Chemical and Mineralogical Characterization of Waste from Abandoned Copper and Manganese Mines in the Iberian Pyrite Belt, Portugal: A First Step Towards the Waste-to-Value Recycling Process
Abstract
1. Introduction
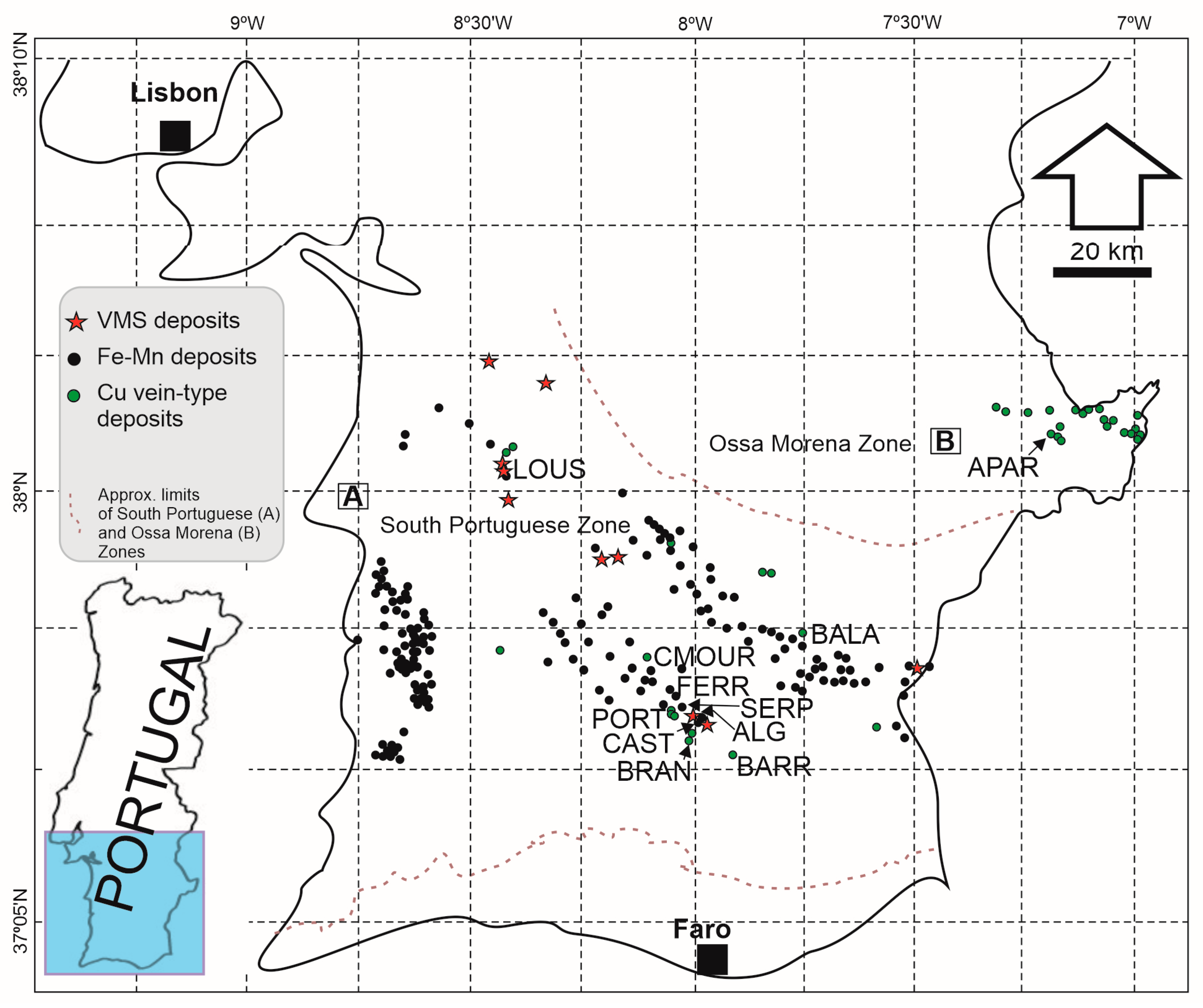
1.1. Historical Mines Overview
1.1.1. VMS-Type Deposits
1.1.2. Cu Vein-Type Deposits
1.1.3. Manganese Lens-Type Deposits
2. Materials and Methods
2.1. Sampling
2.2. Methodology
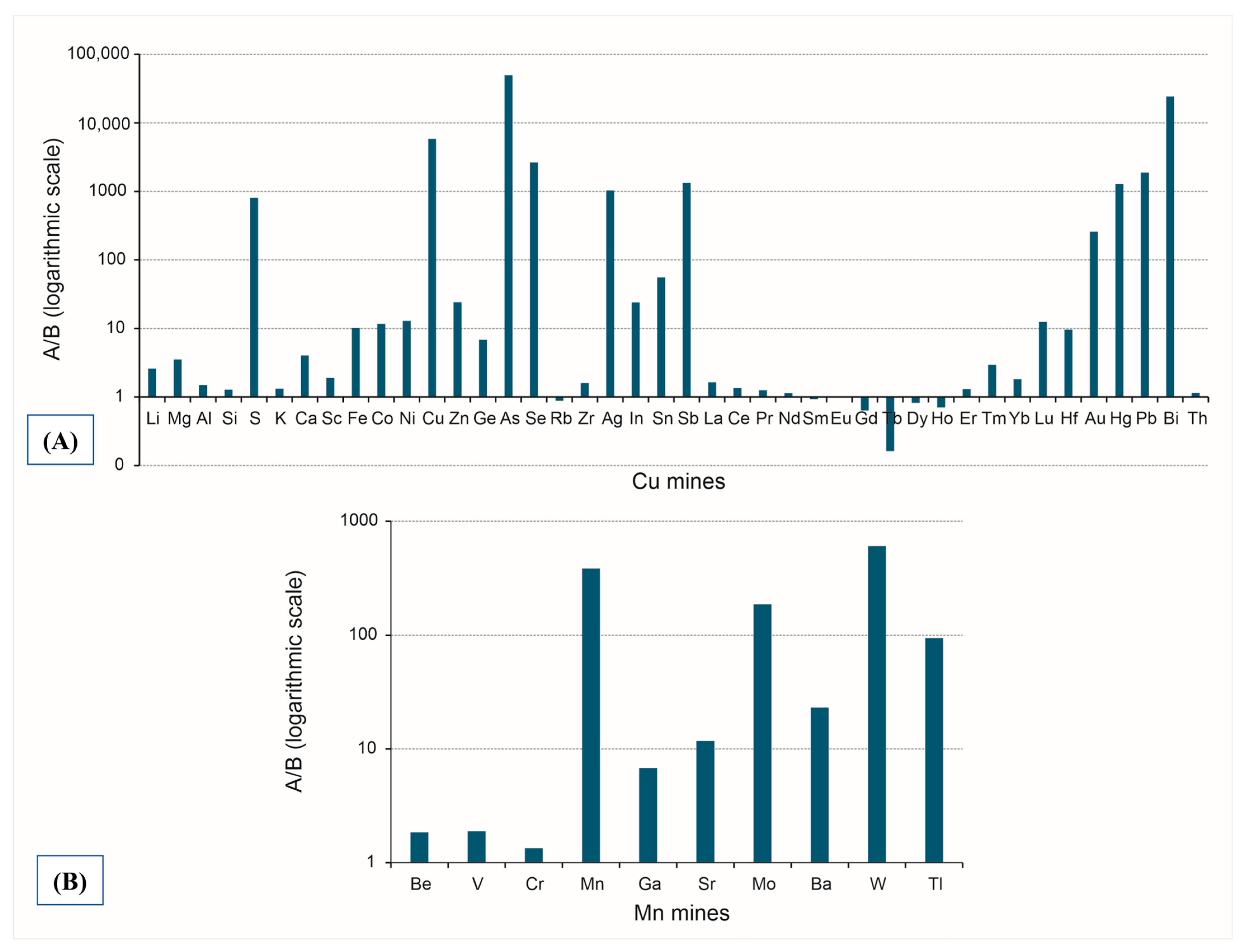
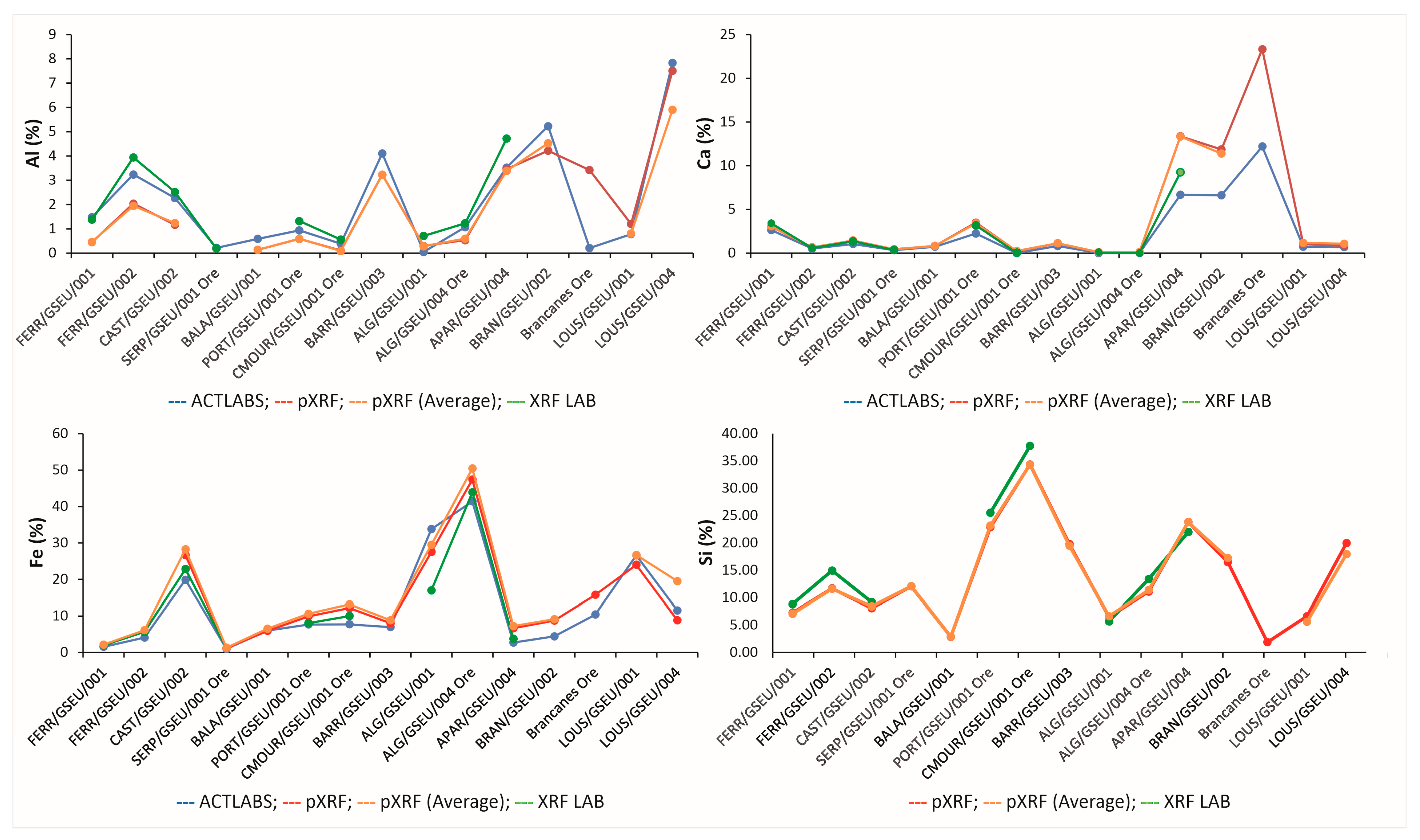
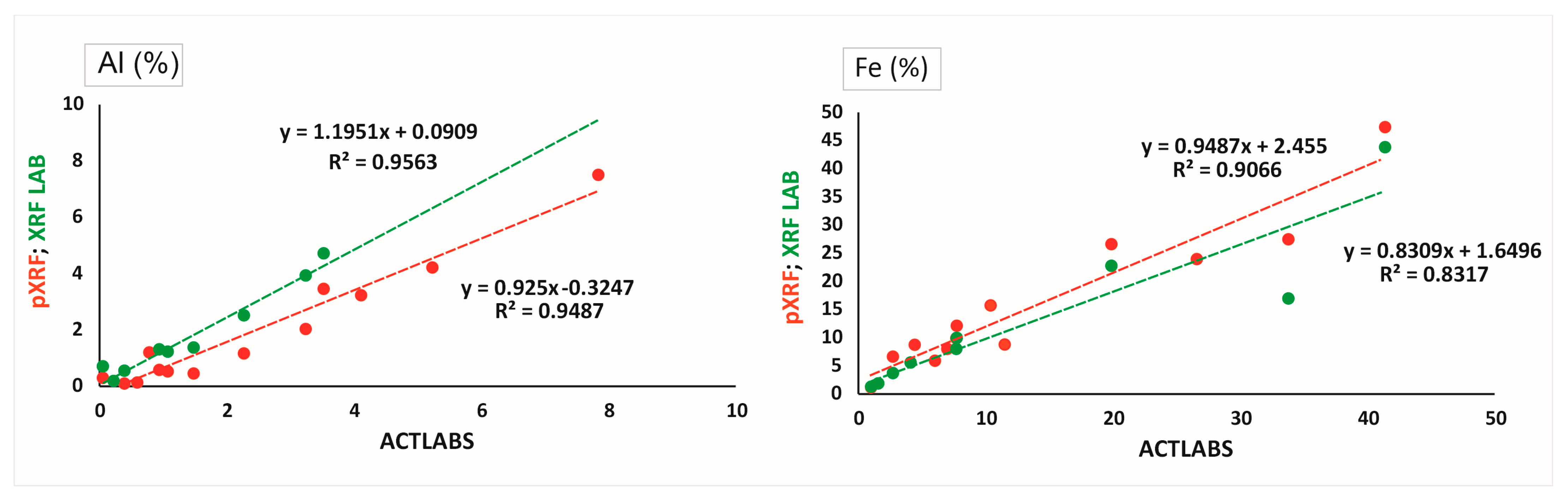
3. Results and Discussion
| Sample Reference | Phase Identification | +++ | ++ | + |
|---|---|---|---|---|
| PORT/GSEU/001 | Ab + Bir + Ccp (vtg) + Chm + Dol + Ms/Bt + Pmlc (vtg) + Qz + Rt (vtg) | Quartz | Muscovite/Biotite, Chamosite, Albite | Birnessite, Dolomite |
| PORT/GSEU/001 Ore | Ank (vtg) + Bir (vtg) + Ccp + Chm (vtg) + Dol + En? (vtg) + Ms/Bt (vtg) + Qz + Var (vtg) | Quartz | Chalcopyrite, Dolomite | |
| CMOUR/GSEU/001 Ore | Bnt (vtg) + Cst + Hem + Mnn (vtg) + Qz + Trd + Zrn (vtg) | Quartz | Cassiterite, Hematite, Tridymite | |
| BARR/GSEU/001 | Ccp (vtg) + Chm + Dol + Gp (vtg) + Kln (vtg) + Ms/Bt + Qz + Tnt-Fe (vtg) | Quartz | Dolomite, Chamosite, Musc./Biotite | |
| BARR/GSEU/002 | Ab + Mul + Qz +Tmgh (vtg) | Quartz | Mullite | Albite |
| BARR/GSEU/003 | Ccp + Chm + Dol + Kln + Ms/Bt + Ncr + Qz + Tnt-Fe | Quartz | Tennantite-(Fe), Chalcopyrite, Kaolinite | Chamosite, Musc./Biotite, Dolomite |
| ALG/GSEU/001 | Alu (vtg) + Ath (vtg) + Btl (vtg) + Phl (vtg) + Prl (vtg) + Py + Qz + Scd | Quartz | Pyrite | Scorodite |
| ALG/GSEU/002 | Chm + Cld (vtg) + Fau-Ca? (vtg) + Gth (vtg) + Hem (vtg) + Kln (vtg) + Mlc (vtg) + Ms/Bt + Qz | Quartz | Chamosite | Musc./Biotite |
| ALG/GSEU/003 | Chm + Fau-Ca? (vtg) + Gth (vtg) + Kln (vtg) + Ms/Bt (vtg) + Qz | Quartz | Chamosite | |
| ALG/GSEU/004 | Cld (vtg) + Gth (vtg) + Hem + Ilm (vtg) + Kln + Ms/Bt (vtg) + Qz | Quartz | Hematite, Kaolinite | |
| ALG/GSEU/004 Ore | Gth + Hem + Kln (vtg) + Ms/Bt (vtg) + Qz | Quartz | Hematite | Goethite |
| Algaré Ore | Py + Qz + Scd | Quartz, Pyrite | Scorodite |
| Sample Reference | Phase Identification | +++ | ++ | + |
|---|---|---|---|---|
| APAR/GSEU/001 | Cal + Dol + Gth + Mlc + Qz | Quartz | Calcite | Goethite, Malachite, Dolomite |
| APAR/GSEU/002 | Bir (vtg) + Ccp + Chm (vtg) + Dol + Ms/Bt (vtg) + Qz | Quartz, Dolomite | Chalcopyrite | |
| APAR/GSEU/003 | Bct (vtg) + Ccp + Chm (vtg) + Dol + Mlc (vtg) + Ms/Bt (vtg) + Qz | Quartz | Dolomite | Chalcopyrite |
| APAR/GSEU/004 | Ank (vtg) + Cal (vtg) + Cer (vtg) + Chm + Cpr (vtg) + Dol + Ms/Bt + Qz | Quartz | Dolomite | Chamosite, Muscovite/Biotite |
| APAR/GSEU/005 | Chm + Dol + Ms/Bt + Qz | Quartz | Dolomite | Chamosite, Muscovite/Biotite |
| APAR/GSEU/006 | Chm + Dol + Ms/Bt + Qz | Quartz | Dolomite | Chamosite, Muscovite/Biotite |
| APAR/GSEU/007 | Chm + Dol + Ms/Bt + Qz | Quartz | Dolomite, Chamosite, Muscovite/Biotite | |
| BRAN/GSEU/001 | Ab + An + Fa + Mag + Mul + Qz + Spl(1) + Spl(2) + Tns | Spinel(1), Anorthite, Albite | Magnetite, Spinel(2), Fayalite | Quartz,Mullite, Ternesite |
| BRAN/GSEU/002 | Ank + Dol + Gp + Kln + Mlc + Ms/Bt + Qz | Quartz | Dolomite, Gypsum, Ankerite | Muscovite/Biotite, Malachite, Kaolinite |
| BRAN/GSEU/003 | Ab (vtg) + Hem (vtg) + Mag + Mul + Qz | Quartz | Mullite, Magnetite | |
| Brancanes Ore | Ccp + Dol + Gp (vtg) + Qz + Tnt? (vtg) + Wwf (vtg) | Dolomite | Chalcopyrite, Quartz |
| Sample Reference | Phase Identification | +++ | ++ | + |
|---|---|---|---|---|
| FERR/GSEU/001 | Ab + Alm calcian + Cbz-Ca (vtg) + Chm + Dpt + Gnp (vtg) + Gp (vtg) + Hsm + Mnt (vtg) + Phl (vtg) + Qz + Rds | Rhodochrosite, Quartz | Albite | Dioptase, Hausmannite, Almandine calcian, Chamosite |
| FERR/GSEU/002 | Ab (vtg) + Alm calcian + Bir (vtg) + Fau-Na (vtg) + Ms/Bt (vtg) + Pyl + Qz + Sps | Quartz | Pyrolusite, Almandine calcian, Spessartine | |
| FERR/GSEU/003 | Ab (vtg) + Bir (vtg) + Chm (vtg) + Mnt (vtg) + Ms/Bt + Qz + Rt (vtg) | Quartz | Muscovite/Biotite | |
| FERR/GSEU/004 | Ab (vtg) + Alm calcian + Bir + Fau-Na + Mnt (vtg) + Ms/Bt (vtg) + Qz + Sps | Quartz | Faujasite-Na, Almandine calcian, Spessartine | Birnessite |
| CAST/GSEU/001 | Ab (vtg) + Bir (vtg) + Chm (vtg) + Mnn + Qz + Rds + Sid (vtg) | Rhodochrosite | Quartz, Manganite | |
| CAST/GSEU/002 | Ab (vtg) + Bir (vtg) + Chm + Clb-Fe? (vtg) + Cpr (vtg) + Hem (vtg) + Ms/Bt (vtg) + Py (vtg) + Pyc (vtg) + Qz + Rds + Rt (vtg) + Sd | Quartz | Chamosite | Rhodochrosite, Siderite |
| CAST/GSEU/003 | Ab (vtg) + Ank (vtg) + Bir (vtg) + Chm + Gh? (vtg) + Hem (vtg) + Qz + Rds + Sd + Whm? (vtg) | Chamosite | Quartz | Rhodochrosite, Siderite |
| CAST/GSEU/004 | Ab (vtg) + Bir (vtg) + Chm + Ms/Bt + Qz + Rt (vtg) | Quartz | Chamosite | Muscovite/Biotite |
| SERP/GSEU/001 Ore | Ab + Bob? (vtg) + Mog (vtg) + Pyl + Qz | Quartz | Albite, Pyrolusite | |
| BALA/GSEU/001 | Ab + Bnt + Hem + Ncr? (vtg) + Pyl + Qz (vtg) + Rds | Rhodochrosite, Braunite | Pyrolusite | Hematite, Albite |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Council of the European Communities. Resolution of the Council of the European Communities and of the Representatives of the Governments of the Member States Meeting Within the Council of May 1977 on the continuation and implementation of a European Community policy and action programme on the environment. Off. J. Eur. Commun. 1977, 139, 1–46. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A41977X0613 (accessed on 5 December 2024).
- European Commission. The Raw Materials Initiative—Meeting Our Critical Needs for Growth and Jobs in Europe, Communication from the Commission to the European Parliament and the Council, COM (2008) 699 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:52008DC0699 (accessed on 21 October 2024).
- European Commission. A Secure and Sustainable Supply of Critical Raw Materials in Support of the Twin Transition, Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, COM (2023) 165 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2023%3A165%3AFIN (accessed on 21 October 2024).
- Rizos, V.; Righetti, E. Low-carbon technologies and Russian imports: How far can recycling reduce the EU’s raw material dependency? CEPS Policy Insight 2022, 2022–17, 36180, Centre for European Policy Studies.. [Google Scholar]
- Righetti, E.; Rizos, V. The EU’s Quest for Strategic Raw Materials: What Role for Mining and Recycling? Intereconomics 2023, 58, 69–73. Available online: https://www.intereconomics.eu/pdf-download/year/2023/number/2/article/the-eu-s-quest-for-strategic-raw-materials-what-role-for-mining-and-recycling.html (accessed on 4 December 2024). [CrossRef]
- Hool, A.; Helbig, C.; Wierink, G. Challenges and opportunities of the European Critical Raw Materials Act. Miner. Econ. 2024, 37, 661–668. [Google Scholar] [CrossRef]
- Grohol, M.; Veeh, C.; European Commission. Study on the Critical Raw Materials for the EU: Final Report, Publications Office of the European Union. 2023. Available online: https://data.europa.eu/doi/10.2873/725585 (accessed on 10 September 2024).
- European Commission. Proposal for a Regulation of the European Parliament and of the Council Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) 168/2013, (EU) 2018/858, 2018/1724 and (EU) 2019/1020, COM/2023/160 Final. 2023. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52023PC0160 (accessed on 4 October 2024).
- Carvalho, J.; Diamantino, C.; Rosa, C.; Carvalho, E. Potential recovery of mineral resources from mining tailings of abandoned mines in Portugal. In Proceedings of the 3rd International Symposium on Enhanced Landfill Mining, Lisbon, Portugal, 8–10 February 2016; Pereira, M.J., Carvalho, M.T., Neves, P.F., Eds.; Instituto Superior Técnico: Lisbon, Portugal, 2016; pp. 501–516, ISBN 978-989-98342-4-8. [Google Scholar]
- Hu, X.; Yang, H.; Wu, F.; Fang, X.; Tan, K. Recovery of copper-dominated resources from copper mine drainage by chemical oxidation and sulfur biocycling: A pilot-scale study. J. Clean. Prod. 2022, 378, 134525. [Google Scholar] [CrossRef]
- Sánchez-Andrea, I.; Stams, A.J.M.; Weijma, J.; Contreras, P.G.; Dijkman, H.; Rozendal, R.A.; Johnson, D.B. A case in support of implementing innovative bio-processes in the metal mining industry. FEMS Microbiol. Lett. 2016, 363, fnw106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mohanty, S.; Ghosh, S.; Bal, B.; Das, A.P. A review of biotechnology processes applied for manganese recovery from wastes. Rev. Environ. Sci. Biotechnol. 2018, 17, 791–811. [Google Scholar] [CrossRef]
- Euro Manganese Inc. Chvaletice Manganese Project. 2024. Available online: https://www.mn25.ca/chvaletice-manganese-project (accessed on 23 September 2024).
- Neves, F.; Esperto, L.; Figueira, I.; Mascarenhas, J.; Salgueiro, R.; Silva, T.P.; Correia, J.B.; Carvalho, P.A.; de Oliveira, D. Mechanochemical synthesis of tetrahedrite materials using mixtures of synthetic and ore samples collected in the Portuguese zone of the Iberian Pyrite Belt. Miner. Eng. 2021, 164, 106833. [Google Scholar] [CrossRef]
- de Oliveira, D.P.S.; Filipe, A.; Gonçalves, P.; Santos, S.; Albardeiro, L. Critical Raw Materials Deposits Map of Mainland Portugal: New Mineral Intelligence in Cartographic Form. Cartog. J. 2021, 58, 222–232. [Google Scholar] [CrossRef]
- Tornos, F.; Inverno, C.M.C.; Casquet, C.; Mateus, A.; Ortiz, G.; Oliveira, O. The metallogenic evolution of the Ossa-Morena Zone. J. Iber. Geol. 2004, 30, 143–181. [Google Scholar]
- Sáez, R.; González, F.; Donaire, T.; Toscano, M.; Yesares, L.; de Almodóvar, G.R.; Moreno, C. Updating Geological Information about the Metallogenesis of the Iberian Pyrite Belt. Minerals 2024, 14, 860. [Google Scholar] [CrossRef]
- Díez-Montes, A.; Matos, J.X.; Dias, R.; Carmona, J.J.H.; Albardeiro, L.; Oliveira, J.T.; Morais, I.; Fernandes, P.; Inverno, C.; Machado, S.; et al. Geological Map of the South Portuguese Zone, Mapa Geológico de la Zona Surportuguesa/Carta Geológica da Zona Sul Portuguesa, Escala 1/400 000. Proj. Geo-FPI/Interreg POCTEP; Instituto Geológico y Minero de España/LNEG/Junta de Andalucía-SGIEM/CM Aljustrel. 2020. Available online: https://info.igme.es/geofpi/docs/mapas/GEOLOGICO_400K_ZSP_2020.pdf (accessed on 10 December 2024).
- Piçarra, J.M. Estudo Estratigráfico do Sector de Estremoz—Barrancos, Zona de Ossa Morena, Portugal. Ph.D. Thesis, University of Évora, Évora, Portugal, 2000. Volume I and II, 268p. [Google Scholar]
- Gaspar, O.C. O Jazigo de Cobre de Aparis. Est. Not. Trab. SFM 1968, 18, 253–290. [Google Scholar]
- Matos, J.X.; Rosa, C. Diagnóstico Preliminar de Minas Abandonadas—Área Sul. In Internal IGM Report; IGM Eds.: Lisbon, Portugal, 2001; 276p. [Google Scholar]
- Matos, J.X.; Martins, L.; Rosa, C. Parque Mineiro da Cova dos Mouros—IGM: Contribution for the sustainable development of the mining park. IGME Pub. Mus. Geom. 2003, 2, 487–494. [Google Scholar]
- Reiser, F.K.M.; Rosa, D.R.N.; Pinto, Á.M.M.; Carvalho, J.R.S.; Matos, J.X.; Guimarães, F.M.G.; Alves, L.C.; de Oliveira, D.P.S. Mineralogy and geochemistry of tin- and germanium-bearing copper ore, Barrigão re-mobilized vein deposit, Iberian Pyrite Belt, Portugal. Int. Geol. Rev. 2011, 53, 1212–1238. [Google Scholar] [CrossRef]
- Matos, J.X. Recursos geológicos—Minérios metálicos. In Notícia Explicativa da Folha 46D Mértola; Oliveira, J.T., Silva, J.B., Eds.; Dep. Geologia INETI: Lisbon, Portugal, 2007; pp. 28–34. [Google Scholar]
- Sáez, R.; Pascual, E.; Toscano, M.; Almodóvar, G. The Iberian type of volcano-sedimentary massive sulphide deposits. Mineral. Depos. 1999, 34, 549–570. [Google Scholar] [CrossRef]
- Matos, J.X.; Martins, L.P.; Oliveira, J.T.; Pereira, Z.; Batista, M.J.; Quental, L. Rota da pirite no sector português da Faixa Piritosa Ibérica, desafios para um desenvolvimento sustentado do turismo geológico e mineiro. In Rutas Minerales en Iberoamérica; Carrion, P., Ed.; Esc. Sup. Politécnica del Litoral: Guayaquil, Equador, 2008; pp. 136–155. [Google Scholar]
- Oliveira, J.T.; Relvas, J.; Pereira, Z.; Matos, J.X.; Rosa, C.; Rosa, D.; Munhá, J.M.; Fernandes, P.; Jorge, R.; Pinto, A. Geologia da Zona Sul Portuguesa, com ênfase na estratigrafia e na vulcanologia física, geoquímica e mineralizações da Faixa Piritosa. In Geologia de Portugal Vol. I—Geologia Pré-Mesozóica de Portugal; Dias, R., Araújo, A., Terrinha, P., Kullberg, J., Eds.; Escolar Editora: Lisbon, Portugal, 2013; pp. 673–767. [Google Scholar]
- Pinedo Vara, I. Piritas de Huelva. Su Historia, Minería y Aprovechamiento; Edit. Summa: Madrid, Spain, 1963; 1003p. [Google Scholar]
- Fernandes, C. Jazigos de Mn do Alentejo. Breve estudo das minas de Mn do concelho de Castro Verde. In Internal IGM Report; IGM Eds.: Lisbon, Portugal, 1947. [Google Scholar]
- Goinhas, F. Minas de Manganês do Baixo Alentejo. Internal Report from DGGM; DGGM Eds.: Lisbon, Portugal, 1986. [Google Scholar]
- Silva, F. Géologie et génese des gisements de Manganese du Baixo Alentejo Portugal. Est. Not. Trab. SFM 1956, XI, 28–66. [Google Scholar]
- Lohmeier, S.; Gallhofer, D.; Lottermoser, B.G. Field-portable X-ray fluorescence analyzer for chemical characterization of carbonate-bearing base metal tailings: Case study from Namib Pb-Zn Mine, Namibia. J. South. Afr. Inst. Min. Metall. 2024, 124, 421–436. [Google Scholar] [CrossRef]
- Figueiredo, M.O.; Silva, T.P.; Veiga, J.P.; de Oliveira, D.; Batista, M.J. Towards the recovery of by-product metals from mine wastes: An X-ray absorption spectroscopy study on the binding state of rhenium in debris from a centennial Iberian Pyrite Belt mine. J. Miner. Mater. Charact. Eng. 2014, 2, 135–143. [Google Scholar] [CrossRef][Green Version]
- de Oliveira, D.; Gonçalves, P.; Morais, I.; Silva, T.P.; Matos, J.X.; Albardeiro, L.; Filipe, A.; Batista, M.J.; Santos, S.; Fernandes, J. Unlocking the secondary critical raw material potential of historical mine sites, Lousal Mine, southern Portugal. Minerals 2024, 14, 127. [Google Scholar] [CrossRef]
- Yaroshevsky, A.A. Abundances of Chemical Elements in the Earth’s Crust. Geochem. Int. 2006, 44, 48–55. [Google Scholar] [CrossRef]
- Abreu, M.M.; Matias, M.J.; Magalhães, M.C.F.; Basto, M.J. Impacts on water, soil and plants from the abandoned Miguel Vacas copper mine, Portugal. J. Geochem. Explor. 2008, 96, 161–170. [Google Scholar] [CrossRef][Green Version]
- De Oliveira, D.; Salgueiro, R.; Silva, T.P.; Reiser, F.; Guimarães, F.; Neves, F. The Barrigão copper deposit: Tennantite-tetrahedrite for thermoelectric and high-technology applications. In Extended Abstracts Boock of the XII Congresso Ibérico de Geoquímica and XX Semana da Geoquímica, Évora, Portugal, 22–26 September 2019; Nogueira, P., Moreira, N., Roseiro, J., Maia, M., Eds.; University of Évora: Évora, Portugal, 2019; pp. 255–258. ISBN 978-972-778-121-8. [Google Scholar]
- Tabelin, C.B.; Corpuz, R.D.; Igarashi, T.; Villacorte-Tabelin, M.; Ito, M.; Hiroyoshi, N. Hematite-catalysed scorodite formation as a novel arsenic immobilisation strategy under ambient conditions. Chemosphere 2019, 233, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Warr, L.N. IMA–CNMNC approved mineral symbols. Mineral. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Moreira, B.; Figueiras, J.; Mateus, A.; Rodrigues, P.; Jorge, R.; Gonçalves, L. A new manganese mineralisation type in the Iberian Pyrite Belt? In Abstract Book of the X Congresso Ibérico de Geoquímica—XVIII Semana de Geoquímica, Lisbon, Portugal, 19–23 October 2015; LNEG, Eds.: Lisbon, Portugal, 2015; pp. 133–136. ISBN 978-989-675-039-8. Available online: https://www.researchgate.net/publication/320945506 (accessed on 10 December 2024).


| Sample Reference | Mine Name | Geotectonic Zone | Mine Type | Material Size | Sample Type | Sample Description |
|---|---|---|---|---|---|---|
| LOUS/001 | Lousal | South Portuguese Zone—Iberian Pyrite Belt | VMS | M | Processing | Crushed ore (mainly pyrite) with clasts of quartz. Oxidation cap with 5 cm |
| LOUS/002 | C | Dump composite | Blocks of mineralization host rocks (felsic volcanic rocks, shales, and quartz) | |||
| LOUS/003 | F | Processing | Tailings inside the acid lagoons. Material very fine, composed of neoformation minerals | |||
| LOUS/004 | C | Dump composite | Blocks of mineralization host rocks (essentially shales) | |||
| LOUS/006 | C | Dump composite | Blocks of mineralization host rocks (essentially shales). Occasionally massive pyrite blocks | |||
| LOUS/007 | C | Dump composite | Blocks of mineralization host rocks (essentially shales and volcanic rocks). Occasionally massive pyrite blocks. Neoformation minerals | |||
| LOUS/008 | F | Processing | Tailings inside the acid lagoons. Material very fine, composed of neoformation minerals | |||
| LOUS/009 | M | Processing | Crushed pyrite with blocks of host rocks (volcanic rocks and shales). Neoformation minerals (sulfates) | |||
| LOUS/010 | C | Processing | Shales with fine pyrite associated. Quartz and neoformation minerals | |||
| LOUS/011 | C | Dump composite | Shales with pyrite blocks. Neoformation minerals | |||
| LOUS/012 | C | Dump composite | Pyrite blocks with rare volcanic rocks and quartz. Neoformation minerals | |||
| LOUS/013 | C | Dump composite | Shales with pyrite blocks | |||
| LOUS/014 | C | Processing | Crushed pyrite | |||
| PORT/001 | Porteirinhos | Cu veins | C | Dump composite | Shales and graywackes with blocks of quartz (pyrite, chalcopyrite, and malachite | |
| PORT/001 Ore | C | Ore | Quartz with primary sulfides | |||
| BARR/001 | Barrigão | C | Dump composite | Shales and graywackes with fine material composed of host rock crushed and cooper neoformation minerals | ||
| BARR/002 | C | Roasting | Slags probably from 19th century. Blocks of coal are observed | |||
| BARR/003 | C | Ore | Ore blocks with host rocks associated | |||
| ALG/001 | Algaré | M | Dump composite | Crushed pyrite with clast of host rocks | ||
| Algaré Ore | C | Ore | Massive pyrite | |||
| ALG/002 | M | Dump composite | Host rocks (shales and volcanic rocks) with neoformation minerals (malachite) | |||
| ALG/003 | C | Processing | Blocks of volcanic rocks, shales, and quartzites with oxidized sulfides | |||
| ALG/004 | C | Dump composite | Quartzites and volcanic rocks with iron and manganese oxides | |||
| ALG/004 Ore | C | Dump composite | Iron and manganese oxides | |||
| BRAN/001 | Brancanes | M | Roasting | Slags probably from 19th century. Blocks of coal are observed | ||
| BRAN/002 | F | Processing | Post flotation tailings | |||
| BRAN/003 | M | Roasting | Slags probably from 19th century. Blocks of host rocks | |||
| Brancanes Ore | C | Ore | Quartz and carbonate veins with sulfides (chalcopyrite, tetrahedrite) | |||
| CMOUR/001 Ore | Cova dos Mouros | C | Dump composite | Volcanic rocks, quartz, and cherts with Fe and Mn concretions | ||
| APAR/001 | Aparis | Ossa Morena Zone | Cu veins | C | Dump composite | Quartz blocks and host rocks (shales). Copper neoformation minerals |
| APAR/002 | C | Dump composite | Quartz blocks and shales. Sulfides impregnation in the quartz | |||
| APAR/003 | C | Dump composite | Quartz blocks with copper neoformation minerals | |||
| APAR/004 | F | Processing | Post flotation tailings | |||
| APAR/005 | F | Processing | Post flotation tailings | |||
| APAR/006 | F | Processing | Post flotation tailings | |||
| APAR/007 | F | Processing | Post flotation tailings | |||
| FERR/001 | Ferragudo | South Portuguese Zone—Iberian Pyrite Belt | Fe-Mn lens | M | Processing | Crushed material composed of iron and manganese oxides |
| FERR/002 | F | Processing | Black material composed of crushed ore | |||
| FERR/003 | C | Dump composite | Blocks of host rocks (shales) and cherts with Fe and Mn impregnation | |||
| FERR/004 | M | Processing | Black material composed of crushed ore | |||
| CAST/001 | Ferrarias and Castelo | C | Dump composite | Jaspers and cherts with iron and manganese mineralization. Pyrite associated with chert facies | ||
| CAST/002 | C | Dump composite | Iron and manganese oxides with pyrite | |||
| CAST/003 | C | Dump composite | Jaspers and cherts with iron and manganese mineralization. Pyrite associated with chert facies | |||
| CAST/004 | C | Dump composite | Shales with iron and manganese impregnation | |||
| SERP/001 Ore | Serpe | C | Dump composite | Jaspers and cherts block with iron and manganese mineralization | ||
| BALA/001 | Balança | C | Dump composite | Jaspers and cherts block with iron and manganese mineralization |
| Sample Reference | Sb | As | Bi | Co | Cu | Ga | Ge | Hf | HREE | Li | LREE | Mg | Mn | Ni | Nb | Sc | Sr | W | V |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PORT/GSEU/001 | X | ||||||||||||||||||
| PORT/GSEU/001 Ore | X | X | X | X | X | X | X | ||||||||||||
| CMOUR/GSEU/001 Ore | X | ||||||||||||||||||
| BARR/GSEU/001 | X | X | X | X | |||||||||||||||
| BARR/GSEU/002 | X | ||||||||||||||||||
| BARR/GSEU/003 | X | X | X | X | X | X | X | X | |||||||||||
| ALG/GSEU/001 | X | X | |||||||||||||||||
| ALG/GSEU/002 | X | X | |||||||||||||||||
| ALG/GSEU/003 | X | ||||||||||||||||||
| ALG/GSEU/004 | X | ||||||||||||||||||
| ALG/GSEU/004 Ore | X | X | X | X | X | X | |||||||||||||
| Algaré Ore | |||||||||||||||||||
| APAR/GSEU/001 | X | ||||||||||||||||||
| APAR/GSEU/002 | X | ||||||||||||||||||
| APAR/GSEU/003 | X | ||||||||||||||||||
| APAR/GSEU/004 | X | X | X | ||||||||||||||||
| APAR/GSEU/005 | |||||||||||||||||||
| APAR/GSEU/007 | |||||||||||||||||||
| BRAN/GSEU/001 | X | ||||||||||||||||||
| BRAN/GSEU/002 | X | X | X | X | X | X | X | ||||||||||||
| BRAN/GSEU/003 | X | X | X | ||||||||||||||||
| Brancanes Ore | X | X | X | X | X | X | |||||||||||||
| LOUS/GSEU/001 | X | X | X | ||||||||||||||||
| LOUS/GSEU/002 | X | ||||||||||||||||||
| LOUS/GSEU/003 | |||||||||||||||||||
| LOUS/GSEU/004 | X | X | X | X | X | X | X | ||||||||||||
| LOUS/GSEU/006 | |||||||||||||||||||
| LOUS/GSEU/007 | |||||||||||||||||||
| LOUS/GSEU/008 | |||||||||||||||||||
| LOUS/GSEU/009 | |||||||||||||||||||
| LOUS/GSEU/010 | |||||||||||||||||||
| LOUS/GSEU/011 | |||||||||||||||||||
| LOUS/GSEU/012 | |||||||||||||||||||
| LOUS/GSEU/013 | |||||||||||||||||||
| LOUS/GSEU/014 | X | ||||||||||||||||||
| FERR/GSEU/001 | X | X | X | X | X | ||||||||||||||
| FERR/GSEU/002 | X | X | X | X | X | X | X | X | |||||||||||
| FERR/GSEU/003 | X | ||||||||||||||||||
| FERR/GSEU/004 | X | ||||||||||||||||||
| CAST/GSEU/001 | X | X | |||||||||||||||||
| CAST/GSEU/002 | X | X | X | X | X | ||||||||||||||
| CAST/GSEU/003 | X | X | |||||||||||||||||
| CAST/GSEU/004 | X | X | |||||||||||||||||
| SERP/GSEU/001 Ore | X | X | X | X | X | ||||||||||||||
| BALA/GSEU/001PORT1 | X | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, D.P.S.; Silva, T.P.; Morais, I.; Fernandes, J.A.E. Chemical and Mineralogical Characterization of Waste from Abandoned Copper and Manganese Mines in the Iberian Pyrite Belt, Portugal: A First Step Towards the Waste-to-Value Recycling Process. Minerals 2025, 15, 58. https://doi.org/10.3390/min15010058
de Oliveira DPS, Silva TP, Morais I, Fernandes JAE. Chemical and Mineralogical Characterization of Waste from Abandoned Copper and Manganese Mines in the Iberian Pyrite Belt, Portugal: A First Step Towards the Waste-to-Value Recycling Process. Minerals. 2025; 15(1):58. https://doi.org/10.3390/min15010058
Chicago/Turabian Stylede Oliveira, Daniel P. S., Teresa P. Silva, Igor Morais, and João A. E. Fernandes. 2025. "Chemical and Mineralogical Characterization of Waste from Abandoned Copper and Manganese Mines in the Iberian Pyrite Belt, Portugal: A First Step Towards the Waste-to-Value Recycling Process" Minerals 15, no. 1: 58. https://doi.org/10.3390/min15010058
APA Stylede Oliveira, D. P. S., Silva, T. P., Morais, I., & Fernandes, J. A. E. (2025). Chemical and Mineralogical Characterization of Waste from Abandoned Copper and Manganese Mines in the Iberian Pyrite Belt, Portugal: A First Step Towards the Waste-to-Value Recycling Process. Minerals, 15(1), 58. https://doi.org/10.3390/min15010058








