The Removal of Arsenic from Contaminated Water: A Critical Review of Adsorbent Materials from Agricultural Wastes to Advanced Metal–Organic Frameworks
Abstract
1. Introduction
2. Natural and Waste-Based Adsorbents
2.1. Agricultural Byproducts
2.2. Industrial Waste
2.3. Bio-Sorbents
2.4. Polysaccharide-Based Adsorbents
3. Engineered Adsorbents
3.1. Metal Oxide Nanoparticles
3.2. Activated Alumina Systems
3.3. Zeolite-Based Adsorbents
4. Advanced Hybrid Materials
4.1. Metal–Organic Frameworks (MOFs)
4.2. Polymer and Gel Adsorbents
4.2.1. Innovative Composites
4.2.2. Cryogel Advancements
5. Adsorption Mechanisms and Modeling
5.1. Physicochemical Mechanisms Regulating Arsenic Adsorption
5.1.1. Surface Complexation
5.1.2. Electrostatic Interactions
5.1.3. Oxidation-Reduction Reactions
5.1.4. Supplementary Mechanisms
5.2. Kinetic and Isothermal Modeling
5.2.1. Kinetics of Adsorption
5.2.2. Adsorption Isotherms
- -
- Langmuir Model: Relevant for monolayer adsorption on uniform surfaces (e.g., activated alumina, Qₘₐₓ = 0.318 mg/g [58]). A high R2 value for the Langmuir model indicates a uniform surface characterized by specific, identical sites. This is frequently an idealization, yet it is applicable to numerous synthetic materials.
- -
- The Freundlich Model accounts for multilayer adsorption on heterogeneous substrates, exemplified by red mud (n = 2.14, K_F = 1.83 L/mg) [23]. The Freundlich exponent *n* greater than 1 signifies a favorable adsorption process, with its value serving as an indicator of heterogeneity.
- -
- The Sips Model presents a hybrid isotherm applicable to advanced materials such as Metal–Organic Frameworks (MOFs). For instance, MIL-53(Al) exhibits a maximum adsorption capacity (Qₘₐₓ) of 105.6 mg/g and a heterogeneity index of 1.2 [78]. The Sips model is significant because it simplifies to the Langmuir model under conditions of low heterogeneity and to the Freundlich model under high heterogeneity, thus proving suitable for complex adsorbents with multiple mechanisms.
5.2.3. Thermodynamic Parameters
6. Challenges and Perspectives
- (i)
- Scalability Limitations
- (ii)
- Concerns regarding Material Stability
- (iii)
- Economic and Logistical Obstacles
7. Emerging Solutions and Future Directions
7.1. Advanced Material Engineering
7.2. Strategies for Process Intensification
7.3. Circular Economy Strategies
8. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mandel, H.G.; Mayersak, J.S.; Riis, M. The action of arsenic on Bacillus cereus. J. Pharm. Pharmacol. 1965, 17, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Costa, M. Arsenic: A Global Environmental Challenge. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Committee on Medical and Biological Effects of Environmental Pollutants. Arsenic Medical and Biologic Effects of Environmental Pollutants; National Academy of Sciences: Washington, DC, USA, 1977; p. 332. Available online: https://www.ncbi.nlm.nih.gov/books/NBK231016/ (accessed on 1 September 2024).
- Dray, R. Arsenic, Metals, Fibres and Dusts: A Review of Human Carcinogens. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; The International Agency for Research on Cancer: Lyon, France, 2012; Volume 100C, p. 501. Available online: https://www.ncbi.nlm.nih.gov/books/NBK304380 (accessed on 1 September 2024).
- Hassan, H.R. A review on different arsenic removal techniques used for decontamination of drinking water. Environ. Pollut. Bioavailab. 2023, 35, 2165964. [Google Scholar] [CrossRef]
- Pezeshki, H.; Hashemi, M.; Rajabi, S. Removal of arsenic as a potentially toxic element from drinking water by filtration: A mini review of nanofiltration and reverse osmosis techniques. Heliyon 2023, 9, e14246. [Google Scholar] [CrossRef]
- Han, D.; E, J.; Deng, Y.; Chen, J.; Leng, E.; Liao, G.; Zhao, X.; Feng, C.; Zhang, F. A review of studies using hydrocarbon adsorption material for reducing hydrocarbon emissions from cold start of gasoline engine. Renew. Sustain. Energy Rev. 2021, 135, 110079. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S. Application of adsorption process for effective removal of emerging contaminants from water and wastewater. Environ. Pollut. 2021, 280, 116995. [Google Scholar] [CrossRef]
- Jekel, M.; Amy, G.L. Interface Science in Drinking Water Treatment—Theory and Application Arsenic removal during drinking water treatment. Interface Sci. Technol. 2006, 10, 193–206. [Google Scholar]
- Hao, L.; Liu, M.; Wang, N.; Li, G. A critical review on arsenic removal from water using iron-based adsorbents. RSC Adv. 2018, 8, 39545–39560. [Google Scholar] [CrossRef]
- Li, D.; Yadav, A.; Zhou, H.; Roy, K.; Thanasekaran, P.; Lee, C. Advances and Applications of Metal-Organic Frameworks (MOFs) in Emerging Technologies: A Comprehensive Review. Glob. Chall. 2024, 8, 2300244. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Academic Press: Cambridge, MA, USA, 1999. [Google Scholar]
- Masel, R.I. Principles of Adsorption and Reaction on Solid Surfaces; Wiley: Hoboken, NJ, USA, 1996. [Google Scholar]
- Srivastav, A.L.; Pham, T.D.; Izah, S.C.; Singh, N.; Singh, P.K. Biochar Adsorbents for Arsenic Removal from Water Environment: A Review. Bull. Environ. Contam. Toxicol. 2022, 108, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, T.; Li, M.; Yang, Y.; Lu, P.; Ning, P.; Wang, Q. Arsenic removal from water/wastewater using layered double hydroxide derived adsorbents, a critical review. RSC Adv. 2018, 8, 22694–22709. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, Z.; Shahid, M.; Natasha; Khalid, S.; Khalid, S.; Imran, M.; Qureshi, M.I.; Niazi, N.K. Use of Agricultural Bio-Wastes to Remove Arsenic from Contaminated Water. Environ. Geochem. Health 2020, 45, 5703–5712. [Google Scholar] [CrossRef]
- Shahid, M.; Niazi, N.K.; Dumat, C.; Naidu, R.; Khalid, S.; Rahman, M.M.; Bibi, I. A meta-analysis of the distribution, sources and health risks of arsenic-contaminated groundwater in Pakistan. Environ. Pollut. 2018, 242 Pt A, 307–319. [Google Scholar] [CrossRef]
- Shah, A.H.; Shahid, M.; Khalid, S.; Natasha; Shabbir, Z.; Bakhat, H.F.; Murtaza, B.; Farooq, A.; Akram, M.; Shah, G.M.; et al. Assessment of arsenic exposure by drinking well water and associated carcinogenic risk in peri-urban areas of Vehari, Pakistan. Environ. Geochem. Health 2020, 42, 121–133. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Shakoor, M.B.; Jilani, A.; Anjum, R. High sorption efficiency for As(III) and As(V) from aqueous solutions using novel almond shell biochar. Chemosphere 2020, 243, 125330. [Google Scholar]
- Vukašinović-Pešić, V.L.; Rajaković-Ognjanović, V.N.; Blagojević, N.Z.; Grudić, V.V.; Jovanović, B.M.; Rajaković, L.V. Enhanced Arsenic Removal from Water by Activated Red Mud Based on Hydrated Iron(Iii) and Titan(Iv) Oxides. Chem. Eng. Commun. 2012, 199, 849–864. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, Z.; Liu, C.; Gao, Y.; Qi, Y. Properties of red mud blended with magnesium phosphate cement paste: Feasibility of grouting material preparation. Constr. Build. Mater. 2020, 260, 119704. [Google Scholar] [CrossRef]
- Lu, Z.; Qi, X.; Zhu, X.; Li, X.; Li, K.; Wang, H. Highly effective remediation of high-arsenic wastewater using red mud through formation of AlAsO4@silicate precipitate. Environ. Pollut. 2021, 287, 117484. [Google Scholar] [CrossRef]
- Lekić, B.M.; Marković, D.D.; Rajaković-Ognjanović, V.N.; Đukić, A.R.; Rajaković, L.V. Arsenic Removal from Water Using Industrial By-Products. J. Chem. 2013, 2013, 9. [Google Scholar] [CrossRef]
- Bhakta, J.N.; Ali, M.M. Biosorption of Arsenic: An Emerging Eco-Technology of Arsenic Detoxification in Drinking Water; Springer International Publishing: Cham, Switzerland, 2020; pp. 207–230. [Google Scholar]
- Giri, D.D.; Jha, J.M.; Srivastava, N.; Hashem, A.; Abd Allah, E.F.; Shah, M.; Pal, D.B. Sustainable removal of arsenic from simulated wastewater using solid waste seed pods biosorbents of Cassia fistula L. Chemosphere 2022, 287 Pt 3, 132308. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Shahid, M.; Sharif, F.; Bashir, S.; Shaheen, M.S.; Wang, H.; Ok, Y.S.; Rinklebe, J. Arsenic removal by natural and chemically modified water melon rind in aqueous solutions and groundwater. Sci. Total Environ. 2018, 645, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.M.; Wang, J.; Bibi, I.; Shahid, M.; Niazi, N.K.; Iqbal, J.; Mian, I.A.; Shaheen, S.M.; Bashir, S.; Shah, N.S.; et al. Arsenic speciation and biotransformation pathways in the aquatic ecosystem: The significance of algae. J. Hazard. Mater. 2021, 403, 124027. [Google Scholar] [CrossRef] [PubMed]
- de Souza, E.C.; Pimenta, A.S.; da Silva, A.J.F.; do Nascimento, P.F.P.; Ighalo, J.O. Oxidized eucalyptus charcoal: A renewable biosorbent for removing heavy metals from aqueous solutions. Biomass Convers. Biorefin. 2021, 13, 4105–4119. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Shahid, M.; Saqib, Z.A.; Nawaz, M.F.; Shaheen, S.M.; Wang, H.; Tsang, D.C.; Bundschuh, J.; et al. Exploring the arsenic removal potential of various biosorbents from water. Environ. Int. 2019, 123, 567–579. [Google Scholar] [CrossRef]
- Letechipia, J.O.; Gonzalez-Trinidad, J.; Junez-Ferreira, H.E.; Bautista-Capetillo, C.; Robles Rovelo, C.O.; Contreras Rodriguez, A.R. Removal of arsenic from semiarid area groundwater using a biosorbent from watermelon peel waste. Heliyon 2023, 9, e13251. [Google Scholar] [CrossRef]
- Chiavola, A.; D’Amatoa, E.; Stoller, M.; Chianese, A.; Boni, M.R. Application of Iron Based Nanoparticles as Adsorbents for Arsenic Removal from Water. Chem. Eng. Trans. 2016, 47, 325–330. [Google Scholar]
- Nguyen, T.H.; Tran, H.N.; Vu, H.A.; Trinh, M.V.; Nguyen, T.V.; Loganathan, P.; Vigneswaran, S.; Nguyen, T.M.; Trinh, V.T.; Vu, D.L.; et al. Laterite as a low-cost adsorbent in a sustainable decentralized filtration system to remove arsenic from groundwater in Vietnam. Sci. Total Environ. 2020, 699, 134267. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nguyen, T.V.; Vigneswaran, S.; Ha, N.T.H.; Ratnaweera, a.H. A Review of Theoretical Knowledge and Practical Applications of Iron-Based Adsorbents for Removing Arsenic from Water. Minerals 2023, 13, 741. [Google Scholar] [CrossRef]
- Asare, E.A.; Dartey, E.; Sarpong, K.; Effah-Yeboah, E.; Amissah-Reynolds, P.K.; Tagoe, S.; Balali, G.I. Adsorption Isotherm, Kinetic and Thermodynamic Modelling of Bacillus subtilis ATCC13952 Mediated Adsorption of Arsenic in Groundwaters of Selected Gold Mining Communities in the Wassa West Municipality of the Western Region of Ghana. Am. J. Anal. Chem 2021, 12, 121–161. [Google Scholar] [CrossRef]
- Amen, R.; Bashir, H.; Bibi, I.; Shaheen, S.M.; Niazi, N.K.; Shahid, M.; Hussain, M.M.; Antoniadis, V.; Shakoor, M.B.; Al-Solaimani, S.G.; et al. A critical review on arsenic removal from water using biochar-based sorbents: The significance of modification and redox reactions. Chem. Eng. J. 2020, 396, 125195. [Google Scholar] [CrossRef]
- Znad, H.; Awual, M.R.; Martini, S. The utilization of algae and seaweed biomass for bioremediation of heavy metal-contaminated wastewater. Molecules 2019, 24, 3651. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Boddu, V.M.; Abburi, K.; Talbott, J.L.; Smith, E.D.; Haasch, R. Removal of arsenic (III) and arsenic (V) from aqueous medium using chitosan-coated biosorbent. Water Res. 2008, 42, 633–642. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chang, T.H.; Kuo, J.T.; Chen, Y.F.; Chung, Y.C. Characteristics of molybdate-impregnated chitosan beads (MICB) in terms of arsenic removal from water and the application of a MICB-packed column to remove arsenic from wastewater. Bioresour. Technol. 2008, 99, 7487–7494. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Etale, A.; Wang, J.; Berglund, L.A.; Li, Y.; Cai, Y.; Chen, C.; Cranston, E.D.; Johns, M.A.; Fang, Z.; et al. Current international research into cellulose as a functional nanomaterial for advanced applications. J. Mater. Sci. 2022, 57, 5697–5767. [Google Scholar] [CrossRef]
- Bessaies, H.; Iftekhar, S.; Doshi, B.; Kheriji, J.; Ncibi, M.C.; Srivastava, V.; Sillanpää, M.; Hamrouni, B. Synthesis of novel adsorbent by intercalation of biopolymer in LDH for the removal of arsenic from synthetic and natural water. J. Environ. Sci. 2020, 91, 246–261. [Google Scholar] [CrossRef]
- Chandan, A.K.; Mallika, G.N.; Narsaiah, T.B. A green approach to arsenic removal using ZnO nanoparticles synthesized from Acacia Catechu leaf extract. Mater. Today Proc. 2023, 72, 110–119. [Google Scholar] [CrossRef]
- Jain, C.K.; Singh, R.D. Technological options for the removal of arsenic with special reference to South East Asia. J. Environ. Manag. 2012, 107, 1–18. [Google Scholar] [CrossRef]
- Gallegos-Garcia, M.; Ramírez-Muñiz, K.; Song, S. Arsenic Removal from Water by Adsorption Using Iron Oxide Minerals as Adsorbents: A Review. Miner. Process. Extr. Metall. Rev. 2012, 33, 301–315. [Google Scholar] [CrossRef]
- Asere, T.G.; Stevens, C.V.; Du Laing, G. Use of (modified) natural adsorbents for arsenic remediation: A review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Chaudhry, S.A. Iron oxide and its modified forms as an adsorbent for arsenic removal: A comprehensive recent advancement. Process. Saf. Environ. Prot. 2017, 111, 592–626. [Google Scholar] [CrossRef]
- Mondal, M.K.; Garg, R. A comprehensive review on removal of arsenic using activated carbon prepared from easily available waste materials. Environ. Sci. Pollut. Res. Int. 2017, 24, 13295–13306. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Muensri, P.; Danwittayakul, S. Removal of Arsenic from Groundwater Using Nano-Metal Oxide Adsorbents. Key Eng. Mater. 2017, 751, 766–772. [Google Scholar] [CrossRef]
- Ashraf, S.; Siddiqa, A.; Shahida, S.; Qaisar, S. Titanium-based nanocomposite materials for arsenic removal from water: A review. Heliyon 2019, 5, e01577. [Google Scholar] [CrossRef]
- Aziz, N.A.A.; Jayasuriya, N.; Fan, L. Adsorption Study on Moringa Oleifera Seeds and Musa Cavendish as Natural Water Purification Agents for Removal of Lead, Nickel and Cadmium from Drinking Water. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2016; Volume 136, p. 012044. [Google Scholar]
- Shan, T.C.; Matar, M.A.; Makky, E.A.; Ali, E.N. The use of Moringa oleifera seed as a natural coagulant for wastewater treatment and heavy metals removal. Appl. Water Sci. 2016, 7, 1369–1376. [Google Scholar] [CrossRef]
- Alo, M.N.; Anyim, C.; Elom, M. Coagulation-and-antimicrobial-activities-of-moringa-oleifera-seedstorage-at-3c-temperature-in-turbid-water. Adv. Appl. Sci. Res. 2012, 3, 887–894. [Google Scholar]
- Soto-Robles, C.A.; Nava, O.J.; Vilchis-Nestor, A.R.; Castro-Beltrán, A.; Gómez-Gutiérrez, C.M.; Lugo-Medina, E.; Olivas, A.; Luque, P.A. Biosynthesized zinc oxide using Lycopersicon esculentum peel extract for methylene blue degradation. J. Mater. Sci. Mater. Electron. 2017, 29, 3722–3729. [Google Scholar] [CrossRef]
- Tripathy, S.S.; Raichur, A.M. Enhanced adsorption capacity of activated alumina by impregnation with alum for removal of As(V) from water. Chem. Eng. J. 2008, 138, 179–186. [Google Scholar] [CrossRef]
- Lee, S.M.; Lalhmunsiama; Thanhmingliana; Tiwari, D. Porous hybrid materials in the remediation of water contaminated with As(III) and As(V). Chem. Eng. J. 2015, 270, 496–507. [Google Scholar] [CrossRef]
- Majumder, C. Arsenic(V) Removal Using Activated Alumina: Kinetics and Modeling by Response Surface. J. Environ. Eng. 2018, 144, 04017115. [Google Scholar] [CrossRef]
- Dhanasekaran, P.; Sahu, O. Arsenate and fluoride removal from groundwater by sawdust impregnated ferric hydroxide and activated alumina (SFAA). Groundw. Sustain. Dev. 2021, 12, 100490. [Google Scholar] [CrossRef]
- Khatamian, M.; Afshar No, N.; Hosseini Nami, S.; Fazli-Shokouhi, S. Synthesis and characterization of zeolite A, Fe3O4/zeolite A, and Fe2O3/zeolite A nanocomposites and investigation of their arsenic removal performance. J. Iran. Chem. Soc. 2023, 20, 1657–1670. [Google Scholar] [CrossRef]
- Salem Attia, T.M.; Hu, X.L.; Yin, D.Q. Synthesised magnetic nanoparticles coated zeolite (MNCZ) for the removal of arsenic (As) from aqueous solution. J. Exp. Nanosci. 2014, 9, 551–560. [Google Scholar] [CrossRef]
- Noroozifar, M.; Khorasani-Motlagh, M.; Naderpour, H. Modified nanocrystalline natural zeolite for adsorption of arsenate from wastewater: Isotherm and kinetic studies. Microporous Mesoporous Mater. 2014, 197, 101–108. [Google Scholar] [CrossRef]
- Dabiri, R.; Shiraz, E.A. Evaluating performance of natural sepiolite and zeolite nanoparticles for nickel, antimony, and arsenic removal from synthetic wastewater. J. Min. Environ. 2018, 9, 1049–1064. [Google Scholar]
- Jian, M.; Liu, B.; Zhang, G.; Liu, R.; Zhang, X. Adsorptive removal of arsenic from aqueous solution by zeolitic imidazolate framework-8 (ZIF-8) nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 465, 67–76. [Google Scholar] [CrossRef]
- Yin, L.; Li, W.; Lin, S.; Owens, G.; Chen, Z. Simultaneous removal of arsenite and arsenate from mining wastewater using ZIF-8 embedded with iron nanoparticles. Chemosphere 2022, 304, 135269. [Google Scholar] [CrossRef]
- Rajni; Taruna; Udayasri, A.; Raghav, N.; Bendi, A.; Tomar, R. Revolutionizing wastewater treatment: Polymeric metal oxide nanocomposites for effective dye and heavy metal removal. Chem. Eng. J. 2025, 511, 161694. [Google Scholar]
- de Jesús Ruíz-Baltazar, Á. Advancements in nanoparticle-modified zeolites for sustainable water treatment: An interdisciplinary review. Sci. Total Environ. 2024, 946, 174373. [Google Scholar] [CrossRef]
- Khatamian, M.; Khodakarampoor, N.; Saket-Oskoui, M. Efficient removal of arsenic using graphene-zeolite based composites. J. Colloid Interface Sci. 2017, 498, 433–441. [Google Scholar] [CrossRef]
- Zhou, C.; Han, C.; Min, X.; Yang, T. Enhancing arsenic removal from acidic wastewater using zeolite-supported sulfide nanoscale zero-valent iron: The role of sulfur and copper. J. Chem. Technol. Biotechnol. 2021, 96, 2042–2052. [Google Scholar] [CrossRef]
- Faalzadeh, M.; Faghihian, H. Separation of arsenic from aqueous solutions by amino-functionalized γ-Fe2O3-β-zeolite. Sep. Sci. Technol. 2015, 50, 958–964. [Google Scholar] [CrossRef]
- Shahsavari, M.; Mohammadzadeh Jahani, P.; Sheikhshoaie, I.; Tajik, S.; Aghaei Afshar, A.; Askari, M.B.; Salarizadeh, P.; Di Bartolomeo, A.; Beitollahi, H. Green Synthesis of Zeolitic Imidazolate Frameworks: A Review of Their Characterization and Industrial and Medical Applications. Materials 2022, 15, 447. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Wang, B.; Zhu, L.; Jiang, L. Properties of Cobalt- and Nickel-Doped Zif-8 Framework Materials and Their Application in Heavy-Metal Removal from Wastewater. Nanomaterials 2020, 10, 1636. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jian, M.; Liu, R.; Yao, J.; Zhang, X. Highly efficient removal of arsenic(III) from aqueous solution by zeolitic imidazolate frameworks with different morphology. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 358–366. [Google Scholar] [CrossRef]
- Samimi, M.; Zakeri, M.; Alobaid, F.; Aghel, B. A Brief Review of Recent Results in Arsenic Adsorption Process from Aquatic Environments by Metal-Organic Frameworks: Classification Based on Kinetics, Isotherms and Thermodynamics Behaviors. Nanomaterials 2022, 13, 60. [Google Scholar] [CrossRef]
- Ehzari, H.; Safari, M.; Samimi, M. Signal amplification of novel sandwich-type genosensor via catalytic redox-recycling on platform MWCNTs/Fe3O4@TMU-21 for BRCA1 gene detection. Talanta 2021, 234, 122698. [Google Scholar] [CrossRef]
- Alam, E. Metal-organic frameworks (MOFs) for arsenic remediation: A brief overview of recent progress. RSC Adv. 2025, 15, 20281–20308. [Google Scholar] [CrossRef]
- Zhu, B.-J.; Yu, X.-Y.; Jia, Y.; Peng, F.-M.; Sun, B.; Zhang, M.-Y.; Luo, T.; Liu, J.-H.; Huang, X.-J. Iron and 1,3,5-Benzenetricarboxylic Metal–Organic Coordination Polymers Prepared by Solvothermal Method and Their Application in Efficient As(V) Removal from Aqueous Solutions. J. Phys. Chem. C 2012, 116, 8601–8607. [Google Scholar]
- Li, J.; Wu, Y.N.; Li, Z.; Zhu, M.; Li, F. Characteristics of arsenate removal from water by metal-organic frameworks (MOFs). Water Sci. Technol. 2014, 70, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-C.; Yin, X.-B. CoFe2O4@MIL-100(Fe) hybrid magnetic nanoparticles exhibit fast and selective adsorption of arsenic with high adsorption capacity. Sci. Rep. 2017, 7, 40955. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Feng, X.; Bao, C.; Lai, Q.; Li, Z.; Tang, W.; Shao, Z.-W.; Zhang, Z.; Dai, Z.; Liu, C. Aquatic arsenic removal with a Zr-MOF constructed via in situ nitroso coupling. Sep. Purif. Technol. 2022, 288, 120700. [Google Scholar] [CrossRef]
- Su, S.; Zhang, R.; Rao, J.; Yu, J.; Jiang, X.; Wang, S.; Yang, X. Fabrication of lanthanum-modified MOF-808 for phosphate and arsenic(V) removal from wastewater. J. Environ. Chem. Eng. 2022, 10, 108527. [Google Scholar]
- Saleh, T.A.; Tuzen, M.; Sarı, A.; Altunay, N. Factorial design, physical studies and rapid arsenic adsorption using newly prepared polymer modified perlite adsorbent. Chem. Eng. Res. Des. 2022, 183, 181–191. [Google Scholar] [CrossRef]
- Imyim, A.; Sirithaweesit, T.; Ruangpornvisuti, V. Arsenite and arsenate removal from wastewater using cationic polymer-modified waste tyre rubber. J. Environ. Manag. 2016, 166, 574–578. [Google Scholar] [CrossRef]
- Katsoyiannis, I.; Zouboulis, A. Removal of arsenic from contaminated water sources by sorption onto iron-oxide-coated polymeric materials. Water Res. 2002, 36, 5141–5155. [Google Scholar] [CrossRef]
- Önnby, L.; Pakade, V.; Mattiasson, B.; Kirsebom, H. Polymer composite adsorbents using particles of molecularly imprinted polymers or aluminium oxide nanoparticles for treatment of arsenic contaminated waters. Water Res. 2012, 46, 4111–4120. [Google Scholar] [CrossRef]
- Gao, Z.; Sui, J.; Xie, X.; Li, X.; Song, S.; Zhang, H.; Hu, Y.; Hong, Y.; Wang, X.; Cui, J. Metal-organic gels of simple chemicals and their high efficacy in removing arsenic(V) in water. AIChE J. 2018, 64, 3719–3727. [Google Scholar] [CrossRef]
- Aremu, J.O.; Lay, M.; Glasgow, G. Kinetic and isotherm studies on adsorption of arsenic using silica based catalytic media. J. Water Process. Eng. 2019, 32, 100939. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, X.; Qi, X.; Shu, B.; Zhang, X.; Li, K.; Wei, Y.; Wang, H. Removal and immobilization of arsenic from copper smelting wastewater using copper slag by in situ encapsulation with silica gel. Chem. Eng. J. 2020, 394, 124833. [Google Scholar] [CrossRef]
- Biswas, B.K.; Inoue, J.-i.; Inoue, K.; Ghimire, K.N.; Harada, H.; Ohto, K.; Kawakita, H. Adsorptive removal of As(V) and As(III) from water by a Zr(IV)-loaded orange waste gel. J. Hazard. Mater. 2008, 154, 1066–1074. [Google Scholar]
- Yang, B.; Zhou, X.; Chen, Y.; Fang, Y.; Luo, H. Preparation of a spindle δ-MnO2@Fe/Co-MOF-74 for effective adsorption of arsenic from water. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127378. [Google Scholar] [CrossRef]
- Min, J.H.; Hering, J.G. Arsenate sorption by Fe(III)-doped alginate gels. Water Res. 1998, 32, 1544–1552. [Google Scholar] [CrossRef]
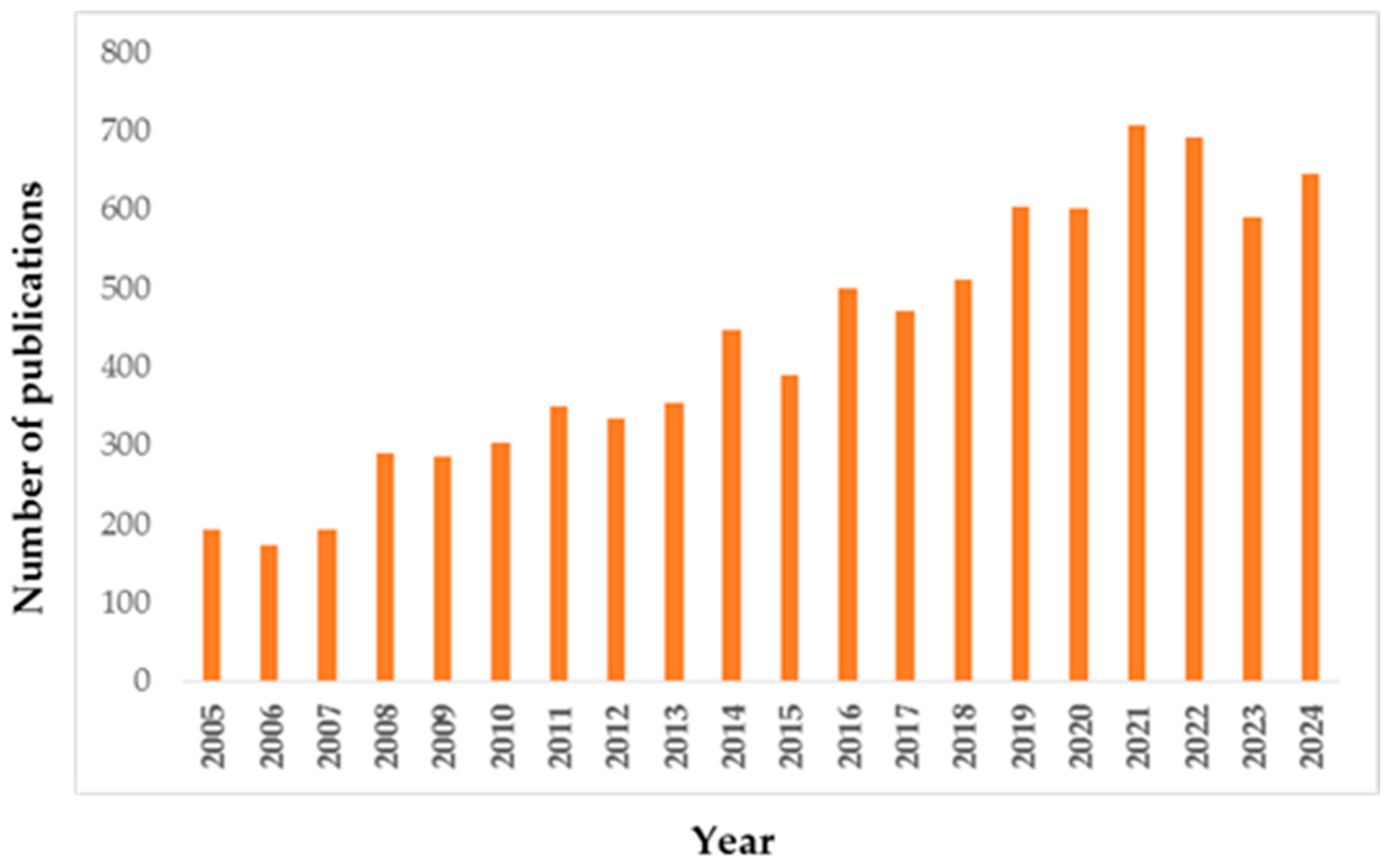
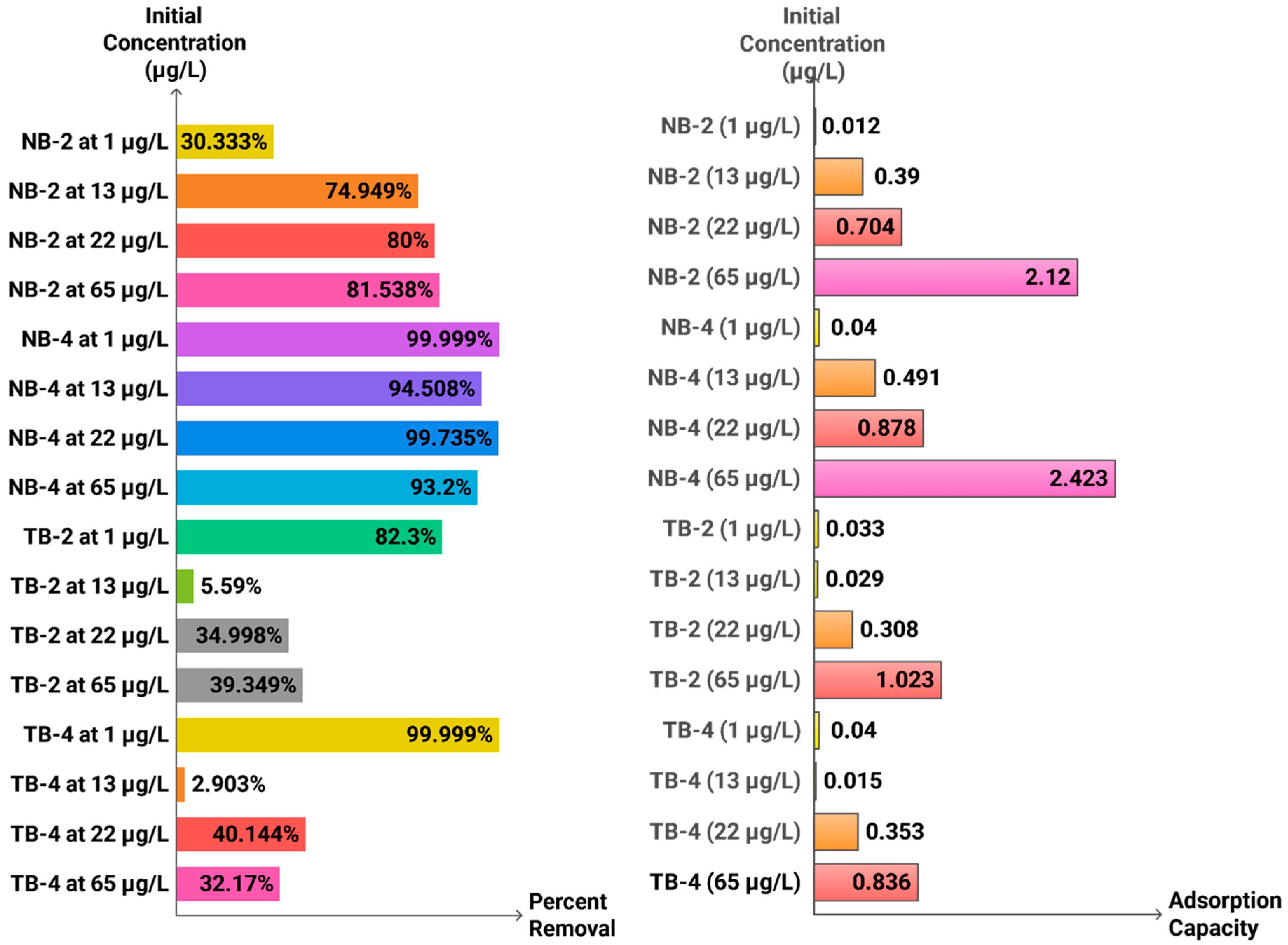
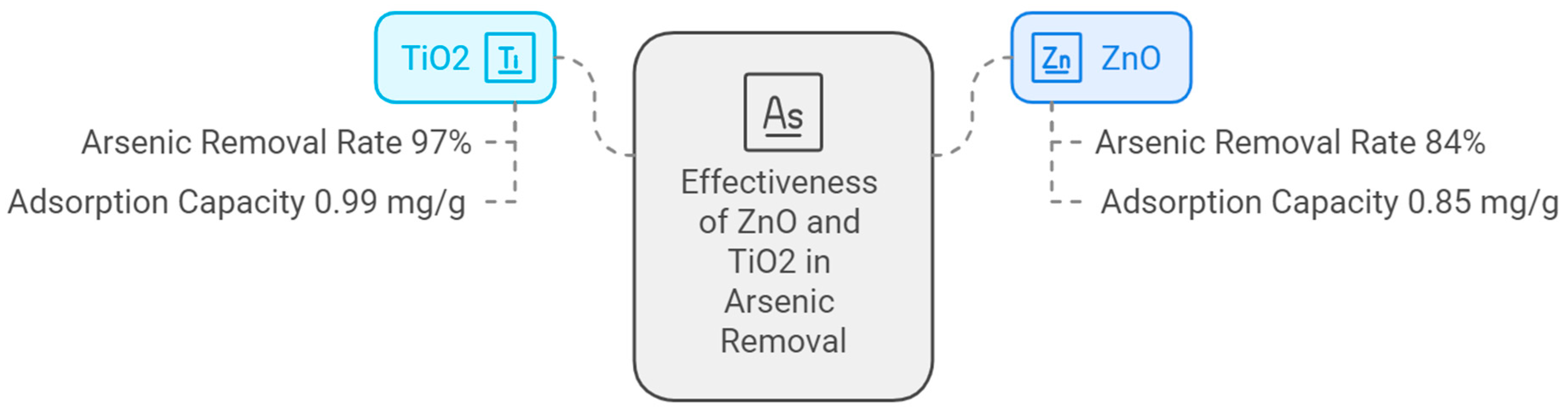
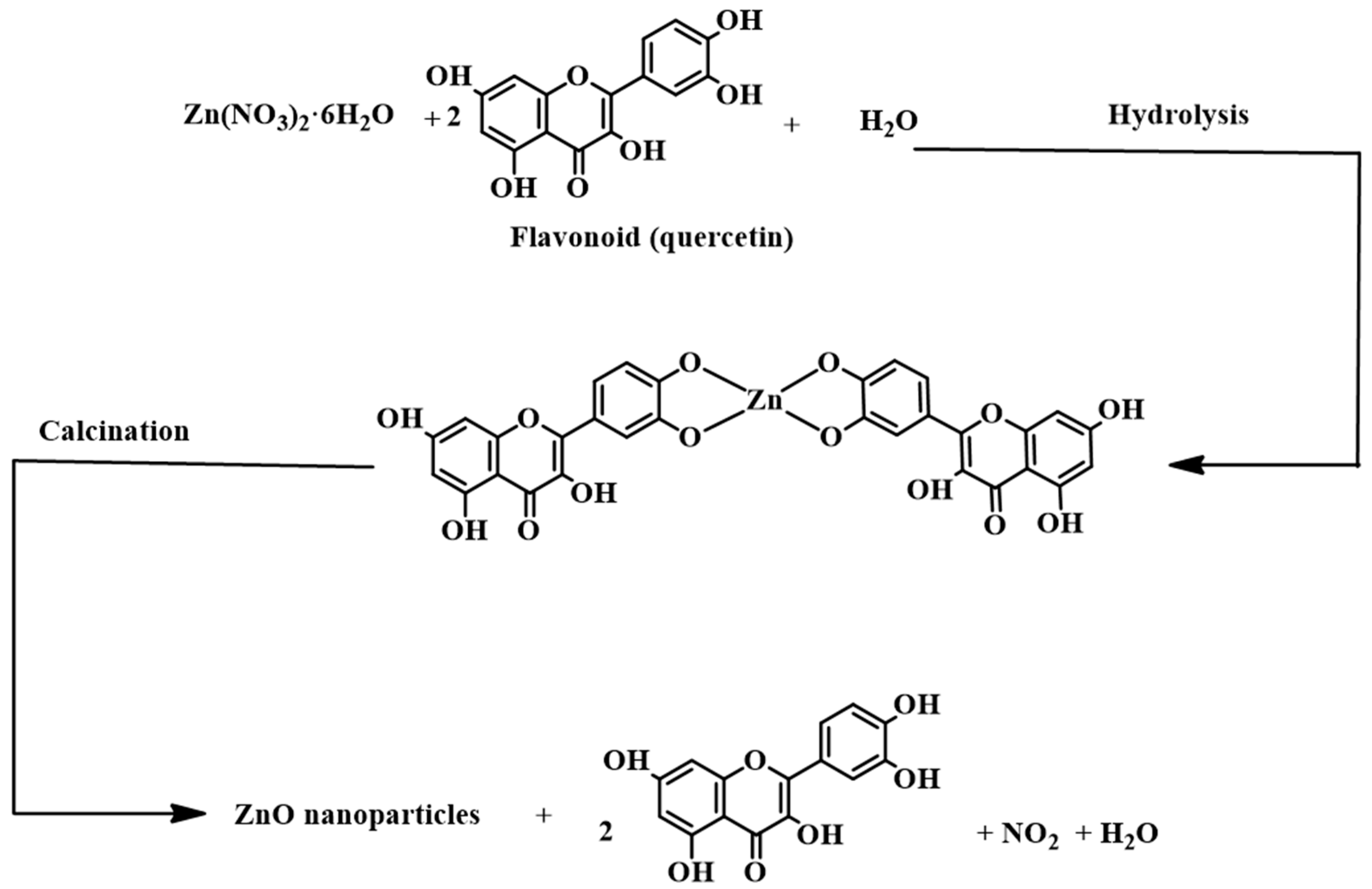

| Characteristic | 1st Generation (Natural/Waste) | 2nd Generation (Engineered) | 3rd Generation (Advanced Hybrids) |
|---|---|---|---|
| Example Materials | Rice husks, red mud, watermelon rind, soybean hulls | Activated alumina, TiO2, ZnO, Fe3O4 nanoparticles | MOFs (e.g., MIL-53, ZIF-8), polymer composites, MXenes |
| Primary Mechanism | Physisorption, electrostatic attraction, ion exchange | Chemisorption, surface complexation, ligand exchange | Synergistic mechanisms: size exclusion, redox, complexation |
| Avg. Capacity (Q_max) | Low (0.1–5 mg/g) | Moderate (5–50 mg/g) | High (50–300+ mg/g) |
| Key Advantage | Very low cost, sustainable, waste valorization | High reliability, proven efficacy, commercial availability | Exceptional capacity & selectivity, tunable properties |
| Key Limitation | Low capacity, low reusability, variable composition | Sensitive to water chemistry (pH, competing ions) | High synthesis cost, poor stability, scalability challenges |
| Element | Fe | Ca | Al | Si | Ti | O | Others |
|---|---|---|---|---|---|---|---|
| Concentration (%) | 20.56 | 11.52 | 10.51 | 7.71 | 3.40 | 37.48 | 8.82 |
| Model Type | Model Name | Primary Application | Mechanistic Insight | Selected Refs. |
|---|---|---|---|---|
| Kinetic | Pseudo-First-Order (PFO) | All types, but often poor fit | Physisorption; pore diffusion | [50,58] |
| Pseudo-Second-Order (PSO) | Engineered & Advanced Materials | Chemisorption is rate-limiting | [26,77,83] | |
| Elovich | Heterogeneous Surfaces (Biosorbents) | Multi-site chemisorption on irregular surfaces | [35] | |
| Isotherm | Langmuir | Homogeneous surfaces (* MOs, * MOFs) | Monolayer coverage on a surface with identical sites | [58,77,78] |
| Freundlich | Heterogeneous surfaces (Biosorbents, Waste) | Multilayer adsorption on a surface with sites of different energies | [21,23] | |
| Sips (Langmuir-Freundlich) | Advanced Materials (MOFs, Composites) | Hybrid model; describes heterogeneous surfaces that approach monolayer capacity | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmakki, M.A.E.; Ghosh, S.; Motente, M.; Ajiboye, T.O.; Venter, J.; Adetunji, A.I. The Removal of Arsenic from Contaminated Water: A Critical Review of Adsorbent Materials from Agricultural Wastes to Advanced Metal–Organic Frameworks. Minerals 2025, 15, 1037. https://doi.org/10.3390/min15101037
Elmakki MAE, Ghosh S, Motente M, Ajiboye TO, Venter J, Adetunji AI. The Removal of Arsenic from Contaminated Water: A Critical Review of Adsorbent Materials from Agricultural Wastes to Advanced Metal–Organic Frameworks. Minerals. 2025; 15(10):1037. https://doi.org/10.3390/min15101037
Chicago/Turabian StyleElmakki, Mohammed A. E., Soumya Ghosh, Mokete Motente, Timothy Oladiran Ajiboye, Johan Venter, and Adegoke Isiaka Adetunji. 2025. "The Removal of Arsenic from Contaminated Water: A Critical Review of Adsorbent Materials from Agricultural Wastes to Advanced Metal–Organic Frameworks" Minerals 15, no. 10: 1037. https://doi.org/10.3390/min15101037
APA StyleElmakki, M. A. E., Ghosh, S., Motente, M., Ajiboye, T. O., Venter, J., & Adetunji, A. I. (2025). The Removal of Arsenic from Contaminated Water: A Critical Review of Adsorbent Materials from Agricultural Wastes to Advanced Metal–Organic Frameworks. Minerals, 15(10), 1037. https://doi.org/10.3390/min15101037











