Color Genesis and Compositional Features of Red-Blue Colored Gem-Quality Corundum from Malipo, China
Abstract
1. Introduction
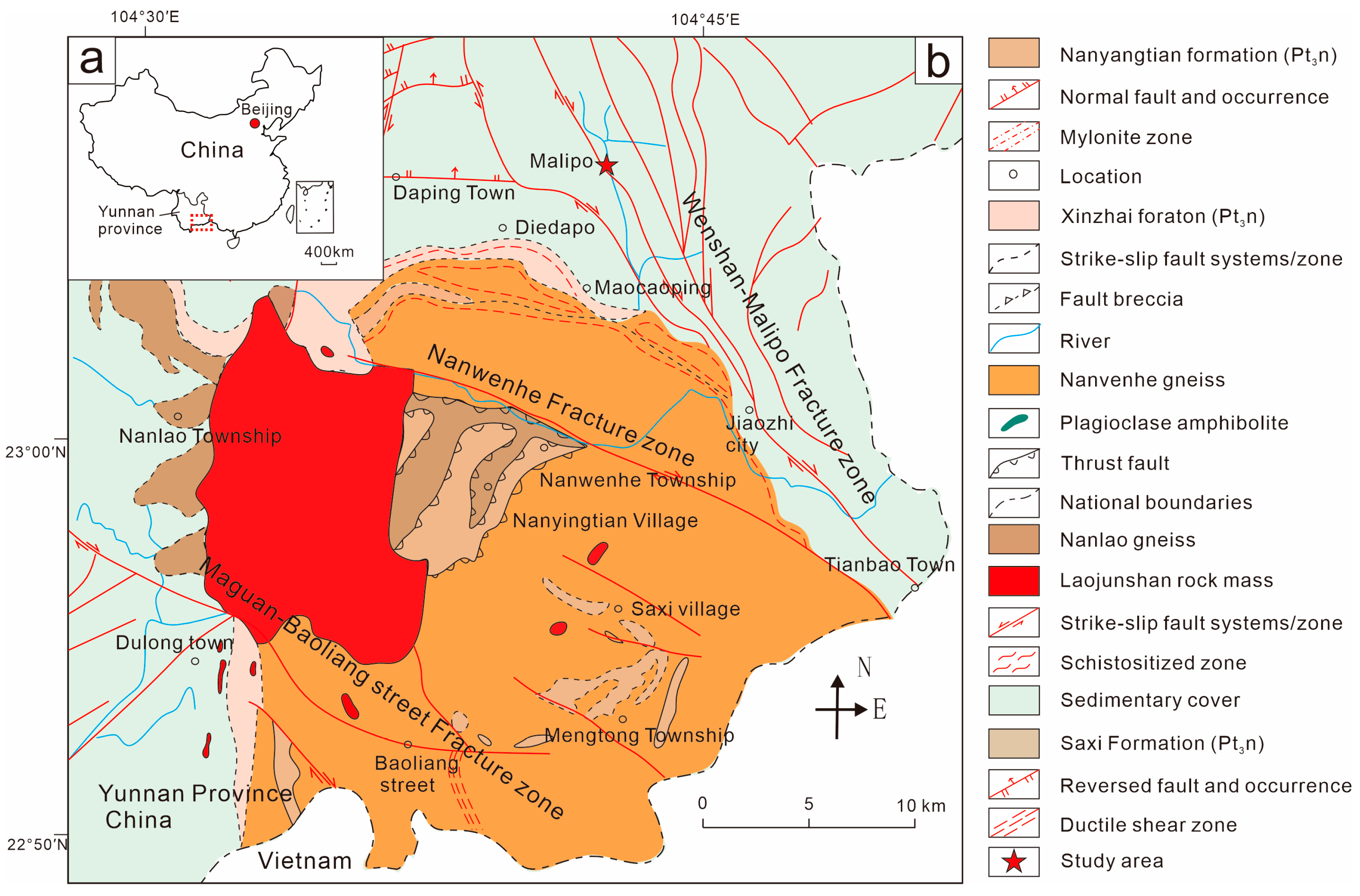
2. Geological Background and Samples
3. Samples and Methods
3.1. Samples
3.2. Methods
4. Results
4.1. Morphological and Physical Characteristics
4.2. UV-Vis Spectroscopic Characteristics of the RBCC Samples
4.3. Chemical Composition of the RBCC Samples
5. Discussion
5.1. Coloration Mechanism of the Red-Blue Colored Corundum
5.1.1. Color Genesis in Red Region
5.1.2. Color Genesis in Blue Region
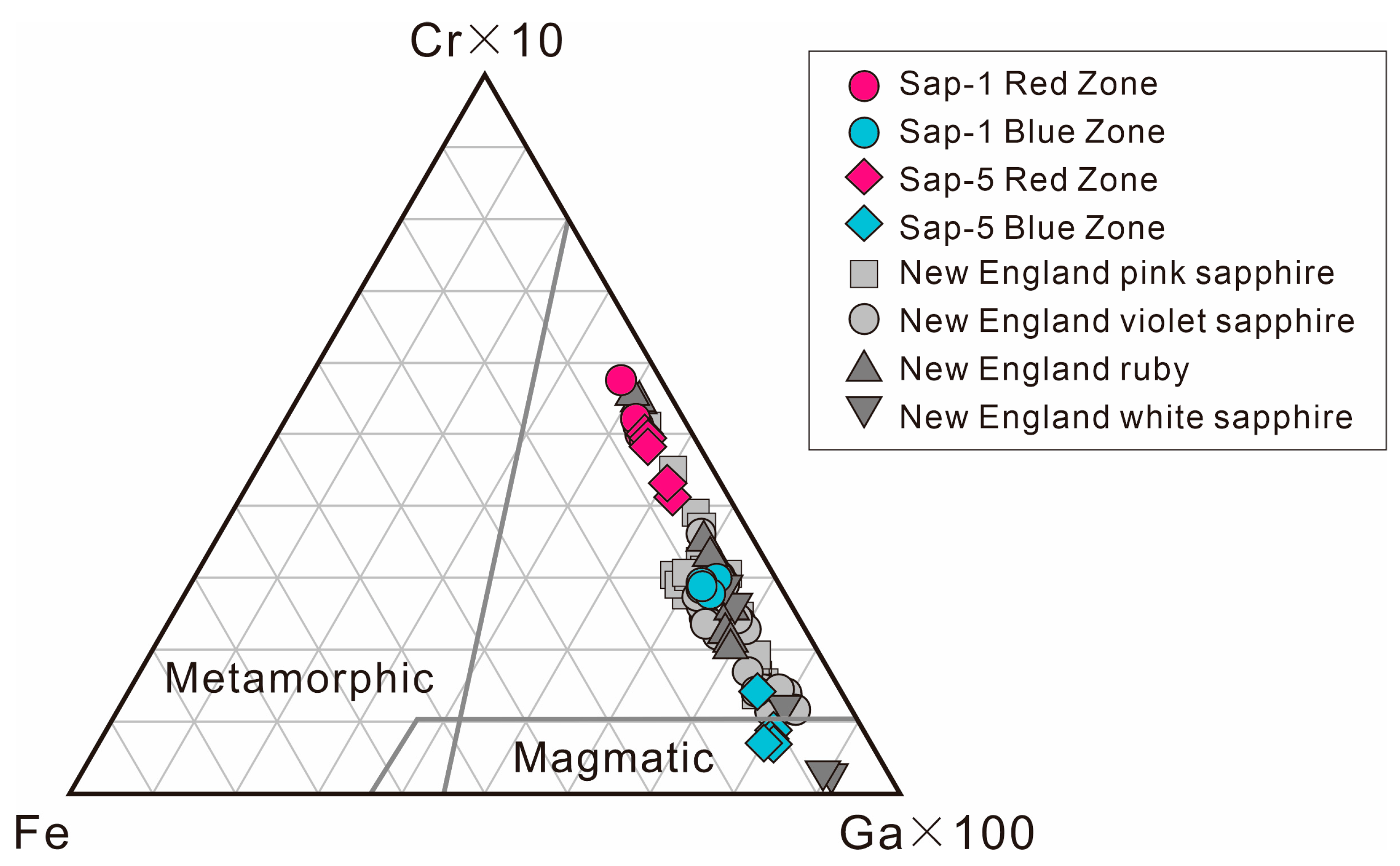
5.2. Genesis of Red-Blue Colored Corundum from the Malipo Alluvial Deposit
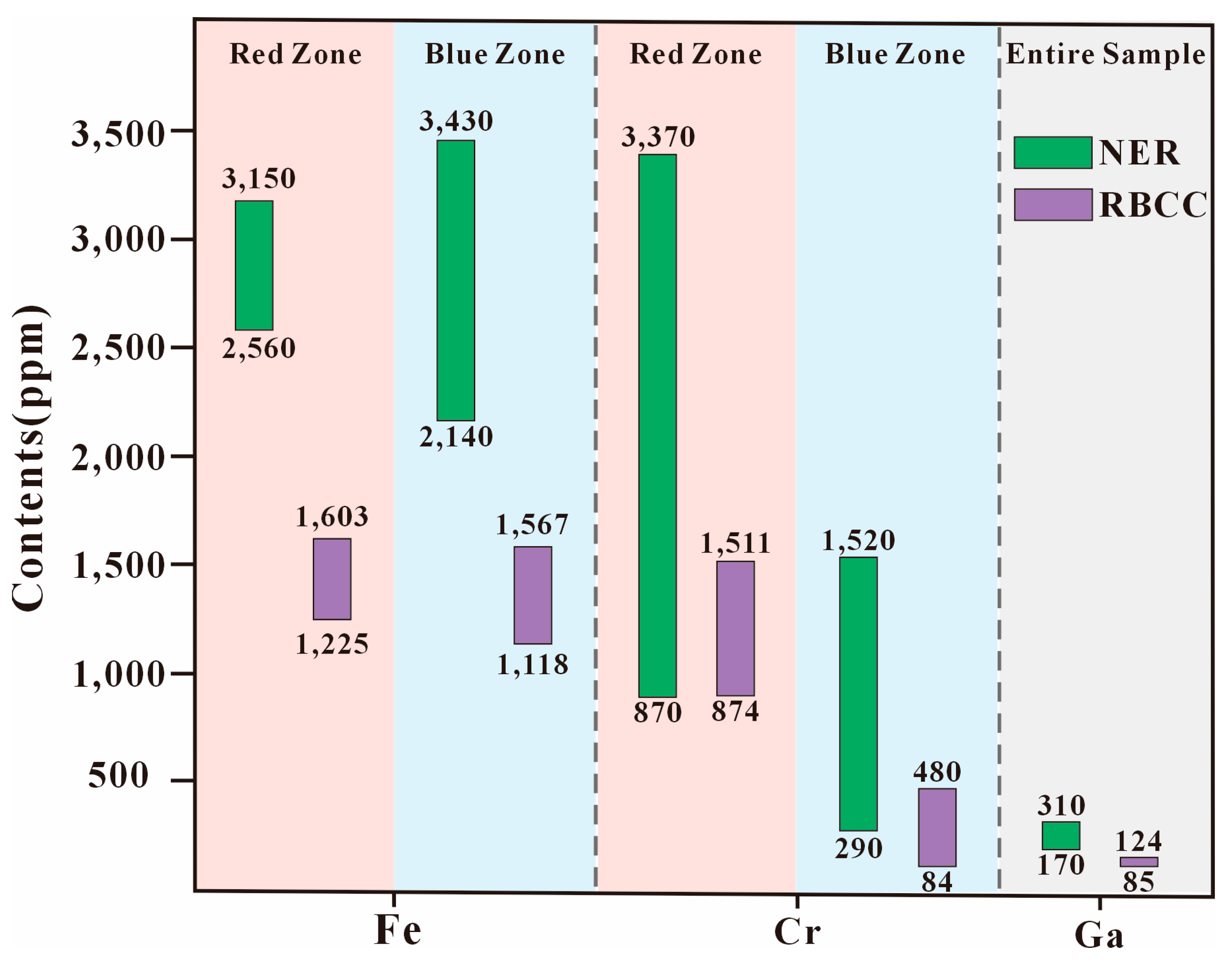
5.3. Comparison of Zoning in RBCC from Malipo and New England Ruby-Violet Sapphires
6. Conclusions
- Multi-colored corundum gems from Malipo, Yunnan, China are typically red, colorless or purplish-blue, with irregular color distributions. They exhibit a refractive index between 1.760 and 1.763 (Ne), 1.770–1.772 (No), and DR = 0.009–0.010. The average specific gravity is 3.79. These sapphires often display twinning wisps and uneven fractures.
- The red regions of the multi-colored corundum are primarily caused by Cr3+, while Fe2+-Ti4+ ion pair and Fe3+-Fe3+ ion pair contribute partially to the genesis of color. Cr3+ is a decisive factor determining the intensity of red coloration. The blue zones are mainly caused by Fe2+-Ti4+ ion transitions, while the ions Cr3+ and Fe3+ play a secondary role. Cr is also responsible for imparting a purple hue to the blue zone.
- The chemical composition of the multi-colored corundum from Malipo, Yunnan, China, generally aligns with the characteristics of metamorphic genesis, with an influence from magma mixing and mingling. The RBCC suite comprises ruby (up to 1512 ppm Cr, 172 ppm V, 3 ppm Ti, and 1604 ppm Fe) and sapphire (up to 481 ppm Cr, 346 ppm V, 22 ppm Ti, and 1568 ppm Fe). The Cr content in the red zone is significantly higher than that in the blue zone, whereas the Ti and V content in the blue zone is notably higher than in the red zone. All color zones demonstrate stability in Ga (up to 125 ppm), with minor fluctuations in Fe. The Mg content is extremely low, with only one detection point indicating its presence.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Che, S.; Ren, L. The color origin of gem diaspore: Correlation to corundum. Gems Gemol. 2018, 54, 326–327. [Google Scholar]
- Dubinsky, E.V.; Stone-Sundberg, J.; Emmett, J.L. A quantitative description of the causes of color in corundum. Gems Gemol. 2020, 56, 1–27. [Google Scholar] [CrossRef]
- Sutherland, F.L.; Schwarz, D.; Jobbins, E.A.; Coenraads, R.R.; Webb, G. Distinctive gem corundum suites from discrete basalt fields: A comparative study of Barrington, Australia, and West Pailin, Cambodia, Gemfields. Gemmology 1998, 26, 65–85. [Google Scholar] [CrossRef]
- McMillan, N.J.; McManus, C.E. Application of Multivariate Analysis to the Problem of the Provenance of Gem Stones (Ruby, Sapphire, Emerald, Diamond). In Laser Induced Breakdown Spectroscopy (LIBS): Concepts, Instrumentation, Data Analysis and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; Volume 1, pp. 287–303. [Google Scholar]
- Wang, H.; Yu, X.Y.; Liu, F.; Alam, M.; Wu, G.C. Color genesis and compositional characteristics of color-change sapphire from Fuping, China. Crystals 2022, 12, 463. [Google Scholar] [CrossRef]
- Yu, X.Y.; Long, Z.Y.; Zhang, Y.; Qin, L.J.; Zhang, C.; Xie, Z.R.; Wu, Y.R.; Yan, Y.; Wu, M.K.; Wan, J.X. Overview of Gemstone Resources in China. Crystals 2021, 11, 1189. [Google Scholar] [CrossRef]
- Chen, J.X. Records of Gemstones in Yunnan in Ancient Books. Yunnan Metall. 1985, 5, 57. [Google Scholar]
- Liu, C.L. Type of Genesis of Bauxite in China. Sci. China (Ser. B Chem. Biol. Agric. Sci. Med. Sci. Earth Sci.) 1987, 5, 535–544. [Google Scholar]
- Sun, K.X. The geological characteristics of gem metallogenesis in Yunnan. Yunnan Geol. 1996, 15, 81–90. [Google Scholar]
- Bo, G.N.; Zhao, J.F. Minerogenetic law and minerogenetic area division of gem-jade in Wenshan area, Yunnan. Yunnan Geol. 2015, 34, 352–358. [Google Scholar]
- Bristow, J.K.; Tiana, D.; Parker, S.C.; Walsh, A. Defect chemistry of Ti and Fe impurities and aggregates in Al2O3. J. Mater. Chem. A 2014, 2, 6198–6208. [Google Scholar] [CrossRef]
- Emmett, J.L.; Stone-Sundberg, J.; Guan, Y.; Sun, Z. The Role of Silicon in the Color of Gem Corundum. Gems Gemol. 2017, 53, 42–47. [Google Scholar] [CrossRef]
- Ji, J.H. A Study of Colour-assuming Mechanism and Modifying Test of Su-Lu Sapphire. Jiangsu Geol. 1999, 23, 162–166. [Google Scholar]
- Gagan, C.; Shi, G.H. Gem News International. Gems Gemol. 2024, 60, 400–429. [Google Scholar]
- Yu, X.Y.; Sun, H.N.; Wang, H. Gemological Properties and Coloration of Metamorphic Type Ruby from Gansu, China. Natl. Gemstone Test. Cent. Gems Jewel. Trade Assoc. China 2009, 4, 79–85. [Google Scholar]
- Hughes, R.W.; Manorotkul, W.; Hughes, E.B. Ruby & Sapphire: A Gemologist’s Guide; RWH Publishing: Boulder, CO, USA, 2017. [Google Scholar]
- Nikolskaya, L.V.; Terekhova, V.M.; Samoilovich, M.I. On the origin of natural sapphire color. Phys. Chem. Miner. 1978, 3, 213–224. [Google Scholar] [CrossRef]
- Guo, K.P.; Zhou, Z.Y.; Zhong, Q.; Lai, M.; Wang, H.; Li, Y.B.; Qiao, X.; Nong, P.Z. Comparative study on elemental content and UV-Vis spectroscopy characteristics of Ruby from Myanmar and Mozambique. Acta Petrol. Mineral. 2018, 37, 1002–1010. [Google Scholar]
- Guo, Q.R.; Li, P.Y.; Wang, M.Y.; Zhao, S.Y.; Yang, S.C.; Shi, G.H. Color Mechanism Analysis and Origin Comparison of Pink-Purple Sapphires from Vietnam and Madagascar. Crystals 2025, 15, 229. [Google Scholar] [CrossRef]
- Li, E.Q.; Zhang, Y.F.; Xu, B. Gemological and Chemical Composition Characteristics of Basalt-Hosted Rubies from Thailand. Geoscience 2023, 37, 486–499. [Google Scholar]
- Wang, Y.Y.; Yang, L.Y.; Li, M.; Yang, P.T.; Shen, A.H.; Wang, C.W. Spectral Characteristics and Color Origin of Unstable Yellow Sapphire. Spectrosc. Spectr. Anal. 2021, 41, 2611–2617. [Google Scholar]
- Soonthorntantikul, W.; Vertriest, W.; Raynaud-Flattot, V.L.; Sangsawong, S.; Atikarnsakul, U.; Khowpong, C.; Weeramonkhonlert, V.; Pardieu, V. An in depth gemological study of blue sapphires from the Baw Mar mine (Mogok, Myanmar). Gemmol. Inst. Am. Rep. 2017, 2, 87. [Google Scholar]
- Liu, Y.M. Classic Gemological Characteristics of Ruby and Sapphire from Muling, Heilongjiang Province, China. Doctoral Dissertation, China University of Geosciences, Wuhan, China, 2023. [Google Scholar]
- Fritsch, E.; Rossman, G.R. An update on color in gems. Part 2: Colors involving multiple atoms and color centers. Gems Gemol. 1988, 24, 3–15. [Google Scholar] [CrossRef]
- Liu, F.k.; Guo, Y.; Zhao, B.; Liu, M.Y. Chromogenic Mechanisms of Rubies with Varying Color Tones. Geoscience 2025, 39, 158–166. [Google Scholar]
- Chen, S.M.; Tan, H.L.; Zhang, C.; Teng, Y.J.; Zu, E.D. Study on Gemological Characteristics of Blue Sapphires from Baw-Mar Mine, Mogok, Myanmar. Crystals 2021, 11, 1275. [Google Scholar] [CrossRef]
- Krebs, J.J.; Maisch, W.G. Exchange effects in the optical-absorption spectrum of Fe3+ in Al2O3. Phys. Rev. B 1971, 4, 757. [Google Scholar] [CrossRef]
- Pluthametwisute, T.; Nasdala, L.; Chutimun, C.N.; Wildner, M.; Libowitzky, E.; Giester, G.; Zoysa, E.G.; Jakkawanvibul, C.; Suwanmanee, W.; Sripoonjan, T.; et al. Luminescence and a New Approach for Detecting Heat Treatment of Geuda Sapphire. Solid Earth 2025, 16, 81–96. [Google Scholar] [CrossRef]
- Zhang, P.Q. Relationship between color and chemical composition of Sapphire in Changle, Shandong province. Shandong Geol. 2000, 16, 36–43. [Google Scholar]
- Emmett, J.L.; Scarratt, K.; McClure, S.F.; Moses, T.; Douthit, T.R.; Hughes, C.; Novak, S.; Shigley, J.E.; Wang, W.Y.; Bordelon, O. Beryllium diffusion of ruby and sapphire. Gems Gemol. 2003, 39, 84–135. [Google Scholar] [CrossRef]
- Xie, Y.H. Analysis on UV-VIS Spectrumand Colouration Mechanism of Sapphire. J. Gems Gemmol. 2004, 6, 9–12. [Google Scholar]
- Muyal, J. Ruby & Sapphire: A Gemologist’s Guide to understanding color. In Gems & Gemology; Gemological Institute of America: Carlsbad, CA, USA, 2025; p. 124. [Google Scholar]
- Ozerov, K. Form of corundum crystals as dependent upon chemical composition of medium. Dolkady Akad. Nauk SSSR 1945, 47, 49–52. [Google Scholar]
- Hughes, R.W. Ruby and Sapphire; RWH Publishing: Boulder, CO, USA, 1997. [Google Scholar]
- Schwarz, D. Aus Basalten, Marmoren und Pegmatiten. Spezielle Ursachen formten in der Erdkruste edle Rubine und Saphire. In Rubin, Saphir, Korund: Schön, Hart, Selten, Kostbar; Weise, C., Ed.; ExtraLapis: München, Germany, 1998; Volume 15, pp. 5–9. [Google Scholar]
- Simonet, C. Géologie des Gisements de Saphir et de Rubis. L’exemple de la John Saul Mine, Mangari, Kenya. Doctoral Dissertation, University of Nantes, Nantes, France, 2000. [Google Scholar]
- Simonet, C.; Fritsch, E.; Lasnier, B. A classification of gem corundum deposits aimed towards gem exploration. Ore Geol. Rev. 2008, 34, 127–133. [Google Scholar] [CrossRef]
- Kievlenko, E.Y. Geology of Gems. Mineral. Rec. 2003, 36, 42–43. [Google Scholar]
- Pham, V.L.; Hoang, Q.V.; Garnier, V.; Giuliani, G.; Ohnenstetter, D. Marblehosted ruby from Vietnam. Can. Gemmol. 2004, 25, 83–95. [Google Scholar]
- Walton, L. Exploration Criteria for Coloured Gemstone Deposits in the Yukon; Yukon Geological Survey: Whitehorse, YT, Canada, 2004; p. 184. [Google Scholar]
- Giuliani, G.; Ohnenstetter, D.; Fallick, A.E.; Groat, L.; Fagan, A.J. The geology and genesis of gem corundum deposits. In The Geology of Gem Deposits; Groat, L.A., Ed.; Mineralogical Association of Canada: Québec City, QC, Canada, 2007; Chapter 2; pp. 23–78. [Google Scholar]
- Giuliani, G.; Fallick, A.E.; Garnier, V.; France, L.C.; Ohnenstetter, D.; Schwarz, D. Oxygen isotope composition as a tracer for the origins of rubies and sapphires. Geology 2005, 332, 249–252. [Google Scholar] [CrossRef]
- Giuliani, G.; Ohnenstetter, D.; Fallick, A.E.; Groat, L.; Feneyrol, J. Geographic origin of gems linked to their geographical history. InColor 2012, 19, 16–27. [Google Scholar]
- Sun, Z.Y.; Michael, J.; Aaron, C.P. Chemical Analysis in the Gemological Laboratory: XRF and LA-ICP-MS. Gems Gemol. 2024, 60, 149–153. [Google Scholar] [CrossRef]
- Baldwin, L.; Tomaschek, F.; Ballhaus, C.; Gerdes, A.; Fonseca, R.; Wirth, R.; Geisler, T.; Nagel, T. Petrogenesis of alkaline basalthosted sapphire megacrysts. Petrological and geochemical investigations of in situ sapphire occurrences from the Siebengebirge Volcanic Field, Germany. Contrib. Mineral. Petrol. 2017, 172, 43. [Google Scholar] [CrossRef]
- Buravleva, S.Y.; Smirnov, S.Z.; Pakhomova, V.; Fedoseev, D. Sapphires from the Sutara Placer in the Russian Far East. Gems Gemol. 2016, 52, 252–264. [Google Scholar] [CrossRef]
- Guang, Y.W.; Xiao, Y.Y.; Liu, F. Genesis of Color Zonation and Chemical Composition of Penglai Sapphire in Hainan Province, China. Minerals 2022, 12, 832. [Google Scholar] [CrossRef]
- Sutherland, F.L.; Graham, I.T.; Harris, S.J.; Coldham, T.; Powell, W.; Belousova, E.A.; Martin, L. Unusual ruby-sapphire transition in alluvial megacrysts, Cenozoic basaltic gem field, New England, New South Wales, Australia. Lithos 2017, 278, 347–360. [Google Scholar] [CrossRef]
- Muhlmeister, S.; Fritsch, E.; Shigley, J.E.; Devouard, B.; Laurs, B.M. Separating natural and synthetic rubies on the basis of trace-element chemistry. Gems Gemol. 1998, 34, 80–101. [Google Scholar] [CrossRef]
- Sutherland, F.L.; Zaw, K.; Meffre, S.; Giuliani, G.; Fallick, A.E.; Graham, I.T.; Webb, G.B. Gem-corundum megacrysts from east Australian basalt fields: Trace elements, oxygen isotopes and origins. Aust. J. Earth Sci. 2009, 56, 1003–1022. [Google Scholar] [CrossRef]
- Abduriyim, A. Determination of the origin of blue sapphire using laser ablation inductively coupled plasma mass spectrome-try (LA-ICP-MS). J. Gemmol. 2006, 30, 23–36. [Google Scholar] [CrossRef]
- Guo, J.; Wang, F.; Yakoumelos, G. Sapphires from Changle in Shandong province, China. Gems Gemol. 1992, 28, 255–260. [Google Scholar] [CrossRef]
- Zaw, K.; Sutherland, L.; Yui, T.F.; Meffre, S.; Thu, K. Vanadium-rich ruby and sapphire within mogok gemfield, myanmar: Implications for gem color and genesis. Miner. Depos. 2015, 50, 25–39. [Google Scholar] [CrossRef]

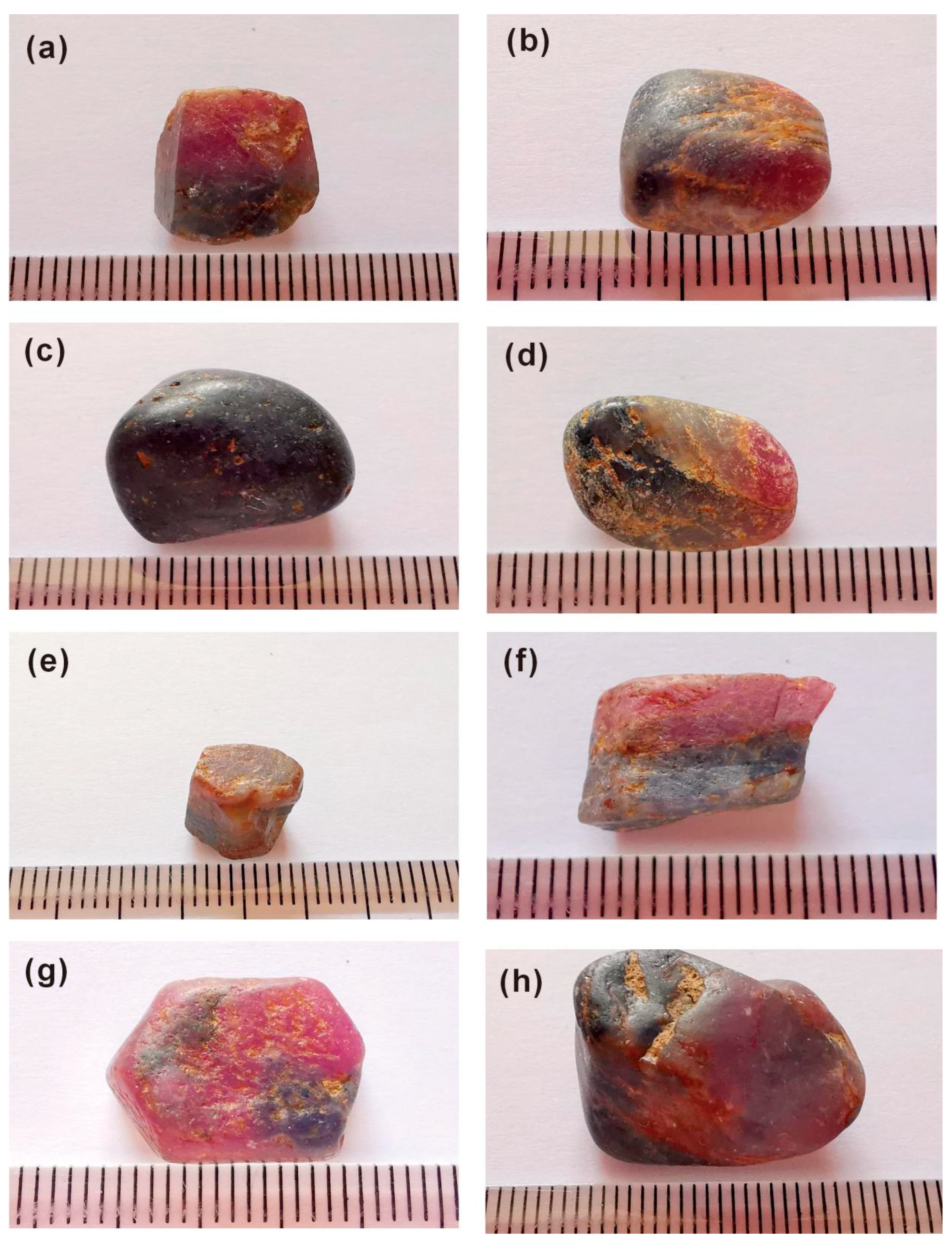



| Sample | Color | Luster | Characteristics Observed Under Magnification | Transparency | Specific Gravity | Refractive Indices | Fluorescence Long Short Wave Wave | |
|---|---|---|---|---|---|---|---|---|
| Sap-1 | Violet-red and violet-blue | Medium glassy luster | Twin lamellae, grayish-black country rock. | Translucent | 3.61 | 1.760–1.770 | Moderate red in the local region | Moderate red in the local region |
| Sap-2 | Pink | Medium glassy luster | Ragged fracture with fine-grained crystals. | Translucent | 3.78 | 1.76 | Moderate red | Moderate red |
| Sap-4 | Dark blue | Weak glassy luster | Brownish holes. | Non-transparent | 3.68 | 1.77 | Inert | Inert |
| Sap-5 | Pink and violet-blue | Medium glassy luster | Twin lamellae, blue color zones. | Translucent | 3.80 | 1.763–1.772 | Moderate red in the local region | Weak red in the local region |
| Sap-7 | Violet-blue | Medium glassy luster | Twin lamellae. | Translucent | 3.72 | —— | Moderate white-blue in the core and moderate red on the edge | Moderate white-blue in the core and moderate red on the edge |
| Sap-9 | Blue and violet-red | Medium glassy luster | twin lamellae. | Translucent | 3.79 | —— | Moderate red | Moderate red |
| Sap-10 | Violet-red | Medium glassy luster | Yellowish-brown mineral dissemination | Translucent | 3.84 | 1.762–1.772 | Moderate red in the local region | Weak red in the local region |
| Sap-12 | Violet-red | Medium glassy luster | Twin lamellae, yellowish-brown mineral dissemination | Translucent | 3.80 | —— | Moderate red in the local region | Weak red in the local region |
| Sample | Color | Spot | Cr | Fe | Ti | V | Ga | Mg | Gr/Ga | Fe/Ti |
|---|---|---|---|---|---|---|---|---|---|---|
| Sap-1 | red | 1−1 | 983 | 1225 | 2 | 53 | 86 | 0 | 11.48 | 625.02 |
| 1−2 | 1151 | 1320 | 0 | 54 | 96 | 0 | 12.01 | —— | ||
| 1−3 | 1512 | 1250 | 2 | 76 | 99 | 0 | 15.31 | 809.00 | ||
| 1−4 | 1291 | 1421 | 1 | 142 | 104 | 0 | 12.43 | 1004.57 | ||
| 1−5 | 1121 | 1377 | 0 | 172 | 101 | 0 | 11.09 | —— | ||
| blue | 1−6 | 441 | 1499 | 22 | 289 | 96 | 0 | 4.62 | 67.70 | |
| 1−7 | 474 | 1118 | 7 | 281 | 99 | 0 | 4.77 | 161.62 | ||
| 1−8 | 437 | 1388 | 21 | 285 | 92 | 0 | 4.77 | 66.87 | ||
| 1−9 | 454 | 1442 | 5 | 330 | 103 | 0 | 4.41 | 284.66 | ||
| 1−10 | 481 | 1568 | 21 | 346 | 103 | 0 | 4.67 | 73.91 | ||
| Sap-5 | red | 5−1 | 1417 | 1604 | 0 | 56 | 125 | 0 | 11.33 | —— |
| 5−2 | 1219 | 1389 | 0 | 63 | 111 | 0 | 10.99 | —— | ||
| 5−3 | 1101 | 1415 | 0 | 94 | 104 | 0 | 10.61 | —— | ||
| 5−4 | 874 | 1428 | 3 | 141 | 111 | 0 | 7.91 | —— | ||
| 5−5 | 938 | 1379 | 0 | 128 | 110 | 0 | 8.54 | —— | ||
| blue | 5−6 | 208 | 1469 | 0 | 262 | 111 | 0 | 1.86 | —— | |
| 5−7 | 124 | 1516 | 4 | 294 | 114 | 0 | 1.09 | 386.63 | ||
| 5−8 | 99 | 1535 | 5 | 288 | 105 | 0 | 0.94 | 322.11 | ||
| 5−9 | 88 | 1527 | 5 | 297 | 105 | 15 | 0.84 | 287.90 | ||
| 5−10 | 84 | 1562 | 21 | 260 | 97 | 0 | 0.87 | 75.26 |
| Color Mechanism | Elements | Sap-1 (Red Zone) | Sap-5 (Red Zone) |
|---|---|---|---|
| Crystal field theory | Fe3+ | ||
| Fe3+-Fe3+ | 398 nm | 395 nm | |
| Electronic transfer References | Fe2+-Ti4+ | 555 nm | 551 nm |
| Cr3+ | 398 nm, 555 nm, 656 nm, 692 nm | 395 nm, 551 nm, 665 nm, 690 nm | |
| Fe2+-Fe3+ | 787 nm |
| Color Mechanism | Elements | Sap-1 (Blue Zone) | Sap-5 (Blue Zone) |
|---|---|---|---|
| Crystal field theory | Fe3+ | 389 nm | |
| Fe3+-Fe3+ | |||
| Electronic transfer References | Fe2+-Ti4+ | 558 nm | 562 nm |
| Cr3+ | 398 nm, 558 nm, 670 nm | 562 nm, 669 nm | |
| Fe2+-Fe3+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Yu, X.-Y.; Wang, G.-Y.; Alam, M.; Mu, L.; Xu, Y.-X.; Liu, F. Color Genesis and Compositional Features of Red-Blue Colored Gem-Quality Corundum from Malipo, China. Minerals 2025, 15, 1099. https://doi.org/10.3390/min15111099
Wang H, Yu X-Y, Wang G-Y, Alam M, Mu L, Xu Y-X, Liu F. Color Genesis and Compositional Features of Red-Blue Colored Gem-Quality Corundum from Malipo, China. Minerals. 2025; 15(11):1099. https://doi.org/10.3390/min15111099
Chicago/Turabian StyleWang, Hui, Xiao-Yan Yu, Guang-Ya Wang, Masroor Alam, Lan Mu, Ying-Xin Xu, and Fei Liu. 2025. "Color Genesis and Compositional Features of Red-Blue Colored Gem-Quality Corundum from Malipo, China" Minerals 15, no. 11: 1099. https://doi.org/10.3390/min15111099
APA StyleWang, H., Yu, X.-Y., Wang, G.-Y., Alam, M., Mu, L., Xu, Y.-X., & Liu, F. (2025). Color Genesis and Compositional Features of Red-Blue Colored Gem-Quality Corundum from Malipo, China. Minerals, 15(11), 1099. https://doi.org/10.3390/min15111099







