From a Scheelite Concentrate (Spanish Origin) to Nanotungsten Derivatives
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Leaching in Sodium Carbonate Medium
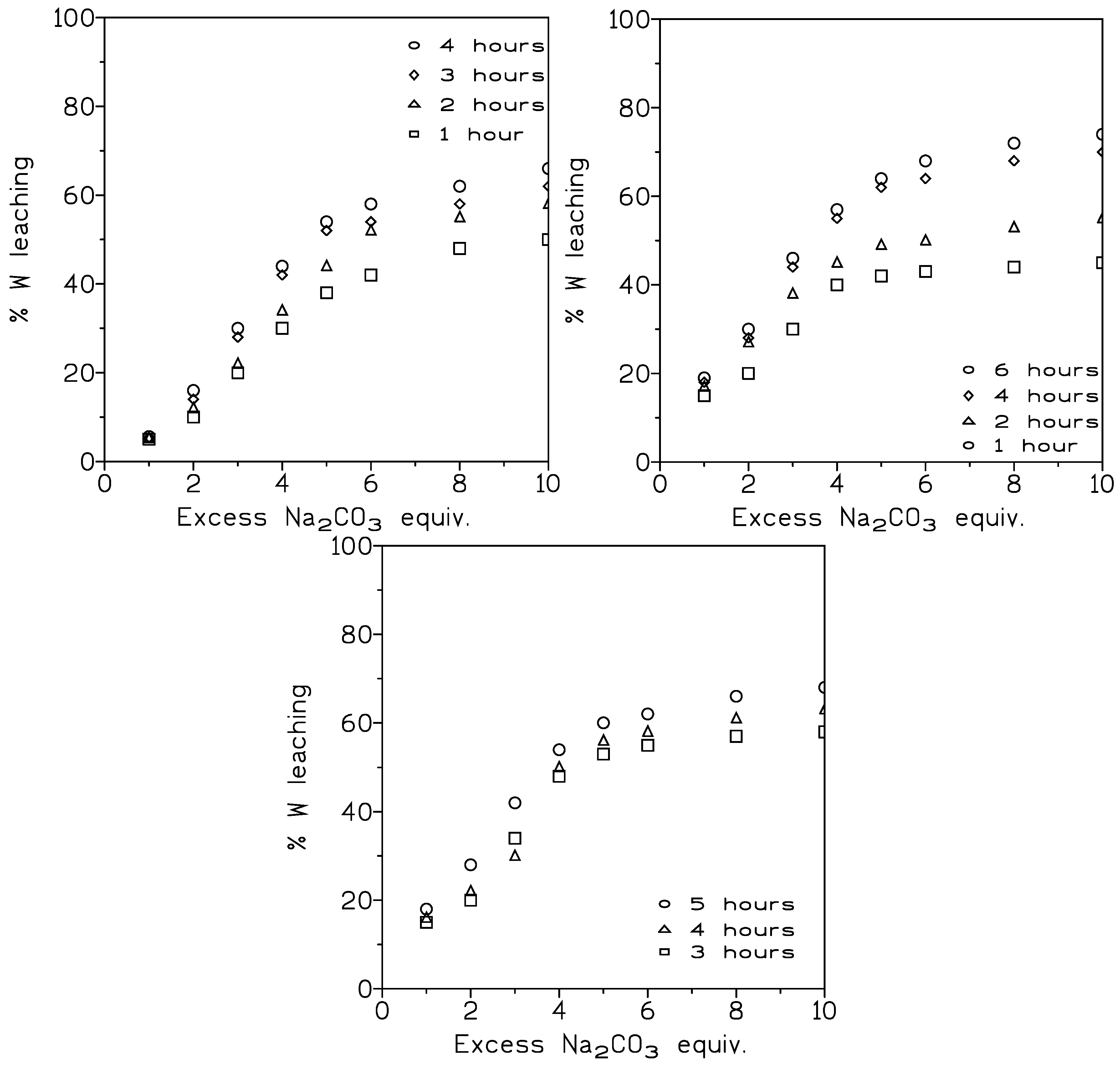
3.1.1. Influence of the Pulp Density and the Sodium Carbonate Concentration on Tungsten Dissolution
3.1.2. Influence of Particle Size on Tungsten Leaching
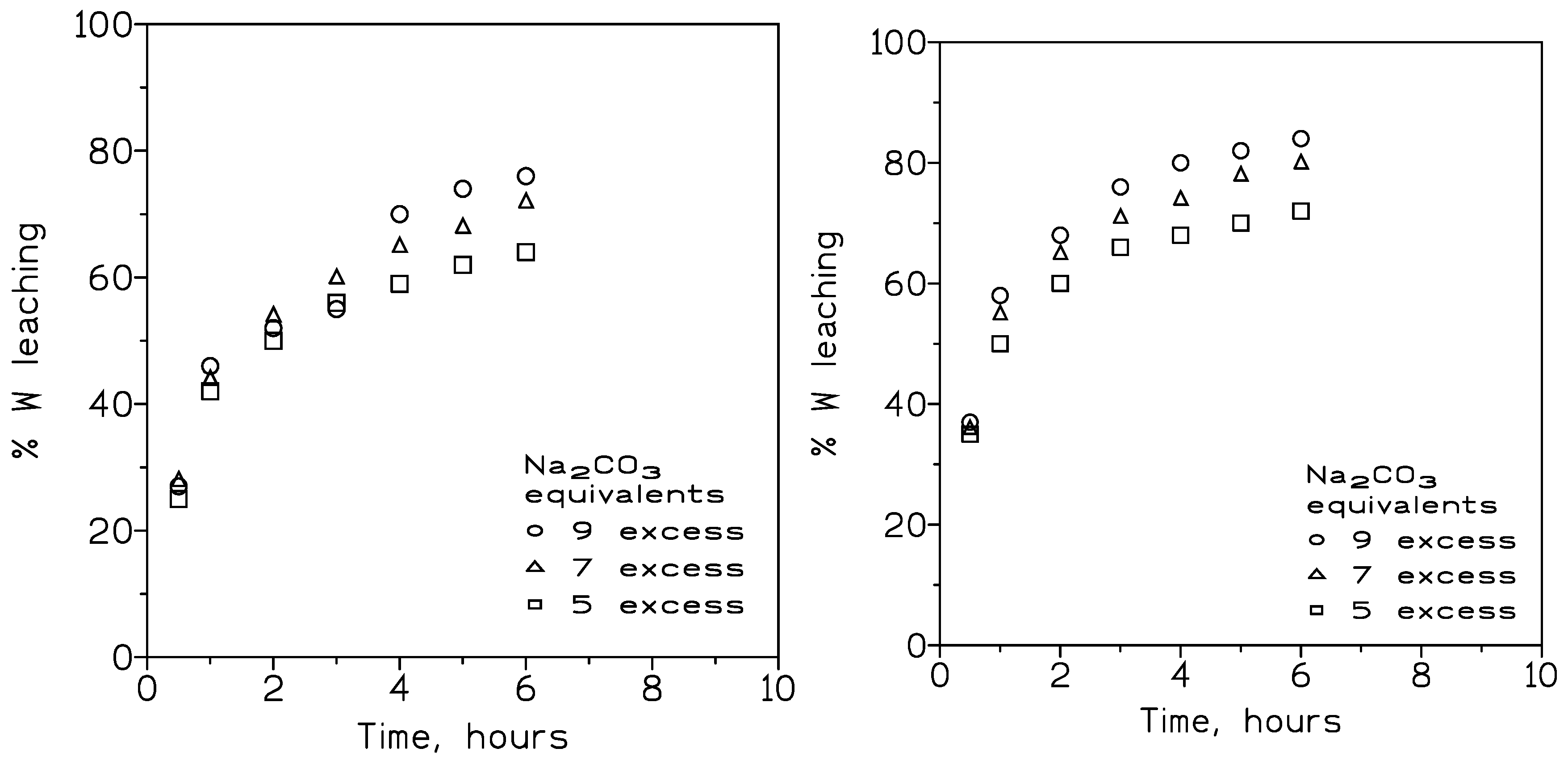
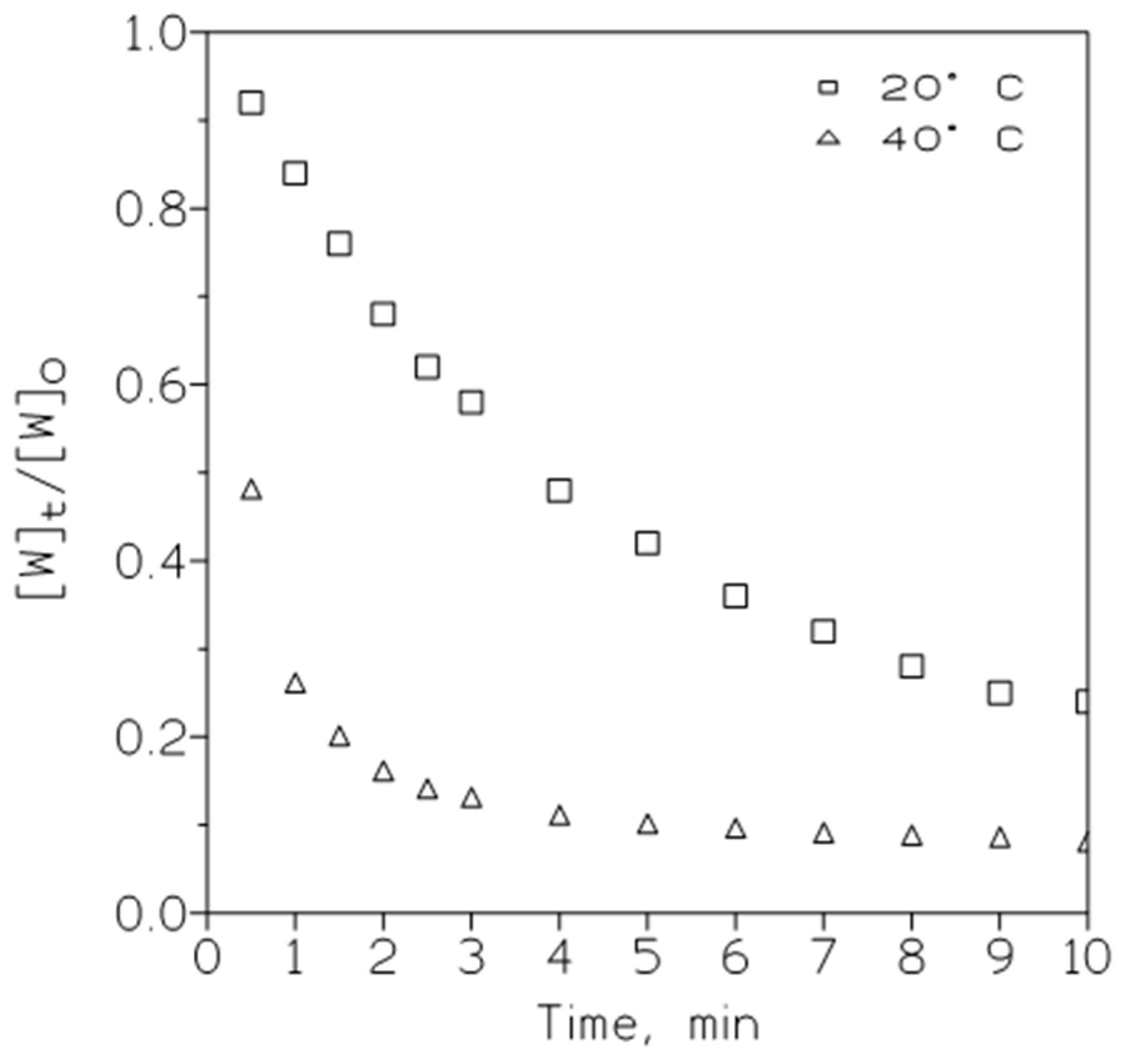
3.1.3. Influence of the Temperature
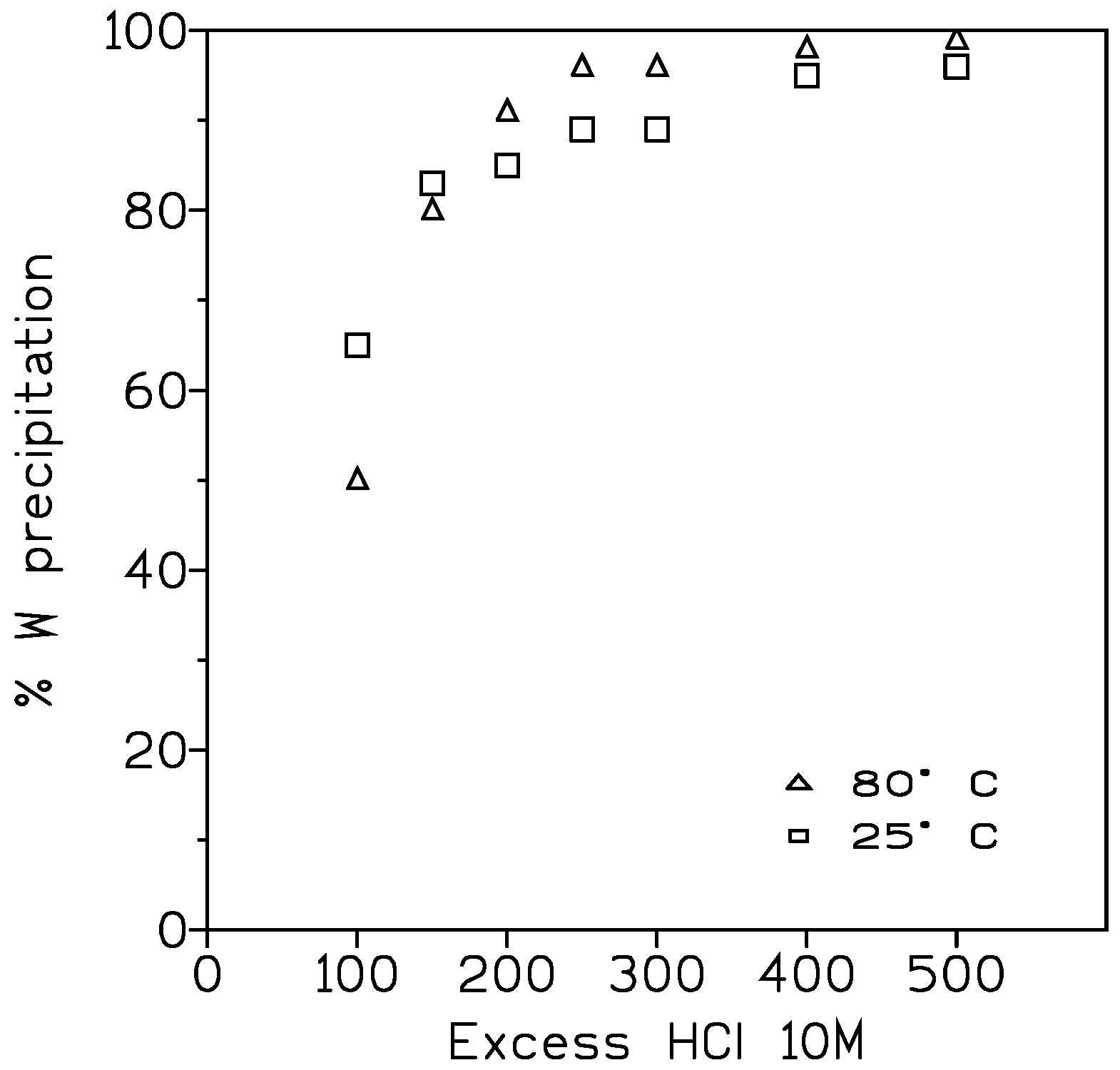
3.1.4. Treatment of the Leachate
3.2. Leaching in Hydrochloric Acid Medium
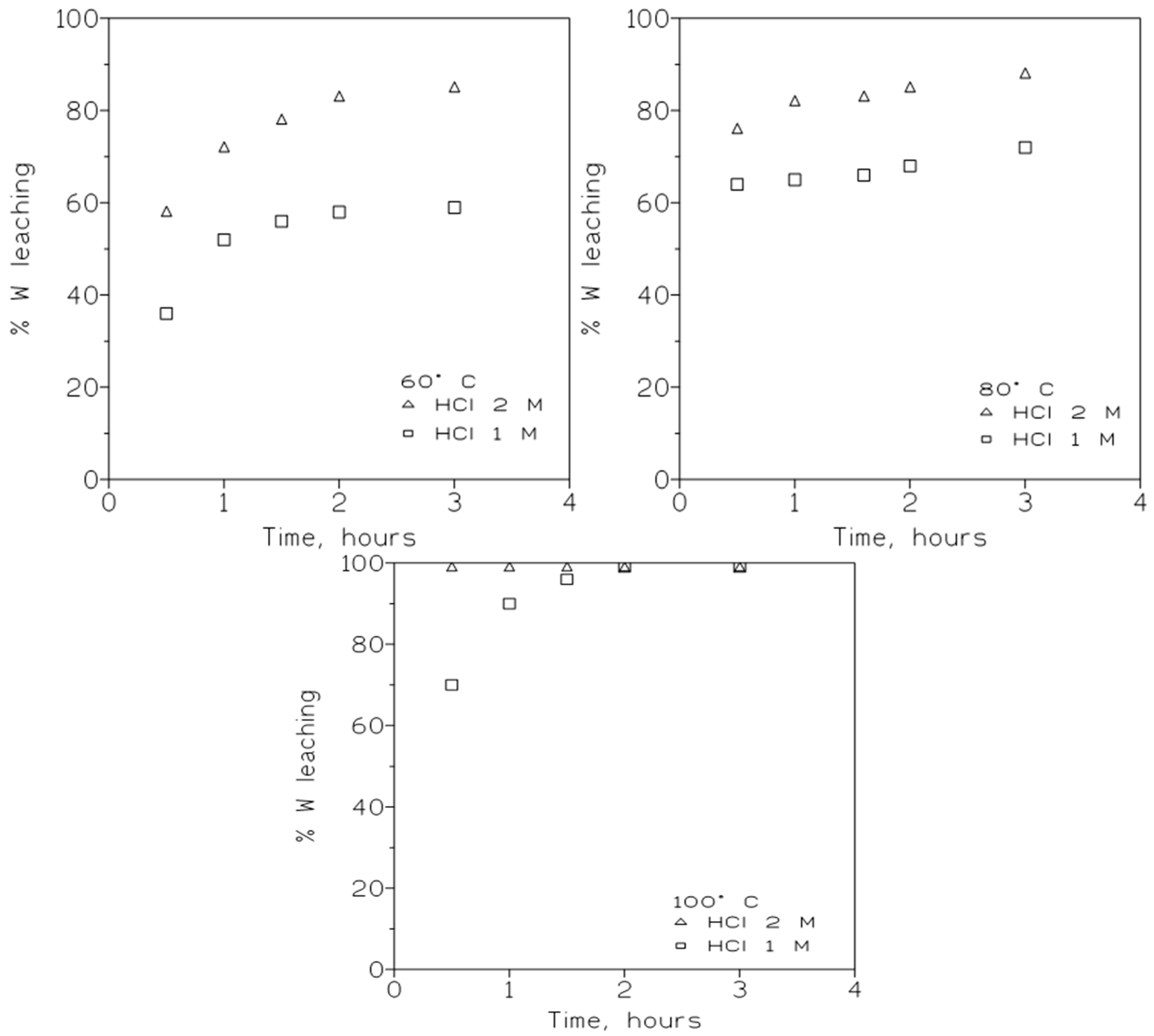
3.2.1. Influence of the HCl Concentration and Temperature on Tungsten Leaching
3.2.2. Influence of the Particle Size on Tungsten Leaching
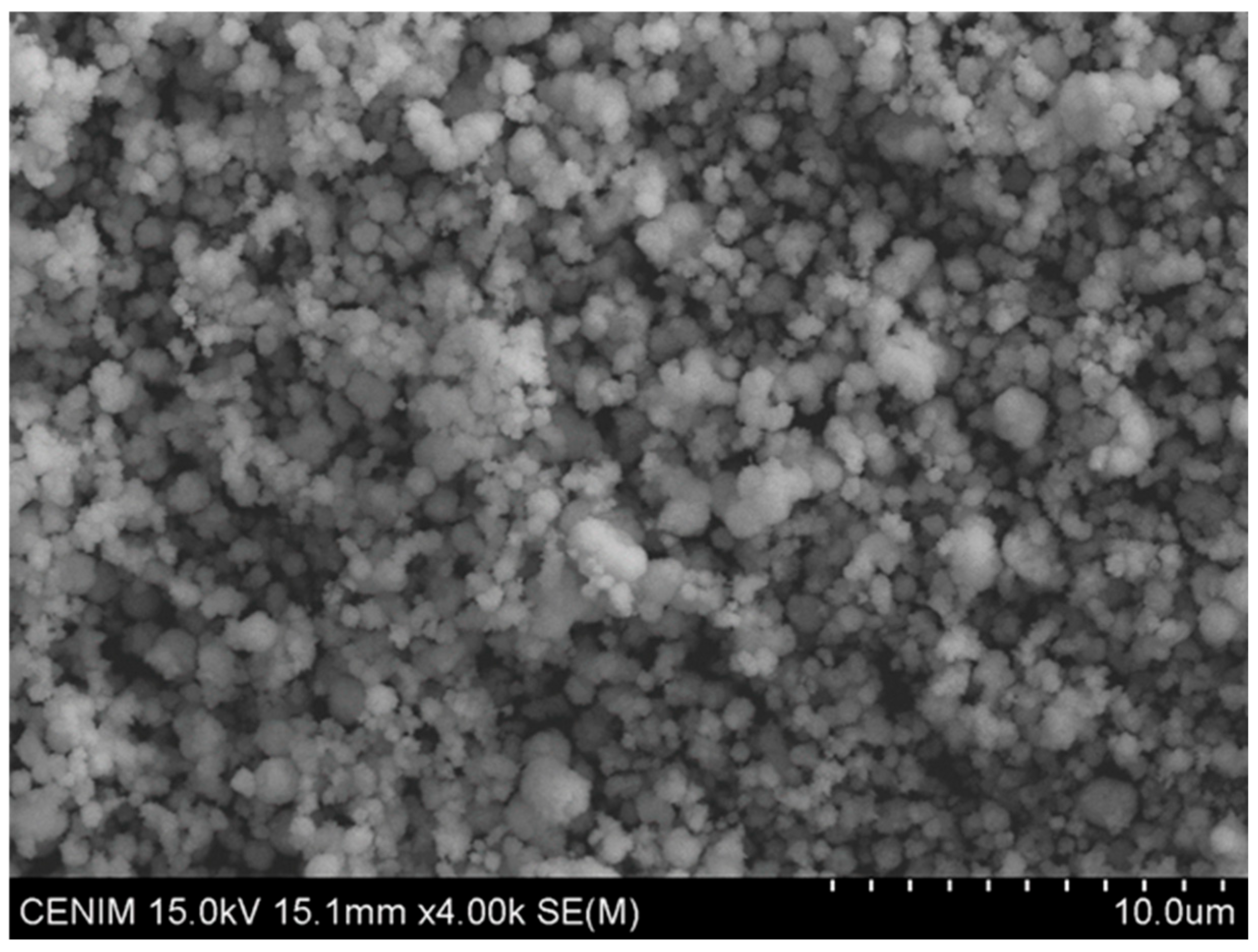
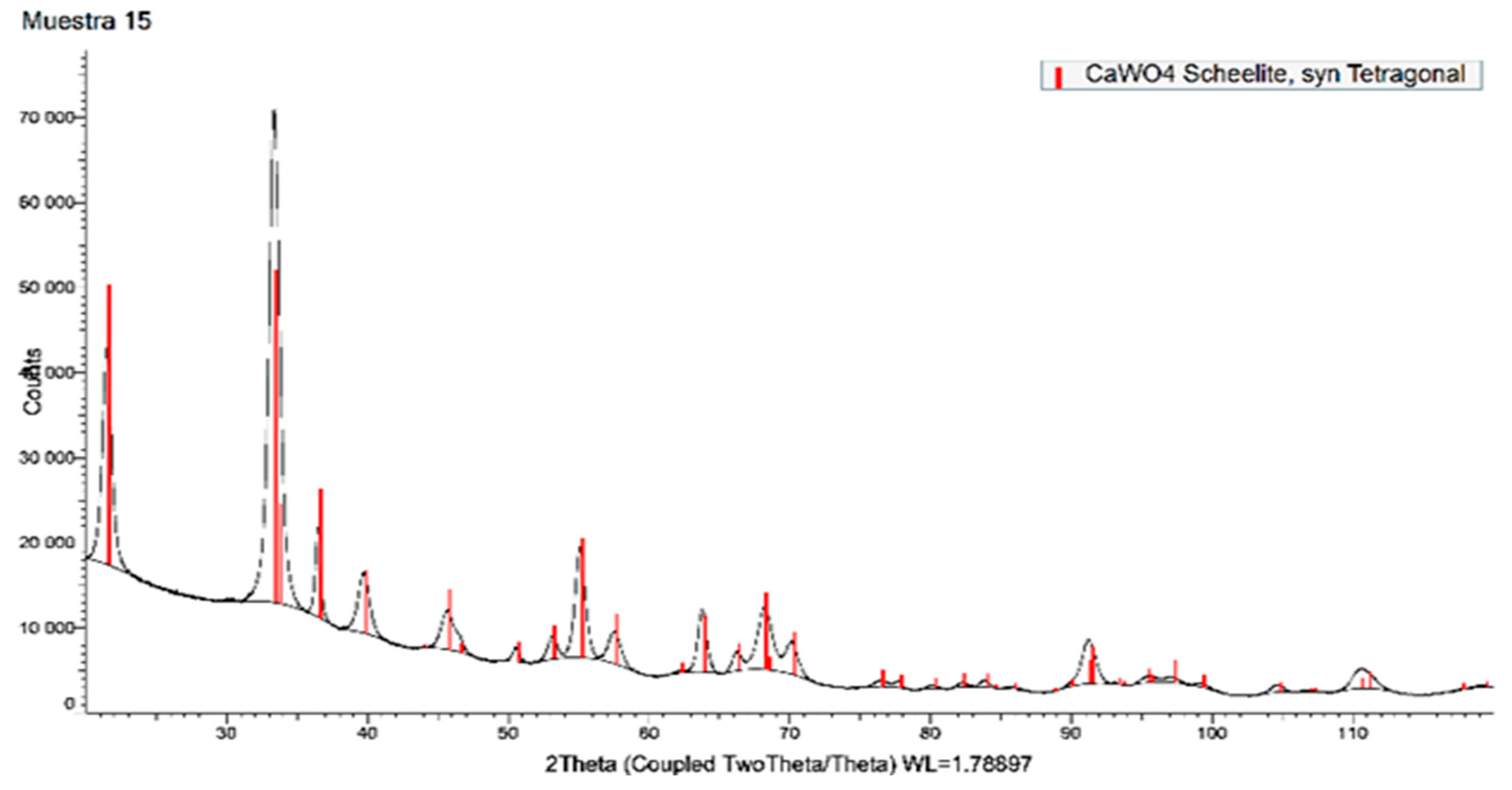
4. Obtaining Advanced Tungsten Compounds (Nanotungsten Derivatives)
4.1. From the Alkaline Solutions
4.2. From Acidic Medium
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grohol, M.; Veeh, C. Study on the Critical Raw Materials for the EU 2023—Final Report; European Commission: Directorate-General for Internal Market, Industry, Entrepreneurship and SMEss; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Alonso, M. Recovery of tungsten from raw and secondary materials using hydrometallurgical processing. Metals 2025, 15, 799. [Google Scholar] [CrossRef]
- Chen, Y.; Huo, G.; Guo, X.; Chen, J. A review of flowsheets for tungsten recovery from scheelite, wolframite and secondary resources and challenges for sustainable production. Hydrometallurgy 2025, 234, 106455. [Google Scholar] [CrossRef]
- Li, X.; Jiang, H.; Peng, Z.; Duan, A.; Zhang, T.; Gong, Z. Leaching of scheelite concentrate for tungsten extraction. Minerals 2025, 15, 475. [Google Scholar] [CrossRef]
- Zhao, J.-Y.; Mu, P.-P.; Liu, H.; Chachina, S.B.; Zhang, X.-G.; Zhang, S.-G.; Pan, D. Extraction and response surface methodology optimization of tungsten and molybdenum from spent fluidized catalytic cracking catalysts. Tungsten 2025, 7, 268–283. [Google Scholar] [CrossRef]
- Li, D.; Chang, J.; Wei, Y.; Qiao, J.; Yue, X.; Fan, H.; Zhang, L.; Guo, X. Green and efficient recovery of tungsten from spent SCR denitration catalyst by Na2S alkali leaching and calcium precipitation. Adv. Sust. Syst. 2025, 9, 2400895. [Google Scholar] [CrossRef]
- Cera, M.; De Gaudenzi, G.P.; Tedeschi, S.; Muntoni, A.; De Gioannis, G.; Serpe, A. Valorisation of hard metal wastes using organic acids in an eco-friendly process. Int. J. Refract. Met. Hard Mater. 2025, 128, 107062. [Google Scholar] [CrossRef]
- Zheng, X.; Li, H.; Liu, X.; Wang, X.; Zhang, Y.; Tang, Q.; Ren, B. Supply risk propagation in international trade networks of the tungsten industry chain. Humanit. Soc. Sci. Commun. 2025, 12, 54. [Google Scholar] [CrossRef]
- Asghar, M.Z.; Saeed, A.; Ain, N.U.; Alarfaji, S.S.; Khan, M.I. Tungsten tetraboride nanosheet as a potential gas adsorption material: A density functional theory (DFT) study. Chem. Phys. Lett. 2025, 870, 142090. [Google Scholar] [CrossRef]
- Zhao, K.; Shi, Y.; Niu, H.; Qinglong Chen, Q.; Liu, J.; Tang, B.; Zheng, C. Polyaniline/tungsten disulfide composite for room-temperature NH3 detection with rapid response and low-ppm sensitivity. Sensors 2025, 25, 3948. [Google Scholar] [CrossRef]
- Verma, A.M.; Prakash, C.; Yadav, N.; Verma, V.; Singh, P.; Ojha, S.; Sanjay Kumar Kedia, S.K.; Singh, F.; Siva Kumar, V.V.; Brajpuriya, R.K.; et al. Enhanced hydrogen gas sensing performance with Ag-doped WO3 thin film. Int. J. Hydrogen Energy 2025, 137, 1161–1170. [Google Scholar] [CrossRef]
- Wu, H.; Peng, Z.; Hao, J.; Tesfaye, F.; Shen, L. Gradient recovery of tungsten, cerium, and titanium from spent W-Ce/TiO2 catalysts. Processes 2025, 13, 1678. [Google Scholar] [CrossRef]
- Sasikumar, K.; Thangabalu, S.; Muthuramamoorthy, M. Ni-doped WO3 nanoparticles: An efficient dual-functional material for photocatalytic and antibacterial applications. Ceram. Int. 2025, 51, 21424–21440. [Google Scholar] [CrossRef]
- Xie, H.; Li, X.; Wang, Z.; Zhao, J.; Chen, Q.; Wang, Y.; Khalifa, M.A.; Zhao, B.; Pei, G.; Zheng, J.; et al. Boosting WO3-based electrochromic performance for practical smart windows: Balancing charge density and matching color based on Mn2+/MnO2 counter-electrode reaction. Adv. Opt. Mater. 2025, 13, 2500015. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Jin, G.; Ma, Y.; Bian, Y.; Hu, M.; Li, C.; Luo, S. Research on the effect of ultrasonic-electric pulse composite milling on the machining performance of tungsten alloy. Int. J. Adv. Manuf. Technol. 2025, 138, 5521–5536. [Google Scholar] [CrossRef]
- Pandey, B.; Mishra, R.; Chauhan, R.K.; Patel, A.K. Performance investigation of double absorber based thin film solar cell with MoS2 and WS2 as absorber layers. Phys. Scr. 2025, 100, 055529. [Google Scholar] [CrossRef]
- Tuncer, I.M.; Yue, H.; Liu, J. An optimisation study for leaching synthetic scheelite in H2SO4 and H2O2 solution. Can. Metall. Q. 2025, 64, 653–663. [Google Scholar] [CrossRef]
- Shen, L.T.; Liu, Y.; Guo, J.-L.; Zhou, Q.-S.; Qi, T.-G.; Peng, Z.-H.; Liu, G.-H.; Xiao-bin Li, X.-L. Leaching of WO3 from sulfuric acid converted product of scheelite in NH3·H2O−NH4HCO3 solution. Trans. Nonferrous Met. Soc. China 2025, 35, 326–337. [Google Scholar] [CrossRef]
- Karimova, L.; Oleinikova, T.; Terentyeva, I.; Korabayev, A.; Tussupbekova, T. Study of the efficiency of using pelletizing poor scheelite ore for leaching tungsten. Acta Metall. Slovaca 2025, 31, 11–15. [Google Scholar] [CrossRef]
- Han, Z.; Edraki, M.; Golev, A. Characterization, potential valuable metals recovery and remediation of historic wolframite mining waste. Sep. Purif. Technol. 2025, 354, 129009. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, Z.; Li, Q.; Liu, L.; Chen, M. The tungsten tailings remediation by soilless plant establishment: Varied aggregation structure, heavy metal mobilization, and microbial community structure. Ecol. Eng. 2025, 213, 107565. [Google Scholar] [CrossRef]
- Chen, K.; Li, L.; Cao, Q. Recovery of rhenium and tungsten from calcium tungstate containing rhenium via hydrochloric acid decomposition. Sep. Sci. Technol. 2025, 60, 480–493. [Google Scholar] [CrossRef]
- Zhang, P.; Ren, Q.; Mi, H.; Kang, S.; Yang, B.; Wang, F. Simultaneous separation and enrichment of tungsten and tin from wolframite by one-step vacuum distillation. Sep. Purif. Technol. 2025, 376, 133966. [Google Scholar] [CrossRef]
- Pu, T.; Chen, Z.; Liu, Y.; Hu, H.; Liang, Y. Factors affecting purity of ammonium paratungstate (APT) prepared from wolframite ((Fe,Mn)WO4) concentrate by NaHSO4·H2O roasting, water/ammonia leaching and evaporation crystallization. Hydrometallurgy 2025, 234, 106467. [Google Scholar] [CrossRef]
- Wang, C.; Wu, J.; Wan, K.; Miao, Z. Synergistic recovery of Ge and W from lignite via subcritical water activation followed by alkaline leaching. Min. Eng. 2025, 232, 109580. [Google Scholar] [CrossRef]
- Shemi, A.; Ndlovu, S.; Sacks, N. An optimized recovery of cobalt and tungsten carbide (WC) from a binary-phase WC-6 wt% Co cemented tungsten carbide hard metal using design of experiments. Min. Eng. 2025, 233, 109597. [Google Scholar] [CrossRef]
- Huo, T.; Sun, F.; Liu, X.; Chen, X.; Li, J.; He, L.; Zhang, W.; Zhao, Z. Synthesis of intermediate valence tungsten oxides via carbon reduction and their dissolution behavior in hydrogen peroxide. Int. J. Refract. Met. Hard Mater. 2025, 131, 107191. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, Y.; Xi, X.; Nie, Z. 1T/2H-Mo1-xWxS2 obtained from secondary resources and its superior piezoelectric catalysis. J. Environ. Chem. Eng. 2025, 13, 115500. [Google Scholar] [CrossRef]
- Wu, T.; Wang, J.; Sun, Q.; Yang, Y.; Si, Q.; Liang, C.; Liu, G.; Mi, A.; Wang, S. Recycling efficiency optimization of tungsten-filled vinyl-methyl-silicone-based flexible gamma ray shielding materials. Nucl. Eng. Technol. 2025, 57, 103163. [Google Scholar] [CrossRef]
- Lin, S.; Yi, B.; Hu, X.; Fang, L.; Yu, S.; Yu, S.; Wu, D. Advanced sequential treatment approach for enhanced recovery in tungsten-nickel electroplating wastewater. J. Water Proc. Eng. 2025, 71, 107421. [Google Scholar] [CrossRef]
- Li, H.; Li, Q.; Zhang, G.; Zeng, L.; Wu, S.; Guan, W.; Wang, M.; Wu, X. A novel pyro-hydrometallurgy process for efficient recovery of tungsten, vanadium, and titanium from spent SCR catalysts. J. Environ. Chem. Eng. 2025, 13, 115253. [Google Scholar] [CrossRef]
- Xie, T.; Pu, T.; Liu, Y.; Zhang, M.; Lei, X.; Hu, H.; Chen, Z. Extraction of tungsten from tungsten fine mud by caustic soda autoclaving. ACS Omega 2025, 10, 6901–6907. [Google Scholar] [CrossRef]
- Zhang, C.; Xi, X.; Ma, L.; Chen, N.; Nie, Z. Highly efficient separation of tungsten and molybdenum by selective adsorption with iron based metal organic framework enhanced by cetyltrimethyl ammonium bromide as complex agent. Desalination 2025, 614, 119122. [Google Scholar] [CrossRef]
- Ahmed, Z.M.A.; Morad, A.R.; Gubari, M.Q.; Mahmoud, M.A.; Eldoma, M.A.; Alomar, M.A.; Abouatia, A.F.F.; Marashdeh, A.; Cheira, M.F.; Hassanein, T.F. Recovery of tungsten from catalytic converter scraps using a Schiff-base adsorbent and subsequent preparation of tungsten oxide nanoparticles. Mater. Chem. Phys. 2025, 344, 131146. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, H.; Tong, L.; Zhang, Q.; Zhang, J. Coupled precipitation enrichment of molybdenum and tungsten for synthesizing AMTOF (artificial molybdenum and tungsten ore first) from a complex trace molybdenum-tungsten mixed solution. Min. Eng. 2025, 228, 109328. [Google Scholar] [CrossRef]
- Zhu, J.; Qiu, Y.; He, J.; Chen, X.; Chen, A.; Liu, X.; Li, J.; He, L.; Sun, F.; Zhao, Z. Research on the ammonia-free solvent extraction of tungsten from sodium tungstate solution using TBP. Chem. Eng. J. 2025, 513, 162869. [Google Scholar] [CrossRef]
- Deng, L.-Q.; Liu, X.-H.; Chen, X.-Y.; Li, J.-T.; He, L.-H.; Sun, F.-L.-; Zhao, Z.-W. Competitive behaviors between tungsten and iron in TBP−HCl−H2O extraction system. Trans. Nonferrous Met. Soc. China 2025, 35, 990–999. [Google Scholar] [CrossRef]
- Wang, C.; Liu, L.; Hu, W.; Wang, C.; Hou, X.; Li, H. Separation of vanadium, tungsten, and arsenic from alkaline leachate of spent SCR catalysts via coextraction and stepwise stripping. Sep. Purif. Technol. 2025, 352, 127991. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Q.; Qi, J.; Liu, G. Probing the improved collecting ability of dodecyl phosphate/poly-amine cation-anion association collector towards scheelite. Adv. Powder Technol. 2025, 36, 104977. [Google Scholar] [CrossRef]
- Wang, X.; Cui, Y. Mechanism of flotation separation of scheelite from calcite using sulfonated phenolic resin as a novel depressant. Min. Eng. 2025, 230, 109393. [Google Scholar] [CrossRef]
- Rodriguez, J.F.; Alguacil, F.J.; Martinez, S.; Caravaca, C. Production of high-grade tin in a new electrolytic plant. In Hydrometallurgy’94; Institution of Mining and Metallurgy, Society of Chemical Industry, Eds.; Chapman & Hall: London, UK, 1994; pp. 939–946. [Google Scholar]
- Ye, L.; Tang, K.; Zhang, L.; Xia, Z.; Liu, S. Separation of cobalt and tungsten from grinding waste of cemented carbide by a midly process of chloridizing roasting and water leaching combining. Int. J. Refract. Met. Hard Mater. 2025, 132, 107303. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Lv, S.; Luo, Y.; Zhao, Z.; Chen, X.; Liu, X.; Lihua He, L.; Sun, F. Mechanism of calcite inhibiting scheelite decomposition during the NaOH decomposition process. JOM 2025, 77, 3074–3081. [Google Scholar] [CrossRef]
- Barton, I.F.; Hiskey, J.B. Chalcopyrite leaching in novel lixiviants. Hydrometallurgy 2022, 207, 105775. [Google Scholar] [CrossRef]
- Ibrahim, A.I.I.; Aboelgamel, M.; Kaan Soylu, K.K.; Top, S.; Kursunoglu, S.; Altiner, M. Production of high-grade antimony oxide from smelter slag via leaching and hydrolysis process. Sep. Purif. Technol. 2025, 355, 129355. [Google Scholar] [CrossRef]
- Grebenkov, D.S. Diffusion-controlled reactions: An overview. Molecules 2023, 28, 7570. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.I.; Moreira, A.; Costa, S.C. Leaching of synthetic scheelite by hydrochloric acid without the formation of tungstic acid. Hydrometallurgy 2003, 70, 131–141. [Google Scholar] [CrossRef]
- Kahruman, C.; Yusufoglu, I. Leaching kinetics of synthetic CaWO4 in HCl solutions containing H3PO4 as chelating agent. Hydrometallurgy 2006, 81, 182–189. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Alonso, M.; Lozano, L.J.; Robla, M. Strategies for the recovery of tungsten from wolframite, scheelite, or wolframite–scheelite mixed concentrates of Spanish origin. Metals 2025, 15, 819. [Google Scholar] [CrossRef]
- Alguacil, F.J. Mechanistic investigation of facilitated transport of gold(III) from HCl media using ionic liquid Cyphos IL102 as carrier across a supported liquid membrane. Gold Bull. 2019, 52, 145–151. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Escudero, E. The removal of toxic metals from liquid effluents by ion exchange resins. Part XII: Mercury(II)/H+/Lewatit SP112. Rev. Metal. 2020, 56, e160. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, J.; Dong, X.; Zeng, Z.; Lin, K.; Li, J. Rapid pyrolysis-based fabrication of high-performance electrochromic WO3 films using polyethylene glycol as a pore-forming agent. J. Mater. Sci. 2025, 60, 2297–2313. [Google Scholar] [CrossRef]
- Jiang, B.; Ning, H.; Li, M.; Yao, R.; Guo, C.; Yucheng, H.; Zijie, G.; Luo, D.; Yuan, D.; Peng, J. Predictive modeling of electrochromic performance in ammonium metatungstate solutions using machine learning algorithms. AIP Adv. 2025, 15, 025308. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, B.; Li, S.; Pan, F.; Zhu, X.; Wei, X.I. N-S co-doped WC nanoparticles show high catalytic activity in hydrogen evolution reaction. Coatings 2025, 15, 630. [Google Scholar] [CrossRef]
- Li, Y.; Long, R.; Tao, J.; Chen, X.; Liu, Y.; Li, C.; Xu, Z.; Yi, J. Regulation of the interfacial structure in CNTs/Cu composites by chemical vapor deposited WC to enhance mechanical properties and conductivity. Ceram. Int. 2025, 51, 23725–23740. [Google Scholar] [CrossRef]
- Zhou, Y.; Ding, W.; Wei, J.; Luo, L.; Ma, B.; Zhang, Y.; Wang, J.; Liu, J.; Hai, W.; Wu, Y. The effect of reduction heat treatment after mechanical alloying on the microstructure and properties of Cu-Y2O3/W composites. J. Alloys Compd. 2025, 1030, 180835. [Google Scholar] [CrossRef]
- Jiang, H.-Y.; Chen, G.-L.; Xin, Y.-Y.; Shen, S.-K.; Deng, Z.-P.; Xu, Y.-M.; Huo, L.-H.-; Gao, S. Honeycomb-like WO3 architecture derived from grapevine boosts efficient detection of trace NO2 gas at near room temperature. Sens. Actuators B Chem. 2025, 436, 137686. [Google Scholar] [CrossRef]
- Chen, G.-L.; Jiang, H.-Y.; Jiang, Y.; Deng, Z.-P.; Xu, Y.-M.; Huo, L.-H.; Gao, S. Temperature-tuned dual-selective NO2/diisopropylamine sensor based on (NH4)0.33WO3 doped γ-WO3 nanosheets-assembled hollow microspheres via simple pyrolysis of commercial ammonium metatungstate. Chem. Eng. J. 2025, 512, 162684. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhou, X.; Yang, L. Boosting oxidative-adsorptive desulfurization of fuel oil by constructing a mesoporous coupled desulfurizer with bimetal of MoO3 and WO3. J. Porous Mater. 2025, 32, 1429–1441. [Google Scholar] [CrossRef]
- Baba, A.A.; Girigisu, S.; Raji, M.A.; Ibrahim, A.S.; Ayinla, K.I.; Adeyemi, C.O.; Abdulkareem, A.Y.; Abdul, M.J.; Alabi, A.G.F. Preparation of high grade ammonium metatungstate (AMT) as precursor for industrial tungsten catalyst. In Rare Metal Technology; Azimi, G., Ouchi, T., Forsberg, K., Kim, H., Alam, S., Baba, A.A., Neelamegg, N.R., Eds.; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Liming, Z.; Leiting, S.; Zhou, Q.; Qi, T.; Peng, Z.; Liu, G.; Li, X. Green and low-carbon preparation of ammonium paratungstate by adding ammonia to ammonium metatungstate solution. Hydrometallurgy 2023, 222, 106196. [Google Scholar] [CrossRef]
- Chinatungsten. Available online: http://news.chinatungsten.com (accessed on 1 June 2025).
- Eder, F.; Weil, M.; Schubert, W.-D.; Gierl-Mayer, C. Ammonium metatungstate, (NH4)6[H2W12O40]: Crystallization and thermal behavior of various hydrous species. Cryst. Growth Des. 2021, 21, 6195–6212. [Google Scholar] [CrossRef]
- Althubayti, M.M.; Abdel-Daiem, A.; Farghal, M.; Loucif, A.; Aida, M.S. Electrical and dielectric properties of sprayed tungsten oxide thin films. Next Mater. 2025, 8, 100672. [Google Scholar] [CrossRef]
- Lourenço, C.S.; Oliveira, G.S.; Morais, L.M.F.; do Nascimento, A.B.G.; Silva, A.S.; Morales, M.A.; Lima, M.J.S.; Gomes, U.U. Influence of temperature on the APT carborreduction process for obtaining nanocrystalline WC powder from scheelite concentrate. Mat. Res. 2025, 28, 0279. [Google Scholar] [CrossRef]
- Brauer, G. Handbuch der Präparativen Anorganischen Chemie; Spanish Edition for Ed. Reverte S.A. Barcelona (Spain); Ferdinand Enke Verlag: Stuttgart, Germany, 1958. [Google Scholar]
- Nakamoto, K. Infrared Spectra of Inorganic Compound and Coordination Compounds; Wiley: New York, NY, USA, 1963. [Google Scholar]
- Nyquist, R.A.; Kagel, R.O. Infrared Spectra of Inorganic Compounds; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Agrawal, A.; Cho, S.H.; Zandi, O.; Ghosh, S.; Johns, R.W.; Milliron, D.L. Localized surface plasmon resonance in semiconductor nanocrystals. Chem. Rev. 2018, 118, 3121–3207. [Google Scholar] [CrossRef] [PubMed]
- Zafari, M.; Shariatmadar Tehrani, F. Enhanced photocatalytic performance of non-stoichiometric WO3−x nanocrystals via near-infrared localized surface plasmon resonance. Sci. Rep. 2025, 15, 14624. [Google Scholar] [CrossRef] [PubMed]
- Mousavi-Zadeh, S.H.; Poursalehi, R.; Yourdkhani, A. Photocatalytic activity mechanism of oxygen vacancy saturated blue WO3−x/exfoliated g-C3N4 Z-scheme heteronanostructures. Appl. Surf. Sci. Adv. 2025, 27, 100755. [Google Scholar] [CrossRef]
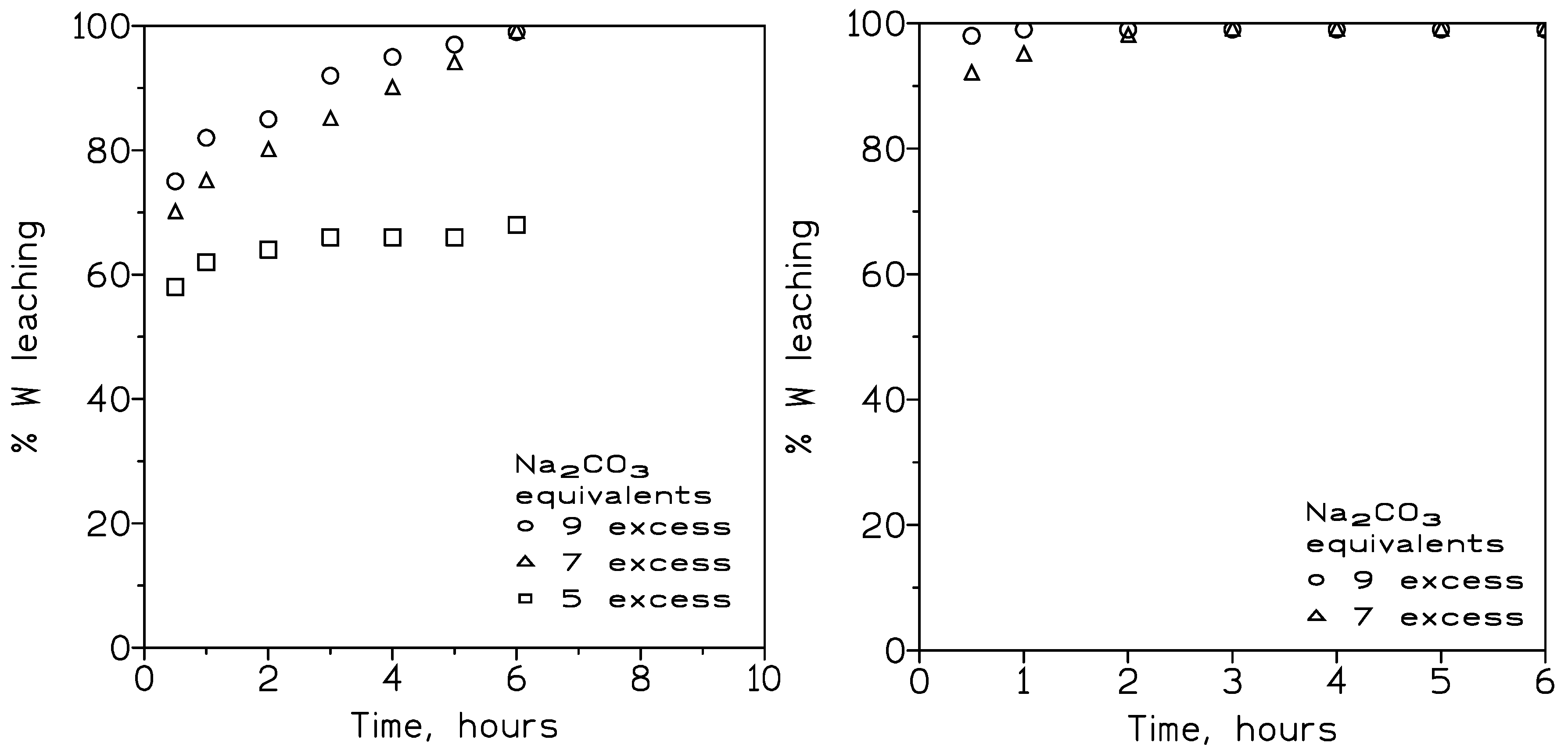





| Time, Hours | 9 wt% | 16 wt% | 26 wt% |
|---|---|---|---|
| 2 | 52 | 50 | 50 |
| 3 | 55 | 58 | 54 |
| 4 | 58 | 62 | 58 |
| [HCl], M | 106 µm < Sample < 149 µm | 53 µm < Sample < 74 µm |
|---|---|---|
| 1 | 71 | 81 |
| 2 | 88 | 92 |
| 3 | 89 | 92 |
| 4 | 75 | 84 |
| 5 | 73 | 83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alguacil, F.J. From a Scheelite Concentrate (Spanish Origin) to Nanotungsten Derivatives. Minerals 2025, 15, 1095. https://doi.org/10.3390/min15101095
Alguacil FJ. From a Scheelite Concentrate (Spanish Origin) to Nanotungsten Derivatives. Minerals. 2025; 15(10):1095. https://doi.org/10.3390/min15101095
Chicago/Turabian StyleAlguacil, Francisco Jose. 2025. "From a Scheelite Concentrate (Spanish Origin) to Nanotungsten Derivatives" Minerals 15, no. 10: 1095. https://doi.org/10.3390/min15101095
APA StyleAlguacil, F. J. (2025). From a Scheelite Concentrate (Spanish Origin) to Nanotungsten Derivatives. Minerals, 15(10), 1095. https://doi.org/10.3390/min15101095







