1. Introduction
An optically anisotropic feldspathoid with a lazurite-related orthorhombic structure was first discovered at the Lyadzhvardara gem lazurite deposit, SE Pamir, Tajikistan [
1]. The crystal structure of this mineral was studied on a single crystal in 1998 [
2]. Later, the lazurite-related orthorhombic mineral was found in significant amounts at the Tultuy gem lazurite deposit, Baikal Lake area, Siberia, Russia. Based on the results of comparative studies, samples from both deposits were assigned to the same new mineral species named vladimirivanovite, with the simplified formula Na
6Ca
2[Al
6Si
6O
24](SO
4,S
3,S
2,Cl)
2·H
2O [
3]. Recently a mineral with orthorhombic unit-cell parameters close to those of vladimirivanovite and presumably assigned to the same mineral species was identified by us in a specimen from the small Slyudyanskoe gem lazurite deposit, Baikal Lake area.
In this work, three newly selected samples from the three abovementioned deposits were studied using single-crystal X-ray diffraction (XRD), electron microprobe analysis (EMPA), and infrared (IR) and Raman spectroscopy. Differences in their chemical composition, the nature of their extra-framework components, and their physical properties were revealed. Based on these data, a refined general crystal-chemical formula of vladimirivanovite has been proposed.
2. Materials
All vladimirivanovite samples studied in this work (
Figure 1) originate from gem lazurite deposits formed in the contact zone between alkaline rocks and marbles.
Sample 1 is a monomineral fraction of vladimirivanovite selected from the holotype specimen of this mineral collected at the Tultuy deposit. This sample was described as an orthorhombic sodalite-group mineral with the simplified general formula Na
6Ca
2(Al
6Si
6O
24)(SO
4,S
3,S
2,Cl)
2·H
2O, space group
Pnaa, and unit-cell parameters
a = 9.066 Å,
b = 12.851 Å, and
c = 38.558 Å (
Z = 6) [
3]. It forms dark blue individuals (crude crystals) up to 1 cm across in a metasomatic rock replacing calciphyre. The associated minerals are calcite, diopside, afghanite, lazurite, tounkite, phlogopite, and marialite.
Sample 2 is the vladimirivanovite cotype originating from the Lyadzhvardara deposit, Pamir, Tajikistan. Vladimirivanovite forms bright blue optically anisotropic zones in individuals of a lazurite-like mineral from a recrystallized metasomatic rock mainly consisting of calcite, diopside, lazurite, fluorapatite, and phlogopite.
Sample 3 originates from the Slyudyanskoe deposit. Vladimirivanovite forms lilac interrupted rims (up to 2 mm thick) around blue haüyne grains. The other associated minerals are calcite, dolomite, meionite, diopside, phlogopite, and chlorite.
3. Methods
The IR spectra were measured at the Federal Research Center of Problems of Chemical Physics and Medicinal Chemistry, Russian Academy of Sciences, Chernogolovka, Russia. In order to obtain IR absorption spectra, powdered samples were mixed with anhydrous KBr (in a KBr-to-mineral ratio of about 150:1), pelletized, and analyzed using an ALPHA FTIR spectrometer (Bruker Optics, Ettlingen, Germany) with a resolution of 4 cm−1. A total of 16 scans were collected for each spectrum. The IR spectrum of an analogous pellet of pure KBr was used as a reference.
The Raman spectra were obtained for randomly oriented grains using an EnSpectr R532 spectrometer based on an OLYMPUS CX 41 microscope (Enhanced Spectrometry, San Jose, CA, USA) coupled with a diode laser (λ = 532 nm) at room temperature (Moscow State University, Faculty of Geology, Dept. of Mineralogy). The spectra were recorded in the range of 100 to 4000 cm−1 with a diffraction grating (1800 gr mm−1) and spectral resolution of about 6 cm−1. The output power of the laser beam was in the range of 5 to 13 mW. The diameter of the focal spot on the sample was 5–10 μm. The backscattered Raman signal was collected with a 40× objective; the signal acquisition time for a single scan of the spectral range was 1 s, and the signal was averaged over 50 scans. Crystalline silicon was used as a standard.
The chemical composition of Sample 2 was studied at the Institute of Experimental Mineralogy RAS on an analytical suite including a digital scanning electron microscope Tescan VEGA-II XMU equipped with an INCA Energy 450 energy-dispersive spectrometer (EDS) with a Link INCA Energy semiconducting Si (Li) detector and an Oxford INCA Wave 700 wave-dispersive spectrometer (WDS), produced by Tescan Orsay Hld., Brno, Czech Republic. The analyses were performed at an accelerating voltage of 20 kV, a current of 120 to 150 pA, and a beam diameter of 120 nm. The diameter of the excitation zone was below 5 μm. The following standards were used: CaF2 for F, albite for Na, synthetic Al2O3 for Al, wollastonite for Ca, potassium feldspar for K, SiO2 for Si, Fe metal for Fe, and FeS2 for S. The contents of other elements with atomic numbers > 6 were below the detection limits.
The chemical composition of Sample 3 was studied at the Laboratory of Analytical Techniques of High Spatial Resolution, Faculty of Geology, Moscow State University, using a JEOL JSM-6480LV scanning electron microscope (EOL LTD, Welwyn Garden, UK) equipped with an X-Max 50energy-dispersive spectrometer. The EMPA conditions were as follows: an acceleration voltage of 20 kV, a beam current of 0.7 nA, and a 5 μm beam diameter. The following standards were used: Na, Cl—NaCl; K—potassic feldspar (NMNH 143966); Ca, Al—anorthite (NMNH 137041); Si—diopside; S—FeS2; O—plagioclase (NMNH 115900). The correctness of the quantitative determination of oxygen was controlled using quartz, as a so-called inner standard, mounted in a polished epoxy resin sample together with tounkite.
The contents of extra-framework CO
2 molecules were determined from IR spectra using the method described in [
4].
Single-crystal XRD data were collected at the Faculty of Geology, Moscow State University, using an Xcalibur CCD diffractometer (Mo
Kα radation). Data reduction was performed using the CrysAlisPro program system [
5]. Single-crystal structure analysis was performed using the SHELX [
6] and Jana [
7] programs.
4. Results
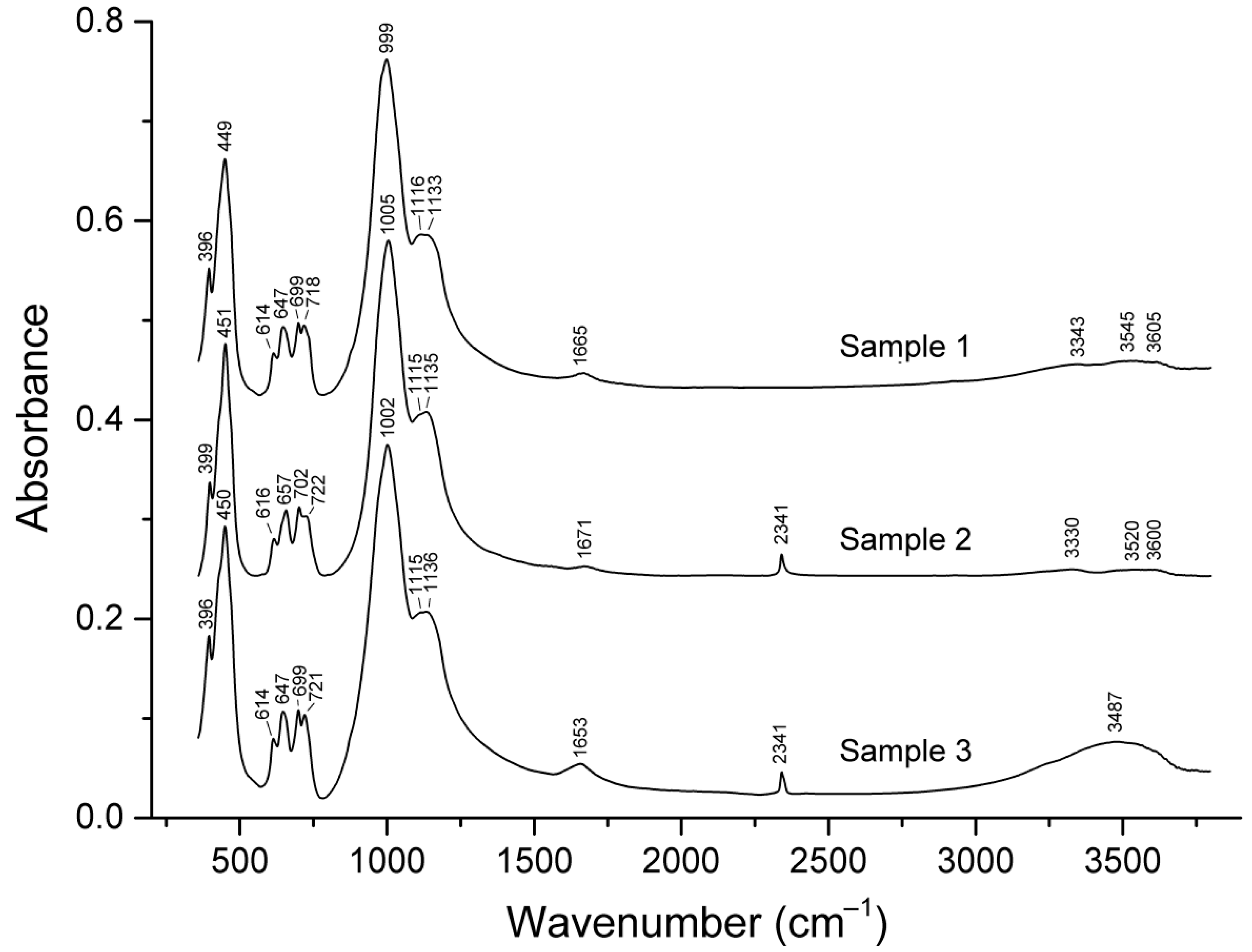
4.1. Infrared Spectroscopy
The IR spectra of the studied vladimirivanovite samples in the range of 300–1200 cm
−1 are similar (
Figure 2). The bands at 999–1005 cm
−1 and below 500 cm
−1 correspond to collective T–O stretching and T–O–T bending vibrations, respectively, where T = Si, Al. The bands at 1115–1116 and 1133–1136 cm
−1 are due to asymmetric stretching vibrations of distorted extra-framework SO
42− anionic groups, related to the F
2(ν
3) mode of the undistorted SO
4 tetrahedron. The band at 614–616 cm
−1 corresponds to bending vibrations of the SO
42− groups, related to the F
2(ν
4) mode of the undistorted SO
4 tetrahedron. Other bands in the so-called finger-print region (610–730 cm
−1) are due to collective O–T–O bending vibrations.
Bands of stretching and bending vibrations of H2O molecules are observed in the ranges of 3300–3700 and 1650–1680 cm−1. The water bands in the IR spectrum of Sample 3 are significantly more intense and have significantly different wave numbers than similar bands in the spectra of the other two samples. The content of H2O in Sample 3 estimated from the IR spectrum using Sample 1 as a standard is 2.6 ± 0.4 molecules per formula unit.
The band at 2341 cm−1 corresponds to stretching vibrations of extra-framework CO2 molecules. These molecules are absent in Sample 1.
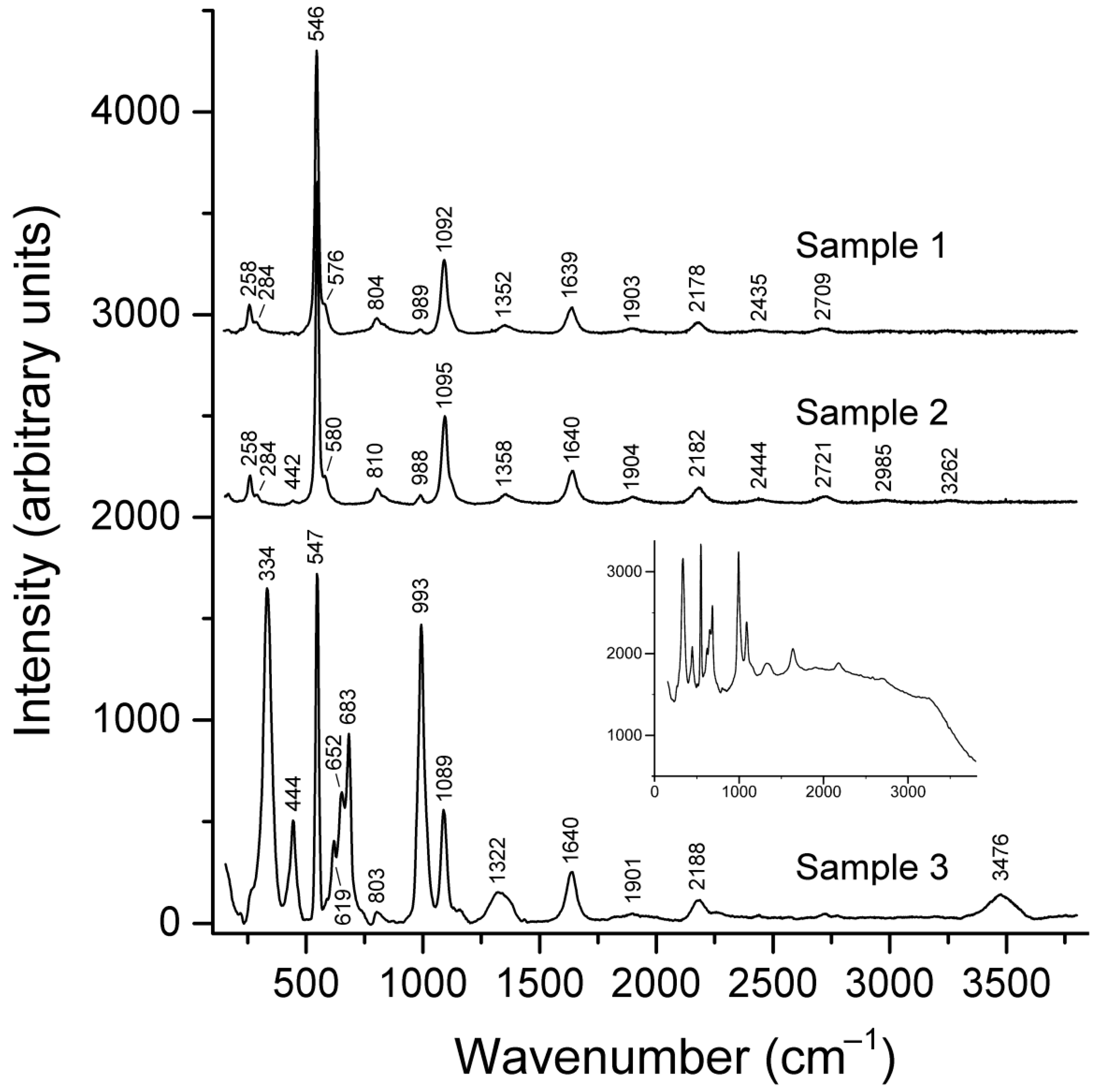
4.2. Raman Spectroscopy
Unlike IR spectroscopy, Raman spectroscopy is very sensitive to different kinds of polysulfide groups. The Raman spectra of the studied vladimirivanovite samples are given in
Figure 3. The assignment of bands in the Raman spectra (
Table 1) was carried out in accordance with [
4,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20] and the references therein. As one can see from these data, the main extra-framework polysulfide component in Sample 1 and Sample 2 is the S
3•− radical anion, whereas most of the sulfide sulfur in Sample 3 is represented by different conformers of the S
4 molecule. In addition, Sample 3 contains a minor amount of S
3•− whose content, estimated using the band of SO
42− symmetric stretching vibrations as an internal standard, is 0.008 ± 0.002 radical anions per formula unit. The Raman spectrum of Sample 3 confirms its higher (in comparison with other vladimirivanovite samples) degree of hydration.
The Raman spectra of Samples 1 and 2 do not show significant luminescence, whereas rather strong luminescence (presumably due to the presence of trace amounts of Fe
3+ and/or S
3•−) is observed for Sample 3 (
Figure 2).
4.3. Chemical Composition
The chemical data of the studied samples are given in
Table 2. The contents of the other elements with atomic numbers >8 are below the detection limits.
Based on the Raman spectra, the main forms of sulfide sulfur are S
3•− (in Sample 1 and Sample 2) and S
4 (in Sample 3). The content of admixed S
3•− in Sample 3 was estimated from the Raman spectrum (see above); the SO
42−:S
3•− and SO
42−:S
4 ratios were calculated based on the charge balance requirement. The calculated fraction of sulfide sulfur in Sample 1 is 0.293, which is close to the value of 0.299 obtained based on the measured contents of sulfate and sulfide sulfur [
3].
The water content in Sample 2 and Sample 3 was not measured due to the scarcity of pure material. The IR spectra of these samples show that the only carbon-bearing species in them are neutral CO2 molecules.
The empirical formulae calculated on the basis of Si + Al = 12 atoms per formula unit (apfu) are as follows.
We avoided an anionic basis of formula calculation because of the presence of different forms of sulfur (both sulfate and polysulfide groups) and uncertainties with O distribution between sulfate anions and water molecules.
4.4. Crystal Structure
All three single-crystal samples studied in this work are orthorhombic (space group
Pnaa) with almost identical unit-cell parameters (
Table 3). In terms of structure, they are similar to each other and to the previously studied structure of vladimirivanovite from the Lyadzhvardara deposit [
2]. A summary table of the atomic coordinates of the samples is provided in
Table S1 to show the high degree of structural similarity.
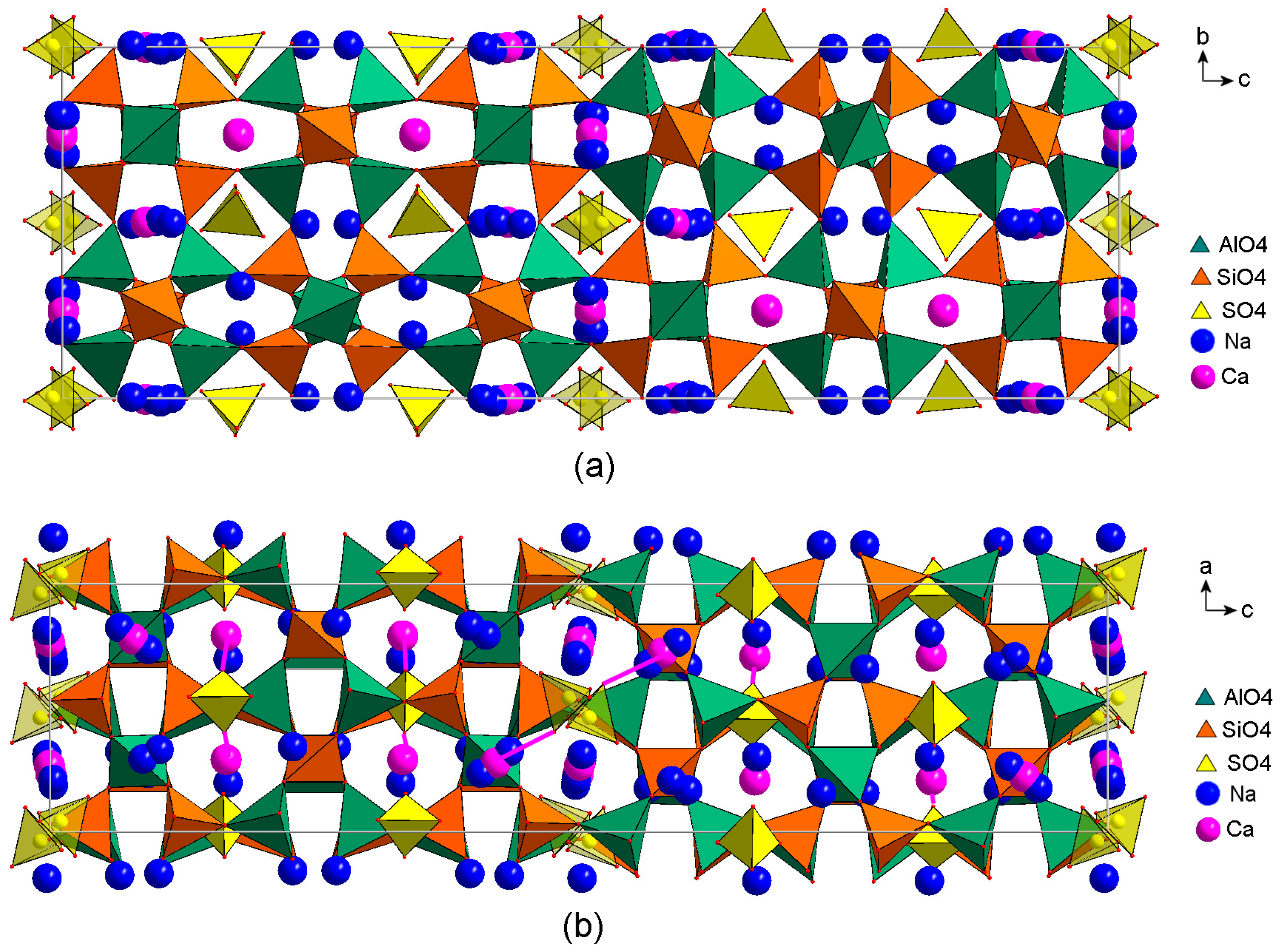
The structure of vladimirivanovite is shown in
Figure 4 in two projections. As usual, in sodalite-like structures, AlO
4 and SiO
4 tetrahedra with ordered Al and Si atoms are connected via common vertices and form an open framework. The cavities of the framework (so-called sodalite cages) are populated by SO
4 tetrahedra in a coordination environment of sodium and calcium cations.
In four of the twelve cages per unit cell, SO
4 tetrahedra are disordered by the inversion center located in the center of the cage. In such cages, the sites of sulfur atoms of the SO
42− anions are partly occupied, with probabilities of 50%, 75%, and 60% in Samples 1, 2, and 3, respectively. The disordering of tetrahedra is preserved upon transition to the non-centrosymmetric subgroup
Pn2
1a of the
Pnaa group, i.e., it really exists, and is not “imposed” by the redundant symmetry of the model. two thirds of the sodalite cages do not contain inversion centers. In these cages, the SO
4 tetrahedra are ordered, with occupancies of 100% in Sample 1, 80% in Sample 2, and 90% in Sample 3. Each SO
4 tetrahedron is oriented in such a way that one of its vertices forms a short (within 2.2–2.4 Å) Ca–O bond with the nearest calcium cation. Some Ca–O bonds are indicated in
Figure 4b. The sites of cations near disordered SO
42− groups are split into two, sometimes even three, closely spaced sites. In the latter case, the Ca
2+ cation occupies the middle of the three sites and occurs in the cage simultaneously with the nearest SO
42− anion, forming a short bond with it. In other cases, when the SO
4 tetrahedron is differently oriented or is absent, Na
+ cations at the other two sites are shifted to neighboring cages. These features are typical for all studied samples of vladimirivanovite, with minor variations in the degree of splitting and occupancies of split sites. The contents of the main extra-framework ions obtained from the structural models of the studied samples, Samples 1, 2, and 3 (
Table 3), are in agreement with their empirical formulae.
The positions of S3•−, S4, CO2, and Cl−, i.e., extra-framework species occurring in these samples in subordinate amounts, and of H2O molecules could not be localized because of their low occupancy factors and disordering. We believe that in Sample 3, large S4 molecules occurring there in three conformation states occupy the most spacious and isometric and the most SO42−-deficient cages, with disordered SO4 tetrahedra. These cages are most suitable for S4 and other neutral and low-charged anionic species (CO2, H2O and/or S3•−), ensuring charge balance.
In Samples 2 and 3, cages with ordered SO4 tetrahedra are also somewhat SO42−-deficient, with occupancies of the sulfate group of 80% and 90%, respectively. These cages can also contain admixtures of other extra-framework anionic and/or neutral groups. It is quite possible that in Sample 2, with the maximum number of both disordered SO4 tetrahedra and anionic S3•− groups, some of the S3•− radical anions are located in the cages of the second group due to a lack of vacancies in the first group.
Although the structural models for the three samples are almost identical and refined with similar R values, the quality of the measured diffraction patterns is not the same. The most complex picture was obtained from Sample 1. In addition to reflections at the lattice sites, the diffraction pattern contains relatively weak additional reflections at interstices along the b* axis. With their participation, it is possible to form a second, presumably twin lattice of vladimirivanovite, rotated 90 degrees relative to the first one. The exact contribution of twinning to the intensities of overlapping reflections is difficult to determine. In addition, the source of additional reflections can be not only twinning, but also structural modulation in two directions (see below). Both factors influence the intensities of reflections. We were able to refine the model with an R value of less than 7% only after rejecting about 40% of the reflections. The diffraction patterns from Samples 2 and 3 do not contain visible signs of twinning or multidimensional modulation. The models for them are refined with an R value of about 6% after rejecting 8% and 5%, respectively, of the total number of reflections averaged in the Laue class mmm.
5. Discussion
5.1. Extra-Framework Components
As one can see from the chemical data (
Table 2), empirical formulae, vibrational spectra, and results of the refinement of the crystal structures, all vladimirivanovite samples studied in this work are similar to each other in terms of the contents of the main extra-framework ions (Na
+, Ca
2+ and SO
42−) but differ in terms of the contents of extra-framework polysulfide groups as well as H
2O and CO
2 molecules.
Sample 1 is H
2O-poor and does not contain CO
2 and S
4. The only detectable polysulfide component in this sample is S
3•−. Taking into account that CO
2 and H
2O are volatile species and escape from sodalite cages at high temperatures (above 600 °C), as well as the fact that S
3•− has the highest thermal stability among polysulfide groups [
21], one can assume that Sample 1 crystallized at high temperatures.
Sample 2 differs from Sample 1 by the presence of extra-framework CO2 molecules. Based on this fact, one can suppose that Sample 2 crystallized at somewhat lower temperatures (at least, below 600 °C) or under lower reducing conditions.
In Sample 3, the S4 molecule, which is unstable at high temperatures, is the main polysulfide component. In addition, this sample is H2O-rich and contains CO2. These facts indicate possible low-temperature and moderately reducing conditions in the crystallization of Sample 3.
The electric charge per one S atom is −2 for SO42−, −1/3 for S3•−, and 0 for S4. Thus, the groups S3•− and S4 replacing some of the SO42− ions in sodalite cages play the role of charge-balancing components. The occurrence of these components in the structure of vladimirivanovite determines its color: Sample 1 and Sample 2 are blue because of the presence of S3•− (a very strong blue chromophore), whereas the lilac color of Sample 3 is due to the presence of S4 (red chromophore) and a minor admixture of S3•−.
The alternation of sodalite cages with different occupancy factors of SO42−, S3•−, and S4 (i.e., particles having different sizes and charges) results in differences in the sizes of the cages and leads to distortions of the structure as a whole, as described below.
5.2. Structure Distortions
The transition from the cubic structure of sodalite to its derivative orthorhombic superstructure of vladimirivanovite is described by the relations a ≈ a
cub, b ≈ a
cub√2, and c ≈ 3a
cub√2. The unit cell shown in
Figure 3 can be divided along the c-axis into three equal parts similar, but not identical, in structure. In other words, the structure of vladimirivanovite is the result of the commensurate modulation of an average (basic) structure with the unit-cell parameters a
0 = a, b
0 = b, and c
0 = c/3. The modulation vector
q =
c0*/3 is directed along the c
0 axis, and the modulation period 3c
0 is equal to the unit-cell parameter of vladimirivanovite.
The formalism of structural modulations was used by us in [
22] to compare three minerals with a sodalite-derived structure: slyudyankaite (
q =
c0*/2), a monoclinic analog of lazurite (
q ≈ 0.43
c0*) and vladimirivanovite (
q =
c0*/3). The structure of the monoclinic analog of lazurite is incommensurately modulated with respect to the period of the basic lattice, and it was studied using superspace (3 + 1)D formalism. It is shown that the modulations of the framework atoms are in good phase agreement, and the amplitudes of their displacements from the average positions change according to a harmonic law, forming a modulation wave.
In the structure of vladimirivanovite, regular alternation of sodalite cages of two types (larger and smaller ones) takes place. Presumably, cages of the first type host large polysulfide groups (S
4 and/or S
3•−). Even more spacious cavities occupied by larger S
6 molecules, as well as S
4 molecules, S
3•− radical anions, and CO
2 and H
2O molecules, were found by us in the structure of slyudyankaite [
9]. Unlike the cavities of vladimirivanovite, such cavities did not contain sulfate sulfur at all. The space for accommodating large molecules was provided by the shifting of Na
+ and Ca
2+ cations into neighboring cavities hosting SO
42− anionic groups.
Harmonic modulations are characteristic of frameworks of sodalite-like structures, but modulations of extra-framework atoms often occur according to more complex laws. Vladimirivanovite is not an exception to this rule. The displacements of framework atoms can be described by harmonics, but the displacements of many extra-framework atoms are clearly not harmonic. For example, the Ca atoms in
Figure 4a move abruptly from the upper left part to the lower right part of the unit cell. The SO
4 tetrahedra in the same figure rotate abruptly around the c axis. These changes cannot be described by harmonics; other techniques have been developed for them. If the modulation period is commensurate with the lattice period and is not too large, as in the case of vladimirivanovite, it is preferable not to complicate the problem and work with the superstructure in 3D, without constructing (3 + 1)D models for modulated structures.
The role of large polysulfide groups in the symmetry and modulation of the structure of sodalite-group minerals was discussed by us in a previous study [
21]. In particular, it was shown that all studied samples of sulfide-free minerals of the sodalite group with the [Al
6Si
6O
24] framework have a cubic unmodulated structure. Samples with low contents of the S
3•− and S
4 groups (below 4 wt.% in total) have lower symmetry and a commensurately modulated structure. As a rule, sodalite-group minerals with the [Al
6Si
6O
24] framework and higher contents of S
3•− and S
4 (more than 4 wt.% in total) are cubic, with incommensurate structure modulations. Their crystallization temperatures are 550–600 °C, whereas less symmetrical lazurite-related minerals crystallize at lower temperatures [
23]. The three single-crystal samples of vladimirivanovite studied in this work are orthorhombic and commensurately modulated. Among them, Sample 3, which crystallized in lower temperature conditions, gives a simpler diffraction pattern and a better structure refinement result.
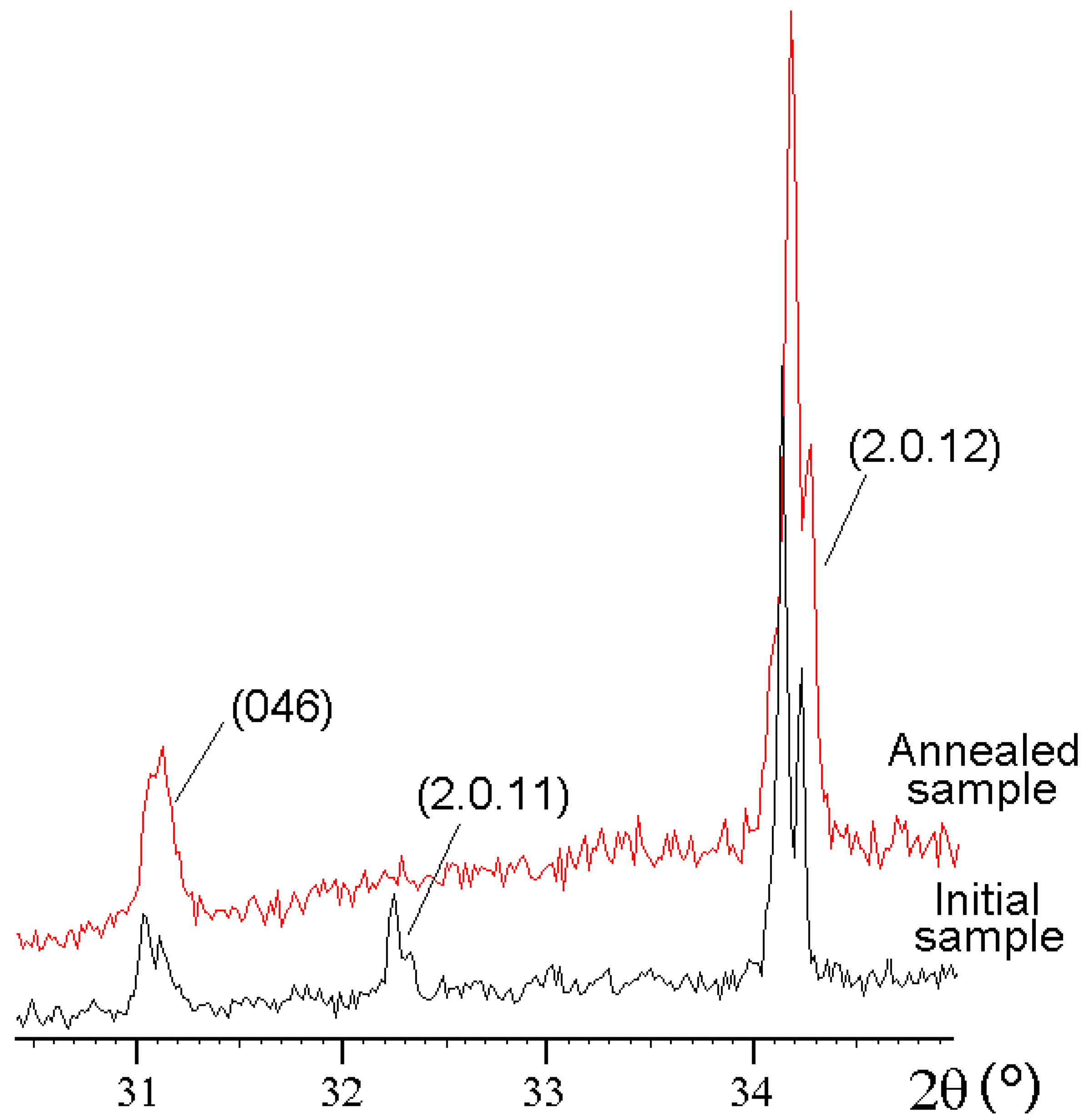
Annealing of vladimirivanovite from the Tultuy deposit in air at 800 °C results in the disappearance of superstructure reflections (
Figure 5) and the transformation of the orthorhombic unit cell into a cubic sodalite-type cell. This process is accompanied by the disordering of extra-framework components and partial transformation of S
3•− into SO
42− according to the scheme S
3•− + 5
e +6O
2(gas) → 3SO
42− [
21].
6. Conclusions and Implications
Based on the compositional, structural, and spectroscopic data obtained in this work, two vladimirivanovite varieties (or, possibly, two different related mineral species) can be distinguished. One of them, presumably crystallized at a relatively high temperature, has the simplified formula (Na+6.0–6.4Ca2+1.5–1.7)(Al6Si6O24)(SO42−,S3•−)1.7–1.9(CO2)0–0.1·H2O and has a deep blue color, whereas another one, with the simplified formula (Na+6.2Ca2+1.6)(Al6Si6O24)(SO42−,S4)1.6(CO2)0.1·nH2O (n > 2), is lilac and has low-temperature origin.
Three single-crystal samples studied in this work have similar commensurately modulated orthorhombic structures, with the unit-cell parameter c equal to the triple period of the basic lattice (c = 3c0), unlike triclinic slyudyankaite, with c = 2c0, and the monoclinic analog of lazurite, with c ≈ 21/3·c0. The decrease in symmetry compared to the basic cubic structure of the sodalite type is associated with the ordering of extra-framework components and the regular alternation of hosting sodalite cages of different sizes. With increasing temperature, the degree of structural order of vladimirivanovite gradually decreases until the complete disorder of the extra-framework components and the transformation of the orthorhombic unit cell into a cubic one.
Supplementary Materials
The following supporting information can be downloaded at:
https://www.mdpi.com/article/10.3390/min14090883/s1, Table S1. Relative atomic coordinates x/a, y/b and z/c in the samples 1, 2 and 3. P is site occupancy and U(eq) is equivalent atomic displacement parameter.
Author Contributions
Conceptualization, N.V.C., N.B.B., I.V.P. and A.N.S.; Methodology, N.V.C., N.B.B., M.F.V., N.V.Z. and D.A.V.; Investigation, N.V.C., N.B.B., M.F.V., N.V.Z., D.A.V., M.O.B., V.O.Y. and D.A.K.; Original Manuscript Draft Preparation, N.V.C. and N.B.B.; Manuscript Review and Editing, A.N.S. and I.V.P.; Figures, N.B.B. and N.V.C. All authors have read and agreed to the published version of the manuscript.
Funding
A major part of this work, including the single-crystal structure analysis, chemical data processing, registration of the Raman spectra of all samples, and the electron microprobe analysis of Sample 3, was supported by the Russian Science Foundation, grant no. 22-17-00006 (for N.B.B., N.V.C., N.V.Z., I.V.P., M.F.V. and F.D.S.). IR spectroscopic investigation and the assignment of Raman bands were carried out in accordance with the state task, state registration no. FFSG-2024-0009 (for N.V.C.). The electron microprobe data of Sample 2 were obtained under Research Program FMUF-2022-0002 of the D.S. Korzhinskii Institute of Experimental Mineralogy (for D.A.V.).
Data Availability Statement
Acknowledgments
The authors are grateful to Ramiza A. Rastsvetaeva for a fruitful discussion.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sapozhnikov, A.N.; Ivanov, V.G.; Levitsky, V.I.; Piskunova, L.F. Structural-mineralogical peculiarities of lasurite from the south-western Pamir. Zapiski Vserossiiskogo Mineralogicheskogo Obshchestva 1993, 122, 108–115. (In Russian) [Google Scholar]
- Evsyunin, V.G.; Sapozhnikov, A.N.; Rastsvetaeva, R.K.; Kashaev, A.A. Modulated structure of orthorhombic lazurite. Crystallogr. Rep. 1998, 43, 999–1002. [Google Scholar]
- Sapozhnikov, A.N.; Kaneva, E.V.; Cherepanov, D.I.; Suvorova, L.F.; Levitsky, V.I.; Ivanova, L.A.; Reznitsky, L.Z. Vladimirivanovite, Na6Ca2[Al6Si6O24](SO4,S3,S2,Cl)2·H2O, a new mineral of sodalite group. Geol. Ore Depos. 2012, 54, 557–564. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Vigasina, M.F.; Zubkova, N.V.; Pekov, I.V.; Schäfer, C.; Kasatkin, A.V.; Yapaskurt, V.O.; Pushcharovsky, D.Y. Extra-framework content in sodalite-group minerals: Complexity and new aspects of its study using infrared and Raman spectroscopy. Minerals 2020, 10, 363. [Google Scholar] [CrossRef]
- Oxford Diffraction CrysAlisPro Software System; v. 1.171.42.49; Rigaku Corporation: Oxford, UK, 2022.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Zeitschrift für Kristallographie Cryst. Mater. [CrossRef]
- Steudel, R.; Chivers, T. The role of polysulfide dianions and radical anions in the chemical, physical and biological sciences, including sulfur-based batteries. Chem. Soc. Rev. 2019, 48, 3279–3319. [Google Scholar] [CrossRef] [PubMed]
- Sapozhnikov, A.N.; Bolotina, N.B.; Chukanov, N.V.; Shendrik, R.Y.; Kaneva, E.V.; Vigasina, M.F.; Ivanova, L.A.; Tauson, V.L.; Lipko, S.V. Slyudyankaite, Na28Ca4(Si24Al24O96)(SO4)6(S6)1/3(CO2)·2H2O, a new sodalite group mineral from the Malo-Bystrinskoe lazurite deposit, Baikal Lake area, Russia. Am. Mineral. 2023, 108, 1805–1817. [Google Scholar] [CrossRef]
- Chivers, T.; Oakley, R.T. Structures and spectroscopic properties of polysulfide radical anions: A theoretical perspective. Molecules 2023, 28, 5654. [Google Scholar] [CrossRef] [PubMed]
- Chukanov, N.V.; Sapozhnikov, A.N.; Shendrik, R.Y.; Zubkova, N.V.; Vigasina, M.F.; Potekhina, N.V.; Ksenofontov, D.A.; Pekov, I.V. Crystal Chemistry, Thermal and Radiation-Induced Conversions and Indicatory Significance of S-Bearing Groups in Balliranoite. Minerals 2023, 13, 822. [Google Scholar] [CrossRef]
- Rejmak, P. Computational refinement of the puzzling red tetrasulfur chromophore in ultramarine pigments. Phys. Chem. Chem. Phys. 2020, 22, 22684–22698. [Google Scholar] [CrossRef] [PubMed]
- Eckert, B.; Steudel, F. Molecular spectra of sulfur molecules and solid sulfur allotropes. Top. Curr. Chem. 2003, 231, 31–97. [Google Scholar] [CrossRef]
- Hettmann, K.; Wenzel, T.; Marks, M.; Markl, G. The sulfur speciation in S-bearing minerals: New constraints by a combination of electron microprobe analysis and DFT calculations with special reference to sodalite-group minerals. Am. Mineral. 2012, 97, 1653–1661. [Google Scholar] [CrossRef]
- Ling, Z.C.; Wang, A.; Jolliff, B.L. Mineralogy and geochemistry of four lunar soils by laser-Raman study. Icarus 2011, 211, 101–113. [Google Scholar] [CrossRef]
- Wong, M.W.; Steudel, R. Structure and spectra of tetrasulfur S4—An ab initio MO study. Chem. Phys. Lett. 2003, 379, 162–169. [Google Scholar] [CrossRef]
- Caggiani, M.C.; Mangone, A.; Aquafredda, P. Blue coloured haüyne from Mt. Vulture (Italy) volcanic rocks: SEM-EDS and Raman investigation of natural and heated crystals. J. Raman Spectrosc. 2022, 53, 956–968. [Google Scholar] [CrossRef]
- Bény, C.; Guilhaumou, N.; Touray, J.-C. Native-sulphur-bearing fluid inclusions in the CO2-H2S-H2O-S system—Microthermometry and Raman microprobe (MOLE) analysis—Thermochemical interpretations. Chem. Geol. 1982, 37, 113–127. [Google Scholar] [CrossRef]
- Dubessy, J.; Boiron, M.-C.; Moissette, A.; Monnion, C.; Sretenskaya, N. Determination of water, hydrates and pH in fluid inclusions by micro-Raman spectrometry. Eur. J. Mineral. 1992, 4, 885–894. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Shendrik, R.Y.; Vigasina, M.F.; Pekov, I.V.; Sapozhnikov, A.N.; Shcherbakov, V.D.; Varlamov, D.A. Crystal Chemistry, Isomorphism, and Thermal Conversions of Extra-Framework Components in Sodalite-Group Minerals. Minerals 2022, 12, 887. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Shchipalkina, N.V.; Shendrik, R.Y.; Vigasina, M.F.; Tauson, V.L.; Lipko, S.V.; Varlamov, D.A.; Shcherbakov, V.D.; Sapozhnikov, A.N.; Kasatkin, A.V.; et al. Isomorphism and mutual transformations of S-bearing components in feldspathoids with microporous structures. Minerals 2022, 12, 1456. [Google Scholar] [CrossRef]
- Bolotina, N.B.; Sapozhnikov, A.N.; Chukanov, N.V.; Vigasina, M.F. Structure modulations and symmetry of lazurite-related sodalite-group minerals. Crystals 2023, 13, 768. [Google Scholar] [CrossRef]
- Ivanov, V.G.; Sapozhnikov, A.N. Lazurites of the USSR; Nauka: Novosibirsk, Russia, 1985; p. 172. (In Russian) [Google Scholar]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).