1. Introduction
Salts have been indispensable to humankind since ancient times. Back then, common salt (NaCl) primarily used for domestic purposes, was directly extracted from rock deposits. However, after the Industrial Revolution, it became necessary to seek other resources such as brines or seawater [
1]. Currently, inorganic salts are not limited only to human consumption but are also used as fertilizers for agriculture (KCl), in the formulation of detergents (Na
2SO
4), in the manufacturing of glass and paper (Na
2CO
3), in the production of batteries (LiCl) [
2], among others. In general, there are four types of brine resources from which a wide range of salts are extracted: oceans and seas, salt lakes, salars, and solution mining [
3]. Brines from salars are one of the most important sources of salt production in the world. Salars are salt lakes that have evaporated over time leaving layers of salts hundreds of meters deep. In these salars, brine is trapped in the interstices and is then pumped to the surface for processing. The Salar de Uyuni in Bolivia and the Salar de Atacama in Chile are excellent examples [
4].
In the industrial extraction of salts, various fractional or sequential crystallization techniques are used [
2,
5]. Among the technologies used, solar evaporation ponds stand out primarily in arid or semi-arid regions [
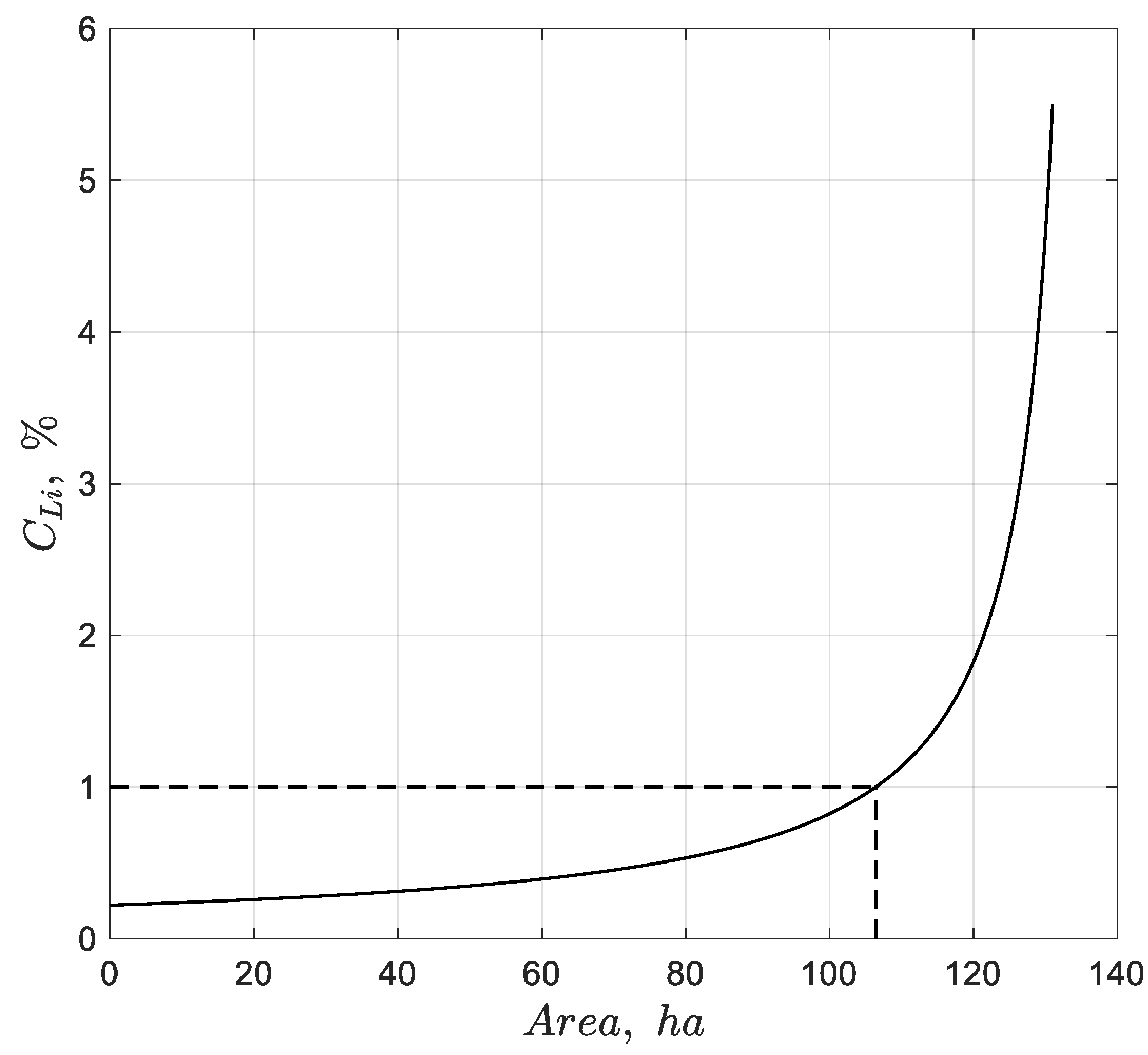
6] because they take advantage of intense solar radiation and low humidity, which are key factors in the evaporation process. Notably, the Salar de Atacama, in northern Chile, has brine with the highest known lithium concentration averaging 0.14% (1.68 g/L) [
7]. This salar also holds approximately 60% of the world’s lithium reserves, and it is the largest lithium producer from brine in the world [
8]. The two companies currently operating at the Salar the Atacama under lease agreement with the Chilean government are SQM and Albemarle, which reported production of lithium carbonate equivalent (LCE) in the year 2023 of 168,000 and 50,400 tons, respectively.
The climatic conditions of the Salar de Atacama favor the efficiency of solar evaporation for the recovery of lithium and other industrial salts present in the brine. The average daily temperature ranges between 22 °C in February and 8 °C in July, with a maximum oscillation of approximately 14 °C. Wind speed varies daily from less than 2 m/s in the morning to 15 m/s in the afternoon. Rainfall corresponds to a desert climate, with an annual average of 11.6 mm at the lithium saltworks [
9]. The average relative air humidity is 19.8%, with a maximum of 27% in February and a minimum of 15% in October.
From the thermodynamic point of view, studying solar evaporation from brines is complex because they are electrolyte solutions; therefore, brines exhibit non-ideal behavior at very low concentrations due to ionic electrostatic interactions [
10]. One of the most advanced thermodynamic models is the OLI Mixed-Solvent Electrolyte model [
11]. This model integrates standard-state thermochemical properties of solution species with an expression for excess Gibbs energy. The excess Gibbs energy model incorporates a long-range electrostatic interaction term (Pitzer–Debye–Hückel equation), a short-range electrostatic interaction term (UNIQUAC model), and a middle-range electrostatic interaction term (second virial coefficient type equation). While long-range forces dominate in dilute electrolyte solutions, chemical and physical forces become significant at moderate and short separation distances between species. As a result, the expression for excess Gibbs energy includes the sum of these three interaction terms.
Besides the thermodynamic complexities, the operation of solar evaporation ponds is influenced by physical phenomena that governs water removal. For instance, it is known that the evaporation rate is proportional to the difference between the saturated vapor pressure at the water temperature and the vapor pressure of the surrounding air [
12]. Furthermore, it has been shown that dissolved solids decrease the saturation vapor pressure of brine [
13]. Additionally, the limited number of studies on infiltration [
1,
14] highlighted that it is still a little understood phenomenon.
Despite the above considerations, several studies have applied solubility phase diagrams to salt lakes and brines [
2,
15,
16,
17,
18,
19]. These studies aimed to establish relationships between ion concentrations and the crystallization points of salts. However, the vast number of ion combinations in brines, which fixes the possible combinations of salts to precipitate, turn into a very complex system. Therefore, phase diagram approaches often face practical drawbacks including difficulty of calibrating parameters for complex brine systems and their limited scalability to large-scale pond operations.
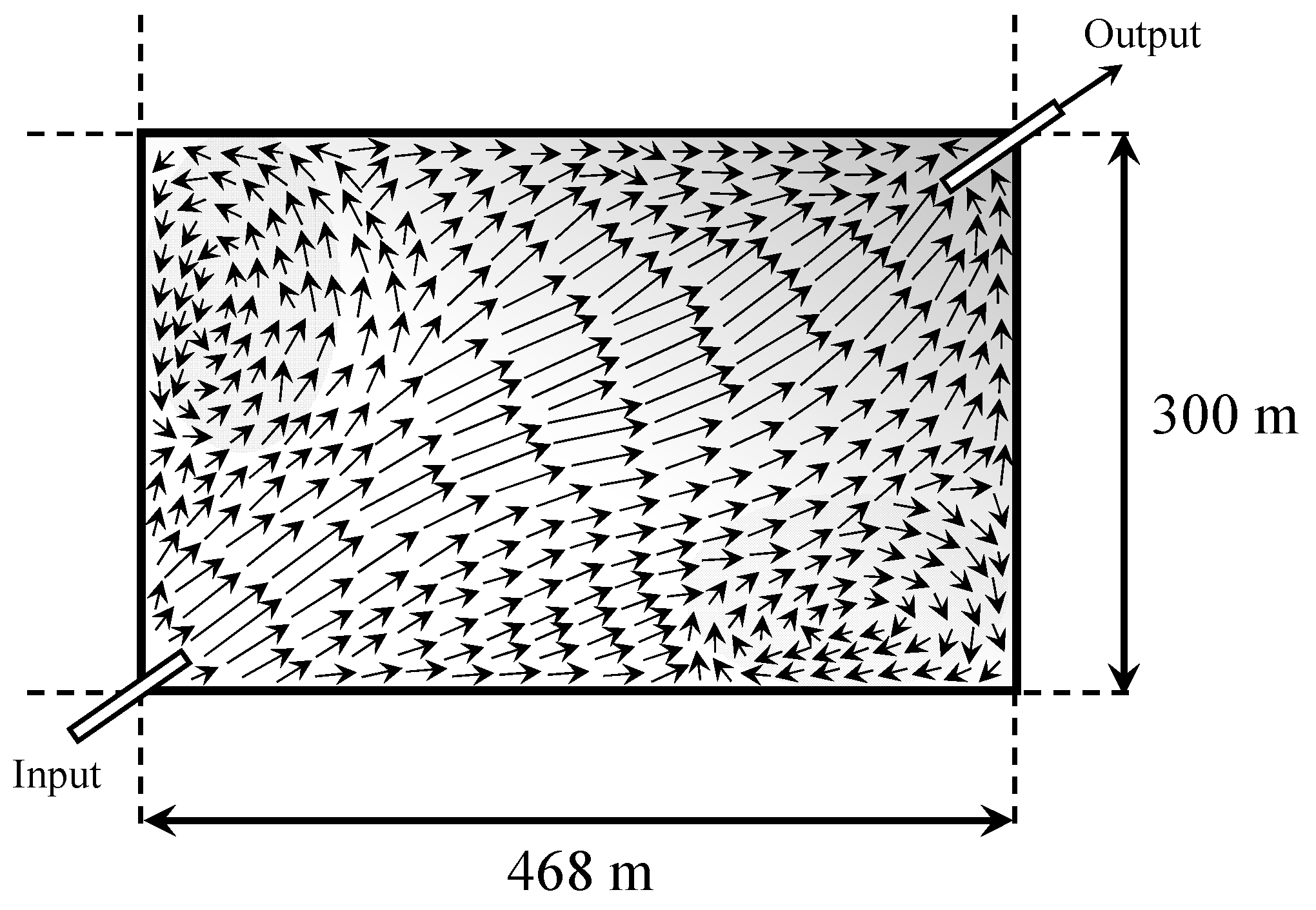
Since the nature of the solar evaporation process is inherently three-dimensional, with various physicochemical phenomena occurring in the ponds, the modeling becomes very demanding. For instance,
Figure 1 qualitatively displays velocity and concentration fields for the simplest type of evaporation ponds without any baffles.
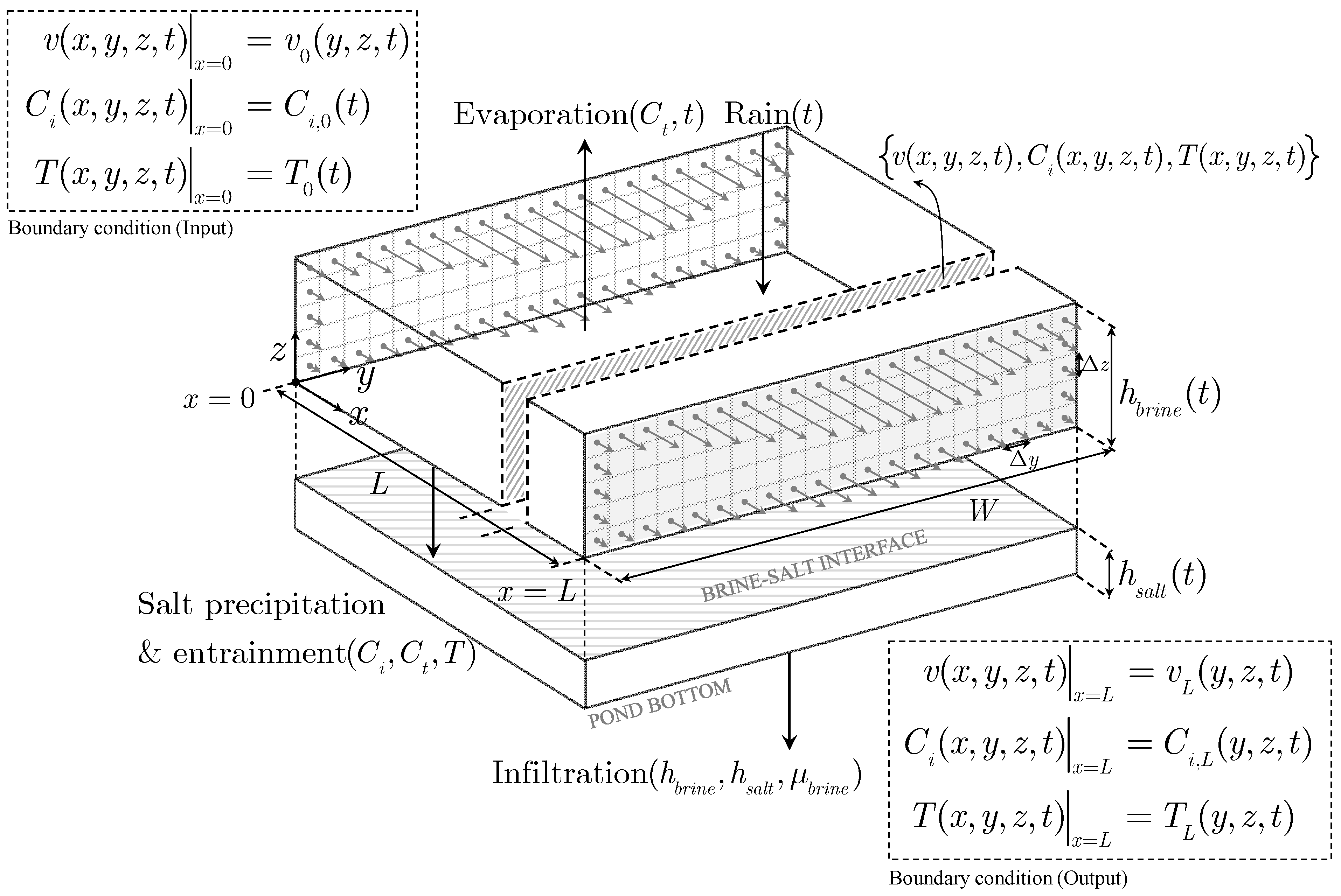
In the figure, velocity arrows indicate how the pond corners trap brine, increasing residence time, and species’ local concentrations (shades of gray). At the same time, the figure shows the length and width of the pond and how the brine is fed and discharged. The depth of the ponds is small, usually less than 1 m. Gradients in concentration also occur in the vertical direction and a layer of solid precipitates will form when the brine concentration reaches the crystallization point of some of the salts. Because the heat capacity of the brine decreases with concentration, an increase in temperature is also anticipated in the zones of higher concentrations. Thus, fields of temperature, concentration, and velocity are established, requiring the formulation and solution of the mass, energy, and momentum balances in three dimensions, together with additional equations to close the system. To illustrate this, we conceptualize a three-dimensional solar evaporation pond as shown in
Figure 2. For simplicity, the depiction considers unidirectional laminar flow. As indicated in the figure, in addition to water evaporation, other physicochemical phenomena need to be considered: salt precipitation, entrainment of brine in the precipitates, infiltration of brine in the soil, and rainfall.
Modeling Efforts on Solar Evaporation Ponds
The modeling efforts on solar evaporation ponds can be classified according to the dimensions considered for the property gradients.
The first and simplest conceptual model is the well-known perfect mixing model. In this model, it is assumed that the pond exhibits perfect mixing, ensuring uniformity in solute concentration and temperature throughout the entire volume. Despite its simplicity and the potential limitations in practical applications, this model has been widely used, as reported in the literature. Some of the models focused solely on either thermodynamic aspects or on mass balance aspects [
20,
21], in some cases, assuming steady-state conditions [
22,
23]. These models use mass balance relationships and constant rates for precipitation, entrainment, and infiltration with empirical adjustments for evaporation based on factors such as seasonality and brine density. Other models explore the system’s dynamics over discrete time intervals and provide thermodynamic closure through simulations of equilibrium conditions using hyperplanes [
24].
The one-dimensional model would be next in complexity, which accounts for concentration, temperature, and velocity gradients along a single spatial dimension (typically, the length of the pond), thus providing a more realistic depiction of how these variables evolve as the fluid moves through the system. Little has been done in this category. The Myers model [
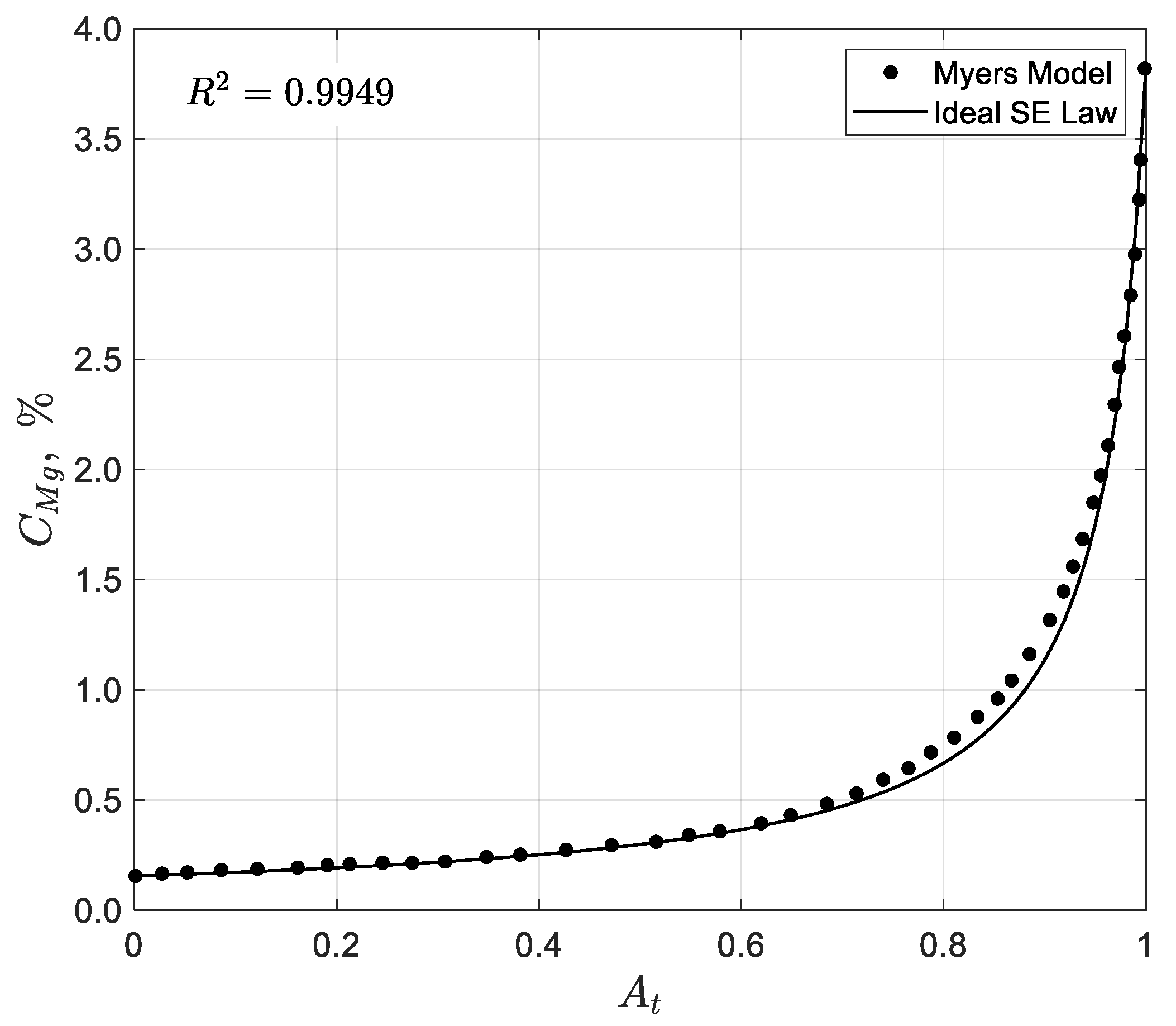
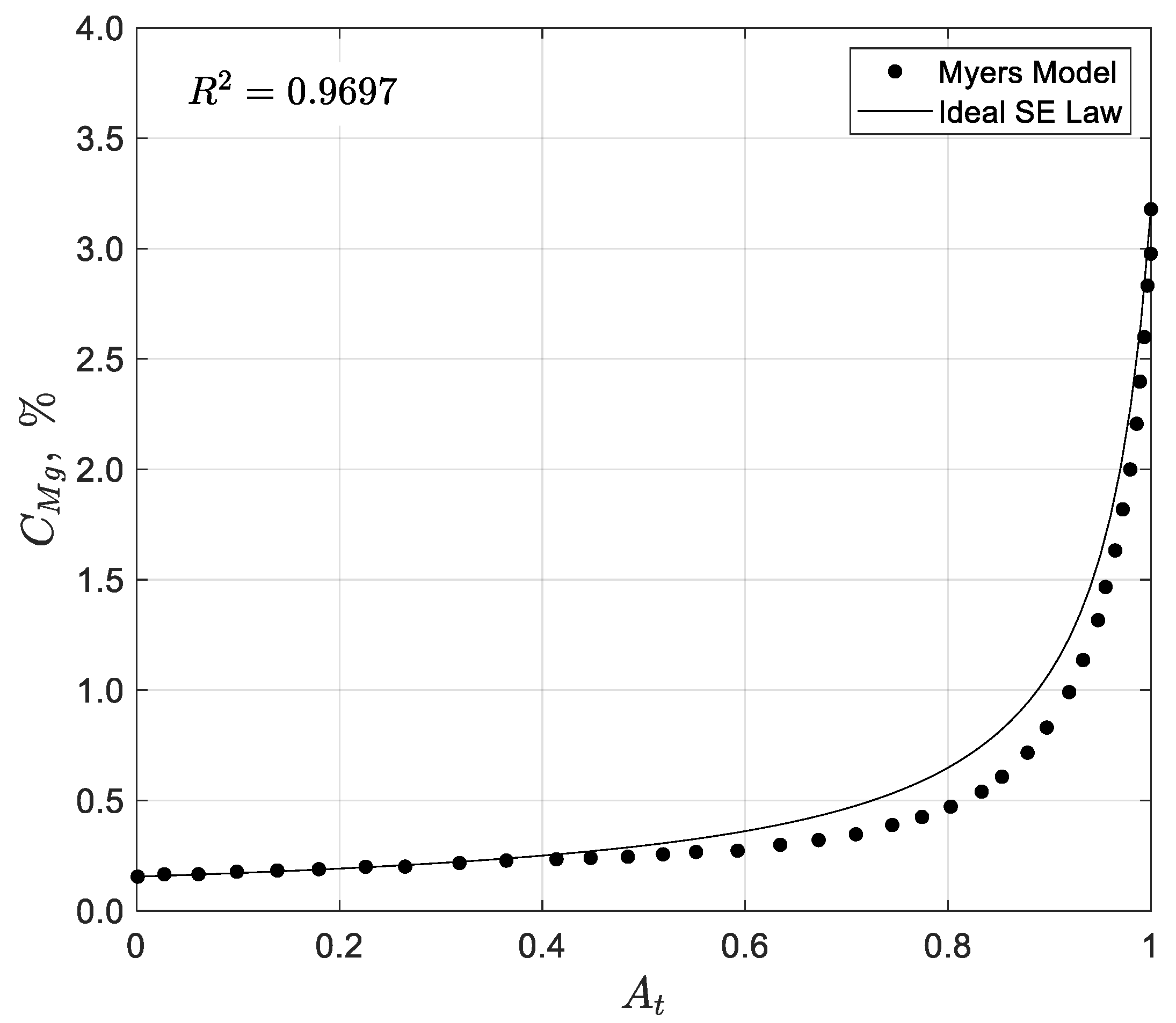
25] is the only documented one-dimensional model in the literature for solar evaporation ponds. The model was developed to be used in a commercial plant for NaCl recovery from seawater. It considers a concentration stage, where seawater is progressively concentrated until reaching NaCl saturation, and a crystallization stage, where NaCl precipitates before other solutes begin to precipitate, ensuring purity. This model does not explicitly track NaCl concentration but models only the concentration of Mg, using the known relationship that NaCl begins to precipitate when Mg salinity reaches approximately 1.275%, corresponding to a brine density of 1215 g/L. After the NaCl crystallization, the final brine reaches a Mg salinity of 3.9% with a density of 1250 g/L. The model assumes that the seawater moves steadily from the inlet to the outlet, increasing gradually in salinity and absence of forward or backward mixing along the ponds. The primary variables considered in the model include the evaporation rate, rainfall, seawater’s salinity, the NaCl saturation concentration, and infiltration rate. Evaporation and infiltration are empirically correlated with the concentration of Mg in the brine, under the assumption that Mg does not precipitate during the process. The model’s objective was to determine the location where NaCl precipitation begins and to quantify the NaCl yield per unit area under varying evaporation rates, rainfall levels, and infiltration conditions. These calculations were based on average meteorological conditions and assumed no entrainment of seawater in the precipitate.
The next step in line would be the two-dimensional model, which could consider the concentration, temperature, and velocity gradients along two spatial dimensions, typically the length and width of the pond. Although this type of model would provide a more realistic representation of how concentrations and temperatures vary not just along the flow direction but also across the width of the pond, there is a lack of published literature on the subject.
The three-dimensional model, on the other hand, could consider the concentration, temperature, and velocity gradients in all three spatial dimensions, providing a detailed and realistic representation of the processes occurring within the pond. This comprehensive approach would allow obtaining vertical gradients and stratification effects, which are crucial for understanding the complete dynamics of the system. Therefore, a three-dimensional model has the potential to deliver accurate predictions for the behavior of solar evaporation ponds. On this subject, Kasedde et al. [
26] reported a simulation of a sodium chloride extraction evaporation pond in Uganda using a tridimensional CFD approach. This pioneering effort is a valuable step in modeling a complex system, where some limitations were noted, particularly the absence of thermodynamic closure equations for sodium chloride precipitation. Additionally, the infiltration and entrainment of brine in the precipitates were also ignored. The simulated temperature and velocity fields showed unexpected variations (20-degree Celsius increase in 5 h), and evaporation rates were not reported. Nevertheless, this study formally remained an important documented contribution of its kind, highlighting the challenges and opportunities that still exist for future developments in this area.
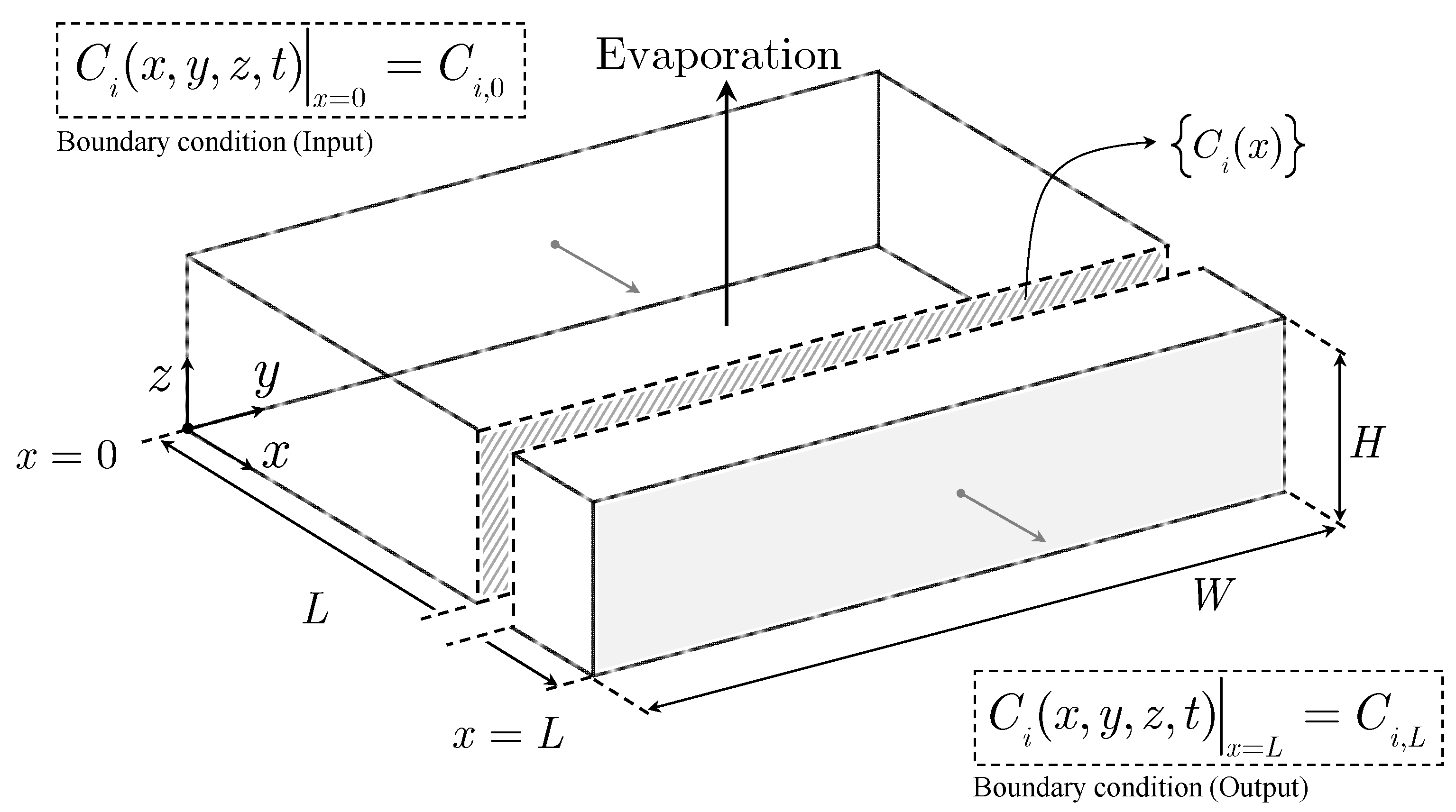
Having discussed above the complexity of modeling a solar evaporation pond, in the present paper, we introduce a surrogate mathematical model based on an ideal solar evaporation pond. The conceptualization of such a pond considers only evaporation excluding phenomena such as solids precipitation and entrainment, rainfall, and infiltration. Additionally, the model assumes that the rate of evaporation per unit area is uniform in the pond. Consequently, evaporation is directly proportional to the pond’s length (or area) and independent of the local concentration of species
i or any other variable. The shape of the ideal pond is assumed to be an orthogonal parallelepiped with the top rectangular area exposed to solar radiation.
Figure 3 illustrates a one-dimensional ideal solar evaporation pond, where the brine flow in the pond is unidirectional along its length, while maintaining a constant brine height, and the brine feed and discharge are continuous. Variables such as velocity and temperature are explicitly excluded, focusing solely on the concentration profile of species
i along the pond’s length as the primary variable of interest.
From this conceptualization, a governing equation was derived in this work—the ideal solar evaporation law—which is novel, not being previously reported in the literature. The model represents a significant advancement in the field of solar evaporation because it is ingenious and applicable to any solar evaporation process as discussed in the following section.
2. Development of a Surrogate Model for a Solar Evaporation Pond
Based on simple mathematical considerations, it is possible to derive a relationship between the steady state ion concentration along the length of the ideal pond and the concentrations of the feed and discharge of the pond. The developed mathematical model is one-dimensional and straightforward.
The model is based on the following assumptions (see
Figure 3):
The area exposed to solar radiation has a length L, and a width W.
The pond is continuously fed and discharged with brine at and , respectively, and the system is at a steady state, maintaining a constant brine height H.
The feed and discharge concentration of species i, , respectively, are known.
Let
n be the number of times when half of the water is removed from
to
. Thus, if
, half of the water is removed once along the length of the pond, resulting in
. If
, half of the water is removed twice, doubling the concentration twice, so that
, and so on. Then, it becomes clear that
Additionally, if , it follows that , meaning there was no evaporation.
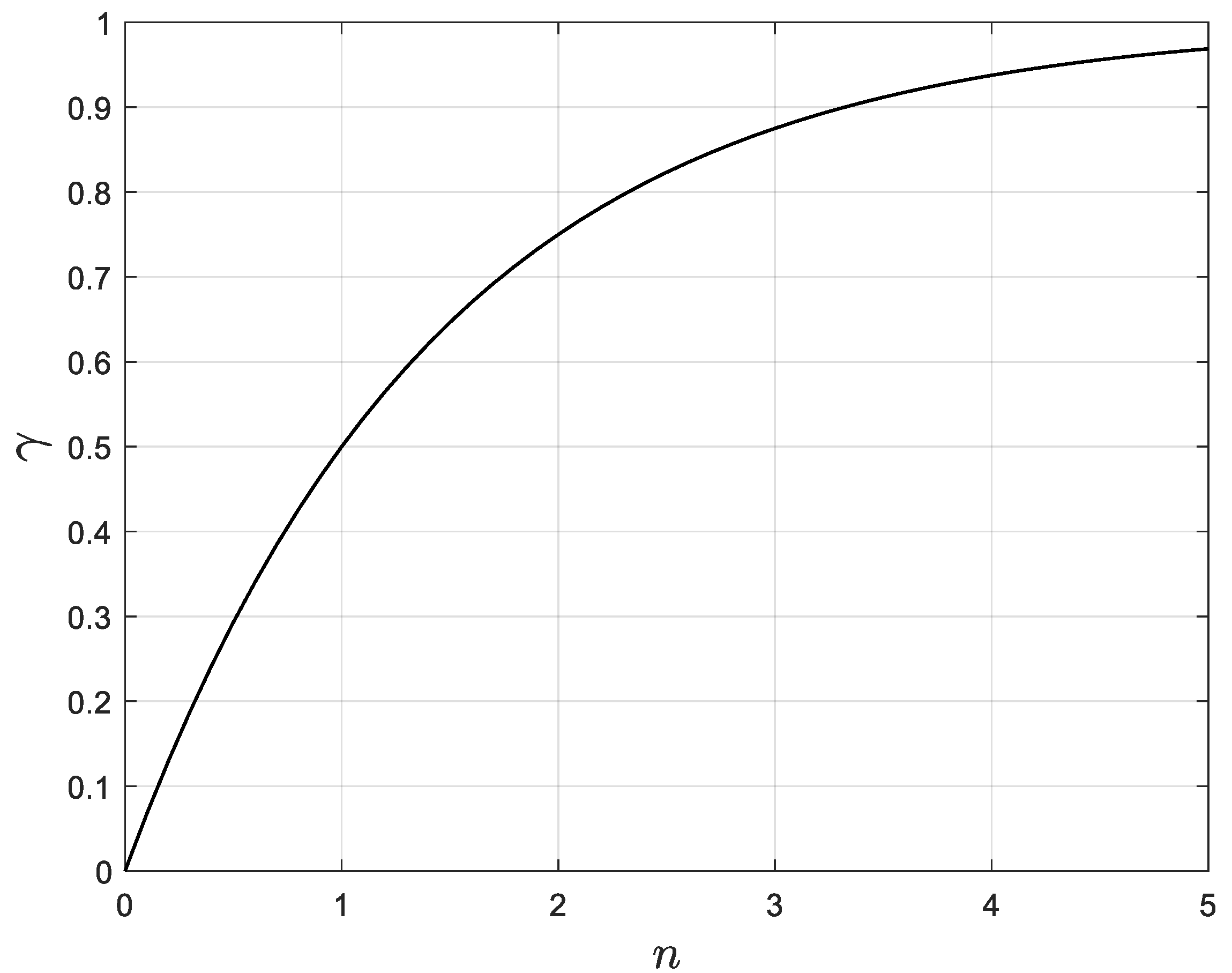
Clearly, there is a relationship between
and the fraction of water remaining in the system. Indeed, if
the fraction of water remaining is 1. If
, the fraction of water remaining is 1/2. Then, if
, the fraction of water remaining is 1/4, and so on. Thus, the fraction of water remaining in the system is
. Moreover, the fraction of water removed,
, plus the fraction of water remaining must always be equal to 1. Thus, the fraction of water removed
must satisfy Equation (2):
This relationship between
and
is shown in
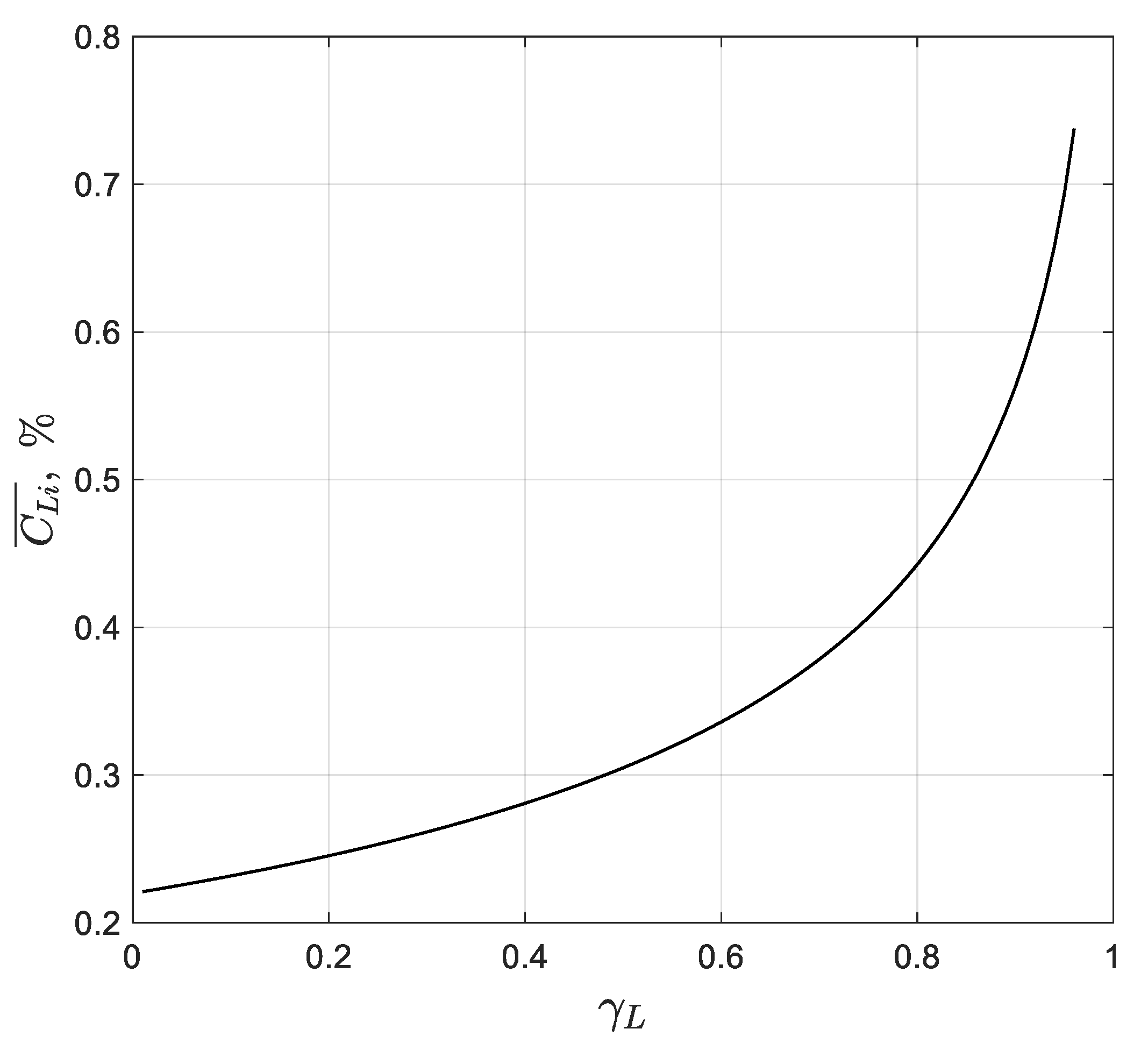
Figure 4.
Solving for
in terms of
Substituting this result in Equation (1), we obtain
Since
, it follows that
Since the fraction of water removed is directly proportional to the length of the pond (see
Figure 3),
and
. Thus
Replacing in Equation (5)
At the feed and discharge locations, Equation (7) is satisfied as follows:
Consequently, from Equation (9), the fraction of water removed in the pond can be written as follows:
Considering that
, Equation (7) can be referred to as the “ideal solar evaporation law” because it uniquely determines the ion concentration profile just given the feed concentration at
and the discharge concentration at
; it is also clear that this equation is independent of the concentration units used for the species
i. Thus, solar evaporation systems following this law must satisfy that
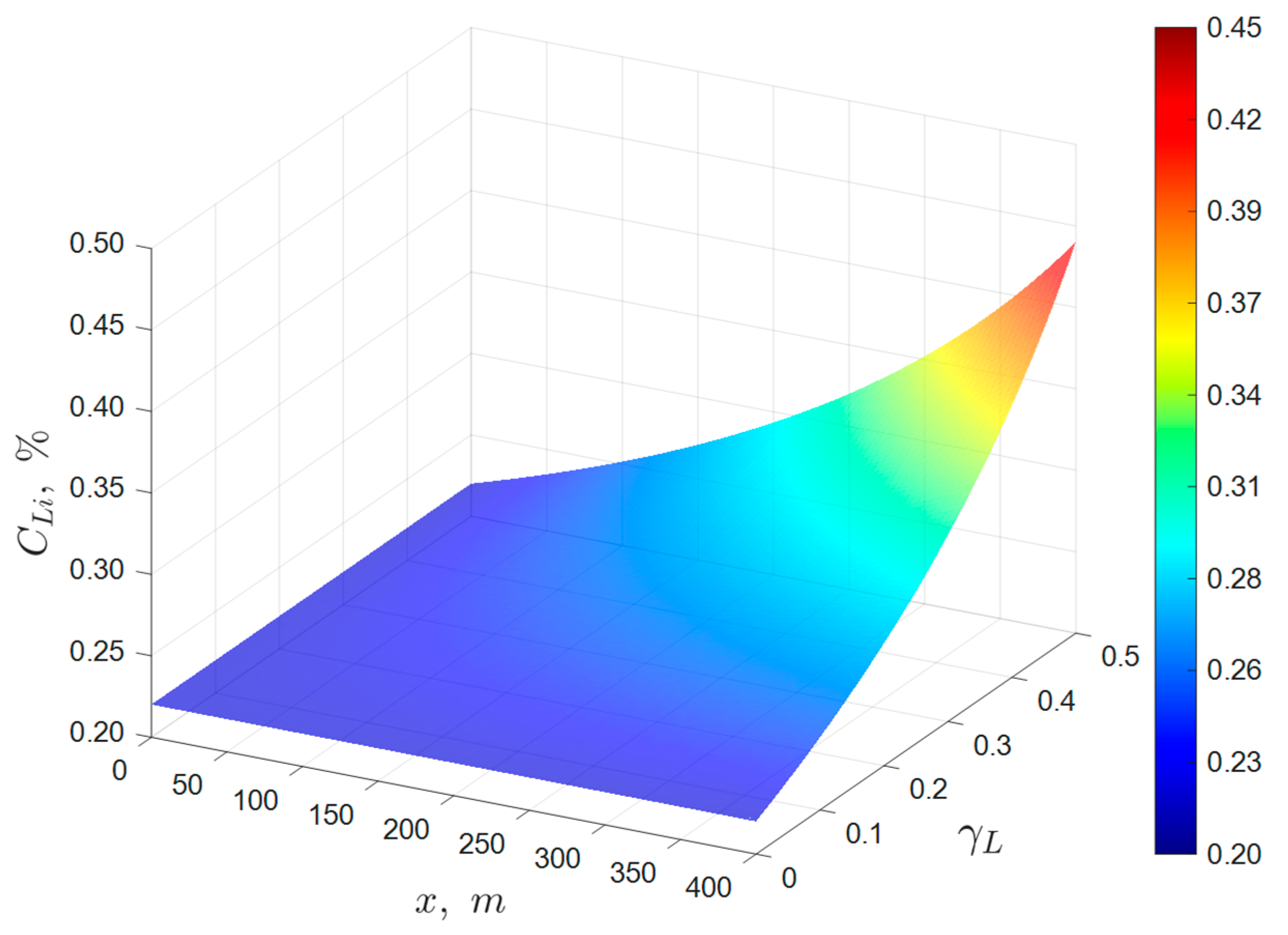
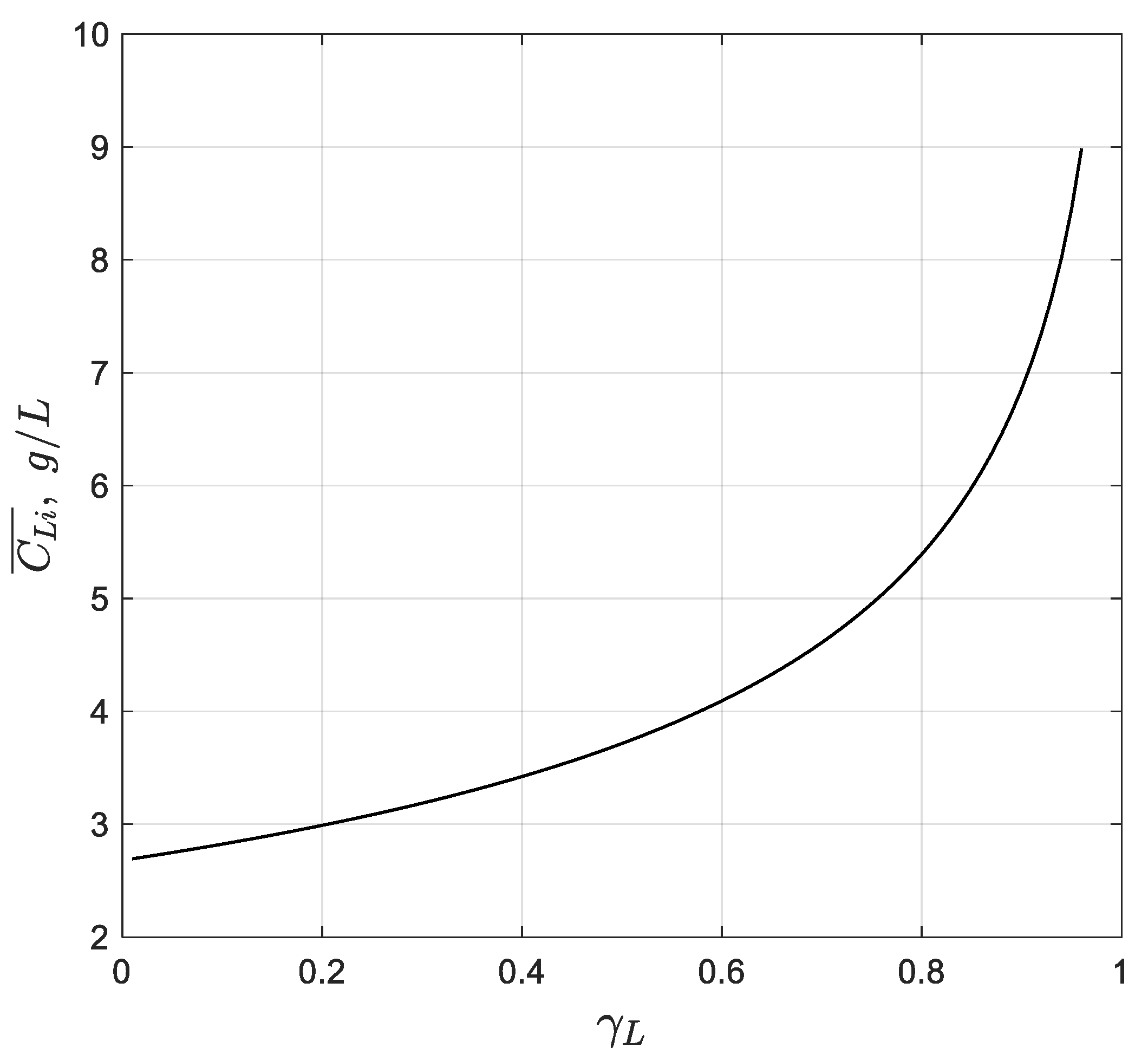
Therefore, plotting the experimental data on a graph as a function of should result in a straight line with an intercept of .
4. Experimental Validation of the Model
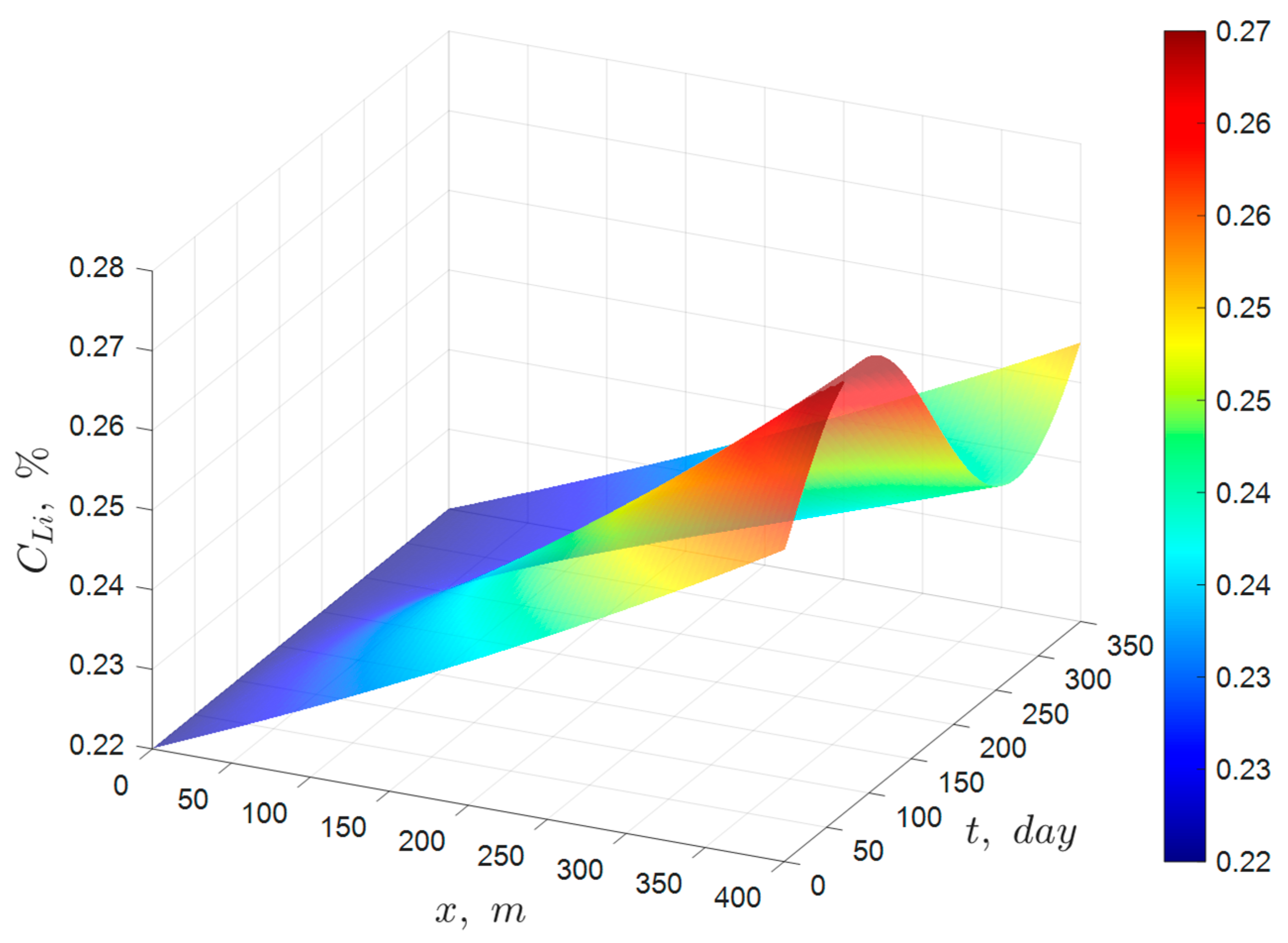
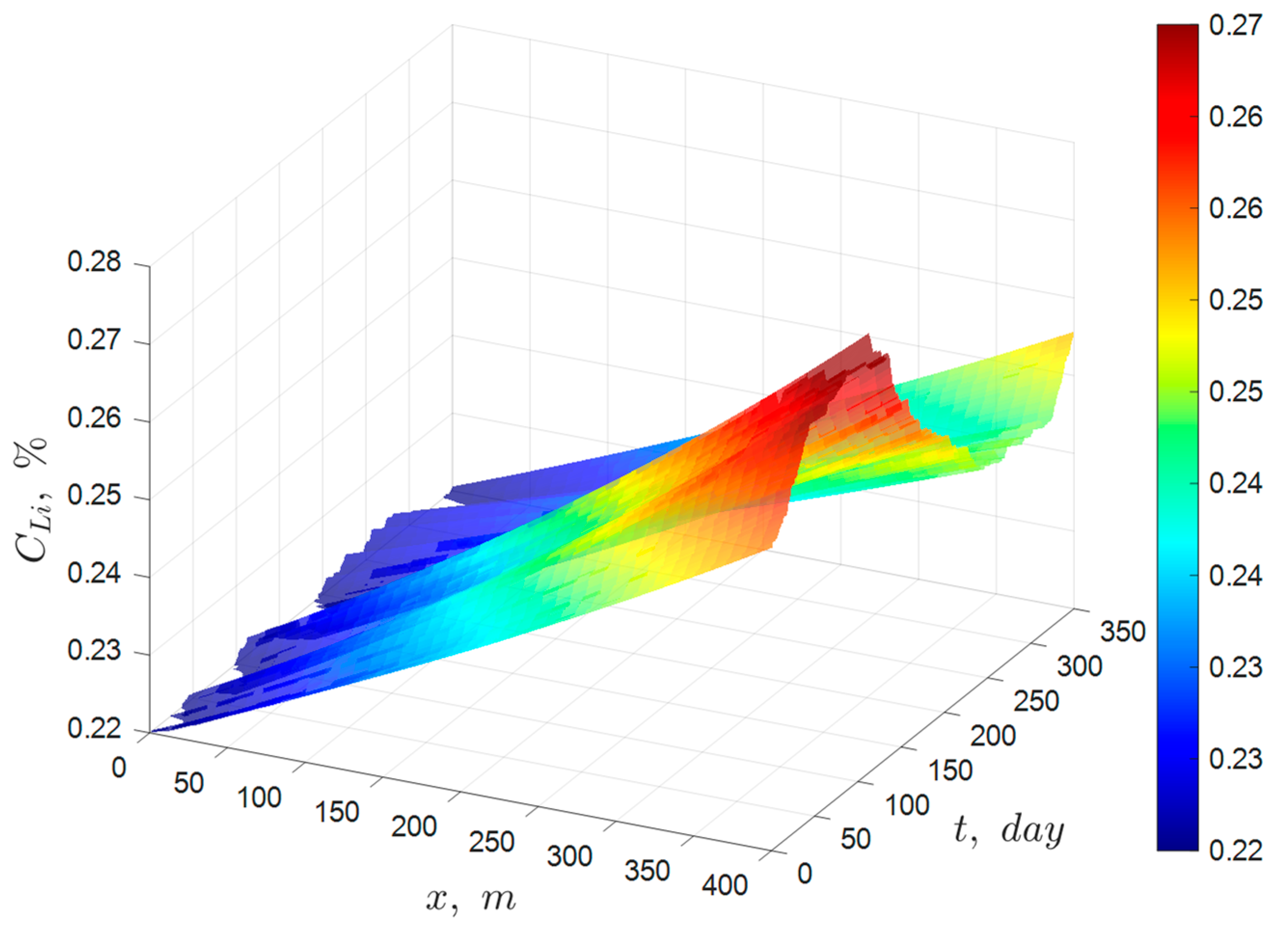
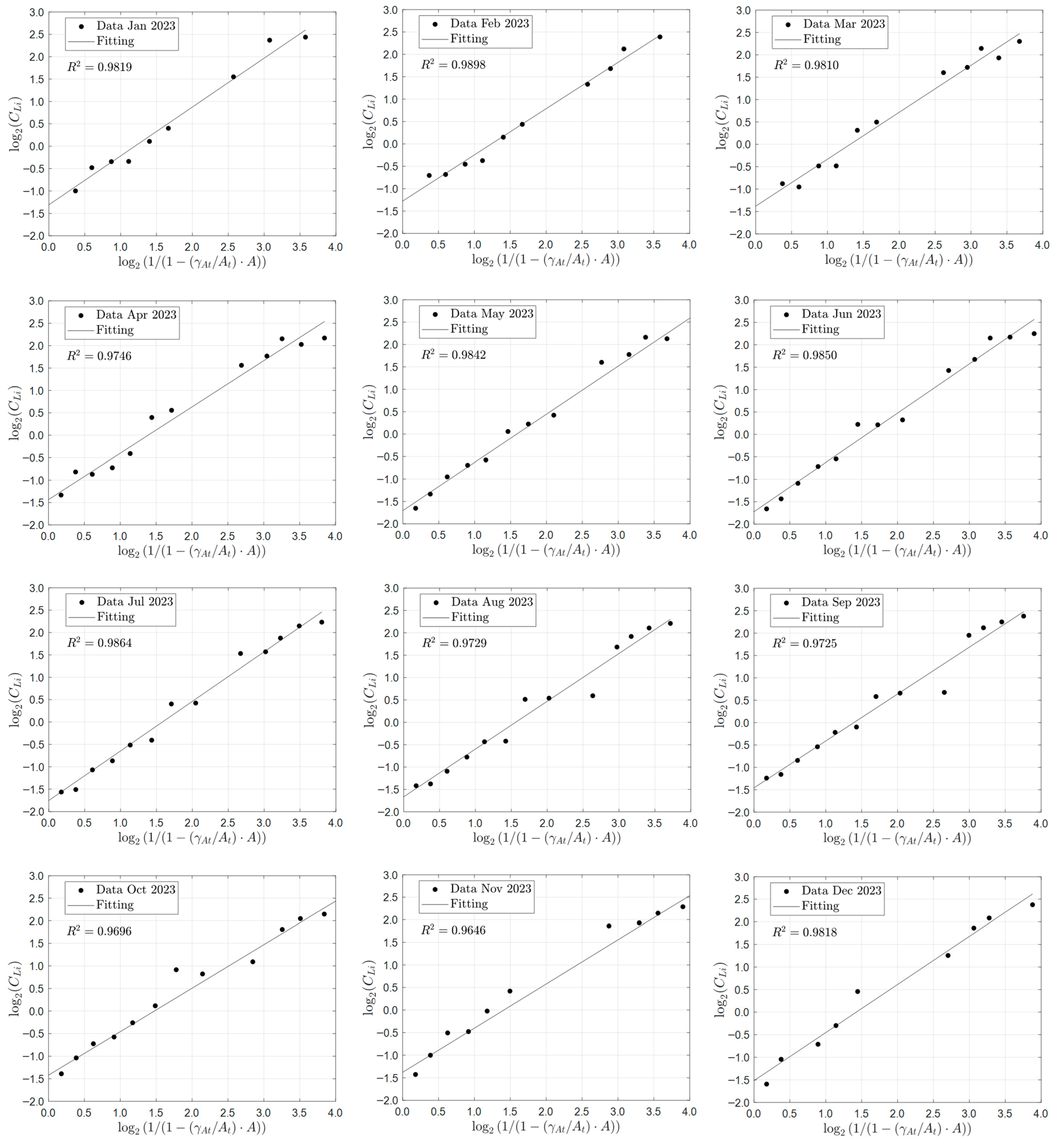
Although the ideal solar evaporation law was developed for an ideal pond that excludes infiltration, rainfall, and precipitation of the species of interest, it should be able to accurately predict the behavior of industrial solar ponds when applied to species that predominantly remain in solution. Therefore, the validation of the ideal solar evaporation law was carried out by comparing the model predictions with averaged monthly industrial data collected in the year 2023 from a pond system of 15 ponds arranged in series at one of Albemarle’s solar evaporation trains in the Salar de Atacama, Chile. The main species of interest in the brine were lithium, magnesium, and boron. The validation results for lithium are summarized below in
Figure 13, which shows the comparison between the predictions of the ideal solar evaporation law and the experimental data for lithium from January to December 2023. The linear Equation (11) was used for the validation to better visualize deviations from a straight-line behavior and to protect Albemarle’s proprietary data. The good agreement observed (R
2 in the range 0.9646 to 0.9864) indicated that low values of infiltration and rainfall in the industrial ponds do not affect appreciably the lithium concentration profile. Therefore, the ideal solar evaporation law can be used to accurately predict the concentration profile of lithium in an industrial evaporation pond system, and consequently, the lithium inventory of the system.
In the case of magnesium and boron in the brine, their concentrations were expected to increase proportionally with lithium according to the model assumptions. However, the experimental outlet concentrations for these species were significantly lower than predicted by the model, indicating that precipitation occurred within the pond. This is consistent with the known precipitation of magnesium primarily as potassium carnallite (KMgCl3·6H2O) and bischofite (MgCl2·6H2O), and boron precipitation mainly as calcium borate (Ca3(BO3)2). As a result, the ideal solar evaporation model cannot be used to determine the magnesium and boron concentration profiles under these conditions. However, it allows predicting the amounts of B and Mg that precipitate in the pond. The concentration behavior of B and Mg will be addressed further in subsequent articles, using more advanced models developed by the authors. Nevertheless, despite the precipitation of B and Mg in the pond system, the possible entrainment of lithium within the solid phases does not notably affect its concentration profile, thereby preserving the validity of the ideal solar evaporation model for lithium.
Overall, the results confirm that the ideal solar evaporation law provides a robust framework for predicting lithium concentration profiles in industrial ponds when the precipitation of lithium does not occur; and infiltration and rainfall, and the precipitation effects of other species in the brine remain low.