and 9.69% of muscovite. The main minerals of the tailings were muscovite (12.9%) and quartz (74.62%).
The mineralogy composing the feed of sample CAR9.1 MCH B is represented by 75.98% of quartz, 9.68% of muscovite, and 3.63% of pyrite. The gravity concentrate proportions are 58.97% of pyrite and 19.84% of quartz. The gravity tailings contain 75.46% of quartz, 10.78% of muscovite, and 2.43% of pyrite. The mineral content of flotation concentrate values are 21.9% for quartz, 16.32% for pyrite, 18.10% for muscovite, and 3.52% for pyrite. The flotation tailings values are 76.87% of quartz and 10.14% of muscovite (
Figure 6).
The following section presents the mineral association analyses (
Table 4), detailing the dominant minerals in the modal mineralogy and their major associated minerals in the feed samples:
The metallurgical results for sample QMD6 MCH showed 0.65% of mass recovery and 22.34% of metal recovery in gravity separation, while flotation achieved 7.63% of mass recovery and 89.86% of metal recovery. For CAR9.1 MCH C, gravity separation yielded 0.65% of mass recovery and 24.53% of metal recovery, with flotation reaching 8.26% of mass recovery and 92.71% of metal recovery. The CAR9.1 MCH B sample demonstrated 0.65% of mass recovery and 42.85% of metal recovery in gravity separation, while flotation produced 4.01% of mass recovery and 44.16% of metal recovery.
3.4. Carbonaceous Phyllite
The samples exhibited a grinding time of 19 min to achieve a particle size passing 74 µm, with an energy consumption of 8.17 kW. The variable K, representing the particle breakage rate per unit of energy, was 0.15.
The carbonaceous phyllite sample QMD6 XG B feed composition consists of quartz (18.59%), pyrite (15.9%), chlorite (23.03%), muscovite (7.23%), and ankerite (9.98%). The flotation concentrate contains 64.05% of pyrite and 7.31% of chlorite, while the flotation tailings show 19.72% of quartz, 23.28% of chlorite, 12.60% of ankerite, 8.32% of muscovite, 5.79% of oxidized sulfides, and 6.67% of pyrite. The QMD6 XG A sample feed is composed of quartz (24.97%), pyrite (15.9%), chlorite (15.44%), and muscovite (22.29%). Its flotation concentrate presents 18.03% of quartz, 21.42% of muscovite, and 16.01% of chlorite, with tailings containing 25.26% of quartz, 15.7% of chlorite, and 21.76% of muscovite.
The CAR4.1 A sample feed contains quartz (52.16%) and muscovite (21.7%). The flotation concentrate has 31.95% of quartz, 28.41% of muscovite, and 5.90% of pyrite, while the tailings show 49.44% of quartz and 23.91% of muscovite (
Figure 7). Metallurgical results for QMD6 XG B indicate 9.56% of mass recovery and 77.21% of metal recovery in flotation. QMD6 XG A achieved 50.13% of mass recovery and 75.89% of metal recovery, while CAR4.1 A showed 29.01% of mass recovery and 83.27% of recovery.
Gold concentration in QMD6 XG B increased 7.97 times from 7.64 g/t in feed to 60.88 g/t in concentrate, with sulfur concentrating 5.14 times from 6.82% to 35.10%. Carbon content remained stable at 3.39% in feed and 3.06% in concentrate. For QMD6 XG A, gold concentration was 1.5x (0.33 g/t to 0.50 g/t), sulfur 2.28x (1.37% to 3.13%), and carbon increased 1.68x (3.41% to 5.72%). The CAR4.1 XG sample showed gold concentration of 2.87x (1.19 g/t to 3.41 g/t), sulfur 3.96x (1.09% to 4.32%), and carbon increasing slightly by 1.14x (2.58% to 2.94%).
The mineral association analyses to be presented below (
Table 5) will illustrate the key minerals observed in the modal mineralogy and their most significant associated minerals in the feed samples.
The mineralogical and metallurgical data demonstrate significant differences in processing behavior between the carbonaceous phyllite samples, particularly in terms of gold and sulfur concentration factors and recovery rates across the different lithologies. The QMD6 XG B sample showed particularly strong gold upgrading potential through flotation, while the CAR4.1 samples exhibited higher overall metal recoveries despite lower initial grades.
4. Discussion
The mineralogy of the studied samples revealed significant variations among the mineralized bodies, particularly in the proportions of carbonates, sulfides, and phyllosilicates. In the case of the metandesite, the predominance of ankerite and dolomite in QMD6 MAN (as opposed to calcite in CAR4.1 MAN) may have negatively impacted flotation selectivity. Carbonates with higher reactivity, such as dolomite and ankerite, are known to compete with sulfides for collector adsorption, thereby reducing process efficiency and potentially diluting the concentrates [
2,
3,
24].
The high concentration of sulfides in the flotation concentrate of QMD6 MAN (30.43% pyrite and 9.38% arsenopyrite) resulted in high recoveries of gold and sulfur (74.09% and 98.46%, respectively), in contrast to the low values observed in CAR4.1 MAN (18.09% and 80.51%), where the pyrite content in the feed was only 1.27%. This trend aligns with the findings of [
4], who emphasized the role of sulfide minerals, especially pyrite, as preferential hosts for submicroscopic gold, which can be efficiently recovered by flotation when sufficiently liberated.
In the BIF samples, the predominance of pyrite, arsenopyrite, pyrrhotite, and goethite in the gravity concentrates indicates efficient recovery of dense minerals. However, the lower recovery observed in QMD6 S suggests that factors such as fine grain size or gold dissemination within the sulfides affected the performance of the gravity concentrator. As demonstrated [
5], gold and sulfide particles in fine size fractions (<38 µm) exhibit low gravity recovery and are better suited to flotation or leaching. The authors [
6] reinforce this limitation, showing that both gravity and flotation performance decrease sharply in tailings with finely disseminated sulfides and heterogeneous matrixes, particularly in complex systems such as those of the Iron Quadrangle.
Despite this, QMD6 S exhibited only moderate gold recovery (42.71%) despite its high sulfide content, possibly due to gold encapsulation or sulfide surface passivation. As discussed [
7], the mode of gold occurrence (free, included, or in solid solution) is critical in selecting the appropriate processing route. This mineralogical variability also highlights the importance of quantitative mineralogical balances as predictive tools for metallurgical performance.
In the smoky quartz group, sample CAR9.1 MCH B showed good gravity performance (42.85%) but poor flotation recovery (44.16%), suggesting the presence of free gold. Such gold, being unassociated with sulfides, has low affinity for conventional flotation reagents and requires well-optimized gravity circuits [
8]. Conversely, CAR9.1 MCH C, with a similar pyrite content, performed better in flotation, likely due to a stronger association between gold and sulfides.
In the carbonaceous phyllite samples, the dominance of muscovite (especially in QMD6 XG A and CAR4.1 XG A) explains the high mass recoveries and low enrichment factors. Muscovite, due to its platy morphology and negative surface charge, interferes with flotation by indiscriminately adsorbing bubbles and reagents [
25], in addition to increasing pulp viscosity and disrupting hydrodynamics. These effects are accentuated in fine and complex ores, as noted by [
26], who also highlight the limitations of automated mineralogy techniques such as QEMSCAN in detecting phyllosilicates and carbonaceous materials, thereby requiring complementary methods such as Raman spectroscopy and thermal analysis.
In the grinding stage, differences in the breakage parameter (K) and energy consumption reflect the mechanical resistance of each lithotype. Smoky quartz, with K = 0.120 and a specific energy of 10.32 kWh/t, exhibited higher resistance to breakage, attributed to the hardness of quartz and fine intergrowths between minerals. In contrast, metandesites and BIF showed intermediate grinding behavior (K ≈ 0.15–0.19), facilitating more efficient mineral liberation. These findings are consistent with Bond’s Third Theory of Comminution (1952), which describes an inverse relationship between energy consumption and ore breakability, with parameter K serving as a practical indicator of resistance.
Ultimately, integrating modal mineralogy with comminution parameters and metallurgical recovery reinforces the need for geometallurgical approaches in process optimization. As emphasized [
7], in-depth knowledge of gold occurrence, along with mineralogical and morphological characterization of gangue minerals, is essential for developing more efficient and cost-effective gold processing flowsheets.