Study on Impurity Removal from Lepidolite Leaching Solution and the Extraction Process of Rubidium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction and Stripping Experiments
3. Results
3.1. Extraction of Iron and Aluminum
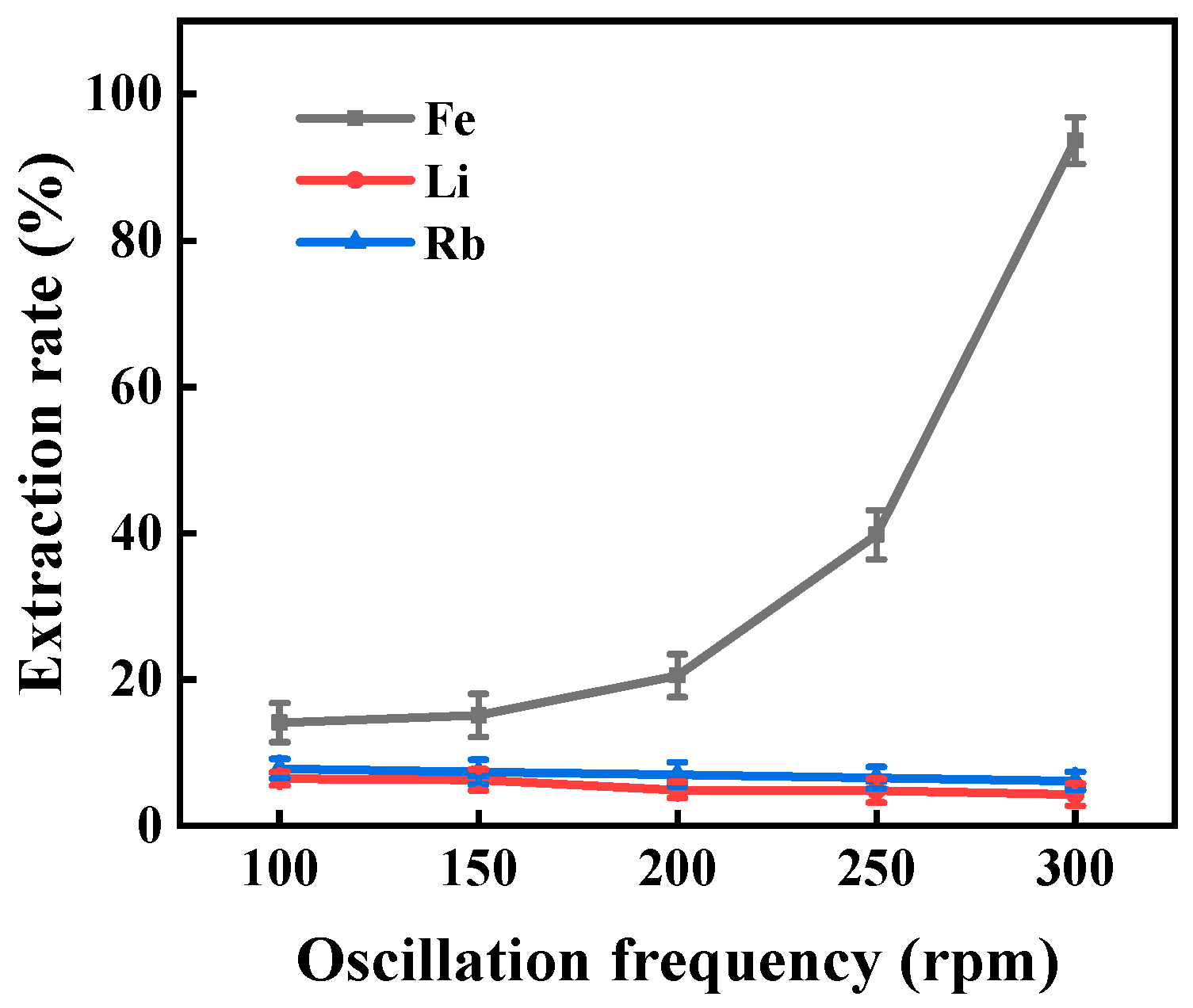
3.1.1. Oscillation Frequency
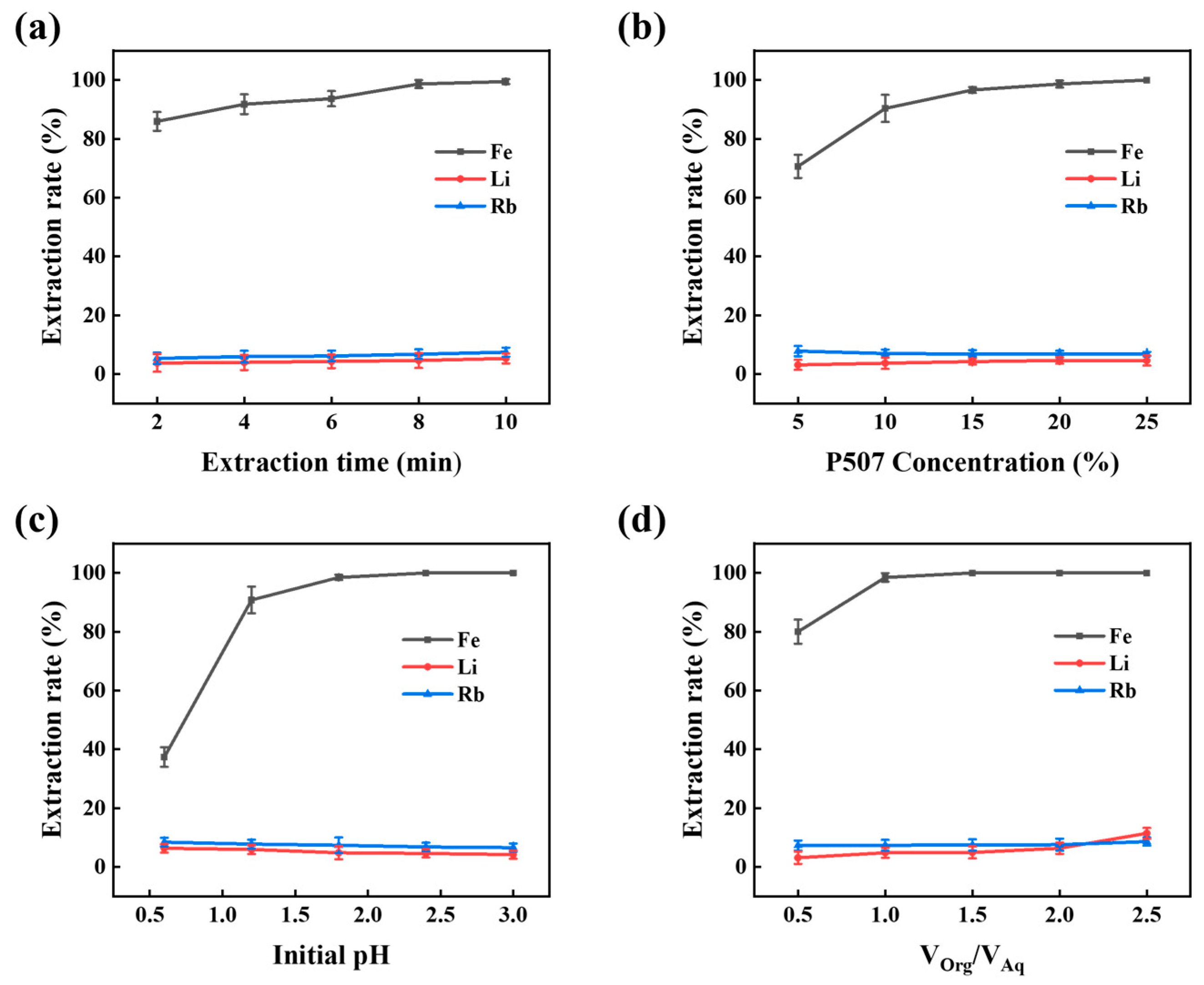
3.1.2. Extraction Time
3.1.3. Extractant Concentration
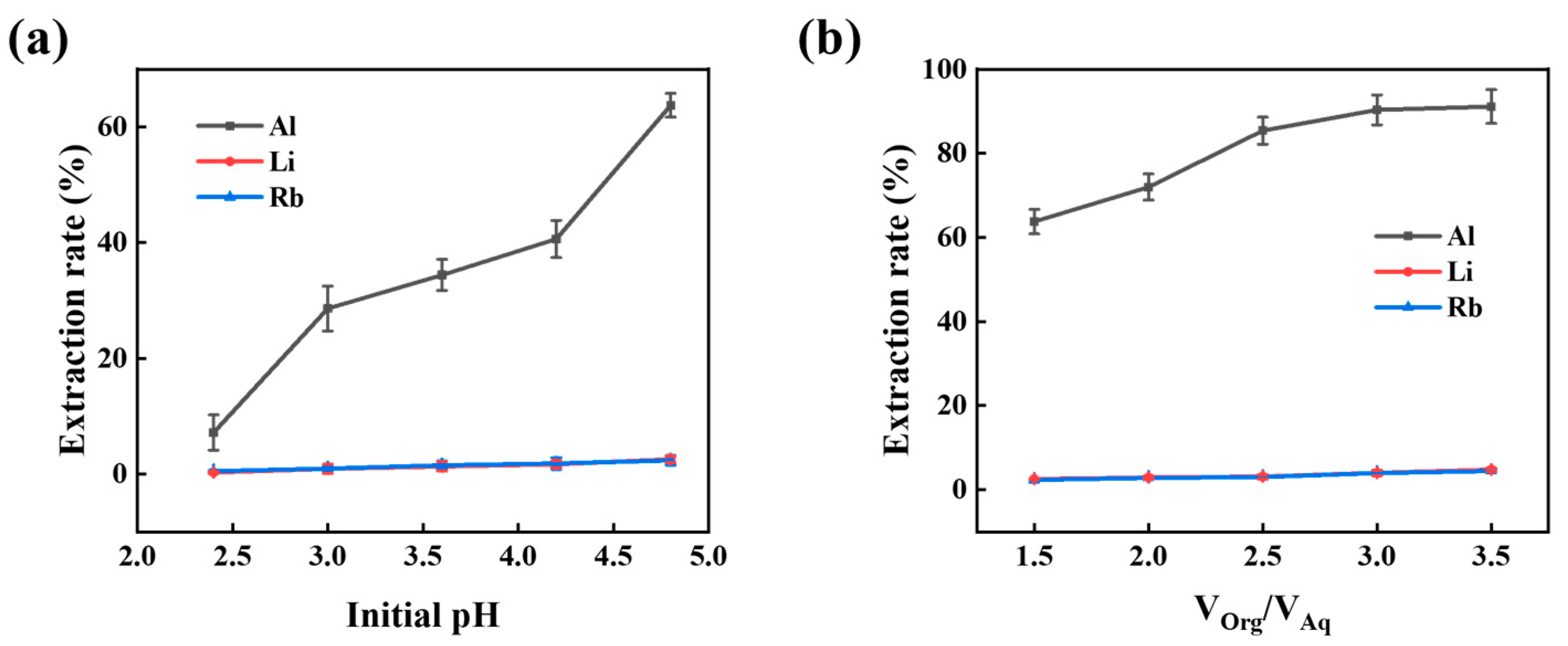
3.1.4. Initial pH Value
3.1.5. O/A Ratio
3.1.6. Two-Stage Extraction of Aluminum
3.2. Stripping of Iron and Aluminum
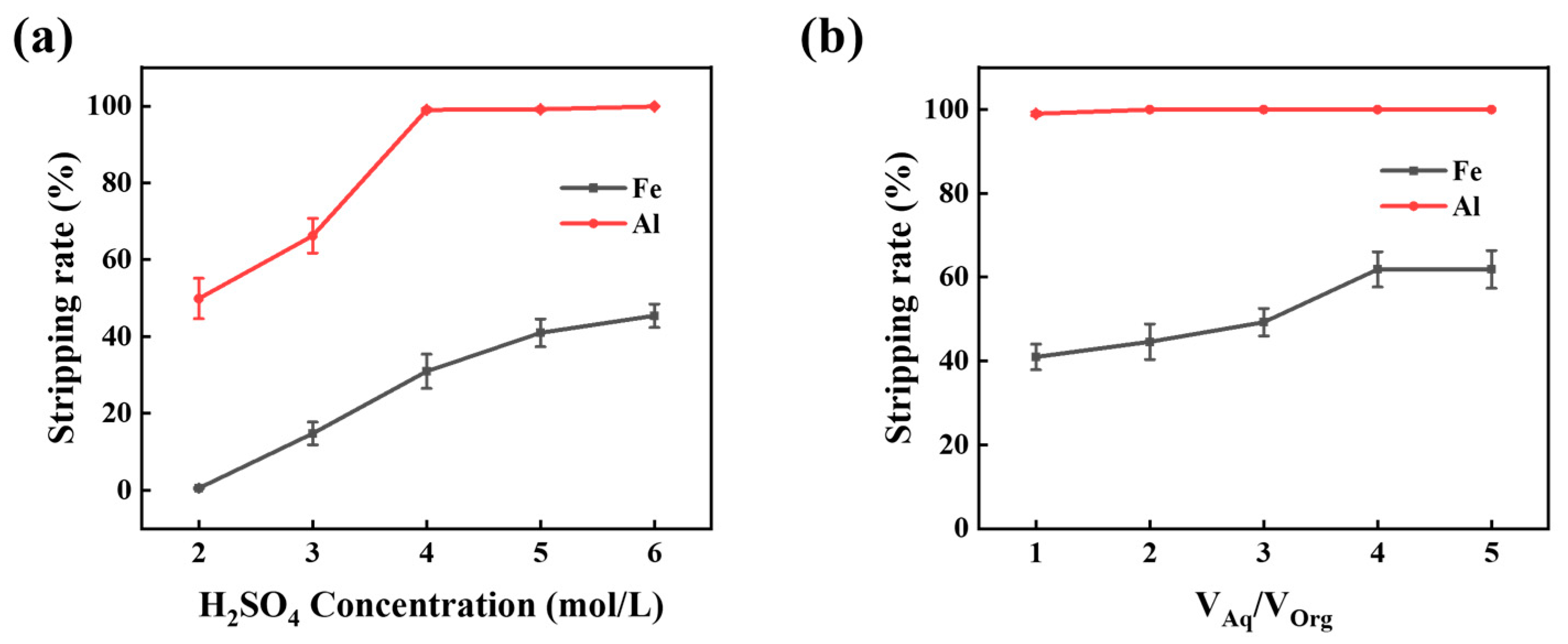
3.2.1. Sulfuric Acid Concentration and A/O Ratio
3.2.2. Stripping of Fe3+ Enhanced by Ascorbic Acid
3.3. Extraction of Rb with T-BAMBP
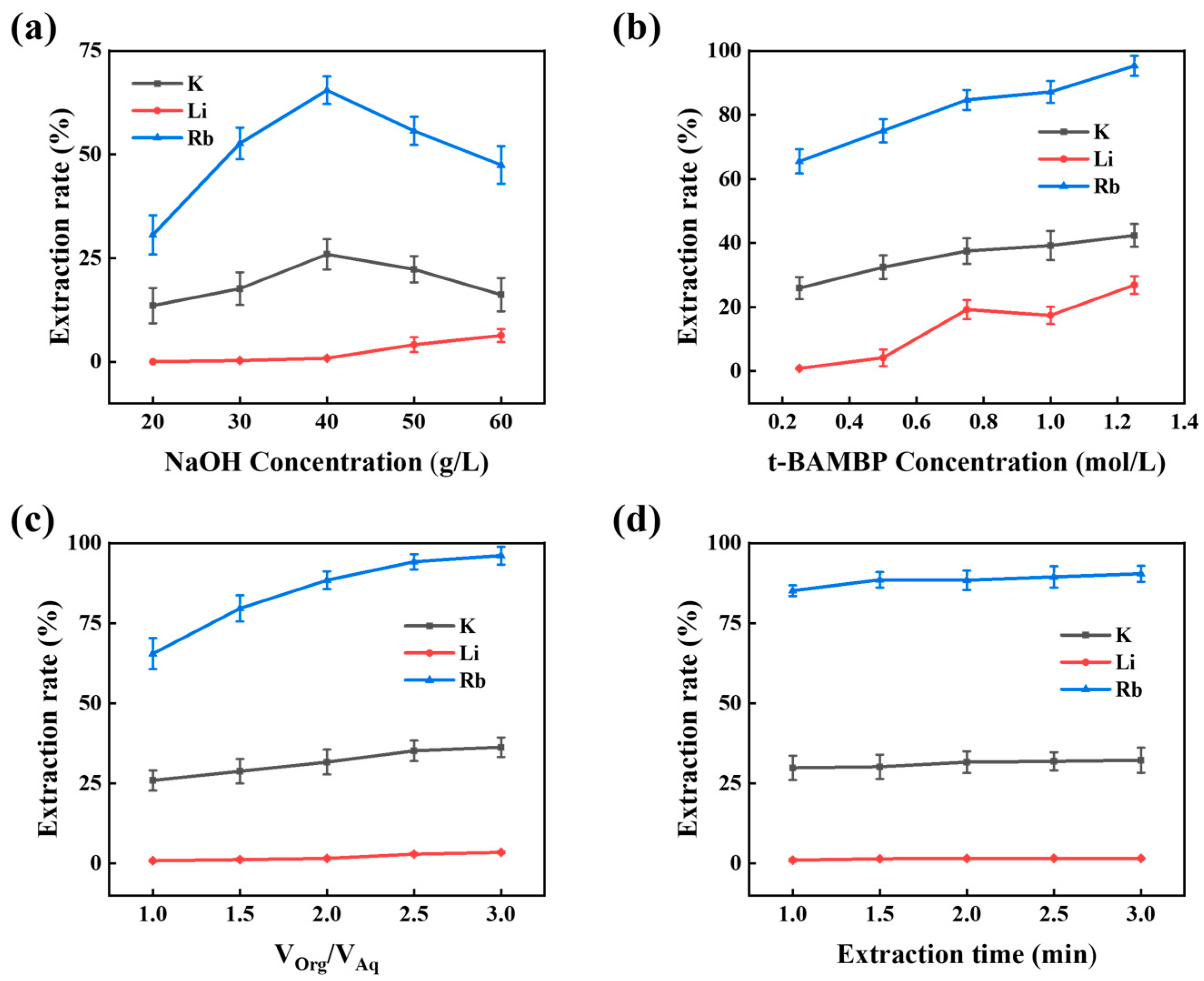
3.3.1. Effect of NaOH Concentration on Rubidium Extraction
3.3.2. Effect of t-BAMBP Concentration on Rubidium Extraction
3.3.3. Effect of O/A Ratio on Rubidium Extraction
3.3.4. Effect of Extraction Time on Rubidium Extraction
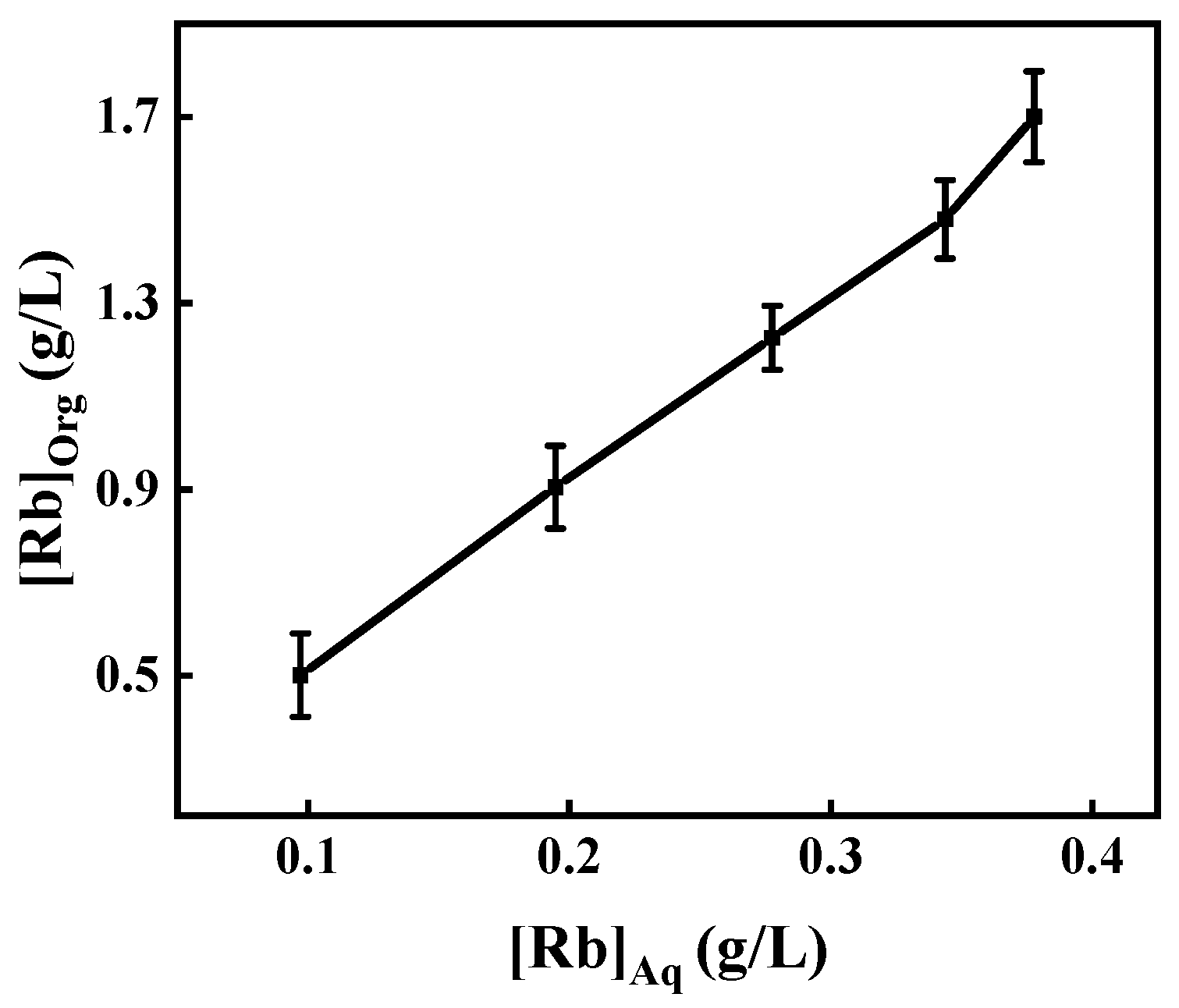
3.3.5. Extraction Equilibrium Isotherms of Rubidium
3.4. Stripping of Rubidium from Organic Phase
3.4.1. Effect of Washing Water pH on the Washing Rate of Metal Ions
3.4.2. Effect of H2SO4 Concentration on the Stripping Rate of Rubidium
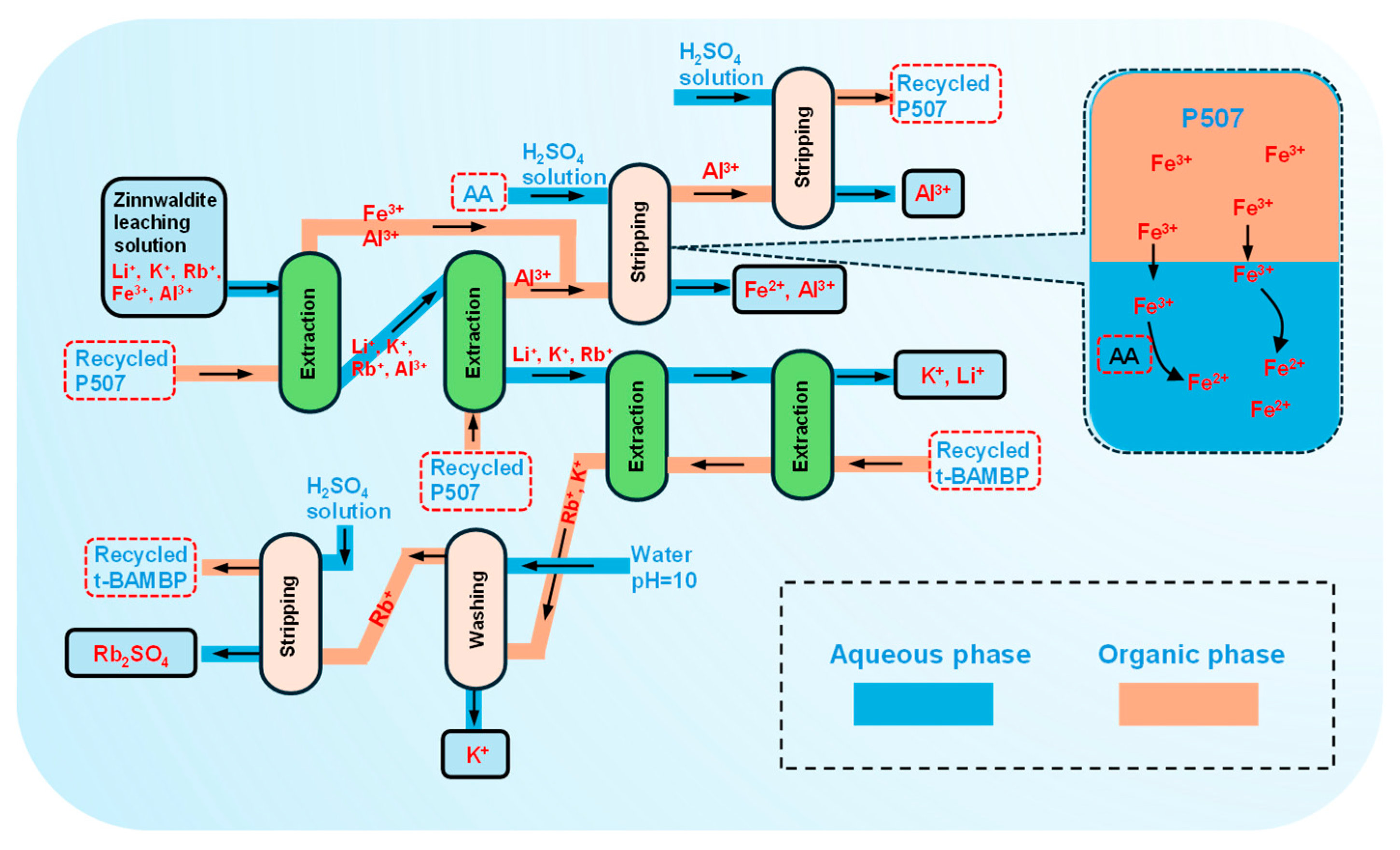
3.5. Scale-Up Experiment
4. Conclusions
- In this study, we removed iron and aluminum impurities and separated rubidium from the leaching solution of zinnwaldite using an extraction process. Specifically, using P507 extractant and sulfonated kerosene as the organic phase, under optimal conditions, a single-stage extraction removed 99.99% of the iron, and a two-stage extraction removed 91.71% of the aluminum. For the purified solution, t-BAMBP was used as the extractant, and under optimal conditions, a two-stage counter-current extraction achieved a 99.99% extraction of rubidium. These processes were validated through comprehensive scale-up tests. As a result, the removal rates of iron and aluminum exceeded 99.99%, and the total recovery rate of rubidium reached 88.53%.
- For the recycling of the extractants, both aluminum and rubidium can be stripped from the organic phase back into the aqueous phase using a simple sulfuric acid process. To address the difficulty of stripping iron from the P507 organic phase, we developed a novel reduction stripping process. Ascorbic acid was used to reduce Fe3⁺ in the aqueous phase to Fe2⁺, breaking the distribution equilibrium of Fe3⁺ between the organic and aqueous phases, thus promoting the transfer of iron from the organic phase to the aqueous phase. After a one-step reduction stripping, 99.99% of the iron was removed from the organic phase. We hope this study provides new insights into the efficient extraction and separation of lithium and associated rubidium resources.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kelly, J.C.; Wang, M.; Dai, Q.; Olumide, W. Energy, Greenhouse Gas, and Water Life Cycle Analysis of Lithium Carbonate and Lithium Hydroxide Monohydrate from Brine and Ore Resources and Their Use in Lithium Ion Battery Cathodes and Lithium Ion Batteries. Resour. Conserv. Recycl. 2021, 174, 105762. [Google Scholar] [CrossRef]
- Shehzad, K.; Zaman, U.; Zaman, B.U.; Liu, X.; Jafri, R.A. Lithium Production, Electricity Consumption, and Greenhouse Gas Emissions: An Imperious Role of Economic Globalization. J. Clean. Prod. 2022, 372, 133689. [Google Scholar] [CrossRef]
- Gu, G.; Gao, T. Sustainable Production of Lithium Salts Extraction from Ores in China: Cleaner Production Assessment. Resour. Policy 2021, 74, 102261. [Google Scholar] [CrossRef]
- Huan, L.; Jacques, E.; Kuang, G. Recovery of Lithium from Mineral Resources: State-of-the-Art and Perspectives—A Review. Hydrometallurgy 2019, 189, 105129. [Google Scholar] [CrossRef]
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Everson, M.P.; Wallington, T.J. Global Lithium Resources: Relative Importance of Pegmatite, Brine and Other Deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Lars, R.; Gunther, M.; Martin, B.; Michael, H. Lithium Extracting from Zinnwaldite: Economical Comparison of an Adapted Spodumene and a Direct-carbonation Process. Chem. Eng. Technol. 2018, 41, 975–982. [Google Scholar] [CrossRef]
- Peng, X.; Chengyan, W.; Yongqiang, C.; Baozhong, M. Rubidium Extraction from Mineral and Brine Resources: A Review. Hydrometallurgy 2021, 203, 105644. [Google Scholar] [CrossRef]
- Martin, G.; Schneider, A.; Voigt, W.; Bertau, M. Lithium Extraction from the Mineral Zinnwaldite: Part II: Lithium Carbonate Recovery by Direct Carbonation of Sintered Zinnwaldite Concentrate. Miner. Eng. 2017, 110, 75–81. [Google Scholar] [CrossRef]
- Hien-Dinh, T.T.; Luong, V.T.; Gieré, R.; Tran, T. Extraction of Lithium from Lepidolite via Iron Sulphide Roasting and Water Leaching. Hydrometallurgy 2015, 153, 154–159. [Google Scholar] [CrossRef]
- Guo, H.; Kuang, G.; Wan, H.; Yang, Y.; Yu, H.; Wang, H. Enhanced Acid Treatment to Extract Lithium from Lepidolite with a Fluorine-Based Chemical Method. Hydrometallurgy 2019, 183, 9–19. [Google Scholar] [CrossRef]
- Kristianová, E.; Vu, N.H.; Dvořák, P.; Tomaško, T.; Vaculíková, L. Extraction of Li, K, and Rb from Zinnwaldite by Chlorination Roasting with CaCl2. Miner. Eng. 2023, 204, 108420. [Google Scholar] [CrossRef]
- Zhong, S.; Liang, D.; Weng, W.; Chi, X.; Zhang, W.; Tan, W. Efficient Extraction of Li and Rb from Zinnwaldite via Thermal Activation and Acid Leaching. Miner. Eng. 2024, 216, 108898. [Google Scholar] [CrossRef]
- Adam, B.; Norman, K.; Toni, H.; Christina, M.; Markus Andreas, R. Separation of Aluminum and Iron from Lanthanum—A Comparative Study of Solvent Extraction and Hydrolysis-Precipitation. Minerals 2020, 10, 556. [Google Scholar] [CrossRef]
- Martin, G.; Pätzold, C.; Bertau, M. Integrated Process for Lithium Recovery from Zinnwaldite. Int. J. Miner. Process. 2017, 160, 8–15. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Y.; Li, B.; Duan, P.; Ren, Z.; Zhou, Z. Selective Leaching and Recovery of Neodymium from NdFeB Carbonyl Residues. Sep. Purif. Technol. 2024, 329, 125137. [Google Scholar] [CrossRef]
- Yang, L.; Liu, R.; Li, H.; Luo, J.; Zhao, Y. Experimental Study on the Mechanism and Thermodynamics of Extraction of Fe3+ from Phosphoric Acid Solution. Chem. Eng. Res. Des. 2024, 206, 151–162. [Google Scholar] [CrossRef]
- Han, Y.; Chen, J.; Deng, Y.; Liu, T.; Li, H. A Leaching, Solvent Extraction, Stripping, Precipitation and Calcination Process for the Recovery of MoO3 and NiO from Spent Hydrofining Catalysts. Hydrometallurgy 2023, 218, 106046. [Google Scholar] [CrossRef]
- Salman, A.D.; Juzsakova, T.; Jalhoom, M.G.; Abdullah, T.A.; Le, P.-C.; Viktor, S.; Domokos, E.; Nguyen, X.C.; La, D.D.; Nadda, A.K.; et al. A Selective Hydrometallurgical Method for Scandium Recovery from a Real Red Mud Leachate: A Comparative Study. Environ. Pollut. 2022, 308, 119596. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhou, S.; Wang, M.; Wang, X. Stripping of Fe(III) from P204 by Oxalic Acid. In Rare Metal Technology 2016; Shafiq, A., Kim, H., Neelameggham, N.R., Ouchi, T., Oosterhof, H., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 175–181. ISBN 978-3-319-48135-7. [Google Scholar]
- Sinha, M.K.; Purcell, W.; Van Der Westhuizen, W.A. Recovery of Manganese from Ferruginous Manganese Ore Using Ascorbic Acid as Reducing Agent. Miner. Eng. 2020, 154, 106406. [Google Scholar] [CrossRef]

| Chemical Composition | Fe | Al | Li | Rb |
|---|---|---|---|---|
| Concentration (g/L) | 1.65 | 4.40 | 0.26 | 0.14 |
| Add Ascorbic Acid | A/O | Stripping Rate of Iron (%) | Stripping Rate of Aluminum (%) |
|---|---|---|---|
| No | 4 | 61.85 | 99.99 |
| Yes | 2 | 66.57 | 99.99 |
| Yes | 3 | 70.18 | 99.99 |
| Yes | 4 | 99.99 | 99.99 |
| Metal Ion | Fe | Al | Li | Rb | K |
|---|---|---|---|---|---|
| Original leaching solution (g/L) | 5.53 | 10.33 | 0.84 | 0.63 | 29.26 |
| Purified solution (g/L) | / | / | 0.81 | 0.60 | 28.48 |
| Proportion (%) | <0.001 | <0.001 | 96.10 | 93.84 | 97.33 |
| Metal Ion | Fe | Al | Li | Rb | K |
|---|---|---|---|---|---|
| Original leaching solution (g/L) | 5.53 | 10.33 | 0.84 | 0.63 | 29.27 |
| Rb2SO4 solution (g/L) | / | / | 0.021 | 0.59 | 1.67 |
| Proportion (%) | <0.001 | <0.001 | 0.25 | 94.34 | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, W.; Yang, Y.; Liang, D.; Weng, W.; Chi, X.; Zhong, S. Study on Impurity Removal from Lepidolite Leaching Solution and the Extraction Process of Rubidium. Minerals 2025, 15, 19. https://doi.org/10.3390/min15010019
Tan W, Yang Y, Liang D, Weng W, Chi X, Zhong S. Study on Impurity Removal from Lepidolite Leaching Solution and the Extraction Process of Rubidium. Minerals. 2025; 15(1):19. https://doi.org/10.3390/min15010019
Chicago/Turabian StyleTan, Wen, Yanbo Yang, Donghui Liang, Wei Weng, Xiaopeng Chi, and Shuiping Zhong. 2025. "Study on Impurity Removal from Lepidolite Leaching Solution and the Extraction Process of Rubidium" Minerals 15, no. 1: 19. https://doi.org/10.3390/min15010019
APA StyleTan, W., Yang, Y., Liang, D., Weng, W., Chi, X., & Zhong, S. (2025). Study on Impurity Removal from Lepidolite Leaching Solution and the Extraction Process of Rubidium. Minerals, 15(1), 19. https://doi.org/10.3390/min15010019






