Enhancement of Arsenic Release from Amorphous Arsenic-Containing Ferric Hydroxides Systems Using Bacterial Reduction: Applicability of Injecting Iron-Reducing Bacteria for Dissolved Arsenic Species and Colloid Phases
Abstract
1. Introduction
2. Materials and Methods
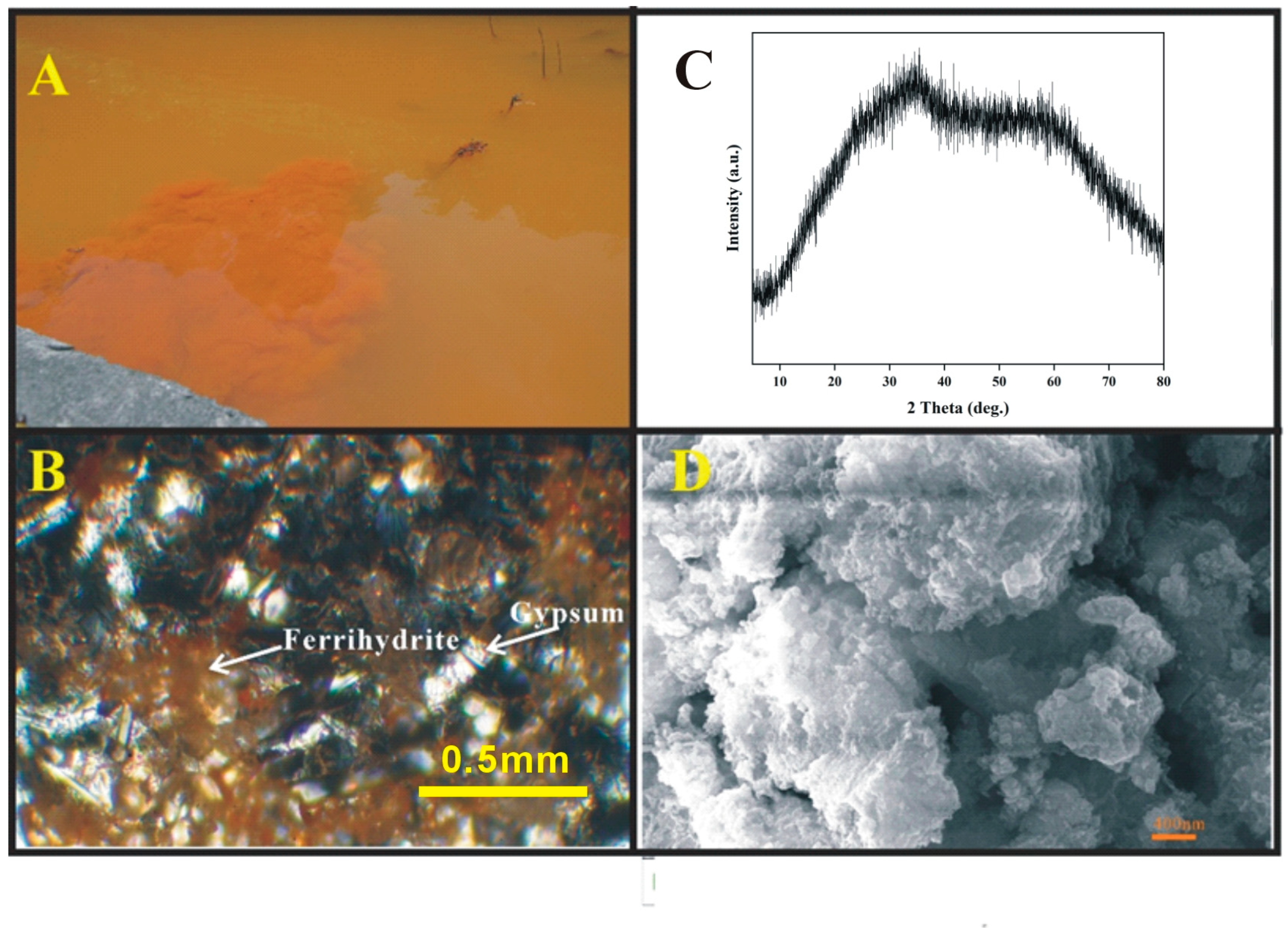
2.1. Sample Collection and Characterization
2.2. Culture of Iron-Reducing Bacteria (A. cryptum JF-5) and Batch Experiment of Enhanced Arsenic Release by Addition of Acetate
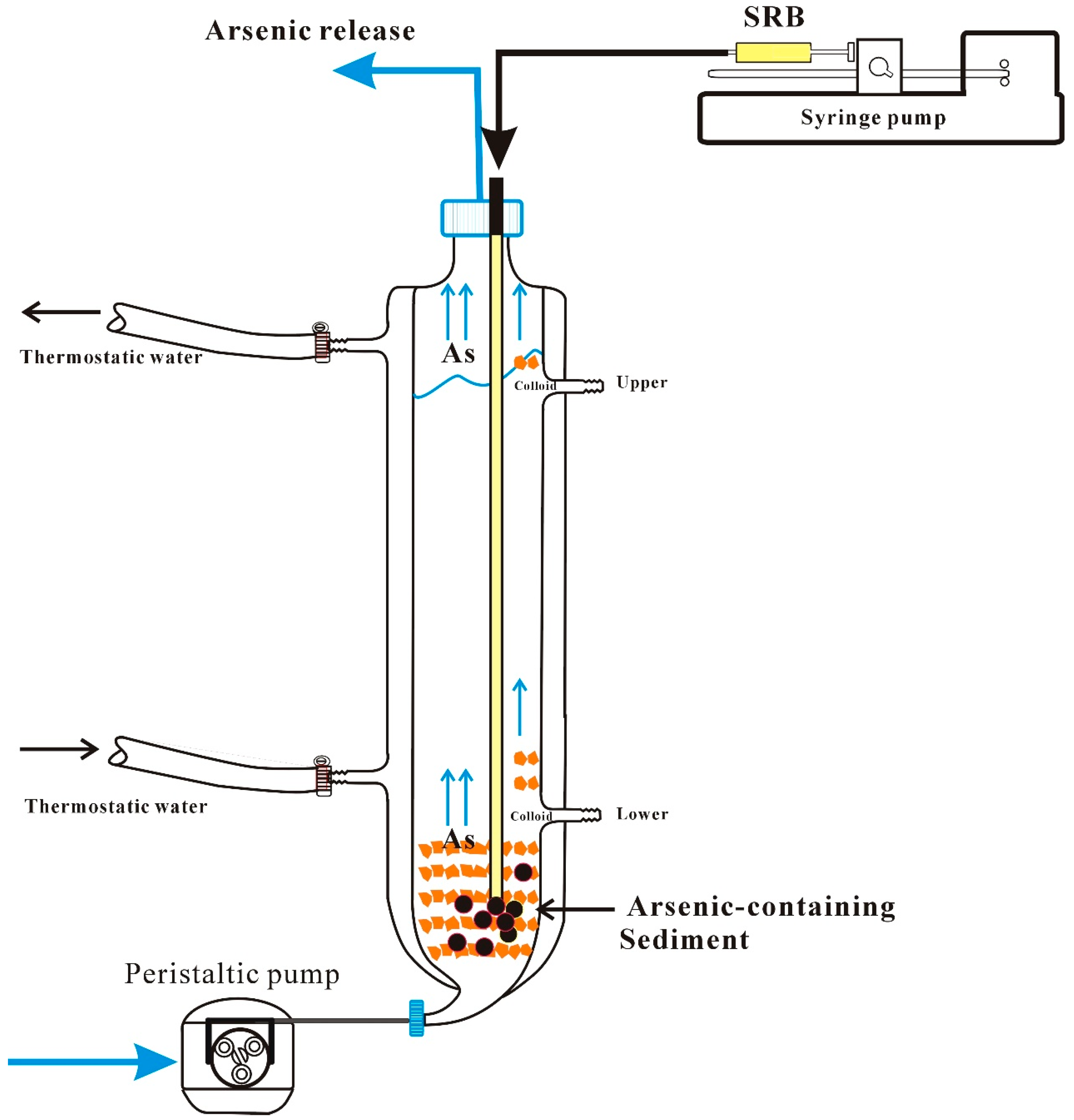
2.3. Arsenic Release from Sediment Column Experiment
2.3.1. Sediment Column
2.3.2. Arsenic Associated with Colloid/Particles
2.4. Characterization of Samples Before and After Interaction with IRB
3. Results and Discussion
3.1. Arsenic-Containing Ferric Hydroxides
3.2. The Effect of Temperature on Arsenic Release
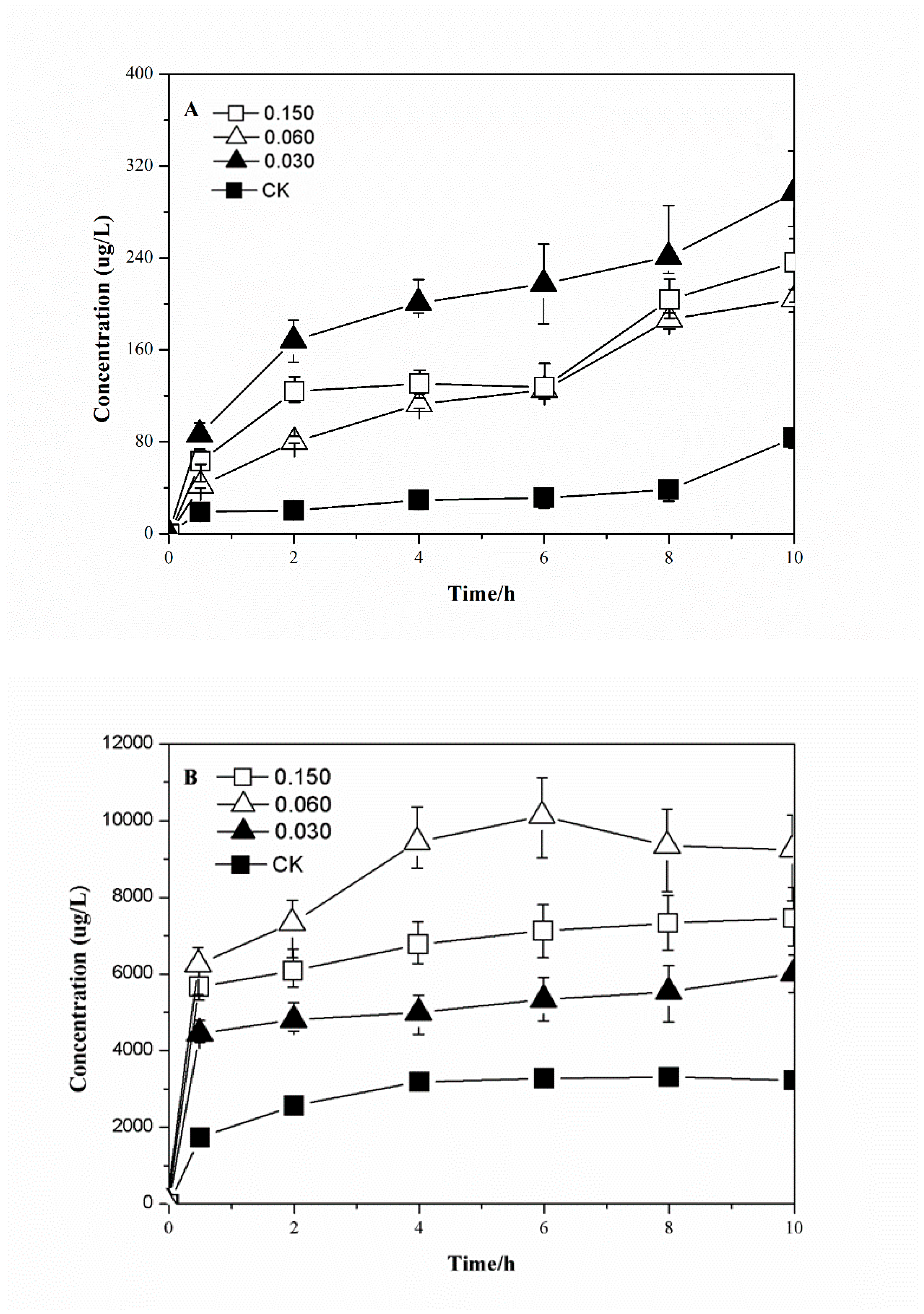
3.3. The Effect of Addition of Acetate on Arsenic Release
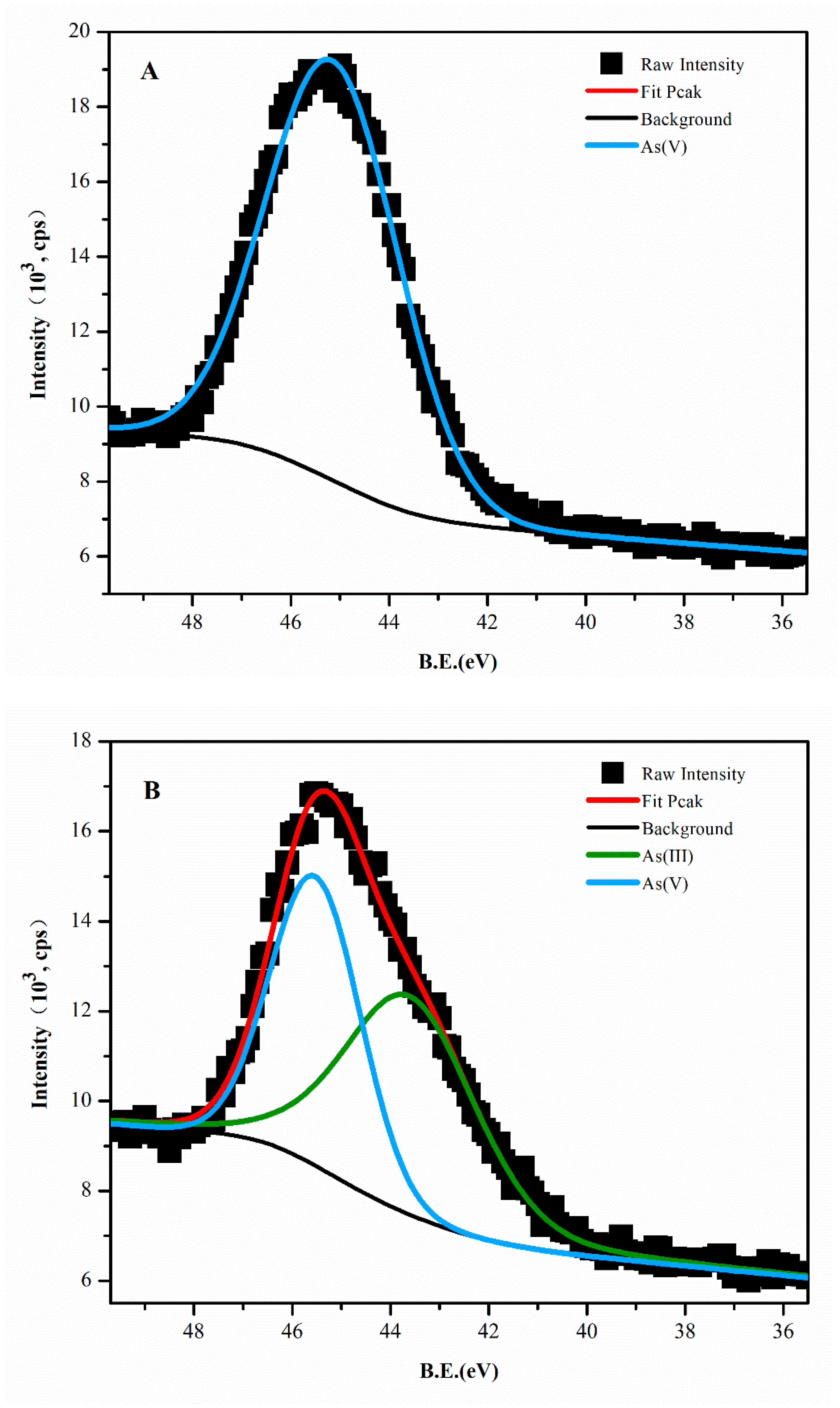
3.4. Comparison of Arsenic Species in Solids Before and After Acted with IRB
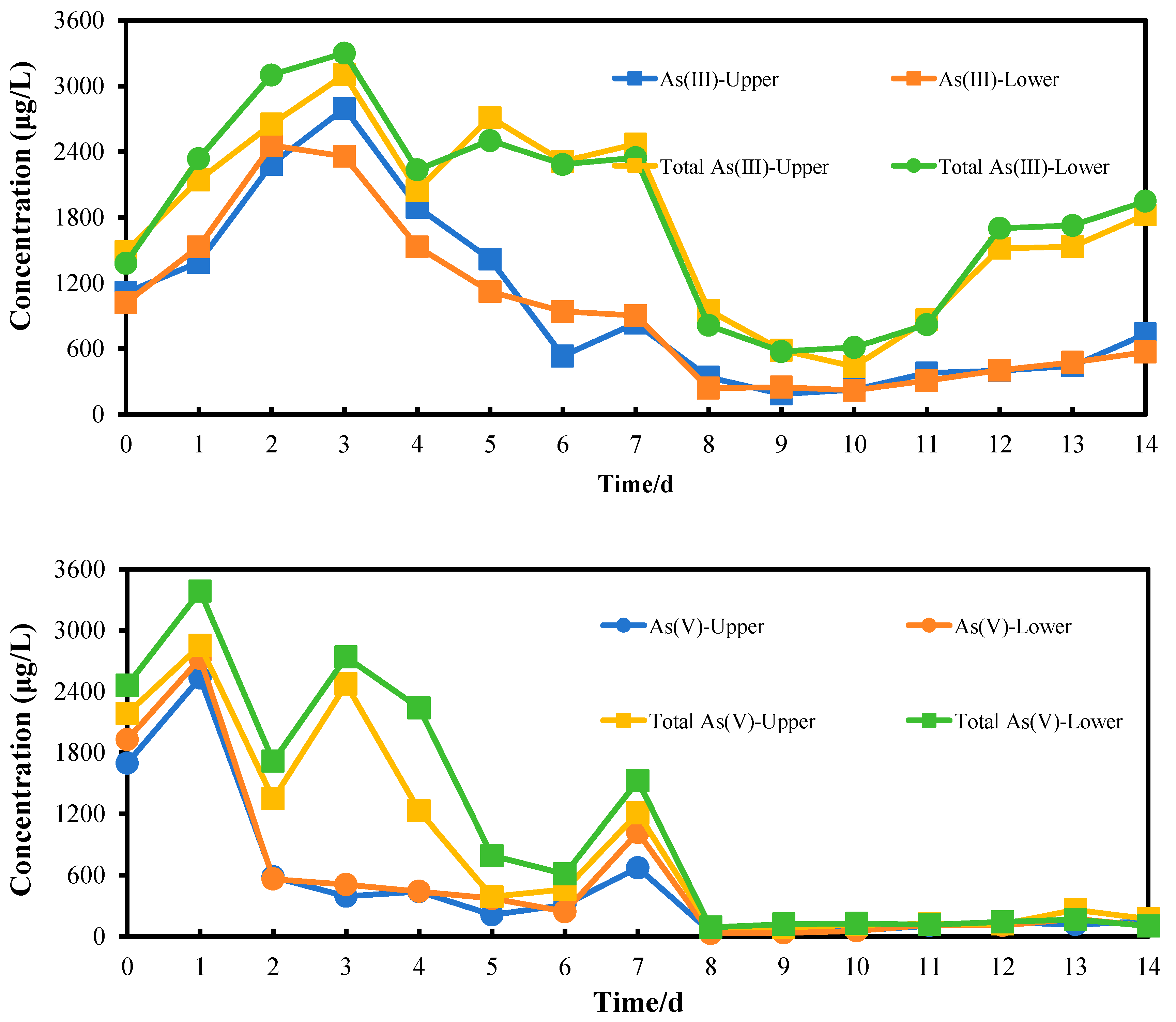
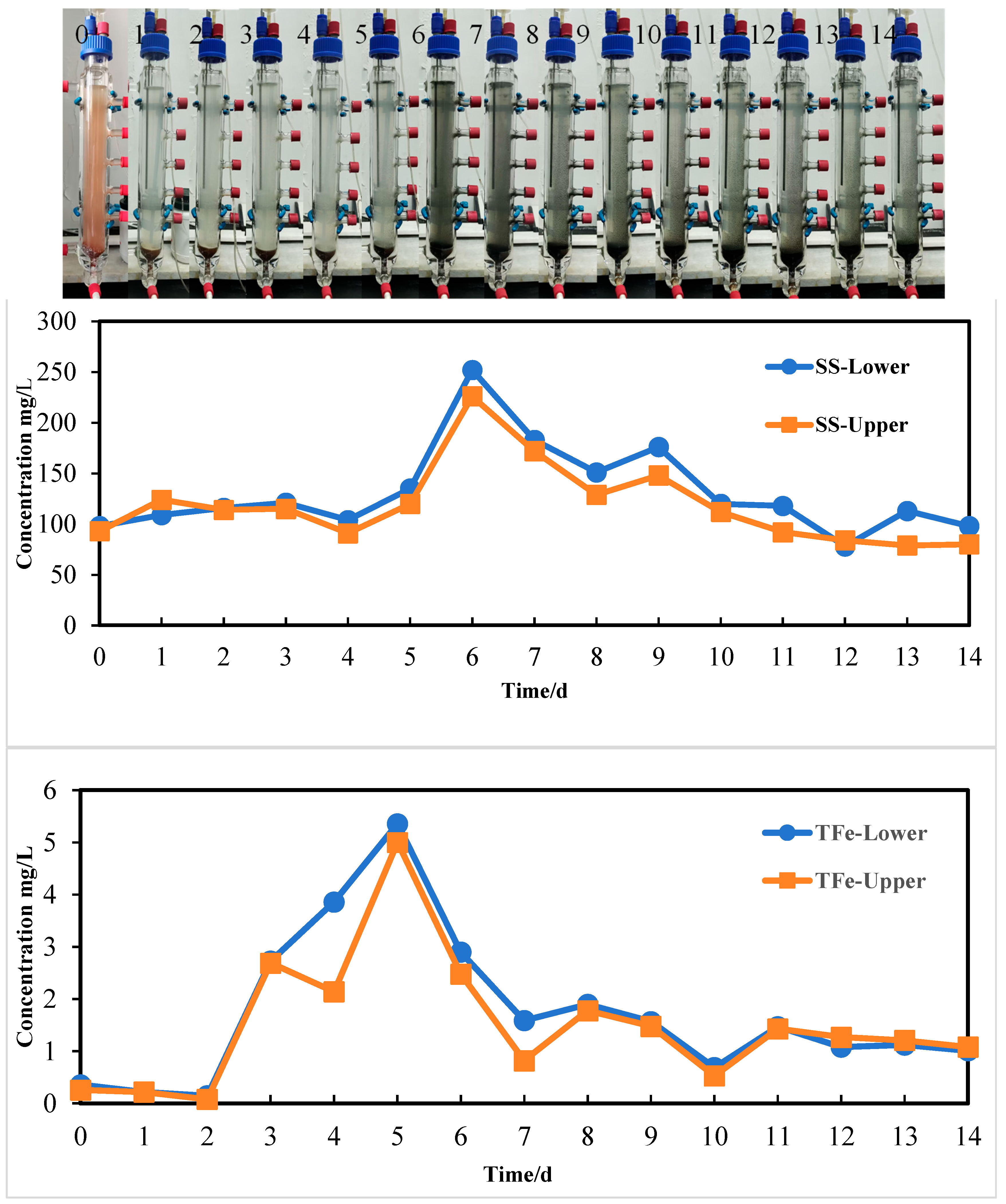
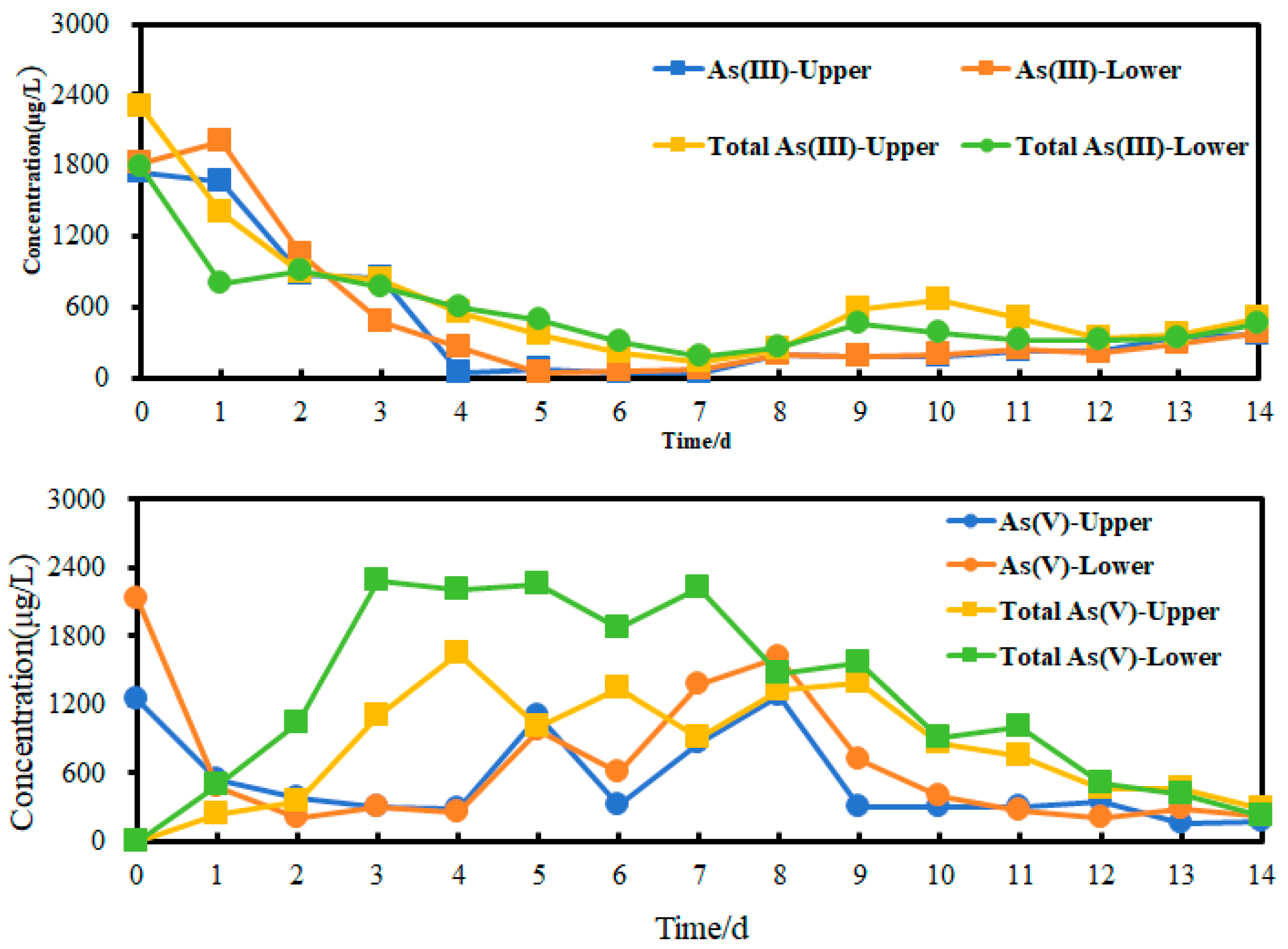
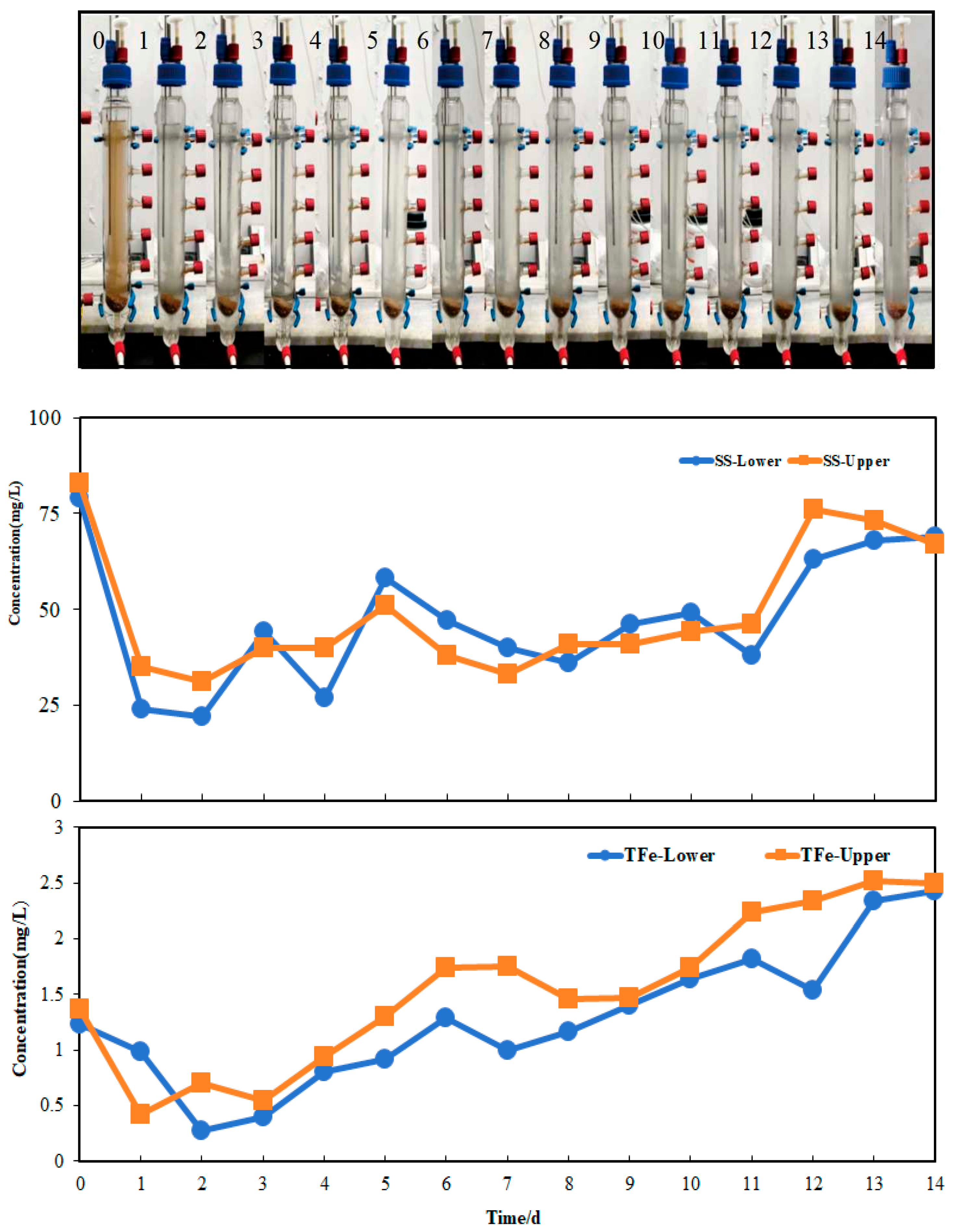
3.5. Arsenic Release from Sediment Column
3.5.1. Arsenic Release Under 35 °C
3.5.2. Arsenic Release Under 8 °C
4. Conclusions
- With an increase in temperature, IRB can promote arsenic release from arsenic-containing amorphous hydroxides. A suitable temperature (35 °C) can cause the release of more than 1.9–2.5 times arsenic(III) and 1.1–1.3 times arsenic(V). Compared to the control group, acetate can enhance arsenic release more than 2.8–6.1 times for arsenic(III) and 1.1–1.3 times for arsenic(V).
- XPS results showed that some arsenic species in solid change into arsenic(III) compared to pristine amorphous hydroxides. Injecting IRB into arsenic-containing amorphous hydroxides layer could cause arsenic release from sediment.
- Both aqueous arsenic(III) and arsenic(V) were observed during arsenic release. Meanwhile, arsenic related to particles/colloid was also released by IRB. From the upper and lower sites, they account for 4%–334% of aqueous arsenic(III) and 6%–332% of aqueous arsenic(V), respectively. These fractions are often underestimated or are not detected. SS data also showed that average values of SS from lower and upper sites are 131 and 118 mg/L, respectively. They are significantly higher compared to the group with low bacterial activity. These fractions contain rich iron. The IRB-assisted arsenic release technology can promote arsenic release and form concentration profile of arsenic and SS.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Craw, D.; Bowell, R.J. The characterization of arsenic in mine waste. Rev. Mineral. Geochem. 2014, 79, 473–505. [Google Scholar] [CrossRef]
- Gieré, R.; Sidenko, N.V.; Lazareva, E.V. The role of secondary minerals in controlling the migration of arsenic and metals from high-sulfide wastes Berikul gold mine, Siberia. Appl. Geochem. 2003, 18, 1347–1359. [Google Scholar] [CrossRef]
- Drahota, P.; Filippi, M. Secondary arsenic minerals in the environment: A review. Environ. Int. 2009, 35, 1243–1255. [Google Scholar] [CrossRef]
- Blowes, D.W.; Ptacek, C.J. Acid neutralization mechanisms in inactive mine tailings. In The Environmental Geochemistry of Sulfide Mine-Wastes; Jambor, J.L., Blowes, D.W., Eds.; Mineralogical Association of Canada: Nepean, ON, Canada, 1994; pp. 271–292. [Google Scholar]
- Henke, K.R. Arsenic in natural environments. In Arsenic Environmental Chemistry, Health Threats and Waste Treatment; Henke, K.R., Ed.; John Wiley& Sons Ltd.: Chichester, UK, 2009; pp. 69–235. [Google Scholar]
- Shih, M.C. An overview of arsenic removal by pressure-driven membrane processes. Desalination 2005, 172, 85–97. [Google Scholar] [CrossRef]
- Hughes, M.F.; Thomas, D.J.; Kenyon, E.M. Toxicology and epidemiology of arsenic and its compounds. In Arsenic Environmental Chemistry, Health Threats and Waste Treatment; Henke, K., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2010; pp. 237–265. [Google Scholar]
- Childs, C.W. Ferrihydrite: A review of structure, properties and occurrence in relation to soils. Pflanzenernährung Bodenkd. 1992, 155, 441–448. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Alpers, C.N. Geochemistry of acid mine water. In The Environmental Geochemistry of Mineral Deposis; Rev. Econ. Geol. V.6A; Society for Economic Geologists Inc.: Littleton, CO, USA, 1999; pp. 133–160. [Google Scholar]
- Childs, C.W.; Matsue, N.; Yoshinaga, N. Ferrihydrite deposits in paddy races, Asodani. Clay Sci. 1990, 8, 9–15. [Google Scholar]
- Schwertmann, U.; Murad, E. Forms and translocation of iron in podzoilized soils. In Proceedings of the 5th International Soil Correlation Meeting (ISCOMIV) Characterization: Classification and Utilization of Spodosols; Meeting on Spodosols; Soil Conservation Service, USDA: Lincoln, NE, USA, 1990; pp. 319–341. [Google Scholar]
- Bruun Hansen, H.C.; Raben-Lange, R.; Raulund-Rasmussen, K.; Borggaard, O.K. Monosilicate adsorption by ferrihydrite and goethite at pH 3–6. Soil Sci. 1994, 158, 40–46. [Google Scholar] [CrossRef]
- Carlson, L.; Schwertmann, U. Natural ferrihydrites in surface deposits from Finlan and their association with silica. Geochim. Cosmochim. Acta 1981, 45, 421–429. [Google Scholar] [CrossRef]
- Carlson, L.; Bigham, J.M.; Schwertmann, U.; Kyek, A.; Wagner, F. Scavenging of As from acid mine drainage by schwertmannite and ferrihydrite: A comparison with synthetic analogues. Environ. Sci. Technol. 2002, 36, 1712–1719. [Google Scholar] [CrossRef]
- Areej, A.; Guillaume, M. Arsenate and arsenite adsorption onto Al-containing ferrihydrite. Implications for arsenic immobilization after neutralization of acid mine drainage. Appl. Geochem. 2016, 64, 2–9. [Google Scholar]
- Areej, A.; Guiilaume, M.; Georges, O.N.; Menguy, N.; Maillot, F.; Casiot, C.; Bruneel, O.; Lebrun, S.; Juillot, F.; Brest, J. Arsenic scavenging by aluminum-substituted ferrihydrites in a circumneutral pH river impacted by acid mine drainage. Environ. Sci. Technol. 2013, 47, 12784–12792. [Google Scholar]
- Regelink, I.C.; Weng, L.; Koopmans, G.F.; van Riemsdijk, W.H. Asymmetric flow field-flow fractionation as a new approach to analyse iron-(Hydr)Oxide nanoparticles in soil extracts. Geoderma 2013, 202–203, 134–141. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Huang, B.J.; Zeng, H.Y. Arsenic extraction from seriously contaminated paddy soils with ferrihydrite-loaded sand columns. Chemosphere 2022, 307, 135744. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, A.; Moyano, A.; Mayorga, P. High arsenic contents in groundwater of central Spain. Environ. Geol. 2005, 47, 847–854. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, F.; Liu, J.; Frost, R.L. The As behavior of natural arsenical-containing colloidal ferric oxyhydroxide reacted with sulfate reducing bacteria. Chem. Eng. J. 2017, 332, 183–191. [Google Scholar] [CrossRef]
- Ren, X.; Yan, N.Z.; Chen, S.; Yao, J.; Liu, J. Remediaiton of arsenic-containing ferrihydrite in soil using iron and sulfa-reducing bacteria: Implications for microbially-assisted clean technology. J. Environ. Chem. Eng. 2022, 10, 108876. [Google Scholar] [CrossRef]
- Serrano, S.; Gomez-Gonzalez, M.A.; O’Day, P.A.; Laborda, F.; Bolea, E.; Garrido, F. Arsenic speciation in the dispersible colloidal fraction of soils from a mine-impacted creek. J. Hazard. Mater. 2015, 286, 30–40. [Google Scholar] [CrossRef]
- Slowey, A.J.; Johnson, S.B.; Newville, M.; Brown, G.E. Speciation and colloid transport of arsenic from mine tailings. Appl. Geochem. 2007, 22, 1884–1898. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, S.; Yao, J.; Liu, J. Arsenic release and colloidal species characterization during microbial reduction of As-containing ferrihydrite. Appl. Geochem. 2023, 153, 105685. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, L.; Dong, F.Q.; Hudson-Edwards, K.A. Enhancing As(V) adsorption and passivation using biologically formed nano-sized FeS coatings on limestone: Implications for acid mine drainage treatment and neutralization. Chemosphere 2017, 168, 529–538. [Google Scholar] [CrossRef]
- Widdel, F.; Pfenning, N. A new anaerobic, Sporing, Acetate-oxidizing, Sulfate-reducing bacterium, Desulfotomaculum (emend.) acetoxidans. Arch. Microbiol. 1977, 112, 119–122. [Google Scholar] [CrossRef]
- Utgika, V.P.; Chen, B.Y.; Chaudhary, N.; Tabak, H.H.; Haines, J.R.; Govind, R. Acute toxicity of heavy metals to acetate-utilizing mixed cultures of sulfate-reducing bacteria: EC100 and EC50. Environ. Toxicol. Chem. 2001, 12, 2662–2669. [Google Scholar] [CrossRef]
- Jakoben, R.; Postma, D. Redozoning, rates of sulfate reduction and interactions with Fe-reduction and methangenesis in a shallow sandy aquifer, Romo, Denmark. Geochim. Cosmochim. Acta 1999, 63, 137–151. [Google Scholar] [CrossRef]
- Jakobsen, R. Redox microniches in groundwater: A model study on the geometric and kinetic conditions required for concomitant Fe oxide reduction, sulfate reduction, and methanogenesis. Water Resour. Res. 2007, 43, 1–11. [Google Scholar] [CrossRef]
- Fan, L.J.; Zhang, X.J. ATR-FTIR spectroscopic characterization of ferric arsenic-containing colloid and gypsum. Desalination Water Treat. 2019, 153, 130–135. [Google Scholar] [CrossRef]
- Corkhill, C.L.; Vaughan, D.J. Arsenopyrite oxidation—A review. Appl. Geochem. 2009, 24, 2342–2361. [Google Scholar] [CrossRef]
- Yam, H.M.; Leong, S.K.W.; Qiu, X.; Zaiden, N. Bioremediaiton of arsenic-contaminated water through application of bioengi neered Shewanella oneidensis. In IRC-SET 2020; Springer: Singapore, 2021; pp. 559–574. [Google Scholar] [CrossRef]
- Wang, J.; Wu, M.Y.; Si, Y.B. Biotransformation and biomethylation of arsenic by Shewanella oneidensis MR-1. Chemosphere 2016, 145, 329–335. [Google Scholar] [CrossRef]
- Zhou, J.M.; Liu, Y.Z.; Bu, H.L. Effects of Fe(II)-induced transformation of scorodite on arsenic solubility. J. Hazard. Mater. 2022, 429, 128274. [Google Scholar] [CrossRef]
- Han, Y.S.; Callegos, T.J.; Domond, A.H.; Hayes, K.F. Fes-coated sand for removal of arsenic(III) under anaerobic conditions in permeable reactive barriers. Water Res. 2011, 45, 593–604. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, D.; Tian, X.; Qin, R. Enhancement of Arsenic Release from Amorphous Arsenic-Containing Ferric Hydroxides Systems Using Bacterial Reduction: Applicability of Injecting Iron-Reducing Bacteria for Dissolved Arsenic Species and Colloid Phases. Minerals 2025, 15, 1115. https://doi.org/10.3390/min15111115
Luo D, Tian X, Qin R. Enhancement of Arsenic Release from Amorphous Arsenic-Containing Ferric Hydroxides Systems Using Bacterial Reduction: Applicability of Injecting Iron-Reducing Bacteria for Dissolved Arsenic Species and Colloid Phases. Minerals. 2025; 15(11):1115. https://doi.org/10.3390/min15111115
Chicago/Turabian StyleLuo, Dayong, Xiaosong Tian, and Ruxiang Qin. 2025. "Enhancement of Arsenic Release from Amorphous Arsenic-Containing Ferric Hydroxides Systems Using Bacterial Reduction: Applicability of Injecting Iron-Reducing Bacteria for Dissolved Arsenic Species and Colloid Phases" Minerals 15, no. 11: 1115. https://doi.org/10.3390/min15111115
APA StyleLuo, D., Tian, X., & Qin, R. (2025). Enhancement of Arsenic Release from Amorphous Arsenic-Containing Ferric Hydroxides Systems Using Bacterial Reduction: Applicability of Injecting Iron-Reducing Bacteria for Dissolved Arsenic Species and Colloid Phases. Minerals, 15(11), 1115. https://doi.org/10.3390/min15111115





