The Esquinzo Ultra-Alkaline Rock Suite of Fuerteventura Basal Complex (Canary Islands): Evidence for Origin of Carbonatites by Fractional Crystallization
Abstract
1. Introduction
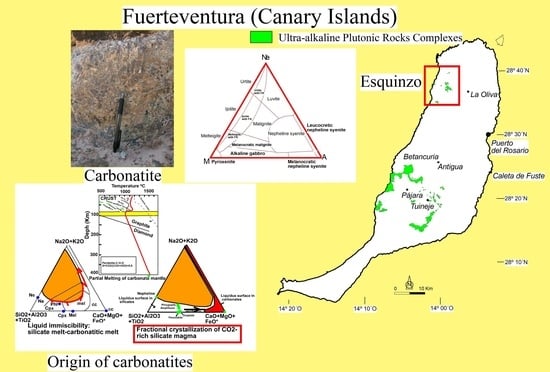
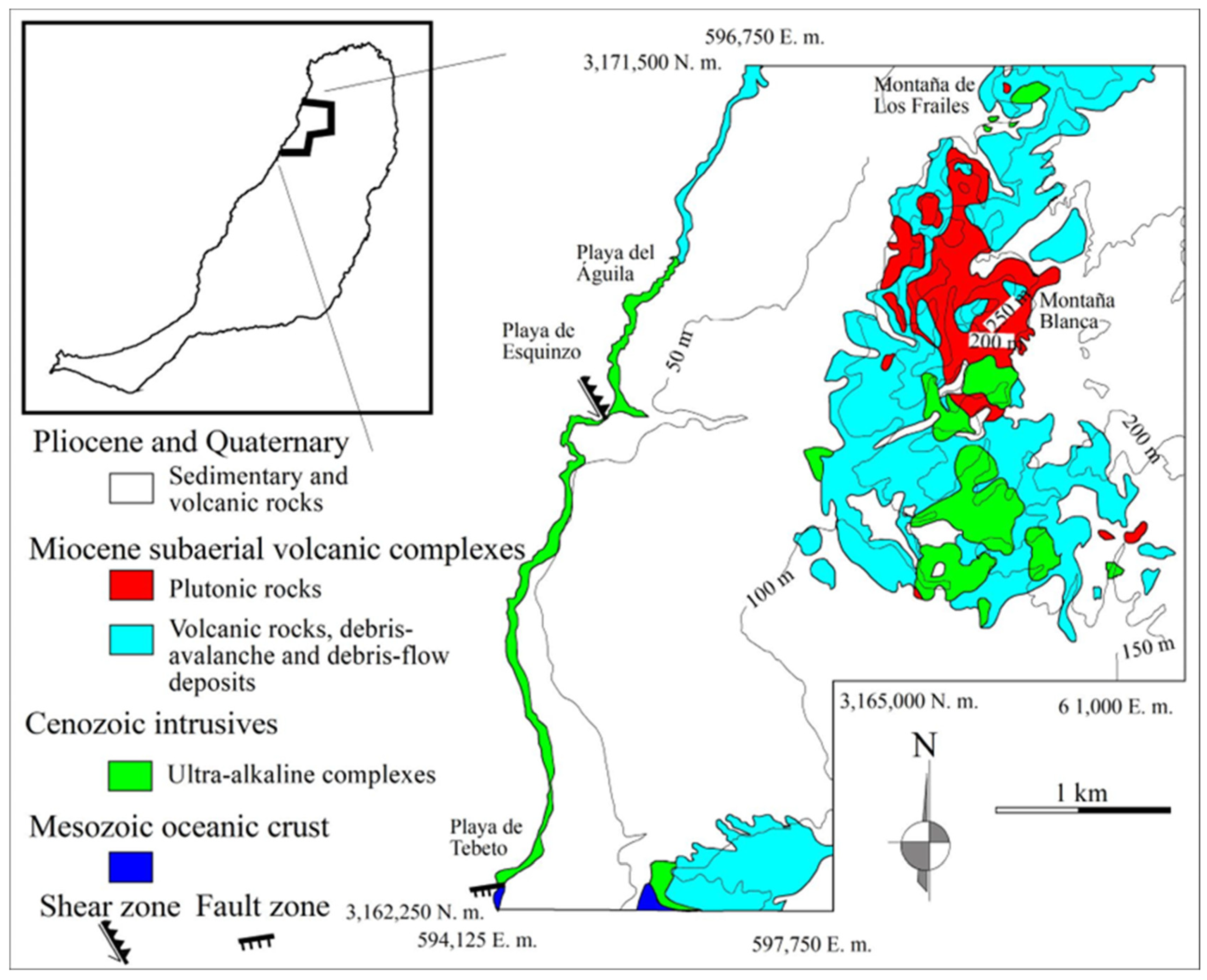
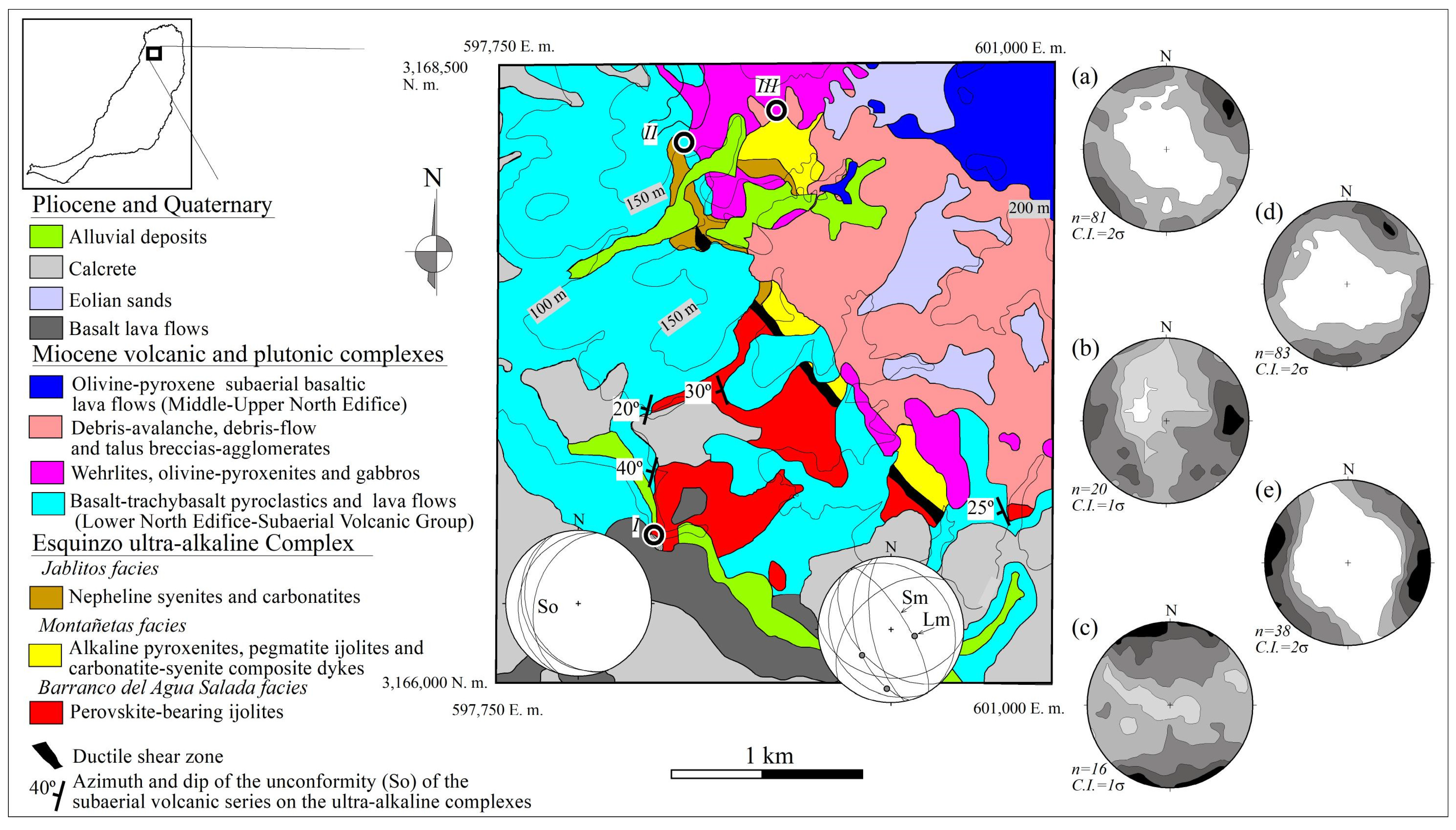
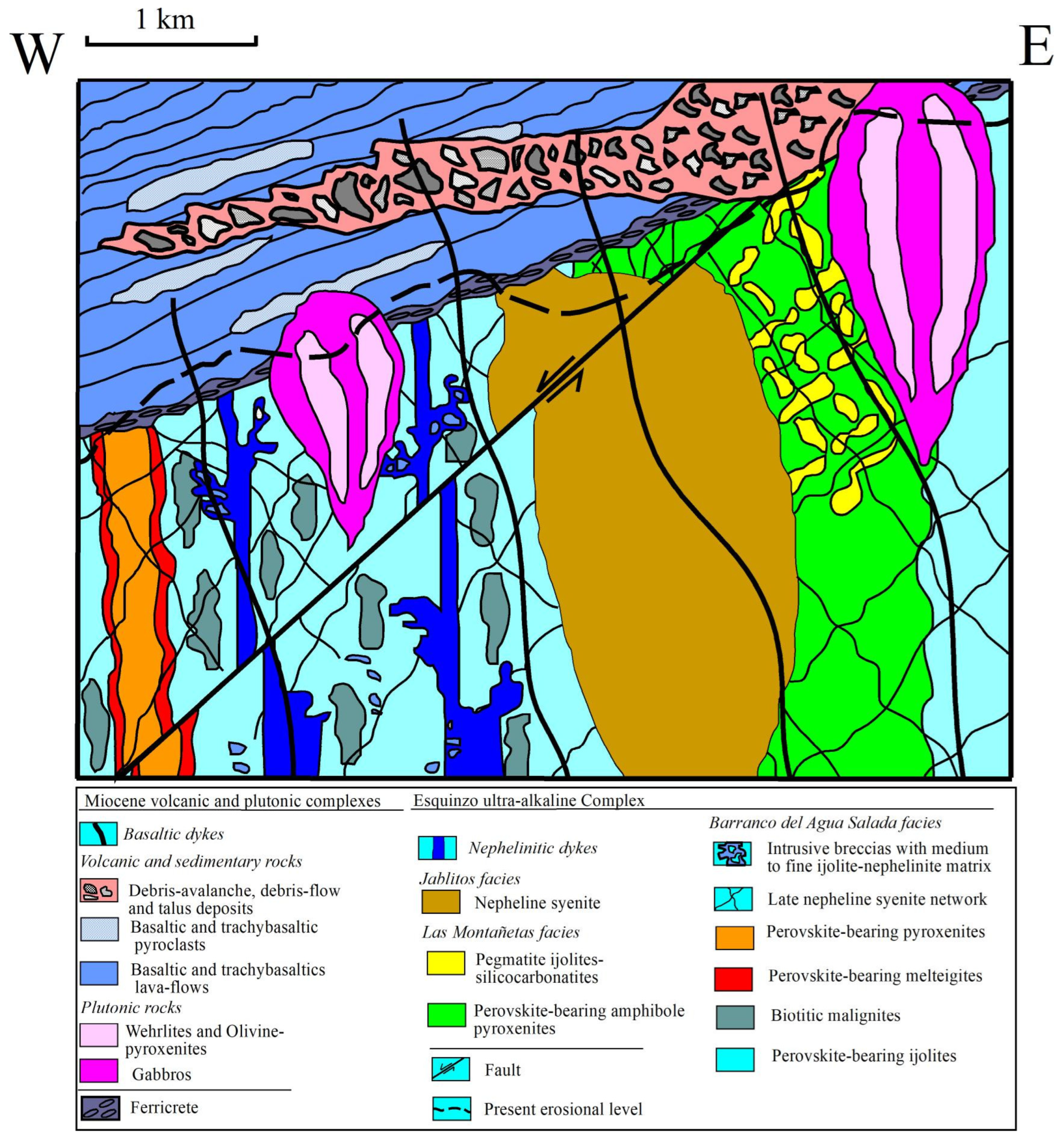
1.1. Geological Background
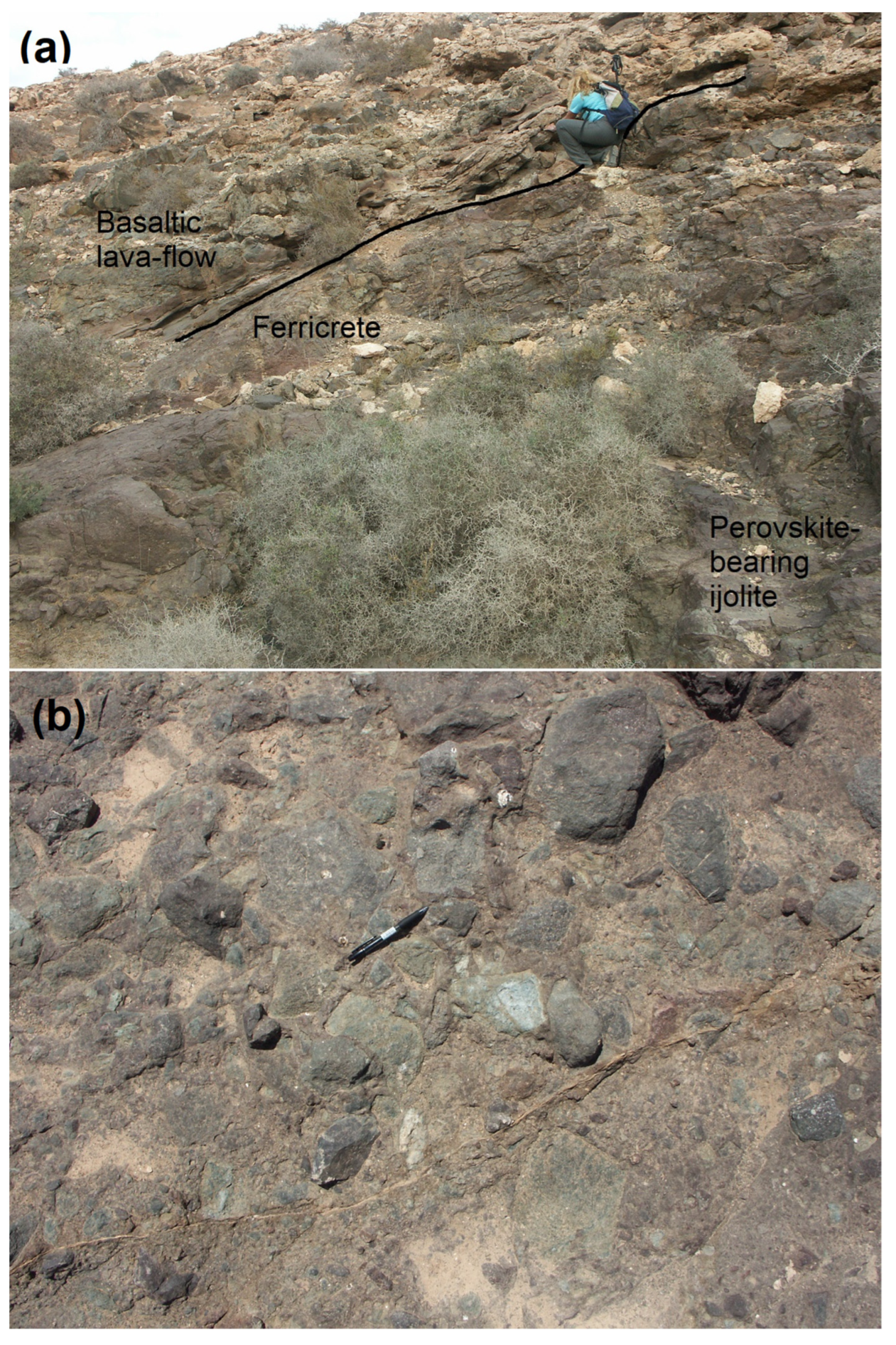
1.2. Field Relations
1.2.1. Northeastern Block
1.2.2. Southwestern Block
2. Materials and Methods
3. Results and Discussion
3.1. Petrography
- (1)
- The early magmatic event (Plutonic Event 1) formed the rocks of Las Montañetas Facies (perovskite-bearing amphibole pyroxenites, pegmatite ijolite, nepheline syenites, silicocarbonatites and carbonatites), Montaña Los Frailes Facies (melteigites-ijolites-urtites and carbonatites) and most of the rocks forming Barranco del Agua Salada Facies (perovskite-bearing pyroxenites, perovskite-bearing ijolites s.l., biotitic malignites and the feldspathic ijolite veins of Playa de Esquinzo and Montaña de la Morriña).
- (2)
- A second magmatic pulse (Plutonic Event 2) formed Jablitos Facies (nepheline syenites), late feldspathic ijolite veins (Barranco del Agua Salada) and the nepheline syenite network in Barranco del Agua Salada, Montaña Los Frailes and Playa del Águila.
- (3)
- The third magma batch (Plutonic Event 3) formed the nephelinite and phonolitic nephelinites dykes and the intrusive breccias with medium- to fine-grained ijolite-nephelinite matrix found in Barranco del Agua Salada Facies.
3.2. Mineral Chemistry
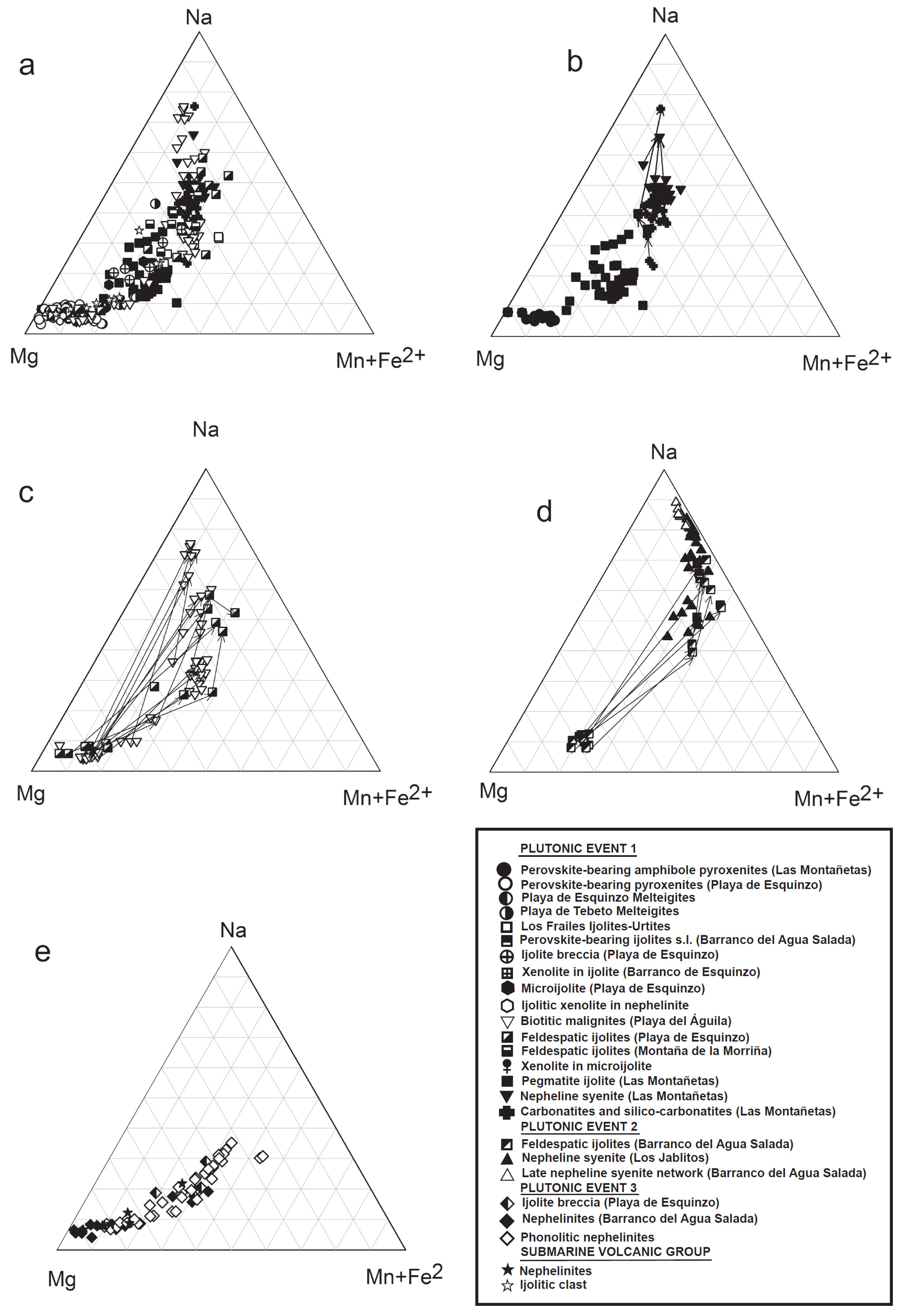
3.2.1. Pyroxene
3.2.2. Micas
3.2.3. Feldspar
3.2.4. Nepheline
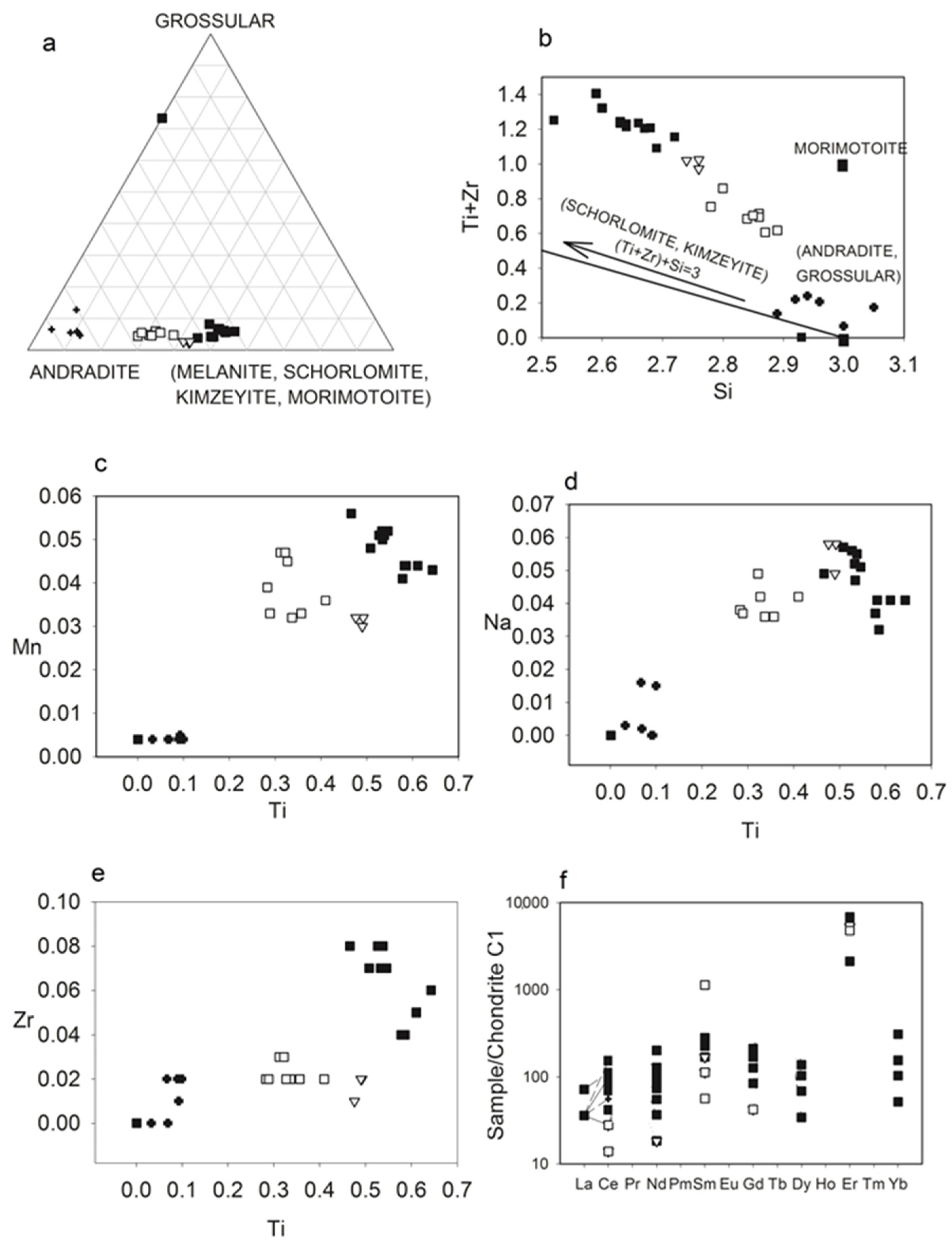
3.2.5. Garnet
- (a)
- Grandite ⇆ Schorlomite:
- (b)
- Grandite ⇆ Kimzeyite:Ca3(Al, Fe3+)y2Si3O12 ⇆ Ca63ry4(Al,Fe3+)z2Si2O12and
- (c)
- Grandite ⇆ Morimotoite:
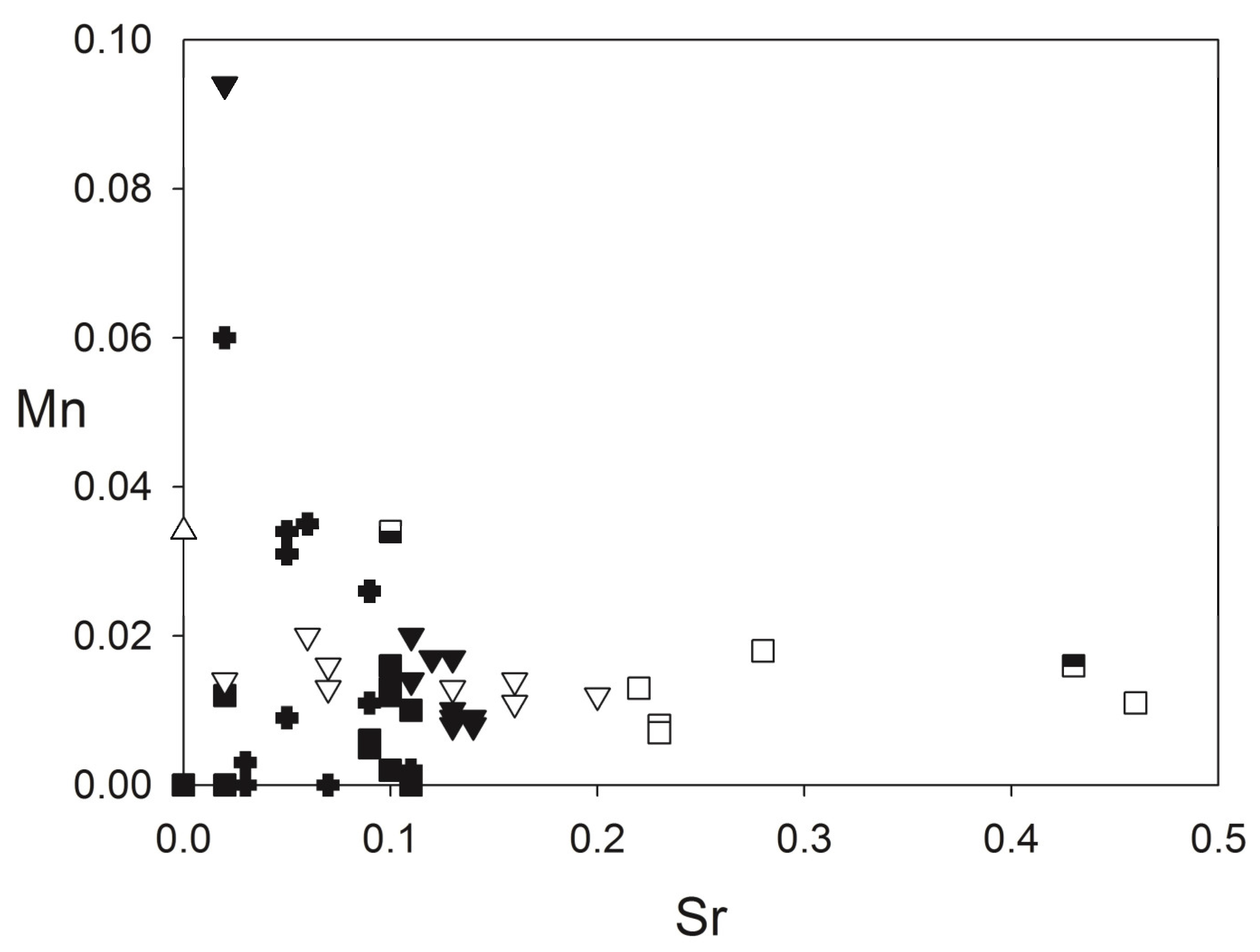
3.2.6. Carbonates
3.2.7. Magnetite and Ilmenite
3.3. Geochemistry
4. Origin of the Different Rocks
4.1. Origin of Silicate Rocks
- The starting point is a thick (87–100 km) lithosphere around 140 Ma old with a Depleted Mantle (DM) composition, near the continental lithosphere with an EM (Enriched Mantle) signature [2].
- In the upper zone of this anomaly or plume, which was volatile-rich and cooler than the inner zone, small-degree partial melting produced olivine melanephelinite magmas.
- The melanephelinite melts ascended through the lithosphere, where olivine fractionation took place in deep chambers emplaced somewhere in the lithospheric mantle or at the base of the crust.
- Melanephelinite melts reaching the crust began their stabilization, assimilation of carbonated sediments from the oceanic crust and differentiation at low pressures forming the Esquinzo ultra-alkaline complex and other equivalent complexes exposed in Fuerteventura (Ajuy-Solapa and Punta del Peñón Blanco).

4.2. Carbonatite Origin
- (1)
- In Las Montañetas facies, there is a spatial-temporal relationship between carbonatites and silicate rocks, especially between ijolite pegmatites and nepheline syenite–carbonatite composite dykes, that indicates a strong genetic link between them. Pegmatite ijolites, nepheline syenites and carbonatites form commonly zoned dykes where carbonatites occupy the inner part. Although, in some outcrops, carbonatite veins cut nepheline syenite dykes, both rocks are coeval [18,33]. Sometimes carbonatites occur as bands and lenses within the syenites, and there are modal gradations between the two rock types, which may indicate that some of the carbonatites are also cumulates.
- (2)
- Although [3] considered some mineral phases in Fuerteventura carbonatites as xenocrysts (nepheline, aegirine augite or titanite), there is evidence that these minerals grew in equilibrium with calcite. Aegirine augite crystals in pegmatite ijolites, nepheline syenites, silicocarbonatites and carbonatites from Las Montañetas contain inclusions of calcite (Figure S5e), indicating their coprecipitation. Moreover, titanite crystals in carbonatites (Figure S4e) show idiomorphic habits and no sign of corrosion. Only in silicocarbonatites located near the wehrlite to gabbro Montaña Blanca intrusion [33], aegirine augite crystals show reaction coronas composed of magnetite, epidote, chlorite, titanite and calcite that can clearly be attributed to the thermal effect produced by the intrusion.
- (3)
- Calcite is ubiquitous as a late accessory mineral in all types of silicate rocks of the first magmatic event (pyroxenites, meltegites, ijolites and syenites). The chemical composition of those is very similar to the calcite crystals forming most of the carbonatite bodies (see Figure 9 and Table S8), which show high SrO concentration. These features are common to carbonatite calcites all over the world [121]. The authors of [12] demonstrated that calcite can be an igneous mineral formed by fractional crystallization of an evolved carbonated alkali-silicate melts. Thus, the common occurrence of calcite in the silicate rocks of the first magma batch can be explained by fractional crystallization of a very CO2-rich silicate melt, related to carbonatite formation.
- (4)
- The similarity of composition of some minerals (diopside-hedenbergite-acmite proportions in pyroxenes, Fe/(Mg + Fe) versus Ti and Fe/(Mg + Fe) versus Al in biotites-phlogopites, Ba-K ratios in alkali feldspar, Figure 6, Figure 7 and Figure S6) present in some silicate rocks (nepheline syenites from the nepheline syenite–carbonatite composite dykes and ijolite pegmatites in Las Montañetas) and in carbonatites and silicocarbonatites. Clinopyroxenes show a continuous increase in Na and FeTOT (aegirine and hedenbergite components) and decrease in Ca (diopside component) with decreasing Mg (see Figure 6), which is consistent with normal closed system fractionation; it is similar to reported trends for other alkaline intrusions [64,65,66,67].
- (5)
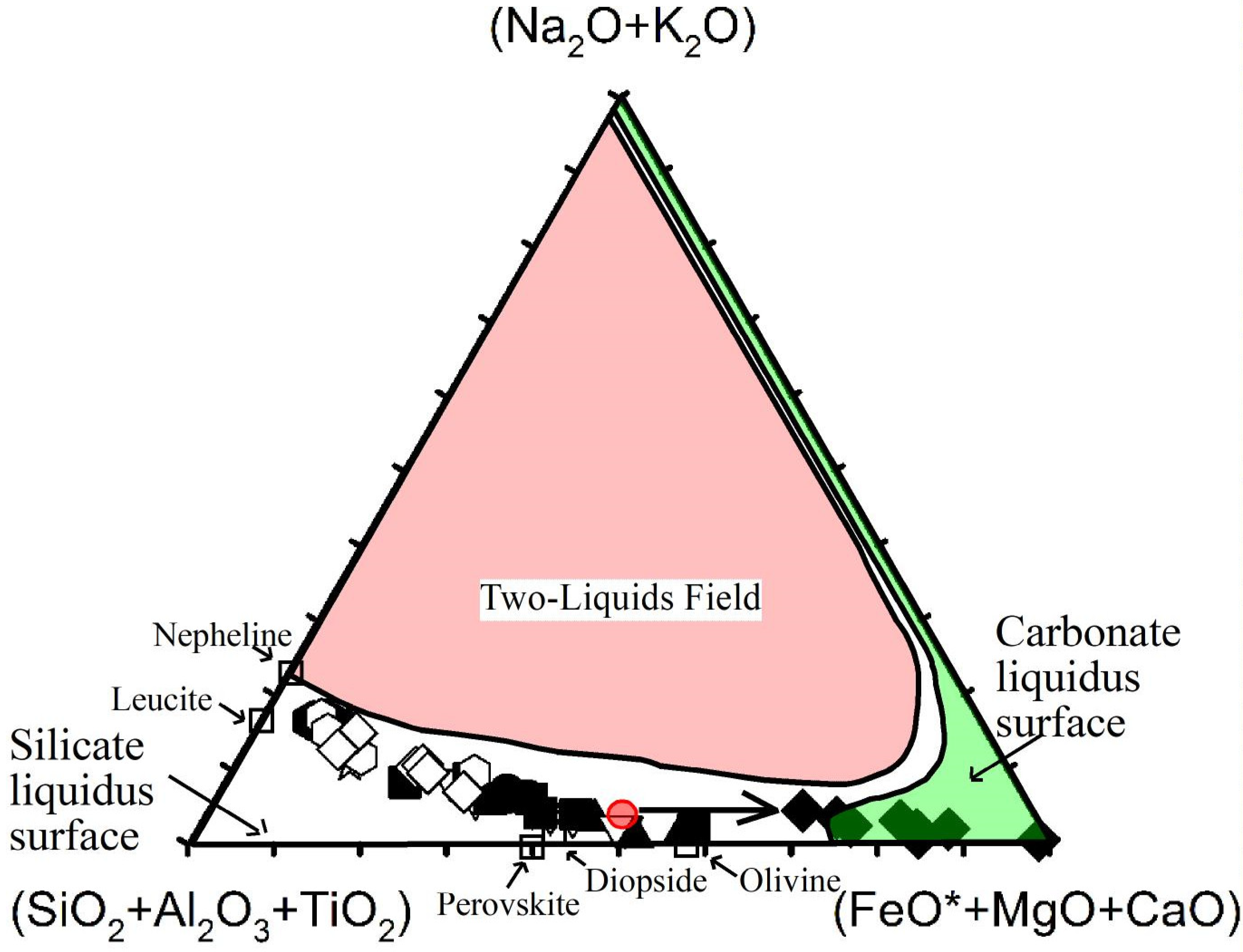
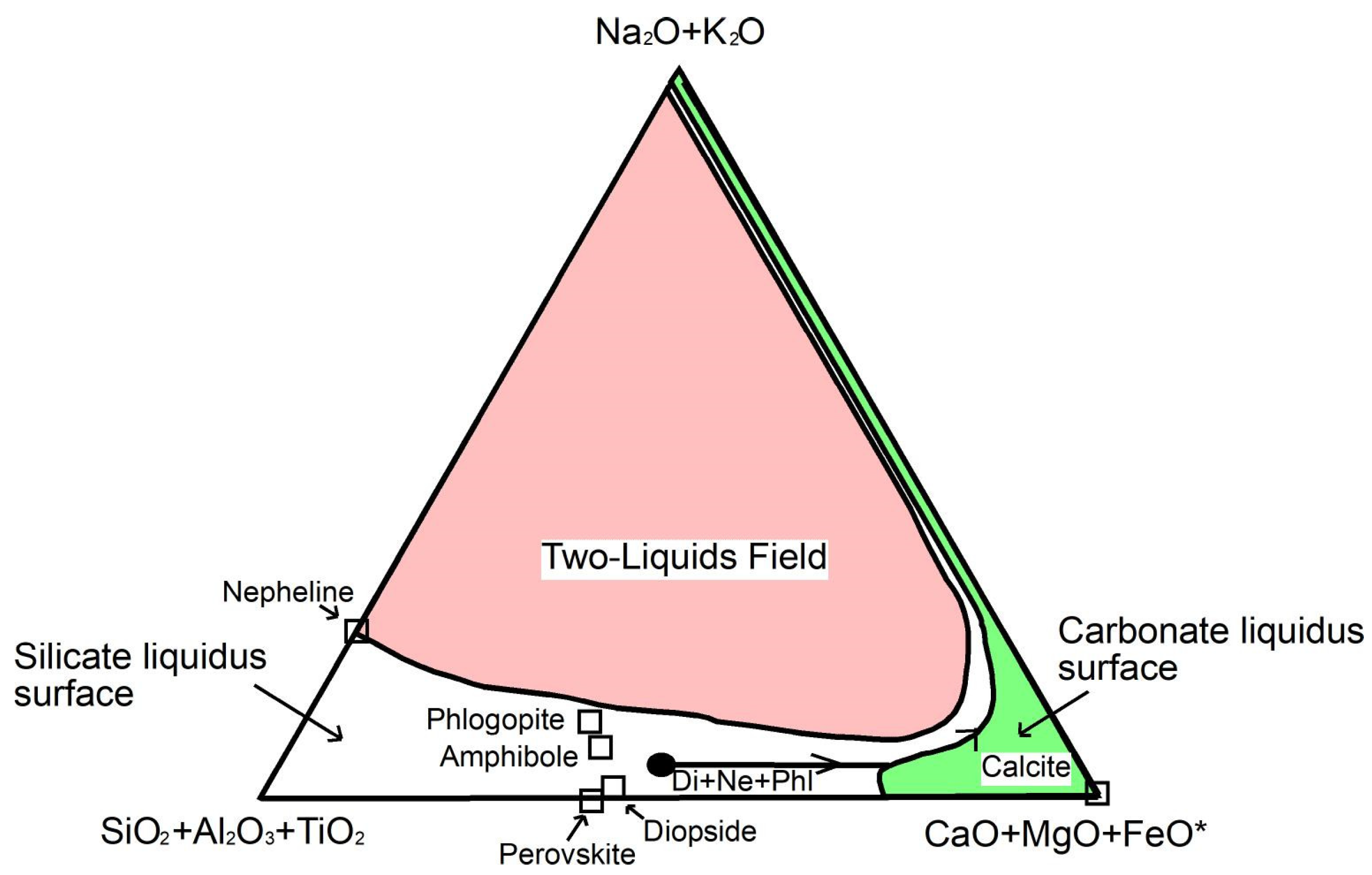
- Isotopic compositions (Sr–Nd–Pb isotope data) of carbonatites are similar to those obtained for spatially related ijolites and nepheline syenites [2,3,109], suggesting a genetic relationship between the silicate and carbonate rocks. The authors of [2] explained the origin of Fuerteventura carbonatites by liquid immiscibility of a carbonated, alkaline silicate parent magma. Nevertheless, the position of these carbonatites and their associated silicate rocks far away from the liquid immiscibility curve [11,12,14] that consider carbonatite melts richer in alkalis and more depleted in CaO, in SiO2 + Al2O3 + TiO2-Na2O + K2O-MgO + FeOT + CaO diagram (Figure 13) excludes the origin of carbonatites by liquid immiscibility between a carbonatitic melt and a nephelinitic or syenitic melt.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Bas, M.J. Oceanic carbonatites. In Kimberlites and Related Rocks; Kornprobst, J., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; pp. 169–178. [Google Scholar]
- Hoernle, K.A.; Tilton, G.R. Sr–Nd–Pb isotope data for Fuerteventura (Canary Islands). Schweiz. Mineral. Und Petrogr. Mitt. 1991, 71, 3–18. [Google Scholar]
- Hoernle, K.; Tilton, G.; Le Bas, M.J.; Duggen, S.; Garbe-Schönberg, D. Geochemistry of oceanic carbonatites compared with continental carbonatites: Mantle recycling of oceanic crustal carbonate. Contrib. Mineral. Petrol. 2002, 142, 520–542. [Google Scholar] [CrossRef]
- Le Bas, M.J. Nephelinites and carbonatites. In Alkaline Igneous Rocks; Fitton, J.G., Upton, B.J.J., Eds.; Geological Society of London: London, UK, 1987; Special Publication 30; pp. 53–83. [Google Scholar]
- Mitchell, R.H. Carbonatites and carbonatites and carbonatites. Can. Mineral. 2005, 43, 2049–2068. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Huang, W.L. Peridotite, kimberlite, and carbonatite explained in the system CaO-MgO-SiO2-CO2. Geology 1975, 3, 621–624. [Google Scholar] [CrossRef]
- Wallace, M.E.; Green, D.H. An experimental determination of primary carbonatite magma composition. Nature 1988, 335, 343–346. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Lee, W.J. Model system controls on conditions for formation of magnesiocarbonatite and calciocarbonatite magmas from the mantle. J. Pet. 1998, 39, 1885–1893. [Google Scholar] [CrossRef]
- Freestone, L.C.; Hamilton, D.L. The role of liquid immiscibility in the genesis of carbonatites-An experimental study. Contrib. Mineral. Petrol. 1980, 73, 105–117. [Google Scholar] [CrossRef]
- Kjarsgaard, B.A.; Hamilton, D.L. Liquid immiscibility and the origin of alkali-poor carbonatites. Mineral. Mag. 1988, 52, 43–55. [Google Scholar] [CrossRef]
- Lee, W.; Wyllie, P.J. Petrogenesis of carbonatite magmas from mantle to crust, constrained by the system CaO-(MgO + FeO*)-(Na2O + K2O) (SiO2 + Al2O3 + TiO2)-CO2. J. Petrol. 1998, 39, 495–577. [Google Scholar] [CrossRef]
- Kjarsgaard, B.A. Phase relations in a carbonated high-CaO nephelinite at 0.2 and 0.5 GPa. J. Petrol. 1998, 39, 2061–2075. [Google Scholar] [CrossRef]
- Veksler, I.V.; Petibon, C.; Jenner, G.A.; Dorfman, A.M.; Dingwel, D.B. Trace element partitioning in immiscible silicate–carbonate liquid systems: An initial experimental study using a centrifuge autoclave. J. Petrol. 1998, 39, 2095–2104. [Google Scholar] [CrossRef]
- Brooker, R.A.; Kjarsgaard, B.A. Silicate-Carbonate Liquid Immiscibility and Phase Relations in the System SiO2-Na2O-Al2O3-CaO-CO2 at 0.1–2.5 G. Pa with Applications to Carbonatite Genesis. J. Petrol. 2010, 52, 1281–1305. [Google Scholar] [CrossRef]
- Weidendorfer, D.; Schmidt, M.W.; Mattsson, H.B. Fractional crystallization of Si-undersaturated alkaline magmas leading to unmixing of carbonatites on Brava Island (Cape Verde) and a general model of carbonatite genesis in alkaline magma suites. Contrib. Mineral. Pet. 2016, 171, 43. [Google Scholar] [CrossRef]
- Watkinson, D.H.; Wyllie, P.J. Experimental study of the join NaAlSiO4-CaCO3-H2O and the genesis of alkalic rock-carbonatite complexes. J. Petrol. 1971, 12, 357–378. [Google Scholar] [CrossRef]
- Lee, W.J.; Wyllie, P.J. Experimental data bearing on liquid immiscibility, crystal fractionation, and the origin of calciocarbonatites and natrocarbonatites. Inter. Geol. Rev. 1994, 36, 797–819. [Google Scholar] [CrossRef]
- Fúster, J.M.; Cendrero, A.; Gastesi, P.; Ibarrola, E.; López Ruiz, J. Geología y Volcanología de las Islas Canarias—Fuerteventura; Instituto ‘Lucas Mallada’; Consejo Superior de Investigaciones Científicas: Madrid, Spain, 1968; pp. 1–239. [Google Scholar]
- Stillman, C.J.; Fúster, J.M.; Bennell-Baker, M.J.; Muñoz, M.; Smewing, J.D.; Sagredo, J. Basal Complex of Fuerteventura (Canary Islands) is an oceanic intrusive complex with rift-system affinities. Nature 1975, 257, 469–471. [Google Scholar] [CrossRef]
- Robertson, A.H.F.; Stillman, C.J. Late Mesozoic sedimentary rocks of Fuerteventura, Canary Islands. Implications for West Africa continental margin evolution. J. Geol. Soc. Lond. 1979, 136, 47–60. [Google Scholar] [CrossRef]
- Robertson, A.H.F.; Stillman, C.J. Submarine volcanic and associated sedimentary rocks of the Fuerteventura Basal Complex, Canary Islands. Geol. Mag. 1979, 116, 203–214. [Google Scholar] [CrossRef]
- Le Bas, M.J.; Rex, D.C.; Stillman, C.J. The early magmatic chronology of Fuerteventura. Geol. Mag. 1986, 123, 287–298. [Google Scholar] [CrossRef]
- Stillman, C.J. A Canary Islands Dyke Swarm: Implications for the formation of oceanic islands by extensional fissural volcanism. In Mafic Dyke Swarms; Halls, H.C., Fahrig, W.J., Eds.; Geological Association of Canada Special paper 34, Geological Association of Canada: San Juan de Terranova, NL, Canada, 1987; pp. 243–255. [Google Scholar]
- Steiner, C.; Hobson, A.; Favre, P.; Stampli, G.M. Early Jurassic sea-floor spreading in the central Atlantic -the Jurassic sequence of Fuerteventura (Canary Islands). Geol. Soc. Am. Bull. 1998, 110, 1304–1317. [Google Scholar] [CrossRef]
- Gutiérrez, M. Estudio Petrológico, Geoquímico y Estructural de la Serie Volcánica Submarina del Complejo Basal de Fuerteventura (Islas Canarias): Caracterización del Crecimiento Submarino y de la Emersión de la Isla. Ph.D. Thesis, Department of Pedology and Geology, Universidad de La Laguna, La Laguna, Spain, 2000; pp. 1–533. [Google Scholar]
- Gutiérrez, M.; Casillas, R.; Fernández, C.; Balogh, K.; Ahijado, A.; Castillo, C. La serie volcánica submarina del Complejo Basal de Fuerteventura: Crecimiento submarino y emersión de la isla. Geogaceta 2002, 32, 250–254. [Google Scholar]
- Gutiérrez, M.; Casillas, R.; Fernández, C.; Balogh, K.; Ahijado, A.; Castillo, C.; Colmenero, J.R.; García-Navarro, E. The submarine volcanic succession of the Basal Complex of Fuerteventura, Canary Islands: A model of submarine growth and emersion of some tectonic volcanic islands. Geol. Soc. Am. Bull. 2006, 118, 785–804. [Google Scholar] [CrossRef]
- Fernández, C.; Casillas, R.; Navarro, E.; Gutiérrez, M.; Camacho, M.; Ahijado, A. Miocene rifting of Fuerteventura (Canary Islands). Tectonics 2006, 5, C6009. [Google Scholar] [CrossRef]
- Balcells, R.J.; Barrera, J.L.; Gómez Sainz de Aja, J.A. Mapa Geológico de España a Escala 1: 100:000; Fuerteven-tura (92); Mapa y Memoria Instituto Geológico y Minero de España: Madrid, Spain, 2006; pp. 1–108. [Google Scholar]
- Sagan, M.; Heaman, L.M.; Pearson, D.G.; Luo, Y.; Stern, R.A. Removal of continental lithosphere beneath the Canary archipelago revealed from a U—Pb Age and Hf/O isotope study of modern sand detrital zircons. Lithos 2020, 362–363, 105448. [Google Scholar] [CrossRef]
- Casillas, R.C.; Fernández, C.; Colmenero, J.R. Crecimiento Temprano y Evolución Geológica de la Isla de Fuerteventura; 8th Meeting of the Iberian Group of Petrology, Geochemistry and Geochronology; Commission of Petrology, Geochemistry and Geochronology of Igneous and Metamorphic Rocks of the Spanish Geological Society: La Laguna, Spain; pp. 1–191.
- Fúster, J.M.; Muñoz, M.; Sagredo, J.; Yébenes, A.; Bravo, T.; Hernández-Pacheco, A. Excursión n° 121 A + c del 26° I.G.C. a las Islas Canarias. Boletín Inst. Geológico Y Min. España 1980, 92, 351–390. [Google Scholar]
- Barrera, J.L.; Fernández Santín, S.; Fúster, J.M.; Ibarrola, E. Ijolitas-Sienitas-Carbonatitas de los Macizos del Norte de Fuerteventura. Boletín Inst. Geológico Y Min. España 1981, 92, 309–321. [Google Scholar]
- Cantagrel, J.M.; Fúster, J.M.; Pin, C.; Renaud, U.; Ibarrola, E. Age Miocène inférieur des carbonatites de Fuerteventura. Comptes Rendus Acad. Sci. Paris 1993, 316, 1147–1153. [Google Scholar]
- Ahijado, A. Las Intrusiones Plutónicas e Hipoabisales del Sector meridional del Complejo Basal de Fuerteventura. Ph.D. Thesis, Department of Petrology and Geochemistry, Universidad Complutense de Madrid, Madrid, Spain, 1999; pp. 1–386. [Google Scholar]
- Muñoz, M.; Sagredo, J.; De Ignacio, C.; Fernández-Santín, S.; Jeffries, T.E. New data (U-Pb, K-Ar) on the geochronology of the alkaline-carbonatitic association of Fuerteventura, Canary Islands, Spain. Lithos 2005, 85, 140–153. [Google Scholar] [CrossRef]
- Casillas, R.; Ahijado, A.; Hernandez-Pacheco, A. Zonas de cizalla dúctil en el Complejo Basal de Fuerteventura. Geogaceta 1994, 15, 117–120. [Google Scholar]
- Fernández, C.; Casillas, R.; Ahijado, A.; Perelló, V.; Hernández-Pacheco, A. Shear zones as result of intraplate tectonics in oceanic crust: An example of the Basal Complex of Fuerteventura (Canary Islands). J. Struct. Geol. 1997, 19, 41–57. [Google Scholar] [CrossRef]
- Balogh, K.; Ahijado, A.; Casillas, R.; Fernández, C. Contributions to the chronology of the Basal Complex of Fuerteventura, Canary Islands. J. Volcanol. Geotherm. Res. 1999, 90, 81–102. [Google Scholar] [CrossRef]
- Coello, J.; Cantagrel, J.M.; Hernán, F.; Fúster, J.M.; Ibarrola, E.; Ancochea, E.; Casquet, C.; Jamond, J.; Cendrero, A. Evolution of the eastern volcanic ridge of the Canary Islands based on new K-Ar data. J. Volcanol. Geotherm. Res. 1992, 53, 251–274. [Google Scholar] [CrossRef]
- Ancochea, E.; Brandle, J.L.; Cubas, C.R.; Hernán, F.; Huertas, M.J. Volcanic complexes in the eastern ridge of the Canary Islands: The Miocene activity of the Island of Fuerteventura. J. Volcanol. Geotherm. Res. 1996, 70, 183–204. [Google Scholar] [CrossRef]
- Muñoz, M. Estudio petrológico de las formaciones alcalinas de Fuerteventura (Islas Canarias). Estud. Geológicos 1969, 25, 257–310. [Google Scholar]
- Hobson, A.; Bussy, F.; Hernández, J. Shallow-level migmatization of gabbros in a metamorphic contact aureole, Fuerteventura Basal Complex, Canary Islands. J. Petrol. 1998, 39, 125–137. [Google Scholar] [CrossRef]
- Ahijado, A.; Casillas, R.; Hernandez-Pacheco, A. The dike swarms of the Amanay Massif, Fuerteventura, Canary Islands. J. Asian Earth Sci. 2001, 19, 333–345. [Google Scholar] [CrossRef]
- Muñoz, M.; Sagredo, J. Reajustes mineralógicos y geoquímicos producidos durante el metamorfismo de contacto de diques basálticos (Fuerteventura, Islas Canarias). Boletín Soc. Española Mineral. 1994, 17, 86–87. [Google Scholar]
- Muñoz, M.; Sagredo, J. Existencia de Metamorfismos Superpuestos en el Complejo Basal de Fuerteventura (Canarias); I National Assembly of Geodesy and Geophysics: Madrid, Spain, 1975; pp. 1287–1288. [Google Scholar]
- Muñoz, M.; Sagredo, J. Características del metamorfismo térmico producido por los eventos plutónicos intrusivos más recientes del Complejo Basal de Fuerteventura. In Proceedings of the ESF Meeting on Canarian Volcanism, Lanzarote, Spain, 30 November–7 December 1989; pp. 104–108. [Google Scholar]
- Holloway, M.I.; Bussy, F.; Vennemann, T.W. Low-pressure, water-assisted anatexis of basic dykes in a contact metamorphic aureole, Fuerteventura (Canary Islands): Oxygen isotope evidence for a meteoric fluid origin. Contrib. Mineral. Petrol. 2007, 155, 111–121. [Google Scholar] [CrossRef]
- Casillas, R.; Nagy, G.; Démeny, A.; Ahijado, A.; Fernández, C. Cuspidine-niocalite-baghdadite solid solutions in the metacarbonatites of the Basal Complex of Fuerteventura (Canary Islands). Lithos 2008, 105, 25–41. [Google Scholar] [CrossRef]
- Casillas, R.; Démeny, A.; Nagy, G.; Ahijado, A.; Fernández, C. Metacarbonatites in the Basal Complex of Fuerteventura (Canary Islands). The role of fluid/rock interactions during contact metamorphism and anatexis. Lithos 2011, 125, 503–520. [Google Scholar] [CrossRef]
- Meco, J.; Pomel, R.S. Les formations marines et continentales intervolcaniques des Iles Canaries Orientales (Grande Canarie, Fuerteventura et Lanzarote): Stratigraphie et signification paleoclimatique. Estud. Geológicos 1985, 41, 223–227. [Google Scholar] [CrossRef]
- Cueto, L.A.; Balcells, R.; Barrera, J.L.; Gómez, J.A.; García, E.; Nieto, M.; Vidal, J.R.; Ruiz, M.T. Mapa y Memoria Explicativa de la Hoja de Punta del Paso Chico (92-76) del Mapa Geológico Nacional; Instituto Geológico y Minero de España: Madrid, Spain, 2004; scale 1:25,000, 1 sheet. [Google Scholar]
- Cueto, L.A.; Balcells, R.; Barrera, J.L.; Gómez, J.A.; Vidal, J.R.; García, E.; Nieto, M.; Ruiz, M.T. Mapa y Memoria Explicativa de la Hoja de la Oliva (93/94-76) del Mapa Geológico Nacional; Instituto Geológico y Minero de España: Madrid, Spain, 2004; scale 1:25,000, 1 sheet. [Google Scholar]
- Kamb, W.B. Ice petrofabric observations from Blue Glacier, Washington; in relation to theory and experiment. J. Geophys. Res. 1959, 64, 1891–1909. [Google Scholar] [CrossRef]
- Demény, A.; Vennemann, T.W.; Hegner, E.; Ahijado, A.; Casillas, R.; Nagy, G.; Homonnay, Z.; Gutierrez, M.; Szabo, C. H, O, Sr, Nd, and Pb isotopic evidence for recycled oceanic crust in the Transitional Volcanic Group of Fuerteventura, Canary Islands, Spain. Chem. Geol. 2004, 205, 37–54. [Google Scholar] [CrossRef]
- Mitchell, R.H. The melilitite clan. In Undersaturated Alkaline Rocks: Mineralogy, Petrogenesis, and Economic Potential; Mitchell, R.H., Ed.; Mineralogical Association Canada: Winnipeg, MB, Canada, 1996; Volume 24, pp. 123–152. [Google Scholar]
- Le Bas, M.J. Carbonatite—Nephelinite Volcanism; J. Wiley and Sons: London, UK, 1997; pp. 1–347. [Google Scholar]
- Woolley, A.R.; Kjarsgaard, B.A. Paragenetic types of carbonatite as indicated by the diversity and relative abundances of associated silicate rocks: Evidence from a global database. Can. Mineral. 2008, 46, 741–752. [Google Scholar] [CrossRef]
- Zhabin, A.J. Primary textural-structural features of carbonatites and their metamorphic evolution. Intern. Geol. Rev. 1971, 13, 1087–1096. [Google Scholar] [CrossRef]
- Cooper, A.F.; Reid, D.L. Textural evidence for calcite-carbonatite magmas, Dicker Willem, south-west Namibia. Geology 1991, 19, 1193–1196. [Google Scholar] [CrossRef]
- Bowden, P.; Wall, F.; Schürmann, L. ‘Spinifex-textured’ pegmatitic crystallisation in carbonatites. J. Afr. Earth Sci. 2000, 32, A11–A12. [Google Scholar] [CrossRef]
- Wyllie, P.J. Experimental studies of carbonatite problems. In The Origin and Differentiation of Carbonatite Magmas, in: Carbonatites; Tuttle, O.F., Gittins, J., Eds.; Wiley-Interscience: New York, NY, USA, 1966; pp. 311–352. [Google Scholar]
- Morimoto, N.; Fabries, J.; Ferguson, J.; Ginzburg, I.V.; Ross, M.; Seifert, F.A.; Zussman, J.; Aoki, K.; Gottardi, G. Nomenclature of pyroxenes. Am. Mineral. 1988, 73, 1123–1133. [Google Scholar]
- Nash, W.P. Mineralogy and Petrology of the Iron Hill carbonatite complex, Colorado. Bull. Geol. Soc. Am. 1972, 83, 361–382. [Google Scholar] [CrossRef]
- Larsen, L.M. Clinopyroxene and coexisting mafic minerals from the alkaline Ilimaussaq intrusion, South Greenland. J. Petrol. 1976, 17, 258–290. [Google Scholar] [CrossRef]
- Mitchell, R.H. Pyroxenes of the Fen alkaline complex, Norway. Am. Miner. 1980, 65, 45–54. [Google Scholar]
- Vuorinen, J.H.; Hålenius, U.; Whitehouse, M.J.; Mansfelda, J.; Skelton, A.D.L. Compositional variations (major and trace elements) of clinopyroxene and Ti-andradite from pyroxenite, ijolite and nepheline syenite, Alno Island, Sweden. Lithos 2005, 81, 55–77. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Schilling, J.; Coulson, I.M.; Wenzel, T.; Markl, G. The alkaline-peralkaline Tamazeght complex, High Atlas Mountains, Morocco: Mineral chemistry and petrological constraints for derivation from a compositionally heterogeneous mantle source. J. Petrol. 2008, 49, 1097–1131. [Google Scholar] [CrossRef]
- Le Bas, M.J.; Srivastava, R.K. Mineralogy and geochemistry of the Mundwara carbonatite dykes, Sirohi District, Rajasthan, India. Neues Jahrb. Für Mineral.-Abh. 1989, 160, 207–227. [Google Scholar]
- Mccormick, G.R.; Le Bas, M.J. Phlogopite crystallization in carbonatitic magmas from Uganda. Can. Mineral. 1996, 34, 469–478. [Google Scholar]
- Hamilton, D.L. Nephelines as crystallization temperature indicators. J. Geol. 1961, 69, 321–329. [Google Scholar] [CrossRef]
- Mcdonough, W.F.; Sun, S.S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. Rock-forming minerals. In Vol lA Orthosilicates, 2nd ed.; Longmans: London, UK, 1982; pp. 1–919. [Google Scholar]
- Dawson, J.B.; Smith, J.V.; Steele, I.M. Petrology and mineral chemistry of plutonic igneous xenoliths from the carbonatite volcano, Oldoinyo-Lengai, Tanzania. J. Petrol. 1995, 36, 797–826. [Google Scholar] [CrossRef]
- Huggins, F.E.; Virgo, D.; Huckenholz, H.G. Titanium-containing silicate garnets: II. The crystal chemistry of melanites and schorlomites. Am. Mineral. 1977, 62, 646–665. [Google Scholar]
- Barros Gomes, C. Electron microprobe analysis of zoned melanites. Am. Mineral. 1969, 54, 1654–1661. [Google Scholar]
- Vuorinen, J.H.; Halenius, U. Nb-, Zr- and LREE-rich titanite from the Alno alkaline complex: Crystal chemistry and its importance as a petrogenetic indicator. Lithos 2005, 83, 128–142. [Google Scholar] [CrossRef]
- Flohr, M.I.K.; Ross, M. Alkaline igneous rocks of Magnet-Cove, Arkansas- metasomatized ijolite xenoliths from Diamond-Jo quarry. Am. Mineral. 1989, 74, 113–131. [Google Scholar]
- Campbell, L.S.; Henderson, P.; Wall, F. Rare earth chemistry of perovskite group minerals from the Gardiner Complex, East Greenland. Mineral. Mag. 1997, 61, 197–212. [Google Scholar] [CrossRef]
- Hogarth, D.D. Classification and nomenclature of pyrochlore group. Am. Mineral. 1977, 62, 403–410. [Google Scholar]
- Atencio, D.; Andrade, M.B.; Christy, A.G.; Gieré, R.; Kartashov, P.M. The pyrochlore supergroup of minerals: Nomenclature. Can. Mineral. 2010, 48, 673–698. [Google Scholar] [CrossRef]
- Möller, P. REE(Y), Nb and Ta enrichment in pegmatites and carbonatite alkalic rock complexes. In Lanthanides, Tantalum and Niobium—Mineralogy, Geochemistry, Characteristics of Primary ore Deposits, Prospecting, Processing and Applications; Möller, P., Cerny, P., Saupé, F., Eds.; Springer: New York, NY, USA, 1989; pp. 103–144. [Google Scholar]
- Woolley, A.R.; Kempe, D.R.C. Carbonatites: Nomenclature, average chemical compositions and element distribution. In Carbonatites: Genesis and Evolution; Bell, K., Ed.; Unwin Hyman: London, UK, 1989; pp. 1–14. [Google Scholar]
- Lumpkin, G.R.; Ewing, R.C. Geochemical alteration of pyrochlore group minerals: Microlite subgroup. Am. Mineral. 1992, 77, 179–188. [Google Scholar]
- Lumpkin, G.R.; Ewing, R.C. Geochemical alteration of pyrochlore group minerals: Pyrochlore subgroup. Am. Mineral. 1995, 80, 732–743. [Google Scholar] [CrossRef]
- Nasraoui, M.; Bilal, E. Pyrochlores from the Lueshe carbonatite complex (Democratic Republic of Congo): A geochemical record of different alteration stages. J. Asian Earth Sci. 2000, 18, 237–251. [Google Scholar] [CrossRef]
- Hogarth, D.D. Pyrochlore, apatite and amphibole: Distinctive minerals in carbonatite. In Carbonatites: Genesis and Evolution; Bell, K., Ed.; Unwin Hyman: London, UK, 1989; pp. 105–148. [Google Scholar]
- Sørensen, H. Alkali syenites, feldspathoidal syenites and related lavas. In The Alkaline Rocks; Sørensen, H., Ed.; John Wiley and Sons: London, UK, 1974; pp. 22–52. [Google Scholar]
- De Ignacio, C.; Muñoz, M.; Sagredo, J.; Fernández-Santín, S. Mineralogía y geoquímica de las sienitas nefelínicas de Montaña Blanca-Esquinzo, NO de Fuerteventura (Islas Canarias). Geotemas 2004, 6, 29–32. [Google Scholar]
- Eby, J.N.; Woolley, A.R.; Din, V.; Platt, G. Geochemistry and Petrogenesis of Nepheline Syenites: Kasungu-Chipala, Ilomba, and Ulindi Nepheline Syenite Intrusions, North Nyasa Alkaline Province, Malawi. J. Petrol. 1998, 39, 1405–1424. [Google Scholar] [CrossRef]
- Downes, H.; Balaganskaya, E.; Beard, A.; Liferovich, R.; Demaiffe, D. Petrogenetic processes in the ultramafic, alkaline and carbonatitic magmatism in the Kola Alkaline Province: A review. Lithos 2005, 85, 48–75. [Google Scholar] [CrossRef]
- Harmer, R.E. The petrogenetic association of carbonatite and alkaline magmatism: Constraints from the Spitskop complex, South Africa. J. Petrol. 1999, 40, 525–548. [Google Scholar] [CrossRef]
- Horning-Kjarsgaard, I. Rare earth elements in sovitic carbonatites and their mineral phases. J. Petrol. 1998, 39, 2105–2121. [Google Scholar] [CrossRef]
- Sun, S.S.; Mcdonough, W.F. Chemical and Isotopic Systematics of OCEANIC Basalts; Implications for Mantle Composition and Processes; Geological Society: London, UK, 1989; Volume 42, pp. 313–345. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Chattopadhyay, B.; Gupta, A.K. Preliminary phase equilibria study of the system nepheline–diopside–sanidine under 1 kbar in presence of excess water. Indian J. Geol. 1999, 71, 81–91. [Google Scholar]
- Gupta, A.K.; Chattopadhyay, S.; Chattopadhyay, B.; Arima, M. Experimental study of the system diopside–nepheline–sanidine at 1, 1 and 2 GPa [P(H2O)=P(Total)]: Its significance in the genesis of alkali-rich basic and ultrabasic rocks. Lithos 2006, 86, 91–109. [Google Scholar] [CrossRef]
- Kjarsgaard, B.A.; Hamilton, D.L. The genesis of carbonatites by immiscibility. In Carbonatites: Genesis and Evolution; Bell, K., Ed.; Unwin Hyman: London, UK, 1989; pp. 388–404. [Google Scholar]
- Cooper, D.L.; Reid, D.L. Nepheline-sövites as parental magmas in carbonatite complexes: Evidence from Dicker Willem, southwest Namibia. J. Petrol. 1998, 39, 2123–2136. [Google Scholar] [CrossRef]
- Demény, A.; Ahijado, A.; Casillas, R.; Boyce, A.J.; Fallick, A.E. Crustal contamination of carbonatites indicated by δ34S-δ13C correlations (Fuerteventura, Canary Islands). Rev. Soc. Geológica España 1998, 12, 453–460. [Google Scholar]
- Demény, A.; Ahijado, A.; Casillas, R.; Vennemann, T.W. Crustal contamination and fluid/rock interaction in the carbonatites of Fuerteventura (Canary Islands, Spain): A C, O, H isotope study. Lithos 1998, 44, 101–115. [Google Scholar] [CrossRef]
- Arculus, R.J. Melting behaviour of the two basanites in the range 10–35 Kb and the effect of TiO2 on the olivine-diopside reactions at high pressures. Year Book—Carnegie Inst. Wash. 1975, 74, 512–515. [Google Scholar]
- Brey, G.P.; Green, D.H. Solubility of CO2 in olivine melilitite at high pressures and role of CO2 in the Earth’s upper mantle. Contrib. Mineral. Petrol. 1976, 55, 217–230. [Google Scholar] [CrossRef]
- Brey, G.; Brice, W.R.; Elli, S.D.J.; Green, D.H.; Harris, K.L.; Ryabchikov, I.D. Pyroxene-carbonate reactions in the upper mantle. Earth Planet. Sci. Lett. 1983, 62, 63–74. [Google Scholar] [CrossRef]
- Edgar, A.D. The genesis of alkaline magmas with emphasis on their source regions: Inferences for experimental studies. In Alkaline Igneous Rocks; Fitton, J.G., Upton, B.J.J., Eds.; Geological Society of London, Special Publication: London, UK, 1987; Volume 30, pp. 29–52. [Google Scholar]
- Stein, C.A.; Stein, S. A model for global variation in oceanic depth and heat flow with lithospheric age. Nature 1992, 359, 123–129. [Google Scholar] [CrossRef]
- Turcotte, D.I.; Schubert, G. Geodynamics, Application of Continuum Physics to Geological Problems; J. Wiley: New York, NY, USA, 1982; pp. 1–450. [Google Scholar]
- Ranelli, G. Rheology of the Earth, 2nd ed.; Chapman and Hall: London, UK, 1995; pp. 1–413. [Google Scholar]
- Wyllie, P.J. Origin of carbonatites: Evidence from phase equilibrium studies. In Carbonatites: Genesis and Evolution; Bell, K., Ed.; Unwin Hyman: London, UK, 1989; pp. 500–545. [Google Scholar]
- De Ignacio, C.; Muñoz, M.; Sagredo, J.; Fernández-Santín, S.; Johansson, A. Isotope geochemistry and FOZO mantle component of the alkaline–carbonatitic association of Fuerteventura, Canary Islands, Spain. Chem. Geol. 2006, 232, 99–113. [Google Scholar] [CrossRef]
- Hoernle, K.; Zhang, Y.S.; Schmincke, H.U. Seismic and geochemical evidence for large-scale mantle upwelling beneath the eastern Atlantic and western and central Europe. Nature 1995, 374, 34–39. [Google Scholar] [CrossRef]
- Carnevale, G.; Caracausi, A.; Correale, A.; Italiano, L.; Rotolo, S.G. An Overview of the Geochemical Characteristics of Oceanic Carbonatites: New Insights from Fuerteventura Carbonatites (Canary Islands). Minerals 2021, 11, 203. [Google Scholar] [CrossRef]
- Ito, K.; Kennedy, G.C. Melting and phase relations in the plane tholeiite-lherzolite-nepheline basanite to 40 kilobars with geological implications. Contrib. Mineral. Petrol. 1968, 19, 177–211. [Google Scholar] [CrossRef]
- Bultitude, R.J.; Green, D.H. Experimental study of crystal-liquid relationships at high pressures in olivine nephelinite and basanite compositions. J. Petrol. 1971, 12, 121–147. [Google Scholar] [CrossRef]
- Von Eckerman, H. The alkaline district of Alno Island. Sver. Geolgiska Unders. 1948, 36, 176. [Google Scholar]
- Von Eckerman, H. The petrogenesis of the Alno alkaline rocks. Bull. Geol. Inst. Univ. Upps. 1961, 40, 25–36. [Google Scholar]
- Koster Van Groos, A.F.; Wyllie, P.J. Experimental data bearing on the role of liquid immiscibility in the genesis of carbonatites. Nature 1963, 199, 801–802. [Google Scholar] [CrossRef]
- King, B.C. The Napak Area of Southern Karamoja, Uganda; Geological Survey Uganda: Kampala, Uganda, 1949; Mem 5; pp. 1–57. [Google Scholar]
- Hamilton, D.L.; Bedson, P.; Esson, J. The behaviour of trace elements in the evolution of carbonatites. In Carbonatites: Genesis and Evolution; Bell, K., Ed.; Unwin Hyman: London, UK, 1989; pp. 405–427. [Google Scholar]
- Vuorinen, H.; Skelton, A.D.L. Origin of silicate minerals in carbonatites from Alno Island, Sweden—Magmatic crystallisation or wall rock assimilation? Terra Nova 2004, 16, 210–215. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Mitchell, R.H. The Role of Assimilated Silica in the Evolution of Carbonatites; 4th Eurocarb Workshop; Lanzarote-Fuerteventura: Canary Islands, Spain, 2003; pp. 10–11. [Google Scholar]
- Kapustin, Y.U.L. Mineralogy of Carbonatites; Amerind Publishing Company Pvt., Ltd.: New Dehli, India, 1980; pp. 1–259, (translated from Mineralogiya Karbonatitov. Nauka Publ. Moscow, 1971). [Google Scholar]
- Veksler, I.V.; Nielsen, T.F.D.; Sokolov, S.V. Mineralogy of Crystallized Melt Inclusions from Gardiner and Kovdor Ultramafic Alkaline Complexes: Implications for Carbonatite Genesis. J. Petrol. 1998, 39, 2015–2031. [Google Scholar] [CrossRef]
- Yoder, H.S. Potassium-rich rocks: Phase analysis and heteromorphic relations. J. Petrol. 1986, 27, 1215–1228. [Google Scholar] [CrossRef]
- Nielsen, T.F.D. Alkaline dike swarms of the Gardiner Complex and the origin of ultramafic alkaline complexes. Geochem. Int. 1995, 31, 37–56. [Google Scholar]
- Fedorchuk, Y.A.M.; Veksler, I.V. Phase equilibria in the system akermanite–F-phlogopite at atmospheric pressure. Geochem. Int. 1997, 35, 197–199. [Google Scholar]
- Veksler, I.V.; Fedorchuk, Y.A.M.; Nielsen, T.F.D. Phase equilibria in the silica-undersaturated part of the KAlSiO4–Mg2SiO4–Ca2SiO4–SiO2–F system at 1 atm and the larnite-normative trend of melt evolution. Contrib. Mineral. Petrol. 1998, 131, 347–363. [Google Scholar] [CrossRef]
- Le Bas, M.J. Ultra-alkaline magmatism with or without rifting. Tectonophysics 1987, 143, 75–84. [Google Scholar] [CrossRef]
- Woolley, A.R. The spatial and temporal distribution of carbonatites. In Carbonatites: Genesis and Evolution; Bell, K., Ed.; Unwin Hyman: London, UK, 1989; pp. 15–37. [Google Scholar]
- Nasir, S.; Klemd, R. New carbonatite occurrences along the Hatta transform fault zone (Northern Oman Mountains, United Arab Emirates). J. Afr. Earth Sci. 1998, 27, 3–10. [Google Scholar] [CrossRef]
- Nasir, S.; Hanna, S.; Al-Hajari, S. The Petrogenetic Association of Carbonatite and Alkaline Magmatism: Constrains from the Masfut- Rawda Ridge, Northern Oman Mountains. Mineral. Petrol. 2003, 77, 235–258. [Google Scholar] [CrossRef]
- Woolley, A.R.; Barr, M.W.C.; Din, V.K.; Jones, G.C.; Wall, F.; Williams, C.T. Extrusive carbonatites from the Uyaynah area, United Arab Emirates. J. Petrol. 1991, 32, 1143–1167. [Google Scholar] [CrossRef]


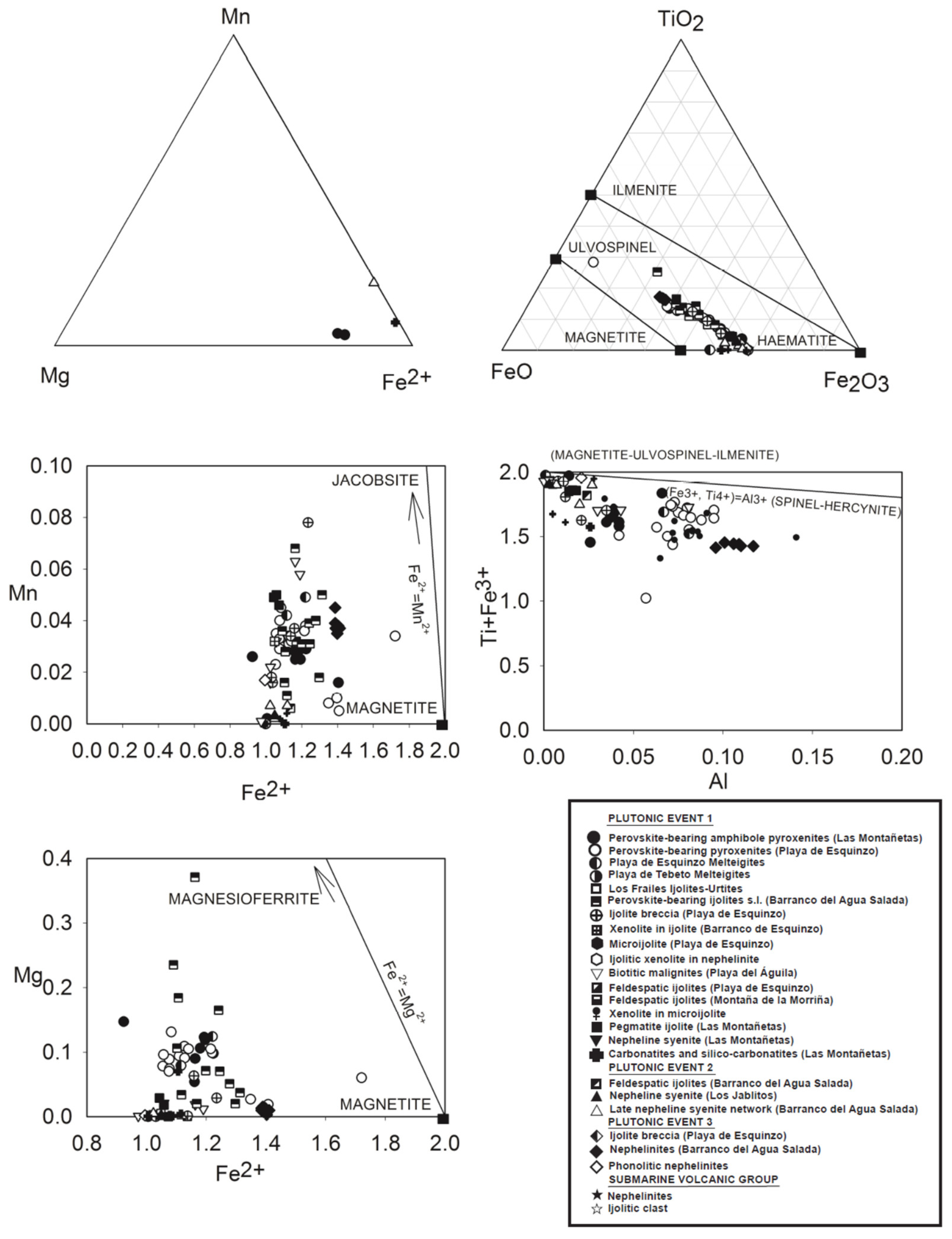

| FACIES/Rocks | % Volume | Plutonic Event | Petrographic Characteristics |
|---|---|---|---|
| LAS MONTAÑETAS | 15 | 1° | |
| Perovskite-bearing amphibole pyroxenites | 70 | 1° | A prismatic pyroxene cumulate (80%) with brown interstitial Mg-hastingsite/pargasite in variable amounts (5%–10%). Minor brown phlogopite growing around pyroxenes (2%). Apatite forms interstitial aggregates (2%). Allotriomorphic perovskite (2%) includes crystals of apatite and titanomagnetite. Titanomagnetite has also an interstitial position (3%). Titanite crystals growing around perovskite (2%). Interstitial nepheline (2%). Pyroxene in the contact with interstitial nepheline has a slightgreenish pleochroism (diopside). |
| Pegmatite ijolite | 20 | 1° | Feldspathic ijolites or even malignites grading to nepheline syenites. Coarse- to very coarse-grained size rocks frequently with comb texture defined by the orientation of large pyroxene (20%) crystals. Subidiomorphic brown micas grow over pyroxene. Nepheline (60%) and alkali feldspar (5%) are usually interstitial. Calcite (5%) is included into pyroxenes or fills interstices between other minerals. Apatite (1%) occurs as inclusions in pyroxene, perovskite (2%), biotite (2%), nepheline and alkali feldspar. Titanite (3%) grows interstitially between pyroxenes or around perovskite. Melanite (2%) occurs growing over titanite. |
| Nepheline syenites | 5 | 1° | Form dykes or masses that grade from pegmatite ijolite to silicocarbonatites or carbonatites. They are medium- to coarse-grained rocks with a general texture defined by large idiomorphic alkali feldspar crystals (40%). Pyroxenes (10%) forming short prisms with calcite (1%) and apatite (1%) inclusions. Biotite (4%) and nepheline (40%) form subidiomorphic crystals. Calcite can form inclusions in pyroxene or fill interstices between alkali feldspars. In contact with alkali feldspar reaction rims composed of garnet, analcime and prehnite can be observed. Apatite, pyrochlore, zircon and titanite are common accessory phases (around the 1%). |
| Carbonatites and silicocarbonatites | 5 | 1° | Coarse-grained sövites are dominant with variable content of silicate minerals. Occasionally silicates reach 50% of the rock (silicocarbonatites). Dykes with lower thickness and magnitude are usually fine- to medium-grained alvikites. In some outcrops spinifex textures can be observed in carbonatites. Calcite forms large platy subidiomorphic crystals commonly with consertal texture. Aegirine augite/aegirine form subidiomorphic crystals with many apatite and calcite inclusions. They occasionally occur intergrown with calcite. Biotite occurs as large primary idiomorphic crystals, frequently zoned. Subidiomophic, very perthitic alkali feldspar crystals (sanidine) and nepheline also occur. Prismatic subidiomorphic or subrounded apatite inclusions are seen in sanidine, aegirine augite, biotite-phlogopite and calcite. Magnetite and Mn-rich ilmenite form discrete subidiomorphic crystals. Titanite is scarce and forms idiomorphic crystals. Zircon forms idiomorphic fractured crystals included into calcite and alkali feldspar. Pyrochlore forms zoned subidiomorphic crystals included in calcite (Supplementary Materials Figure S4b,d) and alkali feldspar. Monazite is included in apatite crystals or around it. Minor crystals of chalcopyrite, sphalerite and pyrite can be found. |
| LOS JABLITOS | 5 | 2° | |
| Nepheline syenite | >99 | 2° | They can show both agpaitic or miaskitic textures. Are characterised by alkali feldspar (43%) and nepheline (43%), together with aegirine augite/aegirine (10%). Apatite (2%) and titanite (2%) are common accessory phases. |
| Carbonatite | >1 | 2° | Medium- to fine-grained alvikite dykes. They are dark pink or purple in color with calcite crystals (96%) full of hematite microexolutions. Apatite (4%) forms subrounded discrete grains or aggregates included or placed around calcite margins. |
| MONTAÑA DE LOS FRAILES | 5 | 1° and 2° | |
| Melteigites-ijolites-urtites | 90 | 1° | Biotite melteigites are dominant and grade locally to ijolites-urtites. The overall rock microstructure of these rocks is that of a prismatic pyroxene cumulate. Pyroxene crystals are subidiomorphic and frequently zoned showing a pink core (titanian diopside) and pale green or uncolored rims (diopside). Minor brown biotite also occurs in some samples growing around pyroxenes. Apatite and titanomagnetite are ubiquitous. Some samples contain titanite, nepheline, andradite garnet and calcite. |
| Carbonatites | 5 | 1° | Calcite crystals (80%) include prismatic alkali feldspar (10%), idiomorphic titanite (3%, ore-rich rims), red pleochroic idiomorphic biotite plates (3%) and prismatic apatite crystals (4%), both isolated and as aggregates. |
| Nepheline syenite network | 50 | 2° | Late nepheline syenites form a network of pinkish dykes and veins. Heterogranular rocks with coarse to very coarse grain size and frequently showing comb textures due to the orientation of alkali-feldspar (43%) and pyroxene (10%) aggregates. Nepheline (43%) and fractured zircon grains (4%) commonly occur. |
| BARRANCO DEL AGUA SALADA. 75%. 1°, 2° and 3° Plutonic Events | 75 | 1°, 2° and 3° | |
| Alkali pyroxenites | 35 | 1° | Alkali pyroxenites grading to melteigites are predominantly medium- to coarse-grained rocks containing usually phlogopite and perovskite (Playa de Esquinzo) or amphibole (Playa de Tebeto). They are commonly adcumulates to mesocumulates with clinopyroxene (75%, titanian diopside and diopside), kaersutitic amphibole (8%), subidiomorphic perovskite (3%) and idiomorphic apatite (3%) as cumulus phases, while phlogopite (2%), titanomagnetite (2%), titanite (1%), nepheline (3%) and calcite (3%) are intercumulus minerals. |
| Perovskite-bearing ijolites s.l | 50 | 1° | Perovskite-bearing ijolites s.l. are meso to orthocumulates, with the nepheline/clinopyroxene ratio increasing from melteigite (3:1) to ijolite (1:3). They also contain brown phlogopite (3%) and kaersutite (3%), abundant apatite (2%), magnetite (2%), perovskite (2%) and allotriomorphic titanite (2%) is seen in place of perovskite. In urtitic ijolites, the occurrence of interstitial melanite crystals (4%) is common. |
| Biotitic malignites (feldspathic ijolite-biotitic malignites-nepheline syenites) | 5 | 1° | In fine-grained types, the rock texture is characterized by the presence of poikilitic crystals, both idiomorphic (titanite and zoned biotite) and allotriomorphic (nepheline, alkali feldspar and calcite), containing small idiomorphic zoned pyroxene crystals (titanian diopside, diopside and aegirine augite), apatite and titanomagnetite. Inside the biotite crystals colorless cores and outer rims with strong red-brown pleochroism are observed. In coarse- to very coarse-grained varieties, textures are equigranular with large idiomorphic zoned pyroxene (titanian diopside, diopside and aegirine augite), apatite, titanite, perovskite and biotite (Supplementary Material Figure S5b). Nepheline, alkali feldspar (Supplementary Material Figure S5c) and calcite occur interstitially. Biotite forms large crystals growing over or as aggregates around pyroxenes. Nepheline is subidiomorphic and occurs interstitially or as inclusions in alkali feldspar |
| Feldspathic ijolite veins | <1 | 1° | Contain aegirine augite (20%) and crystals of zoned nepheline (50%), which is invariably highly altered and pinkish or almost red in color. Pyroxene and nepheline are enclosed by clear “pools” of interstitial or poikilitic alkali feldspar (20%). Orange-brown phlogopite (11%) is also common. Accessory minerals include titanite (3%), apatite (3%) and magnetite (3%). |
| Late nepheline syenite network | <3 | 2° | Network of pinkish dykes and veins that cut all the lithologies They are heterogranular rocks with coarse to very coarse grain size frequently with comb texture due to the orientation of alkali-feldspar (60%) and pyroxene (20%) aggregates. Also include heavily altered nepheline (11%), fractured zircon grains (3%), idiomorphic pyrochlore crystals (3%) and biotite (3%). |
| Intrusive breccias with medium- to fine-grained ijolite-nephelinite matrix | <1 | 3° | Heterogeneous porphyritic rocks containing abundant fragments of xenocrysts and different rock types. The most common phenocrysts are subidiomorphic (diopside and titanian diopside) pyroxenes (30%) and heavily altered nepheline (60%). Biotite phenocrysts (10%) also occur occasionally. The groundmass contains small nepheline and calcite crystals interstitially or as ocelli. |
| Nephelinite and phonolitic nephelinite dykes | <5 | 3° | Nephelinite and phonolitic nephelinite dykes constitute the youngest intrusions. They are strongly porphyritic with pyroxene (50%), nepheline (40%), red-brown garnet (3%), alkali feldspar (3%) and titanite phenocrysts (2%) and minor amounts of oxide minerals (2%). The groundmass consists mainly of fine-grained pyroxene, alkali feldspar, nepheline and titaniferous magnetite. Accessory minerals include apatite, titanite and perovskite. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casillas, R.; Ahijado, A.; Nagy, G.; Demény, A.; Fernández, C. The Esquinzo Ultra-Alkaline Rock Suite of Fuerteventura Basal Complex (Canary Islands): Evidence for Origin of Carbonatites by Fractional Crystallization. Minerals 2024, 14, 295. https://doi.org/10.3390/min14030295
Casillas R, Ahijado A, Nagy G, Demény A, Fernández C. The Esquinzo Ultra-Alkaline Rock Suite of Fuerteventura Basal Complex (Canary Islands): Evidence for Origin of Carbonatites by Fractional Crystallization. Minerals. 2024; 14(3):295. https://doi.org/10.3390/min14030295
Chicago/Turabian StyleCasillas, Ramón, Agustina Ahijado, Géza Nagy, Attila Demény, and Carlos Fernández. 2024. "The Esquinzo Ultra-Alkaline Rock Suite of Fuerteventura Basal Complex (Canary Islands): Evidence for Origin of Carbonatites by Fractional Crystallization" Minerals 14, no. 3: 295. https://doi.org/10.3390/min14030295
APA StyleCasillas, R., Ahijado, A., Nagy, G., Demény, A., & Fernández, C. (2024). The Esquinzo Ultra-Alkaline Rock Suite of Fuerteventura Basal Complex (Canary Islands): Evidence for Origin of Carbonatites by Fractional Crystallization. Minerals, 14(3), 295. https://doi.org/10.3390/min14030295