An Overview of Thermochemical Reduction Processes for Titanium Production
Abstract
1. Introduction
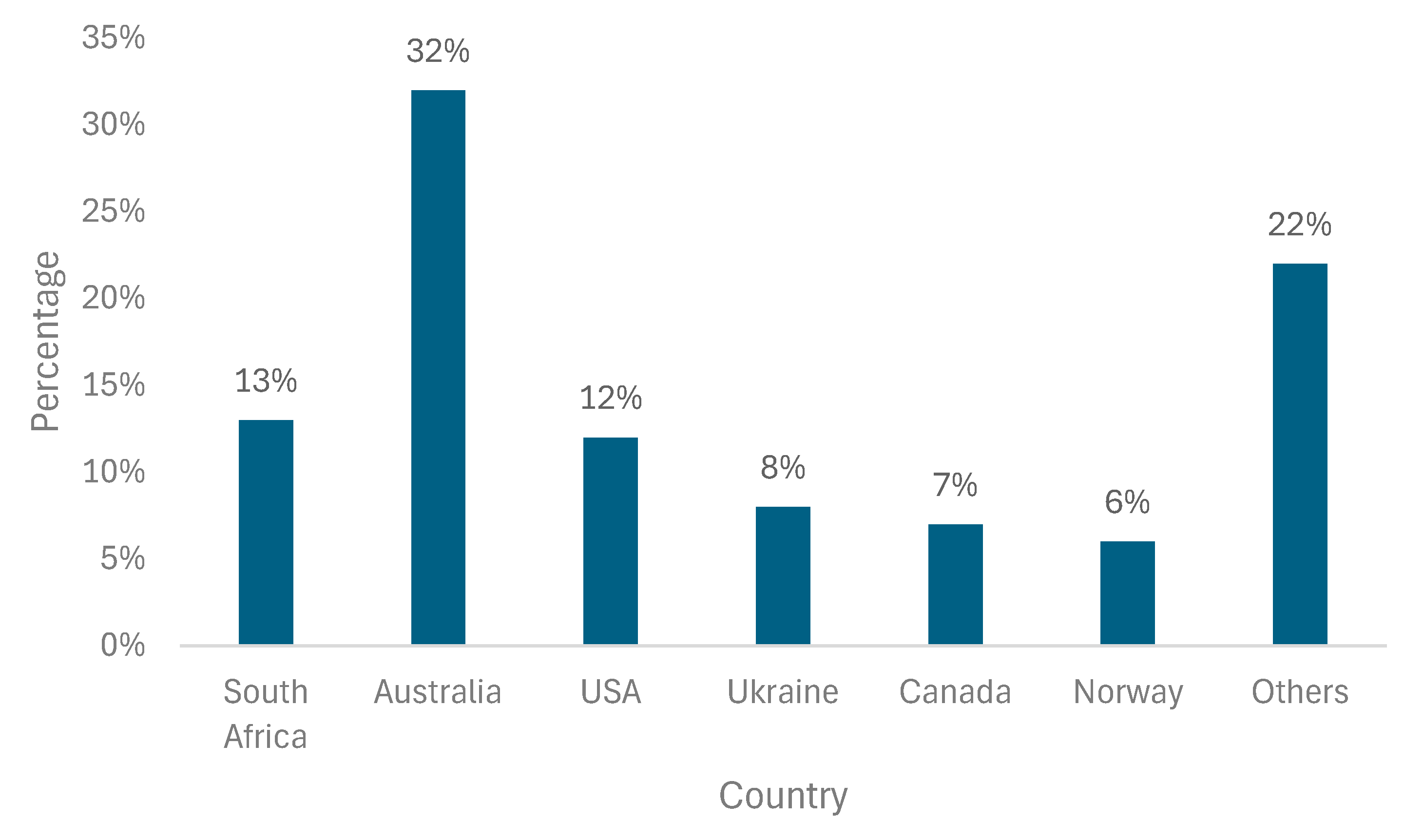
2. Titanium Ores
3. Application of Titanium and Its Alloys
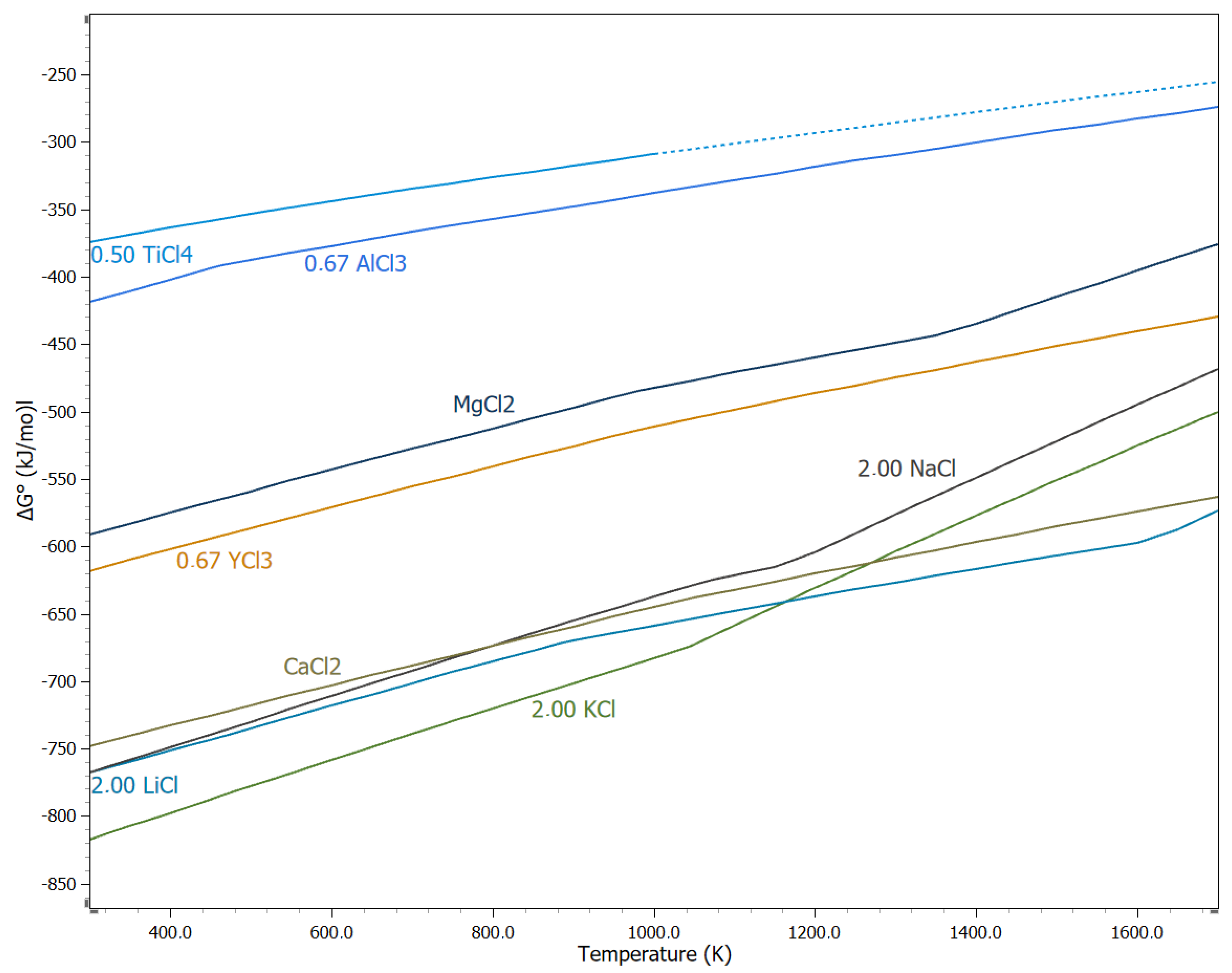
4. Thermochemical Production of Titanium Using TiCl4
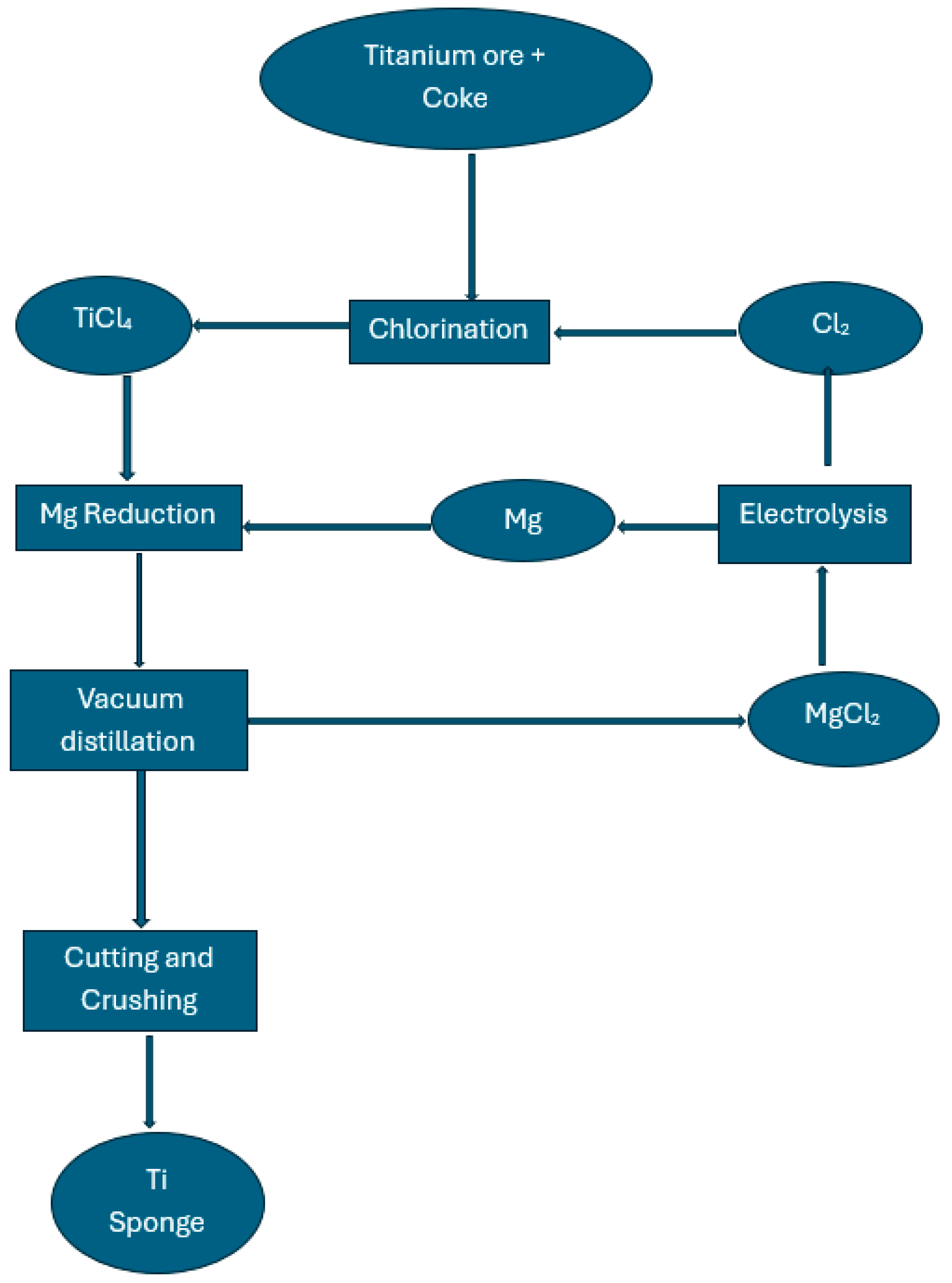
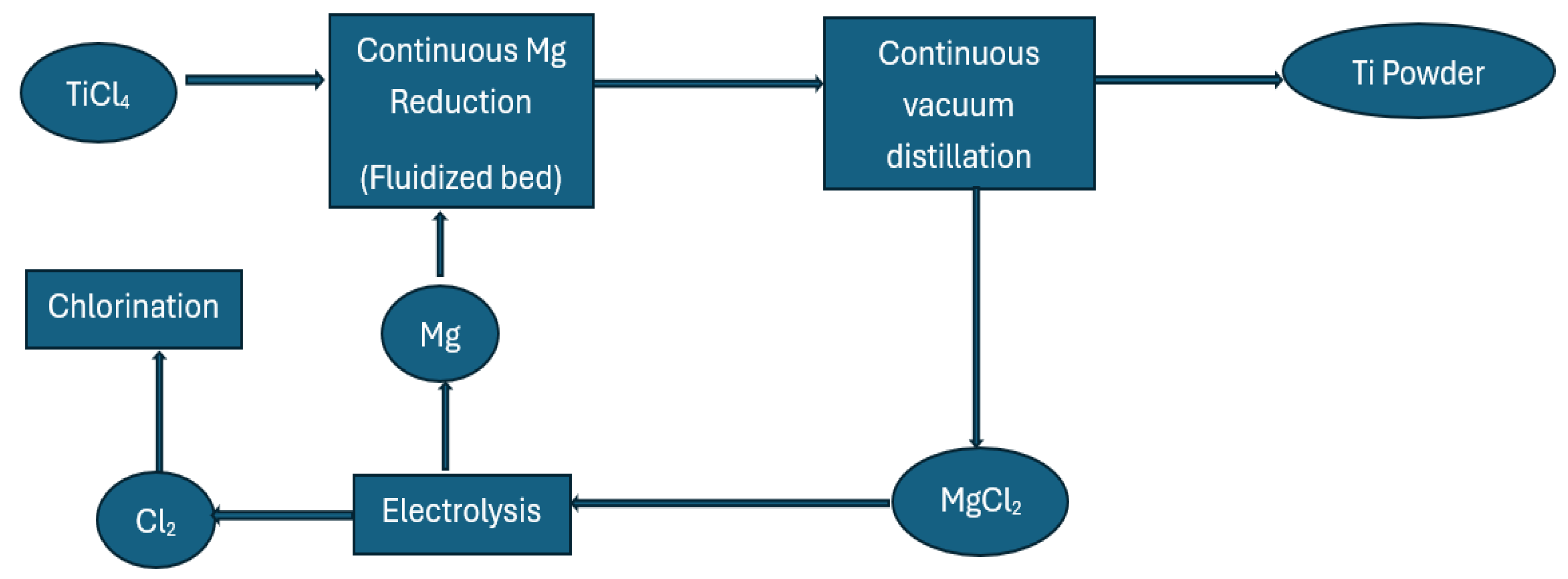
4.1. The Kroll Process
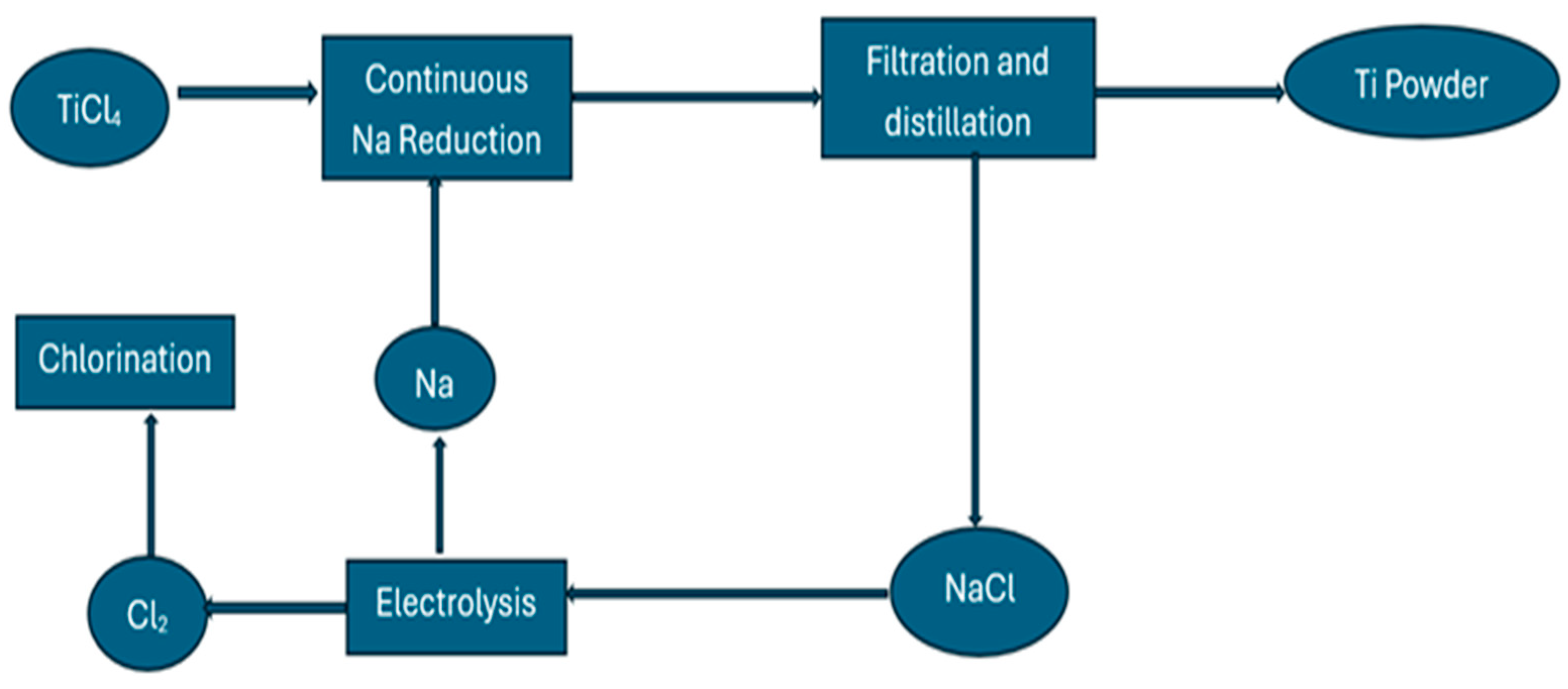
4.2. The Hunter Process
4.3. The Armstrong Process
4.4. The TiRO Process
4.5. The CSIR-Ti Process
4.6. The ADMA Process
4.7. The ARC Process
4.8. The SRI (Stanford Research Institute) Process
4.9. The ITT (Idaho Ti Technologies) Process
4.10. The JTS (Japan Titanium Society) Process
4.11. Vapor Phase Reduction Process
4.12. Aluminothermic Reduction
5. Thermochemical Production of Titanium Using TiO2
5.1. Combustion Synthesis (Magnesiothermic) Reduction
5.2. Self-Propagating High-Temperature Synthesis (SHS)
5.3. Hydrogen-Assisted Magnesium Reduction (HAMR) Process
5.4. Metal Hydride Reduction (MHR) Process
5.5. Electronically Mediated Reduction (EMR) Process
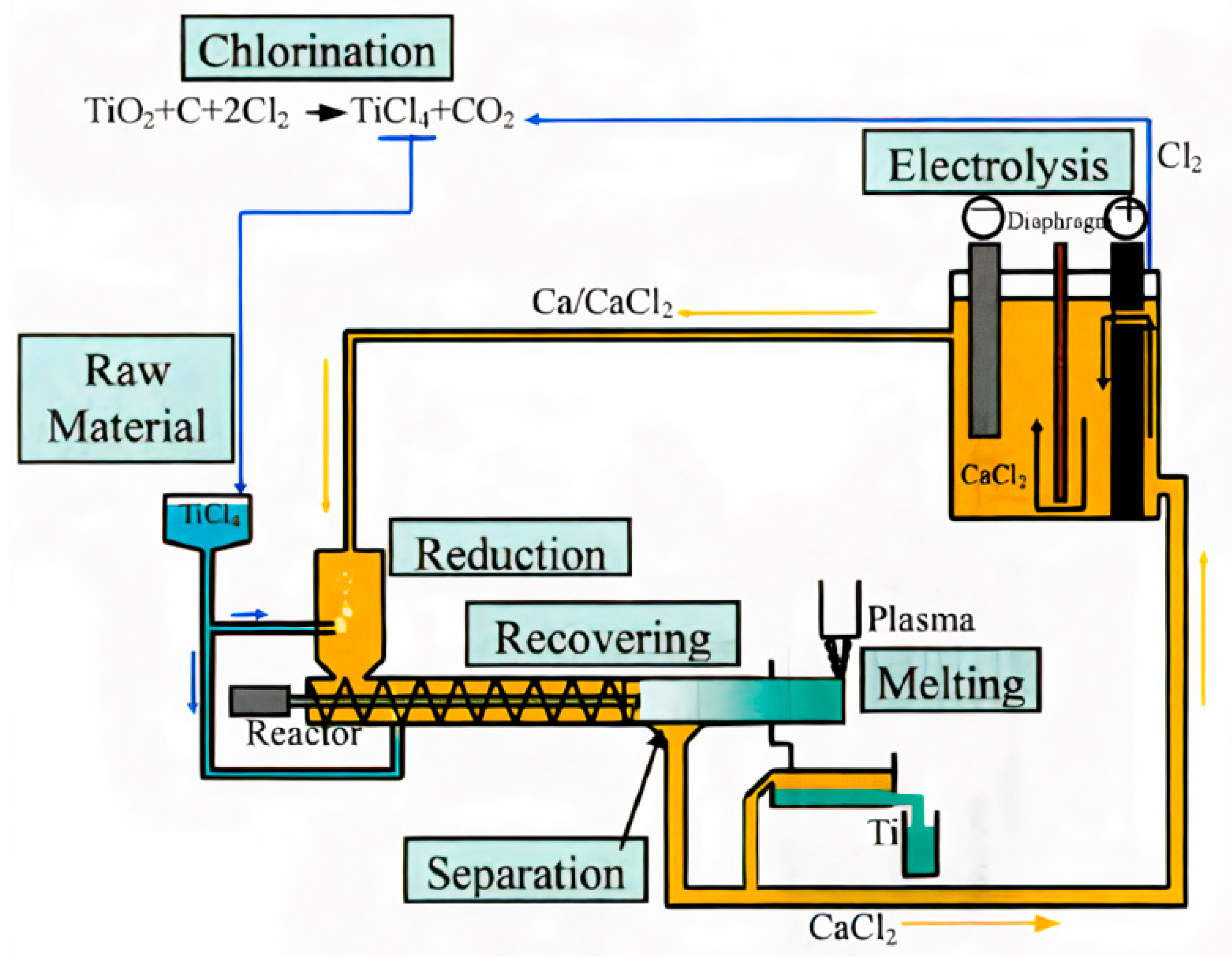
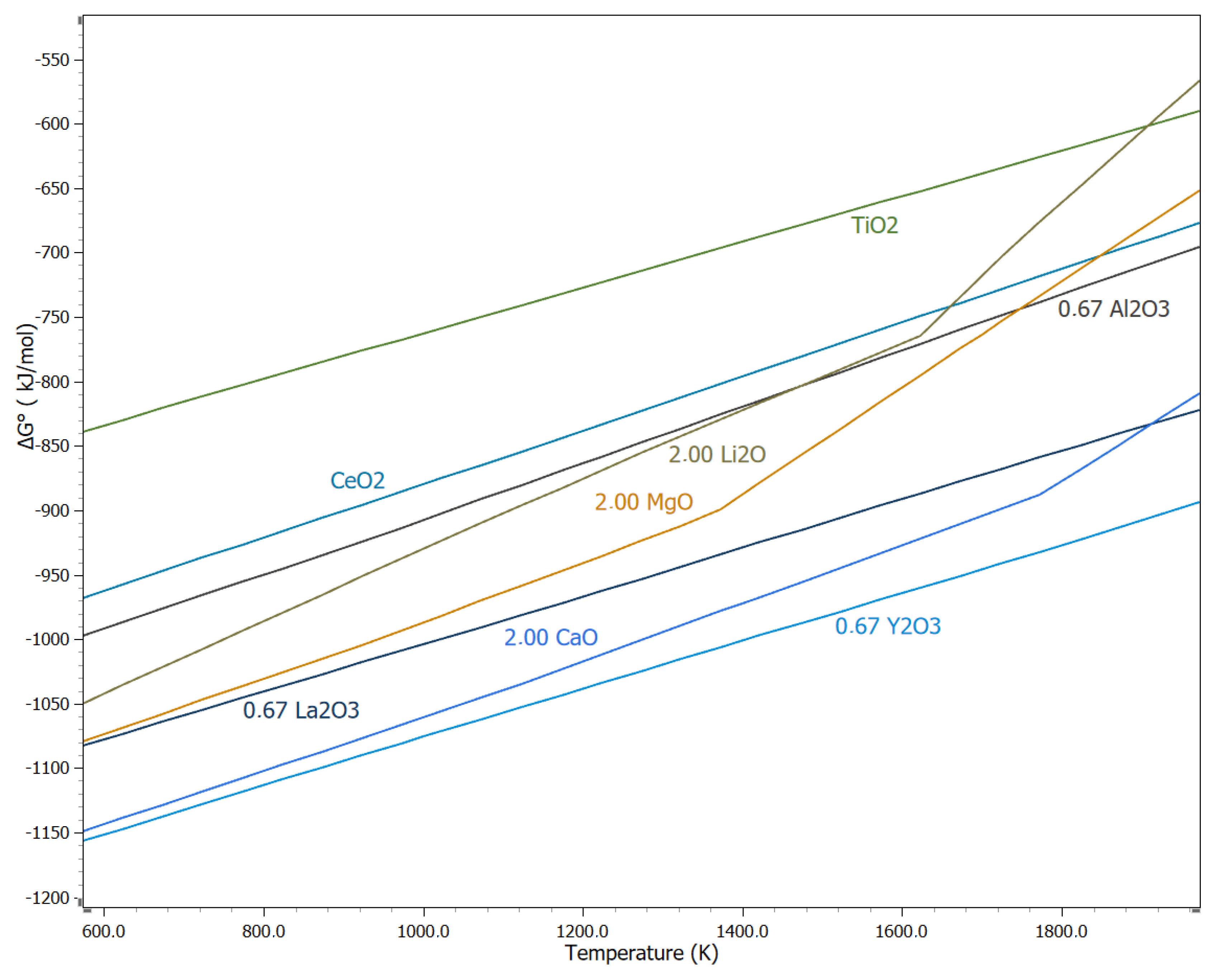
5.6. Calciothermic Reduction
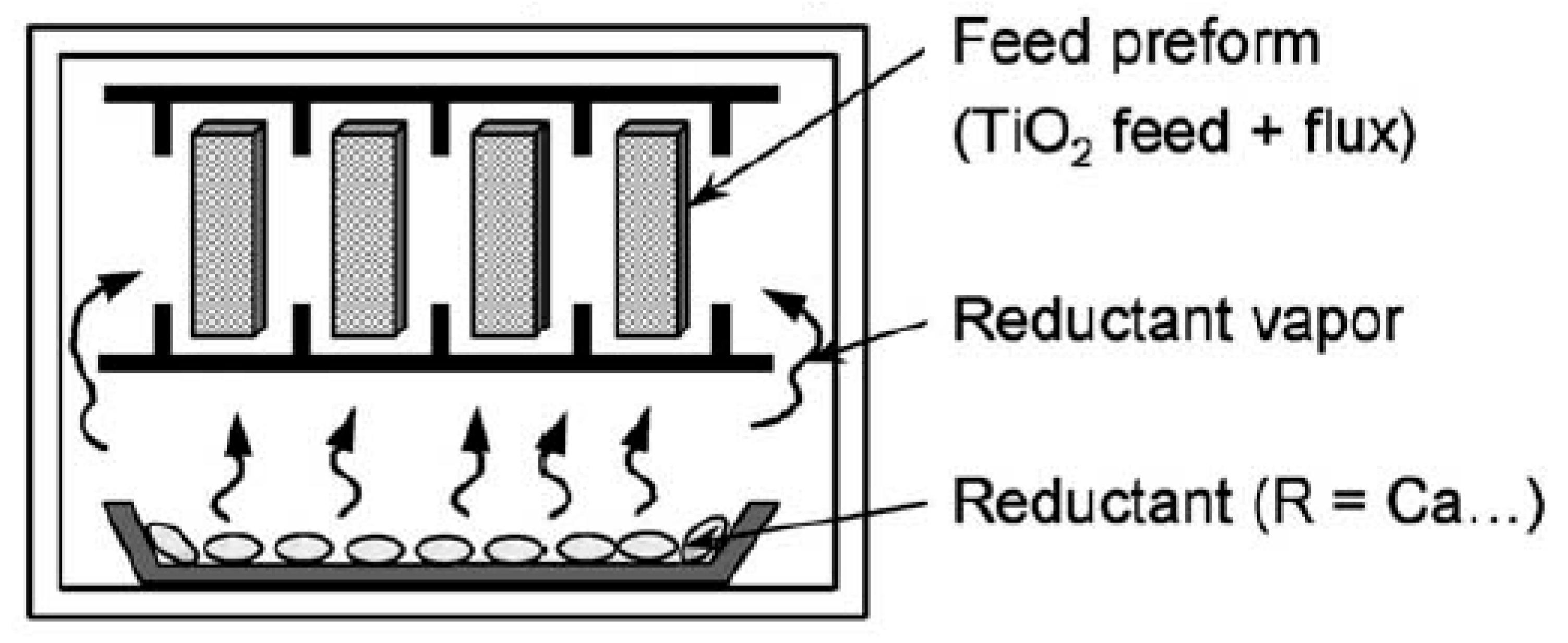
5.7. Preform Reduction Process (PRP)
5.8. Aluminothermic Reduction
6. Environmental Effects of Thermochemical Production of Titanium
7. Comprehensive Analysis of the Thermochemical Reduction of Titanium
8. Recommendations and Future Outlooks
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, Y.; Dou, Z.; Zhang, T.A.; Liu, Y. Research progress on the extractive metallurgy of titanium and its alloys. Miner. Process. Extr. Metall. Rev. 2021, 42, 535–551. [Google Scholar] [CrossRef]
- Cai, T.; Zhang, Y.; Zheng, S.; Yan, P.; Zhang, Y. Precise fabrication of Ti2O from TiO2 by Al reduction towards potentially lower-cost Ti metal powder production. J. Clean. Prod. 2024, 447, 141547. [Google Scholar] [CrossRef]
- Feng, Q.; Lv, M.; Mao, L.; Duan, B.; Yang, Y.; Chen, G.; Lu, X.; Li, C. Research Progress of Titanium Sponge Production: A Review. Metals 2023, 13, 408. [Google Scholar] [CrossRef]
- Li, M.; Liu, C.; Ding, A.; Xiao, C. A review on the extraction and recovery of critical metals using molten salt electrolysis. J. Environ. Chem. Eng. 2023, 11, 109746. [Google Scholar] [CrossRef]
- Miao, Y.; Cai, Y.; Ren, M.; Hu, J. Research Progress of Preparing Titanium Alloy By Molten Salt Method. Int. J. Nat. Resour. Environ. Stud. 2024, 2, 278–291. [Google Scholar] [CrossRef]
- Cao, P.; Zhang, L. Titanium Alloys: Basics and Applications; World Scientific: Singapore, 2024. [Google Scholar] [CrossRef]
- Kroll, W. The production of ductile titanium. Trans. Electrochem. Soc. 1940, 78, 35–47. [Google Scholar] [CrossRef]
- Hunter, M.A. Metallic titanium. J. Am. Chem. Soc. 1910, 32, 330–336. [Google Scholar] [CrossRef]
- Zhu, F.L.; Yuan, H.B.; Yu, Q.C.; Bin, Y.; Xu, B.Q.; Dai, Y.N. Behavior of titanium dioxide in alumina carbothermic reduction-chlorination process in vacuum. Trans. Nonferrous Met. Soc. China 2011, 21, 1855–1859. [Google Scholar] [CrossRef]
- Zheng, H.; Ito, H.; Okabe, T.H. Production of titanium powder by the calciothermic reduction of titanium concentrates or ore using the preform reduction process. Mater. Trans. 2007, 48, 2244–2251. [Google Scholar] [CrossRef]
- Ono, K.; Suzuki, R.O. A new concept for producing Ti sponge: Calciothermic reduction. JOM 2002, 54, 59–61. [Google Scholar] [CrossRef]
- Free, M.L. A brief introduction to production of titanium dioxide and titanium tetrachloride. In Extractive Metallurgy of Titanium: Conventional and Recent Advances in Extraction and Production of Titanium Metal; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Subasinghe, H.C.S.; Ratnayake, A.S. General review of titanium ores in exploitation: Present status and forecast. Comun. Geol. 2022, 109, 21–31. [Google Scholar]
- Daba, K.; Ramakokovhu, M.M.; Mojisola, T.; Shongwe, M.B.; Ntholeng, N. Iron Extraction from South African Ilmenite Concentrate Leaching by Hydrochloric Acid (HCl) in the Presence of Reductant (Metallic Fe) and Additive (MgSO4). Minerals 2022, 12, 1336. [Google Scholar] [CrossRef]
- El Khalloufi, M.; Drevelle, O.; Soucy, G. Titanium: An Overview of Resources and Production Methods. Minerals 2021, 11, 1425. [Google Scholar] [CrossRef]
- Williams, J.C.; Boyer, R.R. Opportunities and Issues in the Application of Titanium Alloys for Aerospace Components. Metals 2020, 10, 705. [Google Scholar] [CrossRef]
- Jang, T.-S.; Kim, D.; Han, G.; Yoon, C.-B.; Jung, H.-D. Powder based additive manufacturing for biomedical application of titanium and its alloys: A review. Biomed. Eng. Lett. 2020, 10, 505–516. [Google Scholar] [CrossRef]
- Pandey, L.M. Design of biocompatible and self-antibacterial titanium surfaces for biomedical applications. Curr. Opin. Biomed. Eng. 2023, 25, 100423. [Google Scholar] [CrossRef]
- Anil Kumar, V.; Gupta, R.; Prasad, M.; Narayana Murty, S. Recent advances in processing of titanium alloys and titanium aluminides for space applications: A review. J. Mater. Res. 2021, 36, 689–716. [Google Scholar] [CrossRef]
- Pushp, P.; Dasharath, S.; Arati, C. Classification and applications of titanium and its alloys. Mater. Today Proc. 2022, 54, 537–542. [Google Scholar] [CrossRef]
- Ponappa, K.; Panchal, Y. Metals and alloys for lightweight automotive structures. In Materials for Lightweight Constructions; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Busarac, N.; Adamovic, D.; Grujovic, N.; Zivic, F. Lightweight Materials for Automobiles. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1271, 012010. [Google Scholar] [CrossRef]
- Fadzil, M.; Abdullah, A.; Samad, Z.; Yusof, F.; Manurung, Y. Application of lightweight materials toward design for sustainability in automotive component development. In Design for Sustainability; Elsevier: Amsterdam, The Netherlands, 2021; pp. 435–463. [Google Scholar] [CrossRef]
- Veiga, C.; Davim, J.P.; Loureiro, A. Properties and applications of titanium alloys: A brief review. Rev. Adv. Mater. Sci. 2012, 32, 133–148. [Google Scholar]
- Takeda, O.; Uda, T.; Okabe, T.H. Rare earth, titanium group metals, and reactive metals production. In Treatise on Process Metallurgy; Elsevier: Amsterdam, The Netherlands, 2024; pp. 697–750. [Google Scholar] [CrossRef]
- Niu, L.; Zhang, T.A.; Lv, G.; Zhang, B. Study on the Direct Oxidation Thermal Decomposition of Magnesium Chloride by Product in the Sponge Titanium Production Process to Prepare Magnesium Oxide. In Magnesium Technology 2017; Springer International Publishing: Cham, Switzerland, 2017; pp. 209–213. [Google Scholar] [CrossRef]
- Turner, P.C.; Hartman, A.D.; Hansen, J.S.; Gerdemann, S.J. Low Cost Titanium—Myth or Reality; No. DOE/ARC-2001-086; Albany Research Center (ARC): Albany, OR, USA, 2001. [Google Scholar]
- Sohn, H.S. Production technology of titanium by Kroll process. Resour. Recycl. 2020, 29, 3–14. [Google Scholar] [CrossRef]
- Reddy, R.G.; Shinde, P.S.; Liu, A. The emerging technologies for producing low-cost titanium. J. Electrochem. Soc. 2021, 168, 042502. [Google Scholar] [CrossRef]
- Nagesh, C.R. Titanium Extraction Metallurgy Developments and Control of Impurity Elements. In Titanium Alloys-Recent Progress in Design, Processing, Characterization, and Applications; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Jena, K.D.; Xu, S.; Hayat, M.D.; Zhang, W.; Cao, P. Aiming at low-oxygen titanium powder: A review. Powder Technol. 2021, 394, 1195–1217. [Google Scholar] [CrossRef]
- Cariola, M. A high-potential sector: Titanium metal: Oligopolistic policies and technological constraints as main limits to its development. Resour. Policy 1999, 25, 151–159. [Google Scholar] [CrossRef]
- Araci, K.; Mangabhai, D.; Akhtar, K. Production of titanium by the Armstrong Process®. Titan. Powder Metall. 2015, 9, 149–162. [Google Scholar] [CrossRef]
- Haapala, K.R.; Atre, S.V.; Enneti, R.; Garretson, I.C.; Zhang, H. Materials processing. In Energy Efficient Manufacturing: Theory and Applications; Scrivener Publishing LLC: Beverly, MA, USA, 2018; pp. 33–64. [Google Scholar] [CrossRef]
- Takeda, O.; Ouchi, T.; Okabe, T.H. Recent progress in titanium extraction and recycling. Metall. Mater. Trans. B 2020, 51, 1315–1328. [Google Scholar] [CrossRef]
- Doblin, C.; Freeman, D.; Richards, M. The TiRO™ Process for the Continuous Direct Production of Titanium Powder. Key Eng. Mater. 2013, 551, 37–43. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, Y.; Fang, Z.Z. Selected processes for Ti production—A cursory review. In Extractive Metallurgy of Titanium; Elsevier: Amsterdam, The Netherlands, 2020; pp. 351–362. [Google Scholar] [CrossRef]
- Serwale, M.R.; Coetsee, T.; Fazluddin, S. Purification of crude titanium powder produced by metallothermic reduction by acid leaching. J. South. Afr. Inst. Min. Metall. 2020, 120, 349–354. [Google Scholar] [CrossRef]
- Van Vuuren, D.S. Direct titanium powder production by metallothermic processes. In Titanium Powder Metallurgy; Butterworth-Heinemann: Oxford, UK, 2015; pp. 69–93. [Google Scholar] [CrossRef]
- Lavender, C.A.; Moxson, V.S.; Duz, V. Cost-Effective Production of Powder Metallurgy Titanium Components for High-Volume Commercial Applications; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2010. [Google Scholar] [CrossRef]
- Ashraf Imam, M. The 13th World Conference on Titanium (Ti-2015). JOM 2016, 68, 2492–2501. [Google Scholar] [CrossRef]
- Panigrahi, M.; Paramguru, R.K.; Gupta, R.C.; Shibata, E.; Nakamura, T. An overview of production of titanium and an attempt to titanium production with ferro-titanium. High Temp. Mater. Process. 2010, 29, 495–514. [Google Scholar] [CrossRef]
- Cordes, R.A.; Donaldson, A. Titanium Metal Powder Production by The Plasma Quench Process; No. DOE/ID/13511; USDOE Idaho Operations Office: Idaho Falls, ID, USA; Idaho Titanium Technologies: Idaho Falls, ID, USA, 2000. [Google Scholar]
- Yamaguchi, M.; Ono, Y.; Kosemura, S.; Kagohashi, W.; Takenaka, T. Development of new titanium production process. In Ti-2007 Science and Technology, Proceedings of the 11th World Conference on Titanium, Kyoto, Japan; The Japan Institute of Metals: Sendai, Japan, 2007; pp. 143–146. [Google Scholar]
- Villechaise, P.; Narushima, T.; Sugizaki, Y.; Appolaire, B.; Castany, P.; Dehmas, M.; Delaunay, C.; Delfosse, J.; Denquin, A.; Gautier, E.; et al. Recent activities of titanium research and development in Japan. MATEC Web Conf. 2020, 321, 01004. [Google Scholar] [CrossRef]
- Hansen, D.A.; Gerdemann, S.J. Producing titanium powder by continuous vapor-phase reduction. JOM 1998, 50, 56–58. [Google Scholar] [CrossRef]
- Tebaldo, V.; Gautier Di Confiengo, G.; Duraccio, D.; Faga, M.G. Sustainable Recovery of Titanium Alloy: From Waste to Feedstock for Additive Manufacturing. Sustainability 2023, 16, 330. [Google Scholar] [CrossRef]
- Varacalle, D. Vapor-phase process cuts cost of producing titanium powders. Adv. Mater. Process. 2003, 161, 24. [Google Scholar]
- Haidar, J.; Gnanarajan, S.; Dunlop, J.B. Direct production of alloys based on titanium aluminides. Intermetallics 2009, 17, 651–656. [Google Scholar] [CrossRef]
- Frolov, J.; Fetzov, V. Synthesis of submicron titanium powder under the combustion mode. In The First All-Union Symposium on Macroscopic Kinetics and Chemical Gasodynamics; Academy of Sciences of the USSR: Chernogolovka, Russia, 1984; pp. 13–14. [Google Scholar]
- Nersisyan, H.; Won, H.; Won, C.; Jo, A.; Kim, J. Direct magnesiothermic reduction of titanium dioxide to titanium powder through combustion synthesis. Chem. Eng. J. 2014, 235, 67–74. [Google Scholar] [CrossRef]
- Merzhanov, A.G. The chemistry of self-propagating high-temperature synthesis. J. Mater. Chem. 2004, 14, 1779–1786. [Google Scholar] [CrossRef]
- Fan, S.G.; Dou, Z.H.; Zhang, T.A.; Yan, J. Sen Self-propagating reaction mechanism of Mg–TiO2 system in preparation process of titanium powder by multi-stage reduction. Rare Met. 2021, 40, 2645–2656. [Google Scholar] [CrossRef]
- Xia, Y.; Fang, Z.Z.; Zhang, Y.; Lefler, H.; Zhang, T.; Sun, P.; Huang, Z. Hydrogen assisted magnesiothermic reduction (HAMR) of commercial TiO2 to produce titanium powder with controlled morphology and particle size. Mater. Trans. 2017, 58, 355–360. [Google Scholar] [CrossRef]
- Shamsuddin, M.; Sohn, H.Y. Role of electrochemical processes in the extraction of metals and alloys—A review. Miner. Process. Extr. Metall. Trans. Inst. Min. Metall. 2023, 132, 193–209. [Google Scholar] [CrossRef]
- Suzuki, R.O.; Inoue, S. Calciothermic reduction of titanium oxide in molten CaCl2. Metall. Mater. Trans. B 2003, 34, 277–285. [Google Scholar] [CrossRef]
- Okabe, T.H.; Oda, T.; Mitsuda, Y. Titanium powder production by preform reduction process (PRP). J. Alloys Compd. 2004, 364, 156–163. [Google Scholar] [CrossRef]
- Okabe, T.H. Metallothermic reduction of TiO2. In Extractive Metallurgy of Titanium: Conventional and Recent Advances in Extraction and Production of Titanium Metal; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Chaikin, L.I.; Kireev, A.E.; Loginova, I.V. Studying the Possibility of Obtaining Titanium Powder of Various Sizes by Alumino-Thermic Reduction. Solid State Phenom. 2021, 316, 619–624. [Google Scholar] [CrossRef]
- Kireev, A.E.; Chaikin, L.I.; Loginova, I.V. Aluminothermic Reduction of Titanium Powder. J. Sib. Fed. University. Eng. Technol. 2024, 17, 297–307. [Google Scholar]
- Fygle, I. Production of Titanium Alloys via Aluminothermic Reduction. Master’s Thesis, NTNU, Trondheim, Norway, 2020. [Google Scholar]
- Farjana, S.H.; Huda, N.; Mahmud, M.P. Life-Cycle environmental impact assessment of mineral industries. IOP Conf. Ser. Mater. Sci. Eng. 2018, 351, 012016. [Google Scholar] [CrossRef]
- Gao, F.; Nie, Z.; Yang, D.; Sun, B.; Liu, Y.; Gong, X.; Wang, Z. Environmental impacts analysis of titanium sponge production using Kroll process in China. J. Clean. Prod. 2018, 174, 771–779. [Google Scholar] [CrossRef]
- Dai, Y.; Dong, H.; Sun, L.; Li, J.; Zhang, T.; Geng, Y.; Liu, Z. Life cycle environmental impact assessment of titanium dioxide production in China. Environ. Impact Assess. Rev. 2024, 105, 107412. [Google Scholar] [CrossRef]
- Landi, D.; Spreafico, C.; Russo, D. LCA of titanium powder: Empirical evidence vs data from patents, possible future applications. Procedia CIRP 2023, 116, 318–323. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Z.; Cheng, C.Y. A literature review of titanium metallurgical processes. Hydrometallurgy 2011, 108, 177–188. [Google Scholar] [CrossRef]
- Feng, Q.; Li, C. Low-Cost Preparation Technologies for Titanium Alloys: A Review. In Titanium Alloys-Recent Progress in Design, Processing, Characterization, and Applications; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Cheng, X.; You, Y.; Fu, J.; Hu, T.; Liu, W.; Su, X.; Yan, Y.; Tang, X. Self-propagating high-temperature synthesis and thermoelectric performances of Cu2SnSe3. J. Alloys Compd. 2018, 750, 965–971. [Google Scholar] [CrossRef]
- Xia, Y.; Lefler, H.D.; Zhang, Y.; Sun, P.; Fang, Z.Z. Hydrogen assisted magnesiothermic reduction (HAMR) of TiO2 to produce titanium metal powder. In Extractive Metallurgy of Titanium; Elsevier: Amsterdam, The Netherlands, 2020; pp. 165–179. [Google Scholar] [CrossRef]
- Duz, V.; Matviychuk, M.; Klevtsov, A.; Moxson, V. Industrial application of titanium hydride powder. Met. Powder Rep. 2017, 72, 30–38. [Google Scholar] [CrossRef]
- Van Vuuren, D.S. A critical evaluation of processes to produce primary titanium. J. South. Afr. Inst. Min. Metall. 2009, 109, 455–461. [Google Scholar]
- Zhao, K.; Huang, X.; Wang, Y.; Zhang, Y.; Liu, K. Low-oxygen Ti-6Al-4V alloy powder synthesized by an aluminothermic reduction combined with deoxidation process. J. Alloys Compd. 2023, 969, 172332. [Google Scholar] [CrossRef]
- Du, D.; Yan, J.; Dou, Z.; Zhang, T.A. A New Low-Cost, Short-Flow, and Clean Preparation Process for Ti6Al4V Alloys. In TMS Annual Meeting & Exhibition; Springer Nature: Cham, Switzerland, 2024; pp. 751–762. [Google Scholar] [CrossRef]
- Kraft, E.H. Summary of Emerging Titanium Cost Reduction Technologies. A Study Performed for US Department of Energy and Oak Ridge National Laboratory, Subcontract 4000023694; EHK Technologies: Vancouver, WA, USA, 2004; 55p. [Google Scholar]


| Process | Cost | Scalability | Environmental Impact | Product Purity | Advantages | Challenges | References |
|---|---|---|---|---|---|---|---|
| Hunter process | High | High | High (chemical waste) | High | Produces ultra-high-purity titanium | High cost of Na Batch process High energy consumption | [3,66] |
| Kroll process | High | High | High (Chlorine waste) | High | Lower cost of Mg than Na Industry standard, widely used | Batch process Energy-intensive, large carbon footprint | [3,66] |
| ARC | Medium | High | Moderate | High | Continuous production Controllable reaction speed Effective for melting and refining titanium alloys; scalable | High energy consumption; needs inert atmosphere to avoid contamination | [1] |
| Vapor-phase reduction process | High | Moderate | High (chemical waste) | Very high | Continuous production Produces high-purity titanium; versatile applications | Titanium powder has high oxygen, magnesium, or chlorine content; expensive infrastructure and high energy use | [3] |
| TiRo | Medium | High | Moderate | High | Efficient powder production, lower cost than Kroll | Limited adoption, oxygen contamination risks | [36] |
| EMR | Medium | Moderate | Low | Very high | Continuous production Energy efficient, scalable for certain applications | Onerous separation of metal and salt Requires extensive R&D for optimization | [3] |
| MHR | Medium-High | Moderate | Moderate | High | Single-step process Potential for lower cost titanium | Complex reactor design High energy consumption and pollution | [31,67] |
| SHS | Low | Low | Moderate | Moderate | Simple setup, low cost High efficiency | Uncontrollable process Limited scalability and product uniformity | [68] |
| HAMR | Low | Moderate | Low | High | Low-cost reduction process | Limited data on large-scale use | [31,69] |
| ADMA | Medium | High | Moderate | High | Uses novel techniques for cost-effective production | Relatively new, high setup cost | [70] |
| CSIR-Ti | Medium | Moderate | Moderate | High | Alternative to Kroll, promising for industrial use Continuous production | Oxygen content difficult to control Requires development for widespread adoption | [1,3] |
| JTS | High | Low | High | Very High | Ultra-high purity titanium for niche markets | Extremely high cost Harsh separation conditions | [71] |
| SRI | Medium | Moderate | Moderate | High | Lower energy process | Large gas recycling loop Requires process optimization for wider use | [71] |
| PRP | Low | Moderate | Low | Moderate | Simple, cost-efficient High reduction efficiency | Limited to small-scale production | [57] |
| Aluminothermic | Low | Moderate | Moderate | High | Cost-efficient for certain applications | Residual aluminum impurity risks | [37,72] |
| Calciothermic reduction | Medium | Moderate | Low | High | Low production cost High product purity | Handling calcium metal safely is challenging | [15,73] |
| Armstrong | Medium-High | Moderate | Low | High | Continuous production of titanium powder with uniform size Controllable reaction speed | Requires proprietary equipment, scalability challenge Expensive reductant and residual impurities | [3,33] |
| ITT | Medium | High | Moderate | High | Integrated production with lower cost and improved scalability | Harsh plasma process Requires significant technological integration for implementation | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsanga, N.; Wa Kalenga, M.; Nheta, W. An Overview of Thermochemical Reduction Processes for Titanium Production. Minerals 2025, 15, 17. https://doi.org/10.3390/min15010017
Matsanga N, Wa Kalenga M, Nheta W. An Overview of Thermochemical Reduction Processes for Titanium Production. Minerals. 2025; 15(1):17. https://doi.org/10.3390/min15010017
Chicago/Turabian StyleMatsanga, Nyasha, Michel Wa Kalenga, and Willie Nheta. 2025. "An Overview of Thermochemical Reduction Processes for Titanium Production" Minerals 15, no. 1: 17. https://doi.org/10.3390/min15010017
APA StyleMatsanga, N., Wa Kalenga, M., & Nheta, W. (2025). An Overview of Thermochemical Reduction Processes for Titanium Production. Minerals, 15(1), 17. https://doi.org/10.3390/min15010017






