Engineered Artificial Minerals (EnAMs): Concept, Design Strategies, and Case Studies
Abstract
1. Introduction
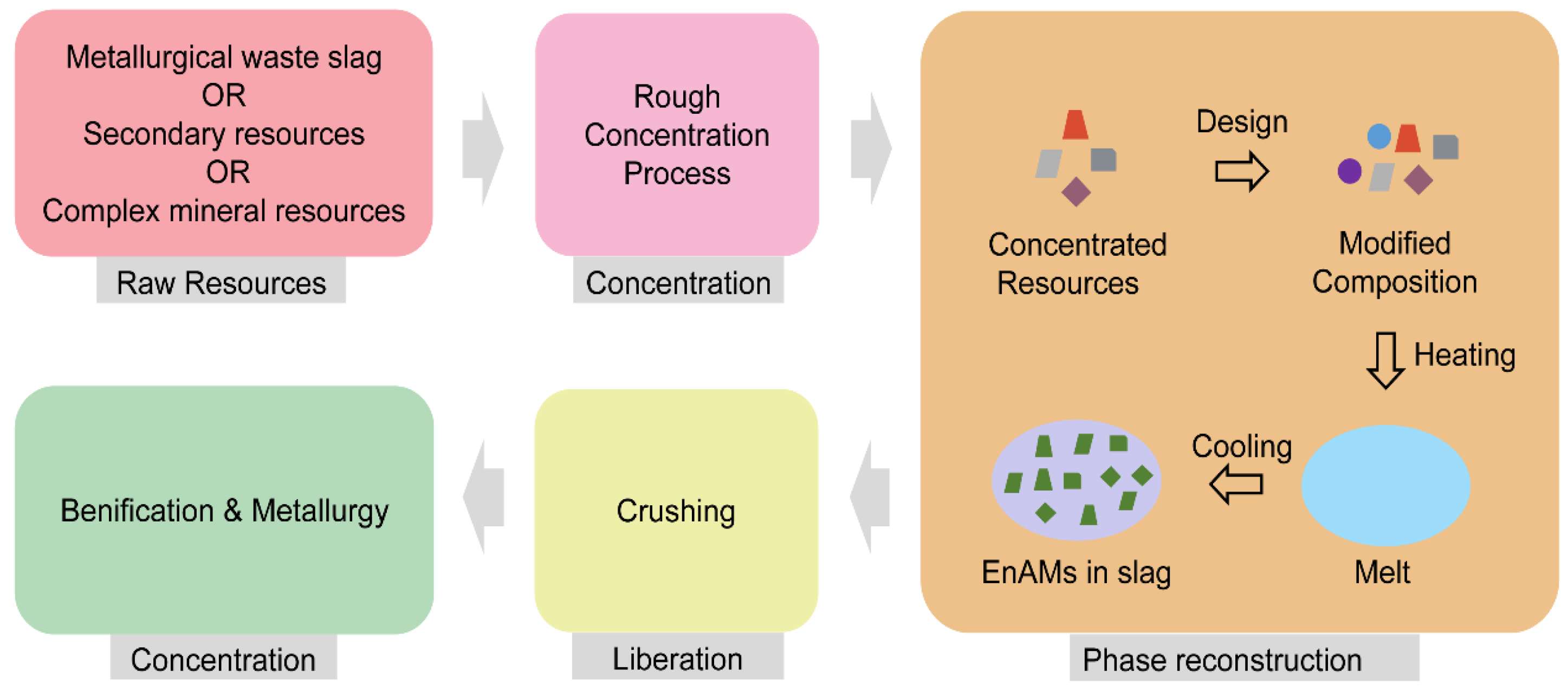
2. Concept of EnAMs
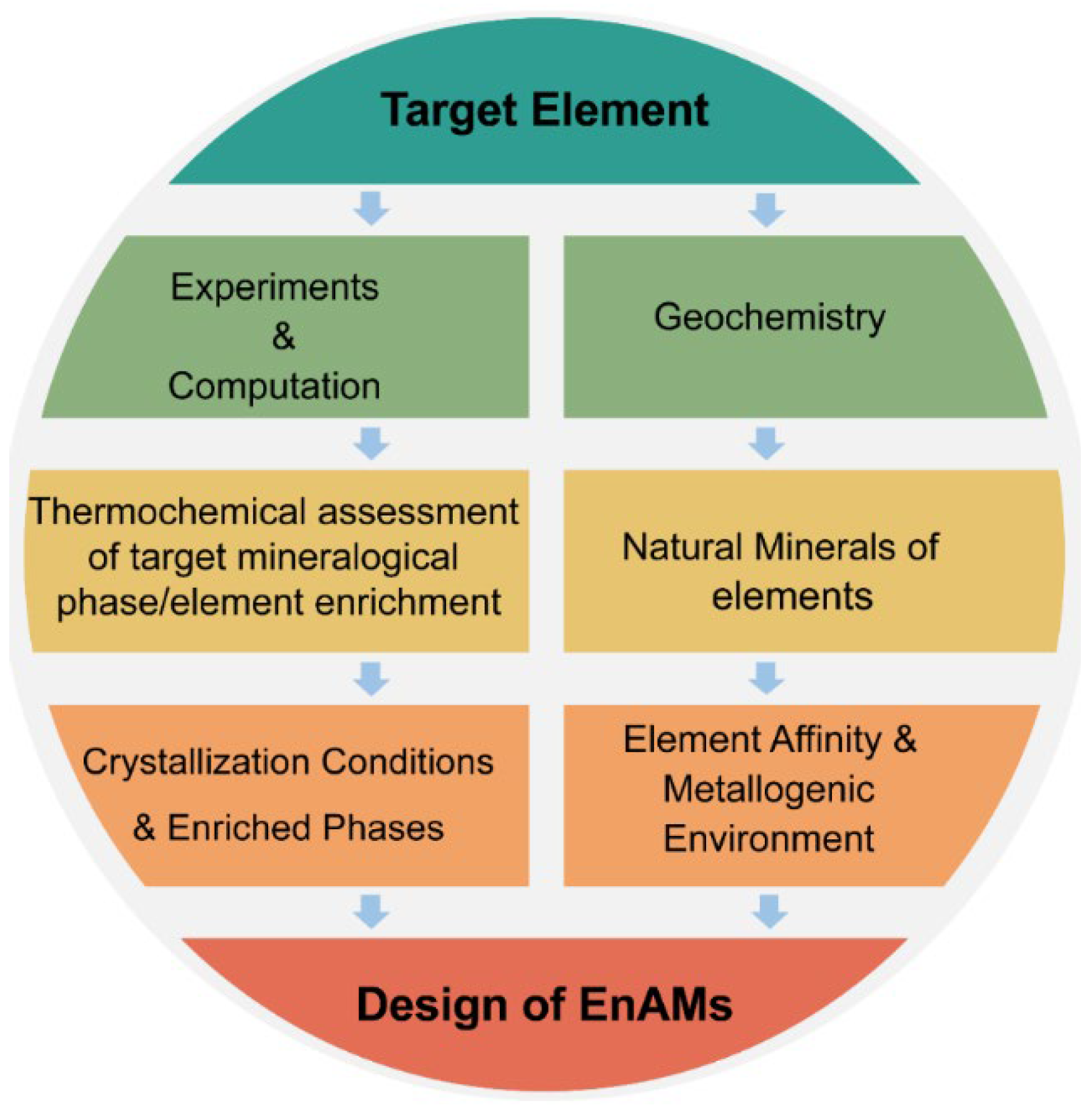
3. Design of EnAMs
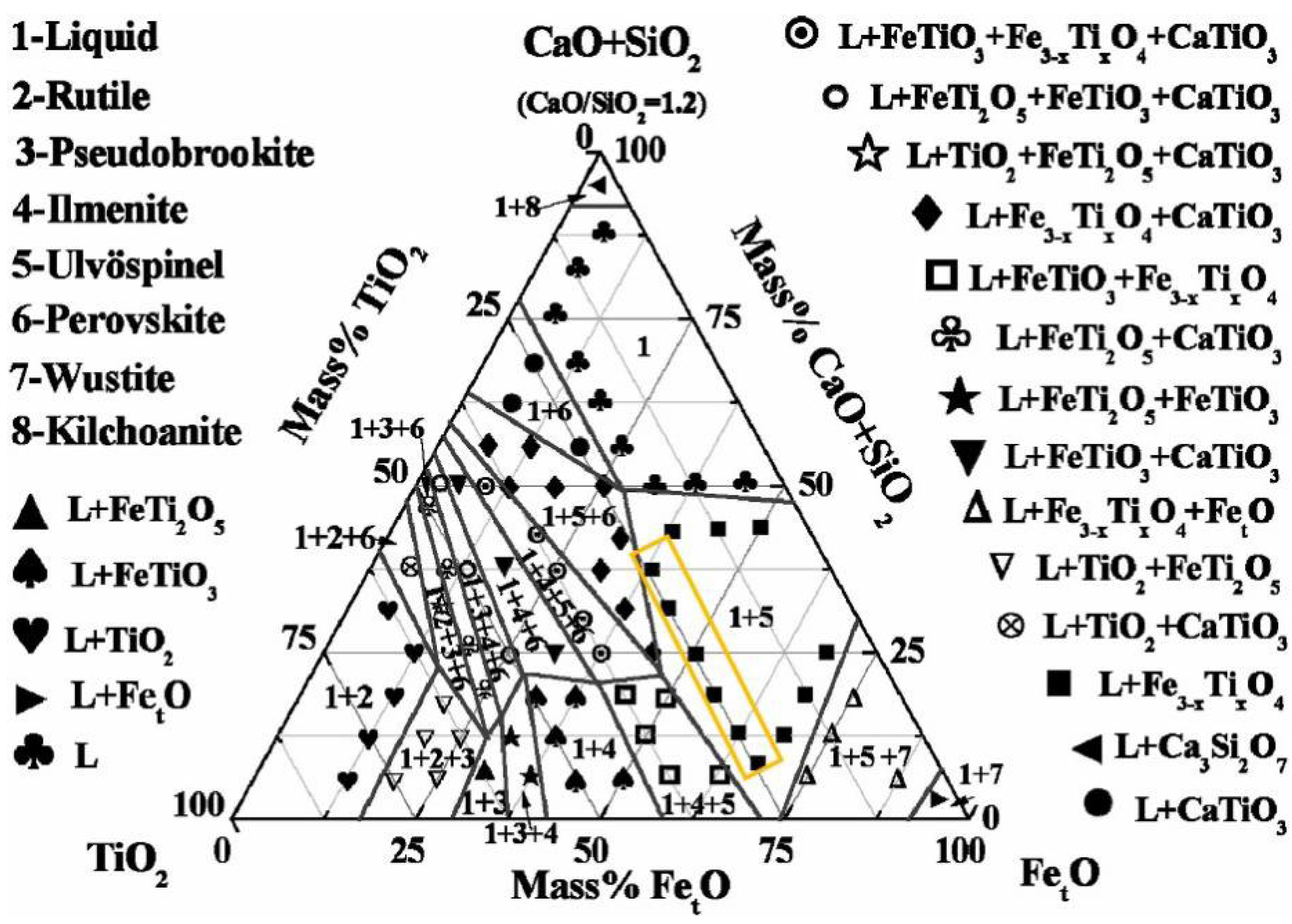
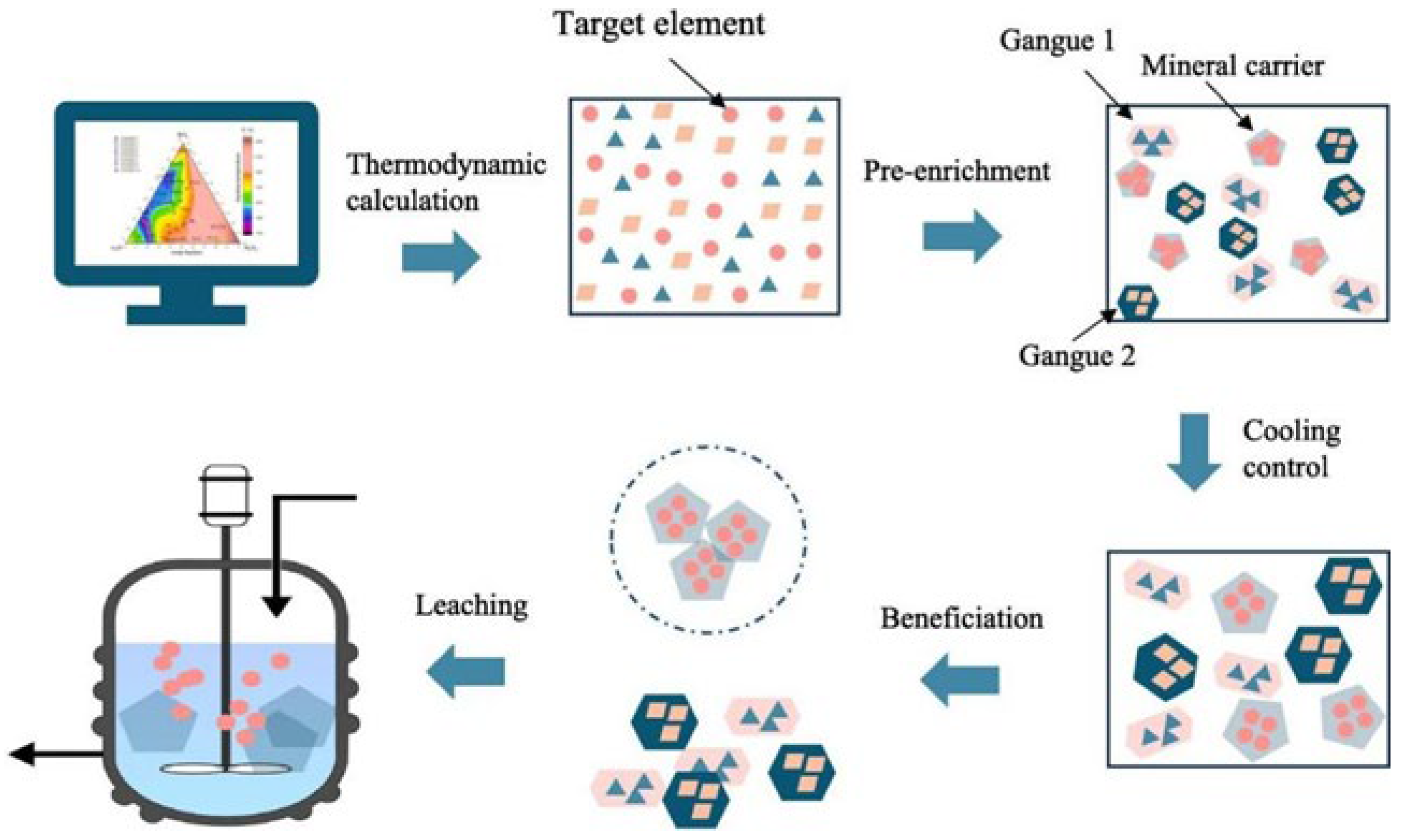
3.1. Phase Equilibrium Study
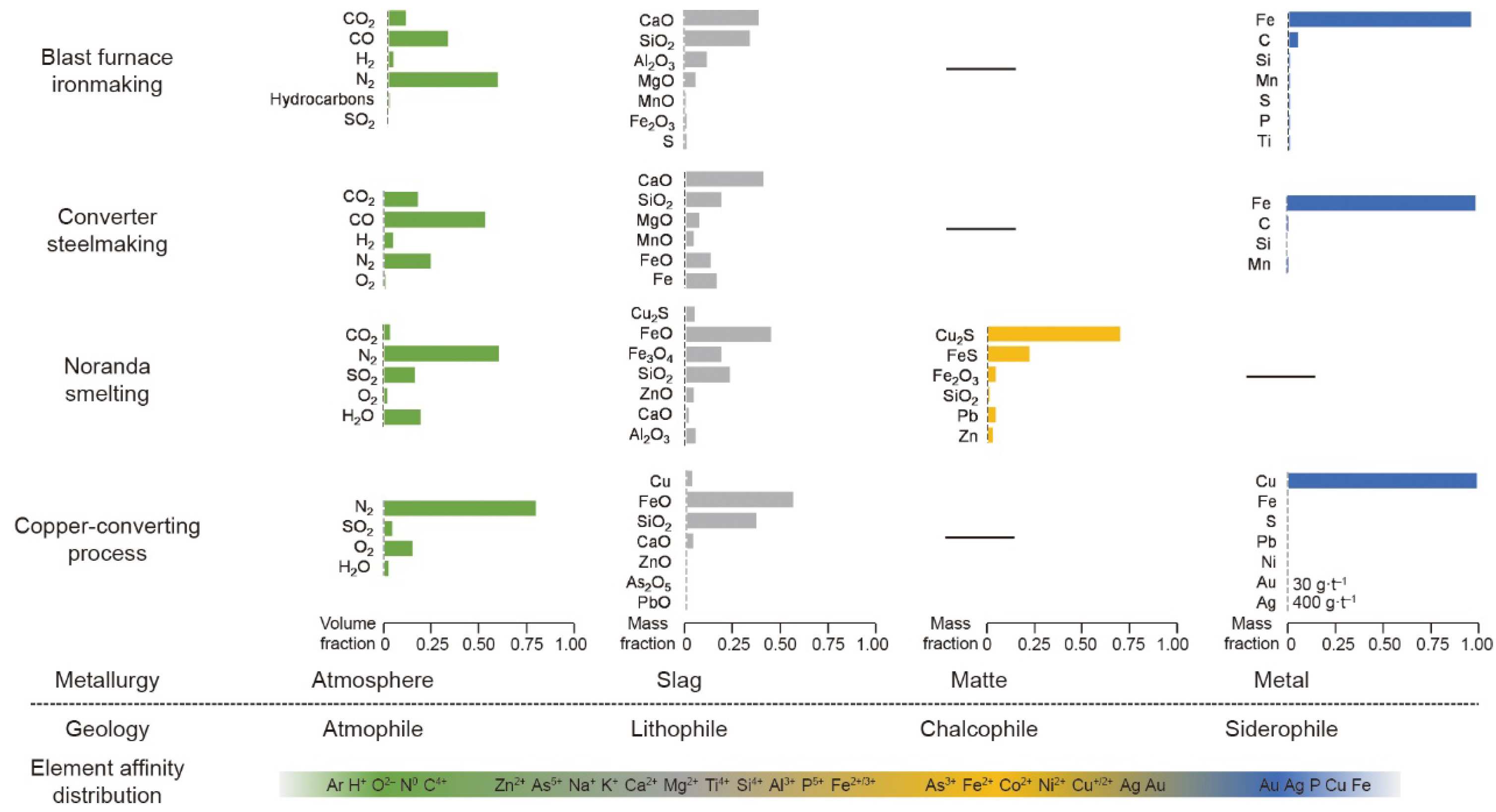
3.2. Utilizing Geochemical Behaviors
- 1.
- Simulating Element Affinity
- 2.
- Simulating Geochemical Environments of Ore Formation
4. Applications and Case Studies
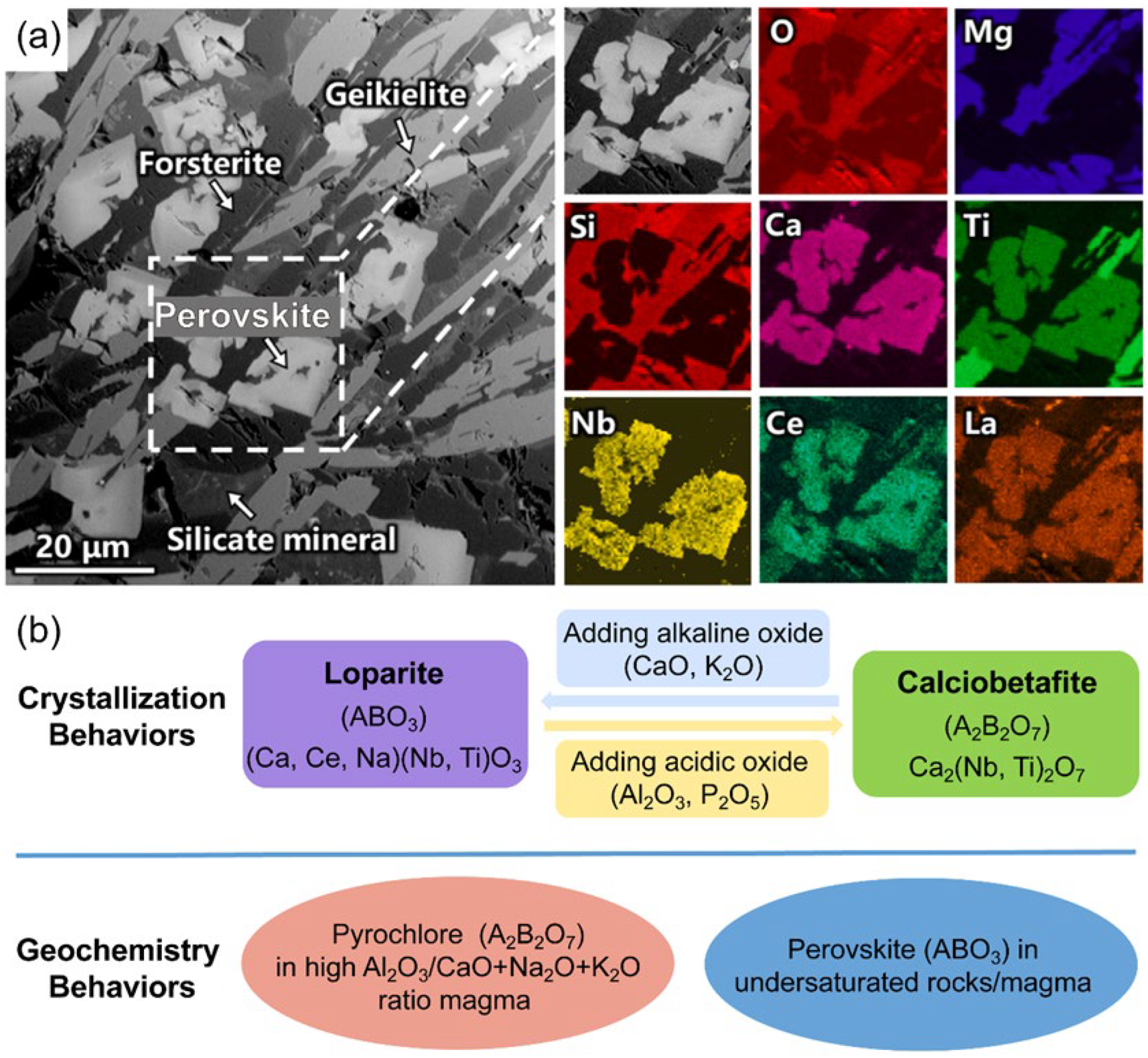
4.1. Challenging Niobium Resources
4.2. Secondary Lithium Resource Recycling
4.3. Recovery and Crystallization of Rare Earth from Slag
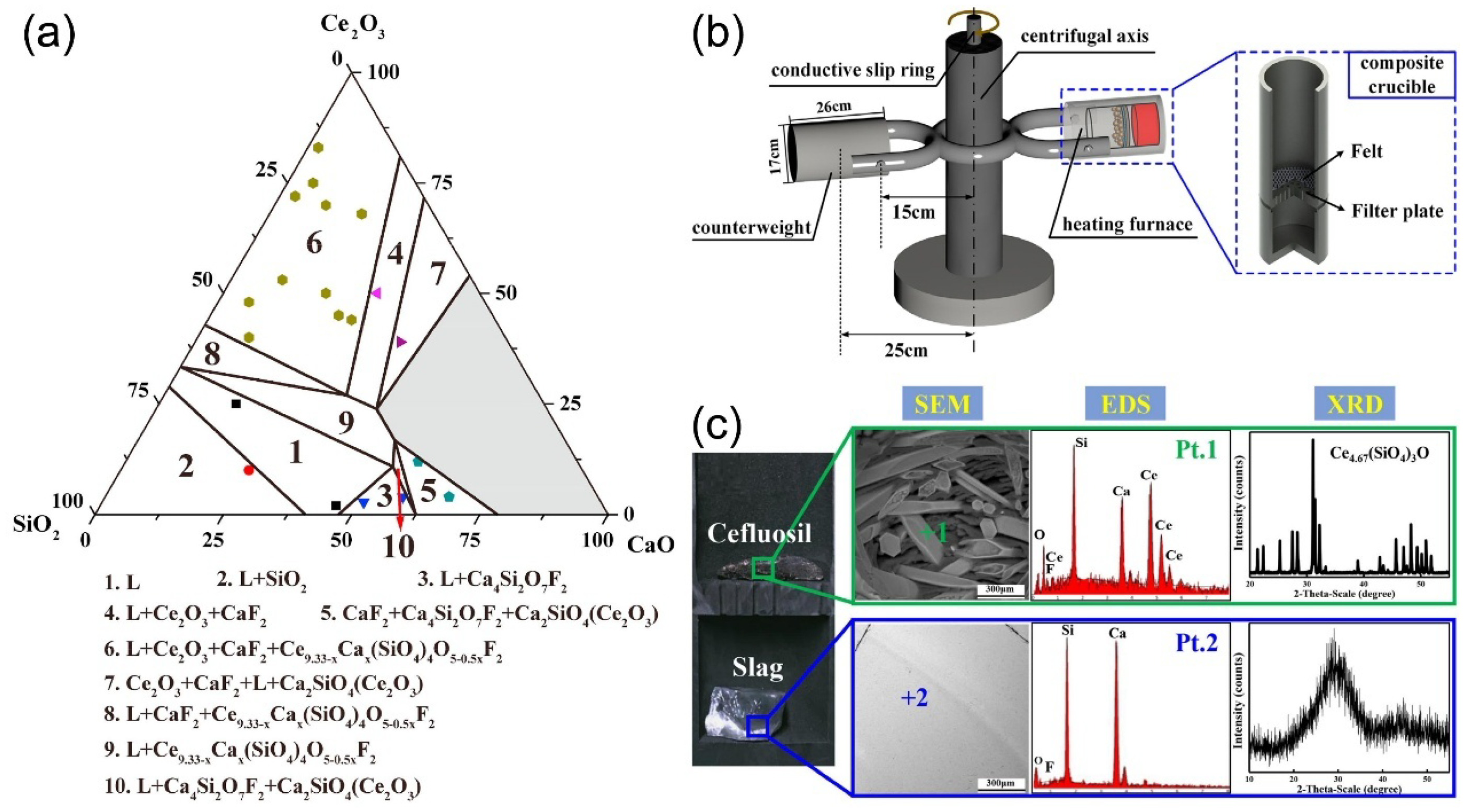
5. Challenges and Future Perspectives
5.1. Optimization of EnAMs Design
5.2. Improving Economic Viability
5.3. Physical Separation of Fine-Grained (<20 μm) EnAMs
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EnAMs | Engineered Artificial Minerals |
| EnANMs | Engineered Artificial Niobium-Bearing Minerals |
| HEV | Hybrid Electric Vehicle |
| PGMs | Platinum-Group Metals |
| REEs | Rare Earth Elements |
| WEEE | Electrical and Electronic Equipment Waste |
References
- Li, Z.; Wang, C.; Chen, J. Supply and demand of lithium in China based on dynamic material flow analysis. Renew. Sustain. Energy Rev. 2024, 203, 114786. [Google Scholar] [CrossRef]
- Tang, H.; Peng, Z.; Tian, R.; Ye, L.; Zhang, J.; Rao, M.; Li, G. Platinum-group metals: Demand, supply, applications and their recycling from spent automotive catalysts. J. Environ. Chem. Eng. 2023, 11, 110237. [Google Scholar] [CrossRef]
- Pan, D.; Yuming, C.; Jinhua, Y.E.; Weibo, Z. Study on the resource distribution and industry development of global niobium and tantalum. China Min. Mag. 2019, 28, 63–68. [Google Scholar]
- Mackay, D.A.R.; Simandl, G.J. Geology, market and supply chain of niobium and tantalum—A review. Miner. Depos. 2014, 49, 1025–1047. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, D.; Li, Y.; Ke, C.; Yu, H.; Chen, Z.; She, H.; Wang, R.; Hu, H.; Zhao, Y.; et al. Detail mineralogical study and geochronological framework of Bayan Obo (China) Nb mineralization recorded by in situ U-Pb dating of columbite. Ore Geol. Rev. 2024, 165, 105874. [Google Scholar] [CrossRef]
- Rene, E.R.; Sethurajan, M.; Kumar Ponnusamy, V.; Kumar, G.; Bao Dung, T.N.; Brindhadevi, K.; Pugazhendhi, A. Electronic waste generation, recycling and resource recovery: Technological perspectives and trends. J. Hazard. Mater. 2021, 416, 125664. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Uddin, M.N.; Chowdhury, J.I.; Ahmed, S.F.; Uddin, M.N.; Mofijur, M.; Uddin, M.A. A review of the recent development, challenges, and opportunities of electronic waste (e-waste). Int. J. Environ. Sci. Technol. 2023, 20, 4513–4520. [Google Scholar] [CrossRef]
- Botelho, A.B., Jr.; da Silva, M.; Camargo, P.S.S.; Munchen, D.D.; Cenci, M.P.; Bertuol, D.A.; Veit, H.M.; Tenório, J.A.S.; Espinosa, D.C.R. Electronic waste in emerging countries: Current scenario of generation, policies, and recycling technologies regarding the coronavirus pandemic. Int. J. Environ. Sci. Technol. 2024, 21, 1121–1140. [Google Scholar] [CrossRef]
- Li, P.W.; Luo, S.H.; Zhang, L.; Liu, Q.Y.; Wang, Y.K.; Lin, Y.C.; Xu, C.; Guo, J.; Cheali, P.; Xia, X.N. Progress, challenges, and prospects of spent lithium-ion batteries recycling: A review. J. Energy Chem. 2024, 89, 144–171. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Abdullah, M.F.; Dawood, M.K.; Wei, B.; Subramanian, Y.; Azad, A.T.; Nourin, S.; Afroze, S.; Taweekun, J.; Azad, A.K. Innovative lithium-ion battery recycling: Sustainable process for recovery of critical materials from lithium-ion batteries. J. Energy Storage 2023, 67, 107551. [Google Scholar] [CrossRef]
- Sun, S.; Jin, C.; He, W.; Li, G.; Zhu, H.; Huang, J. A review on management of waste three-way catalysts and strategies for recovery of platinum group metals from them. J. Environ. Manag. 2022, 305, 114383. [Google Scholar] [CrossRef]
- Xolo, L.; Moleko-Boyce, P.; Makelane, H.; Faleni, N.; Tshentu, Z.R. Status of recovery of strategic metals from spent secondary products. Minerals 2021, 11, 673. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Kaya, M. Recovery of lithium from secondary resources. Chem. Eng. Prog. 2024, 120, 55. [Google Scholar]
- Hagelüken, B.C. Recycling the platinum group metals: A european perspective. Platin. Met. Rev. 2012, 56, 29–35. [Google Scholar] [CrossRef]
- Yakoumis, I.; Moschovi, A.M.; Giannopoulou, I.; Panias, D. Real life experimental determination of platinum group metals content in automotive catalytic converters. IOP Conf. Ser. Mater. Sci. Eng. 2018, 329, 012009. [Google Scholar] [CrossRef]
- Korkmaz, K.; Alemrajabi, M.; Rasmuson, Å.; Forsberg, K. Recoveries of valuable metals from spent nickel metal hydride vehicle batteries via sulfation, selective roasting, and water leaching. J. Sustain. Metall. 2018, 4, 313–325. [Google Scholar] [CrossRef]
- Meshram, P.; Somani, H.; Pandey, B.D.; Mankhand, T.R.; Deveci, H. Two stage leaching process for selective metal extraction from spent nickel metal hydride batteries. J. Clean. Prod. 2017, 157, 322–332. [Google Scholar] [CrossRef]
- El Ouardi, Y.; Virolainen, S.; Massima Mouele, E.S.; Laatikainen, M.; Repo, E.; Laatikainen, K. The recent progress of ion exchange for the separation of rare earths from secondary resources – A review. Hydrometallurgy 2023, 218, 106047. [Google Scholar] [CrossRef]
- Firdaus, M.; Rhamdhani, M.A.; Durandet, Y.; Rankin, W.J.; McGregor, K. Review of high-temperature recovery of rare earth (Nd/Dy) from magnet waste. J. Sustain. Metall. 2016, 2, 276–295. [Google Scholar] [CrossRef]
- Holuszko, M.E.; Kumar, A.; Espinosa, D.C.R. Electronic Waste: Recycling and Reprocessing for a Sustainable Future; Wiley-VCH GmbH: Weinheim, Germany, 2022. [Google Scholar]
- Menad, N.; Kana, N.; Kanari, N.; Pereira, F.; Seron, A. Process for enhancing the valuable metal recovery from “electric arc furnace” (EAF) slags. Waste Biomass Valorization 2021, 12, 5187–5200. [Google Scholar] [CrossRef]
- Vuppaladadiyam, S.S.V.; Thomas, B.S.; Kundu, C.; Vuppaladadiyam, A.K.; Duan, H.; Bhattacharya, S. Can e-waste recycling provide a solution to the scarcity of rare earth metals? An overview of e-waste recycling methods. Sci. Total Environ. 2024, 924, 171453. [Google Scholar] [CrossRef] [PubMed]
- Tawonezvi, T.; Nomnqa, M.; Petrik, L.; Bladergroen, B.J. Recovery and recycling of valuable metals from spent lithium-ion batteries: A comprehensive review and analysis. Energies 2023, 16, 1365. [Google Scholar] [CrossRef]
- Critical Raw Materials Resilience: Charting a Path Towards Greater Security and Sustainability. Available online: https://www.eesc.europa.eu/en/our-work/opinions-information-reports/opinions/critical-raw-materials-resilience-charting-path-towards-greater-security-and-sustainability (accessed on 24 March 2021).
- Generation of Electronic Waste Worldwide in the Years from 2014 to 2019 and a Forecast Until 2030. Available online: https://de.statista.com/statistik/daten/studie/792541/umfrage/erzeugung-von-elektroschrott-weltweit/ (accessed on 31 July 2020).
- Latacz, D.; Diaz, F.; Birich, A.; Flerus, B.; Friedrich, B. WEEE recycling at IME – RWTH Aachen: From basic metal recovery to resource efficiency. World Metall. Erzmetall 2020, 3, 155–162. [Google Scholar]
- Ji, R.; Liu, T.-J.; Kang, L.-L.; Wang, Y.-T.; Li, J.-G.; Wang, F.-P.; Yu, Q.; Wang, X.-M.; Liu, H.; Guo, H.-W.; et al. A review of metallurgical slag for efficient wastewater treatment: Pretreatment, performance and mechanism. J. Clean. Prod. 2022, 380, 135076. [Google Scholar] [CrossRef]
- Rachmawati, C.; Weiss, J.; Lucas, H.I.; Löwer, E.; Leißner, T.; Ebert, D.; Möckel, R.; Friedrich, B.; Peuker, U.A. Characterisation of the grain morphology of artificial minerals (EnAMs) in lithium slags by correlating multi-dimensional 2D and 3D methods. Minerals 2024, 14, 130. [Google Scholar] [CrossRef]
- Schirmer, T.; Hiller, J.; Weiss, J.; Munchen, D.; Lucas, H.; Fittschen, U.E.A.; Friedrich, B. Behavior of tantalum in a Fe-Dominated synthetic fayalitic slag system—Phase analysis and incorporation. Minerals 2024, 14, 262. [Google Scholar] [CrossRef]
- Javadi, M.; Rachmawati, C.; Wollmann, A.; Weiss, J.; Lucas, H.; Möckel, R.; Friedrich, B.; Peuker, U.; Weber, A.P. Enhancing lithium recovery from slag through dry forced triboelectric separation: A sustainable recycling approach. Minerals 2024, 14, 1254. [Google Scholar] [CrossRef]
- Dohrn, R.; Fonseca, J.M.S.; Peper, S. Experimental methods for phase equilibria at high pressures. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 343–367. [Google Scholar] [CrossRef]
- Xu, K.; Zhan, C.C.; Lou, M.; Xiao, X.L.; Zhou, R.A.; Wang, F.M.; Hu, X.F.; Yuan, Y.; Chang, K.K. Design of the rare-earth-containing materials based on the micro-alloying phase equilibria, phase diagrams and phase transformations. J. Mater. Sci. Technol. 2023, 151, 119–149. [Google Scholar] [CrossRef]
- Dimian, A.C.; Bildea, C.S.; Kiss, A.A. Phase equilibria. Integr. Des. Simul. Chem. Process 2014, 35, 201–251. [Google Scholar]
- Lv, N.N.; Qiu, Y.C.; Hu, Y.M.; Shi, H.K.; Shi, J.J.; Ding, X.; Li, H.L. Equilibrium phase relationships of CaO-SiO2-TiO2 system with 5 wt% Cr2O3 addition for titania-bearing slag recycling. J. Sustain. Metall. 2023, 9, 1303–1314. [Google Scholar] [CrossRef]
- Shi, J.J.; Chen, M.; Wan, X.B.; Taskinen, P.; Jokilaakso, A. Phase equilibrium study of the CaO-SiO2-MgO-Al2O3-TiO2 system at 1300 °C and 1400 °C in Air. JOM 2020, 72, 3204–3212. [Google Scholar] [CrossRef]
- Chen, M.; Shi, J.J.; Taskinen, P.; Jokilaakso, A. Phase equilibria of the CaO-SiO2-TiO2-Al2O3-MgO system in air at 1250–1400 °C. Ceram. Int. 2020, 46, 27702–27710. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, R.Z. Phase equilibria of TiO2-SiO2-CaO-10% Al2O3-7.5% MgO at 1773 K and effects of MgO on the distribution of phase fields. Ceram. Int. 2024, 50, 29975–29986. [Google Scholar] [CrossRef]
- Kaußen, F.; Friedrich, B. Reductive smelting of red mud for iron recovery. Chem. Ing. Tech. 2015, 87, 1535–1542. [Google Scholar] [CrossRef]
- Wan, X.; Shi, J.; Klemettinen, L.; Chen, M.; Taskinen, P.; Jokilaakso, A. Equilibrium phase relations of CaO–SiO2–TiO2 system at 1400 °C and oxygen partial pressure of 10−10 atm. J. Alloys Compd. 2020, 847, 156472. [Google Scholar] [CrossRef]
- Shi, J.J.; Qiu, Y.C.; Wan, X.B.; Yu, B.; Chen, M.; Zhao, F.; Li, J.Z.; Liu, C.S.; Taskinen, P. Equilibrium Phase Relations of the CaO-SiO2-Ti3O5 System at 1400 °C and a p(O2) of 10−16 atm. JOM 2022, 74, 668–675. [Google Scholar] [CrossRef]
- Wan, X.; Chen, M.; Qiu, Y.; Shi, J.; Li, J.; Liu, C.; Taskinen, P.; Jokilaakso, A. Influence of manganese oxide on the liquid-perovskite equilibrium in the CaO–SiO2–TiO2 system at 1400 °C in air. Ceram. Int. 2021, 47, 11176–11182. [Google Scholar] [CrossRef]
- Wittkowski, A.; Schirmer, T.; Qiu, H.; Goldmann, D.; Fittschen, U.E.A. Speciation of manganese in a synthetic recycling slag relevant for lithium recycling from lithium-ion batteries. Metals 2021, 11, 188. [Google Scholar] [CrossRef]
- Fittschen, U.E.A.; Hampel, S.; Schirmer, T.; Merkert, N. Multimodal spectroscopy and molecular dynamic simulations to understand redox-chemistry and compound formation in pyrometallurgical slags: Example of manganese oxidation state with respect to lithium recycling. Appl. Spectrosc. Rev. 2024, 59, 780–797. [Google Scholar] [CrossRef]
- Schirmer, T.; Qiu, H.; Li, H.; Goldmann, D.; Fischlschweiger, M. Li-distribution in compounds of the Li2O-MgO-Al2O3-SiO2-CaO System—A first survey. Metals 2020, 10, 1633. [Google Scholar] [CrossRef]
- Qiu, H.; Li, H.; Fischlschweiger, M.; Ranneberg, M.; Graupner, T.; Lucas, H.; Stallmeister, C.; Friedrich, B.; Yagmurlu, B.; Goldmann, D. Valorization of lithium containing slags from pyrometallurgical recycling route of spent lithium-ion batteries: The enrichment of γ-LiAlO2 phase from thermodynamic controlled and modified slags. Miner. Eng. 2024, 217, 108918. [Google Scholar] [CrossRef]
- Li, Y.; Yan, B. Phase equilibria relationship in the FetO-TiO2-CaO-SiO2 system with CaO/SiO2 weight ratio of 12 at, 1673 K. Calphad 2024, 87, 102768. [Google Scholar] [CrossRef]
- Ma, H.; Jiao, K.; Zhang, J.; Zong, Y.; Zhang, J.; Meng, S. Viscosity of CaO-MgO-Al2O3-SiO2-TiO2-FeO slag with varying TiO2 content: The Effect of Crystallization on Viscosity Abrupt Behavior. Ceram. Int. 2021, 47, 17445–17454. [Google Scholar] [CrossRef]
- Lan, X.; Gao, J.T.; Li, Y.; Guo, Z.C. Phase equilibria of CaO-SiO2-CaF2-P2O5-Ce2O3 system and formation mechanism of britholite. Ceram. Int. 2021, 47, 11966–11972. [Google Scholar] [CrossRef]
- Coquerel, G. Phase diagrams for process design. In Engineering Crystallography: From Molecule to Crystal to Functional Form; Roberts, K.J., Docherty, R., Tamura, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 215–233. [Google Scholar]
- Fan, Y.; Shibata, E.; Iizuka, A.; Nakamura, T. Crystallization behaviors of copper smelter slag studied using time-temperature-transformation diagram. Mater. Trans. 2014, 55, 958–963. [Google Scholar] [CrossRef]
- Weiss, J.; Munchen, D.; Richter, S.; Friedrich, B. Crystallization study of a synthetic fayalitic slag system with ta based on thermochemical modeling. In Proceedings of the 63rd Conference of Metallurgists, COM 2024, Toronto, ON, Canada, 21–24 August 2024; Springer Nature: Cham, Switzerland, 2025; pp. 1361–1368. [Google Scholar]
- Wu, S.-w.; Zhang, Y.-l.; Zhang, S. Chromium enrichment in different crystalline phases of Cr-containing slag under various basicities and equilibrium temperatures. J. Iron Steel Res. Int. 2022, 29, 1412–1422. [Google Scholar] [CrossRef]
- Gibson, C.E.; Kelebek, S.; Aghamirian, M. Niobium oxide mineral flotation: A review of relevant literature and the current state of industrial operations. Int. J. Miner. Process. 2015, 137, 82–97. [Google Scholar] [CrossRef]
- Qi, H.; Gong, N.; Zhang, S.-Q.; Li, J.; Yuan, G.-L.; Liu, X.-F. Research progress on the enrichment of gallium in bauxite. Ore Geol. Rev. 2023, 160, 105609. [Google Scholar] [CrossRef]
- Guan, T.; Guo, M.; Wang, L.; Liu, J. Production and recycling of the cutting edge material of gallium: A review. Sci. Total Environ. 2025, 971, 179046. [Google Scholar] [CrossRef]
- Mollo, S.; Hammer, J.E. Dynamic crystallization in magmas. In Mineral Reaction Kinetics: Microstructures, Textures, Chemical and Isotopic Signatures; Heinrich, W., Abart, R., Eds.; European Mineralogical Union and the Mineralogical Society of Great Britain & Ireland: London, UK, 2017; Volume 16, pp. 373–412. [Google Scholar]
- Encyclopedia of Geochemistry: A comprehensive reference source on the chemistry of the earth. In Encyclopedia of Geochemistry: A Comprehensive Reference Source on the Chemistry of the Earth; White, W.M., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–1557. [Google Scholar]
- Vance, D.; Little, S.H. The history, relevance, and applications of the periodic system in geochemistry. In Periodic Table I: Historical Development and Essential Features; Mingos, D.M.P., Ed.; Springer International Publishing: Cham, Switzerland, 2019; Volume 181, pp. 111–156. [Google Scholar]
- Lei, Y.; Sun, F.; Zhao, Z. Interdisciplinarity of geochemistry and extractive metallurgy- Taking platinum group metals and gold as examples. Chin. J. Nonferrous Met. 2023, 33, 2957–2974. [Google Scholar]
- Sun, F.L.; Zhao, Z.W. An interdisciplinary perspective from the earth scientist’s periodic table: Similarity and connection between geochemistry and metallurgy. Engineering 2020, 6, 707–715. [Google Scholar] [CrossRef]
- Volodin, V.; Trebukhov, S.; Nitsenko, A.; Linnik, X.; Tuleutay, F.; Trebukhov, A.; Ruzakhunova, G. Pyrometallurgical scheme intended to process arsenic-containing concentrates with recovery of precious metals. Metals 2023, 13, 540. [Google Scholar] [CrossRef]
- Yu, B.Q.; Kou, J.; Sun, C.B.; Sun, F.; Gui, C.L.; Liu, J. Development of special magnetic separator to recover precious metal-bearing alloy from high-grade nickel matte and its magnetic field simulation. Min. Metall. Explor. 2022, 39, 1687–1692. [Google Scholar] [CrossRef]
- Li, Z.C.; Tian, Q.H.; Wang, Q.M.; Guo, X.Y. Recovery of copper, lead and zinc from copper flash converting slag by the sulfurization-reduction process. JOM 2023, 75, 1107–1118. [Google Scholar] [CrossRef]
- Liao, Y.L.; Ji, G.X.; Shi, G.C.; Xi, J.J. A study on the selective leaching of valuable metals and the configuration of iron silicon phases in copper smelting slag by oxidative pressure leaching. J. Sustain. Metall. 2021, 7, 1143–1153. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Zhang, J.; Li, G. Oxidation kinetics and oxygen capacity of ti-bearing blast furnace slag under dynamic oxidation conditions. Metals 2016, 6, 105. [Google Scholar] [CrossRef]
- Huang, L.; An, S.; Zhang, F.; Peng, J.; Chen, Y.; Ping, X.; Liu, C. Synergistic treatment of blast furnace slag and basic oxygen furnace slag for efficient recovery of iron: Phase transformation and oxidation mechanisms. J. Mater. Res. Technol. 2024, 28, 2347–2362. [Google Scholar] [CrossRef]
- Long, T.V.; Palacios, J.; Sanches, M. Recovery of molybdenum from copper slag. Tetsu Hagane-J. Iron Steel Inst. Jpn. 2012, 98, 48–54. [Google Scholar] [CrossRef]
- Fan, H.R.; Niu, H.C.; Li, X.C.; Yang, K.F.; Yang, Z.F.; Wang, Q.W. The types, ore genesis and resource perspective of endogenic REE deposits in China. Chin. Sci. Bull.-Chin. 2020, 65, 3778–3793. [Google Scholar] [CrossRef]
- Lobach-Zhuchenko, S.B.; Baltybaev, S.K.; Egorova, Y.S.; Sergeev, S.A.; Kaulina, T.; Saltykova, T.E. Stages of paleoarchean to paleoproterozoic basic-ultrabasic magmatism in the sarmatian craton. Russ. Geol. Geophys. 2022, 63, 225–244. [Google Scholar] [CrossRef]
- Raza, M.; Giebel, R.J.; Staude, S.; Beranoaguirre, A.; Kolb, J.; Markl, G.; Walter, B.F. The magmatic to post-magmatic evolution of the Nooitgedacht Carbonatite Complex, South Africa. Geochemistry 2025, 85, 126249. [Google Scholar] [CrossRef]
- Emad, B.M. Alkaline igneous rocks, a potential source of rare metals and radioactive minerals: Case study at Amreit area, south Eastern Desert, Egypt. Acta Geochim. 2025, 44, 189–214. [Google Scholar] [CrossRef]
- Xi, Z.-C.; Qiu, K.-F.; Zhi, C.-L.; Li, S.-S.; Shang, Z.; Huang, Y.-Q. Partial melting of lithospheric mantle and formation of the early cretaceous alkaline rocks in the Guandimiao ree deposit, Luxi Terrane, Eastern China. Minerals 2022, 12, 670. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Vasyukova, O.V. Niobium, critical metal, and progeny of the mantle. Econ. Geol. 2023, 118, 837–856. [Google Scholar] [CrossRef]
- Yong, T.; Linnen, R.L.; McNeil, A.G. An experimental study of pyrochlore solubility in peralkaline granitic melts. Econ. Geol. 2023, 118, 209–223. [Google Scholar] [CrossRef]
- Han, W.S.; Ran, M.J.; Lua, X.; Jiang, C.L.; Chen, W. The effects of multielement basicity on niobium-bearing phase reconstruction in low grade niobium rougher concentrate. Miner. Eng. 2024, 217, 108951. [Google Scholar] [CrossRef]
- Zurevinski, S.E.; Mitchell, R.H. Extreme compositional variation of pyrochlore-group minerals at the oka carbonatite complex, quebec: Evidence of magma mixing? Can. Mineral. 2004, 42, 1159–1168. [Google Scholar] [CrossRef]
- Yaroshevskii, A.A.; Bagdasarov, Y.A. Geochemical diversity of minerals of the pyrochlore group. Geochem. Int. 2009, 46, 1245–1266. [Google Scholar] [CrossRef]
- Han, W.; Ran, M.; Lu, X.; Jiang, C.; Zhou, Y.; Chen, W. Effects of oxygen on the phase reconstruction of low-grade complicated niobium resources during cooling process. JOM 2025, 77, 6443–6453. [Google Scholar] [CrossRef]
- Wu, B.; Hu, Y.-Q.; Bonnetti, C.; Xu, C.; Wang, R.-C.; Zhang, Z.-S.; Li, Z.-Y.; Yin, R. Hydrothermal alteration of pyrochlore group minerals from the Miaoya carbonatite complex, central China and its implications for Nb mineralization. Ore Geol. Rev. 2021, 132, 104059. [Google Scholar] [CrossRef]
- Han, W.; Ran, M.; Lu, X.; Chen, W. Study on the transformation and growth behavior of niobium-bearing phase in slag during cooling process. In Proceedings of the Asia Steel 2024 Conference, Changsha, China, 4–7 September 2024. [Google Scholar]
- Fan, H.-R.; Yang, K.-F.; Hu, F.-F.; Liu, S.; Wang, K.-Y. The giant Bayan Obo REE-Nb-Fe deposit, China: Controversy and ore genesis. Geosci. Front. 2016, 7, 335–344. [Google Scholar] [CrossRef]
- Liu, S.; Ding, L.; Fan, H.-R.; Yang, K.-F.; Tang, Y.-W.; She, H.-D.; Hao, M.-Z. Hydrothermal genesis of Nb mineralization in the giant Bayan Obo REE-Nb-Fe deposit (China): Implicated by petrography and geochemistry of Nb-bearing minerals. Precambrian Res. 2020, 348, 105864. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, X.; Yang, X.; Ling, M.; Liu, Y. Mineralogical study on the distribution regularity of niobium in various types of ores in the giant Bayan Obo Fe-REE-Nb deposit. Ore Geol. Rev. 2023, 161, 105602. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, X.; Wang, X. Occurrence of niobium in biotite-type Fe-REE-Nb ore in the Bayan Obo deposit. Solid Earth Sci. 2023, 8, 25–28. [Google Scholar] [CrossRef]
- Yang, K.-F.; Fan, H.-R.; Santosh, M.; Hu, F.-F.; Wang, K.-Y. Mesoproterozoic carbonatitic magmatism in the Bayan Obo deposit, Inner Mongolia, North China: Constraints for the mechanism of super accumulation of rare earth elements. Ore Geol. Rev. 2011, 40, 122–131. [Google Scholar] [CrossRef]
- Congzhong, T.; Wenjie, Z.; Fengyang, L.; Yuxin, L.; Zhijun, Z.; Zhengyao, L. A comprehensive review on recent progress in beneficiation of Nb-bearing minerals /Nb ores. Miner. Eng. 2024, 212, 108710. [Google Scholar] [CrossRef]
- Liu, C.J.; Qiu, J.Y.; Liu, Z.Y. Phase equilibria in the system CaO-SiO2-La2O3-Nb2O5 at 1400 °C. Metals 2021, 11, 1892. [Google Scholar] [CrossRef]
- Liu, C.; Qiu, J.; Liu, Z.; Zhu, D.; Wang, Y.; Jiang, M. Adjacent relations of primary phase fields and invariant reactions of the system CaO-SiO2-Nb2O5-La2O3. J. Am. Ceram. Soc. 2021, 104, 2398–2409. [Google Scholar] [CrossRef]
- Qiu, J.Y.; Liu, C.J.; Liu, Z.Y.; Zheu, D.Y. Isothermal phase diagram of CaO-SiO2-Nb2O5-La2O3 system at 1300 °C, 1200 °C. Ceram. Int. 2020, 46, 4832–4842. [Google Scholar] [CrossRef]
- Qiu, J.Y.; Liu, C.J.; Liu, Z.Y.; Zhu, D.Y. Liquidus phase diagram of CaO-SiO2-La2O3-Nb2O5 system with w(La2O3)=15 to 25 Pct. Metall. Mater. Trans. B-Process Metall. Mater. Process. Sci. 2020, 51, 1190–1200. [Google Scholar] [CrossRef]
- Qiu, J.Y.; Liu, C.J.; Liu, Z.Y.; Yu, Z. Phase equilibria in low basicity region of CaO-SiO2-Nb2O5-(5 wt%, 10 wt%, 15 wt%) La2O3 system. J. Rare Earths 2020, 38, 100–107. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Z.; Jiang, M. Phase equilibria of CaO-SiO2-La2O3-Nb2O5 system in reducing atmosphere. Metals 2022, 12, 768. [Google Scholar] [CrossRef]
- Liu, C.J.; Qiu, J.Y.; Liu, Z.Y.; Zhu, D.Y.; Wang, Y.G. Phase equilibria in the system CaO-SiO2-Nb2O5-La2O3 at 1473 K with pO2=10-15.47 atm. Ceram. Int. 2020, 46, 7711–7718. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Sun, L.F.; Zhao, H.; Xiang, T.Y.; Qiu, J.Y.; Jiang, M.F. Isothermal phase diagram of CaO-SiO2-Nb2O5-5wt% Fe2O3-TiO2 system at 1200 °C. Ceram. Int. 2022, 48, 31636–31651. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, L.; Pan, X.; Jiang, M. Isothermal phase diagram of CaO-SiO2-Nb2O5-5wt% Fe2O3-TiO2 system at 1100 °C. J. Phase Equilibria Diffus. 2024, 45, 849–862. [Google Scholar] [CrossRef]
- Zhang, B.; Ma, C.; Wang, R.; Liu, C.; Jiang, M. Selective smelting reduction of metal oxides in REE-Nb-Fe deposit. JOM 2022, 74, 993–1001. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, J.; Jia, X.; Xing, T.; Liu, C.; Jiang, M. Mineral phase reconstruction of Bayan Obo tailings by smelting reduction and crystallization control of molten slag containing niobium, rare earth and titanium. Miner. Eng. 2024, 214, 108780. [Google Scholar] [CrossRef]
- Jia, X.-L.; Zhang, B.; Cui, L.; Liu, C.-J.; Jiang, M.-F. Separation and enrichment of valuable elements from slag containing rare earth, niobium and titanium via Fe–Si bath smelting reduction. J. Iron Steel Res. Int. 2024, 32, 1990–2000. [Google Scholar] [CrossRef]
- Beyer, C.; Berndt, J.; Tappe, S.; Klemme, S. Trace element partitioning between perovskite and kimberlite to carbonatite melt: New experimental constraints. Chem. Geol. 2013, 353, 132–139. [Google Scholar] [CrossRef]
- Han, W.; Ran, M.; Lu, X.; Chen, W. Growth behaviors of calciobetafite during phase reconstruction process of Bayan Obo niobium rougher concentrate. J. Cent. South Univ. (Sci. Technol.) 2025, 56, 468–477. [Google Scholar]
- Roy, J.J.; Rarotra, S.; Krikstolaityte, V.; Zhuoran, K.W.; Cindy, Y.D.I.; Tan, X.Y.; Carboni, M.; Meyer, D.; Yan, Q.Y.; Srinivasan, M. Green recycling methods to treat lithium-ion batteries e-waste: A circular approach to sustainability. Adv. Mater. 2022, 34, 2103346. [Google Scholar] [CrossRef]
- Rautela, R.; Yadav, B.R.; Kumar, S. A review on technologies for recovery of metals from waste lithium-ion batteries. J. Power Sources 2023, 580, 233428. [Google Scholar] [CrossRef]
- Liu, C.; Lin, J.; Cao, H.; Zhang, Y.; Sun, Z. Recycling of spent lithium-ion batteries in view of lithium recovery: A critical review. J. Clean. Prod. 2019, 228, 801–813. [Google Scholar] [CrossRef]
- Schirmer, T.; Qiu, H.; Goldmann, D.; Stallmeister, C.; Friedrich, B. Influence of P and Ti on phase formation at solidification of synthetic slag containing Li, Zr, La, and Ta. Minerals 2022, 12, 310. [Google Scholar] [CrossRef]
- Hampel, S.; Alhafez, I.A.; Schirmer, T.; Merkert, N.; Wunderlich, S.; Schnickmann, A.; Li, H.; Fischlschweiger, M.; Fittschen, U.E.A. Engineering compounds for the recovery of critical elements from slags: Melt characteristics of Li5AlO4, LiAlO2, and LiAl5O8. ACS Omega 2024, 9, 24584–24592. [Google Scholar] [CrossRef]
- Strube, F.; Guy, B.M.; Pereira, L.; Ebert, D.; Zgheib, A.; Fischer, M.; Möckel, R.; Schmidt, A.; Rudolph, M. Batch flotation of lithium-bearing slag—A special focus on the phase properties of engineered artificial minerals for enhancing the recycling of end-of-life lithium-ion batteries. Minerals 2025, 15, 334. [Google Scholar] [CrossRef]
- Abreu, D.A.d.; Schnickmann, A.; Chakrabarty, S.; Fischlschweiger, M.J.; Schirmer, T.; Fabrichnaya, O. Stability of crystalline compounds in slag systems mainly composed of Li2O-SiO2-CaO-MnOx. JOM 2024, 76, 6472–6486. [Google Scholar] [CrossRef]
- Acker, S.; Namyslo, J.C.; Rudolph, M.; Strube, F.; Fittschen, U.E.A.; Qiu, H.; Goldmann, D.; Schmidt, A. Polyether-tethered imidazole-2-thiones, imidazole-2-selenones and imidazolium salts as collectors for the flotation of lithium aluminate and spodumene. RSC Adv. 2023, 13, 6593–6605. [Google Scholar] [CrossRef]
- Zakeri, A.; Tafaghodi, L. A review of the current progress in high-temperature recycling strategies for recovery of rare-earth elements from magnet waste. J. Sustain. Metall. 2025, 11, 88–113. [Google Scholar] [CrossRef]
- Gao, J.T.; Xue, K.R.; Lan, X.; Guo, Z.C. Phase equilibrium relationship of CaO-SiO2-La2O3 system and crystallization behaviors of RE phases. JOM 2023, 75, 3521–3531. [Google Scholar] [CrossRef]
- Gao, J.T.; Xu, H.H.; Lan, X.; Guo, Z.C. Phase equilibrium of the CaO-SiO2-Al2O3-Ce2O3 system: A basis for recovering REEs from RE-Containing slag. Ceram. Int. 2022, 48, 34907–34914. [Google Scholar] [CrossRef]
- Li, M.C.; Li, R.S.; Zhang, T.S. Phase equilibria of SiO2-Ce2O3-CaO-25 wt% Al2O3 system at 1673 K-1773 K. Ceram. Int. 2022, 48, 31614–31626. [Google Scholar] [CrossRef]
- Lan, X.; Gao, J.T.; Li, Y.; Guo, Z.C. Thermodynamics and kinetics of REEs in CaO-SiO2-CaF2-Ce2O3 system: A theoretical basis toward sustainable utilization of REEs in REE-Bearing slag. Ceram. Int. 2021, 47, 6130–6138. [Google Scholar] [CrossRef]
- Shi, J.J.; Zhai, Y.M.; Qiu, Y.C.; Jiang, C.L.; Hou, C.L.; Dong, J.J.; Li, J.Z. Phase equilibria of the CaO-SiO2-CeO2-Al2O3-MgOsystem at 1300 °C, 1400 °C. JOM 2024, 76, 4598–4607. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, Z.W.; Guo, W.T.; Guo, X.Q. Effect of TiO2 addition cooling rate on crystallization behavior of separated slag containing low-grade, R.E. ISIJ Int. 2023, 63, 1274–1280. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, Z.W.; Guo, X.Q.; Guo, W.T. Selective precipitation and non-isothermal crystallization kinetics of britholite from low grade REE-bearing slag. J. Rare Earths 2023, 41, 1812–1818. [Google Scholar] [CrossRef]
- Zhao, F.H.; Deng, Y.C.; Xin, W.B.; Zhang, J.; Jiang, Y.J.; Cao, Z.J.; Wang, L.Y. Investigation of the effect of P2O5 and CaF2 on the crystallization behavior of La2O3-bearing calcium-silicate-aluminum slags using the single hot thermocouple technique. Metall. Res. Technol. 2024, 121, 503. [Google Scholar] [CrossRef]
- Guo, W.T.; Shi, K.H.; Liu, X.K.; Sun, Z.L.; Liu, X.J. Effects of phosphorus structures on the crystallization behavior of rare earth phase in CaO-SiO2-CaF2-La2O3 Slag. Trans. Indian Inst. Met. 2024, 77, 697–705. [Google Scholar] [CrossRef]
- Lan, X.; Gao, J.T.; Xue, K.R.; Guo, Z.C. Selective separation and crystal characterization of RE-phases from RE-bearing slag systems: Calcium cerite and cefluosil. J. Am. Ceram. Soc. 2023, 106, 2130–2138. [Google Scholar] [CrossRef]
- Lan, X.; Gao, J.T.; Xu, H.H.; Guo, Z.C. In situ separation and characterization of britholite crystals in RE-bearing slag via supergravity. J. Am. Ceram. Soc. 2022, 105, 5966–5974. [Google Scholar] [CrossRef]
- Lan, X.; Gao, J.T.; Xue, K.R.; Xu, H.H.; Guo, Z.C. A new finding and technology for selective separation of different REEs from CaO-SiO2-CaF2-P2O5-Fe3O4-RE2O3 system. Sep. Purif. Technol. 2022, 293, 121121. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, K.; Jiang, J.; Zhang, S.; Xu, G. Effect of operating parameters on high-temperature selective enrichment and precipitation of titanium component in Ti-bearing blast furnace slag and the precipitation mechanism of perovskite. J. Mater. Res. Technol. 2021, 15, 2686–2696. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.N.; Wang, M.Y.; Li, G.Q.; Sui, Z.T. Precipitation selectivity of perovskite phase from Ti-bearing blast furnace slag under dynamic oxidation conditions. J. Non-Cryst. Solids 2007, 353, 2214–2220. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.N.; Wang, M.Y.; Li, G.Q.; Sui, Z.T. Recovery of titanium compounds from molten Ti-bearing blast furnace slag under the dynamic oxidation condition. Miner. Eng. 2007, 20, 684–693. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, H.-Y.; Zhu, Q.-S. Effects of the continuous cooling process conditions on the crystallization and liberation characteristics of anosovite in Ti-bearing titanomagnetite smelting slag. Int. J. Miner. Metall. Mater. 2019, 26, 1120–1128. [Google Scholar] [CrossRef]
- Ma, G.C.; Tian, J.K.; Shen, Y.Y. Structure and magnetic properties of (Ni,Fe)Fe2O4 derived from nickel slag via molten oxidation. Mater. Today Commun. 2024, 40, 109537. [Google Scholar] [CrossRef]
- Li, B.; Du, X.Y.; Shen, Y.Y.; Zhang, Z.L.; Rong, T.L. Nonisothermal crystallization, growth, and shape control of magnetite crystals in molten nickel slag during continuous cooling. Metall. Mater. Trans. B-Process Metall. Mater. Process. Sci. 2022, 53, 1816–1826. [Google Scholar] [CrossRef]
- Shen, Y.Y.; Chong, J.K.; Huang, Z.N.; Tian, J.K.; Zhang, W.J.; Tang, X.C.; Ding, W.W.; Du, X.Y. Thermodynamics and kinetics of FeO transferred to Fe3O4 in modified nickel slag during molten oxidation process. Mater. Res. Express 2019, 6, 096551. [Google Scholar] [CrossRef]
- Shen, Y.Y.; Huang, Z.N.; Zhang, Y.Y.; Zhong, J.K.; Zhang, W.J.; Yang, Y.; Chen, M.; Du, X.Y. Transfer behavior of Fe element in nickel slag during molten oxidation and magnetic separation processes. Mater. Trans. 2018, 59, 1659–1664. [Google Scholar] [CrossRef]
- Yan, Z.H.; Zhao, Q.; Han, C.Z.; Mei, X.H.; Liu, C.J.; Jiang, M.F. Effects of iron oxide on crystallization behavior and spatial distribution of spinel in stainless steel slag. Int. J. Miner. Metall. Mater. 2024, 31, 292–300. [Google Scholar] [CrossRef]
- Huo, X.T.; Zhang, X.; Ding, Z.H.; Zhang, M.; Guo, M. A clean approach for detoxification of industrial chromium-bearing stainless steel slag: Selective crystallization control and binary basicity effect. J. Hazard. Mater. 2023, 446, 130746. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Zhang, X.-P.; Zhang, J.-B.; Peng, L.-J.; Li, J.-G. Optimizing vanadium converter slag utilization: Targeted enrichment and stabilization of vanadium through non-equilibrium solidification. Mater. Tehnol. 2024, 58, 339–348. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, W.; Weiss, J.; Lu, X.; Munchen, D.; Jiang, C.; Lucas, H.; Ran, M.; Chen, W.; Friedrich, B. Engineered Artificial Minerals (EnAMs): Concept, Design Strategies, and Case Studies. Minerals 2025, 15, 1129. https://doi.org/10.3390/min15111129
Han W, Weiss J, Lu X, Munchen D, Jiang C, Lucas H, Ran M, Chen W, Friedrich B. Engineered Artificial Minerals (EnAMs): Concept, Design Strategies, and Case Studies. Minerals. 2025; 15(11):1129. https://doi.org/10.3390/min15111129
Chicago/Turabian StyleHan, Wensheng, Joao Weiss, Xiang Lu, Daniel Munchen, Chuling Jiang, Hugo Lucas, Mengjie Ran, Wen Chen, and Bernd Friedrich. 2025. "Engineered Artificial Minerals (EnAMs): Concept, Design Strategies, and Case Studies" Minerals 15, no. 11: 1129. https://doi.org/10.3390/min15111129
APA StyleHan, W., Weiss, J., Lu, X., Munchen, D., Jiang, C., Lucas, H., Ran, M., Chen, W., & Friedrich, B. (2025). Engineered Artificial Minerals (EnAMs): Concept, Design Strategies, and Case Studies. Minerals, 15(11), 1129. https://doi.org/10.3390/min15111129






