Thermal Behavior of Ceramic Bodies Based on Fly Ash and Smectites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Chemical and Mineralogical Composition of Smectites and PT/Smectite Mixtures
3.1.1. X-ray Fluorescence
3.1.2. X-ray Powder Diffraction
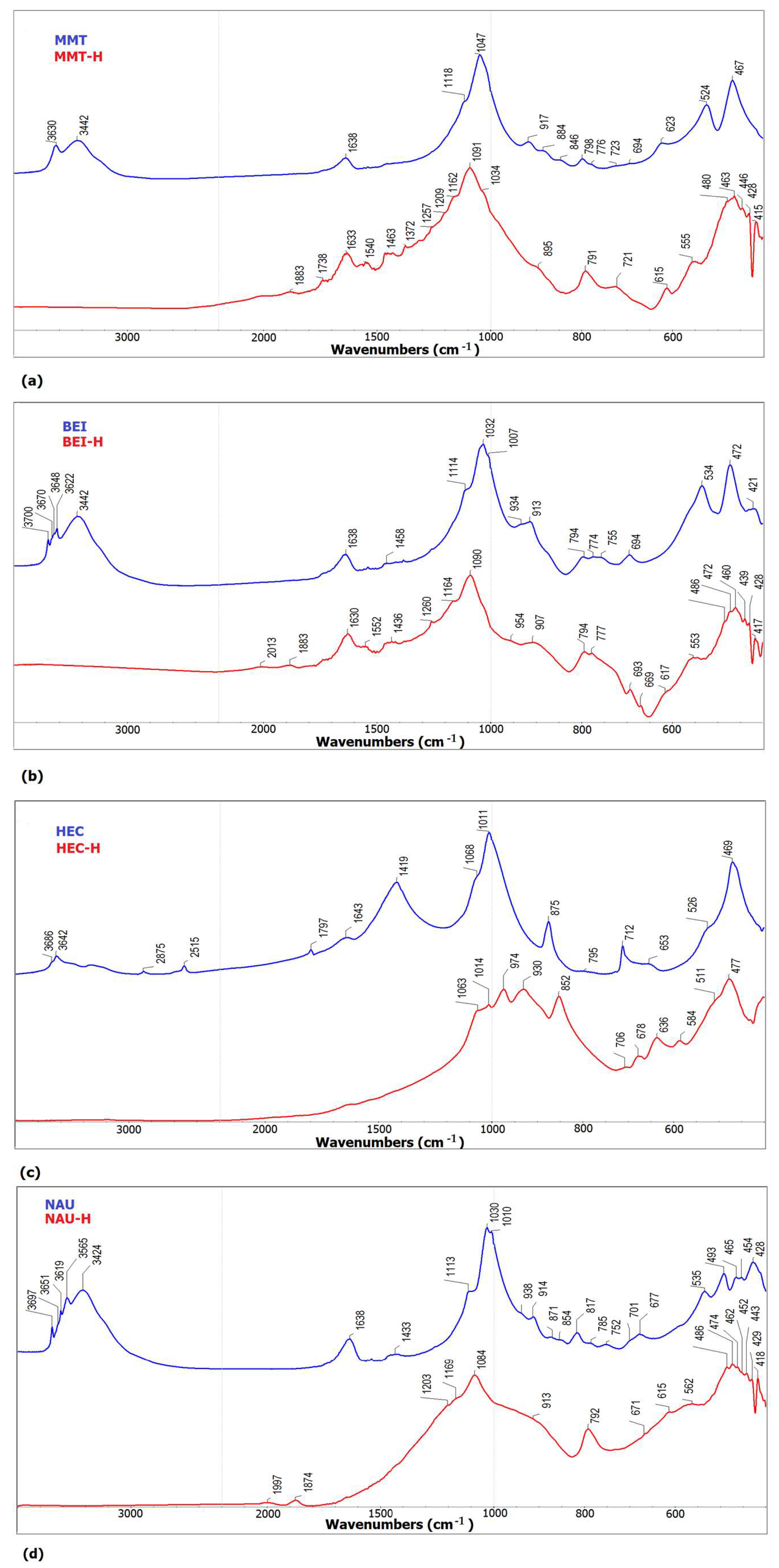
3.1.3. FT-IR Spectroscopy
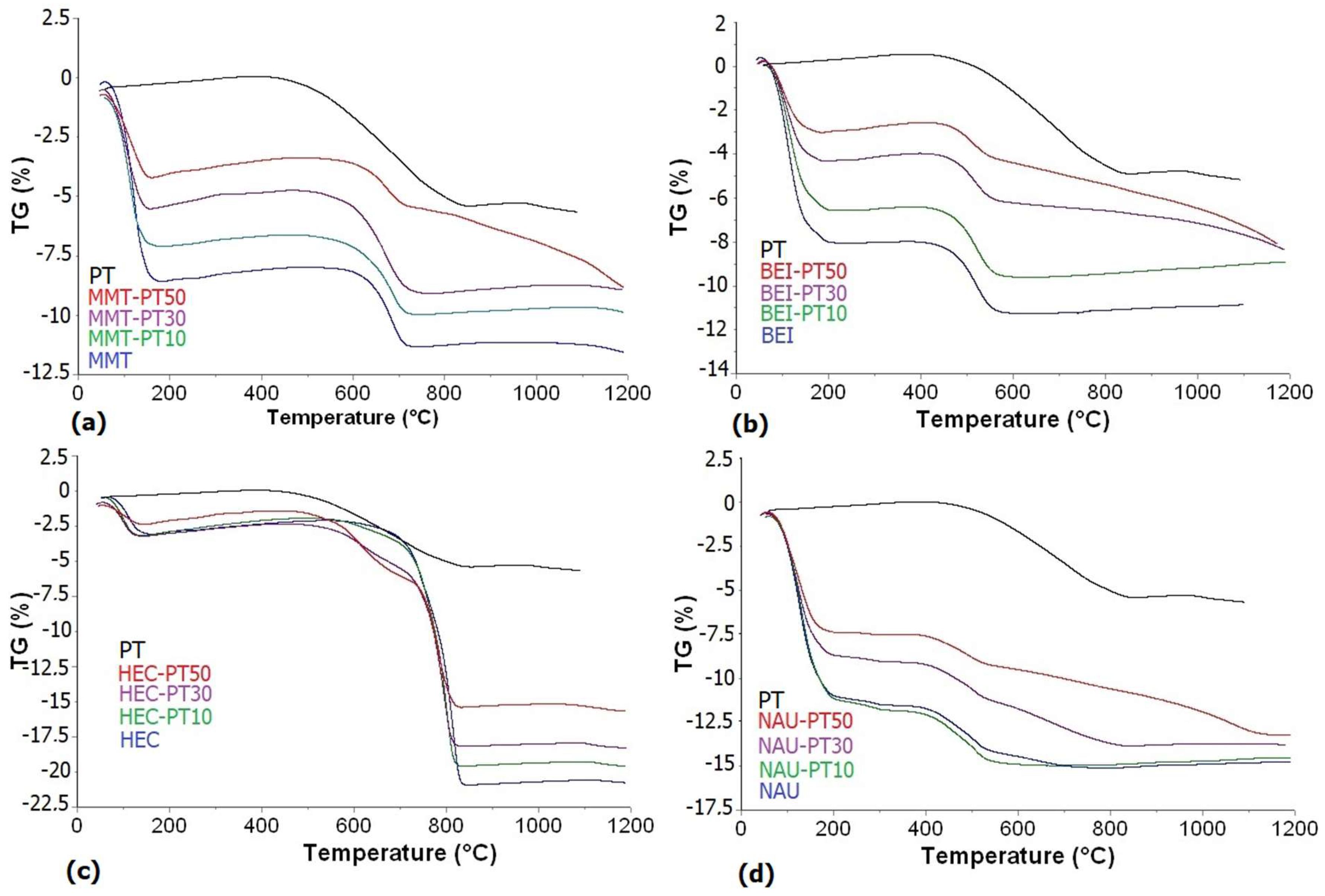
3.2. Thermal Analysis and Transformation of Smectites and PT/Smectite Mixtures
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murali, K.; Sambath, K.; Hashir, S.A. Review on Clay and its Engineering Significance. Int. J. Sci. Res. Public 2018, 8, 8–11. [Google Scholar]
- Singh, N.B. Clays and clay minerals in construction industry. Minerals 2022, 12, 301. [Google Scholar] [CrossRef]
- Laird, D.A. Layer charge influences on the hydration of expandable 2:1 phyllosilicates. Clay Clay Miner. 1999, 47, 630–636. [Google Scholar] [CrossRef]
- Luo, W.; Zeng, Z.; Bian, L. Effect of Lattice Substitution on Adsorption of Hexavalent Chromium by Montmorillonite, Nontronite, and Beidellite. Minerals 2021, 11, 1407. [Google Scholar] [CrossRef]
- Dellisanti, F.; Minguzzi, V.; Valdre, G. Thermal and structural properties of Ca-rich Montmorillonite mechanically deformed by compaction and shear. Appl. Clay Sci. 2006, 31, 282–289. [Google Scholar] [CrossRef]
- Viani, B.E.; Low, P.F.; Roth, C.B. Direct measurement of the relation between interlayer force and interlayer distance in the swelling of montmorillonite. J. Colloid Interface Sci. 1983, 96, 229–244. [Google Scholar] [CrossRef]
- Wu, J.; Low, P.F.; Roth, C.B. Effects of octahedral-iron reduction and swelling pressure on interlayer distances in Na-montmorillonite. Clay Clay Miner. 1989, 37, 211–218. [Google Scholar]
- Guggenheim, S.; Martin, R.T. Definition of Clay and Clay Mineral: Joint Report of the Aipea Nomenclature and CMS Nomenclature Committees. Clay Clay Miner. 1995, 43, 255–256. [Google Scholar] [CrossRef]
- Velde, B. Introduction to Clay Minerals: Chemistry—Origins, Uses and Environmental Significance, 1st ed.; Chapman&Hall: London, UK, 1992; p. 92. [Google Scholar]
- Tao, L.; Xiao-Feng, T.; Yu, Z.; Tao, G. Swelling of K+, Na+ and Ca2+-montmorillonites and hydration of interlayer cations: A molecular dynamics simulation. Chin. Phys. B 2010, 19, 109101. [Google Scholar] [CrossRef]
- Grim, R.E. Clay Mineralogy. International Series in the Earth and Planetary Sciences, 1st ed.; McGraw-Hill Book Company: New York, NY, USA, 1986; pp. 77–92. [Google Scholar]
- Odom, I.E. Smectite clay minerals: Properties and uses. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1984, 311, 391–409. [Google Scholar]
- Ombaka, O. Characterization and classification of clay minerals for potential applications in Rugi Ward, Kenya. Afr. J. Environ. Sci. Technol. 2016, 10, 415–431. [Google Scholar]
- Murray, H.H. Overview, clay mineral applications. Appl. Clay Sci. 1991, 5, 379–395. [Google Scholar] [CrossRef]
- El Hammouti, A.; Charai, M.; Channouf, S.; Horma, O.; Nasri, H.; Mezrhab, A.; Karkri, M.; Tankari, M.A. Laboratory-testing and industrial scale performance of different clays from eastern Morocco for brick manufacturing. Constr. Build. Mater. 2023, 370, 130624. [Google Scholar] [CrossRef]
- Zhang, L.Y. Production of bricks from waste materials—A review. Constr. Build. Mater. 2013, 47, 643–655. [Google Scholar] [CrossRef]
- Wang, S.; Gainey, L.; Mackinnon, I.D.R.; Allen, C.; Gu, Y.; Xi, Y. Thermal behaviors of clay minerals as key components and additives for fired brick properties: A review. J. Build. Eng. 2023, 66, 105802. [Google Scholar] [CrossRef]
- Kijo-Kleczkowska, A.; Szumera, M.; Gnatowski, A.; Sadkowski, D. Comparative thermal analysis of coal fuels, biomass, fly ash and polyamide. Energy 2022, 259, 124840. [Google Scholar] [CrossRef]
- Zacco, A.; Borgese, L.; Giaconcelli, A.; Struis, R.P.W.J.; Depero, L.E.; Bontempi, E. Review of fly ash inertisation treatments and recycling. Environ. Chem. Lett. 2014, 12, 153–175. [Google Scholar] [CrossRef]
- Wang, H.; Yhu, M.; Sun, Z.; Ji, R.; Liu, L.; Wang, X. Synthesis of a ceramic tile base based on high-alumina fly ash. Constr. Build. Mater. 2017, 155, 930–938. [Google Scholar] [CrossRef]
- Hossain, S.S.; Roy, P. Sustainable ceramics derived from solid wastes: A review. J. Asian Ceram. Soc. 2020, 8, 984–1009. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, H.; Jiang, H.H.; Zhang, W.Y.; Mao, L.Q. Recycling municipal solid waste incineration fly ash in fired bricks: An evaluation of physical-mechanical and environmental properties. Construct. Build. Mater. 2021, 294, 1234765. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, F.; Zhou, H.; Li, Q.; Shang, S. Study on the performance and reaction mechanism of alkali-activated clay brick with steel slag and fly ash. Constr. Build. Mater. 2024, 411, 134406. [Google Scholar] [CrossRef]
- Muñoz Velasco, P.; Morales Ortíz, M.P.; Mendívil Giró, M.A.; Muńoz Velasco, L. Fired clay bricks manufactured by adding wastes as sustainable construction material—A review. Constr. Build. Mater. 2014, 63, 97–107. [Google Scholar] [CrossRef]
- Jimenez-Garcia, E.; Arellano-Vazquez, D.; Titotto, S.; Vilchis-Nestor, A.; Mayorga, M.; Romero-Salazar, L.; Arteaga-Arcos, J. A low environmental impact admixture for the elaboration of unfired clay building bricks. Constr. Build. Mater. 2023, 407, 133470. [Google Scholar] [CrossRef]
- Ibrahim, J.E.; Tihtih, M.; Tihtih, M.; Şahin, E.; Basyooni, M.A.; Kocserha, I. Sustainable zeolitic tuff incorporating tea waste fired ceramic bricks: Development and investigation. Case Stud. Construct. Mater. 2023, 19, e02238. [Google Scholar] [CrossRef]
- Hmeid, H.A.; Akokad, M.; Baghour, M. Preliminary characterization and potential use of different clay materials from North-Eastern Morocco in the ceramic industry. Mater. Today—Proc. 2022, 58, 1277–1284. [Google Scholar] [CrossRef]
- Lahcen, D.; Hicham, E.E.; Latifa, S.; Abderrahmane, A.; Jamal, B.; Mohamed, W.; Meriam, E.; Nathalie, F. Characteristics and ceramic properties of clayey materials from Amezmiz region (Western High Atlas, Morocco). Appl. Clay Sci. 2014, 102, 139–147. [Google Scholar] [CrossRef]
- Wang, S.; Gainey, L.; Baxter, D.; Wang, X.; Mackinnon, I.D.; Xi, Y. Thermal behaviours of clay mixtures during brick firing: A combined study of in-situ XRD, TGA and thermal dilatometry. Constr. Build. Mater. 2021, 299, 124319. [Google Scholar] [CrossRef]
- Vasić, M.V.; Pezo, L.; Zdravković, J.; Bačkalić, Z.; Radojević, Z. The study of thermal behavior of montmorillonite and hydromica brick clays in predicting tunnel kiln firing curve. Constr. Build. Mater. 2017, 150, 872–879. [Google Scholar] [CrossRef]
- Baccour, H.; Medhioub, M.; Jamoussi, F.; Mhiri, T. Influence of firing temperature on the ceramic properties of Triassic clays from Tunisia. J. Mater. Process. Technol. 2009, 209, 2812–2817. [Google Scholar] [CrossRef]
- Wang, S.; Gainey, L.; Wang, X.; Mackinnon, I.D.; Xi, Y. Influence of palygorskite on in-situ thermal behaviours of clay mixtures and properties of fired bricks. Appl. Clay Sci. 2022, 216, 106384. [Google Scholar] [CrossRef]
- Christogerou, A.; Lampropoulou, P.; Papoulis, D.; Angelopoulos, G.N. Feasibility Study on the Potential Replacement of Primary Raw Materials in Traditional Ceramics by Clayey Overburden Sterile from the Prosilio Region (Western Macedonia, Greece). Minerals 2021, 11, 961. [Google Scholar] [CrossRef]
- Moll, W.F. Baseline studies of the clay minerals society source clays: Geological origin. Clay Clay Miner. 2001, 49, 374–380. [Google Scholar] [CrossRef]
- Mermut, A.; Cano, A.F. Studies of the clay minerals society source clays: Chemical analysis of major elements. Clay Clay Miner. 2001, 49, 381–386. [Google Scholar] [CrossRef]
- Guggenheim, S.; Koster van Groos, A.F. Baseline studies of The Clay Minerals Society Source Clays: Thermal analysis. Clay Clay Miner. 2001, 49, 430–440. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Frost, R.L.; Kickey, L. Infrared emission spectroscopic study of the dehydroxylation of some hectorites. Thermochim. Acta 2000, 345, 145–156. [Google Scholar] [CrossRef]
- Chipera, S.J.; Bish, D.L. Studies of the clay minerals society source clays: Powder X-ray diffraction analysis. Clay Clay Miner. 2001, 49, 398–409. [Google Scholar] [CrossRef]
- Mukasa-Tebandeke, I.Z.; Mukasa-Tebandeke, I.Z.; Ssebuwufu, P.J.M.; Nyanzi, S.A.; Schumann, A.; Nyakairu, G.W.A.; Ntale, M.; Lugolobi, F. The Elemental, Mineralogical, IR, DTA and XRD Analyses Characterized Clays and Clay Minerals of Central and Eastern Uganda. Adv. Mater. Phys. Chem. 2015, 5, 67–86. [Google Scholar] [CrossRef]
- Madejová, J.; Bujdák, J.; Janek, M.; Komadel, P. Comparative FT-IR study of structural modifications during acid treatment of dioctahedral smectites and hectorite. Spectrochim. Acta A 1998, 54, 1397–1406. [Google Scholar] [CrossRef]
- Vaculíková, L.; Plevová, E.; Ritz, M. Characterization of Montmorillonites by Infrared and Raman Spectroscopy for Preparation of Polymer-Clay Nanocomposites. J. Nanosci. Nanotechnol. 2019, 5, 2775–2781. [Google Scholar] [CrossRef]
- Apeiranthitis, N.; Greenwell, H.C.; Carteret, C. Far- and mid-infrared examination of nontronite-1 clay mineral Redox and cation saturation effects. Appl. Clay Sci. 2022, 228, 106628. [Google Scholar] [CrossRef]
- Madejová, J.; Komadel, P. Baseline study of the clay minerals society source clays: Infrared methods. Clay Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Che, C.; Greenwell, H.C.; Carteret, C. Spectroscopic study of the dehydration and/or dihydroxylation of phyllosilicate and zeolite minerals. J. Geophys. Res. 2011, 116, E05007. [Google Scholar] [CrossRef]
- Yahagi, Y.; Yagi, T. Infrared absorption spectra of the high/pressure phases of cristobalite and their coordination numbers of silicon atoms. Solid State Commun. 1994, 89, 945–948. [Google Scholar] [CrossRef]
- Gören, R.; Ersoy, B.; Özgür, C.; Alp, T. Colloidal stability–slip casting behavior relationship in slurry of mullite synthesized by the USP method. Ceram. Int. 2012, 38, 679–685. [Google Scholar] [CrossRef]
- Xi, H.J.; Gao, L.; Guo, J.K. The structural change of diphasic mullite gel studied by XRD and IR spectrum analysis. J. Eur. Ceram. Soc. 2002, 22, 1307–1311. [Google Scholar]
- Ritz, M. Infrared and raman spectroscopy of mullite ceramics synthesized from fly ash and kaolin. Minerals 2023, 13, 864. [Google Scholar] [CrossRef]
- Giggis, B.S.; Felix, N.S.; Barawy, K.A. Dehydroxylation kinetics of some pure smectites. Thermochim. Acta 1987, 112, 265–274. [Google Scholar] [CrossRef]
- Vaculíková, L.; Plevová, E. Identification of clay minerals and micas in sedimentary rocks. Acta Geodyn. Geomater. 2005, 2, 167–175. [Google Scholar]
- Blažek, A. Book of Thermal Analysis, 1st ed.; SNTL: Prague, Czech Republic, 1974; pp. 208–209. [Google Scholar]
- Šajnor, V.S.; Jesenak, K. Differential thermal analysis of montmorillonite. J. Therm. Anal. Calorim. 1996, 46, 489–493. [Google Scholar]
- Ding, Z.; Frost, L.R. Controlled rate thermal analysis of nontronite. Thermochim. Acta 2002, 389, 185–193. [Google Scholar] [CrossRef]
- Gavin, P.; Chevrier, V.; Rochette, P.; Keck, W.M. Thermally transformed nontronite as a component of red dust layer on Mars. In Proceedings of the 38th Lunar and Planetary Science Conference, (Lunar and Planetary Science XXXVIII), League City, TX, USA, 12–16 March 2007; Volume 38, p. 2295. [Google Scholar]
- Plevová, E.; Vaculíková, L.; Valovičová, V. Thermal analysis and FTIR spectroscopy of synthetic clay mineral mixtures. J. Therm. Anal. Calorim. 2020, 142, 507–518. [Google Scholar] [CrossRef]
- Achik, M.; Benmoussa, H.; Oulmekki, A.; Ijjaali, M.; El Moudden, N.; Touache, A.; Álvaro, G.G.; Rivera, F.G.; Infantes-Molina, A.; Eliche-Quesada, D.; et al. Evaluation of technological properties of fired clay bricks containing pyrrhotite ash. Constr. Build. Mater. 2021, 269, 121312. [Google Scholar] [CrossRef]
- Valášková, M.; Blahůšková, V.; Vlček, J. Effects of Kaolin Additives in Fly Ash on Sintering and Properties of Mullite Ceramics. Minerals 2021, 11, 887. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, K.K.; Ramachandrarao, P. Effects of fly ash additions on the mechanical and other properties of porcelainised stoneware tiles. J. Mater. Sci. 2001, 36, 5917–5922. [Google Scholar] [CrossRef]
- Koukouzas, N.; Ketikidis, C.; Itskos, G.; Spiliotis, X.; Karayannis, V.; Papapolymerou, G. Synthesis of CFB-Coal Fly Ash Clay Bricks and Their Characterisation. Waste Biomass Valoriz. 2011, 2, 87–94. [Google Scholar] [CrossRef]
- Pranav, S.; Singhal, A.; Routroy, S.; Bhunia, D.; Rotta Loria, F.A.; Lahoti, M. Fired clay bricks synergistically valorizing hazardous nickel chrome-plating sludge and fly ash: Performance assessment. Constr. Build. Mater. 2024, 423, 135817. [Google Scholar]


| Sample | Mineral | Locality | Origin | Description |
|---|---|---|---|---|
| MMT | Montmorillonite | Crook County, Wyoming, USA | Newcastle formation (Cretaceous) | Na-rich montmorillonite |
| BEI | Beidelite | Idaho, USA | USA, stratigraphy uncertain | Beidelite with crystalline silica |
| HEC | Hectorite | San Bernardino County, California, USA | Red Mountain Andesite formation (Pliocene) | Li-bearing trioctahedral hectorite |
| NAU | Nontronite | Uley Mine—South Australia | Graphite mine, South Australia | Green color, Al-enriched |
| Sample | Mass % | |||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | MgO | Na2O | CaO | Fe2O3 | TiO2 | K2O | |
| MMT | 61.2 | 17.8 | 2.5 | 1.4 | 1.5 | 3.7 | 0.1 | 0.6 |
| BEI | 60.0 | 21.4 | 0.5 | <0.05 | 0.7 | 1.5 | 0.8 | 0.9 |
| HEC | 34.9 | 0.8 | 14.2 | 0.9 | 23.5 | 0.3 | 0.1 | 0.5 |
| NAU | 42.2 | 13.2 | 0.6 | <0.05 | 1.3 | 24.2 | 0.8 | 0.4 |
| PT | 44.5 | 23.2 | 1.6 | 0.6 | 2.3 | 7.5 | 1.1 | 3.2 |
| Sample | Tm1 (°C) | Tm2 (°C) | Tm3 (°C) | Tm4 (°C) | Tm5 (°C) |
|---|---|---|---|---|---|
| MMT | 127 | 265 | – | 693 | 941 |
| MMT-PT10 | 119 | 249 | – | 691 | 936 |
| MMT-PT30 | 116 | 239 | – | 691 | 934 |
| MMT-PT50 | 111 | 238 | – | 694 | 929 |
| BEI | 112 | 177 | – | 523 | 999 |
| BEI-PT10 | 115 | 175 | 514 | 998 | |
| BEI-PT30 | 117 | 175 | 514 | 996 | |
| BEI-PT50 | 117 | 170 | – | 501 | 998 |
| HEC | 122 | 150 | 751 | 817 | 1092 |
| HEC-PT10 | 106 | 149 | 757 | 803 | 1072 |
| HEC-PT30 | 106 | 144 | 759 | 801 | 1069 |
| HEC-PT50 | 102 | 143 | 762 | 799 | 1063 |
| NAU | 135 | 196 | 448 | 512 | 881 |
| NAU-PT10 | 127 | 187 | 445 | 511 | 881 |
| NAU-PT30 | 124 | 187 | 439 | 509 | – |
| NAU-PT50 | 120 | 183 | 434 | 500 | – |
| Sample | ∆ mtot (%) | ∆ m1 20–500 °C (%) | ∆ m2 500–850 °C (%) |
|---|---|---|---|
| PT | –5.1 | 0.1 | −4.7 |
| MMT | –11.2 | −7.7 | −3.2 |
| MMT-PT10 | −9.1 | −5.7 | −3.1 |
| MMT-PT30 | −8.4 | −4.1 | −3.7 |
| MMT-PT50 | −8.0 | −2.6 | −4.2 |
| BEI | −11.4 | −9.3 | −2.0 |
| BEI-PT10 | −8.9 | −7.7 | −1.9 |
| BEI-PT30 | −8.4 | −4.8 | −2.5 |
| BEI-PT50 | −8.1 | −3.4 | −2.9 |
| HEC | −20.2 | −1.6 | −18.2 |
| HEC-PT10 | −19.1 | −1.5 | −17.3 |
| HEC-PT30 | −17.3 | −1.5 | −15.7 |
| HEC-PT50 | −14.2 | −0.6 | −13.3 |
| NAU | −14.6 | −13.5 | −0.6 |
| NAU-PT10 | −13.7 | −12.6 | −1.0 |
| NAU-PT30 | −12.9 | −9.8 | −2.7 |
| NAU-PT50 | −12.1 | −8.6 | −1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plevová, E.; Vaculíková, L. Thermal Behavior of Ceramic Bodies Based on Fly Ash and Smectites. Minerals 2024, 14, 334. https://doi.org/10.3390/min14040334
Plevová E, Vaculíková L. Thermal Behavior of Ceramic Bodies Based on Fly Ash and Smectites. Minerals. 2024; 14(4):334. https://doi.org/10.3390/min14040334
Chicago/Turabian StylePlevová, Eva, and Lenka Vaculíková. 2024. "Thermal Behavior of Ceramic Bodies Based on Fly Ash and Smectites" Minerals 14, no. 4: 334. https://doi.org/10.3390/min14040334
APA StylePlevová, E., & Vaculíková, L. (2024). Thermal Behavior of Ceramic Bodies Based on Fly Ash and Smectites. Minerals, 14(4), 334. https://doi.org/10.3390/min14040334





