Optimizing Mineral Resources with Automated Mineralogy Techniques: The Case of Colquiri in the Central Andean Tin Belt
Abstract
1. Introduction
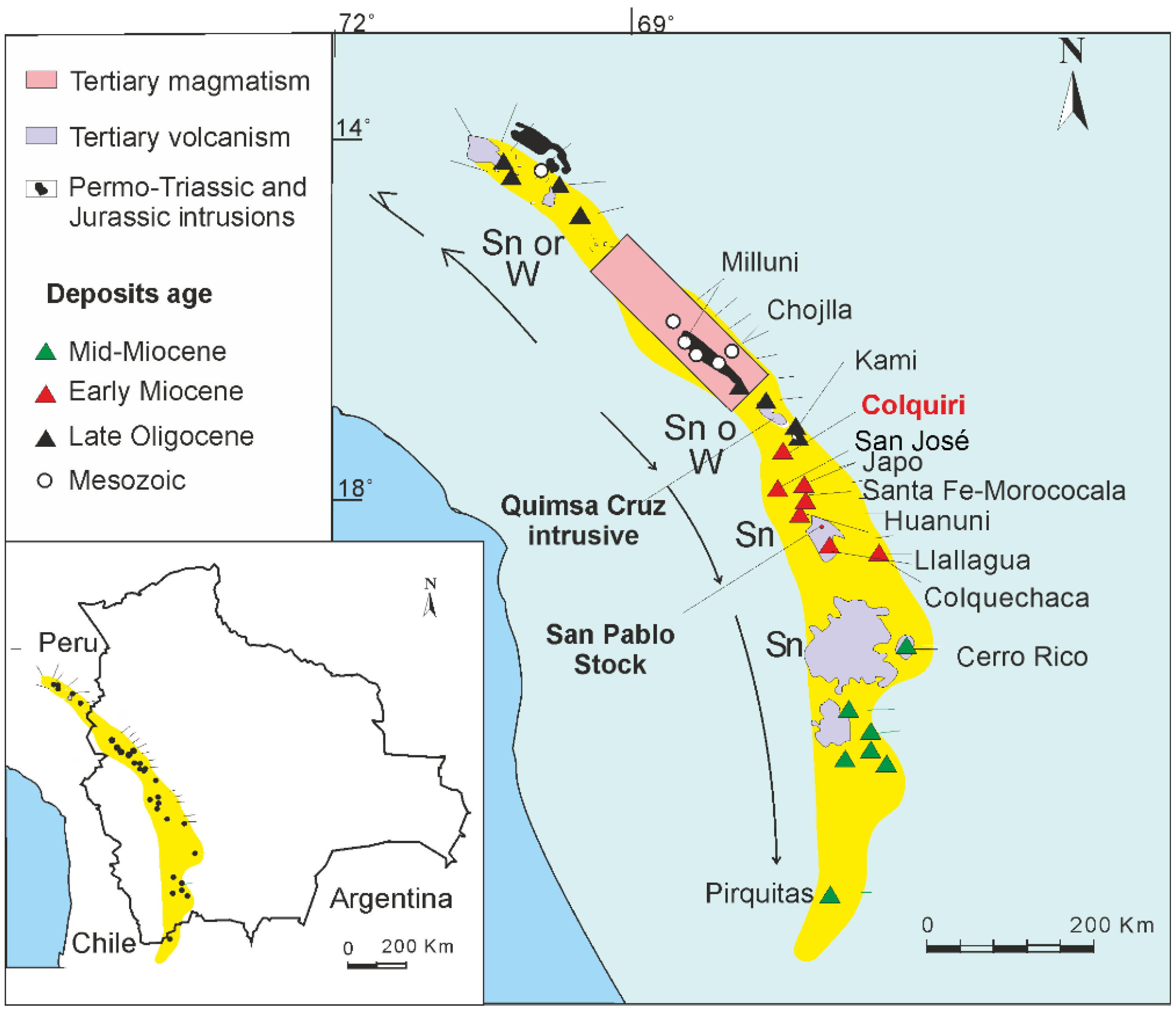
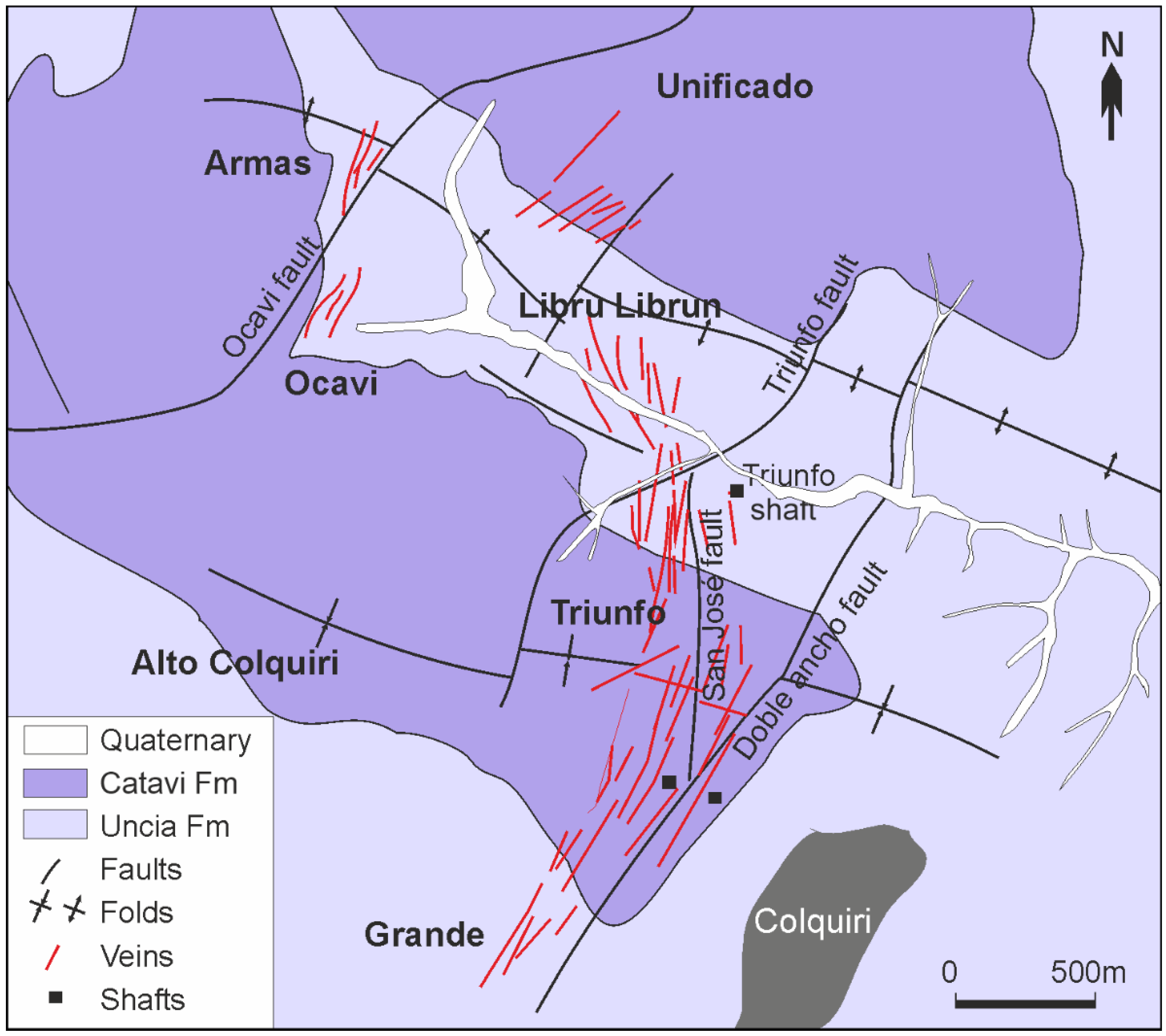
2. Geological Setting
3. Materials and Methods
3.1. Materials
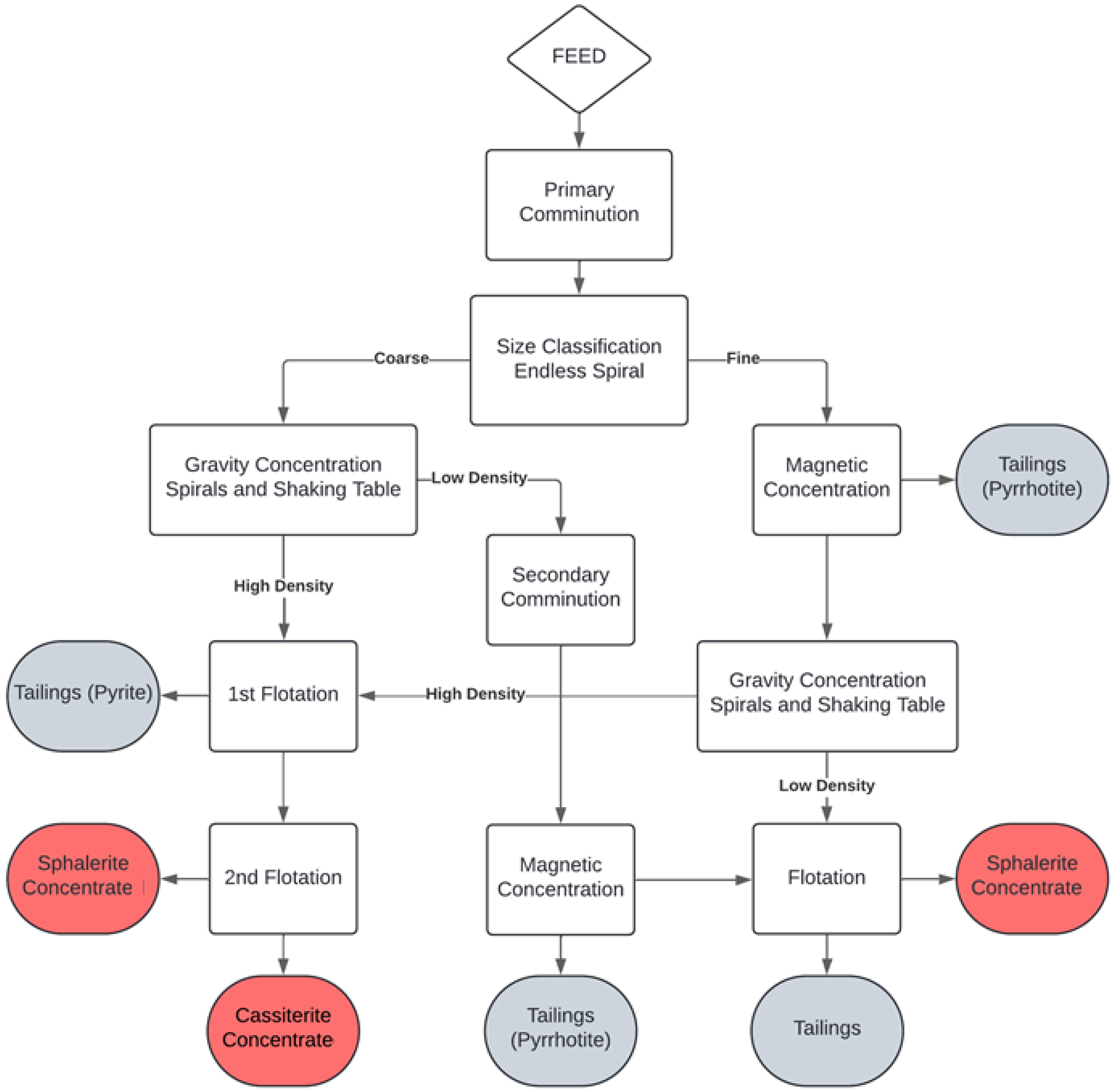
3.2. Analytical Methods
4. Results
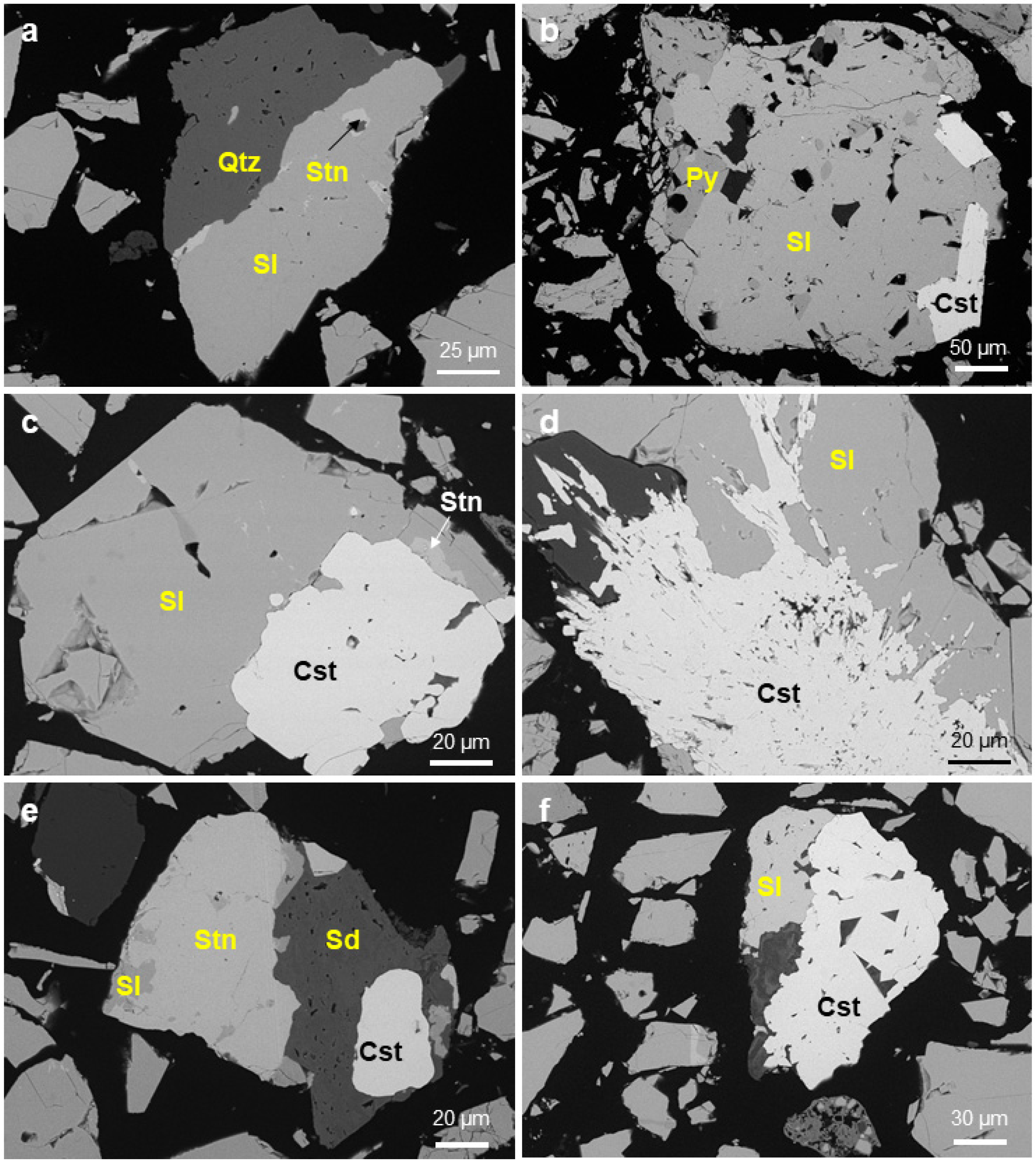
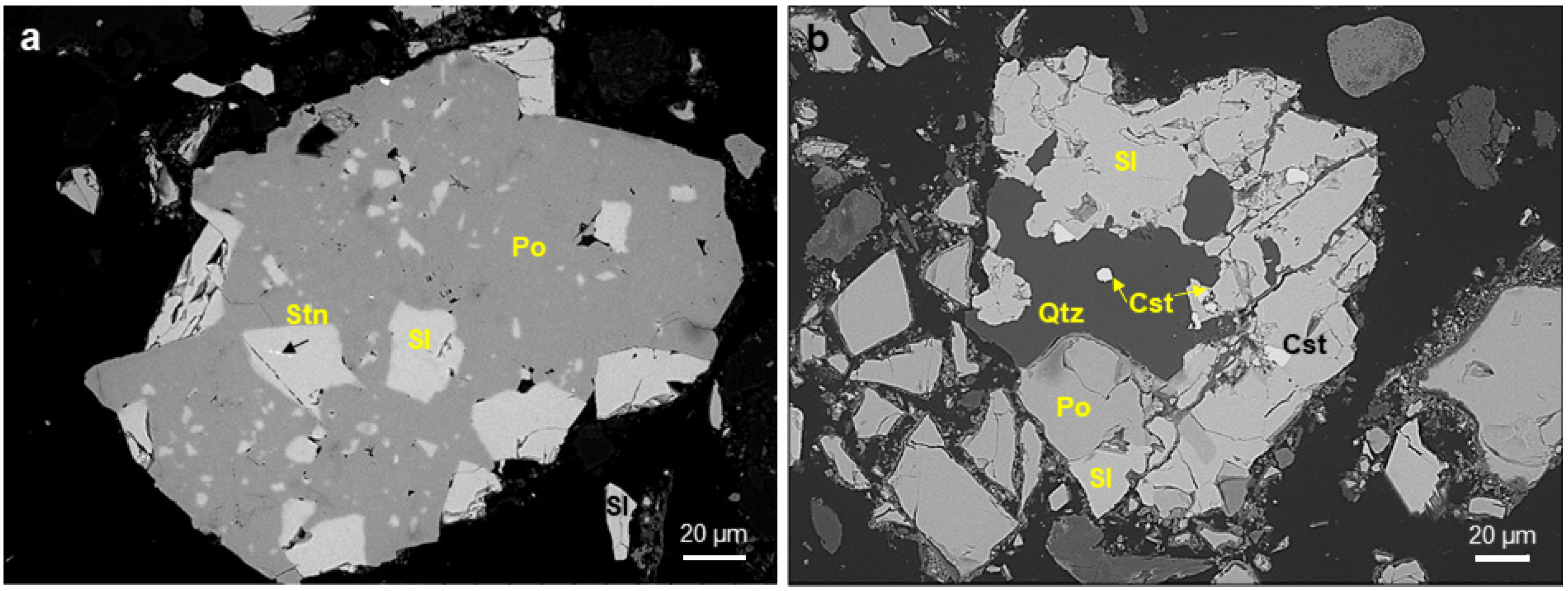
4.1. Mineralogy of Ores
4.2. Mineralogy of Concentrates and Tailings
4.3. Chemical Composition
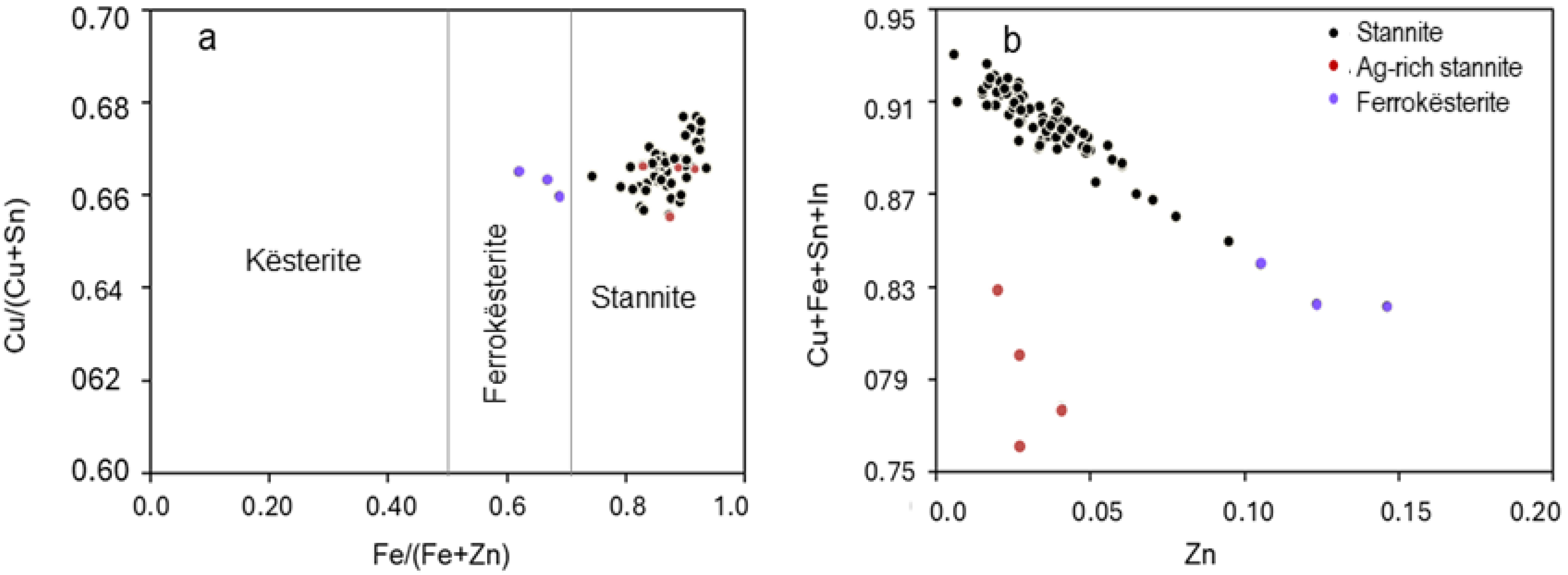
4.4. Mineral Chemistry
4.5. Mineral Liberation
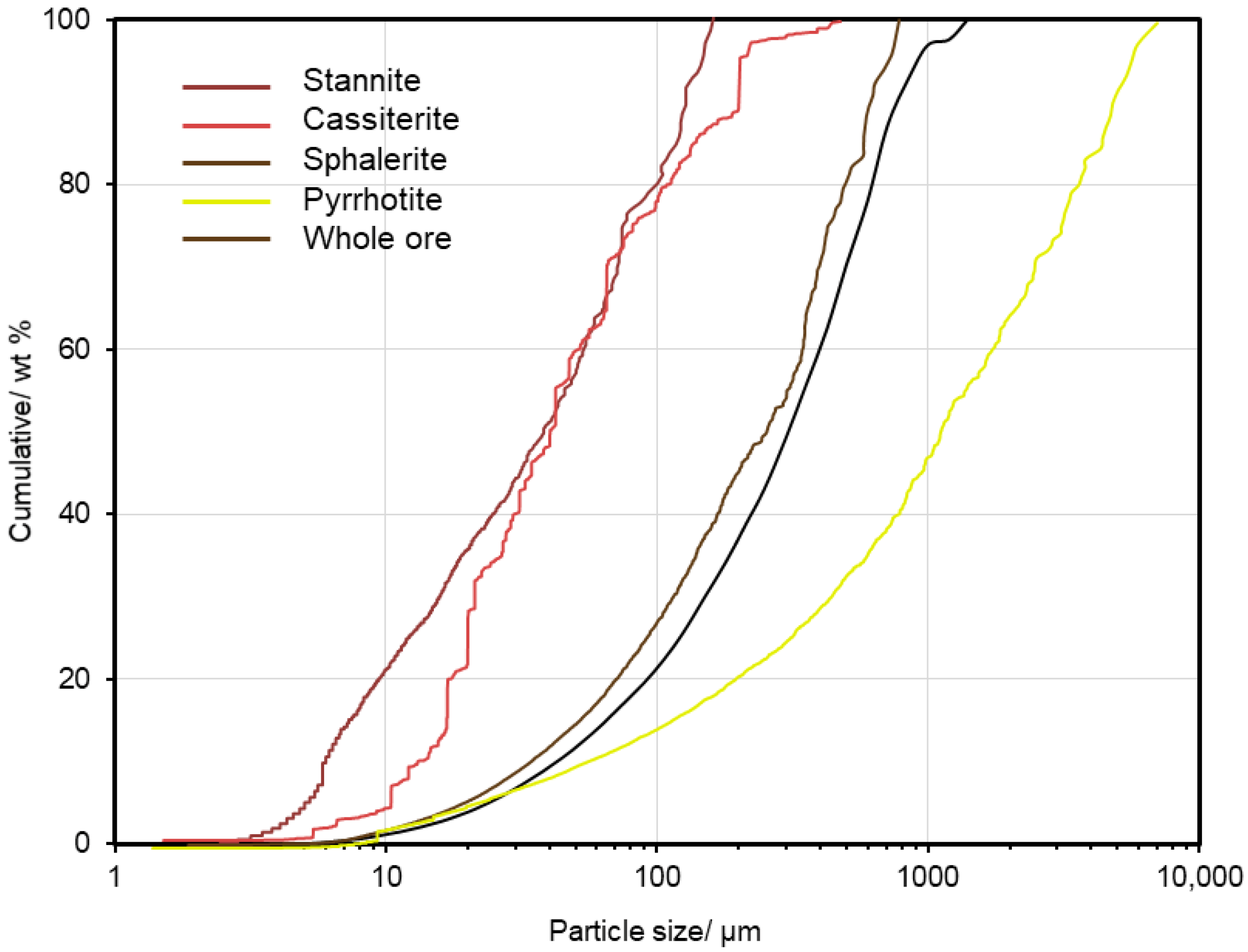
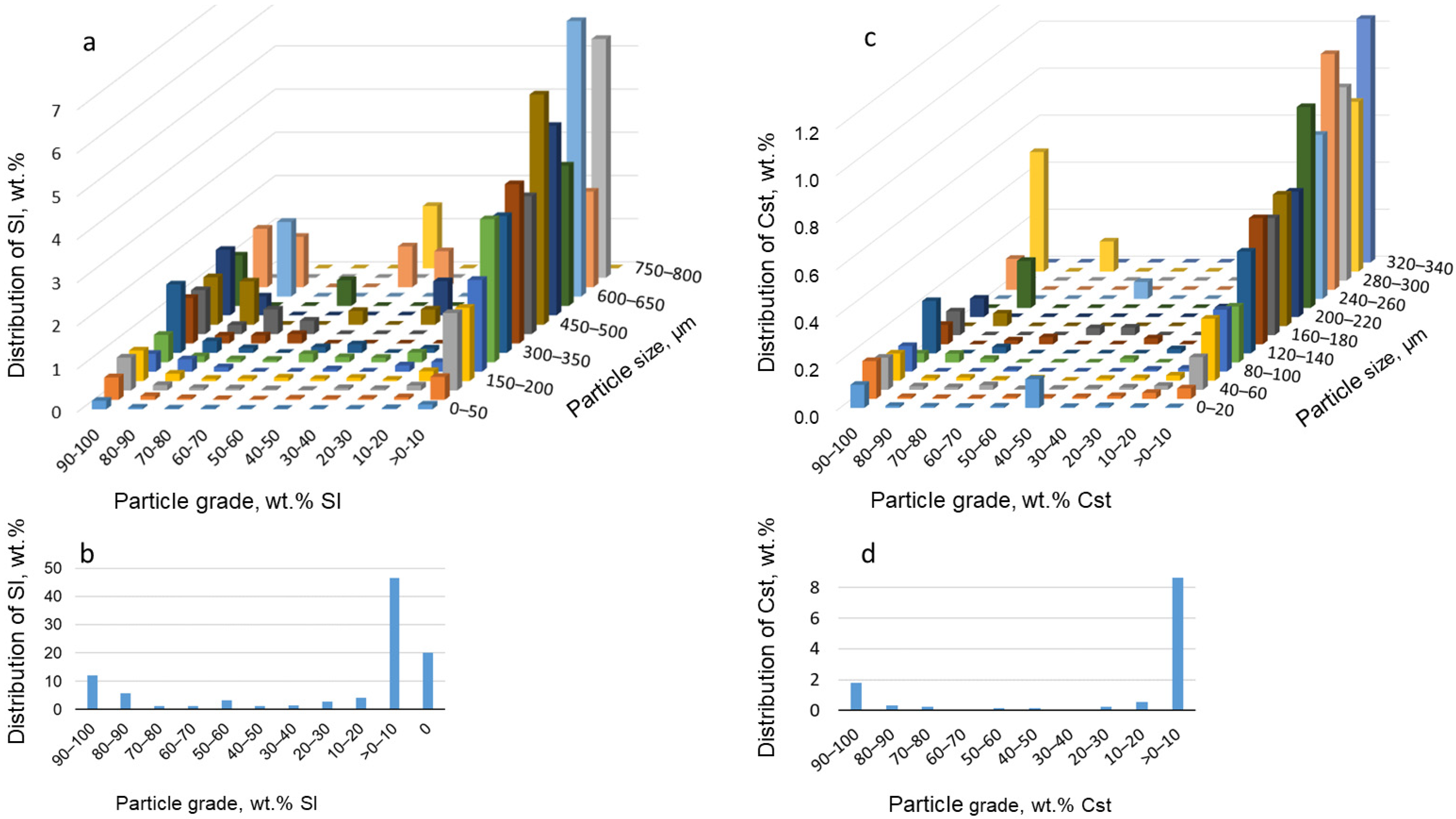
4.5.1. Particle and Grain Size Distribution
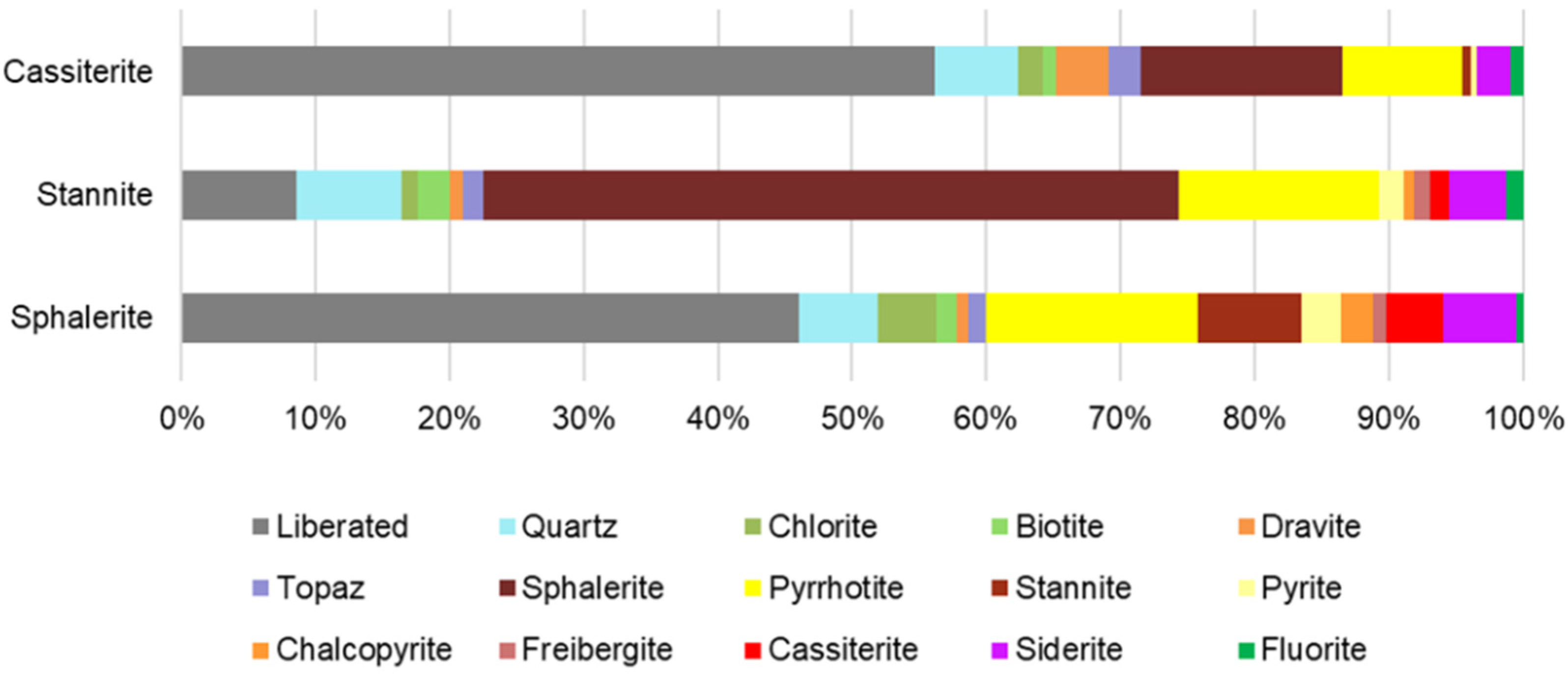
4.5.2. Mineral Association
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henckens, T. Scarce mineral resources: Extraction, consumption and limits of sustainability. Resour. Conserv. Recycl. 2021, 169, 105511. [Google Scholar] [CrossRef]
- Lehmann, B. Formation of tin ore deposits: A reassessment. Lithos 2021, 402, 105756. [Google Scholar] [CrossRef]
- Aydın, E.B.; Sezgintürk, M.K. Indium tin oxide (ITO): A promising material in biosensing technology. TrAC Trends Anal. Chem. 2017, 97, 309–315. [Google Scholar] [CrossRef]
- Manca, P.P.; Massacci, G.; Pintus, D.; Sogos, G. The flotation of sphalerite mine tailings as a remediation method. Miner. Eng. 2021, 165, 106862. [Google Scholar] [CrossRef]
- Mejías, O.; Parbhakar-Fox, A.; Jackson, L.; Valenta, R.; Townley, B. Indium in ore deposits and mine waste environments: Geochemistry, mineralogy, and opportunities for recovery. J. Geochem. Explor. 2023, 255, 107312. [Google Scholar] [CrossRef]
- Martin, M.; Janneck, E.; Kermer, R.; Patzig, A.; Reichel, S. Recovery of indium from sphalerite ore and flotation tailings by bioleaching and subsequent precipitation processes. Miner. Eng. 2015, 75, 94–99. [Google Scholar] [CrossRef]
- Kinnunen, P.; Karhu, M.; Yli-Rantala, E.; Kivikytö-Reponen, P.; Mäkinen, J. A review of circular economy strategies for mine tailings. Clean. Eng. Technol. 2022, 8, 100499. [Google Scholar] [CrossRef]
- Wills, B.A.; Finch, J. Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 7th ed.; Butterworth-Heinemann: Oxford, UK, 2015; pp. 1–27. [Google Scholar]
- Lotter, N.O. Modern Process Mineralogy: An integrated multi-disciplined approach to flowsheeting. Miner. Eng. 2011, 24, 1229–1237. [Google Scholar] [CrossRef]
- Becker, M. The contribution of applied mineralogy to sustainability in the mine life cycle. Miner. Eng. 2023, 199, 108121. [Google Scholar] [CrossRef]
- García, S.; Camus, L.; Gonzalez-Diaz, E.; Collao, R.; Townley, B.; Parviainen, A.; Caraballo, M.A. The importance of geochemical and mineralogical characterization of fresh Cu-Porphyry mine tailings in mineral processing plants to optimize their revalorization potential. J. Geochem. Explor. 2024, 259, 107439. [Google Scholar] [CrossRef]
- Alfonso, P.; Ruiz, M.; Zambrana, R.N.; Sendrós, M.; Garcia-Valles, M.; Anticoi, H.; Sidki-Rius, N.; Salas, A. Process mineralogy of the tailings from Llallagua: Towards a sustainable activity. Minerals 2022, 12, 214. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Williams, T. The mineralogy and mineral chemistry of indium in sulphide deposits and implications for mineral processing. Hydrometallurgy 2011, 108, 226–228. [Google Scholar] [CrossRef]
- Schulz, B.; Sandmann, D.; Gilbricht, S. SEM-based automated mineralogy and its application in geo-and material sciences. Minerals 2020, 10, 1004. [Google Scholar] [CrossRef]
- Blannin, R.; Frenzel, M.; Tuşa, L.; Birtel, S.; Ivăşcanu, P.; Baker, T.; Gutzmer, J. Uncertainties in quantitative mineralogical studies using scanning electron microscope-based image analysis. Miner. Eng. 2021, 167, 106836. [Google Scholar] [CrossRef]
- Lastra, R.; Paktunc, D. An estimation of the variability in automated quantitative mineralogy measurements through inter-laboratory testing. Miner. Eng. 2016, 95, 138–145. [Google Scholar] [CrossRef]
- Ueda, T.; Oki, T.; Koyanaka, S. Experimental analysis of mineral liberation and stereological bias based on X-ray computed tomography and artificial binary particles. Adv. Powder Technol. 2018, 29, 462–470. [Google Scholar] [CrossRef]
- Spencer, S.; Sutherland, D. Stereological correction of mineral liberation grade distributions estimated by single sectioning of particles. Image Anal. Stereol. 2000, 19, 175–182. [Google Scholar] [CrossRef]
- Lotter, N.O.; Whiteman, E.; Hoffman, M.C.; Nkuna, V.; Amos, S.R.; Groenewald, A. Flowsheet development for and commissioning of the Kamoa-Kakula project. Miner. Eng. 2024, 210, 108671. [Google Scholar] [CrossRef]
- Holwell, D.A.; Adeyemi, Z.; Ward, L.A.; Smith, D.J.; Graham, S.D.; McDonald, I.; Smith, J.W. Low temperature alteration of magmatic Ni-Cu-PGE sulfides as a source for hydrothermal Ni and PGE ores: A quantitative approach using automated mineralogy. Ore Geol. Rev. 2017, 91, 718–740. [Google Scholar] [CrossRef]
- Shan, P.; Cao, M.; Evans, N.J.; Gao, H.; Mao, Y.; Gao, Y.; Salazar, L.; Zhao, Y.; Qin, K. Automated quantitative mineralogy analysis reveals characteristics of Co occurrence in the Jinchang porphyry deposit, NE China. Ore Geol. Rev. 2023, 158, 105524. [Google Scholar] [CrossRef]
- Kern, M.; Kästner, J.; Tolosana-Delgado, R.; Jeske, T.; Gutzmer, J. The inherent link between ore formation and geometallurgy as documented by complex tin mineralization at the Hämmerlein deposit (Erzgebirge, Germany). Miner. Depos. 2019, 54, 683–698. [Google Scholar] [CrossRef]
- USGS (United States Geological Survey), 2024. Mineral Commodity Summaries 2024. Available online: https://pubs.usgs.gov/periodicals/mcs2024/mcs2024-indium.pdf (accessed on 20 April 2025).
- Lehmann, B.; Ishihara, S.; Michel, H.; Miller, J.; Rapela, C.W.; Sanchez, A.; Tistl, M.; Winkelmann, L. The Bolivian tin province and regional tin distribution in the Central Andes; a reassessment. Econ. Geol. 1990, 85, 1044–1058. [Google Scholar] [CrossRef]
- Jiménez-Franco, A.; Alfonso, P.; Canet, C.; Trujillo, J.E. Mineral chemistry of In-bearing minerals in the Santa Fe mining district, Bolivia. Andean Geol. 2018, 45, 410–432. [Google Scholar] [CrossRef]
- Ishihara, S.; Murakami, H.; Marquez-Zavalia, M.F. Inferred indium resources of the Bolivian tin-polymetallic deposits. Resour. Geol. 2011, 61, 174–191. [Google Scholar] [CrossRef]
- Cacho, A.; Melgarejo, J.C.; Camprubí, A.; Torró, L.; Castillo-Oliver, M.; Torres, B.; Artiaga, D.; Tauler, E.; Martinez, A.; Campeny, M.; et al. Mineralogy and distribution of critical elements in the Sn–W–Pb–Ag–Zn Huanuni deposit, Bolivia. Minerals 2019, 9, 753. [Google Scholar] [CrossRef]
- Torres, B.; Melgarejo, J.C.; Torró, L.; Camprubí, A.; Castillo-Oliver, M.; Artiaga, D.; Campeny, M.; Tauler, E.; Jiménez-Franco, A.; Alfonso, P.; et al. The Poopó polymetallic epithermal deposit, Bolivia: Mineralogy, genetic constraints, and distribution of critical elements. Minerals 2019, 9, 472. [Google Scholar] [CrossRef]
- Torró, L.; Cazorla, M.; Melgarejo, J.C.; Camprubí, A.; Tarrés, M.; Gemmrich, L.; Campeny, M.; Artiaga, D.; Torres, B.; Martínez, A.; et al. Indium mineralization in the volcanic dome-hosted Ánimas–Chocaya–Siete Suyos polymetallic deposit, Potosí, Bolivia. Minerals 2019, 9, 604. [Google Scholar] [CrossRef]
- Ahlfeld, F. Supergene cassiterite in tin veins; discussion. Econ. Geol. 1930, 25, 546–548. [Google Scholar] [CrossRef]
- Campbell, D.F. Geology of the Colquiri tin mine, Bolivia. Econ. Geol. 1947, 42, 1–21. [Google Scholar] [CrossRef]
- Kelly, W.C.; Turneaure, F.S. Mineralogy, paragenesis and geothermometry of the tin and tungsten deposits of the eastern Andes, Bolivia. Econ. Geol. 1970, 65, 609–680. [Google Scholar] [CrossRef]
- Sugaki, A.; Ueno, H.; Kitakaze, A.; Hayashi, K.; Shimada, N.; Kusachi, I. Geological study on the ore deposits in the La Paz district, Bolivia. ci. Rept. Tohoku Univ. Ser. III 1985, 16, 131–198. [Google Scholar]
- Hanus, D. The Colquiri tin deposit: A contribution to its genesis. In Ore Genesis: The State of the Art; Springer: Berlin/Heidelberg, Germany, 1982; pp. 308–318. [Google Scholar]
- Clark, A.H.; Palma, V.V.; Archibald, D.A.; Farrar, E.; Arenas, F.M.J.; Robertson, R.C. Occurrence and age of tin mineralization in the Cordillera Oriental, southern Peru. Econ. Geol. 1983, 78, 514–520. [Google Scholar] [CrossRef]
- Mlynarczyk, M.S.; Williams-Jones, A.E. The role of collisional tectonics in the metallogeny of the Central Andean tin belt. Earth Planet. Sci. Lett. 2005, 240, 656–667. [Google Scholar] [CrossRef]
- Arce-Burgoa, O.R.; Goldfarb, R.J. Metallogeny of Bolivia. SEG Newsl. 2009, 79, 1–15. [Google Scholar] [CrossRef]
- Avila-Salinas, W.A. Tin-bearing granites from the Cordillera Real, Bolivia; a petrological and geochemical review. In Plutonism from Antarctica to Alaska; Special Paper 241; GeoScienceWorld: McLean, VA, USA, 1990. [Google Scholar]
- Schneider, H.J.; Lehmann, B. Contribution to a new genetical concept on the Bolivian tin province. In Time-and Strata-Bound Ore Deposits; Springer: Berlin/Heidelberg, Germany, 1977; pp. 153–168. [Google Scholar]
- Jiao, Y.; Qiu, K.H.; Zhang, P.C.; Li, J.F.; Zhang, W.T.; Chen, X.F. Process mineralogy of Dalucao rare earth ore and design of beneficiation process based on AMICS. Rare Met. 2020, 39, 959–966. [Google Scholar] [CrossRef]
- Sugaki, A.; Kitakaze, A. Tin-bearing minerals from Bolivian polymetallic deposits and their mineralization stages. Min. Geol. 1988, 38, 419–435. [Google Scholar]
- Schwarz-Schampera, U.; Herzig, P.M. Indium: Geology, Mineralogy and Economics; Springer: Berlin/Heidelberg, Germany, 2002; pp. 147–151. [Google Scholar]
- Murakami, H.; Ishihara, S. Trace elements of Indium-bearing sphalerite from tin-polymetallic deposits in Bolivia, China and Japan: A femto-second LA-ICPMS study. Ore Geol. Rev. 2013, 53, 223–243. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Shimizu, M.; Danyushevsky, L.; Saini-Eidukat, B.; Melcher, F. Trace and minor elements in sphalerite: A LA-ICPMS study. Geochim. Cosmochim. Acta 2009, 73, 4761–4791. [Google Scholar] [CrossRef]
- Frenzel, M.; Hirsch, T.; Gutzmer, J. Gallium, germanium, indium, and other trace and minor elements in sphalerite as a function of deposit type—A meta-analysis. Ore Geol. Rev. 2016, 76, 52–78. [Google Scholar] [CrossRef]
- Bonazzi, P.; Bindi, L.; Bernardini, G.P.; Menchetti, S. A model for the mechanism of incorporation of Cu, Fe and Zn in the stannite–kësterite series, Cu2FeSnS4–Cu2ZnSnS4. Can. Mineral. 2003, 41, 639–647. [Google Scholar] [CrossRef]
- Watanabe, M.; Hoshino, K.I.; Myint, K.K.; Miyazaki, K.; Nishido, H. Stannite from the Otoge kaolin-pyrophyllite deposits, Yamagata Prefecture, NE Japan and its genetical significance. Shigen-Chishitsu 1994, 44, 439–444. [Google Scholar]
- McQueen, K.G.; Box, S. Mineralization at the Wallah Wallah Silver Mine, Rye Park, New South Wales and its metallogenic significance. Mineral. Petrol. 1988, 39, 289–305. [Google Scholar] [CrossRef]
- Murao, S.; Deb, M.; Furuno, M. Mineralogical evolution of indium in high grade tin-polymetallic hydrothermal veins—A comparative study from Tosham, Haryana state, India and Goka, Naegi district, Japan. Ore Geol. Rev. 2008, 33, 490–504. [Google Scholar] [CrossRef]
- Benites, D.; Torró, L.; Vallance, J.; Laurent, O.; Quispe, P.; Rosas, S.; Uzieda, M.F.; Holm-Denoma, S.; Pianowki, L.S.; Camprubí, A.; et al. Geology, mineralogy, and cassiterite geochronology of the Ayawilca Zn-Pb-Ag-In-Sn-Cu deposit, Pasco, Peru. Miner. Depos. 2022, 57, 481–507. [Google Scholar] [CrossRef]
- Petruk, W. Tin sulphides from the deposit of Brunswick Tin Mines Limited. Can. Mineral. 1973, 12, 46–54. [Google Scholar]
- Hayashi, K.; Kitakaze, A.; Sugaki, A. A re-examination of herzenbergite–teallite solid solution at temperatures between 300 and 700 C. Mineral. Mag. 2001, 65, 645–651. [Google Scholar] [CrossRef]
- Pavlova, G.G.; Palessky, S.V.; Borisenko, A.S.; Vladimirov, A.G.; Seifert, T.; Phan, L.A. Indium in cassiterite and ores of tin deposits. Ore Geol. Rev. 2015, 66, 99–113. [Google Scholar] [CrossRef]
- Lerouge, C.; Gloaguen, E.; Wille, G.; Bailly, L. Distribution of In and other rare metals in cassiterite and associated minerals in Sn±W ore deposits of the western Variscan Belt. Eur. J. Mineral. 2017, 29, 739–753. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–51. [Google Scholar]
- Zhao, T.; Chen, C.; He, X.; Meng, L.; Xu, J.; Liu, W. A synthesis of the geology, spatial–temporal distribution and enrichment mechanism of granite-related indium deposits in China. Ore Geol. Rev. 2022, 146, 104932. [Google Scholar] [CrossRef]
- Torró, L.; Melgarejo, J.C.; Gemmrich, L.; Mollinedo, D.; Cazorla, M.; Martínez, A.; Pujol-Solà, N.; Farré-de-Pablo, J.; Camprubí, A.; Artiaga, D.; et al. Spatial and temporal controls on the distribution of indium in xenothermal vein-deposits: The Huari Huari District, Potosí, Bolivia. Minerals 2019, 9, 304. [Google Scholar] [CrossRef]
- Lastra, R.; Petruk, W. Mineralogical characterization of sieved and un-sieved samples. JMMCE 2014, 2, 40–48. [Google Scholar] [CrossRef]
- Rahman, R.M.; Ata, S.; Jameson, G.J. The effect of flotation variables on the recovery of different particle size fractions in the froth and the pulp. Int. J. Mineral. Process. 2012, 106, 70–77. [Google Scholar] [CrossRef]
- Angadi, S.I.; Sreenivas, T.; Jeon, H.S.; Baek, S.H.; Mishra, B.K. A review of cassiterite beneficiation fundamentals and plant practices. Minerals Eng. 2015, 70, 178–200. [Google Scholar] [CrossRef]
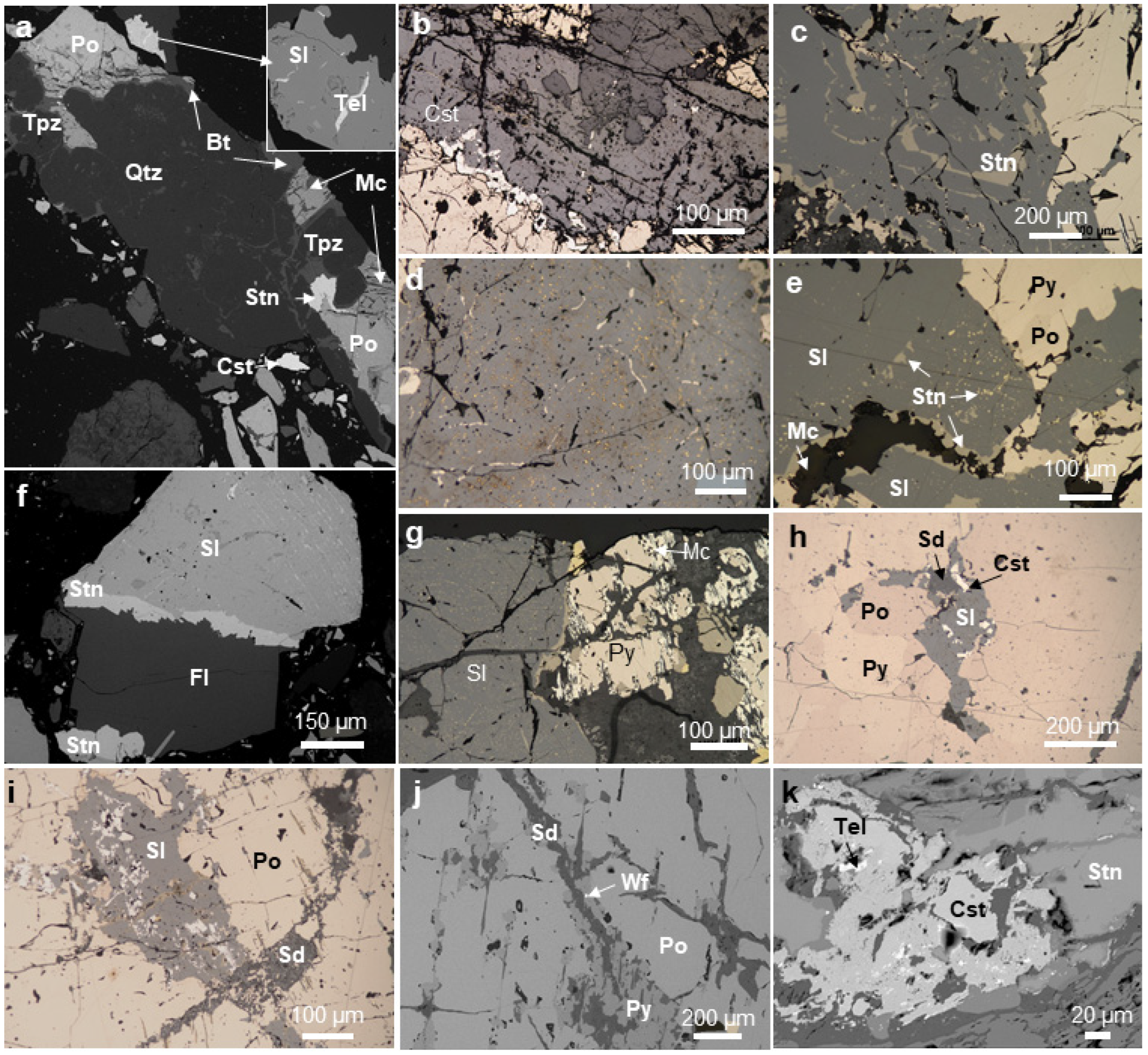
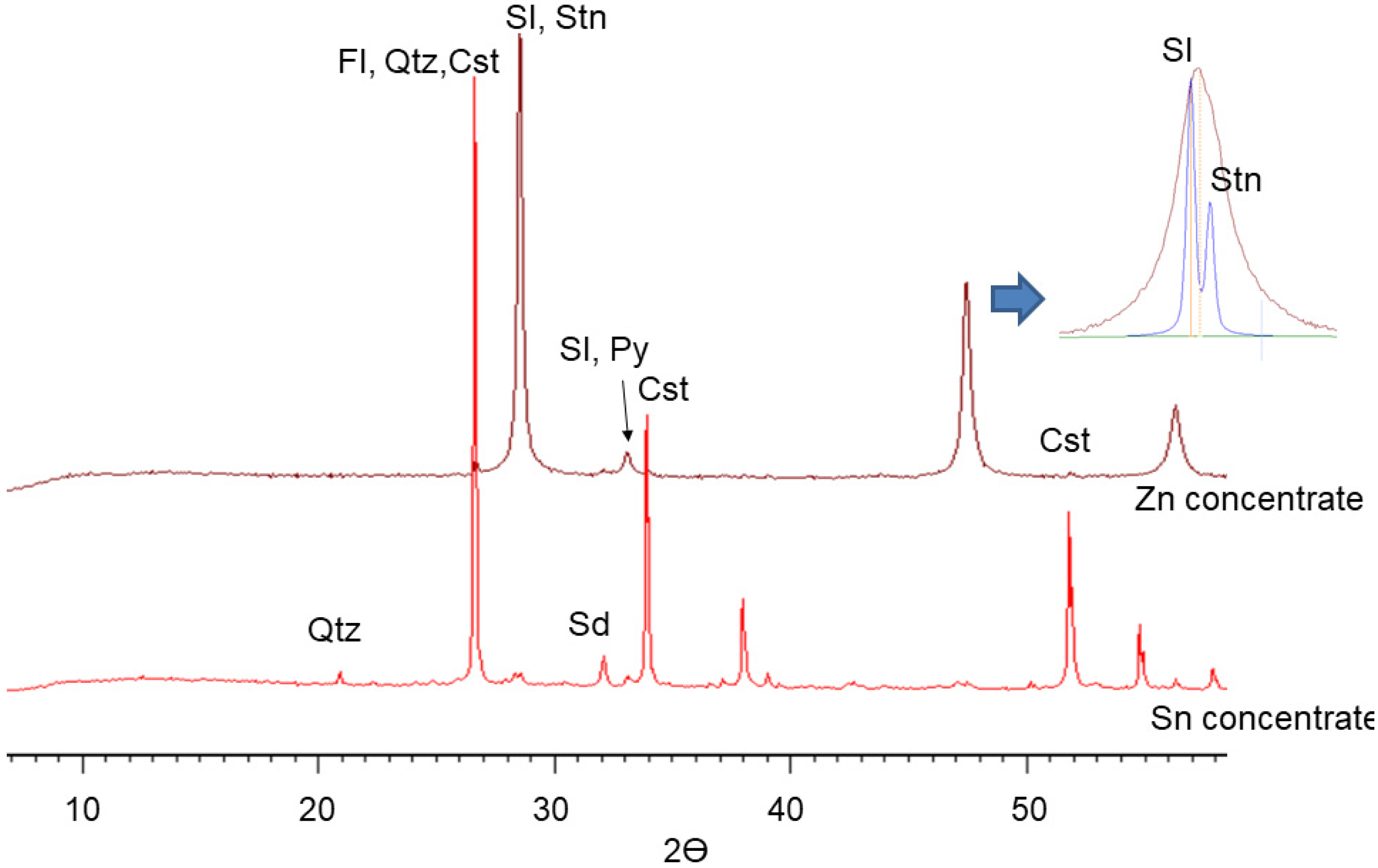

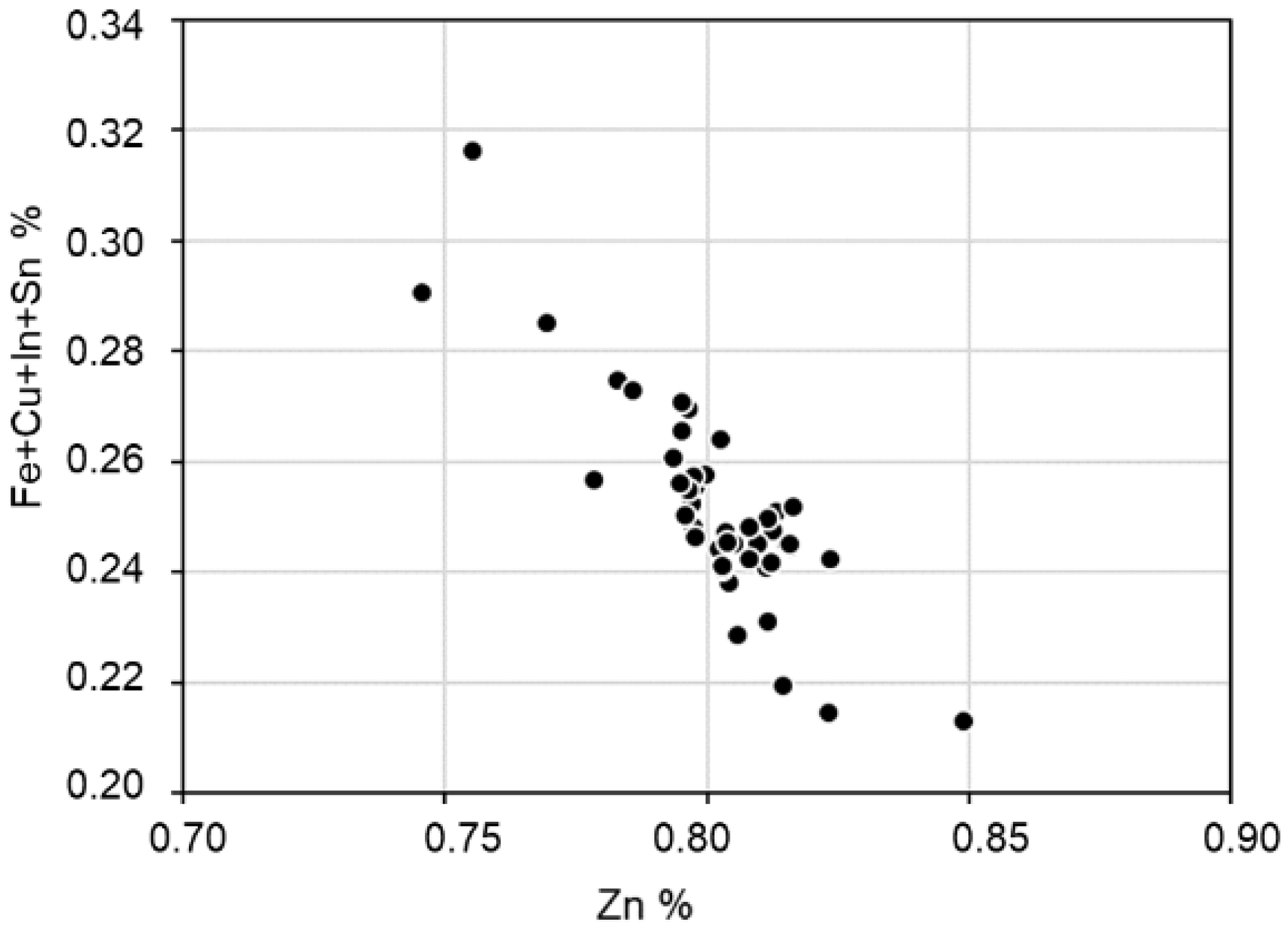
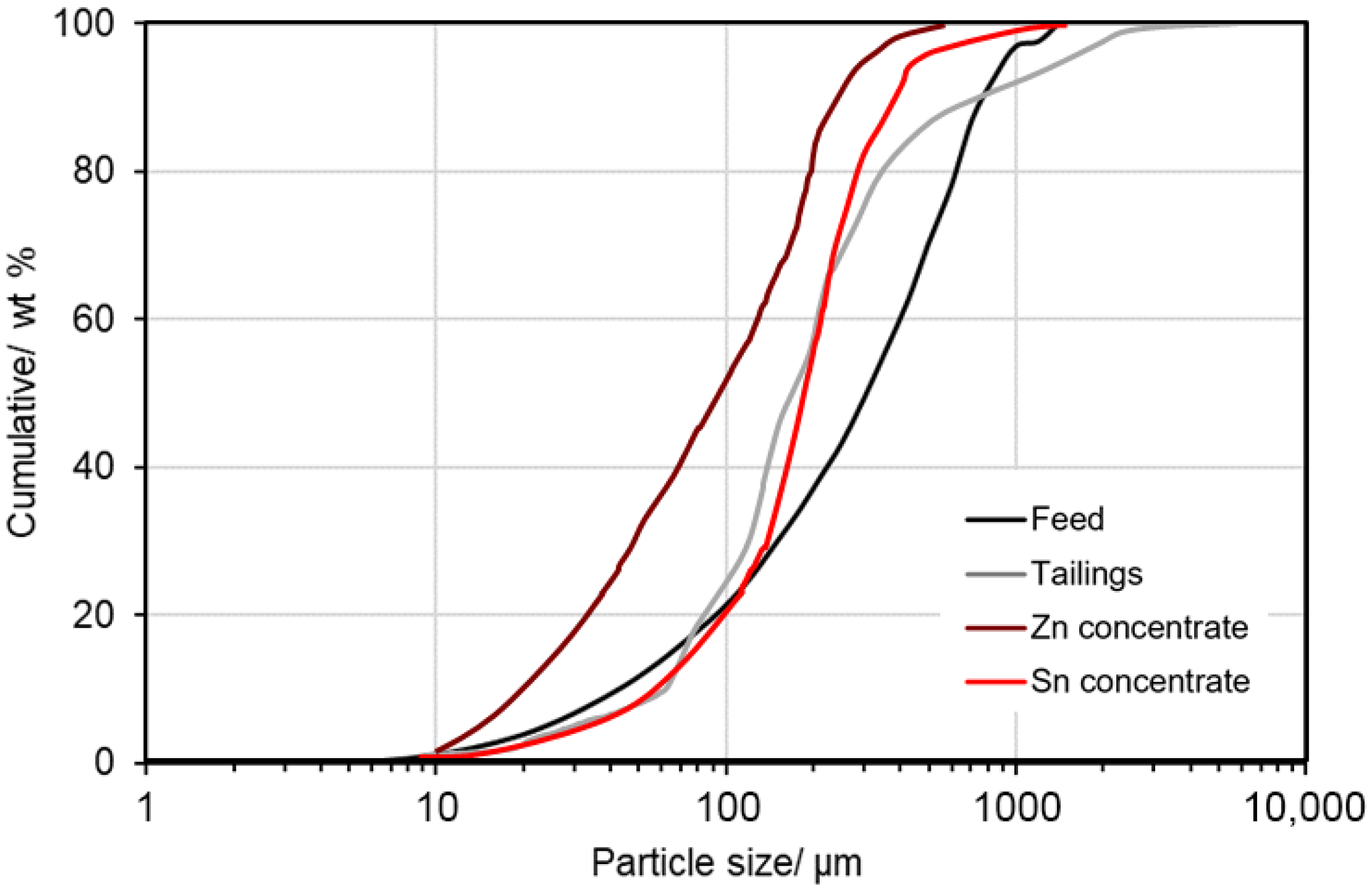
| Mineral, wt.% | Formula | AMICS | MLA | XRD | OM | EPMA |
|---|---|---|---|---|---|---|
| Quartz | SiO2 | 12.62 | 10.63 | x | x | |
| Dravite–schorl | Na(Mg,Fe)3Al6(BO3)3Si6O18(OH)4 | 2.45 | 2.63 | x | x | |
| Topaz | Al2SiO4F2 | 1.54 | 1.19 | x | ||
| Biotite | K(Mg,Fe)3(AlSi3O10)(OH,F)2 | 0.25 | 1.91 | x | x | |
| Albite | NaAlSi3O8 | 0.13 | 0.68 | |||
| K-feldspar | KAlSi3O8 | 0.06 | 0.8 | |||
| Chlorite | (Mg,Fe)3(Si,Al)4O10(OH)2·(Mg,Fe)3(OH)6 | 1.14 | 2.56 | x | ||
| Kaolinite–dickite | Al2Si2O5(OH)4 | 0.26 | 0.26 | x | ||
| Pyrophyllite | Al2Si4O10(OH)2 | 0.97 | 0.97 | |||
| Siderite | FeCO3 | 4.26 | 5.47 | x | x | |
| Apatite | Ca5(PO4)3(F,Cl,OH) | 0.02 | 0.02 | |||
| Fluorite | CaF2 | 1.1 | 1.12 | x | x | |
| Pyrite/marcasite | FeS2 | 7.86 | 6.33 | x | x | |
| Pyrrhotite | Fe(1−x)S | 28.22 | 31.05 | x | x | |
| Arsenopyrite | FeAsS | 1.35 | 0.76 | x | ||
| Sphalerite | (Zn,Fe) S | 24.69 | 24.46 | x | ||
| Stannite | Cu2FeSnS4 | 0.83 | 0.95 | x | x | |
| Chalcopyrite | FeCuS2 | 0.12 | 0.21 | x | ||
| Galena | PbS | 0.04 | 0.05 | x | x | |
| Cassiterite | SnO2 | 2.87 | 2.91 | x | x | x |
| Rutile | TiO2 | 0.05 | 0.04 | |||
| Magnetite | Fe3O4 | 0.37 | x | |||
| Hematite | Fe2O3 | 1.47 | x | |||
| Fe-oxide/hydroxide | 1.18 | x | ||||
| Goethite | FeO(OH) | 0.51 | x | |||
| Wolframite | (Fe,Mn)WO4 | 0.01 | x | x | ||
| Freibergite | (Ag,Cu,Fe)12(Sb,As)4S13 | 0.18 | x | |||
| Pyrargyrite | Ag3SbS3 | x | ||||
| Tetrahedrite | (Cu,Fe)12Sb4S13 | 0.03 | ||||
| Teallite | PbSnS2 | x | ||||
| Bismuthinite | Bi2S3 | x | x | |||
| Other | 3.83 | 0.26 |
| Element | CQ26 | CQ16 | CQ19 | CQ17 | CQ25 |
|---|---|---|---|---|---|
| Feed | Feed | Zn Concentrate | Sn Concentrate | Tailings | |
| Wt.% | |||||
| SiO2 | 13.50 | 18.61 | 1.50 | 22.50 | 23.50 |
| Al2O3 | 3.82 | 5.34 | 0.49 | 6.17 | 5.63 |
| TiO2 | 0.13 | 0.15 | 0.02 | 0.22 | 0.22 |
| MgO | 0.61 | 0.22 | 0.13 | 0.92 | 0.58 |
| CaO | 1.19 | 0.51 | 0.24 | 2.23 | 1.65 |
| Na2O | 0.01 | 0.02 | 0.00 | 0.01 | 0.01 |
| K2O | 0.63 | 0.18 | 0.02 | 0.22 | 0.52 |
| SnO2 | 2.96 | 3.76 | 1.35 | 52.56 | 1.1 |
| Fe | 28.60 | 28.50 | 14.35 | 7.63 | 30.5 |
| Zn | 13.25 | 12.25 | 43.90 | 2.08 | 5.03 |
| S | 25.60 | 32.10 | 4.21 | 22.10 | |
| ppm | |||||
| Mn | 641 | 1140 | 621 | 2410 | 852 |
| Cd | 592 | 503 | >1000 | 88 | 151 |
| Cu | 3760 | 3740 | 7790 | 688 | 1800 |
| In | 172 | 155 | 481 | 18 | 47 |
| Ga | 41.2 | 40.3 | 125.5 | 9.28 | 13.3 |
| Ge | 0.54 | 0.48 | 0.28 | 0.09 | 0.5 |
| Pb | 569 | 627 | 1375 | 289 | 409 |
| As | 1455 | 3800 | 972 | 963 | 2160 |
| Sb | 25.9 | 60.4 | 27.1 | 26.3 | 30.1 |
| Ag | 58.9 | 82.1 | >100 | 12.3 | 28.7 |
| Nb | 0.26 | 0.22 | 0.10 | 0.21 | 0.23 |
| Ta | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| W | 11.3 | 400 | 18.2 | 160 | 26.3 |
| Mineral | DL | Sphalerite, n = 51 | Stannite, n = 79 | Ag-Rich Stannite, n = 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Element, wt.% | Av | MIN | MAX | Av | MIN | MAX | Av | MIN | MAX | |
| S | 0.01 | 32.68 | 31.35 | 33.74 | 28.35 | 23.90 | 29.78 | 26.54 | 26.07 | 26.96 |
| Zn | 0.03 | 52.45 | 48.76 | 55.52 | 2.53 | 0.37 | 9.55 | 1.86 | 1.27 | 2.64 |
| Fe | 0.02 | 13.62 | 11.36 | 15.76 | 12.90 | 10.79 | 14.18 | 11.01 | 10.23 | 11.74 |
| Cu | 0.02 | 0.40 | 0.03 | 2.15 | 28.19 | 24.25 | 29.41 | 25.08 | 24.09 | 26.59 |
| Sn | 0.04 | 0.10 | 0.00 | 0.61 | 26.33 | 22.99 | 27.66 | 23.74 | 23.03 | 24.92 |
| Ag | 0.06 | 0.02 | 0.00 | 0.10 | 0.34 | 0.01 | 1.31 | 8.68 | 6.95 | 10.38 |
| Cd | 0.02 | 0.22 | 0.00 | 0.49 | 0.00 | 0.00 | 0.07 | 0.00 | 0.00 | 0.00 |
| In | 0.02 | 0.04 | 0.00 | 0.22 | 0.06 | 0.00 | 0.26 | 0.00 | 0.00 | 0.00 |
| As | 0.02 | 0.01 | 0.00 | 0.09 | 0.01 | 0.00 | 0.08 | 0.04 | 0.01 | 0.08 |
| Mineral | Sphalerite | Stannite | Cassiterite |
|---|---|---|---|
| Liberated | 45.38 | 8.45 | 54.68 |
| Quartz | 5.71 | 7.77 | 6.04 |
| Chlorite | 4.37 | 1.24 | 1.80 |
| Biotite | 1.41 | 2.30 | 0.88 |
| Dravite | 0.89 | 1.02 | 3.87 |
| Topaz | 1.27 | 1.51 | 2.36 |
| Sphalerite | 51.43 | 14.58 | |
| Pyrrhotite | 15.57 | 14.80 | 8.72 |
| Stannite | 7.66 | 0.61 | |
| Pyrite | 2.84 | 1.84 | 0.40 |
| Chalcopyrite | 2.33 | 0.72 | |
| Freibergite | 0.97 | 1.19 | 0.01 |
| Cassiterite | 4.25 | 1.37 | |
| Siderite | 5.30 | 4.21 | 2.44 |
| Fluorite | 0.53 | 1.27 | 0.94 |
| Total | 98.48 | 99.13 | 97.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfonso, P.; Ruiz, M.; Terricabras, M.; Martínez, A.; Garcia-Valles, M.; Anticoi, H.; Yubero, M.T.; Valls, S. Optimizing Mineral Resources with Automated Mineralogy Techniques: The Case of Colquiri in the Central Andean Tin Belt. Minerals 2025, 15, 1017. https://doi.org/10.3390/min15101017
Alfonso P, Ruiz M, Terricabras M, Martínez A, Garcia-Valles M, Anticoi H, Yubero MT, Valls S. Optimizing Mineral Resources with Automated Mineralogy Techniques: The Case of Colquiri in the Central Andean Tin Belt. Minerals. 2025; 15(10):1017. https://doi.org/10.3390/min15101017
Chicago/Turabian StyleAlfonso, Pura, Miguel Ruiz, Marçal Terricabras, Arnau Martínez, Maite Garcia-Valles, Hernan Anticoi, Maria Teresa Yubero, and Susanna Valls. 2025. "Optimizing Mineral Resources with Automated Mineralogy Techniques: The Case of Colquiri in the Central Andean Tin Belt" Minerals 15, no. 10: 1017. https://doi.org/10.3390/min15101017
APA StyleAlfonso, P., Ruiz, M., Terricabras, M., Martínez, A., Garcia-Valles, M., Anticoi, H., Yubero, M. T., & Valls, S. (2025). Optimizing Mineral Resources with Automated Mineralogy Techniques: The Case of Colquiri in the Central Andean Tin Belt. Minerals, 15(10), 1017. https://doi.org/10.3390/min15101017











