Abstract
The fossil record demonstrates the preservation of porphyrins (e.g., heme) in organic sediments and the fossilized remains of animals. These molecules are essential components in modern metabolic processes, such as electron transport (cytochromes) and oxygen transport (hemoglobin), and likely originated before the emergence of life. The integration and adaptation of porphyrins and structurally similar molecules (e.g., chlorophylls) are key aspects in the evolution of energy production (i.e., aerobic respiration and photosynthesis) and complex life (i.e., eukaryotes and multicellularity). Here, we discuss the evolution and functional diversity of heme-bound hemoglobin proteins in vertebrates, along with the preservation of these molecules in the fossil record. By elucidating the pivotal role of these molecules in the evolution of life, this review lays the groundwork necessary to explore hemoglobin as a means to investigate the paleobiology of extinct taxa, including non-avian dinosaurs.
Keywords:
molecular paleontology; heme; porphyrin; hemoglobin; evolution; chemical evolution; preservation 1. Introduction
Porphyrin-containing proteins (e.g., cytochromes, hemoglobin, and myoglobin) have been essential components of metabolism since the earliest form of life emerged at least 3.4 billion years ago [1,2], and are required for the metabolism of virtually all taxa, from bacteria to plants to humans, in the modern era. This review will focus on heme–hemoglobin interactions. The oxygen-binding activities of heme porphyrin in particular make hemoglobin critical for respiration in most extant vertebrate taxa. Composed of four pyrrole (C4H4NH) rings connected via methine bridges (R1-CH=R2) (Figure 1), heme is responsible for the mediation of oxygen and carbon dioxide (CO2) exchange throughout vertebrate tissues. Some plants also use hemoglobin—in the form of leghemoglobin—to facilitate the activity of anoxic nitrogen-fixing bacteria in symbiosis with plant tissues [3]. Hemoglobins contain a wealth of information on the physiology of taxa bearing them, their phylogenetic history, and their environments. Thus, the successful characterization of hemoglobin from extinct organisms could open an unprecedented window into their paleobiology unattainable from gross skeletal morphology, or even more abundant molecules (e.g., collagen).
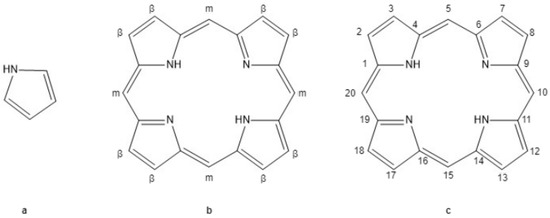
Figure 1.
Pyrrole and base porphyrin ring. (a) Pyrrole (C4H4NH) is a five-member nitrogen-containing ring that forms tetrapyrrole molecules. (b) Unsubstituted porphin tetrapyrrole ring with beta (β) and meso (m) positions labeled along the periphery of the ring. (c) Numeric labels of the 20 carbon molecules forming the porphyrin rings.
Proteins and other biomolecules are assumed to be labile, and most begin to degrade shortly after an organism dies. However, molecular techniques, including paleoproteomics and various forms of spectroscopy (e.g., Raman Spectroscopy), suggest that small concentrations of endogenous organic material are preserved in fossil tissues [4,5,6]. Because their intrinsic structural properties greatly enhance stability, heme porphyrins and similar molecules (e.g., chlorophyll-α) have been reported in plant-derived organic sediments (e.g., oil shales [7,8,9] and asphalts [9]) and fossil tissue of animals [10,11,12,13,14]. Peptide sequences of hemoglobin have also been reported from archaeological and Pleistocene taxa [6,15,16,17,18,19,20,21], as well as specimens from deep time (i.e., greater than 1 Ma in age) [10,22,23]. Further, immunohistology methods have demonstrated immunolocalization of hemoglobin in fossil samples of various ages [5,22,24,25,26,27,28].
It has been hypothesized that hemes recovered from preserved animal taxa may have derived from hemoglobin [10,11,14], and therefore may serve as a proxy for inferring its presence in fossil tissues. However, confident identification of hemoglobin-derived heme must also rule out other potential sources of this porphyrin, such as bacterial and vertebrate cytochromes or peroxidases. Here, we review the structure and function of porphyrins and other tetrapyrrole molecules in the early metabolism of life and the evolution of hemoglobin (beginning with its earliest hypothesized precursors), and discuss the preservation potential of this molecule in the fossil record. We examine methods to confidently identify heme in fossil remains of vertebrates through the lens of its unique molecular structure and function. We start with an overview of the structure of porphyrins molecules followed by the conditions on the early Earth, under which life first evolved, and the role porphyrins and other tetrapyrroles played in the oxygenation of the planet and the evolution of aerobic life. We then delve into the function, structure, and diversity of vertebrate heme-bound proteins, with a specific focus on hemoglobin and its role in respiratory adaptations to environmental pressures. Next, we discuss the physical and chemical properties that contribute to the persistence of heme and/or hemoglobin in the fossil record, the diagenetic alterations that may occur at the molecular level, and how we may distinguish between heme derived from hemoglobin versus other heme-bound proteins. Finally, we explore the ways preserved hemoglobin may be used to investigate aspects of paleobiology that are currently inaccessible.
2. Porphyrins and Other Tetrapyrroles
2.1. Porphyrin Structure and Biological Function
Heme (Figure 2) belongs to a class of molecules called porphyrins, which are a type of tetrapyrrole. A pyrrole (C4H4NH) is a five-member ring that contains nitrogen (Figure 1a) [29,30]. When four of these rings are interconnected by methine bridges (R1-CH=R2) into a larger, macrocyclic ring, they form a tetrapyrrole (Figure 1b). These large, tetrapyrrole rings contain an alternating series of single and double bonds [29,30]. The electron pairs forming these bonds can shift freely across the molecule (i.e., they are delocalized), in such a way that connections between its constituent atoms oscillate between single and double-bond states [29,31,32]. This arrangement of atoms is known as an aromatic ring [29,31,32]. Because of the orientation of the nitrogen in its pyrrole sub-units, tetrapyrroles commonly form complexes with metal cations, such as iron (Fe), magnesium (Mg), copper (Cu), zinc (Zn), cobalt, (Co), and nickel (Ni), coordinated in the molecule’s core [30,33,34,35]. At the periphery of the ring, tetrapyrroles possess sites for potential substitutions [29,30]. In the heme tetrapyrrole, the core binds ferrous iron (Fe2+) at its center, giving hemoglobin its familiar red color, and the substitutions at the periphery of the ring result in several distinct types of heme (e.g., heme b, and heme c) [29,33,36,37].
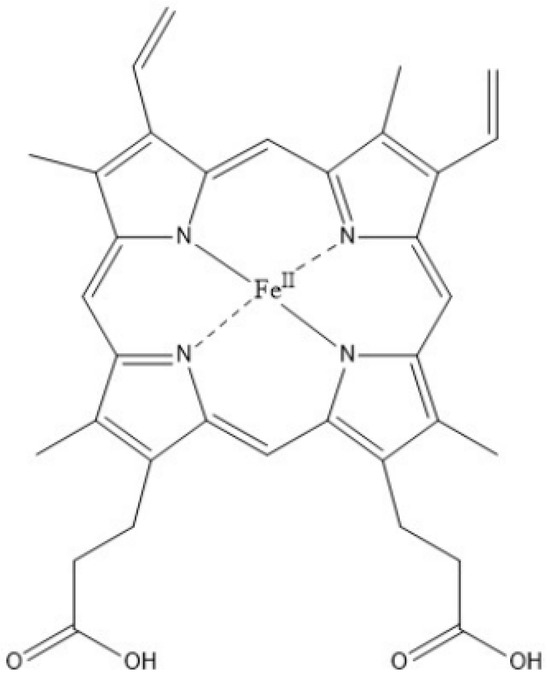
Figure 2.
Heme b. The heme b variant is bound to the hydrophobic pocket of hemoglobin. Ferrous iron (Fe2+) is bound to the center of the heme porphyrin through an ionic bond. Histidine residues of hemoglobin bind to the iron ion. Substitution along the porphin ring includes methyl (-CH3) at C’s 2, 7, 12, and 18, vinyl group (-CH=CH2) at 3 and 8, and propionic acids (-CH2CH2COOH) at 3 and 17.
The aromatic nature of tetrapyrroles, along with their ability to bind metal ions, gives rise to diverse molecules with properties that are essential in many biological pathways. For example, heme is utilized for oxygen transport in hemoglobin [3,34,36], electron transport in cytochromes [34,38,39,40], and chlorophyll for light absorption in photosynthesis [36,41,42]. These features are also thought to contribute to the stability of its molecular structure [31]. In particular, the free-moving nature of the bonded electron pairs in aromatic tetrapyrroles makes them relatively stable molecules [31,32]. This is because the oscillation of their bonds between single and double makes all bonds essentially “one and a half”, conferring greater stability than static single bonds [31]. In particular, the stability of heme is further heightened by the presence of the additional double bond compared to other types. This extra double bond is what distinguishes porphyrins such as heme from other biologically important tetrapyrroles such as chlorins (e.g., chlorophyll) and bacteriochlorin (a photosynthetic pigment found in non-oxygen-producing bacteria) (Figure 3) [32,43]. The aromatic nature of heme, along with its additional porphyrin double bond and its affinity to bind iron, are all stabilizing factors that are thought to increase its potential for preservation in deep time [33,44].
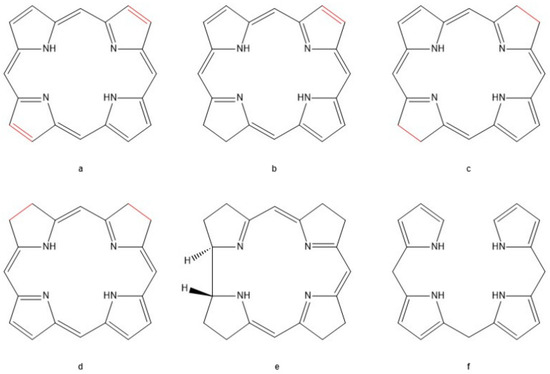
Figure 3.
Tetrapyrrole pigments variants. (a) Porphin ring highlighting the two double bonds on opposite sides of the aromatic molecule. (b) Chlorin, the parent molecule for plant pigments like chlorophyll-α. (c) Bacteriochlorin, the parent molecule of photosynthetic pigments in bacteria in anoxic photosynthesis. (d) Isobacteriochlorin, an isomer of bacteriochlorin. (e) Corrin, the parent pigment of vitamin B12. (f) Linear bilane, pigment molecules formed during the synthesis and degradation of heme molecules [35].
2.2. Role of Porphyrins in Early Life
Porphyrins are ubiquitous across modern taxa and are present in both prokaryotes and eukaryotes [34]. These molecules were likely present in the metabolism of organisms prior to the divergence of eukaryotes and prokaryotes, a hypothesis supported by the utilization of the same precursor molecules (5-aminolevulinic acid) in all observed biological synthesis pathways [34] and the presence of the intermediate molecule uroporphyrin in all biologically active tetrapyrroles (e.g., heme porphyrins and chlorophyll chlorins) [7,36]. Because their incorporation into organisms is thought to have occurred extremely early in the development of life [45], it is necessary to go as far back as the origin of life itself to understand their biological significance and role in biological processes.
2.2.1. The Abiotic Formation of Porphyrin and Chemical Origin of Life
Following the formation of the earth, environmental conditions during the early Hadean era (4.6 Ga) were hostile and destructive to the formation of life. Greatly increased volcanic activity (compared to modern conditions) and frequent meteor impacts created an environment too hot for life to flourish [46,47]. However, as primordial oceans formed and the planet cooled during the Archean (4.0–2.5 Ga), conditions on Earth changed permitting the formation of prebiotic biopolymers (e.g., amino acids, carbohydrates, and RNA) from inorganic molecules such as CO2 and ammonia [46,48,49]. There was little free oxygen present in the Earth’s early atmosphere, which was rich in CO2, nitrogen (N2), and methane gases [34,36,47,48,50]. These reducing conditions led to an ocean rich in ferrous iron and sulfur [51,52,53]. Because free oxygen drives the rapid oxidation of most biomolecules, its presence in the ancient atmosphere would break apart the chemical bonds of prebiotic compounds as soon as they formed, creating an environment toxic to life [54,55]. Therefore, the anaerobic or dysaerobic (low free oxygen) and reducing conditions of the early Earth would favor the abiotic formation of biopolymers and their precursors [48,49], setting the stage for self-organizing molecules (i.e., RNA world hypothesis [56]) that would eventually form the earliest organisms [55]. Porphyrins were likely among the earliest prebiotic molecules to form and contributed to the development of life 3.8–3.4 Ga [2,56,57,58,59,60].
Like the early biopolymers that form the constituents of life even today, porphyrins may have formed abiotically [2,60,61,62], along the shores of primordial volcanic islands. The prebiotic oceans of the late Hadean and early Archean are hypothesized to retain a greater concentration of inorganic salts and amino acids compared to the modern oceans [62,63,64]. These amino acids were potentially generated abiotically via ultraviolet radiation [45,65,66]. The interaction between the salty ocean water containing various abiotic amino acids and volcanic eruptions provided the thermal and acidic conditions required for porphyrin synthesis, driving the conversion of amino acids into pyrroles [62,63,64]. Repeated cycles of heating and cooling condense pyrroles into porphyrins [63]. This abiotic synthesis has successfully generated porphyrins under conditions simulating that of early Earth [2,60,61,62].
Among these abiotically generated porphyrins was uroporphyrin, which is a possible prebiotic photosensitive compound that is also the universal porphyrin intermediate in all biologically relevant tetrapyrroles [2,36,67]. The global extreme heat provided by volcanic activity required for the abiotic generation of porphyrins in the Hadean and early Archean slowly gave way to less harsh conditions, making it unlikely that the porphyrins preserved in organic sediment and fossil tissue have an abiotic origin. However, the abiotically generated porphyrin co-factors may have been assimilated into the proto-metabolisms of early organisms [63].
The earliest organisms to evolve during the Archean were most likely anaerobic prokaryotes [53,59]. One of the earliest cofactors hypothesized to associate with anaerobic enzymes for energy transfer in such early life forms is iron–sulfur (Fe-S) clusters [59,66,68,69]. The reducing conditions of the Archean oceans would have permitted the spontaneous generation and environmental availability of Fe-S clusters [53,69]. Along with these Fe-S clusters, abiotically formed porphyrin molecules were also environmentally available and employed in early metabolic pathways, although it is uncertain when and how this association first occurred. It is hypothesized that early organisms must have developed ways to compensate for oxidative stress and/or light absorption in initial forms of photosynthesis, and porphyrins may have filled this role [2,61]. The different forms of tetrapyrroles, including porphyrins (heme), chlorins (chlorophylls), and bacteriochlorin, are all hypothesized to derive from uroporphyrins [36,41,66], and distinct forms of these molecules may have arisen from variations in redox potentials [42,66,70]. For example, heme c of cytochromes in the electron transport chains (ETC) possess a wider redox potential that is more conducive for electron transport than heme b [71].
2.2.2. This Distribution and Function of Porphyrins as Life Evolves
In our modern, aerobic world, porphyrins are incorporated into heme-containing cytochromes, which are essential components of the ETC in anaerobic [72,73,74] and aerobic respiration [40,73,75], as well as in photosynthesis [39,40]. In aerobic respiration, cytochromes pass electrons through the ETC, resulting in the reduction of oxygen into water by cytochrome oxidases [73,75,76]. Although oxygen is a crucial part of this process, molecular evidence suggests that cytochrome oxidases originated when Earth’s environment was still anaerobic, or at least dysaerobic, and prior to the divergence of bacteria and archaea [1,72,75,76,77,78]. It is thought that cytochrome oxidases evolved from enzymes that reduce nitric oxide (NO) to N2 and that aerobic respiration is derived from this process [72,75]. NO and small concentrations of free oxygen (created by the photolysis of water) present in the early Earth’s atmosphere [48,75,79] would have had the potential to generate toxic reactive nitrogen species (RNS) and reactive oxygen species (ROS) [54,80], respectively. Ancestral cytochrome oxidase may have utilized NO as the terminal electron acceptor instead of oxygen when the atmosphere was still anaerobic and little free oxygen was present [75,76]. In this way, early cytochrome oxidases would serve as a defense against RNSs for early organisms. Accordingly, when the earth’s atmosphere became oxygenated (see below), it is thought cytochrome oxidases transitioned to using oxygen as their final electron acceptor, as oxygen is more electronegative and therefore more efficient in this role [73,75]. The reduction of oxygen by cytochrome oxidases into water is thought to be the basis from which aerobic respiration was derived [76]. This change, then, would have allowed cytochrome oxidases to protect early aerobic life from ROSs in the same way ancestral forms (i.e., NO-reducing enzymes) guarded against RNSs [76]. Early organisms with ETC competent to the utilization of oxygen may have had a selective advantage once atmospheric oxygen levels increased during the Great Oxygenation Event (GOE) [69,76].
The dispersal of aerobic metabolism in early organisms was likely facilitated by the transition from a reduced, anaerobic environment to an oxidized, aerobic environment [76,81]. This transition in the environment was likely driven by photosynthesis, a process contingent on tetrapyrrole molecules, bacteriochlorin, and chlorins. Photosynthesis is essentially the chemical reaction opposite of aerobic respiration; instead of breaking down organic molecules, photosynthesis captures solar energy through the implementation of bacteriochlorin and chlorin tetrapyrrole pigments to produce organic molecules [42,65,82,83].
2.2.3. The Origin of Photosynthesis and the Oxygenation of the Earth
The first photosynthetic organisms were likely anaerobic prokaryotes utilizing protein complexes that were unable to generate oxygen [42]. Modern anoxygenic photosystems utilize bacteriochlorophylls to capture light and have redox potential sufficient to oxidize electron donors such as hydrogen gas (H2), Fe2+, hydrogen sulfide (H2S), formate, or oxalate [70], and it is hypothesized that early forms may have been similar [42,70]. In particular, graphite carbon isotopes from sediments 3.8 Ga provide evidence of early anoxygenic photosynthesis utilizing hydrogen as its electron donor [83]. Conversely, oxygenic photosynthesis, which oxidizes water molecules into molecular oxygen, requires a wider redox potential than possessed by bacteriochlorophylls [42,70] and is thought to have arisen through the development of chlorophyll-α (i.e., a chlorin tetrapyrrole) [70]. The evolution of chlorophyll, and thus oxygenic photosynthesis, in the ancestors of cyanobacteria and modern plants is hypothesized to be the main driver of the oxygenation of the environment during the GOE [42,47,81,84].
The GOE altered the redox chemistry of the environment on a global scale, transforming the planet to an oxidative state [68]. Evidence of the oxygenation of the atmosphere includes banded-iron formations (BIFS) [47,68,85] and mass-independent sulfur isotopes [68,86]. In the presence of oxygen, iron is converted from its ferrous state, Fe2+, to its water-insoluble ferric state, Fe3+. These ferric iron oxides then precipitated out of the water column to form sedimentary structures alternating in iron oxide-rich and iron-poor chert sediments [68,85], giving these structures a banded appearance. It is hypothesized that once ferrous iron was depleted from the oceans, the generation of ferric iron oxides subsided, consistent with the disappearance of banded-iron formations from the rock record (~2.3 Ga). With the oceans saturated with oxygen, free oxygen would have then been able to diffuse into the atmosphere. Additionally, there was a shift in sulfur isotopic signatures that coincided with the disappearance of BIFs between 2.3 to 2.4 Ga. Preservation of mass-independent sulfur isotope signal occurs in the rock record before the GOE. Free oxygen accumulation in ancient atmosphere, resulting in a shift to a mass-dependent sulfur isotopes, and greatly increased oxidative continental weathering [86].
2.2.4. Early Organismal Compensation for Oxygen Toxicity and the Evolution of Multicellularity
The accumulation of dissolved and atmospheric oxygen during the GOE was a critical transition to the evolution of eukaryotic, and, eventually, multicellular, life [53,58,81]. Oxygen functions as the final electron acceptor in the aerobic respiration pathways of prokaryotes and eukaryotes [87]. However, oxygen poses a hazard to both anaerobic and aerobic organisms because it generates ROSs, including superoxide and hydroxyl ions [54,55]. Oxygen and its ROS byproducts are highly reactive molecules that can interact with proteins, lipids, and nucleic acids, disrupting enzymatic processes, destroying lipid membranes, forming cross-linkages that disable proteins [88], and damaging DNA [54,89,90]. Ancient anaerobes likely exhibited metabolisms relatively similar to their modern counterparts [45], which are rich in Fe-S cluster proteins [69]. In the presence of oxygen species, these Fe-S clusters become irreversibly denatured or destabilized [69]. Nitrogenase, an enzyme responsible for the fixation of dissolved atmospheric N2 into ammonia for amino acid synthesis in bacteria and archaea, utilized Fe-S clusters as a cofactor, making it vulnerable in oxygenated environments [91]. Additionally, oxygenation limits the bioavailability of soluble Fe2+, the most widely used metal cofactor in proteins, by oxidizing and precipitating iron [69]. The greatly increased oxidative stress generated by environmental oxygenation required anaerobic organisms to develop mitigation strategies to cope with this change. These may have included escape to low-oxygen environments (i.e., deep-sea hydrothermal vents [55]), development of tolerance, or the evolution of antioxidant defenses [55,69,70,87], such as heme-containing proteins [3].
In aerobic organisms, damage caused by ROSs generated from the environment or through metabolic processes is compensated for by peroxidase hemoproteins [57,92,93,94]. Coupled with cytochromes to generate energy gradients capable of producing 16–18 times more energy (i.e., adenosine triphosphate, ATP) compared to anaerobes [57], hemoproteins are critical to both the efficiency of aerobic respiration and as a guard against oxidative toxicity. Thus, the interaction between hemoproteins and the increased availability of oxygen after the GOE may have driven the dispersal of aerobic respiration. The widespread incorporation of aerobic respiration, in turn, allowed the evolution of more complex life forms [57,95]. For example, the energy surplus provided by aerobic respiration may have been favorable to the incorporation of the aerobic ancestor of modern mitochondria by anaerobic archaea, the endosymbiotic relationship of which is hypothesized to have given rise to eukaryotes 2 Ga ago [57,76,96,97]. Increases in energy output may have also permitted the formation of more complex biomolecules, such as steroids and fatty acids, giving rise to internal lipid membranes [57]. Lipid membranes, in turn, would have allowed the compartmentalization of eukaryotic cells (i.e., formation of organelles) [57], permitting greater control and complexity of signaling pathways within cells [57]. Ultimately, the energetic efficiency of aerobic respiration may have made cooperation and resource sharing between organisms viable, leading to the evolution of multicellular life during the Ediacaran (~600 Ma) [57,95].
With increased energy availability to drive proton pumps and other cellular mechanisms, multicellular life was favored. The emergence of multicellular organisms meant life was no longer restricted to isolated cells. Organisms could achieve greater masses and invade previously unviable environments. But, the development of tissues necessitated a means to transport materials from the organism’s exterior to internal cells no longer in direct contact with the external environment. Aerobic respiration, in particular, would require a means to transport oxygen to distal and/or internal cells once passive diffusion was not sufficient to meet energetic demands. Globin hemoproteins may have provided a mechanism for such capture and transport of oxygen [3].
2.3. The Diversification of Globin Proteins, and Divergences within Vertebrata
The globin superfamily of hemoproteins is a wide and diverse group of proteins. They are typically composed of six to eight alpha-helical domains and are found in both prokaryotic and eukaryotic organisms [3,80,98,99]. Across taxa, globin proteins vary in structure and function, but all possess iron-heme porphyrins that facilitate the reversible binding of oxygen molecules [100]. Vertebrates express seven globin proteins, including myoglobin (Figure 4a) and hemoglobin (Figure 4b), which are tissue-specific proteins expressed in the muscle and blood tissue, respectively [101,102]. An ancestral globin is suspected to have existed before the divergence of prokaryotes and eukaryotes, derived from the hemoproteins used in redox metabolism (i.e., cytochromes) or to mitigate oxygen toxicity (peroxidases) [3,98,100,103].
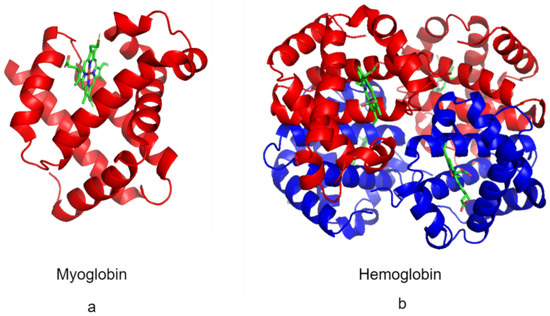
Figure 4.
Myoglobin and hemoglobin. (a) Structure of myoglobin (Protein Data Bank ID: 3RGK) monomer (red) [104] and the heme cofactor (green). (b) The structure of the hemoglobin (Protein Data Bank ID: 1A3N) tetramer is composed of α subunits (red) and β subunits (blue) and the heme cofactor (green) [104].
Outside of vertebrata, organisms possess a variety of globin and “hemoglobin” proteins that are expressed with diverse functions and structures (see [99] and references therein). For example, flavohemoglobin is a chimeric globin protein expressed in bacteria and yeast. Composed of a heme domain and a flavin (a non-tetrapyrrole pigment) domain, they are utilized in denitrification and redox biochemical processes [80,99,105]. Leghemoglobin, found in legume plants (e.g., peanuts) functions as an oxygen scavenger in the roots of plants, allowing for nitrogen fixation by symbiotic anaerobic microbes [98,99]. Interestingly, based on gene sequencing data, plant globin is hypothesized to share ancestry with animal hemoglobin [98,99]; thus, this molecule originated before the divergence of plants and animals in deep time, about 1.6 Ga ago [99]. Neuroglobins (nerve globin) function in oxygen sensing and were likely present in the common ancestor of invertebrates and vertebrates prior to the divergence of these groups during the Ediacaran [106].
Within vertebrata, there is a divergence in globin structure between Agnatha (jawless vertebrates) and Gnathostomata (jawed vertebrates), lineages that diverged 510 Ma [107]. Agnathans possess globin with either one or two domains, and gnathostomes possess a four-domain (tetrameric) hemoglobin (Figure 4). The tetrameric molecules of hemoglobin in jawed vertebrates permit greater efficiency in oxygen transport compared to jawless vertebrates (e.g., lampreys) (see below) [108].
3. Structure and Function of Vertebrate Hemoglobin
In the red blood cells (RBCs) of jawed vertebrates, hemoglobin is a tetramer composed of four globin protein subunits (i.e., domains) that possess heme cofactors and are structurally similar but distinguishable by differing peptide sequences [102,109]. The most common composition of adult vertebrate hemoglobin is a combination of two α-like and two β-like subunits [102,108,109,110]. Each α and β subunit join together or dimerize to form an αβ protein complex or heterodimer, which then binds to another αβ-heterodimer to form a α2 β2 tetramer [111]. Some vertebrates have alternate compositions that incorporate different subunits, such as the β/δ hybrid subunits of paenungulate clade (i.e., elephants, hyraxes, and manatees) [110,112,113]. Subunit expression may also vary depending on the stages of development (e.g., human fetal hemoglobin α2γ2) [114].
Within a highly conserved, hydrophobic pocket of each of the four subunits resides an iron-containing heme prosthetic group, or cofactor. The heme is anchored in this domain pocket by the interaction of its constituent iron with a histidine amino acid residue [111,115]. This heme-bound pocket is the binding site for oxygen or CO2 during respiration [109].
3.1. The Physiological Role of Hemoglobin and Its Limitations
The binding of oxygen induces a conformational change (i.e., changes the molecular shape in response to the environment) that alters the oxygen-binding affinity of the hemoglobin subunit. In the deoxygenated ‘relaxed’ state (R-state), hemoglobin possesses a high oxygen-binding affinity. Once oxygen is bound to a subunit, a conformational change occurs that transitions the bound unit into a low oxygen-binding affinity ‘tense’ state (T-state). Across the hemoglobin molecule, the individual subunits experience a phenomenon known as cooperative binding, in which the loading of oxygen in one subunit induces a conformational change that increases the oxygen-binding affinity of the remaining, unloaded subunits [108,115,116] until all reach the T-state.
The reversible binding of oxygen to hemoglobin is facilitated by the difference in oxygen partial pressure (PO2) between respiratory tissues (gills, skin, and lungs), RBCs, and distal body tissue (Figure 5) [117]. At the interface between respiratory tissue and RBCs, waste CO2 is released from the body, and oxygen is loaded. RBCs then transport oxygen to the distal, reduced PO2 tissues, unloading it for cytochrome-mediated energy production in the mitochondria. The difference in oxygen partial pressure between the RBCs and distal tissues is not sufficient to unload oxygen and requires the aid of “helper molecules” (i.e., allosteric effectors; see Table 1) [109]. Allosteric effectors bind to the periphery of hemoglobin subunits, outside of the heme oxygen-binding pocket. The conformational change they induce stabilizes the T-state, reducing the oxygen-binding affinity of the subunit and promoting the offloading of oxygen [115,118].
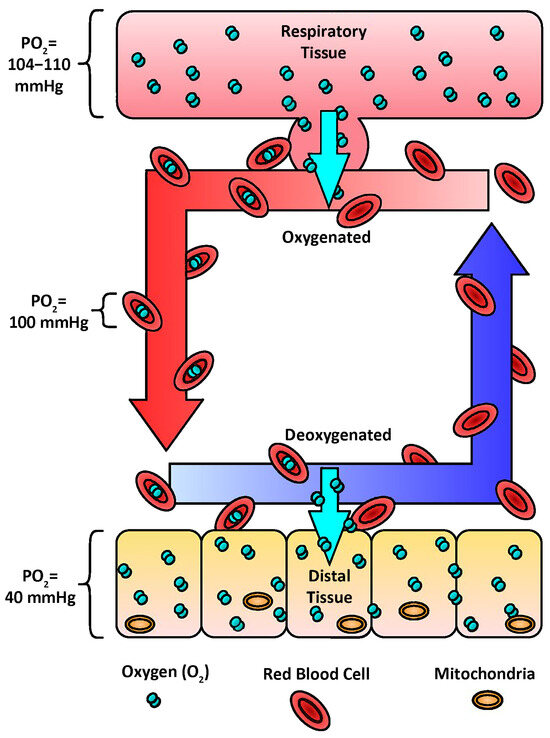
Figure 5.
Oxygen transport. The transport of oxygen to distal tissues of the body is mediated by the difference in the partial pressure of oxygen (PO2). In human lungs, the PO2 ranges from 104–110 mm Hg. Oxygen diffuses down the PO2 gradient from the respiratory tissue into the red blood cells (RBC) (100 mm Hg). The RBCs travel throughout the body to deliver oxygen to the mitochondria of distal tissue (40 mm Hg). Allosteric effectors support the offloading of oxygen.

Table 1.
Properties and allosteric effectors of vertebrate hemoglobin. ‘X’ marks the expression of hemoglobin properties or sensitivity to allosteric effectors. A blue ‘X’ represents the primary allosteric effector of the vertebrate taxa. Listed allosteric effectors include adenosine triphosphate (ATP), guanosine triphosphate (GTP), Bicarbonate (HCO3−), 2,3-Bisphosphoglycerate (2,3-BPG), inositol phosphates (IP), and chlorine ion (Cl−).
3.2. Allosteric Effector Variants
The most basal allosteric effector in vertebrate hemoglobin is ATP, which all gnathostomes share varying levels of sensitivity [108,116,119]. This effector remains the primary ligand regulating oxygen-binding affinity in basal vertebrates such as teleost fish [108,116] and is also the main ligand employed in embryonic hemoglobin across many taxa [120]. However, different tetrapod lineages possess additional distinct effectors that occupy this role. Amphibians and mammals (i.e., extant synapsids) utilize 2,3-bisphosphogylcerate (2,3-BPG) as their primary oxygen affinity regulator [108]. Conversely, in extant archosaurs (i.e., modern birds and crocodilians), the only time they possess 2,3-BPG in their RBCs is during the late stages of embryonic development [120,121], after which they become more sensitive to different organic phosphate effectors. In birds, there is a shift towards sensitivity to inositol phosphates (IPs). Compared to 2,3-BPG, IPs are more effective at reducing the oxygen-binding affinity in hemoglobin and are therefore more efficient promoters of oxygen offloading [122]. Adult crocodilians are insensitive to both 2,3-BPG and IPs and instead are primarily sensitive to HCO3− and acidic conditions [119,123,124]. This is an adaptation that favors their diving and ambush behavior, enabling prolonged periods underwater without the risk of muscle tissue starvation or the accumulation of lactic acid [124]. Dissolved CO2 in RBCs and blood plasma interacts with water to create bicarbonate (HCO3−) and hydrogen ions (H+) [70]. The H+ increases the acidity of the surrounding physiological environment and binds to hemoglobin residues, further lowering oxygen-binding affinity [115,116,125], a process known as the Bohr effect [108,125]. Although birds and mammals are not as dependent on HCO3− and the Bohr effect as crocodiles, these biochemical interactions promote the release of oxygen in distal tissues [125] in all three groups.
3.3. Adaptation to Hemoglobin in Response to Extreme Environments
Because oxygen offloading is affected by factors such as environmental oxygen concentration and temperature, some vertebrates possess molecular or physiological adaptations to offset extreme environmental conditions. In the case of low oxygen environments, such as high altitudes or diving, vertebrates possess adaptations that maintain aerobic demands. One common, metabolically inexpensive strategy is to increase the production of RBCs, thereby increasing the concentration of hemoglobin available to bind the low supply of oxygen molecules [125,126,127]. This has been observed in human populations indigenous to high-altitude environments, such as the Andean peoples of South America [128,129]. Another strategy is to increase hemoglobin’s oxygen-binding affinity, for which both bar-headed geese and Andean camelids have hemoglobin sequence mutations [126,130,131]. Penguins, which are adapted for diving, display hemoglobin mutations that both increase their oxygen-binding affinity and additionally promote oxygen unloading via an increase in acidity (i.e., the Bohr effect) [125].
In addition to high altitude and hypoxia, extreme temperatures can affect hemoglobin’s capacity for oxygen transport. Oxygen binding in hemoglobin is an exothermic (heat-releasing) process [112,132], and, as temperatures rise, hemoglobin’s affinity for oxygen decreases. This effect is utilized by muscles to promote the unloading of oxygen at these tissues where exertion creates small increases in temperature [127,133,134,135]. However, substantial temperature increases can denature proteins, and in hemoglobin, they can greatly reduce oxygen affinity [135,136,137]. Some species, such as the ostrich, have developed high-temperature resistance by increasing the proportion of hydrophobic or uncharged amino acid residues in their hemoglobin sequence [138], in turn increasing their resistance to hydrolysis [139]. Conversely, in cold environments, taxa must overcome barriers to the release of oxygen to dysoxic tissue, by decreasing oxygen affinity. Therefore, cold-adapted species possess mutations that lower the energy threshold for the release of oxygen (i.e., lowering the heat of hemoglobin oxygenation; ΔH) [112,113], allowing them to function over a wider range of environmental temperatures. This adaptation has been observed in reindeer, musk-ox, and reconstructed mammoth hemoglobin [112,113].
Adaptation to Hemoglobin in Transitions to Endothermy
The effect of temperature on oxygen offloading has implications for respiratory changes in lineages that transition from ectothermic to endothermic metabolisms. Ectothermy, a state in which an organism relies on environmental sources for body heat, is the ancestral trait of all vertebrates [133]. Endothermy, the self-generation of body heat, has evolved independently at various times across vertebrate taxa, most notably in mammals and birds [133]. Compared to ectotherms, endotherms have higher aerobic and metabolic demands, as well as an increased body temperature that is less dependent on the surrounding environment [127,133]. As such, the onset of endothermy necessitated greater respiratory efficiency and stability at sustained, high metabolic temperatures. This is exemplified in birds, which have transitioned from the ectothermic metabolism of their basal archosaurian ancestors to the endothermic metabolism observed in living avians [133]. The increased metabolic demand associated with flight is provided for in birds by their complex, unidirectional airflow respiratory system [133,140,141] coupled with the efficiency of IPs for their primary oxygen offloading allosteric effector. The transition between the basal ectothermic state and the derived endothermy of birds must have occurred at some point in the avian lineage, potentially in their non-avian dinosaur ancestors. The hypothesis that endothermy predates the origin of Avialae (i.e., all birds, living and extinct) is supported by the appearance of insulatory coverings (e.g., feathers) [142] and endotherm-specific behaviors (e.g., brooding) [143] in non-avian dinosaurs. Conversely, endothermy may have been present in Archosauria prior to the emergence of dinosaurs. It has been suggested that modern crocodilians are secondary ectotherms that have reverted to ectothermy from endothermic, bipedal pseudosuchian ancestors [144]. However, the timing and number of independent transitions to endothermy that occurred within Archosauria are still unclear and can only be investigated through the fossil record.
4. Hemes and Hemoglobin in the Fossil Record
4.1. Examples of Molecular Preservation, including Heme and Hemoglobin
Various studies in archeology and paleontology unveil compelling evidence of the long-term preservation of the original organic material in both relatively recent [6,15,16,17,18,19,20,21] remains and deep time [10,14,22,23,24] remains. These studies have identified DNA [145], lipids [146], proteins [15,147], amino acids [148], and associated molecules (e.g., heme or metal cofactors) [11]. Hemoglobin has been recovered from the 5200-year-old frozen mummy, Ötzi [6,18], as well as a 10 Ka skull from Castoroides (i.e., giant beaver) [19]. Studies conducted on mammoth remains have revealed the presence of hemoglobin peptides among hundreds of other proteins [21,149].
The preservation of original organic compounds in deep-time fossils older than one million years old is contentious [150,151,152]. Conventional wisdom held that endogenous organic material could persist for less than 100,000 years for DNA or one million years for proteins [88]. However, in more recent decades, the development and utilization of molecular paleontology and paleoproteomic techniques have provided evidence of endogenous proteins across various animal taxa and geological strata. Most recently, the recovery of DNA from a 1.2 Ma mammoth has highlighted the need to reassess predictions for how long organic molecules may preserve [153].
The utilization of mass spectrometry (MS) techniques (e.g., tandem MS, or MS/MS) has recovered peptide sequences from fossil tissue over a wide age range (1 k–80 Ma) [15,21,22,24,154,155]. These include hemoglobin [19,23,149,156] and the preservation of heme cofactors [11,14] in ancient tissues. Independent of MS, immunohistology techniques (employing rigorous specificity controls to rule out cross-reactivity and bacterial contamination) [154] have demonstrated the preservation of hemoglobin in vertebrate fossil tissue [14,24,25,157,158,159]. As a potential proxy of endogenous hemoglobin, heme has been identified in the abdomen of a 46 Ma mosquito fossil [11], a 48 Ma fish from the Messel pit [13], a 56 Ma juvenile sea turtle [14], and in the trabecular bone of a 65 Ma Tyrannosaurus rex [10] (for a review, see [6]). Although hemoglobin is not the only heme-containing protein, it is unlikely these instances of heme detected in fossil tissues are derived from cytochromes, other hemeproteins, or bacteria for the following reasons: (1) there has been an observed lack of bacteria-specific peptidoglycan [14] or cytochrome [10] immunoreactivity; (2) hemoglobin is more abundant than cytochrome proteins in blood vessels [10,160] and trabecular bone [10], giving them relatively higher preservation and detection potential [161]; (3) hemoglobin demonstrates properties that inhibit bacterial growth [88,162], suggesting that tissues abundant in hemoglobin would be less susceptible to bacterial growth; (4) microbial (e.g., bacteria) structures were not observed under scanning electron microscopy [14]; (5) fossilization is likely to occur in anaerobic conditions, and anaerobic microbes exhibit a low expression of cytochrome [11]; and (6) resonance Raman Spectroscopy, which has been used to identify heme in fossil tissue [10,163,164], is capable of distinguishing hemoglobin-derived heme and cytochrome-derived heme in modern tissues [164].
4.2. The Preservation Potential of Heme-Based Proteins
Several factors influence the potential preservation of proteins in fossil tissue. The majority of ancient peptides are derived from preserved soft tissues (e.g., blood vessels) isolated from fossilized bones [6,19,24,155,159,165,166]. Bone and its constituent mineral, hydroxyapatite, may shield soft tissue and peptides from degradation, either by acting as a physical barrier or by providing an adsorptive surface that stabilizes proteins [161,167,168,169]. Proteins that are abundant within a given tissue, such as collagen in bone proteins, also have an increased likelihood of persisting across deep time [161]. Collagen in particular also benefits from a triple helical structure (i.e., tertiary structure) as the tightly complexed fibrils are resistant to enzymatic degradation [170,171]. Other factors, like the formation of cross-linkages and aggregation of peptides, enhance their survivability in deep time [5,25,88,172]. This process may be mediated by the addition of diagenetic modifiers (e.g., advanced glycation end-products, AGEs) [173] or metal cations [25,88]. Environmental and burial conditions may also influence preservation. Fossil formation favors anaerobic conditions, which reduces the impact of bacterial scavenging and growth [174,175]. Alternatively, oxidative environments may promote preservation by inducing polymerization [88,176].
Similarly, the fundamental characteristics of heme-based proteins enhance preservation potential. Schweitzer et al. (2014) proposed a chemical mechanism in which iron and oxygen derived from hemoglobin degradation increase the likelihood of soft tissue and peptide preservation [88]. Free iron and free heme released from hemoglobin along with oxygen have the potential to generate ROSs [88,177,178,179]. These ROS free radicals may interact with proteins and induce the formation of cross-linkages in peptides, increasing the molecule’s resistance to degradation [88]. It may be even feasible that peptides attached to a cofactor (e.g., heme) may enhance peptide survivability [136].
In vivo, hemoglobin proteins degrade into individual globin chains, from which heme is separated. The globin is further broken down into amino acids and heme is converted into a noncyclic tetrapyrrole, bilirubin, by the enzyme heme oxygenase [90,178]. At death, the disruption of this enzymatic process may facilitate the incorporation of intact hemoglobin into the rock record at burial, allowing the possibility of further geochemical modification and, potentially, preservation in fossils.
Tahoun et al. review the chemical properties of heme that are hypothesized to contribute to molecular persistence in the fossil record, based on its intrinsic structure. This potential may be enhanced further by its association with hemoproteins [33]. The macrocyclic tetrapyrrole ring of the porphyrins (as discussed above) is characterized by alternating areas of high and low electron densities, which allows the molecule to dissipate energy from the environment and potentially stabilizes the molecule across deep time [29,33]. The formation of a metalloporphyrin complex with a metal cation further stabilizes the macrocyclic structure against geochemical degradation [33,43,44]. Heme and heme-like porphyrins have been described as resistant to changes in environmental pH and remain relatively unchanged at both low (e.g., pH = 0) and high pH (e.g., pH = 13.5) [180]. These molecules have also demonstrated resistance to degradation by high temperatures (330 °C) [180,181,182]. The hydrophobic nature of porphyrins also contributes to their stability in the rock record by increasing the molecule’s resistance to hydrolysis [33,183]. In addition to their intrinsic properties, the tertiary structure and resulting resonance of hemoglobin may shield the porphyrin against enzymatic degradation and hydrolysis, increasing their likelihood of persistence in the rock record [33,183]. The chemical properties of porphyrins allow these molecules to persist across geological times and thus make porphyrins the ideal biomarker in deep-time analysis on Earth and potential applications for the search for life on other planets [184,185,186].
4.3. Degradation and Diagenesis of Heme
The heme porphyrin has been identified in organic sediment and fossil tissue samples as old as 1.1 Ga [33,187]. Tetrapyrroles (i.e., heme and chlorophylls) buried in sediment are altered by diagenetic processes into products referred to as geophorphyrins [7,8,9,13]. Depending on the depositional environment, these porphyrins may undergo processes such as hydrolysis, reduction, oxidation, demetallation, and remetallation [33,43]. The Treibs scheme [188] suggests that heme and chlorophylls are the biogenetic sources of the geoporphyrins etioporphyrin III and deoxophyloerythoerthoetioporphyrin (DPEP), respectively (Figure 6). It has been suggested that the diagenetic products of heme may also include the geoporphyrins di-DPEP, Rhodo-DPEP, and Rhodo-etioporphyrin, which have been described from various oils shales (Figure 7) [7,8,9,13,189].
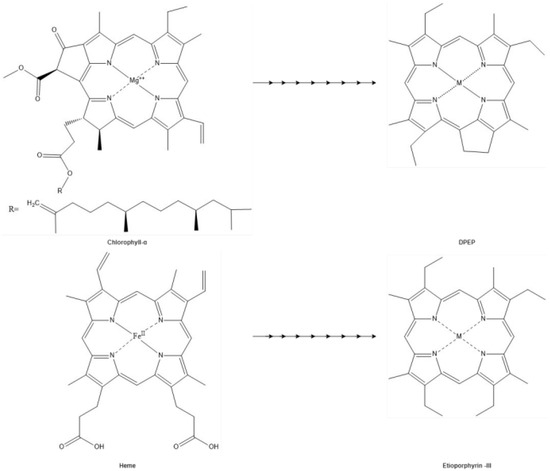
Figure 6.
Triebs scheme. The hypothesis of DPEP and Etioporphyrin-III are geoporphyrins derived from chlorophyll and heme proposed by Triebs in 1936.
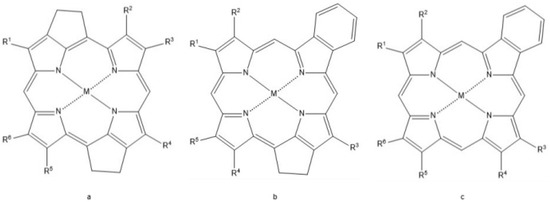
Figure 7.
Other geoporphyrins. (a) Di-Deoxophyloerythoetioporphyrin (Di-DPEP), (b) Rhodo-DPEP, (c) Rhodo-etioporphyrin, porphyrins observed in the fossil records likely derived from the diagenetic alteration of heme and chlorophyll porphyrins.
Diagenetic processes that heme may potentially be subjected to, including demetallation (i.e., the removal of the metal cation) and subsequent remetallation, are of particular interest. During demetallation, the metal cation originally bound to the heme molecule is lost [43,44]. This is typically observed under acidic [43,190] and extreme heat (>300 °C) conditions [44]. In general, Fe2+ is less susceptible to demetallation than more labile cations, such as NA, Li, and K [35]. Among the cations observed in biological systems, including those bound to proteins, iron is more stable than magnesium, and iron-bound heme is hypothesized to be more resistant to demetallation compared to the magnesium metal cation of chlorophylls [43]. Additionally, iron cations that are oxidized (i.e., Fe3+ when oxygen binds to Fe2+ are more resistant to demetallation [35].
Following the diagenetic removal of the original metal cations, remetallation with exogenous metal cations such as nickel (Ni) and vanadyl (VO2+) may occur [44]. In sediment rich in organic material, nickel ions are concentrated in the reduced state (Ni2+) [44]. Because this state is similar to the Fe2+ ion originally bound to the heme, nickel readily occupies the vacant space at the center of the porphyrin ring [44]. The occupation of the vacant porphyrin by transition metals [44] stabilizes it [9,189]. Nickel-bound porphyrins are thermally stable and commonly occur in reduced environments [44]. VO2+ may also chelate to heme, but this substitution is thought to occur less frequently [9,44,189]. VO2+-bound diagenetic porphyrins are easily reduced compared to those bound to Ni2+ but are more resistant to oxidation [9,189,191]. In chlorophylls, the Mg2+ of their porphyrin ring is subject to the same substitution with either nickel or vanadyl ions [9,44,189].
4.4. Diagenesis of Globin
The globin component of hemoglobin is itself susceptible to diagenetic alterations, beyond those described for its heme cofactor, and these may impact the preservation potential of this protein. In general, all proteins undergo a variety of diagenetic changes, producing an altered molecule called a diagenetiform [192]. These changes may include fragmentation through processes such as hydrolysis [192], deamidation of asparagine and glutamine residues [15,193], the formation of AGEs [6,15,173] or other aggregates through cross-linkages with exogenous molecules (e.g., metal ions [88,194,195]).
Despite these potential avenues of degradation, hemoglobin also possesses aspects that may increase its likelihood of persistence. For example, hemoglobin synthesis occurs within vertebrate bone marrow [37], where it may be partially shielded from the environment [176] deep below the periosteal surface. Molecular components ingrained in the structure of hemoglobin (e.g., heme, iron) have also been demonstrated to enhance preservation. For example, iron derived from heme may induce cross-linkages in hemoglobin peptides, increasing their resistance to hydrolysis and enzymatic degradation, and hemoglobin bound to oxygen has been demonstrated to enhance soft tissue preservation and possess an anti-microbial effect [88]. Because hemoglobin is a protein both specific to, and most abundant in, vertebrate RBCs [160], preserved blood vessels [165,166] and the RBC-like structures [196] found within them are an ideal target for concerted efforts at hemoglobin characterization [165,166,196].
5. Looking Forward: The Potential Utility of Preserved Hemoglobin
Porphyrin molecules embedded in proteins have been essential molecules for the development of life. All forms of life require the transformation and translation of energy from one form to another. During the Archean and into the Proterozoic, the earliest anaerobic metabolisms likely utilized free porphyrins, Fe-S metalloproteins, and cytochrome-like hemoproteins [55,59,69,77,180]. The evolution of oxygenic photosynthesis through the incorporation of these elements forever transformed the conditions of the planet not just in the environment but also in the metabolism of life [70,85]. As a more energetically favorable process, aerobic respiration capitalized on the increased presence of oxygen allowing for the emergence of complex life including eukaryotes and multicellular life [96,97]. With greater physical complexity aerobic organisms require a method to supplement their aerobic demands in the form of globin proteins and eventually the tetrameric hemoglobin of vertebrates [102]. The diversity of vertebrate hemoglobin is widely adaptive among taxa, allowing organisms to occupy different habitats and niches [101,108,127,132,197].
The fossil record reveals evidence of proteins, including hemoglobin, in ancient remains of vertebrates. The identification of heme-related compounds in the fossil record spans relatively recent to deep time beyond 1 Ma [6,10]. These ancient molecules have the potential to reveal information on the phylogeny, physiology, evolutionary stages, and/or processes, ecology, and burial environment of ancient organisms and ecosystems. However, to fully understand these aspects of ancient life, the process of degradation and diagenesis on proteins, peptides, and co-factors must be considered. Hemoglobin has the potential to survive diagenesis due to its protection by bone, its inherited greater concentration in RBCs, and its association with iron, heme, and oxygen.
The preservation of hemoglobin provides an invaluable opportunity to bridge the gap between the ancient past and the present. The endurance of hemoglobin into deep time allows a deeper understanding of evolutionary history and provides invaluable insights into the metabolism of extinct organisms and the influence of environmental conditions on aerobic demands.
Applying molecular paleontology techniques, including paleoproteomics and vibrational methods such as resonance Raman (RR) Spectroscopy, to well-preserved ancient tissues, including non-avian dinosaurs, holds the potential to increase our understanding of the advent and evolution of endothermy. However, the study of non-avian dinosaur hemoglobin and metabolism presents unique challenges as well, including the paucity of data for comparisons. Although non-avian dinosaurs are related to both extant birds and crocodilians [147,198], they no doubt had their own unique adaptations [133]. Birds, being endothermic, are highly active creatures adapted for flight [133], with inositol phosphate acting as their primary allosteric effector [108,130]. On the other hand, although crocodilians potentially evolved from endothermic ancestors [133,144], modern crocodiles are ectotherms. These aquatic ambush predators are known for their diving behaviors, with bicarbonate serving as their primary allosteric effector [119,124]. The divergence between these archosaur lineages makes it challenging to use phylogenetic bracketing to effectively deduce shared traits in dinosaurs. However, the analysis of fossilized hemoglobin may shed light on the metabolic processes of dinosaurs, especially maniraptoran dinosaurs, the group that eventually gave rise to modern birds [133]. In contrast, hemoglobin derived from (relatively) distantly related ornithischians may potentially demonstrate a wider diversity of dinosaur metabolism [199]. Fossil hemoglobin research holds the potential to unravel the questions that still surround the metabolic and physiological adaptations of these long-dead taxa.
Author Contributions
Conceptualization, J.D.A. and M.H.S.; writing—original draft preparation, J.D.A., E.R.S. and M.H.S.; writing—review and editing, J.D.A., E.R.S. and M.H.S.; visualization, J.D.A.; supervision, E.R.S. and M.H.S.; funding acquisition, M.H.S. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this research was provided by Lynn Orr, Susan Packard, and Vance and Gail Mullis (M.H.S.) and the Biological Sciences Department at North Carolina State University (J.D.A., E.R.S.).
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank W. Zheng and R. Sharma for logistical support, and two anonymous reviewers.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nelson, D.R.; Kamataki, T.; Waxman, D.J.; Guengerich, F.P.; Estabrook, R.W.; Feyereisen, R.; Gonzalez, F.J.; Coon, M.J.; Gunsalus, I.C.; Gotoh, O.; et al. The P450 Superfamily: Update on New Sequences, Gene Mapping, Accession Numbers, Early Trivial Names of Enzymes, and Nomenclature. DNA Cell Biol. 1993, 12, 1–51. [Google Scholar] [CrossRef]
- Lindsey, J.S.; Chandrashaker, V.; Taniguchi, M.; Ptaszek, M. Abiotic Formation of Uroporphyrinogen and Coproporphyrinogen from Acyclic Reactants. New J. Chem. 2011, 35, 65–75. [Google Scholar] [CrossRef]
- Hardison, R.C. A Brief History of Hemoglobins: Plant, Animal, Protist, and Bacteria. Proc. Natl. Acad. Sci. USA 1996, 93, 5675–5679. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, E.R.; Cleland, T.P.; Schweitzer, M.H. Deep Time Paleoproteomics: Looking Forward. J. Proteome Res. 2022, 21, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, M.H. Soft Tissue Preservation in Terrestrial Mesozoic Vertebrates. Annu. Rev. Earth Planet. Sci. 2011, 39, 187–216. [Google Scholar] [CrossRef]
- Greenwalt, D. Blood to Molecules: The Fossil Record of Blood and Its Constituents. In The Evolution and Fossil Record of Parasitism: Coevolution and Paleoparasitological Techniques; De Baets, K., Huntley, J.W., Eds.; Topics in Geobiology; Springer International Publishing: Cham, Switzerland, 2021; pp. 377–416. ISBN 978-3-030-52233-9. [Google Scholar]
- Ocampo, R.; Bauder, C.; Callot, H.J.; Albrecht, P. Porphyrins from Messel Oil Shale (Eocene, Germany): Structure Elucidation, Geochemical and Biological Significance, and Distribution as a Function of Depth. Geochim. Cosmochim. Acta 1992, 56, 745–761. [Google Scholar] [CrossRef]
- Bauder, C.; Ocampo, R.; Callot, H.J.; Albrecht, P. Structural Evidence for Heme Fossils in Messel Oil Shale (FRG). Naturwissenschaften 1990, 77, 378–379. [Google Scholar] [CrossRef]
- Barwise, A.J.G.; Roberts, I. Diagenetic and Catagenetic Pathways for Porphyrins in Sediments. Org. Geochem. 1984, 6, 167–176. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Marshall, M.; Carron, K.; Bohle, D.S.; Busse, S.C.; Arnold, E.V.; Barnard, D.; Horner, J.R.; Starkey, J.R. Heme Compounds in Dinosaur Trabecular Bone. Proc. Natl. Acad. Sci. USA 1997, 94, 6291–6296. [Google Scholar] [CrossRef] [PubMed]
- Greenwalt, D.E.; Goreva, Y.S.; Siljeström, S.M.; Rose, T.; Harbach, R.E. Hemoglobin-Derived Porphyrins Preserved in a Middle Eocene Blood-Engorged Mosquito. Proc. Natl. Acad. Sci. USA 2013, 110, 18496–18500. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, J.; Yang, T.-R.; Norell, M.A. Dinosaur Egg Colour Had a Single Evolutionary Origin. Nature 2018, 563, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Siljeström, S.; Neubeck, A.; Steele, A. Detection of Porphyrins in Vertebrate Fossils from the Messel and Implications for Organic Preservation in the Fossil Record. PLoS ONE 2022, 17, e0269568. [Google Scholar] [CrossRef]
- Lindgren, J.; Kuriyama, T.; Madsen, H.; Sjövall, P.; Zheng, W.; Uvdal, P.; Engdahl, A.; Moyer, A.E.; Gren, J.A.; Kamezaki, N.; et al. Biochemistry and Adaptive Colouration of an Exceptionally Preserved Juvenile Fossil Sea Turtle. Sci. Rep. 2017, 7, 13324. [Google Scholar] [CrossRef] [PubMed]
- Cleland, T.P.; Schroeter, E.R.; Schweitzer, M.H. Biologically and Diagenetically Derived Peptide Modifications in Moa Collagens. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150015. [Google Scholar] [CrossRef]
- Schroeter, E.R.; Blackburn, K.; Goshe, M.B.; Schweitzer, M.H. Proteomic Method to Extract, Concentrate, Digest and Enrich Peptides from Fossils with Coloured (Humic) Substances for Mass Spectrometry Analyses. R. Soc. Open Sci. 2019, 6, 181433. [Google Scholar] [CrossRef]
- Ntasi, G.; Palomo, I.R.; Marino, G.; Piaz, F.D.; Cappellini, E.; Birolo, L.; Petrone, P. Molecular Signatures Written in Bone Proteins of 79 AD Victims from Herculaneum and Pompeii. Sci. Rep. 2022, 12, 8401. [Google Scholar] [CrossRef]
- Maixner, F.; Overath, T.; Linke, D.; Janko, M.; Guerriero, G.; van den Berg, B.H.J.; Stade, B.; Leidinger, P.; Backes, C.; Jaremek, M.; et al. Paleoproteomic Study of the Iceman’s Brain Tissue. Cell. Mol. Life Sci. 2013, 70, 3709–3722. [Google Scholar] [CrossRef] [PubMed]
- Cleland, T.P.; Schroeter, E.R.; Feranec, R.S.; Vashishth, D. Peptide Sequences from the First Castoroides Ohioensis Skull and the Utility of Old Museum Collections for Palaeoproteomics. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160593. [Google Scholar] [CrossRef]
- Loy, T.H.; Dixon, E.J. Blood Residues on Fluted Points from Eastern Beringia. Am. Antiq. 1998, 63, 21–46. [Google Scholar] [CrossRef]
- Cappellini, E.; Jensen, L.J.; Szklarczyk, D.; Ginolhac, A.; da Fonseca, R.A.R.; Stafford, T.W., Jr.; Holen, S.R.; Collins, M.J.; Orlando, L.; Willerslev, E.; et al. Proteomic Analysis of a Pleistocene Mammoth Femur Reveals More than One Hundred Ancient Bone Proteins. J. Proteome Res. 2012, 11, 917–926. [Google Scholar] [CrossRef]
- Asara, J.M.; Schweitzer, M.H.; Freimark, L.M.; Phillips, M.; Cantley, L.C. Protein Sequences from Mastodon and Tyrannosaurus Rex Revealed by Mass Spectrometry. Science 2007, 316, 280–285. [Google Scholar] [CrossRef]
- Bern, M.; Phinney, B.S.; Goldberg, D. Reanalysis of Tyrannosaurus Rex Mass Spectra. J. Proteome Res. 2009, 8, 4328–4332. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, M.H.; Zheng, W.; Organ, C.L.; Avci, R.; Suo, Z.; Freimark, L.M.; Lebleu, V.S.; Duncan, M.B.; Vander Heiden, M.G.; Neveu, J.M.; et al. Biomolecular Characterization and Protein Sequences of the Campanian Hadrosaur B. Canadensis. Science 2009, 324, 626–631. [Google Scholar] [CrossRef]
- Boatman, E.M.; Goodwin, M.B.; Holman, H.-Y.N.; Fakra, S.; Zheng, W.; Gronsky, R.; Schweitzer, M.H. Mechanisms of Soft Tissue and Protein Preservation in Tyrannosaurus Rex. Sci. Rep. 2019, 9, 15678. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, E.R.; Ullmann, P.V.; Macauley, K.; Ash, R.D.; Zheng, W.; Schweitzer, M.H.; Lacovara, K.J. Soft-Tissue, Rare Earth Element, and Molecular Analyses of Dreadnoughtus Schrani, an Exceptionally Complete Titanosaur from Argentina. Biology 2022, 11, 1158. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Suo, Z.; Avci, R.; Asara, J.M.; Allen, M.A.; Arce, F.T.; Horner, J.R. Analyses of Soft Tissue from Tyrannosaurus Rex Suggest the Presence of Protein. Science 2007, 316, 277–280. [Google Scholar] [CrossRef]
- Lindgren, J.; Kaddumi, H.; Polcyn, M. Soft Tissue Preservation in a Fossil Marine Lizard with a Bilobed Tail Fin. Nat. Commun. 2013, 4, 2423. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.N. Chapter II—Physico-Chemical Properties of Porphyrins. In Comprehensive Biochemistry; Florkin, M., Stotz, E.H., Eds.; Pyrrole Pigments, Isoprenoid Compounds and Phenolic Plant Constituents; Elsevier: Amsterdam, The Netherlands, 1963; Volume 9, pp. 34–72. [Google Scholar]
- Falk, J.E. Chapter I—Chemistry and Biochemistry of Porphyrins and Metalloporphyrins. In Comprehensive Biochemistry; Florkin, M., Stotz, E.H., Eds.; Pyrrole Pigments, Isoprenoid Compounds and Phenolic Plant Constituents; Elsevier: Amsterdam, The Netherlands, 1963; Volume 9, pp. 3–33. [Google Scholar]
- Balaban, A.T.; Oniciu, D.C.; Katritzky, A.R. Aromaticity as a Cornerstone of Heterocyclic Chemistry. Chem. Rev. 2004, 104, 2777–2812. [Google Scholar] [CrossRef] [PubMed]
- Lash, T.D. Origin of Aromatic Character in Porphyrinoid Systems. J. Porphyr. Phthalocyanines 2011, 15, 1093–1115. [Google Scholar] [CrossRef]
- Tahoun, M.; Gee, C.T.; McCoy, V.E.; Sander, P.M.; Müller, C.E. Chemistry of Porphyrins in Fossil Plants and Animals. RSC Adv. 2021, 11, 7552–7563. [Google Scholar] [CrossRef]
- Kořený, L.; Oborník, M.; Horáková, E.; Waller, R.F.; Lukeš, J. The Convoluted History of Haem Biosynthesis. Biol. Rev. 2022, 97, 141–162. [Google Scholar] [CrossRef]
- Falk, J.E.; Smith, K.M. (Eds.) Porphyrins and Metalloporphyrins: A New Edition Based on the Original Volume by J. E. Falk; Elsevier Scientific Publishing Co., Ltd.: Amsterdam, The Netherlands; Elsevier Scientific Publishing Co., Ltd.: New York, NY, USA, 1975; ISBN 978-0-444-41375-8. [Google Scholar]
- Granick, S. Evolution of Heme and Chlorophyll. In Evolving Genes and Proteins; Elsevier: New York, NY, USA, 1965; pp. 67–88. ISBN 978-1-4832-2734-4. [Google Scholar]
- Barupala, D.P.; Dzul, S.P.; Riggs-Gelasco, P.J.; Stemmler, T.L. Synthesis, Delivery and Regulation of Eukaryotic Heme and Fe-S Cluster Cofactors. Arch. Biochem. Biophys. 2016, 592, 60–75. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. The Evolution of Electron-Transport Chains. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Goldman, A.D.; Weber, J.M.; LaRowe, D.E.; Barge, L.M. Electron Transport Chains as a Window into the Earliest Stages of Evolution. Proc. Natl. Acad. Sci. USA 2023, 120, e2210924120. [Google Scholar] [CrossRef]
- Scott Mathews, F. The Structure, Function and Evolution of Cytochromes. Prog. Biophys. Mol. Biol. 1985, 45, 1–56. [Google Scholar] [CrossRef]
- Hendry, G.A.; Jones, O.T. Haems and Chlorophylls: Comparison of Function and Formation. J. Med. Genet. 1980, 17, 1–14. [Google Scholar] [CrossRef]
- Björn, L.O.; Govindjee. The Evolution of Photosynthesis and Chloroplasts. Curr. Sci. 2009, 96, 1466–1474. [Google Scholar]
- Hodgson, G.W. Geochemistry of Porphyrins—Reactions During Diagenesis. Ann. N. Y. Acad. Sci. 1973, 206, 670–684. [Google Scholar] [CrossRef]
- Ventura, G.T.; Gall, L.; Siebert, C.; Prytulak, J.; Szatmari, P.; Hürlimann, M.; Halliday, A.N. The Stable Isotope Composition of Vanadium, Nickel, and Molybdenum in Crude Oils. Appl. Geochem. 2015, 59, 104–117. [Google Scholar] [CrossRef]
- Aylward, N.; Bofinger, N. Possible origin for porphin derivatives in prebiotic chemistry—A computational study. Orig. Life Evol. Biospheres 2005, 35, 345–368. [Google Scholar] [CrossRef] [PubMed]
- Sleep, N.H. The Hadean-Archaean Environment. Cold Spring Harb. Perspect. Biol. 2010, 2, a002527. [Google Scholar] [CrossRef] [PubMed]
- Kasting, J.F. Earth’s Early Atmosphere. Science 1993, 259, 920–926. [Google Scholar] [CrossRef]
- Jortner, J. Conditions for the Emergence of Life on the Early Earth: Summary and Reflections. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1877–1891. [Google Scholar] [CrossRef]
- Benner, S.A.; Kim, H.-J.; Kim, M.-J.; Ricardo, A. Planetary Organic Chemistry and the Origins of Biomolecules. Cold Spring Harb. Perspect. Biol. 2010, 2, a003467. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.L.; Bryant, D.A.; Macalady, J.L. The Role of Biology in Planetary Evolution: Cyanobacterial Primary Production in Low-oxygen Proterozoic Oceans. Environ. Microbiol. 2016, 18, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.E. From Minerals to Metabolisms: Evidence for Life before Oxygen from the Geological Record. Free Radic. Biol. Med. 2019, 140, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Sperling, E.A.; Wolock, C.J.; Morgan, A.S.; Gill, B.C.; Kunzmann, M.; Halverson, G.P.; Macdonald, F.A.; Knoll, A.H.; Johnston, D.T. Statistical Analysis of Iron Geochemical Data Suggests Limited Late Proterozoic Oxygenation. Nature 2015, 523, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Planavsky, N.J.; McGoldrick, P.; Scott, C.T.; Li, C.; Reinhard, C.T.; Kelly, A.E.; Chu, X.; Bekker, A.; Love, G.D.; Lyons, T.W. Widespread Iron-Rich Conditions in the Mid-Proterozoic Ocean. Nature 2011, 477, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Thomson, L.; Paton, J. Oxygen Toxicity. Paediatr. Respir. Rev. 2014, 15, 120–123. [Google Scholar] [CrossRef]
- Lu, Z.; Imlay, J.A. When Anaerobes Encounter Oxygen: Mechanisms of Oxygen Toxicity, Tolerance and Defence. Nat. Rev. Microbiol. 2021, 19, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.D.; Kacar, B. Cofactors Are Remnants of Life’s Origin and Early Evolution. J. Mol. Evol. 2021, 89, 127–133. [Google Scholar] [CrossRef]
- Stamati, K.; Mudera, V.; Cheema, U. Evolution of Oxygen Utilization in Multicellular Organisms and Implications for Cell Signalling in Tissue Engineering. J. Tissue Eng. 2011, 2, 2041731411432365. [Google Scholar] [CrossRef] [PubMed]
- Popall, R.M.; Bolhuis, H.; Muyzer, G.; Sánchez-Román, M. Stromatolites as Biosignatures of Atmospheric Oxygenation: Carbonate Biomineralization and UV-C Resilience in a Geitlerinema Sp.—Dominated Culture. Front. Microbiol. 2020, 11, 948. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.C.; Sousa, F.L.; Mrnjavac, N.; Neukirchen, S.; Roettger, M.; Nelson-Sathi, S.; Martin, W.F. The Physiology and Habitat of the Last Universal Common Ancestor. Nat. Microbiol. 2016, 1, 16116. [Google Scholar] [CrossRef] [PubMed]
- Simionescu, C.I.; Simionescu, B.C.; Mora, R.; Leancâ, M. Porphyrin-like Compounds Genesis under Simulated Abiotic Conditions. Orig. Life 1978, 9, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, J.S.; Ptaszek, M.; Taniguchi, M. Simple Formation of an Abiotic Porphyrinogen in Aqueous Solution. Orig. Life Evol. Biospheres 2009, 39, 495–515. [Google Scholar] [CrossRef]
- Fox, S.; Strasdeit, H. Abiotic Synthesis of Porphyrins and Other Oligopyrroles on the Early Earth and Earth-like Planets. Eur. Planet. Sci. Congr. 2013, 8, EPSC2013-104. [Google Scholar]
- Pleyer, H.L.; Strasdeit, H.; Fox, S. A Possible Prebiotic Ancestry of Porphyrin-Type Protein Cofactors. Orig. Life Evol. Biospheres 2018, 48, 347–371. [Google Scholar] [CrossRef]
- Fox, S.; Strasdeit, H. A Possible Prebiotic Origin on Volcanic Islands of Oligopyrrole-Type Photopigments and Electron Transfer Cofactors. Astrobiology 2013, 13, 578–595. [Google Scholar] [CrossRef]
- Granick, S. Speculations on the Origins and Evolution of Photosynthesis. Ann. N. Y. Acad. Sci. 1957, 69, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Mauzerall, D. Light, Iron, Sam Granick and the Origin of Life. Photosynth. Res. 1992, 33, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.W.U.; Montgomery, B.L. Interdependence of Tetrapyrrole Metabolism, the Generation of Oxidative Stress and the Mitigative Oxidative Stress Response. Redox Biol. 2015, 4, 260–271. [Google Scholar] [CrossRef]
- Olejarz, J.; Iwasa, Y.; Knoll, A.H.; Nowak, M.A. The Great Oxygenation Event as a Consequence of Ecological Dynamics Modulated by Planetary Change. Nat. Commun. 2021, 12, 3985. [Google Scholar] [CrossRef]
- Imlay, J.A. Iron-Sulphur Clusters and the Problem with Oxygen. Mol. Microbiol. 2006, 59, 1073–1082. [Google Scholar] [CrossRef]
- Dismukes, G.C.; Klimov, V.V.; Baranov, S.V.; Kozlov, Y.N.; Das Gupta, J.; Tyryshkin, A. The Origin of Atmospheric Oxygen on Earth: The Innovation of Oxygenic Photosynthesis. Proc. Natl. Acad. Sci. USA 2001, 98, 2170–2175. [Google Scholar] [CrossRef]
- Kleingardner, J.G.; Bren, K.L. Biological Significance and Applications of Heme c Proteins and Peptides. Acc. Chem. Res. 2015, 48, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.J. Bacterial Respiration: A Flexible Process for a Changing Environment. Microbiol. Read. Engl. 2000, 146 Pt. 3, 551–571. [Google Scholar] [CrossRef]
- Bertini, I.; Cavallaro, G.; Rosato, A. Cytochrome c: Occurrence and Functions. Chem. Rev. 2006, 106, 90–115. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.J.; Richardson, D.J.; Paquete, C.M.; Clarke, T.A. Role of Multiheme Cytochromes Involved in Extracellular Anaerobic Respiration in Bacteria. Protein Sci. 2020, 29, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Castresana, J.; Saraste, M. Evolution of Energetic Metabolism: The Respiration-Early Hypothesis. Trends Biochem. Sci. 1995, 20, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Castresana, J.; Lübben, M.; Saraste, M.; Higgins, D.G. Evolution of Cytochrome Oxidase, an Enzyme Older than Atmospheric Oxygen. EMBO J. 1994, 13, 2516–2525. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chakraborty, S.; Hosseinzadeh, P.; Yu, Y.; Tian, S.; Petrik, I.; Bhagi, A.; Lu, Y. Metalloproteins Containing Cytochrome, Iron–Sulfur, or Copper Redox Centers. Chem. Rev. 2014, 114, 4366–4469. [Google Scholar] [CrossRef]
- Pereira, M.M.; Santana, M.; Teixeira, M. A Novel Scenario for the Evolution of Haem–Copper Oxygen Reductases. Biochim. Biophys. Acta BBA-Bioenerg. 2001, 1505, 185–208. [Google Scholar] [CrossRef]
- Ligrone, R. The Great Oxygenation Event. In Biological Innovations that Built the World: A Four-Billion-Year Journey through Life and Earth History; Ligrone, R., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 129–154. ISBN 978-3-030-16057-9. [Google Scholar]
- Frey, A.D.; Kallio, P.T. Bacterial Hemoglobins and Flavohemoglobins: Versatile Proteins and Their Impact on Microbiology and Biotechnology. FEMS Microbiol. Rev. 2003, 27, 525–545. [Google Scholar] [CrossRef]
- Shestakov, S.V.; Karbysheva, E.A. The Origin and Evolution of Cyanobacteria. Biol. Bull. Rev. 2017, 7, 259–272. [Google Scholar] [CrossRef]
- Evstigneev, V.B. On the Evolution of the Photosynthetic Pigments. Orig. Life 1975, 6, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.M. Photosynthesis in the Archean Era. Photosynth. Res. 2006, 88, 109–117. [Google Scholar] [CrossRef]
- Farquhar, J.; Zerkle, A.L.; Bekker, A. Geological Constraints on the Origin of Oxygenic Photosynthesis. Photosynth. Res. 2011, 107, 11–36. [Google Scholar] [CrossRef]
- Holland, H.D. The Oxygenation of the Atmosphere and Oceans. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Babikov, D. Recombination Reactions as a Possible Mechanism of Mass-Independent Fractionation of Sulfur Isotopes in the Archean Atmosphere of Earth. Proc. Natl. Acad. Sci. USA 2017, 114, 3062–3067. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.; Segrè, D. The Effect of Oxygen on Biochemical Networks and the Evolution of Complex Life. Science 2006, 311, 1764–1767. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Zheng, W.; Cleland, T.P.; Goodwin, M.B.; Boatman, E.; Theil, E.; Marcus, M.A.; Fakra, S.C. A Role for Iron and Oxygen Chemistry in Preserving Soft Tissues, Cells and Molecules from Deep Time. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132741. [Google Scholar] [CrossRef] [PubMed]
- Nagababu, E.; Rifkind, J.M. Heme Degradation by Reactive Oxygen Species. Antioxid. Redox Signal. 2004, 6, 967–978. [Google Scholar] [CrossRef]
- Ryter, S.W.; Tyrrell, R.M. The Heme Synthesis and Degradation Pathways: Role in Oxidant Sensitivity: Heme Oxygenase Has Both pro- and Antioxidant Properties. Free Radic. Biol. Med. 2000, 28, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.F.; Thake, B.; Martin, W.F. Nitrogenase Inhibition Limited Oxygenation of Earth’s Proterozoic Atmosphere. Trends Plant Sci. 2019, 24, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. The Biological Chemistry of Hydrogen Peroxide. In Methods in Enzymology; Elsevier: Waltham, MA, USA, 2013; Volume 528, pp. 3–25. ISBN 978-0-12-405881-1. [Google Scholar]
- Zámocký, M.; Hofbauer, S.; Schaffner, I.; Gasselhuber, B.; Nicolussi, A.; Soudi, M.; Pirker, K.F.; Furtmüller, P.G.; Obinger, C. Independent Evolution of Four Heme Peroxidase Superfamilies. Arch. Biochem. Biophys. 2015, 574, 108–119. [Google Scholar] [CrossRef]
- Zamocky, M.; Furtmüller, P.G.; Obinger, C. Evolution of Catalases from Bacteria to Humans. Antioxid. Redox Signal. 2008, 10, 1527–1548. [Google Scholar] [CrossRef] [PubMed]
- Costa, K.M.; Accorsi-Mendonça, D.; Moraes, D.J.A.; Machado, B.H. Evolution and Physiology of Neural Oxygen Sensing. Front. Physiol. 2014, 5, 302. [Google Scholar] [CrossRef] [PubMed]
- Ligrone, R. Eukaryotes. In Biological Innovations That Built the World: A Four-Billion-Year Journey through Life and Earth History; Ligrone, R., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 155–231. ISBN 978-3-030-16057-9. [Google Scholar]
- Ligrone, R. Multicellularity. In Biological Innovations That Built the World: A Four-Billion-Year JOURNEY through Life and Earth History; Ligrone, R., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 251–267. ISBN 978-3-030-16057-9. [Google Scholar]
- Hoy, J.A.; Robinson, H.; Trent, J.T.; Kakar, S.; Smagghe, B.J.; Hargrove, M.S. Plant Hemoglobins: A Molecular Fossil Record for the Evolution of Oxygen Transport. J. Mol. Biol. 2007, 371, 168–179. [Google Scholar] [CrossRef]
- Weber, R.E.; Vinogradov, S.N. Nonvertebrate Hemoglobins: Functions and Molecular Adaptations. Physiol. Rev. 2001, 81, 569–628. [Google Scholar] [CrossRef]
- Suzuki, T.; Imai, K. Evolution of Myoglobin. Cell. Mol. Life Sci. CMLS 1998, 54, 979–1004. [Google Scholar] [CrossRef]
- Götting, M.; Nikinmaa, M. More than Hemoglobin—The Unexpected Diversity of Globins in Vertebrate Red Blood Cells. Physiol. Rep. 2015, 3, e12284. [Google Scholar] [CrossRef]
- Hardison, R.C. Evolution of Hemoglobin and Its Genes. Cold Spring Harb. Perspect. Med. 2012, 2, a011627. [Google Scholar] [CrossRef]
- Runnegar, B. Derivation of the Globins from Type b Cytochromes. J. Mol. Evol. 1984, 21, 33–41. [Google Scholar] [CrossRef]
- Hubbard, S.R.; Hendrickson, W.A.; Lambright, D.G.; Boxer, S.G. X-Ray Crystal Structure of a Recombinant Human Myoglobin Mutant at 2·8 Å Resolution. J. Mol. Biol. 1990, 213, 215–218. [Google Scholar] [CrossRef]
- Zhu, H.; Riggs, A.F. Yeast Flavohemoglobin Is an Ancient Protein Related to Globins and a Reductase Family. Proc. Natl. Acad. Sci. USA 1992, 89, 5015–5019. [Google Scholar] [CrossRef] [PubMed]
- Dröge, J.; Pande, A.; Englander, E.W.; Makałowski, W. Comparative Genomics of Neuroglobin Reveals Its Early Origins. PLoS ONE 2012, 7, e47972. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.; Pedwaydon, J.; Czelusniak, J.; Suzuki, T.; Gotoh, T.; Moens, L.; Shishikura, F.; Walz, D.; Vinogradov, S. An Evolutionary Tree for Invertebrate Globin Sequences. J. Mol. Evol. 1988, 27, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Coates, M.L. Hemoglobin Function in the Vertebrates: An Evolutionary Model. J. Mol. Evol. 1975, 6, 285–307. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.H.; Ghatge, M.S.; Safo, M.K. Hemoglobin: Structure, Function and Allostery. Subcell. Biochem. 2020, 94, 345–382. [Google Scholar] [CrossRef] [PubMed]
- Opazo, J.C.; Sloan, A.M.; Campbell, K.L.; Storz, J.F. Origin and Ascendancy of a Chimeric Fusion Gene: The β/δ-Globin Gene of Paenungulate Mammals. Mol. Biol. Evol. 2009, 26, 1469–1478. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pillai, A.S.; Chandler, S.A.; Liu, Y.; Signore, A.V.; Cortez-Romero, C.R.; Benesch, J.L.P.; Laganowsky, A.; Storz, J.F.; Hochberg, G.K.A.; Thornton, J.W. Origin of Complexity in Haemoglobin Evolution. Nature 2020, 581, 480–485. [Google Scholar] [CrossRef]
- Yuan, Y.; Shen, T.-J.; Gupta, P.; Ho, N.T.; Simplaceanu, V.; Tam, T.C.S.; Hofreiter, M.; Cooper, A.; Campbell, K.L.; Ho, C. A Biochemical-Biophysical Study of Hemoglobins from Woolly Mammoth, Asian Elephant, and Humans. Biochemistry 2011, 50, 7350–7360. [Google Scholar] [CrossRef]
- Campbell, K.L.; Roberts, J.E.E.; Watson, L.N.; Stetefeld, J.; Sloan, A.M.; Signore, A.V.; Howatt, J.W.; Tame, J.R.H.; Rohland, N.; Shen, T.-J.; et al. Substitutions in Woolly Mammoth Hemoglobin Confer Biochemical Properties Adaptive for Cold Tolerance. Nat. Genet. 2010, 42, 536–540. [Google Scholar] [CrossRef]
- Manning, L.R.; Russell, J.E.; Padovan, J.C.; Chait, B.T.; Popowicz, A.; Manning, R.S.; Manning, J.M. Human Embryonic, Fetal, and Adult Hemoglobins Have Different Subunit Interface Strengths. Correlation with Lifespan in the Red Cell. Protein Sci. 2007, 16, 1641–1658. [Google Scholar] [CrossRef]
- Perutz, M.F. Stereochemistry of Cooperative Effects in Haemoglobin: Haem–Haem Interaction and the Problem of Allostery. Nature 1970, 228, 726–734. [Google Scholar] [CrossRef]
- de Souza, P.C.; Bonilla-Rodriguez, G.O. Fish Hemoglobins. Braz. J. Med. Biol. Res. 2007, 40, 769–778. [Google Scholar] [CrossRef]
- Ortiz-Prado, E.; Dunn, J.F.; Vasconez, J.; Castillo, D.; Viscor, G. Partial Pressure of Oxygen in the Human Body: A General Review. Am. J. Blood Res. 2019, 9, 1–14. [Google Scholar] [PubMed]
- Brunori, M. Variations on the Theme: Allosteric Control in Hemoglobin. FEBS J. 2014, 281, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Fago, A.; Natarajan, C.; Pettinati, M.; Hoffmann, F.G.; Wang, T.; Weber, R.E.; Drusin, S.I.; Issoglio, F.; Martí, M.A.; Estrin, D.; et al. Structure and Function of Crocodilian Hemoglobins and Allosteric Regulation by Chloride, ATP, and CO2. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2020, 318, R657–R667. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, G.R. Phosphate Compounds in Reptilian and Avian Red Blood Cells; Developmental Changes. Comp. Biochem. Physiol. A Physiol. 1978, 61, 191–202. [Google Scholar] [CrossRef]
- Bartlett, G.R. Phosphate Compounds in Red Cells of Reptiles, Amphibians and Fish. Comp. Biochem. Physiol. A Physiol. 1976, 55, 211–214. [Google Scholar] [CrossRef]
- Ochiai, T.; Gotoh, T.; Shikama, K. Effect of Intracellular Organic Phosphates on the Oxygen Equilibrium Curve of Chicken Hemoglobin. Arch. Biochem. Biophys. 1972, 149, 316–322. [Google Scholar] [CrossRef]
- Komiyama, N.H.; Miyazaki, G.; Tame, J.; Nagai, K. Transplanting a Unique Allosteric Effect from Crocodile into Human Haemoglobin. Nature 1995, 373, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.E.; White, F.N. Oxygen Binding in Alligator Blood Related to Temperature, Diving, and “Alkaline Tide”. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1986, 251, R901–R908. [Google Scholar] [CrossRef]
- Signore, A.V.; Tift, M.S.; Hoffmann, F.G.; Schmitt, T.L.; Moriyama, H.; Storz, J.F. Evolved Increases in Hemoglobin-Oxygen Affinity and the Bohr Effect Coincided with the Aquatic Specialization of Penguins. Proc. Natl. Acad. Sci. USA 2021, 118, e2023936118. [Google Scholar] [CrossRef]
- Storz, J.F. Hemoglobin Function and Physiological Adaptation to Hypoxia in High-Altitude Mammals. J. Mammal. 2007, 88, 24–31. [Google Scholar] [CrossRef]
- Weber, R.E.; Jensen, F.B. Functional Adaptations in Hemoglobins from Ectothermic Vertebrates. Annu. Rev. Physiol. 1988, 50, 161–179. [Google Scholar] [CrossRef]
- Bigham, A.W. Genetics Of Human Origin and Evolution: High-Altitude Adaptations. Curr. Opin. Genet. Dev. 2016, 41, 8–13. [Google Scholar] [CrossRef]
- León-Velarde, F.; Gamboa, A.; Chuquiza, J.A.; Esteba, W.A.; Rivera-Chira, M.; Monge, C.C. Hematological Parameters in High Altitude Residents Living at 4355, 4660, and 5500 Meters Above Sea Level. High Alt. Med. Biol. 2000, 1, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Rollema, H.S.; Bauer, C. The Interaction of Inositol Pentaphosphate with the Hemoglobins of Highland and Lowland Geese. J. Biol. Chem. 1979, 254, 12038–12043. [Google Scholar] [CrossRef]
- Natarajan, C.; Jendroszek, A.; Kumar, A.; Weber, R.E.; Tame, J.R.H.; Fago, A.; Storz, J.F. Molecular Basis of Hemoglobin Adaptation in the High-Flying Bar-Headed Goose. PLoS Genet. 2018, 14, e1007331. [Google Scholar] [CrossRef] [PubMed]
- Clementi, M.E.; Condò, S.G.; Castagnola, M.; Giardina, B. Hemoglobin Function under Extreme Life Conditions. Eur. J. Biochem. 1994, 223, 309–317. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Marshall, C.L. A Molecular Model for the Evolution of Endothermy in the Theropod-Bird Lineage. J. Exp. Zool. 2001, 291, 317–338. [Google Scholar] [CrossRef]
- Morrison, P.R.; Bernal, D.; Sepulveda, C.A.; Brauner, C.J. The Effect of Temperature on Haemoglobin–Oxygen Binding Affinity in Regionally Endothermic and Ectothermic Sharks. J. Exp. Biol. 2023, 226, jeb244979. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.E.; Campbell, K.L. Temperature Dependence of Haemoglobin-Oxygen Affinity in Heterothermic Vertebrates: Mechanisms and Biological Significance. Acta Physiol. Oxf. Engl. 2011, 202, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Flanders, K.G.; Pessagno, L.R.; Cerda, J.F. Hemoglobin and Myoglobin Stabilization by Heme-Fluoride Complexes. Polyhedron 2021, 203, 115238. [Google Scholar] [CrossRef]
- Collins, M.J.; Waite, E.R.; van Duin, A.C. Predicting Protein Decomposition: The Case of Aspartic-Acid Racemization Kinetics. Philos. Trans. R. Soc. B Biol. Sci. 1999, 354, 51–64. [Google Scholar] [CrossRef]
- Ramesh, P.; Sundaresan, S.S.; Shobana, N.; Vinuchakkaravarthy, T.; Sivakumar, K.; Yasien, S.; Ponnuswamy, M.N.G. Structural Studies of Hemoglobin from Two Flightless Birds, Ostrich and Turkey: Insights into Their Differing Oxygen-Binding Properties. Acta Crystallogr. Sect. Struct. Biol. 2021, 77, 690–702. [Google Scholar] [CrossRef]
- Pace, C.N.; Fu, H.; Fryar, K.L.; Landua, J.; Trevino, S.R.; Shirley, B.A.; Hendricks, M.M.; Iimura, S.; Gajiwala, K.; Scholtz, J.M.; et al. Contribution of Hydrophobic Interactions to Protein Stability. J. Mol. Biol. 2011, 408, 514–528. [Google Scholar] [CrossRef]
- Wedel, M.J. Vertebral Pneumaticity, Air Sacs, and the Physiology of Sauropod Dinosaurs. Paleobiology 2003, 29, 243–255. [Google Scholar] [CrossRef]
- Bernstein, M.H. Ventilation and Respiratory Evaporation in the Flying Crow, Corvus Ossifragus. Respir. Physiol. 1976, 26, 371–382. [Google Scholar] [CrossRef]
- Lingham-Soliar, T. The Evolution of the Feather: Sinosauropteryx, a Colourful Tail. J. Ornithol. 2011, 152, 567–577. [Google Scholar] [CrossRef]
- Norell, M.A.; Clark, J.M.; Chiappe, L.M. Brooding Behavior in a Non-Avian Dinosaur, Oviraptor (Theropoda, Oviraptoridae). Paleontol. Soc. Spec. Publ. 1996, 8, 290. [Google Scholar] [CrossRef]
- Seymour, R.S.; Bennett-Stamper, C.L.; Johnston, S.D.; Carrier, D.R.; Grigg, G.C. Evidence for Endothermic Ancestors of Crocodiles at the Stem of Archosaur Evolution. Physiol. Biochem. Zool. PBZ 2004, 77, 1051–1067. [Google Scholar] [CrossRef]
- Nielsen-Marsh, C.M.; Ostrom, P.H.; Gandhi, H.; Shapiro, B.; Cooper, A.; Hauschka, P.V.; Collins, M.J. Sequence Preservation of Osteocalcin Protein and Mitochondrial DNA in Bison Bones Older than 55 Ka. Geology 2002, 30, 1099–1102. [Google Scholar] [CrossRef]
- Briggs, D.E.G.; Summons, R.E. Ancient Biomolecules: Their Origins, Fossilization, and Role in Revealing the History of Life. BioEssays 2014, 36, 482–490. [Google Scholar] [CrossRef]
- Schroeter, E.R.; DeHart, C.J.; Cleland, T.P.; Zheng, W.; Thomas, P.M.; Kelleher, N.L.; Bern, M.; Schweitzer, M.H. Expansion for the Brachylophosaurus canadensis Collagen I Sequence and Additional Evidence of the Preservation of Cretaceous Protein. J. Proteome Res. 2017, 16, 920–932. [Google Scholar] [CrossRef]
- McCoy, V.E.; Gabbott, S.E.; Penkman, K.; Collins, M.J.; Presslee, S.; Holt, J.; Grossman, H.; Wang, B.; Solórzano Kraemer, M.M.; Delclòs, X.; et al. Ancient Amino Acids from Fossil Feathers in Amber. Sci. Rep. 2019, 9, 6420. [Google Scholar] [CrossRef] [PubMed]
- Cucina, A.; Cunsolo, V.; Di Francesco, A.; Saletti, R.; Zilberstein, G.; Zilberstein, S.; Tikhonov, A.; Bublichenko, A.G.; Righetti, P.G.; Foti, S. Meta-Proteomic Analysis of the Shandrin Mammoth by EVA Technology and High-Resolution Mass Spectrometry: What Is Its Gut Microbiota Telling Us? Amino Acids 2021, 53, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Pevzner, P.A.; Kim, S.; Ng, J. Comment on “Protein Sequences from Mastodon and Tyrannosaurus Rex Revealed by Mass Spectrometry”. Science 2008, 321, 1040. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.T.P.; Bandelt, H.-J.; Hofreiter, M.; Barnes, I. Assessing Ancient DNA Studies. Trends Ecol. Evol. 2005, 20, 541–544. [Google Scholar] [CrossRef]
- Buckley, M.; Warwood, S.; Van Dongen, B.; Kitchener, A.C.; Manning, P.L. A Fossil Protein Chimera; Difficulties in Discriminating Dinosaur Peptide Sequences from Modern Cross-Contamination. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170544. [Google Scholar] [CrossRef] [PubMed]
- van der Valk, T.; Pečnerová, P.; Díez-del-Molino, D.; Bergström, A.; Oppenheimer, J.; Hartmann, S.; Xenikoudakis, G.; Thomas, J.A.; Dehasque, M.; Sağlıcan, E.; et al. Million-Year-Old DNA Sheds Light on the Genomic History of Mammoths. Nature 2021, 591, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, M.H.; Schroeter, E.R.; Cleland, T.P.; Zheng, W. Paleoproteomics of Mesozoic Dinosaurs and Other Mesozoic Fossils. Proteomics 2019, 19, 1800251. [Google Scholar] [CrossRef] [PubMed]
- Cleland, T.P.; Schroeter, E.R.; Zamdborg, L.; Zheng, W.; Lee, J.E.; Tran, J.C.; Bern, M.; Duncan, M.B.; Lebleu, V.S.; Ahlf, D.R.; et al. Mass Spectrometry and Antibody-Based Characterization of Blood Vessels from Brachylophosaurus canadensis. J. Proteome Res. 2015, 14, 5252–5262. [Google Scholar] [CrossRef] [PubMed]
- Brandt, L.Ø.; Schmidt, A.L.; Mannering, U.; Sarret, M.; Kelstrup, C.D.; Olsen, J.V.; Cappellini, E. Species Identification of Archaeological Skin Objects from Danish Bogs: Comparison between Mass Spectrometry-Based Peptide Sequencing and Microscopy-Based Methods. PLoS ONE 2014, 9, e106875. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, J.; Sjövall, P.; Thiel, V.; Zheng, W.; Ito, S.; Wakamatsu, K.; Hauff, R.; Kear, B.P.; Engdahl, A.; Alwmark, C.; et al. Soft-Tissue Evidence for Homeothermy and Crypsis in a Jurassic Ichthyosaur. Nature 2018, 564, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, M.; Hill, C.L.; Asara, J.M.; Lane, W.S.; Pincus, S.H. Identification of Immunoreactive Material in Mammoth Fossils. J. Mol. Evol. 2002, 55, 696–705. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Moyer, A.E.; Zheng, W. Testing the Hypothesis of Biofilm as a Source for Soft Tissue and Cell-Like Structures Preserved in Dinosaur Bone. PLoS ONE 2016, 11, e0150238. [Google Scholar] [CrossRef]
- Bryk, A.H.; Wiśniewski, J.R. Quantitative Analysis of Human Red Blood Cell Proteome. J. Proteome Res. 2017, 16, 2752–2761. [Google Scholar] [CrossRef]
- Wadsworth, C.; Buckley, M. Proteome Degradation in Fossils: Investigating the Longevity of Protein Survival in Ancient Bone. Rapid Commun. Mass Spectrom. 2014, 28, 605–615. [Google Scholar] [CrossRef]
- Hobson, D.; Hirsch, J.G. The antibacterial activity of hemoglobin. J. Exp. Med. 1958, 107, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Hallen, H.D.; Long, B.J.N. Resonance Raman Techniques for Complex Biological Systems. In Proceedings of the Ultrafast Nonlinear Imaging and Spectroscopy VII, SPIE, San Diego, CA, USA, 9 September 2019; Volume 11122, pp. 16–25. [Google Scholar]
- Spiro, T.G.; Strekas, T.C. Resonance Raman Spectra of Hemoglobin and Cytochrome c: Inverse Polarization and Vibronic Scattering. Proc. Natl. Acad. Sci. USA 1972, 69, 2622–2626. [Google Scholar] [CrossRef] [PubMed]
- Pawlicki, R.; Nowogrodzka-Zagorska, M. Blood Vessels and Red Blood Cells Preserved in Dinosaur Bones. Ann. Anat.-Anat. Anz. 1998, 180, 73–77. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Wittmeyer, J.L.; Horner, J.R. Soft Tissue and Cellular Preservation in Vertebrate Skeletal Elements from the Cretaceous to the Present. Proc. R. Soc. B Biol. Sci. 2007, 274, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Poser, J.W.; Price, P.A. A Method for Decarboxylation of Gamma-Carboxyglutamic Acid in Proteins. Properties of the Decarboxylated Gamma-Carboxyglutamic Acid Protein from Calf Bone. J. Biol. Chem. 1979, 254, 431–436. [Google Scholar] [CrossRef]
- Owen, M.; Triffitt, J.T. Extravascular Albumin in Bone Tissue. J. Physiol. 1976, 257, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.J.; Gernaey, A.M.; Nielsen-Marsh, C.M.; Vermeer, C.; Westbroek, P. Slow Rates of Degradation of Osteocalcin: Green Light for Fossil Bone Protein? Geology 2000, 28, 1139. [Google Scholar] [CrossRef]
- San Antonio, J.D.; Schweitzer, M.H.; Jensen, S.T.; Kalluri, R.; Buckley, M.; Orgel, J.P.R.O. Dinosaur Peptides Suggest Mechanisms of Protein Survival. PLoS ONE 2011, 6, e20381. [Google Scholar] [CrossRef]
- Perumal, S.; Antipova, O.; Orgel, J.P.R.O. Collagen Fibril Architecture, Domain Organization, and Triple-Helical Conformation Govern Its Proteolysis. Proc. Natl. Acad. Sci. USA 2008, 105, 2824–2829. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Chiang, C.-C.; Huang, P.-Y.; Chung, C.-Y.; Huang, T.D.; Wang, C.-C.; Chen, C.-I.; Chang, R.-S.; Liao, C.-H.; Reisz, R.R. Evidence of Preserved Collagen in an Early Jurassic Sauropodomorph Dinosaur Revealed by Synchrotron FTIR Microspectroscopy. Nat. Commun. 2017, 8, 14220. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, B.P.; Owan, T.E.; Mohammed, S.F.; Meyer, D.M.; Mills, L.D.; Schalkwijk, C.G.; Redfield, M.M. Advanced Glycation End Products Accumulate in Vascular Smooth Muscle and Modify Vascular but Not Ventricular Properties in Elderly Hypertensive Canines. Circulation 2008, 118, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.; Benton, D.A.T.H. Introduction to Paleobiology and the Fossil Record; Wiley-Blackwell: Chichester, UK; Wiley-Blackwell: Hoboken, NJ, USA, 2009; ISBN 978-1-4051-8646-9. [Google Scholar]
- Iniesto, M.; Villalba, I.; Buscalioni, A.D.; Guerrero, M.C.; López-Archilla, A.I. The Effect Of Microbial Mats In The Decay Of Anurans With Implications For Understanding Taphonomic Processes In The Fossil Record. Sci. Rep. 2017, 7, 45160. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, J.; Fabbri, M.; Yang, T.-R.; Stein, K.; Sander, P.M.; Norell, M.A.; Briggs, D.E.G. Fossilization Transforms Vertebrate Hard Tissue Proteins into N-Heterocyclic Polymers. Nat. Commun. 2018, 9, 4741. [Google Scholar] [CrossRef]
- Bennett, B.D.; Gralnick, J.A. Mechanisms of Toxicity by and Resistance to Ferrous Iron in Anaerobic Systems. Free Radic. Biol. Med. 2019, 140, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Dennery, P.A.; Spitz, D.R.; Yang, G.; Tatarov, A.; Lee, C.S.; Shegog, M.L.; Poss, K.D. Oxygen Toxicity and Iron Accumulation in the Lungs of Mice Lacking Heme Oxygenase-2. Available online: https://www.jci.org/articles/view/448/pdf (accessed on 23 May 2023).
- Kumar, S.; Bandyopadhyay, U. Free Heme Toxicity and Its Detoxification Systems in Human. Toxicol. Lett. 2005, 157, 175–188. [Google Scholar] [CrossRef]
- Pleyer, H.L.; Moeller, R.; Fujimori, A.; Fox, S.; Strasdeit, H. Chemical, Thermal, and Radiation Resistance of an Iron Porphyrin: A Model Study of Biosignature Stability. Astrobiology 2022, 22, 776–799. [Google Scholar] [CrossRef]
- Cook, L.P.; Brewer, G.; Wong-Ng, W. Structural Aspects of Porphyrins for Functional Materials Applications. Crystals 2017, 7, 223. [Google Scholar] [CrossRef]
- Perlovich, G.L.; Golubchikov, O.A.; Klueva, M.E. Thermodynamics of Porphyrin Sublimation. J. Porphyr. Phthalocyanines 2000, 4, 699–706. [Google Scholar] [CrossRef]
- Eglinton, G.; Logan, G.A. Molecular Preservation. Philos. Trans. R. Soc. Lond. 1991, 333, 315–328. [Google Scholar]
- Baqué, M.; Backhaus, T.; Meeßen, J.; Hanke, F.; Böttger, U.; Ramkissoon, N.; Olsson-Francis, K.; Baumgärtner, M.; Billi, D.; Cassaro, A.; et al. Biosignature Stability in Space Enables Their Use for Life Detection on Mars. Sci. Adv. 2022, 8, eabn7412. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Eglinton, G.; Fu, J.; Sheng, G. Biological Markers in Chinese Ancient Sediments. 1. Geoporphyrins. Energy Fuels 1992, 6, 215–225. [Google Scholar] [CrossRef]
- Suo, Z.; Avci, R.; Schweitzer, M.H.; Deliorman, M. Porphyrin as an Ideal Biomarker in the Search for Extraterrestrial Life. Astrobiology 2007, 7, 605–615. [Google Scholar] [CrossRef]
- Gueneli, N.; McKenna, A.M.; Ohkouchi, N.; Boreham, C.J.; Beghin, J.; Javaux, E.J.; Brocks, J.J. 1.1-Billion-Year-Old Porphyrins Establish a Marine Ecosystem Dominated by Bacterial Primary Producers. Proc. Natl. Acad. Sci. USA 2018, 115, E6978–E6986. [Google Scholar] [CrossRef] [PubMed]
- Treibs, A. Chlorophyll- und Häminderivate in organischen Mineralstoffen. Angew. Chem. 1936, 49, 682–686. [Google Scholar] [CrossRef]
- Grosjean, E.; Adam, P.; Connan, J.; Albrecht, P. Effects of Weathering on Nickel and Vanadyl Porphyrins of a Lower Toarcian Shale of the Paris Basin. Geochim. Cosmochim. Acta 2004, 68, 789–804. [Google Scholar] [CrossRef]
- Espenson, J.H.; Christensen, R.J. Kinetics and Mechanism of the Demetalation of Iron(III) Porphyrins Catalyzed by Iron(II). Inorg. Chem. 1977, 16, 2561–2564. [Google Scholar] [CrossRef]
- Hodgson, G.W.; Flop, J.; Baker, B. The origin of petroleum porphyrins: Preliminary evidence for protein fragments associated with porphyrins. Geochim. Cosmochim. Acta 1969, 33, 532–535. [Google Scholar] [CrossRef]
- Cleland, T.P.; Schroeter, E.R.; Colleary, C. Diagenetiforms: A New Term to Explain Protein Changes as a Result of Diagenesis in Paleoproteomics. J. Proteomics 2021, 230, 103992. [Google Scholar] [CrossRef]
- van Doorn, N.L.; Wilson, J.; Hollund, H.; Soressi, M.; Collins, M.J. Site-Specific Deamidation of Glutamine: A New Marker of Bone Collagen Deterioration. Rapid Commun. Mass Spectrom. 2012, 26, 2319–2327. [Google Scholar] [CrossRef]
- Brown, D.R. Metalloproteins and Neuronal Death. Met. Integr. Biometal Sci. 2010, 2, 186–194. [Google Scholar] [CrossRef]
- Bush, A.I. The Metallobiology of Alzheimer’s Disease. Trends Neurosci. 2003, 26, 207–214. [Google Scholar] [CrossRef]
- Higby Schweitzer, M.; Horner, J.R. Intravascular Microstructures in Trabecular Bone Tissues of Tyrannosaurus Rex. Ann. Paléontol. 1999, 85, 179–192. [Google Scholar] [CrossRef]
- Awasthi, G.; Srivastava, G.; Das, A. Comparative Evolutionary Analyses of Beta Globin Gene in Eutherian, Dinosaurian and Neopterygii Taxa. J. Vector Borne Dis. 2011, 48, 27–36. [Google Scholar] [PubMed]
- Organ, C.L.; Schweitzer, M.H.; Zheng, W.; Freimark, L.M.; Cantley, L.C.; Asara, J.M. Molecular Phylogenetics of Mastodon and Tyrannosaurus Rex. Science 2008, 320, 499. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, J.; Menéndez, I.; Crawford, J.M.; Fabbri, M.; Gauthier, J.A.; Hull, P.M.; Norell, M.A.; Briggs, D.E.G. Fossil Biomolecules Reveal an Avian Metabolism in the Ancestral Dinosaur. Nature 2022, 606, 522–526. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).