Construction and Demolition Waste Ballast as a Pozzolanic Addition in Binary Cements: Characterization and Thermodynamic Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Ordinary Portland Cement (OPC)
3.2. Rock Samples
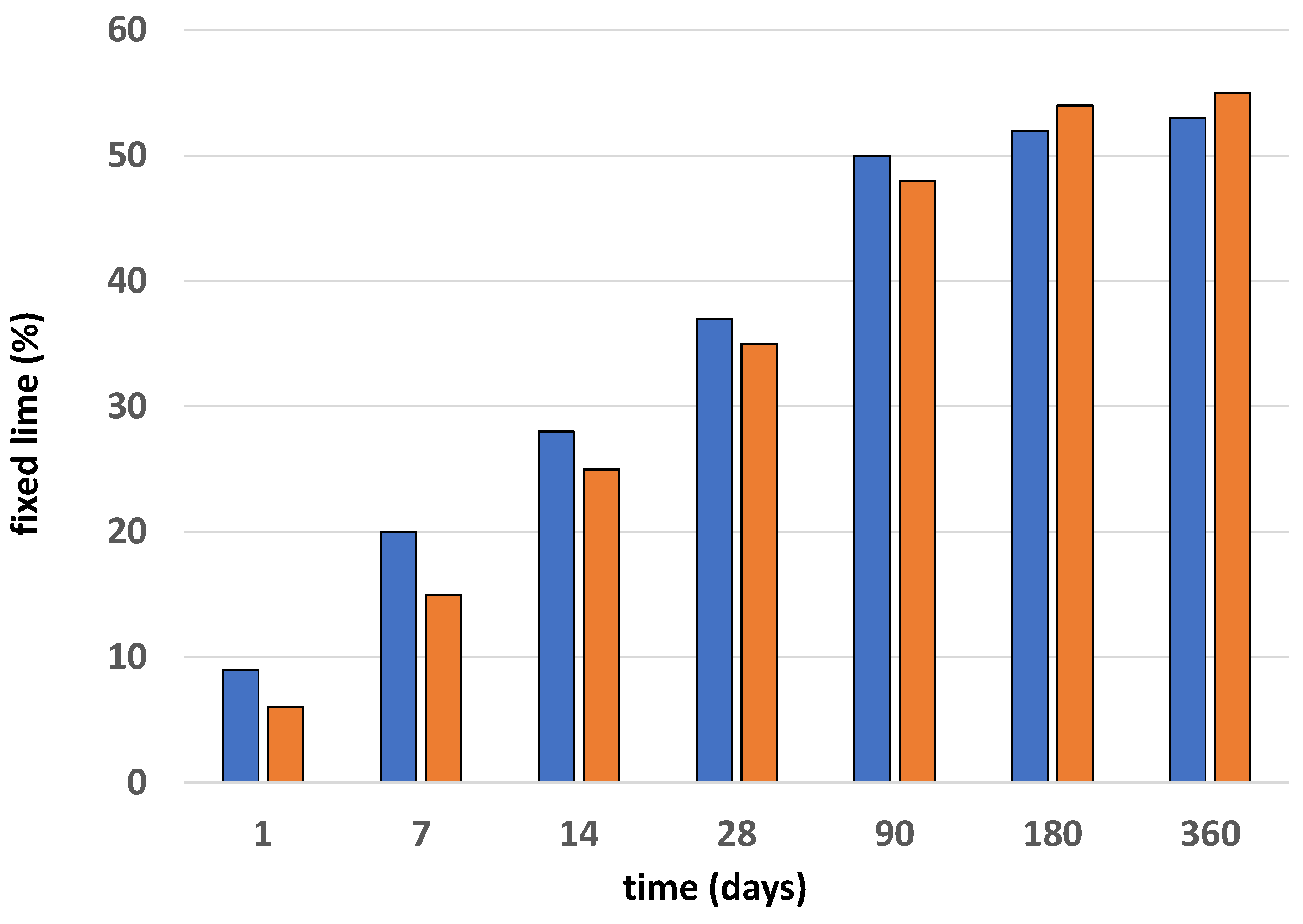
3.3. Pozzolanic Reaction in the Cement Ballast Waste System
3.3.1. Solid Phase
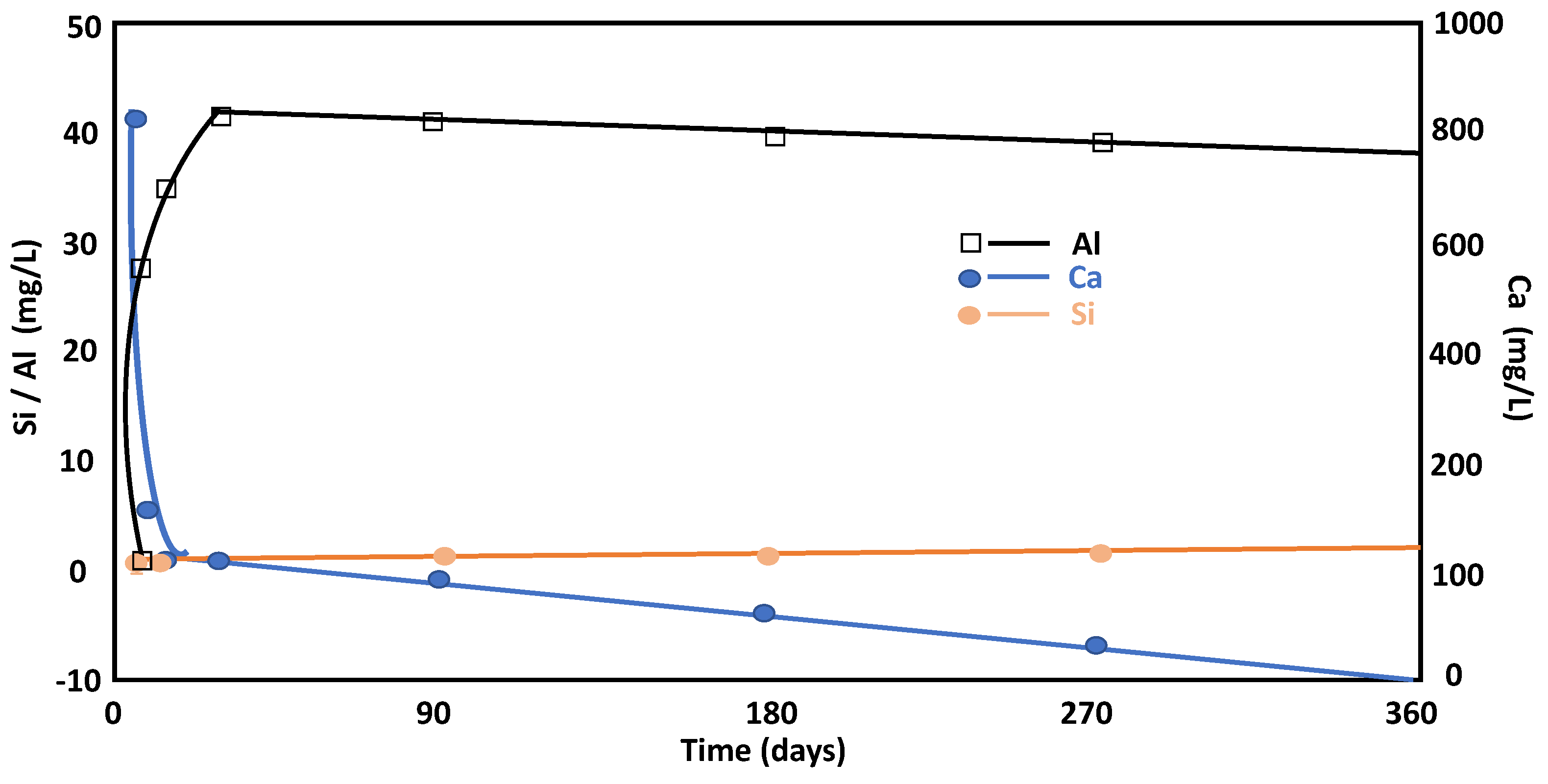
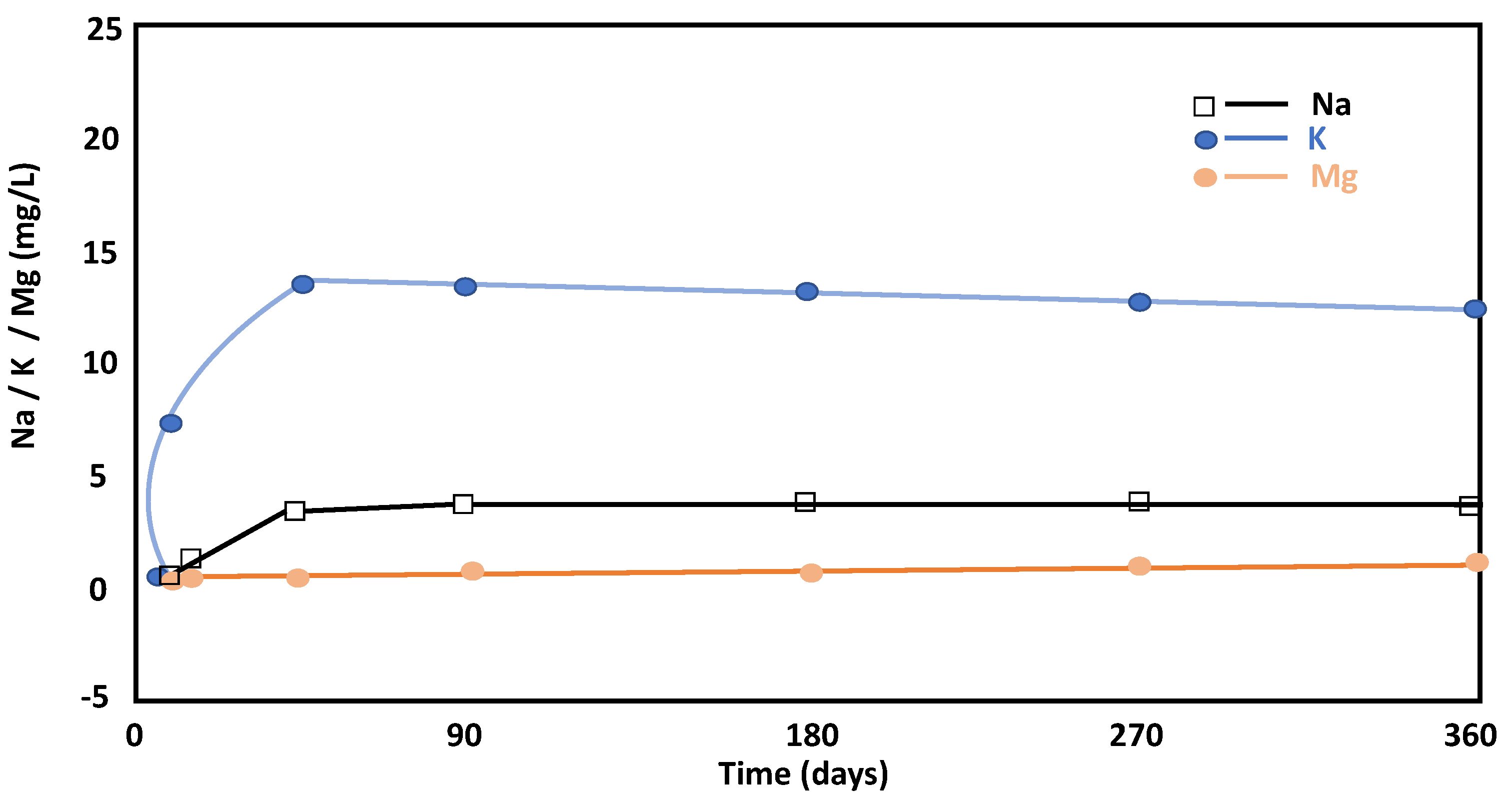
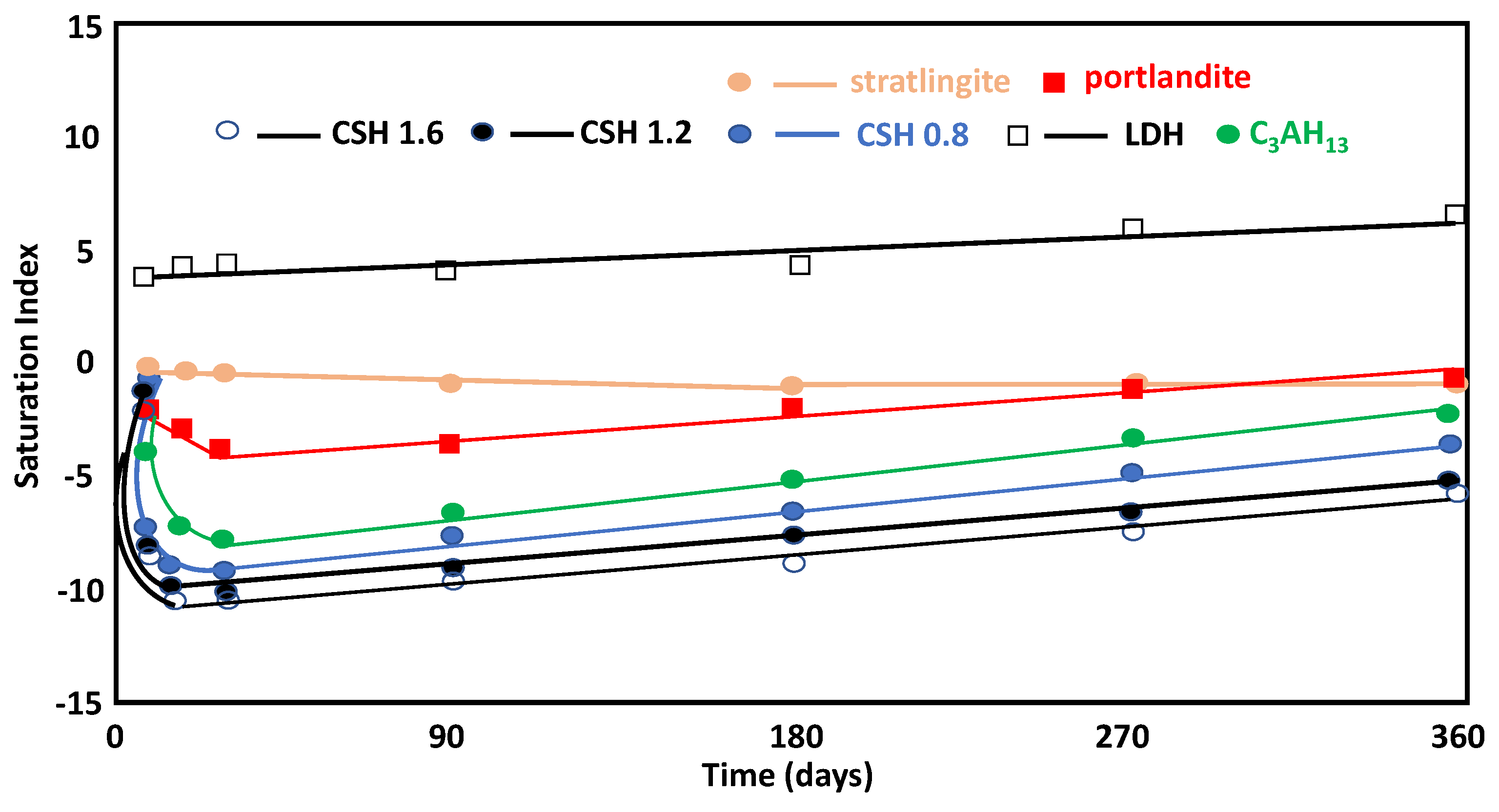
3.3.2. Liquid Phase
4. Conclusions
- The conclusions drawn from the experimental results were as follows. The ballast waste is made up of silicate minerals, such as phyllosilicates 2:1 (biotite), quartz, potassium-rich feldspars in (orthoclase), and plagioclase (labradorite).
- The reactions products were formed from the original materials, except for quartz, which is very stable.
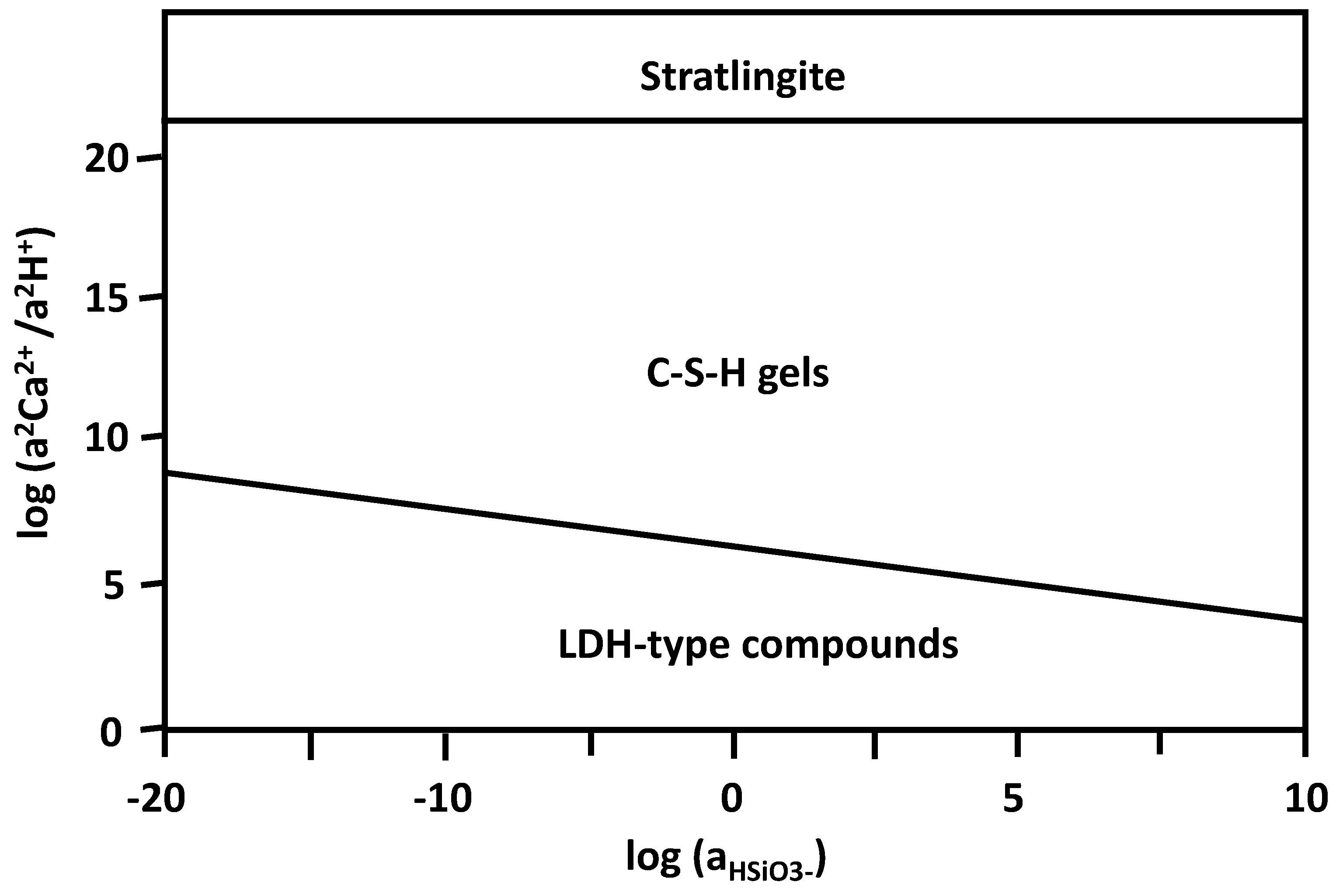
- Stratlingite, LDH-type compounds, and C4AH13 are the main crystalline products of the pozzolanic reaction detected using XRD analysis throughout the reaction.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mvelase, G.; Gräbe, P.; Anochie-Boateng, J. The use of laser technology to investigate the effect of railway ballast roundness on shear strength. Transp. Geotech. 2017, 11, 97–106. [Google Scholar] [CrossRef]
- Kim, J.; Park, B.S.; Woo, S.I.; Choy, Y.T. Evaluation of ballast-track condition based on aggregate-shape characterization. Constr. Build. Mater. 2020, 232, 117082. [Google Scholar] [CrossRef]
- Jawed Roshan, M.; Safuan, A.; Rashid, A.; Abdul Wahab, N.; Tamassoki, S.; Jusoh, S.N.; Azril Hezmi, M.; Nik Daud, N.; Mohd Apandi, N.; Azmi, M. Improved methods to prevent railway embankment failure and subgrade degradation: A review. Transp. Geotech. 2022, 37, 100834. [Google Scholar] [CrossRef]
- Tobón, J.I.; Payá, J.J.; Borrachero, M.V.; Restrepo, O.J. Mineralogical evolution of Portland cement blended with silica nanoparticles and its effect on mechanical strength. Constr. Build. Mater. 2012, 36, 736–742. [Google Scholar] [CrossRef]
- Yagüe García, S.; González Gaya, C. Reusing Discarded Ballast Waste in Ecological Cements. Materials 2019, 12, 3887. [Google Scholar] [CrossRef] [PubMed]
- Yagüe García, S.; Gonzalez Gaya, C. Durability analysis of pozzolanic cements containing recycled track ballast: Sustainability under extreme environmental conditions. Constr. Build. Mater. 2020, 242, 117999. [Google Scholar] [CrossRef]
- Yagüe, S.; Sánchez, I.; Vigil de la Villa, R.; García Giménez, R.; Zapardiel, A.; Frías, M. Coal Mining Tailings as a Pozzolanic Material in Cement Industry. Minerals 2018, 8, 46. [Google Scholar] [CrossRef]
- Lanas, J.; Sirera, R.; Álvarez, J. Compositional changes in lime based mortars exposed to different environments. Thermochim. Acta 2005, 429, 219–226. [Google Scholar] [CrossRef]
- Mehrjardi, G.T.; Alireza Azizi, A.; Haji-Azizi, A.; Asdollafardi, G. Evaluating and improving the construction and demolition waste technical properties to use in road construction. Transp. Geotech. 2020, 23, 100349. [Google Scholar] [CrossRef]
- Bian, Z.; Dong, J.; Lei, S.; Leng, H. The impact of disposal and treatment of coal mining wastes on environment and farmland. Environ. Geol. 2009, 58, 625–634. [Google Scholar] [CrossRef]
- Damidot, D.; Stronach, S.; Kindness, A.; Atkins, M.; Glasser, F.P. Thermodynamic investigation of the CaO_Al2O3_CaCO3-H2O closed system at 25 °C and the influence of Na2O. Cem. Concr. Res. 1994, 24, 563–572. [Google Scholar] [CrossRef]
- Qian, Y.; Boler, H.; Moaveni, M.; Tutumluer, E.; Hashash, Y.; Ghaboussi, J. Characterizing ballast degradation through Los Angeles abrasion test and image analysis. Transp. Res. Rec. 2014, 2448, 142–151. [Google Scholar] [CrossRef]
- Guo, Y.; Markine, V.; Song, J.; Jing, G. Ballast degradation: Effect of particle size and shape using Los Angeles abrasion test and image analysis. Constr. Build. Mater. 2018, 169, 414–424. [Google Scholar] [CrossRef]
- UNE-EN 1097-2; Ensayos Para Determinar Las Propiedades Mecánicas y Físicas de los Áridos. Parte 2: Métodos Para la Determinación de la Resistencia a la Fragmentación. Asociación Española de Normalización y Certificación: Madrid, Spain, 2010.
- Ostrowski, K.; Stefaniuk, D.; Sadowski, L.; Krzywinski, K.; Rozanska, M. Potential use of granite waste sourced from rock processing for the application as coarse aggregate in high-performance self-compacting concrete. Constr. Build. Mater. 2020, 238, 117–794. [Google Scholar] [CrossRef]
- Yagüe, S.; González Gaya, C.; Rosales Prieto, V.; Sánchez Lite, A. Sustainable Ecocements: Chemical and Morphological Analysis of Granite Sawdust Waste as Pozzolan Material. Materials 2020, 13, 4941. [Google Scholar] [CrossRef]
- UNE-EN 197-1; Cement Part 1: Composition, Specifications and Conformity Criteria for Common Cements. Asociación Española de Normalización y Certificación: Madrid, Spain, 2011.
- Parkhurst, D.J.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-reaction, One-dimensional Transport and Inverse Geochemical Calculations. US Geol. Surv. Tech. Methods 2013, 6, 497. [Google Scholar]
- Blanc, P.; Lassin, A.; Piantone, P.; Burnol, A. Thermoddem a Database Devoted to Waste Minerals; BRGM: Orleans, France, 2007; p. 6009. Available online: http://thermoddem.brgm.fr (accessed on 10 June 2022).
- Mosaferi, M.; Fahiminia, M.; Nabizadeh, R. Environmental management of the solid wastes of stone cutting industries: A case study of Qom Province. Int. J. Environ. Sci. Technol. 2007, 9, 65–73. [Google Scholar]
- Nasserdine, K.; Mimi, Z.; Bevan, B.; Elian, B. Environmental management of the stone cutting industry. J. Environ. Manag. 2009, 90, 466–470. [Google Scholar] [CrossRef]
- Medina, G.; Sáez del Bosque, I.; Frías, M.; Sánchez de Rojas, M.I.; Medina, C. Mineralogical study of granite waste in a pozzolan/Ca(OH)2 system. Influence of the activation process. Appl. Clay Sci. 2017, 135, 362–371. [Google Scholar] [CrossRef]
- Gallucci, E.; Zhang, X.; Scrivener, K. Effect of temperature on the microstructure of calcium silicate hydrate (C-S-H). Cem. Concr. Res. 2013, 53, 185–195. [Google Scholar] [CrossRef]
- García, R.; Vigil de la Villa, R.; Frías, M.; Rodríguez, O.; Martínez-Ramírez, S.; Fernández-Carrasco, L.; de Soto, I.S.; Villar-Cociña, E. Mineralogical study of calcined coal waste in a pozzolan/Ca(OH)2 system. Appl. Clay Sci. 2015, 108, 45–54. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford: London, UK, 1997. [Google Scholar]
- Peng, Z.; Shi, C.; Shi, Z.; Lu, B.; Wan, S.; Zhang, Z.; Chang, J.; Zhang, T. Alkali-aggregate reaction in recycled aggregate concrete. J. Clean. Prod. 2020, 255, 120238. [Google Scholar] [CrossRef]
- De Silva, P.S.; Glasser, F.P. Phase relations in the system CaO-Al2O3-SiO2-H2O relevant to MK-Calcium Hydroxide. Cem. Concr. Res. 1993, 23, 627–639. [Google Scholar] [CrossRef]
- Madadi, A.; Wei, J. Characterization of Calcium Silicate Hydrate Gels with Different Calcium to Silica Ratios and Polymer Modifications. Gels 2022, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, D.; Thomas, J.J.; Christensen, B.J.; Jennings, H.M. Solubility behavior of Ca-, S-, Al-, and Si-bearing solid phases in Portland cement pore solutions as a function of hydration time. Cem. Concr. Res. 2002, 32, 1663–1671. [Google Scholar] [CrossRef]
- Lothenbach, B.; Le Saout, G.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Tironi, A.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Potential use of Argentine kaolinitic clays as pozzolanic material. Appl. Clay Sci. 2014, 101, 468–476. [Google Scholar] [CrossRef]
- Holmes, N.; Tyrer, M.; West, R.P.; Lowe, A.; Kelliher, D. Using PHREEQC to model cement hydration. Constr. Build Mater. 2022, 319, 126–129. [Google Scholar] [CrossRef]
- Fernández, R.; Nebreda, B.; Vigil de la Villa, R.; García, R.; Frías, M. Mineralogical and chemical evolution of hydrated phases in the pozzolanic reaction of calcined paper sludge. Cem. Concr. Comp. 2020, 32, 775–782. [Google Scholar] [CrossRef]


| Oxide | SiO2 | Al2O3 | Fe2O3 | CaO | Na2O | K2O | TiO2 | P2O5 | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | 20.16 | 4.36 | 2.52 | 63.41 | 0.35 | 0.91 | 0.21 | 0.14 | 3.57 | 1.9 |
| Sample | Biotite (%) | Quartz (%) | Orthoclase (%) | Albite (%) | Labradorite (%) | Kaolinite (%) | Hematite (%) | Amorphous Material (%) | RB | χ2 |
|---|---|---|---|---|---|---|---|---|---|---|
| BW1 | 30 | 29 | 6 | 18 | n.d. | 7 | 4 | 6 | 16.5 | 3.3 |
| BW2 | 35 | 21 | 5 | n.d. | 23 | 6 | 4 | 6 | 12.3 | 3.5 |
| Oxides (%) | BW2 | BW1 |
|---|---|---|
| Na2O | 4.78 | 2.97 |
| MgO | 0.95 | 1.72 |
| Al2O3 | 19.60 | 13.79 |
| K2O | 4.06 | 2.85 |
| CaO | 3.26 | 2.77 |
| MnO | 0.03 | 0.11 |
| TiO2 | 0.37 | 0.80 |
| Fe2O3 | 3.49 | 6.72 |
| SiO2 | 63.49 | 68.27 |
| LOI | 1.22 | 0.67 |
| Sample | Reaction Time (Days) | Q (%) | F (%) | Fi (%) | LDH (%) | St (%) | C3AH13 (%) | P (%) | Ca (%) | AM (%) | X2 | RB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW1 | 1 | 25 | 18 | 15 | 4 | n.d. | 1 | 27 | 6 | 4 | 11.2 | 4.3 |
| BW2 | 1 | 21 | 24 | 27 | traces | n.d. | n.d. | 23 | 3 | 2 | 11.5 | 6.2 |
| BW1 | 7 | 26 | 15 | 12 | 4 | n.d. | 1 | 24 | 8 | 10 | 13.6 | 5.8 |
| BW2 | 7 | 21 | 22 | 25 | traces | n.d. | n.d. | 18 | 4 | 10 | 14.8 | 6.3 |
| BW1 | 28 | 30 | 14 | 10 | 4 | n.d. | 4 | 16 | 10 | 12 | 12.6 | 4.2 |
| BW2 | 28 | 25 | 15 | 20 | 5 | n.d. | 5 | 11 | 7 | 12 | 11.9 | 5.7 |
| BW1 | 90 | 29 | 12 | 8 | 4 | 2 | 6 | 13 | 11 | 15 | 13.4 | 4.2 |
| BW2 | 90 | 30 | 11 | 7 | 5 | 1 | 6 | 15 | 13 | 12 | 14.8 | 5.6 |
| BW1 | 180 | 30 | 9 | 4 | 7 | 2 | 10 | 12 | 10 | 16 | 15.8 | 3.9 |
| BW2 | 180 | 29 | 8 | 4 | 7 | 3 | 10 | 14 | 9 | 16 | 16.3 | 6.2 |
| BW1 | 360 | 30 | 8 | 4 | 10 | 2 | 11 | 9 | 6 | 18 | 14.3 | 4.8 |
| BW2 | 360 | 29 | 8 | 3 | 11 | 3 | 12 | 7 | 5 | 22 | 15.0 | 5.3 |
| Oxides (%) | C-S-H Gels | LDH | Stratlingite | Portlandite | Aluminate |
|---|---|---|---|---|---|
| Al2O3 | 18.53 ± 0.43 | 24.59 ± 1.15 | 20.72 ± 0.54 | n.d. | 24.32 ± 0.41 |
| SiO2 | 26.59 ± 1.18 | 5129 ± 1.12 | 15.56 ± 0.81 | n.d. | 14.50 ± 1.22 |
| CaO | 44.53 ± 1.59 | 32.20 ± 1.15 | 63.77 ± 2.36 | 100 | 61.18 ± 2.06 |
| CaO/Al2O3 | 2.42 | 1.18 | 3.07 | - | 2.51 |
| CaO/SiO2 | 1.58 | 0.82 | 4.09 | - | 4.21 |
| SiO2/Al2O3 | 1.47 | 1.39 | 0.75 | - | 0.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yagüe-García, S.; García-Giménez, R. Construction and Demolition Waste Ballast as a Pozzolanic Addition in Binary Cements: Characterization and Thermodynamic Stability. Minerals 2024, 14, 402. https://doi.org/10.3390/min14040402
Yagüe-García S, García-Giménez R. Construction and Demolition Waste Ballast as a Pozzolanic Addition in Binary Cements: Characterization and Thermodynamic Stability. Minerals. 2024; 14(4):402. https://doi.org/10.3390/min14040402
Chicago/Turabian StyleYagüe-García, Santiago, and Rosario García-Giménez. 2024. "Construction and Demolition Waste Ballast as a Pozzolanic Addition in Binary Cements: Characterization and Thermodynamic Stability" Minerals 14, no. 4: 402. https://doi.org/10.3390/min14040402
APA StyleYagüe-García, S., & García-Giménez, R. (2024). Construction and Demolition Waste Ballast as a Pozzolanic Addition in Binary Cements: Characterization and Thermodynamic Stability. Minerals, 14(4), 402. https://doi.org/10.3390/min14040402







