From Solid to Solution: How Surface-Active Agents Influence Bioleaching Efficiency and Bacteria–Mineral Interactions
Abstract
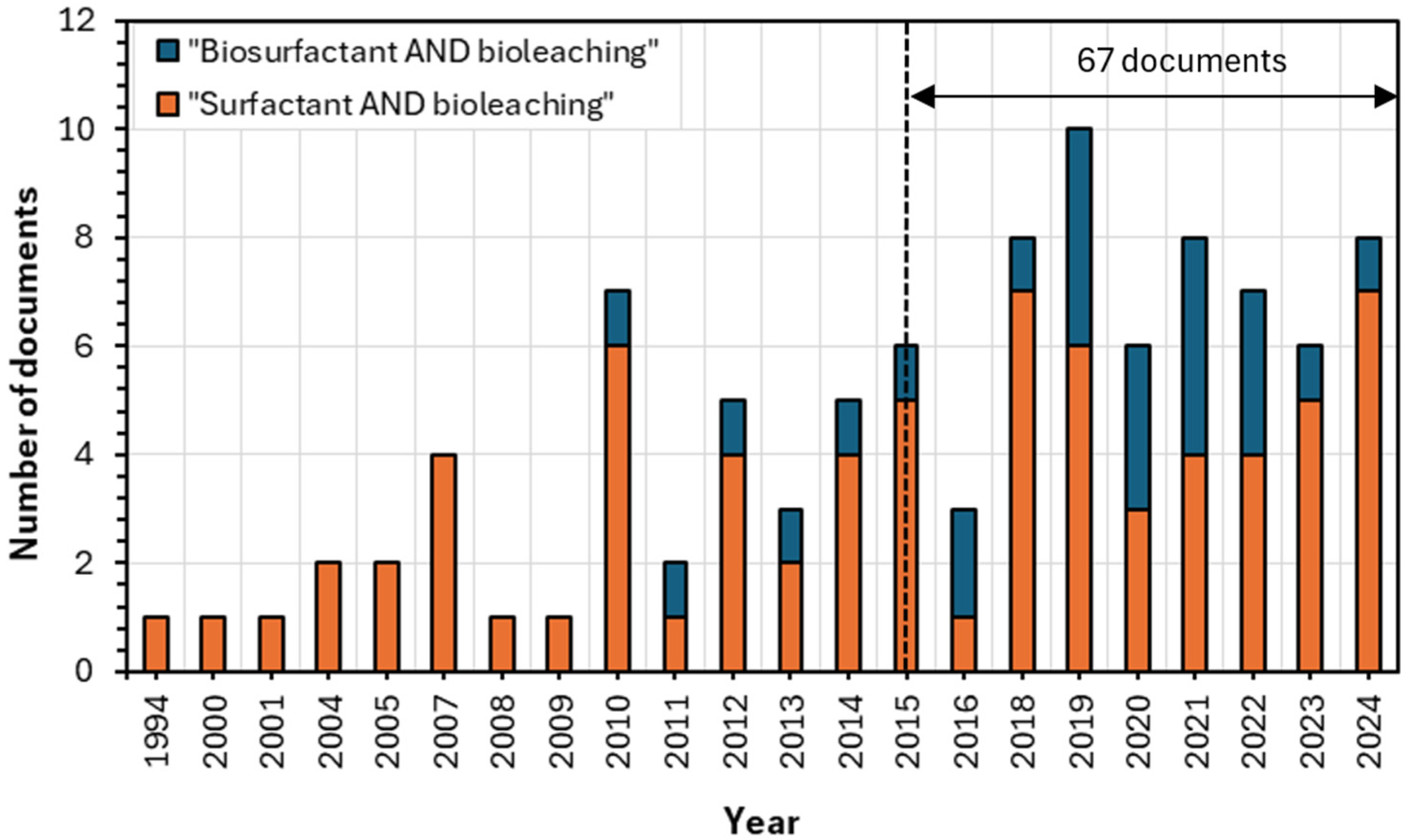
1. Introduction
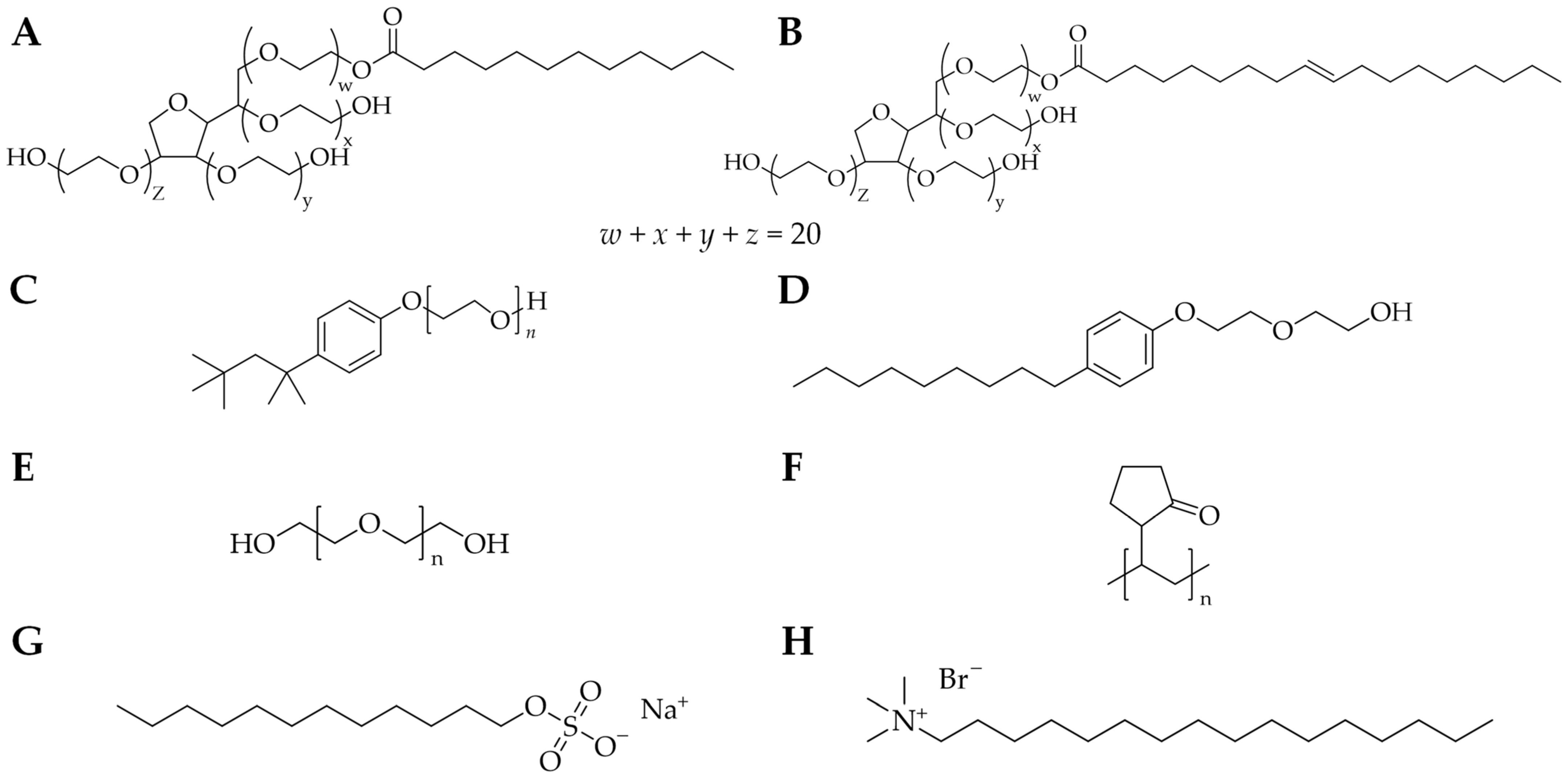
2. Surface-Active Agents Used in Bioleaching
2.1. Surfactants and Polymers
2.2. Biosurfactants and Lignin-Based Polymers
2.3. Adsorption at Hydrophobic/Hydrophilic Surfaces
3. Effect of Various Reagents on Bacterial Growth
- -
- 0.01 g/L: NaEX > KIPX > MIBC/KIBX > PO/KAX > Aero3477;
- -
- 0.1 g/L: NaEX > KIPX > MIBC > KIBX > KAX > PO > Aero3477;
- -
- 1.0 g/L: NaEX > KIPX > MIBC > PO > KIBX > KAX > Aero3477.
4. Interactions with Minerals and Microorganisms
4.1. Mineral Surface Wettability
4.2. Interactions and the Role of Zeta Potential
5. Influence on Metal Recovery
6. Summary
6.1. Current Knowledge Limitations
6.2. Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aero3477 | Isobutyl sodium phosphorodithioate (flotation reagent) |
| CaLS | Calcium lignosulfonate (lignin-based polymer) |
| CMC | Critical micelle concentration |
| CTAB | Cetyltrimethylammonium bromide (cationic surfactant) |
| DTAB | Dodecyltrimethylammonium bromide (cationic surfactant) |
| EO | Ethylene oxide |
| HLB | Hydrophilic–lipophilic balance |
| KAX | Potassium amylxanthate (flotation reagent) |
| KIBX | Potassium isobutylxanthate (flotation reagent) |
| KIPX | Potassium isopropylxanthate (flotation reagent) |
| MIBC | Methyl isobutyl carbinol (nonionic surfactant) |
| NaEX | Sodium ethylxanthate (flotation reagent) |
| NaLS | Sodium lignosulfonate (lignin-based polymer) |
| NHD | Polyethylene glycol dimethyl ether (polymer) |
| NP-12 | Polyoxyethylene (12) nonyl phenyl ether (nonionic surfactant) |
| NP-15 | Polyoxyethylene (15) nonyl phenyl ether (nonionic surfactant) |
| OPD | O-phenylenediamine (reagent, aromatic diamine) |
| PEG | Polyethylene glycol (polymer) |
| PO | Pine oil (natural surfactant) |
| PVP | Polyvinylpyrrolidone (polymer) |
| SDS | Sodium dodecyl sulfate (anionic surfactant) |
References
- Svanedal, I.; Eivazi, A.; Norgren, M.; Edlund, H. Exploring the Versatility of Chelating Surfactants: A Review. Curr. Opin. Colloid. Interface Sci. 2024, 73, 101833. [Google Scholar] [CrossRef]
- Pawlowska, A.; Sadowski, Z. The Role of Biomodification in Mineral Processing. Minerals 2023, 13, 1246. [Google Scholar] [CrossRef]
- Oulkhir, A.; Lyamlouli; Danouche, M.; Ouazzani, J.; Benhida, R. A Critical Review on Natural Surfactants and Their Potential for Sustainable Mineral Flotation. Rev. Environ. Sci. Biotechnol. 2023, 22, 105–131. [Google Scholar] [CrossRef]
- Peng, W.; Chang, L.; Li, P.; Han, G.; Huang, Y.; Cao, Y. An Overview on the Surfactants Used in Ion Flotation. J. Mol. Liq. 2019, 286, 110955. [Google Scholar] [CrossRef]
- Grzywaczyk, A.; Smułek, W.; Smułek, G.; Ślachciński, M.; Kaczorek, E. Application of Natural Surfactants for Improving the Leaching of Zinc and Copper from Different Soils. Environ. Technol. Innov. 2021, 24, 101926. [Google Scholar] [CrossRef]
- Bisht, K.; Tyagi, U.; Bajpai Tripathy, D. Biosurfactants—An Overview. Macromol. Symp. 2024, 413, 2200203. [Google Scholar] [CrossRef]
- Jiao, J. Polyoxyethylated Nonionic Surfactants and Their Applications in Topical Ocular Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1663–1673. [Google Scholar] [CrossRef]
- Nakama, Y. Surfactants. In Cosmetic Science and Technology: Theoretical Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 231–244. [Google Scholar] [CrossRef]
- Hong, I.K.; Kim, S.I.; Lee, S.B. Effects of HLB Value on Oil-in-Water Emulsions: Droplet Size, Rheological Behavior, Zeta-Potential, and Creaming Index. J. Ind. Eng. Chem. 2018, 67, 123–131. [Google Scholar] [CrossRef]
- Peng, A.A.; Liu, H.C.; Nie, Z.Y.; Xia, J.L. Effect of Surfactant Tween-80 on Sulfur Oxidation and Expression of Sulfur Metabolism Relevant Genes of Acidithiobacillus ferrooxidans. Trans. Nonferrous Met. Soc. China 2012, 22, 3147–3155. [Google Scholar] [CrossRef]
- Chen, J.; Xie, H.; Zhu, K.; Liu, Y.; Zhang, P.; Jin, Y.; Zeng, P.; Liu, D. Electrochemical Characteristic Analysis for Surface Passivation Layer of Galena and Chalcopyrite in Acid Corrosion. Miner. Eng. 2023, 199, 108129. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Zhong, C.; Wang, Z.; Yang, Q.; Tan, Q.; Ruan, R.; Qu, J. Comparison Study of Surfactants on Chalcopyrite Ore Bioleaching: Linking Microbiology with Mineral Oxidation. Min. Metall. Explor. 2025, 42, 1285–1293. [Google Scholar] [CrossRef]
- Liu, W.; Yang, H.Y.; Song, Y.; Tong, L.L. Catalytic Effects of Activated Carbon and Surfactants on Bioleaching of Cobalt Ore. Hydrometallurgy 2015, 152, 69–75. [Google Scholar] [CrossRef]
- Su, G.; Li, S.; Deng, X.; Hu, L.; Praburaman, L.; He, Z.; Zhong, H.; Sun, W. Low Concentration of Tween-20 Enhanced the Adhesion and Biofilm Formation of Acidianus manzaensis YN-25 on Chalcopyrite Surface. Chemosphere 2021, 284, 131403. [Google Scholar] [CrossRef]
- Pan, W.; Jin, H.; Liu, Z.; Tang, J.; Cheng, S. Experimental and Theoretical Study on Strengthening Leaching of Sulfide Ores by Surfactants. Process Saf. Environ. Prot. 2020, 137, 289–299. [Google Scholar] [CrossRef]
- Marine, J.E.; Menon, S.R.; Rumbelow, S.J. Surfactants (Polysorbate and Poloxamer): Synthesis, Characterization, and Degradation. In Surfactants in Biopharmaceutical Development; Academic Press: Cambridge, MA, USA, 2023; pp. 23–57. [Google Scholar] [CrossRef]
- Bąk, A.; Podgórska, W. Interfacial and Surface Tensions of Toluene/Water and Air/Water Systems with Nonionic Surfactants Tween 20 and Tween 80. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 414–425. [Google Scholar] [CrossRef]
- Liang, Z.; Marshall, A.G.; Westmoreland, D.G. Determination of Molecular Weight Distributions of Tert-Octylphenol Ethoxylate Surfactant Polymers by Laser Desorption Fourier Transform Ion Cyclotron Resonance Mass Spectrometry and High-Performance Liquid Chromatography. Anal. Chem. 1991, 63, 815–818. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, G.; Xin, X.; Zhang, H.; Shi, X. Surface Tension and Dilational Viscoelasticity of Water in the Presence of Surfactants Tyloxapol and Triton X-100 with Cetyl Trimethylammonium Bromide at 25 °C. J. Chem. Eng. Data 2009, 54, 989–995. [Google Scholar] [CrossRef]
- Kon-no, K.; Kitahara, A. Solubilization of Water and Secondary Solubilization of Electrolytes by Oil-Soluble Surfactant Solutions in Nonaqueous Media: II. Polyoxyethylene Nonylphenyl Ethers. J. Colloid. Interface Sci. 1970, 34, 221–227. [Google Scholar] [CrossRef]
- Zhang, R.; Wei, D.; Shen, Y.; Liu, W.; Lu, T.; Han, C. Catalytic Effect of Polyethylene Glycol on Sulfur Oxidation in Chalcopyrite Bioleaching by Acidithiobacillus ferrooxidans. Miner. Eng. 2016, 95, 74–78. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, D.; Liu, W.; Hou, D.; Zhang, R. Effect of Polyvinyl Pyrrolidone on Chalcopyrite Bioleaching with Acidithiobacillus ferrooxidans. Hydrometallurgy 2021, 205, 105753. [Google Scholar] [CrossRef]
- Gaballa, S.A.; Naguib, Y.W.; Mady, F.M.; Khaled, K.A. Polyethylene Glycol: Properties, Applications, and Challenges. J. Adv. Biomed. Pharm. Sci. J. Adv. Biomed. Pharm. Sci. 2024, 7, 26–36. [Google Scholar] [CrossRef]
- Haaf, F.; Sanner, A.; Straub, F. Polymers of N-Vinylpyrrolidone: Synthesis, Characterization and Uses. Polym. J. 1985, 17, 143–152. [Google Scholar] [CrossRef]
- Águila-Hernández, J.; Trejo, A.; García-Flores, B.E. Volumetric and Surface Tension Behavior of Aqueous Solutions of Polyvinylpyrrolidone in the Range (288 to 303) K. J. Chem. Eng. Data 2011, 56, 2371–2378. [Google Scholar] [CrossRef]
- Pawlowska, A.; Sadowski, Z. Effect of Schwertmannite Surface Modification by Surfactants on Adhesion of Acidophilic Bacteria. Microorganisms 2020, 8, 1725. [Google Scholar] [CrossRef]
- Pawlowska, A.; Sadowski, Z.; Winiarska, K. Bioleaching of Sulfide Containing Material from the Wiśniówka Quarry: Stability and Adhesion of Secondary Products. Physicochem. Probl. Miner. Process. 2022, 58, 149884. [Google Scholar] [CrossRef]
- Siebert, H.M.; Marmulla, R.; Stahmann, K.P. Effect of SDS on Planctonic Acidithiobacillus thiooxidans and Bioleaching of Sand Samples. Miner. Eng. 2011, 24, 1128–1131. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Cao, J.; Zeng, Z.; Liu, X.; Zhang, R.; Li, Q.; Sand, W. Bioleaching of Chalcopyrite Waste Rock in the Presence of the Copper Solvent Extractant LIX984N. Front. Microbiol. 2022, 13, 820052. [Google Scholar] [CrossRef] [PubMed]
- Markande, A.R.; Patel, D.; Varjani, S. A Review on Biosurfactants: Properties, Applications and Current Developments. Bioresour. Technol. 2021, 330, 124963. [Google Scholar] [CrossRef]
- Legawiec, K.J.; Kruszelnicki, M.; Bastrzyk, A.; Polowczyk, I. Rhamnolipids as Effective Green Agents in the Destabilisation of Dolomite Suspension. Int. J. Mol. Sci. 2021, 22, 10591. [Google Scholar] [CrossRef]
- Abbasi, H.; Noghabi, K.A.; Hamedi, M.M.; Zahiri, H.S.; Moosavi-Movahedi, A.A.; Amanlou, M.; Teruel, J.A.; Ortiz, A. Physicochemical Characterization of a Monorhamnolipid Secreted by Pseudomonas aeruginosa MA01 in Aqueous Media. An Experimental and Molecular Dynamics Study. Colloids Surf. B Biointerfaces 2013, 101, 256–265. [Google Scholar] [CrossRef]
- Legawiec, K.J.; Kruszelnicki, M.; Zawadzka, M.; Basařová, P.; Zawala, J.; Polowczyk, I. Towards Green Flotation: Investigating the Effect of Rhamnolipid Biosurfactant on Single Bubble Adhesion Dynamics. J. Mol. Liq. 2023, 388, 122759. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of Structures, Microbial Origins and Roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef]
- Kłosowska-Chomiczewska, I.E.; Mędrzycka, K.; Hallmann, E.; Karpenko, E.; Pokynbroda, T.; Macierzanka, A.; Jungnickel, C. Rhamnolipid CMC Prediction. J. Colloid. Interface Sci. 2017, 488, 10–19. [Google Scholar] [CrossRef]
- Pawlowska, A.; Sadowski, Z.; Winiarska, K. Effect of Rhamnolipids and Lipopolysaccharides on the Bioleaching of Arsenic-Bearing Waste. Minerals 2021, 11, 1303. [Google Scholar] [CrossRef]
- Liu, B.; Sun, S.; Zhang, X.; Zhang, S.; Kong, L. Synergistic Effect of Rhamnolipid and Bacillus mucilaginosus on Detoxification and Activation of Municipal Solid Waste Incineration Fly Ash. Chemosphere 2024, 366, 143461. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Chatterjee, N.; Das, A.K.; McClements, D.J.; Dhar, P. Sophorolipids: A Comprehensive Review on Properties and Applications. Adv. Colloid. Interface Sci. 2023, 313, 102856. [Google Scholar] [CrossRef] [PubMed]
- Castelein, M.; Verbruggen, F.; Van Renterghem, L.; Spooren, J.; Yurramendi, L.; Du Laing, G.; Boon, N.; Soetaert, W.; Hennebel, T.; Roelants, S.; et al. Bioleaching of Metals from Secondary Materials Using Glycolipid Biosurfactants. Miner. Eng. 2021, 163, 106665. [Google Scholar] [CrossRef]
- Singh, N.K.; Baranwal, J.; Pati, S.; Barse, B.; Khan, R.H.; Kumar, A. Application of Plant Products in the Synthesis and Functionalisation of Biopolymers. Int. J. Biol. Macromol. 2023, 237, 124174. [Google Scholar] [CrossRef] [PubMed]
- Imre, B.; Pukánszky, B. Compatibilization in Bio-Based and Biodegradable Polymer Blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef]
- Gangwar, A.K.S.; Singh, M.K.; Vishnoi, P.; Shakyawar, D.B.; Maity, S. Sodium Lignosulfonate: An Industrial Bio-Waste for the Colouration and UV Protective Finish of Nylon Fabric. Fibres Text. East. Eur. 2022, 30, 77–85. [Google Scholar] [CrossRef]
- Khajeh, A.; Nazari, Z.; Movahedrad, M.; Vakili, A.H. A State-of-the-Art Review on the Application of Lignosulfonate as a Green Alternative in Soil Stabilization. Sci. Total Environ. 2024, 943, 173500. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Yang, D. Adsorption Mechanism of Lignosulfonate at the Air/Liquid Interface. J. Braz. Chem. Soc. 2015, 26, 555–561. [Google Scholar] [CrossRef]
- Jorjani, E.; Ghahreman, A. Challenges with Elemental Sulfur Removal during the Leaching of Copper and Zinc Sulfides, and from the Residues; a Review. Hydrometallurgy 2017, 171, 333–343. [Google Scholar] [CrossRef]
- Nazrun, T.; Hassan, M.K.; Hossain, M.D.; Ahmed, B.; Hasnat, M.R.; Saha, S. Application of Biopolymers as Sustainable Cladding Materials: A Review. Sustainability 2024, 16, 27. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef]
- Simões, C.R.; da Silva, M.W.P.; de Souza, R.F.M.; Hacha, R.R.; Merma, A.G.; Torem, M.L.; Silvas, F.P.C. Biosurfactants: An Overview of Their Properties, Production, and Application in Mineral Flotation. Resources 2024, 13, 81. [Google Scholar] [CrossRef]
- Kruszelnicki, M.; Kowalczuk, P.B.; Polowczyk, I. Three-Phase Contact Formation between an Air Bubble and Solid Surfaces with Different Hydrophobicity Degrees in Liquid. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132067. [Google Scholar] [CrossRef]
- Drelich, J.W.; Marmur, A. Meaningful Contact Angles in Flotation Systems: Critical Analysis and Recommendations. Surf. Innov. 2018, 6, 19–30. [Google Scholar] [CrossRef]
- Błaszków, A.; Ratajczak, T.; Szyszka, D. Flotation of Hydrophobic Minerals in Hallimond Tube. Min. Sci. 2024, 31, 219–227. [Google Scholar] [CrossRef]
- Rao, S.R. Hydrophobicity and Contact Angle. In Surface Chemistry of Froth Flotation; Springer Science + Business Media: New York, NY, USA, 2004; pp. 351–384. [Google Scholar] [CrossRef]
- Kalam, S.; Abu-Khamsin, S.A.; Kamal, M.S.; Patil, S. Surfactant Adsorption Isotherms: A Review. ACS Omega 2021, 6, 32342–32348. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.O.; Srinivas, G.; Lopez, C.F.; Klein, M.L. Modeling Surfactant Adsorption on Hydrophobic Surfaces. Phys. Rev. Lett. 2005, 94, 228301. [Google Scholar] [CrossRef]
- Paria, S.; Khilar, K.C. A Review on Experimental Studies of Surfactant Adsorption at the Hydrophilic Solid–Water Interface. Adv. Colloid. Interface Sci. 2004, 110, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Maestro, A. Tailoring the Interfacial Assembly of Colloidal Particles by Engineering the Mechanical Properties of the Interface. Curr. Opin. Colloid. Interface Sci. 2019, 39, 232–250. [Google Scholar] [CrossRef]
- Maestro, A.; Guzmán, E. Colloids at Fluid Interfaces. Processes 2019, 7, 942. [Google Scholar] [CrossRef]
- Biswal, N.R.; Paria, S. Effect of Electrolyte Solutions on the Adsorption of Surfactants at PTFE—Water Interface. Ind. Eng. Chem. Res. 2010, 49, 7060–7067. [Google Scholar] [CrossRef]
- Jafari, M.; Shafaie, S.Z.; Abdollahi, H.; Gharabaghi, M.; Chehreh Chelgani, S. Study of the Effects of Conventional Reagents for Sulfide Flotation on Bio-Oxidation Activity of Acidithiobacillus ferrooxidans. Chem. Eng. Commun. 2019, 206, 365–377. [Google Scholar] [CrossRef]
- Xiong, H.; Peng, S.; Zhang, B. Tween 80 and Triton X-100 Effects on Morphological Evolvement and Stability of Schwertmannite during Bacterial Reproduction. Int. J. Environ. Sci. Technol. 2023, 20, 13399–13410. [Google Scholar] [CrossRef]
- Kingma, J.G.; Silver, M. Autotrophic Growth of Thiobacillus acidophilus in the Presence of a Surface-Active Agent, Tween 80. Appl. Environ. Microbiol. 1979, 38, 795–799. [Google Scholar] [CrossRef]
- Mathivanan, K.; Zhang, R.; Chandirika, J.U.; Mathimani, T.; Wang, C.; Duan, J. Bacterial Biofilm-Based Bioleaching: Sustainable Mitigation and Potential Management of e-Waste Pollution. Waste Manag. 2025, 193, 221–236. [Google Scholar] [CrossRef]
- Fathollahzadeh, H.; Becker, T.; Eksteen, J.J.; Kaksonen, A.H.; Watkin, E.L.J. Microbial Contact Enhances Bioleaching of Rare Earth Elements. Bioresour. Technol. Rep. 2018, 3, 102–108. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, J.; Fang, X. Rare Earth Element Recycling from Waste Nickel-Metal Hydride Batteries. J. Hazard. Mater. 2014, 279, 384–388. [Google Scholar] [CrossRef]
- Adam, N.K. Use of the Term “Young’s Equation” for Contact Angles. Nature 1957, 180, 809–810. [Google Scholar] [CrossRef]
- Petersen, J. From Understanding the Rate Limitations of Bioleaching Mechanisms to Improved Bioleach Process Design. Hydrometallurgy 2023, 221, 106148. [Google Scholar] [CrossRef]
- Ai, C.; Wang, S.; Liu, C.; Li, T. Experimental Study on the Influence of Surfactants on Ore Surface Wettability. ACS Omega 2024, 9, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Saneie, R.; Dixon, D.G.; Asselin, E. Effect of Organic Surfactant TRITON CG-110 on Bioleaching of Chalcopyrite. Miner. Eng. 2025, 232, 109467. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, C.; Kou, J.; Zhao, H.; Wei, D.; Xing, Y. Enhancing the Leaching of Chalcopyrite Using Acidithiobacillus ferrooxidans under the Induction of Surfactant Triton X-100. Minerals 2019, 9, 11. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.J.; Sun, F.; Liu, C. Catalytic Effect of a Combined Silver and Surfactant Catalyst on Cobalt Ore Bioleaching. JOM 2018, 70, 2819–2824. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, X.; Duan, H.; Gu, W.; Zhang, D.; Wang, R.; Lu, X. Mechanism of Surfactant Effect on Bacterial Adsorption during Bioleaching of Lepidolite. Appl. Clay Sci. 2025, 264, 107646. [Google Scholar] [CrossRef]
- Sanwani, E.; Lamandhi, N.B.; Husni, H.; Chaerun, S.K.; Astuti, W.; Mufakhir, F.R. Influence of Indigenous Mixotrophic Bacteria on Pyrite Surface Chemistry: Implications for Bioflotation. Microbiol. Indones. 2020, 14, 1. [Google Scholar] [CrossRef]
- van Oss, C.J. Acid—Base Interfacial Interactions in Aqueous Media. Colloids Surf. A Physicochem. Eng. Asp. 1993, 78, 1–49. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, W.; Hou, J.; You, Z. Review on Physical and Chemical Factors Affecting Fines Migration in Porous Media. Water Res. 2022, 214, 118172. [Google Scholar] [CrossRef]
- Hermansson, M. The DLVO Theory in Microbial Adhesion. Colloids Surf. B Biointerfaces 1999, 14, 105–119. [Google Scholar] [CrossRef]
- Kamble, S.; Agrawal, S.; Cherumukkil, S.; Sharma, V.; Jasra, R.V.; Munshi, P. Revisiting Zeta Potential, the Key Feature of Interfacial Phenomena, with Applications and Recent Advancements. ChemistrySelect 2022, 7, e202103084. [Google Scholar] [CrossRef]
- Chen, M.L.; Zhang, L.; Gu, G.H.; Hu, Y.H.; Su, L.J. Effects of Microorganisms on Surface Properties of Chalcopyrite and Bioleaching. Trans. Nonferrous Met. Soc. China 2008, 18, 1421–1426. [Google Scholar] [CrossRef]
- Martín, F.S.; Aguilar, C. Study of the Adhesion Mechanism of Acidithiobacillus ferrooxidans to Pyrite in Fresh and Saline Water. Minerals 2019, 9, 306. [Google Scholar] [CrossRef]
- Ghadiri, M.; Harrison, S.T.L.; Fagan-Endres, M.A. Effect of Surfactant on the Growth and Activity of Microorganisms in a Heap Bioleaching System. Miner. Eng. 2019, 138, 43–51. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, J.; Zhang, C.; He, P.; Chen, D.; Zhong, G.; Liu, Q.; Hu, W.; Chen, Y.; Zhu, J. Effect of Calcium Lignosulfonate on Surface Modification and Bioleaching of Chalcopyrite. Biochem. Eng. J. 2024, 207, 109329. [Google Scholar] [CrossRef]
- Fang, F.; Zhong, H.; Jiang, F.M.; Li, Z.H.; Chen, Y.F.; Zhan, X.H. Influence of Surfactants on Bioleaching of Arsenic-Containing Gold Concentrate. J. Cent. South. Univ. 2014, 21, 3963–3969. [Google Scholar] [CrossRef]
- Jafari, M.; Chehreh Chelgani, S.; Shafaie, S.Z.; Abdollahi, H.; Hadavandi, E. Study Effects of Conventional Flotation Reagents on Bioleaching of Zinc Sulfide. J. Ind. Eng. Chem. 2019, 78, 364–371. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, Y.; Zhang, Y.; Zheng, Q. Improvement on Bioleaching Interfacial Behavior between Bacillus mucilaginosus and Vanadium-Bearing Shale by Surfactant Additive. J. Environ. Chem. Eng. 2022, 10, 108911. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, Y.; Zhang, Y.; Tian, H. Effects of Surfactant on Bacillus mucilaginosus Adsorption Characteristics during Vanadium Bioleaching Process. J. Environ. Chem. Eng. 2022, 10, 108961. [Google Scholar] [CrossRef]
- Castro, L.; Gómez-Álvarez, H.; Carmona, M.; González, F.; Muñoz, J.A. Influence of Biosurfactants in the Recovery of REE from Monazite Using Burkholderia Thailandensis. Hydrometallurgy 2023, 222, 106178. [Google Scholar] [CrossRef]
- Su, C.; Cai, J.; Lai, H.; Zheng, Q.; Shen, P.; Liu, D. Extracellular Polymeric Substances—Mediated Hydrophobic/Hydrophilic Differentiation of Chalcopyrite and Pyrite by Acidithiobacillus ferrooxidans for Enhanced Selective Flotation. Colloids Surf. A Physicochem. Eng. Asp. 2025, 727, 138443. [Google Scholar] [CrossRef]
- Christopher Nnaemeka, I.; T. O, C.; Callistus Nonso, U.; Maxwell Ikechukwu, O.; Francis Anezichukwu, A.; A. Ikechukwu, N.; M, O.; Benedith Chukwudi, E.; Kosoluchi Chisom, M.; Tina Ifeanyichukwu, O.; et al. Examining the Efficiency of Microbe-Assisted Metal Extraction: A Review of Bio-Hydrometallurgical Leaching Techniques. Hybrid. Adv. 2025, 9, 100407. [Google Scholar] [CrossRef]
- EUR-LEX. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02006R1907-20250901 (accessed on 7 October 2025).
- Naseri, T.; Beiki, V.; Mousavi, S.M.; Farnaud, S. A Comprehensive Review of Bioleaching Optimization by Statistical Approaches: Recycling Mechanisms, Factors Affecting, Challenges, and Sustainability. RSC Adv. 2023, 13, 23570–23589. [Google Scholar] [CrossRef]
- Cardoso, A.C.; Dias, C.S.; de Moura, C.H.R.; Ferreira, J.L.; Rodrigues, E.C.; Macêdo, E.N.; Estumano, D.C.; Viegas, B.M. Use of Bayesian Methods in the Process of Uranium Bioleaching by Acidithiobacillus ferrooxidans. Appl. Sci. 2024, 14, 109. [Google Scholar] [CrossRef]
- Statistics|Eurostat. Available online: https://ec.europa.eu/eurostat/databrowser/view/env_wasgen/default/table?lang=en (accessed on 7 October 2025).


| Mineral | Surfactant | Changes in Contact Angle | Ref. | ||
|---|---|---|---|---|---|
| Type | Concentration [mg/L] | From [°] | to [°] | ||
| Chalcopyrite | PVP | 120 | 77 | 44 | [22] |
| Chalcopyrite (fresh) | Triton CG-110 | 100 | 81 | 69 | [68] |
| 500 | 54 | ||||
| 2000 | 41 | ||||
| Chalcopyrite (oxidized) | 600 | 72 | 50 | ||
| Sulfur (elemental) | Triton X-100 | 120 | 105 | 60 | [69] |
| Cobalt ore | Tween 20 | 150 | 64 | 41 | [70] |
| Tween 60 | 150 | 43 | |||
| Tween 80 | 150 | 45 | |||
| Lepidolite | SDS | 100 | 75 | 7 | [71] |
| Tween 20 | 100 | 43 | |||
| Rhamnolipid | 300 | 11 | |||
| Reagent | Concentration [mg/L] | Bacteria Concentration ×107 cells/mL | Reference |
|---|---|---|---|
| Without reagent | 0 | 27.8 | [13] |
| Tween 20 | 100 | 26.5 | |
| 250 | 22.6 | ||
| 500 | 2.2 | ||
| Tween 80 | 100 | 27.8 | |
| 250 | 23.4 | ||
| 500 | 2.90 | ||
| Without reagent | 0 | 25.6 | [70] |
| Tween 20 | 150 | 23.8 | |
| 300 | 24.3 | ||
| 450 | 8.50 | ||
| Tween 60 | 150 | 25.4 | |
| 300 | 26.2 | ||
| 450 | 7.8 | ||
| Tween 80 | 150 | 24.3 | |
| 300 | 22.8 | ||
| 450 | 8.30 |
| Reagent | Conc. [mg/L] | Co [%] | Cu [%] |
|---|---|---|---|
| - | 0 | 71.3 | 56.2 |
| Tween 20 | 100 | 93.2 | 65.7 |
| 250 | 82.6 | 58.1 | |
| Tween 80 | 100 | 92.4 | 64.3 |
| 250 | 84.4 | 62.9 |
| Reagent | NaEX | KAX | KIBX | KIPX | MIBC | PO | Aero3477 |
|---|---|---|---|---|---|---|---|
| Conc. [mg/L] | Zinc Recovery [%] | ||||||
| 0 | 79.10 | ||||||
| 10 | 71.10 | 79.70 | 76.88 | 33.22 | 72.31 | 76.78 | 75.41 |
| 100 | 63.42 | 66.76 | 78.77 | 88.28 | 71.25 | 68.27 | 67.55 |
| 1000 | 37.55 | 60.23 | 64.31 | 24.43 | 6.92 | 6.59 | 81.61 |
| Reagent | NaEX | KAX | KIBX | KIPX | MIBC | PO | Aero3477 |
|---|---|---|---|---|---|---|---|
| Conc. [mg/L] | Cell Concentration [cells/mL × 107] | ||||||
| 0 | 12.0 | ||||||
| 10 | 8.0 | 12.4 | 11.2 | 10 | 13.2 | 10 | 9.2 |
| 100 | 6.0 | 12.0 | 7.20 | 8.4 | 8.0 | 12.8 | 10.8 |
| 1000 | 0.4 | 10.0 | 12.0 | 0.6 | 0.8 | 1.6 | 10.8 |
| Mineral Material | Conditions | Microorganisms | Reagent | Conc. [mg/L] | Metal Leached | Process Efficiency | Effect on Microorganisms | Ref. |
|---|---|---|---|---|---|---|---|---|
| Chalcopyrite | Solid 10% w/v; pH 1.12–1.20; inoculum 10% v/v (1.2 × 108 cells/mL); 35 °C; 200 rpm; time: 123 d | Sulfobacillus Ferroplasma Acidithiobacillus | SLS CTAB NP12 Tween 80 Span 80 PGA NHD | 100 | Cu | 91.8% 76.5% 70.5% 33.6% 26.7% 38.7% 40.1% | SLS, CTAB—minimal effect on bacterial cells; SP80, NHD, and PEG inhibited activity of around 80%. Tween 80 and NP12 delayed cell growth not as significant as above. | [12] |
| Cobalt ore | inoculum 10% v/v; solid 10% w/v; pH 1.5; 45 °C; 180 rpm; time 15 days | A. ferrooxidans, A. thiooxidans, L. ferrooxidans | Tween 20 Tween 80 | 100 and 250 | Co Cu | Control: Co 71.3% Cu 56.2% Tween 20 (100 mg/L): Co 92.4% Cu 64.3% Tween 80 (100 mg/L): Co 93.2% Cu 65.7% Higher surfactant concentrations gave lower results. | No visible negative effect on bacterial activity within tested surfactant concentrations. | [13] |
| Chalcopyrite | solid 1% w/v; pH 2.0; 160 rpm; 30 °C; inoculum 1 × 107 cell/mL; time: 21 d | A. ferrooxidans | PEG 2000 | 0–180 | Cu | PEG (90 mg/L): 411.04 mg/L; control: 187.35 mg/L | The presence of PEG improved bacterial attachment to sulfur. | [21] |
| Chalcopyrite | solid 1% w/v; inoculum 1 × 107 cell/mL; 30 °C; 160 rpm; pH 2.0; time 30 d | A. ferrooxidans | PVP | 100 | Cu | Increased metal recovery in the presence of PVP (786.5 mg/L); control: 385.6 mg/L | PVP slightly inhibited bacterial growth compared to control sample. | [22] |
| Chalcopyrite concentrate | Solid 1 wt%; inoculum 10% v/v; 34 °C; pH 1.70; 300 rpm; time: 30 d | A. ferrooxidans | CG-110 | 10–2000 | Cu | Bio-Fe-CG (69.1%), Bio-CG (59.7%) Bio-Fe (56.6%), | CG-110 20 and 100 mg/L increased biooxidation ability of bacteria. Reagent dosage over 500 mg/L inhibited metabolic activity. | [68] |
| Chalcopyrite concentrate | Solid 3% w/v; 65 °C; inoculum 1–4 × 108 cell/mL; time: 18 d | M. hakonensis A. cupricumulans | Tween 20 Tween 80 Plurafac LF 120 Plurafac LF 600 Lutensol XL 90 | 5–10 | Cu | Tween 20 (10 mg/L) showed an enhancement of the copper recovery by 2.4% relative to the biotic control. | Microorganisms able to grow in the presence of: 5–10 mg/L Tween 20, 5 mg/L Tween 80, 5 mg/L Plurafac LF 120, 5–10 mg/L Lutensol XL 90. Inhibititory effect for: 10 mg/L of Tween 80, 10 mg/L of Plurafac LF 120, 5–10 mg/L of Plurafac LF 600 | [79] |
| Chalcopyrite, high purity | Inoculum 5% v/v; solid 2% w/v; 30 °C; 180 rpm; pH 2.0; time 30 days | A. ferrooxidans | CaLS | 0, 10, 20, 30, 35 | Cu | CaLS (20 mg/L): 1700 mg/L; control: 1648 mg/L | Bacterial cell population was increased only when 20 mg/L CaLS were used: 102.45 × 107 cells/mL vs. 94.12 × 107 cells/mL for control. | [80] |
| Surface-Active Reagent Effect | ||
|---|---|---|
| Mineral Surface | Bacterial Activity | Leaching Efficiency |
| Surfactants form a layer on the mineral surface, the thickness of which depends on the surfactant concentration in the solution and its molecular structure | Nonionic surfactants at lower concentrations are less toxic to microorganisms than ionic ones | A higher molecular weight surfactant resulted in the leaching efficiency reduction (e.g., copper) |
| Moderate bacterial cell attachment. Ionic surfactant enhances bacterial adhesion due to electrostatic interactions | High concentration inhibits bacterial growth and causes cell disruption | Improvement solution penetration into small pores and cracks of large particles by decreasing surface tension |
| Rhamnolipids increase the contact between bacteria and minerals by forming a wetting film on a solid surface | Acceleration of sulfur oxidation and dissolution generated during bioleaching, which provides an additional energy source for bacterial growth (e.g., PEG) | An increase in leaching efficiency is mainly attributed to its ability to enhance bacterial adhesion to the mineral surface |
| Its presence improves hydrophilicity and reduces surface tension (e.g., SDS, CTAB, NaLS) | The addition of PEG increased bacterial attachment to sulfur | |
| SDS reduce the EPS secretion of bacteria and weakens cell agglomeration | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlowska, A.; Legawiec, K.J. From Solid to Solution: How Surface-Active Agents Influence Bioleaching Efficiency and Bacteria–Mineral Interactions. Minerals 2025, 15, 1094. https://doi.org/10.3390/min15101094
Pawlowska A, Legawiec KJ. From Solid to Solution: How Surface-Active Agents Influence Bioleaching Efficiency and Bacteria–Mineral Interactions. Minerals. 2025; 15(10):1094. https://doi.org/10.3390/min15101094
Chicago/Turabian StylePawlowska, Agnieszka, and Krzysztof Jan Legawiec. 2025. "From Solid to Solution: How Surface-Active Agents Influence Bioleaching Efficiency and Bacteria–Mineral Interactions" Minerals 15, no. 10: 1094. https://doi.org/10.3390/min15101094
APA StylePawlowska, A., & Legawiec, K. J. (2025). From Solid to Solution: How Surface-Active Agents Influence Bioleaching Efficiency and Bacteria–Mineral Interactions. Minerals, 15(10), 1094. https://doi.org/10.3390/min15101094







