Life Cycle Assessment of Fluoride Removal from Mining Effluents Using Electrocoagulation and Biogenic CO2
Abstract
1. Introduction
2. Description of System and Conditions
2.1. LCA Methodology
2.2. Goal and Scope of the Study
2.3. Life Cycle Inventory (LCI)
2.4. Electrocoagulation
2.5. Sedimentation Unit
2.6. pH Adjustment Unit
2.7. Pumping Unit
2.8. Transport and Use of Sludge
2.9. System Constraints and Uncertainties
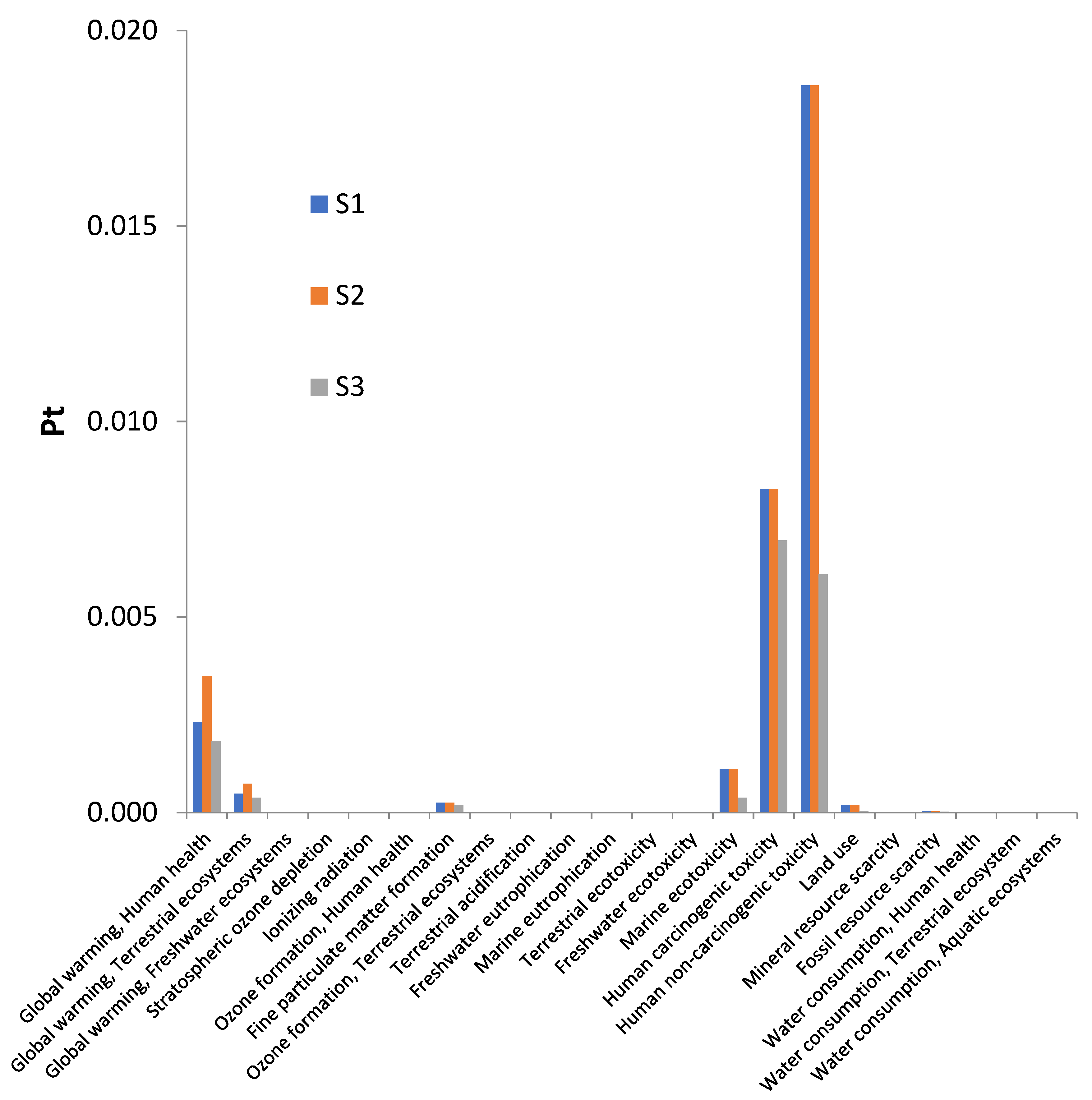
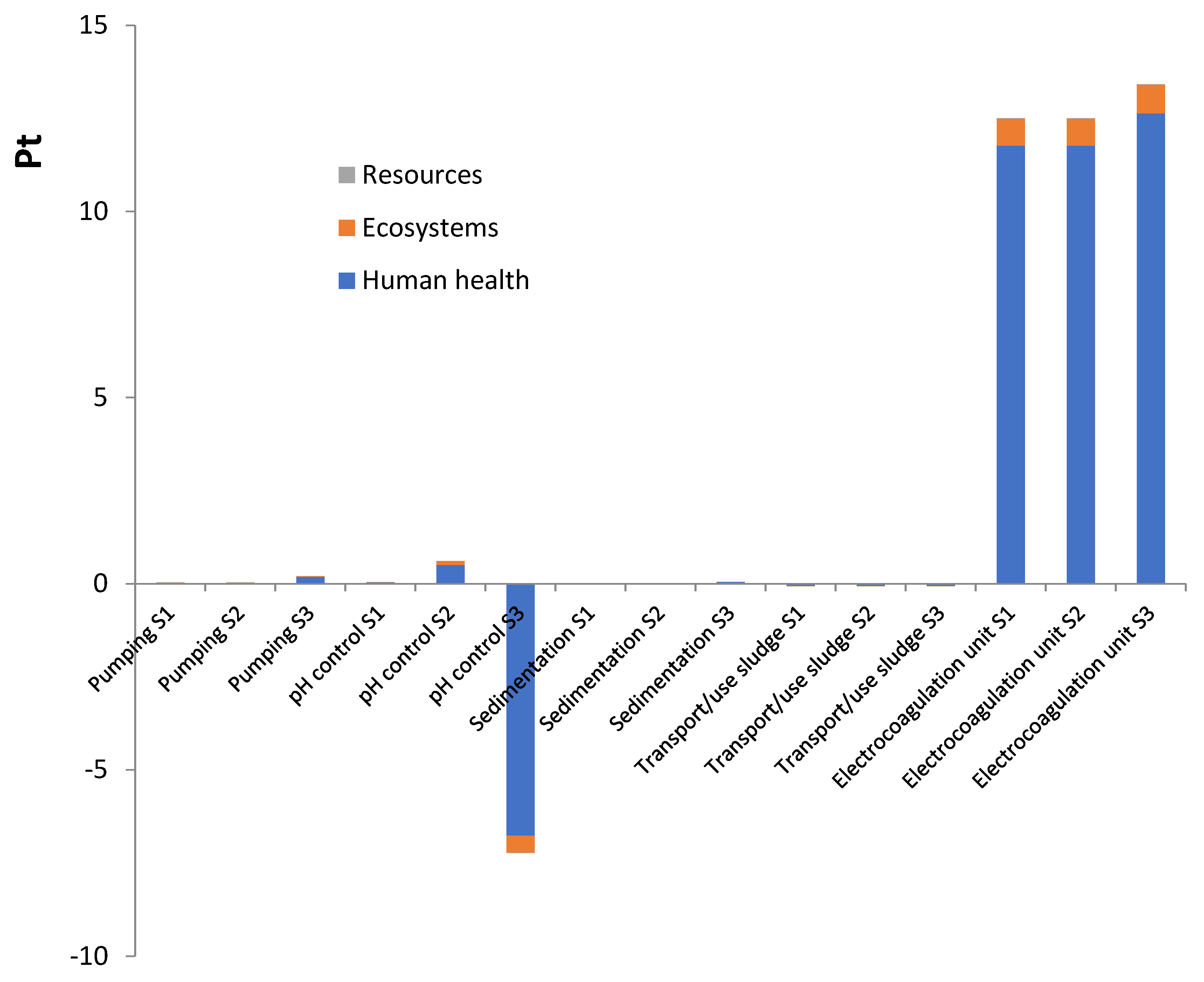
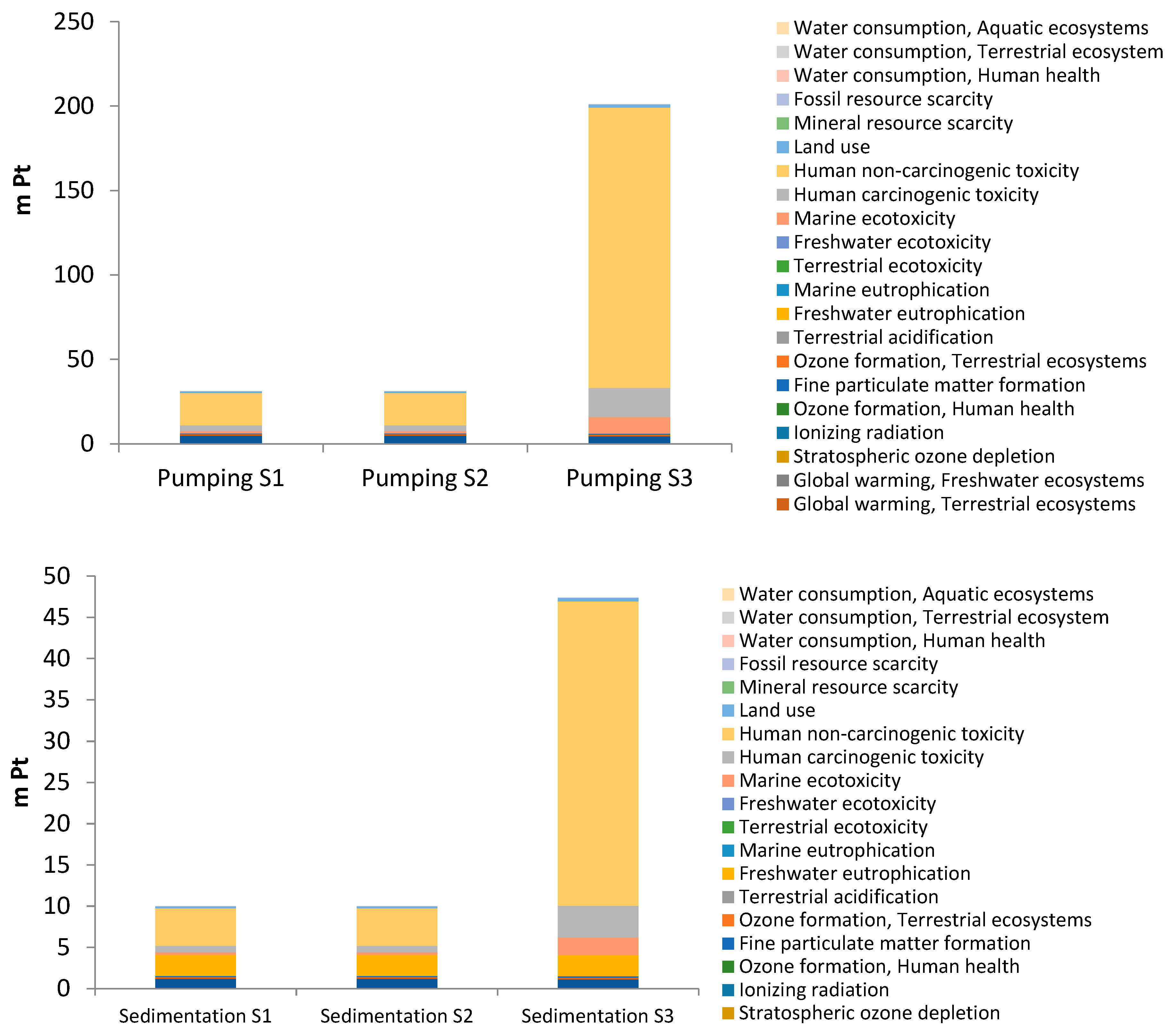
3. Results and Discussion
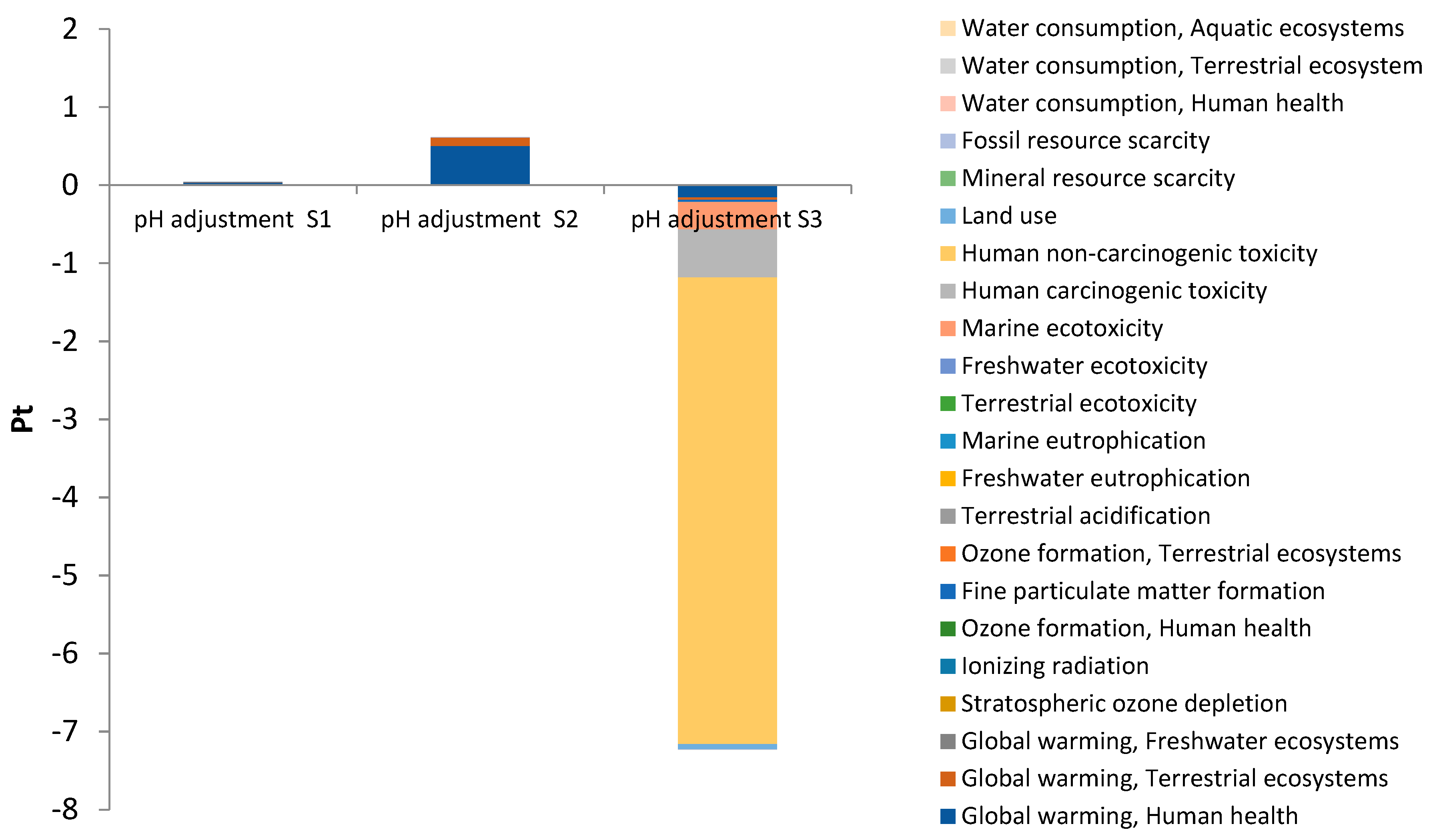
3.1. Environmental Impacts Assessed by Scenario
3.2. Environmental Impacts by Subsystems
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santhi, V.M.; Periasamy, D.; Perumal, M.; Sekar, P.M.; Varatharajan, V.; Aravind, D.; Senthilkumar, K.; Kumaran, S.T.; Ali, S.; Sankar, S.; et al. The global challenge of fluoride contamination: A comprehensive review of removal processes and implications for human health and ecosystems. Sustainability 2024, 16, 11056. [Google Scholar] [CrossRef]
- Ahmad, S.; Singh, R.; Arfin, T.; Neeti, K. Fluoride contamination, consequences and removal techniques in water: A review. Environ. Sci. Adv. 2022, 1, 620–661. [Google Scholar] [CrossRef]
- Reardon, E.J.; Wang, Y. A limestone reactor for fluoride removal from wastewaters. Environ. Sci. Technol. 2000, 34, 3247–3253. [Google Scholar] [CrossRef]
- de Luna, M.D.G.; Warmadewanthi; Liu, J.C. Combined treatment of polishing wastewater and fluoride-containing wastewater from a semiconductor manufacturer. Colloids Surf. A Physicochem. Eng. Asp. 2009, 347, 64–68. [Google Scholar] [CrossRef]
- Chiu, T.C.; Huang, H.-C.; Chen, L.J. Treatment of semiconductor wastewater by dissolved air flotation. J. Environ. Eng. 2002, 128, 974–980. [Google Scholar] [CrossRef]
- Drouiche, N.; Ghaffour, N.; Aoudj, S.; Hecini, M.; Ouslimane, T. Fluoride removal from photovoltaic wastewater by aluminum electrocoagulation and characteristics of products. Chem. Eng. Trans. 2009, 17, 1651–1656. [Google Scholar] [CrossRef]
- Ezzeddine, A.; Bedoui, A.; Hannachi, A.; Bensalah, N. Removal of fluoride from aluminum fluoride manufacturing wastewater by precipitation and adsorption processes. Desalin. Water Treat. 2015, 54, 2280–2292. [Google Scholar] [CrossRef]
- Khatibikamal, V.; Torabian, A.; Janpoor, F.; Hoshyaripour, G. Fluoride removal from industrial wastewater using electrocoagulation and its adsorption kinetics. J. Hazard. Mater. 2010, 179, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, A.; Taira, T. A new method for treating fluorine wastewater to reduce sludge and running costs. IEEE Trans. Semicond. Manuf. 2000, 13, 305–309. [Google Scholar] [CrossRef]
- Harrison, P.T.C. Fluoride in water: A UK perspective. J. Fluor. Chem. 2005, 126, 1448–1456. [Google Scholar] [CrossRef]
- Tripathy, S.S.; Bersillon, J.-L.; Gopal, K. Removal of fluoride from drinking water by adsorption onto alum impregnated activated alumina. Sep. Purif. Technol. 2006, 50, 310–317. [Google Scholar] [CrossRef]
- Xiong, X.; Liu, J.; He, W.; Xia, T.; He, P.; Chen, X.; Yang, K.; Wang, A. Dose–effect relationship between drinking water fluoride levels and damage to liver and kidney functions in children. Environ. Res. 2007, 103, 112–116. [Google Scholar] [CrossRef]
- Sharma, P.; Verma, P.K.; Sood, S.; Singh, M.; Verma, D. Impact of chronic sodium fluoride toxicity on antioxidant capacity, biochemical parameters, and histomorphology in cardiac, hepatic, and renal tissues of Wistar rats. Biol. Trace Elem. Res. 2022, 200, 229–241. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, C.; Ma, J.; Sun, W.; Shah, K.J. Review of fluoride removal technology from wastewater environment. Desalin. Water Treat. 2023, 299, 90–101. [Google Scholar] [CrossRef]
- Mameri, N.; Yeddou, A.R.; Lounici, H.; Belhocine, D.; Grib, H.; Bariou, B. Defluoridation of septentrional Sahara water of North Africa by electrocoagulation process using bipolar aluminum electrodes. Water Res. 1998, 32, 1604–1612. [Google Scholar] [CrossRef]
- Guzmán, A.; Nava, J.L.; Coreño, O.; Rodríguez, I.; Gutiérrez, S. Arsenic and fluoride removal from groundwater by electrocoagulation using a continuous filter press reactor. Chemosphere 2016, 144, 2113–2120. [Google Scholar] [CrossRef]
- Drondina, R.V.; Drako, I.V. Electrochemical technology of fluorine removal from underground and waste waters. J. Hazard. Mater. 1994, 37, 91–100. [Google Scholar] [CrossRef]
- Yang, C.-L.; Dluhy, R. Electrochemical generation of aluminum sorbent for fluoride adsorption. J. Hazard. Mater. 2002, 94, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Chen, X.; Gao, P.; Chen, G. Electrochemical removal of fluoride ions from industrial wastewater. Chem. Eng. Sci. 2003, 58, 987–993. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Sivakumar, M. An empirical model for defluoridation by batch monopolar electrocoagulation/flotation (ECF) process. J. Hazard. Mater. 2006, 131, 118–125. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, H.; Ni, J. Fluoride distribution in electrocoagulation defluoridation process. Sep. Purif. Technol. 2007, 56, 184–191. [Google Scholar] [CrossRef]
- Tezcan Un, U.; Koparal, A.S.; Ogutveren, U.B. Fluoride removal from water and wastewater with a batch cylindrical electrode using electrocoagulation. Chem. Eng. J. 2013, 223, 110–115. [Google Scholar] [CrossRef]
- Sandoval, M.A.; Fuentes, R.; Nava, J.L.; Rodríguez, I. Fluoride removal from drinking water by electrocoagulation in a continuous filter press reactor coupled to a flocculator and clarifier. Sep. Purif. Technol. 2014, 134, 163–170. [Google Scholar] [CrossRef]
- Yetilmezsoy, K.; Dehghani, M.H.; Saeedi, R.; Dalvand, A.; Heibati, B.; Askari, M.; Alimohammadi, M.; McKay, G. Elimination of natural organic matter by electrocoagulation using bipolar and monopolar arrangements of iron and aluminum electrodes. Int. J. Environ. Sci. Technol. 2017, 14, 2125–2134. [Google Scholar] [CrossRef]
- Garg, U.K.; Sharma, C.; Garg, U.K. Electrocoagulation: Promising technology for removal of fluoride from drinking water—A review. Biol. Forum 2016, 8, 248–254. Available online: http://www.researchtrend.net (accessed on 18 March 2019).
- Nigri, E.; Santos, A.; Rocha, S. Electrocoagulation associated with CO2 mineralization applied to fluoride removal from mining industry wastewater. Desalin. Water Treat. 2021, 209, 58–70. [Google Scholar] [CrossRef]
- Astuti, A.R.A.; Mutiara, S.; Saputera, N. Advances in carbon control technologies for flue gas: A step towards sustainable industrial CO2 capture. Int. J. Greenh. Gas Control. 2024, 123, 103587. [Google Scholar]
- Beiron, J.; Normann, F.; Kristoferson, L.; Strömberg, L.; Gardarsdóttir, S.Ö.; Johnsson, F. Enhancement of CO2 absorption in water through pH control and carbonic anhydrase—A technical assessment. Ind. Eng. Chem. Res. 2019, 58, 14275–14283. [Google Scholar] [CrossRef]
- Werkneh, A.A. Biogas impurities: Environmental and health implications, removal technologies and future perspectives. Heliyon 2022, 8, e10929. [Google Scholar] [CrossRef]
- Ahangarnokolaei, R.; Rahmanian, N.; Ramezani, M.; Khataee, A. Environmental performance of electrocoagulation and ozonation for wastewater treatment: A life cycle assessment approach. J. Environ. Manag. 2021, 287, 112345. [Google Scholar] [CrossRef]
- Choudhary, N.; Singh, P.; Kumar, R.; Gupta, S. Life cycle assessment of electrocoagulation processes: Energy consumption and global warming potential. J. Clean. Prod. 2023, 408, 137137. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Chen, Q.; Wang, J. Performance of aluminum electrodes in electrocoagulation for removal of heavy metals and phosphorus. Chemosphere 2024, 351, 139876. [Google Scholar] [CrossRef]
- Zailani, S.; Zin, R.M. Electrocoagulation for wastewater treatment: Influence of electrode material and process parameters. Environ. Technol. Innov. 2018, 10, 123–131. [Google Scholar]
- Li, Y.; Zhang, S.; Zhang, W.; Xiong, W.; Ye, Q.; Hou, X.; Wang, C.; Wang, P. Life cycle assessment of advanced wastewater treatment processes: Involving 126 pharmaceuticals and personal care products in life cycle inventory. J. Environ. Manag. 2019, 238, 442–450. [Google Scholar] [CrossRef]
- Pell, R.; Tijsseling, L.; Palmer, L.W.; Glass, H.J.; Yan, X.; Wall, F.; Zeng, X.; Li, J. Environmental optimisation of mine scheduling through life cycle assessment integration. Resour. Conserv. Recycl. 2019, 142, 267–276. [Google Scholar] [CrossRef]
- Rahman, S.M.; Eckelman, M.J.; Onnis Hayden, A.; Gu, A.Z. Comparative life cycle assessment of advanced wastewater treatment processes for removal of chemicals of emerging concern. Environ. Sci. Technol. 2018, 52, 11346–11358. [Google Scholar] [CrossRef]
- Opher, T.; Friedler, E. Comparative LCA of decentralized wastewater treatment alternatives for non-potable urban reuse. J. Environ. Manag. 2016, 182, 464–476. [Google Scholar] [CrossRef]
- Castellet, L.; Molinos-Senante, M. Efficiency assessment of wastewater treatment plants: A data envelopment analysis approach integrating technical, economic, and environmental issues. J. Environ. Manag. 2016, 167, 160–166. [Google Scholar] [CrossRef]
- Corominas, L.; Foley, J.; Guest, J.S.; Hospido, A.; Larsen, H.F.; Morera, S.; Shaw, A. Life cycle assessment applied to wastewater treatment: State of the art. Water Res. 2013, 47, 5480–5492. [Google Scholar] [CrossRef]
- Goyal, H.; Mondal, P. Life cycle assessment (LCA) of the arsenic and fluoride removal from groundwater through adsorption and electrocoagulation: A comparative study. Chemosphere 2022, 304, 135243. [Google Scholar] [CrossRef]
- Thakur, L.S.; Goyal, H.; Mondal, P. Simultaneous removal of arsenic and fluoride from synthetic solution through continuous electrocoagulation: Operating cost and sludge utilization. J. Environ. Chem. Eng. 2019, 7, 102829. [Google Scholar] [CrossRef]
- Çetinkaya, A.Y. Integration of electrocoagulation and solar energy for sustainable wastewater treatment: A thermodynamic and life cycle assessment study. Environ. Monit. Assess. 2025, 197, 224. [Google Scholar] [CrossRef]
- Sharma, P.; Joshi, H. Utilization of electrocoagulation treated spent wash sludge in making building blocks. Int. J. Environ. Sci. Technol. 2016, 13, 349–358. [Google Scholar] [CrossRef]
- Rajaniemi, K.; Tuomikoski, S.; Lassi, U. Electrocoagulation sludge valorization—A review. Resources 2021, 10, 127. [Google Scholar] [CrossRef]
- Zhu, P.; Li, J.; Wang, L.; Zhou, R.; Liu, Y. Reuse of hazardous calcium fluoride sludge in ceramic products. J. Hazard. Mater. 2013, 260, 855–860. [Google Scholar] [CrossRef]
- Yilmaz, A.E.; Boncukcuoğlu, R.; Kocakerim, M.; Karakaş, İ.H. Waste utilization: The removal of textile dye (Bomaplex Red CR-L) from aqueous solution on sludge waste from electrocoagulation as adsorbent. Desalination 2011, 277, 156–163. [Google Scholar] [CrossRef]
- García Gómez, C.; Rivera Huerta, M.L.; Almazán García, F.; Martín Domínguez, A.; Romero Soto, I.C.; Burboa Charis, V.A.; Gortáres Moroyoqui, P. Electrocoagulated metal hydroxide sludge for fluoride and arsenic removal in aqueous solution: Characterization, kinetic, and equilibrium studies. Water Air Soil Pollut. 2016, 227, 96. [Google Scholar] [CrossRef]
- Golder, A.K.; Samanta, A.N.; Ray, S. Removal of phosphate from aqueous solutions using calcined metal hydroxides sludge waste generated from electrocoagulation. Sep. Purif. Technol. 2006, 52, 102–109. [Google Scholar] [CrossRef]
- Santana, J.J.; Mena, V.F.; Betancor Abreu, A.; Rodríguez Raposo, R.; Izquierdo, J.; Souto, R.M. Use of alumina sludge arising from an electrocoagulation process as functional mesoporous microcapsules for active corrosion protection of aluminum. Prog. Org. Coat. 2021, 151, 106044. [Google Scholar] [CrossRef]
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework (2nd ed.). International Organization for Standardization: Geneva, Switzerland, 2006. Available online: https://www.iso.org/standard/37456.html (accessed on 15 September 2025).
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Requirements and Guidelines (1st ed.). International Organization for Standardization: Geneva, Switzerland, 2006. Available online: https://www.iso.org/standard/38498.html (accessed on 15 September 2025).
- Bonton, A.; Bouchard, C.; Barbeau, B.; Jedrzejak, S. Comparative life cycle assessment of water treatment plants. Desalination 2012, 284, 42–54. [Google Scholar] [CrossRef]
- Goedkoop, M.; Heijungs, R.; Huijbregts, M.; de Schryver, A.; Struijs, J.; van Zelm, R. ReCiPe 2008: A Life Cycle Impact Assessment Method Which Comprises Harmonised Category Indicators at the Midpoint and the Endpoint Level; Report I: Characterisation; Ministry of Housing, Spatial Planning and the Environment (VROM): The Hague, The Netherlands, 2009; Available online: https://www.rivm.nl/en/life-cycle-assessment-lca/recipe (accessed on 15 September 2025).
- Zepon Tarpani, R.R.; Azapagic, A. Life cycle environmental impacts of advanced wastewater treatment techniques for removal of pharmaceuticals and personal care products (PPCPs). J. Environ. Manag. 2018, 215, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Nakhate, P.H.; Moradiya, K.K.; Patil, H.G.; Marathe, K.V.; Yadav, G.D. Case study on sustainability of textile wastewater treatment plant based on life cycle assessment approach. J. Clean. Prod. 2020, 245, 118929. [Google Scholar] [CrossRef]
- Hauschild, M.; Rosenbaum, R.K.; Olsen, S.I. Life Cycle Assessment: Theory and Practice; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Singh, P.; Carliell-Marquet, C.; Kansal, A. Energy pattern analysis of a wastewater treatment plant. Appl. Water Sci. 2012, 2, 221–226. [Google Scholar] [CrossRef]
- CONAMA. Resolução 430. 2011. Available online: http://www.suape.pe.gov.br/images/publicacoes/CONAMA_n.430.2011.pdf (accessed on 15 September 2025).
- Cashman, S.; Gaglione, A.; Mosley, J.; Weiss, L.; Hawkins, T.; Ashbolt, N.; Cashdollar, J.; Xue, X.; Ma, C.; Arden, S. Environmental and Cost Life Cycle Assessment of Disinfection Options for Municipal Drinking Water Treatment; Report; U.S. Environmental Protection Agency, Office of Research and Development: Cincinnati, OH, USA, 2014. Available online: https://cfpub.epa.gov/si/si_public_record_report.cfm?dirEntryId=298570 (accessed on 15 September 2025).
- Dalpaz, R. Avaliação Energética do Biogás com Diferentes Percentuais de Metano na Produção de Energia Térmica e Elétrica. Master’s Thesis, Universidade do Vale do Taquari—Univates, Lajeado, Brazil, 2019. Available online: https://www.univates.br/bduserver/api/core/bitstreams/025b6593-53da-4343-862a-1416feb4b694/content (accessed on 15 September 2025).

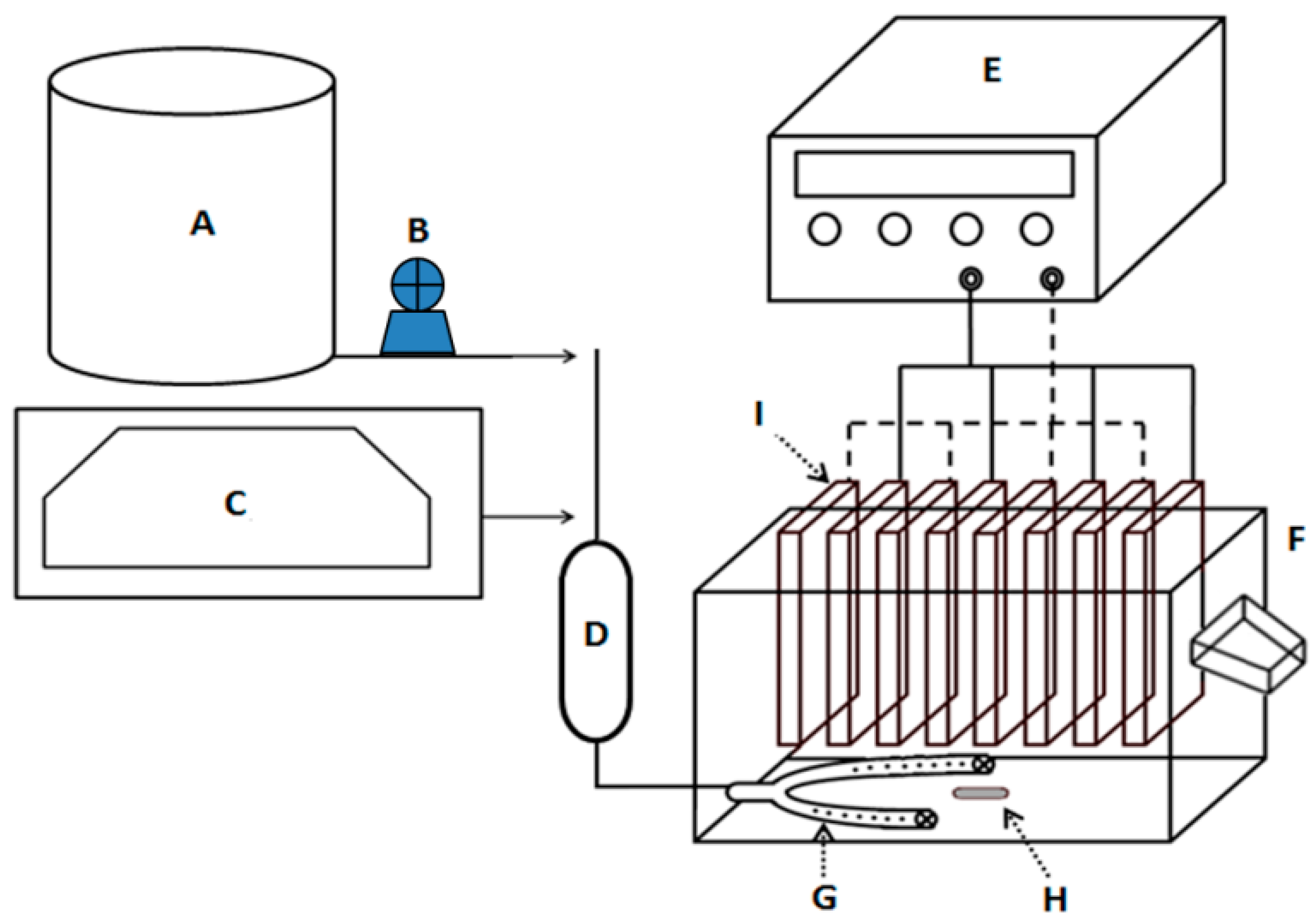
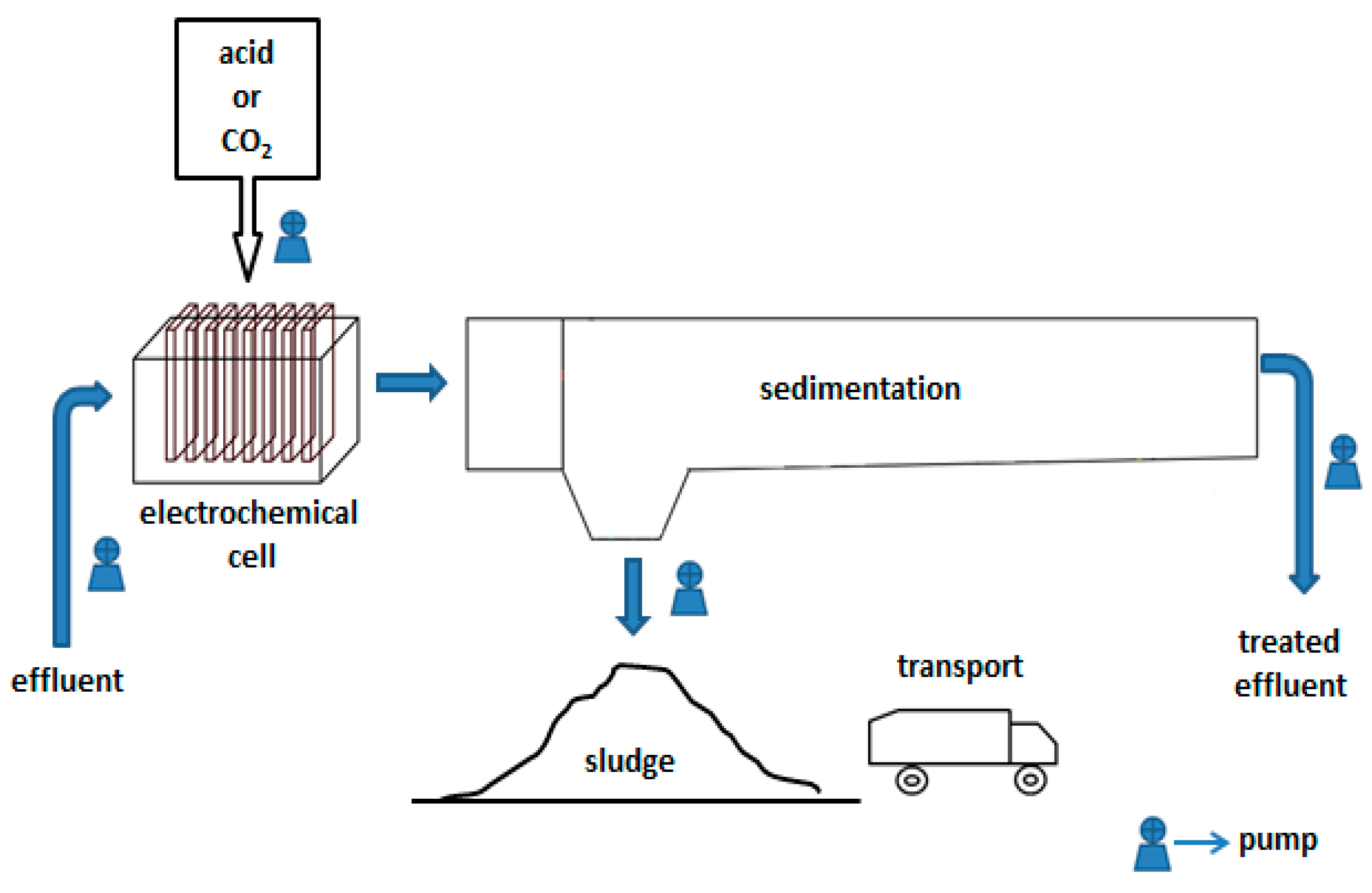
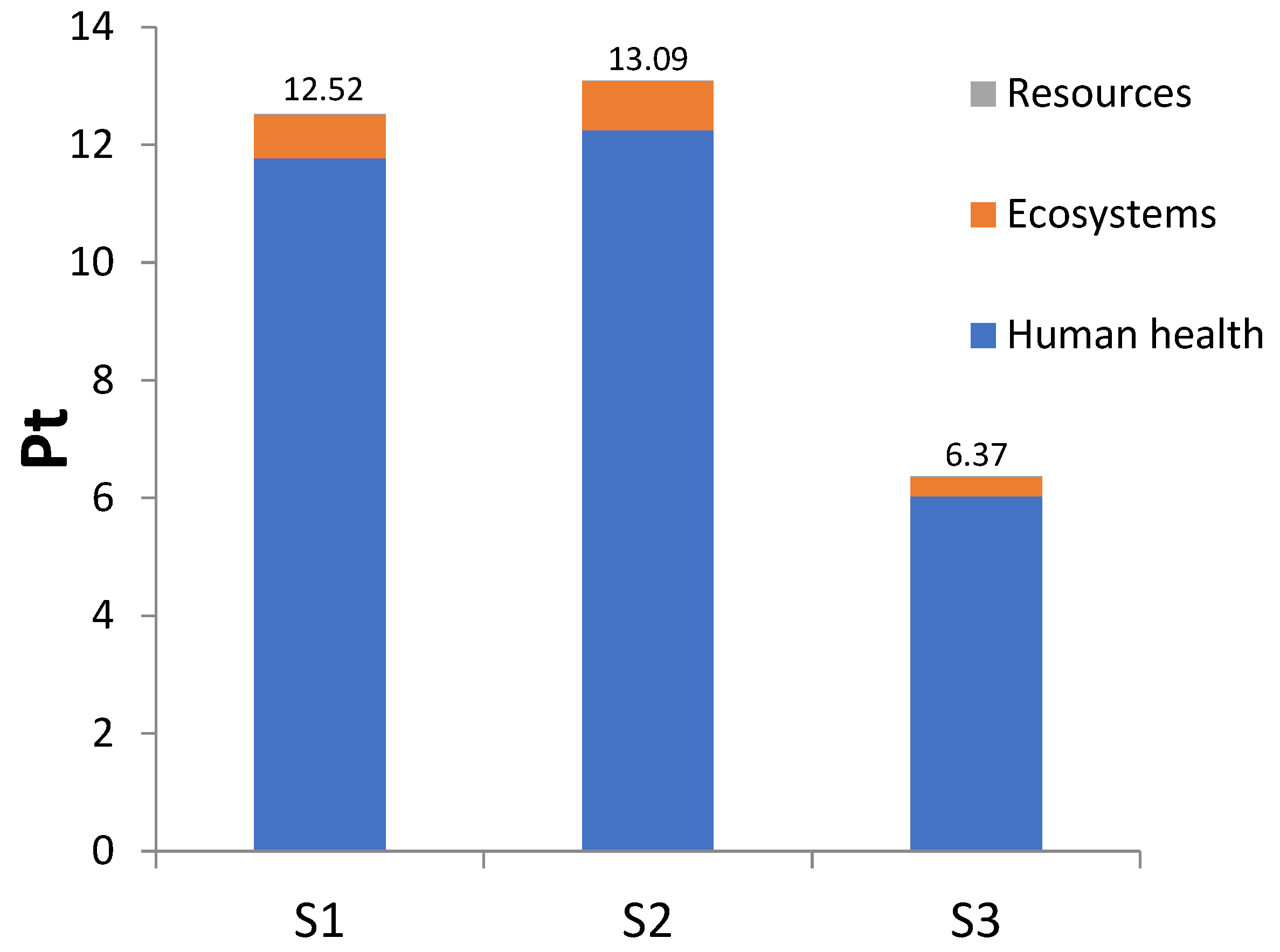
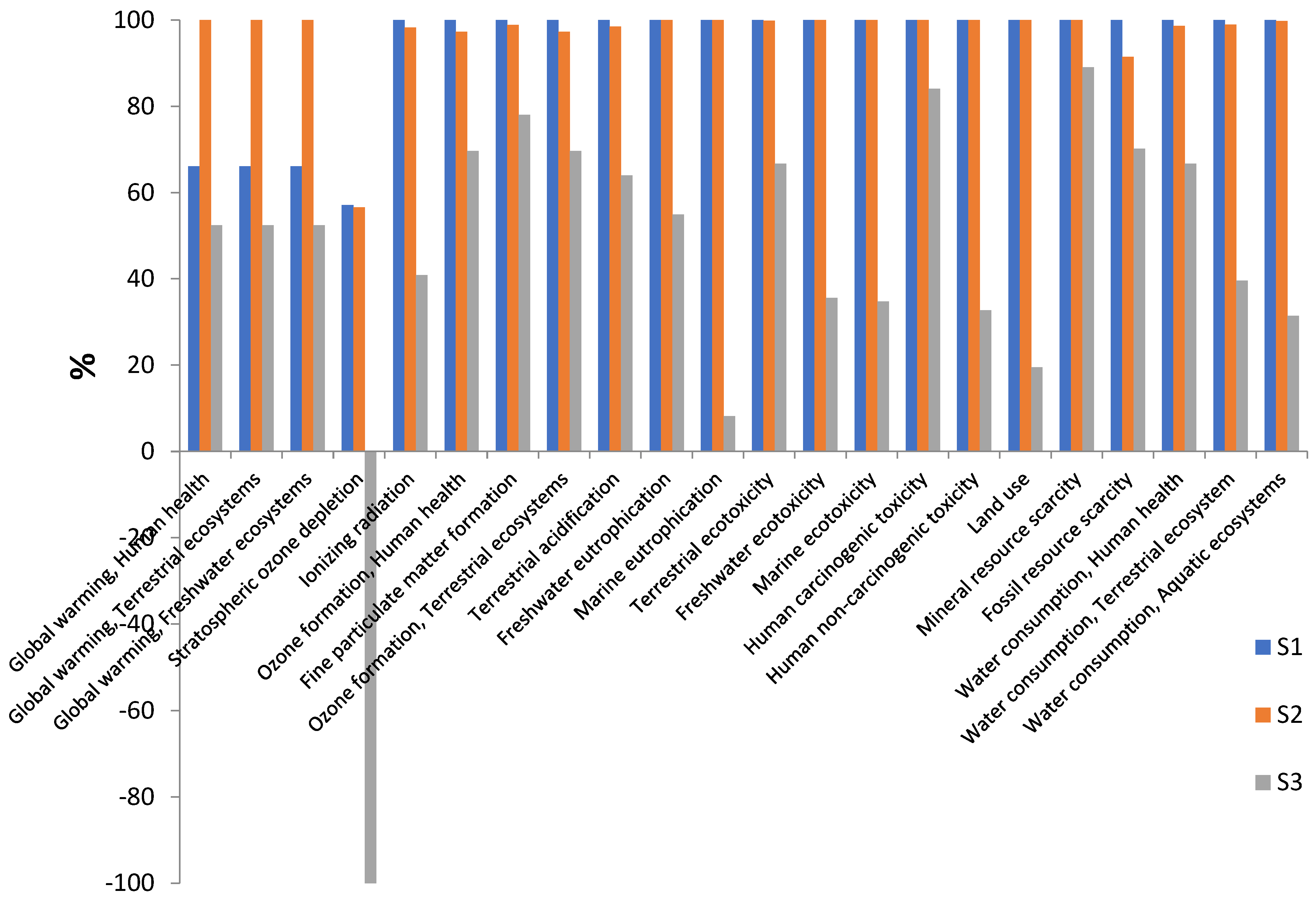
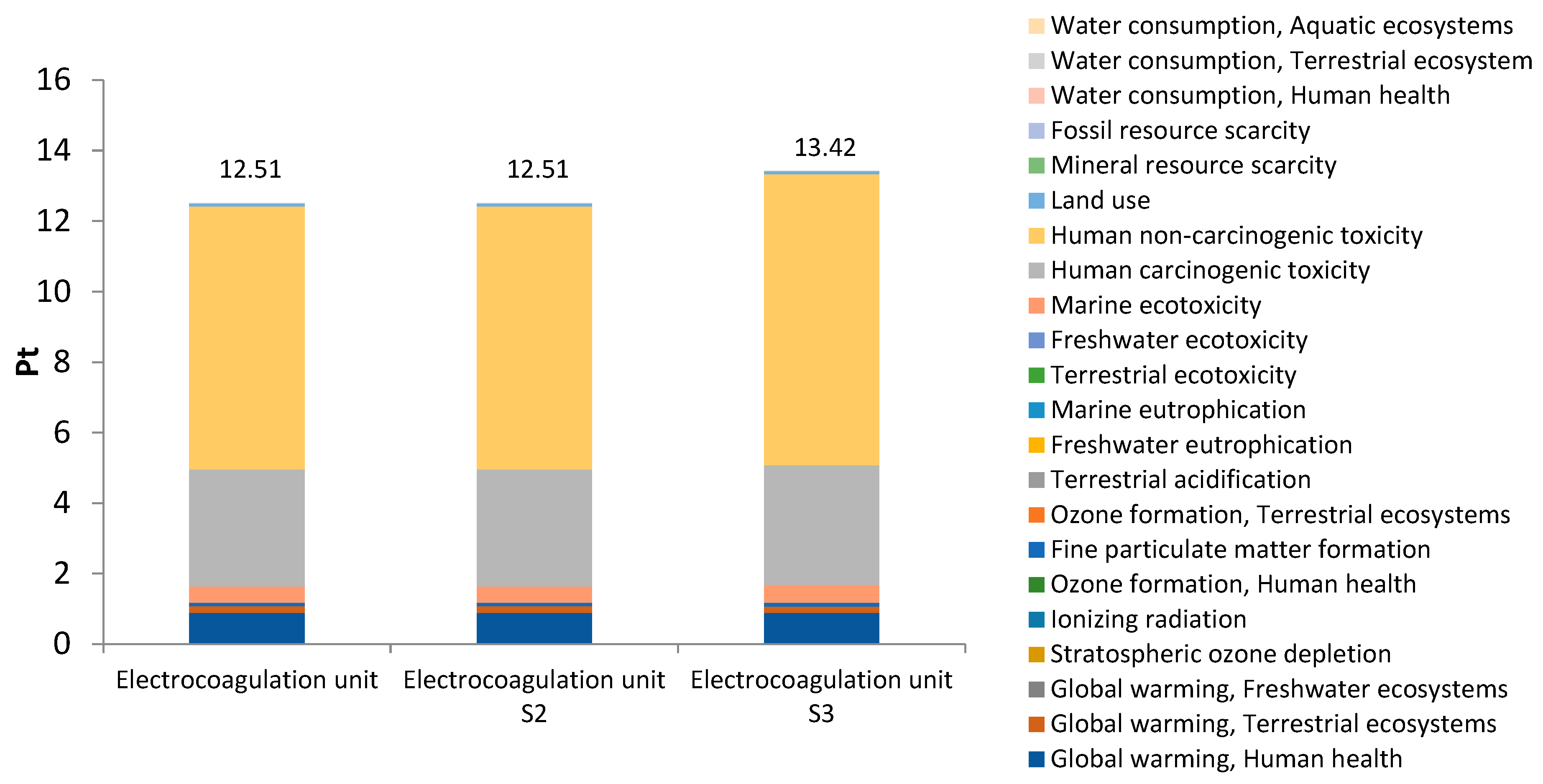

| Scenario | Reagent | Function |
|---|---|---|
| S1 | HCl | pH control |
| S2 | CO2 from biogas | pH control |
| S3 | CO2 from biogas | pH control and energy source |
| Parameter | Value |
|---|---|
| pH | 7.8 |
| Conductivity (µS.cm−1) | 6056.0 |
| Fluoride (mg.L−1) | 134.0 |
| Calcium (mg.L−1) | 4.4 |
| Sodium (mg.L−1) | 632.0 |
| Aluminum (mg.L−1) | <5.0 * |
| Chloride (mg.L−1) | 1424.0 |
| Sulfate (mg.L−1) | 69.0 |
| Alkalinity carbonates and hydroxides (mg.L−1) | 0.0 |
| Alkalinity bicarbonates (mg.L−1) | 660.0 |
| Total phosphorus (mg.L−1) | 8.0 |
| COD (mgO2.L−1) | 106.0 |
| Scenario | Input | Quantity | SimaPro® Data |
|---|---|---|---|
| S1, S2, S3 | Polyethylene (kg) | 2.4948 × 10−4 | Polyethylene high-density granulates (PE-HD), production mix, at-plant RER |
| S1, S2, S3 | Polyethylene molding (kg) | 2.4948 × 10−4 | Injection molding (CA-QC)—APOS, S |
| S1, S2, S3 | Aluminum plate (kg) | 2.52 | Aluminum, cast alloy GLO market for APOS, S |
| S1, S2 | Electricity (kWh) | 2.25 | Electricity, low-voltage (BR—south-eastern grid) market for electricity, low-voltage APOS, S |
| S3 | Electricity (kWh) | 2.25 | Electricity, low-voltage (CH) biogas, burned in micro gas turbine 100 kWe—APOS, S |
| Scenario | Input | Quantity | SimaPro® Data | |
|---|---|---|---|---|
| Sedimentation tank structure | S1, S2, S3 | Steel (kg) | 8.8 × 10−4 | Iron and steel, production mix/US |
| S1, S2, S3 | HDPE (kg) | 5.8 × 10−6 | Polyethylene high-density granulates (PE-HD), production mix, at-plant RER | |
| S1, S2, S3 | Concrete (m3) | 1.0 × 10−5 | Concrete, normal (BR) market for concrete, normal—APOS, S | |
| Motor | S1, S2, S3 | Steel (kg) | 2.4 × 10−8 | Iron and steel, production mix/US |
| S1, S2, S3 | Steel (kg) | 6.0 × 10−9 | Iron and steel, production mix/US | |
| S1, S2, S3 | Cast iron (kg) | 1.8 × 10−7 | Cast iron (GLO) market—APOS, S | |
| S1, S2, S3 | Aluminum (kg) | 5.4 × 10−9 | Aluminum alloy, AlLi (GLO) market—APOS, S | |
| S1, S2, S3 | Cooper (kg) | 4.6 × 10−9 | Copper sheet, technology mix, consumption mix, at-plant, 0.6 mm thickness EU-15 S | |
| S1, S2 | Electricity (kWh) | 0.092 | Electricity, low-voltage (BR—south-eastern grid) market for electricity, low-voltage APOS, S | |
| S3 | Electricity (kWh) | 0.092 | Electricity, low-voltage (CH) biogas, burned in micro gas turbine 100 kWe—APOS, S |
| Scenario | Input | Output | Quantity | SimaPro® Data |
|---|---|---|---|---|
| S1, S2, S3 | Polyethylene (g) | 0.044074 | Polyethylene high-density granulates (PE-HD), production mix, at-plant RER | |
| S1, S2, S3 | Polyethylene molding (g) | 0.044074 | Injection molding (CA-QC) injection molding—APOS, S | |
| S1 | HCl (L) | 2.627 | Hydrochloric acid, Mannheim process (30% HCl), at-plant/RER Mass | |
| S2, S3 | Biogas (L) | 5.3966 | Biogas, from grass (CH) biogas production from grass—APOS S | |
| S3 | Electricity (kwh) | * 15.04857 | Electricity, low-voltage (CH) biogas, burned in micro gas turbine 100 kWe—APOS, S |
| Scenario | Input | Quantity | SimaPro® Data | |
|---|---|---|---|---|
| Pickup pump | S1, S2, S3 | Cast iron (kg) | 2.4 × 10−5 | Cast iron (GLO) market—APOS, S |
| S1, S2, S3 | Stainless steel (kg) | 2.2 × 10−6 | Steel, stainless 304, flat rolled coil/kg/RNA | |
| S1, S2 | Electricity (kWh) | 0.203 | Electricity, low-voltage (BR—south-eastern grid) market for electricity, low-voltage APOS, S | |
| S3 | Electricity (kWh) | 0.203 | Electricity, low-voltage (CH) biogas, burned in micro gas turbine 100 kWe—APOS, S | |
| Acid or biogas injection pump | S1, S2, S3 | Cast iron (kg) | 1.3 × 10−7 | Cast iron (GLO) market—APOS, S |
| S1, S2, S3 | Stainless steel (kg) | 9.1 × 10−8 | Steel, stainless 304, flat rolled coil/kg/RNA | |
| S1, S2 | Electricity (kWh) | 0.0156 | Electricity, low-voltage (BR—south-eastern grid) market for electricity, low-voltage APOS, S | |
| S3 | Electricity (kWh) | 0.0156 | Electricity, low-voltage (CH) biogas, burned in micro gas turbine 100 kWe—APOS, S | |
| Sludge pump | S1, S2, S3 | Cast iron (kg) | 2.8 × 10−7 | Cast iron (GLO) market—APOS, S |
| S1, S2, S3 | Stainless steel (kg) | 1.9 × 10−7 | Steel, stainless 304, flat rolled coil/kg/RNA | |
| S1, S2 | Electricity (kWh) | 0.0165 | Electricity, low-voltage (BR—south-eastern grid) market for electricity, low-voltage APOS, S | |
| S3 | Electricity (kWh) | 0.0165 | Electricity, low-voltage (CH) biogas, burned in micro gas turbine 100 kWe—APOS, S | |
| Treated effluent pump | S1, S2, S3 | Cast iron (kg) | 2.4 × 10−5 | Cast iron (GLO) market—APOS, S |
| S1, S2, S3 | Stainless steel (kg) | 2.2 × 10−6 | Steel, stainless 304, flat rolled coil/kg/RNA | |
| S1, S2 | Electricity (kWh) | 0.183 | Electricity, low-voltage (BR—south-eastern grid) market for electricity, low-voltage APOS, S | |
| S3 | Electricity (kWh) | 0.183 | Electricity, low-voltage (CH) biogas, burned in micro gas turbine 100 kWe—APOS, S | |
| Pipeline | S1, S2, S3 | PVC pipeline (m) | 3.0983 × 10−5 | PVC pipe E |
| Scenario | Input | Output | Quantity | SimaPro® Data |
|---|---|---|---|---|
| S1, S2, S3 | Transport (t.km) | 0.360045 | Transport, truck 10–20 t, EURO5, 80%LF, empty return/GLO Mass | |
| S1, S2, S3 | Sludge (kg) | 7.41 | Clay (RoW) market for clay—APOS.U |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nigri, E.M.; Santos, A.L.A.; Rocha, S.D.F. Life Cycle Assessment of Fluoride Removal from Mining Effluents Using Electrocoagulation and Biogenic CO2. Minerals 2025, 15, 1016. https://doi.org/10.3390/min15101016
Nigri EM, Santos ALA, Rocha SDF. Life Cycle Assessment of Fluoride Removal from Mining Effluents Using Electrocoagulation and Biogenic CO2. Minerals. 2025; 15(10):1016. https://doi.org/10.3390/min15101016
Chicago/Turabian StyleNigri, Elbert Muller, André Luiz Alvarenga Santos, and Sônia Denise Ferreira Rocha. 2025. "Life Cycle Assessment of Fluoride Removal from Mining Effluents Using Electrocoagulation and Biogenic CO2" Minerals 15, no. 10: 1016. https://doi.org/10.3390/min15101016
APA StyleNigri, E. M., Santos, A. L. A., & Rocha, S. D. F. (2025). Life Cycle Assessment of Fluoride Removal from Mining Effluents Using Electrocoagulation and Biogenic CO2. Minerals, 15(10), 1016. https://doi.org/10.3390/min15101016









