Microbial Reduction of Geogenic and Synthetic Goethite and Hematite
Abstract
1. Introduction
2. Materials and Methods
2.1. Geogenic and Synthetic Goethite and Hematite
2.2. Bioreduction Experiments
3. Results and Discussion
3.1. Characterization of Geogenic and Synthetic Goethite and Hematite
3.2. Bioreduction of Geogenic and Synthetic Goethite and Hematite
3.3. Secondary Minerals
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Weber, K.A.; Achenbach, L.A.; Coates, J.D. Microorganisms pumping iron: Anaerobic microbial iron oxidation and reduction. Nat. Rev. Microbiol. 2006, 4, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.G.; Konhauser, K.O. Iron in Earth Surface Systems: A Major Player in Chemical and Biological Processes. Elements 2011, 7, 83–88. [Google Scholar] [CrossRef]
- Emerson, D.; Roden, E.; Twining, B.S. The microbial ferrous wheel: Iron cycling in terrestrial, freshwater, and marine environments. Front. Microbiol. 2012, 3, 383. [Google Scholar] [CrossRef]
- Kappler, A.; Bryce, C.; Mansor, M.; Lueder, U.; Byrne, J.M.; Swanner, E.D. An evolving view on biogeochemical cycling of iron. Nat. Rev. Microbiol. 2021, 19, 360–374. [Google Scholar] [CrossRef]
- Liu, H.; Liu, T.; Chen, S.; Liu, X.; Li, N.; Huang, T.; Ma, B.; Liu, X.; Pan, S.; Zhang, H. Biogeochemical cycles of iron: Processes, mechanisms, and environmental implications. Sci. Total Environ. 2024, 951, 175722. [Google Scholar] [CrossRef]
- Lovley, D.R.; Giovanoli, S.J.; White, D.C.; Champine, J.E.; Phillips, E.J.P.; Gorby, Y.A.; Goodwin, S. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch. Microbiol. 1993, 159, 336–344. [Google Scholar] [CrossRef]
- Roden, E.E.; Lovley, D.R. Dissimilatory Fe(III) reduction by the marine microorganism Desulfuromonas acetoxidans. Appl. Environ. Microbiol. 1993, 59, 734–742. [Google Scholar] [CrossRef]
- Caccavo, F., Jr.; Coates, J.D.; Rossello-Mora, R.A.; Ludwig, W.; Schleifer, K.H.; Lovley, D.R.; McInerney, M.J. Geovibrio ferrireducens, a phylogenetically distinct dissimilatory Fe(III)-reducing bacterium. Arch. Microbiol. 1996, 165, 370–376. [Google Scholar] [CrossRef]
- Greene, A.C.; Patel, B.K.; Sheehy, A.J. Deferribacter thermophilus gen. nov., sp. nov., a novel thermophilic manganese- and iron-reducing bacterium isolated from a petroleum reservoir. Int. J. Syst. Bacteriol. 1997, 47, 505–509. [Google Scholar] [CrossRef]
- Slobodkin, A.; Reysenbach, A.L.; Strutz, N.; Dreier, M.; Wiegel, J. Thermoterrabacterium ferrireducens gen. nov., sp. nov., a thermophilic anaerobic dissimilatory Fe(III)-reducing bacterium from a continental hot spring. Int. J. Syst. Bacteriol. 1997, 47, 541–547. [Google Scholar] [CrossRef]
- Fredrickson, J.K.; Zachara, J.M.; Kennedy, D.W.; Dong, H.; Onstott, T.C.; Hinman, N.W.; Li, S.-M. Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochim. Cosmochim. Acta 1998, 62, 3239–3257. [Google Scholar] [CrossRef]
- Coates, J.D.; Ellis, D.J.; Gaw, C.V.; Lovley, D.R. Geothrix fermentans gen. nov., sp. nov., a novel Fe(III)-reducing bacterium from a hydrocarbon-contaminated aquifer. Int. J. Syst. Bacteriol. 1999, 49, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Kashefi, K.; Lovley, D.R. Reduction of Fe(III), Mn(IV), and toxic metals at 100 °C by Pyrobacterium islandicum. Appl. Environ. Microbiol. 2000, 66, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Coates, J.D.; Bhupathiraju, V.K.; Achenbach, L.; McInerney, M.J.; Lovley, D.R. Geobacter hydrogenophilus, Geobacter chapelli and Geobacter grbiciae, three new, strictly anaerobic, dissimilatory Fe(III)-reducers. Int. J. Syst. Evolut. Microbiol. 2001, 51, 581–588. [Google Scholar] [CrossRef]
- Roh, Y.; Liu, S.V.; Li, G.; Huang, H.; Phelps, T.J.; Zhou, J. Isolation and characterization of metal-reducing Thermoanaerobacter strains from deep subsurface environments of the Piceance Basin, Colorado. Appl. Environ. Microbiol. 2002, 68, 6013–6020. [Google Scholar] [CrossRef]
- Sanford, R.A.; Cole, J.R.; Tiedje, J.M. Characterization and description of Anaeromyxobacter dehalogens gen. nov., sp. nov., an Aryl-halorespiring facultative anaerobic myxobacterium. Appl. Environ. Microbiol. 2002, 68, 893–900. [Google Scholar] [CrossRef]
- Roh, Y.; Chon, C.-M.; Moon, J.-W. Metal reduction and biomineralization by an alkaliphilic metal-reducing bacterium, Alkaliphilus metalliredigens (QYMF). Geosci. J. 2007, 11, 415–423. [Google Scholar] [CrossRef]
- Dong, Y.; Sanford, R.A.; Boyanov, M.I.; Kemner, K.M.; Flynn, T.M.; O’Loughlin, E.J.; Chang, Y.J.; Locke, R.A., Jr.; Weber, J.R.; Egan, S.M.; et al. Orenia metallireducens sp. nov. strain Z6, a novel metal-reducing member of the phylum firmicutes from the deep subsurface. Appl. Environ. Microbiol. 2016, 82, 6440–6453. [Google Scholar] [CrossRef]
- Dong, Y.; Sanford, R.A.; Boyanov, M.I.; Kemner, K.M.; Flynn, T.M.; O’Loughlin, E.J.; Locke Ii, R.A.; Weber, J.R.; Egan, S.M.; Fouke, B.W. Tepidibacillus decaturensis sp. nov.: A microaerophilic, moderately thermophilic iron-reducing bacterium isolated from a depth of 1.7 km in the Illinois Basin, USA. Int. J. Syst. Evol. Microbiol. 2016, 66, 3964–3971. [Google Scholar] [CrossRef]
- Hwang, J.; Song, J.; Lim, Y.; Joung, Y.; Cho, J.C. Ferrimonas sediminicola sp. nov. and Ferrimonas aestuarii sp. nov., Fe(III)-reducing bacteria isolated from marine environments. Int. J. Syst. Evol. Microbiol. 2020, 70, 4927–4934. [Google Scholar] [CrossRef]
- Li, X.; Qiu, D.; Zeng, X.; Shao, Z. Complete genome sequence of Anoxybacter fermentans DY22613(T), a piezophilic dissimilatory Fe(III)-reducing bacterium isolated from East Pacific Rise hydrothermal sulfides. Mar. Genom. 2020, 53, 100755. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.; Flynn, J.R.; Schuler, C.J.; Santelli, C.M.; Toner, B.M.; Bond, D.R.; Gralnick, J.A. Isolation and genomic analysis of “Metallumcola ferriviriculae” MK1, a Gram-positive, Fe(III)-reducing bacterium from the Soudan Underground Mine, an iron-rich Martian analog site. Appl. Environ. Microbiol. 2024, 90, e0004424. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Tang, R.; Yang, S.; Xie, C.-J.; Narsing Rao, M.P.; Rensing, C.; Liu, G.-H.; Zhou, S.-G. Geothrix oryzisoli sp. nov., a ferric iron-reducing bacterium isolated from paddy soil. Antonie Leeuwenhoek 2023, 116, 477–486. [Google Scholar] [CrossRef]
- Johnson, D.B.; Holmes, D.S.; Vergara, E.; Holanda, R.; Pakostova, E. Sulfoacidibacillus ferrooxidans, gen. nov., sp. nov., Sulfoacidibacillus thermotolerans, gen. nov., sp. nov., and Ferroacidibacillus organovorans, gen. nov., sp. nov.: Extremely acidophilic chemolitho-heterotrophic Firmicutes. Res. Microbiol. 2023, 174, 104008. [Google Scholar] [CrossRef]
- Zhao, N.; Ding, H.; Zhou, X.; Guillemot, T.; Zhang, Z.; Zhou, N.; Wang, H. Dissimilatory iron-reducing microorganisms: The phylogeny, physiology, applications and outlook. Crit. Rev. Environ. Sci. Technol. 2024, 1–26. [Google Scholar] [CrossRef]
- Lovley, D.R.; Stolz, J.F.; Nord, G.L., Jr.; Phillips, E.J.P. Anaerobic production of magnetite by a dissimilatory iron-reducing microorganism. Nature 1987, 330, 252–254. [Google Scholar] [CrossRef]
- Myers, C.R.; Nealson, K.H. Respiration-linked proton translocation coupled to anaerobic reduction of Manganese(IV) and iron(III) in Shewanella putrefaciens MR-1. J. Bacteriol. 1990, 172, 6232–6238. [Google Scholar] [CrossRef]
- Urrutia, M.M.; Roden, E.E.; Fredrickson, J.K.; Zachara, J.M. Microbial and surface chemistry controls on reduction of synthetic Fe(III) oxide minerals by the dissimilatory iron-reducing bacterium Shewanella alga. Geomicrobiology 1998, 15, 269–291. [Google Scholar] [CrossRef]
- Zachara, J.M.; Fredrickson, J.K.; Li, S.-M.; Kennedy, D.W.; Smith, S.C.; Gassman, P.L. Bacterial reduction of crystalline Fe3+ oxides in single phase suspension and subsurface materials. Am. Mineral. 1998, 83, 1426–1443. [Google Scholar] [CrossRef]
- Kostka, J.E.; Haefele, E.; Viehweger, R.; Stucki, J.W. Respiration and dissolution of iron(III)-containing clay minerals by bacteria. Environ. Sci. Technol. 1999, 33, 3127–3133. [Google Scholar] [CrossRef]
- Benner, S.G.; Hansel, C.M.; Wielinga, B.W.; Barber, T.M.; Fendorf, S. Reductive dissolution and biomineralization of iron hydroxide under dynamic flow conditions. Environ. Sci. Technol. 2002, 36, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Ona-Nguema, G.; Abdelmoula, M.; Jorand, F.; Benali, O.; Géhin, A.; Block, J.-C.; Génin, J.-M.R. Iron(II,III) hydroxycarbonate green rust formation and stabilization from lepidocrocite bioreduction. Environ. Sci. Technol. 2002, 36, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.; Zhang, C.-L.; Vali, H.; Lauf, R.J.; Zhou, J.; Phelps, T.J. Biogeochemical and environmental factors in Fe biomineralization: Magnetite and siderite formation. Clays Clay Miner. 2003, 51, 83–95. [Google Scholar] [CrossRef]
- Shelobolina, E.S.; Vanpraagh, C.G.; Lovley, D.R. Use of ferric and ferrous iron containing minerals for respiration by Desulfitobacterium frappieri. Geomicrobiol. J. 2003, 20, 143–156. [Google Scholar] [CrossRef]
- Roden, E.E. Geochemical and microbiological controls on dissimilatory iron reduction. Comptes Rendus Geosci. 2006, 338, 456–467. [Google Scholar] [CrossRef]
- Seabaugh, J.L.; Dong, H.; Kukkadapu, R.K.; Eberl, D.D.; Morton, J.P.; Kim, J. Microbial reduction of Fe(III) in the Fithian and Muloorina illites: Contrasting extents and rates of bioreduction. Clays Clay Miner. 2006, 54, 67–79. [Google Scholar] [CrossRef]
- Jorand, F.; Zegeye, A.; Landry, F.; Ruby, C. Reduction of ferric green rust by Shewanella putrefaciens. Lett. Appl. Microbiol. 2007, 45, 515–521. [Google Scholar] [CrossRef]
- Cutting, R.S.; Coker, V.S.; Fellowes, J.W.; Lloyd, J.R.; Vaughan, D.J. Mineralogical and morphological constraints on the reduction of Fe(III) minerals by Geobacter sulfurreducens. Geochim. Cosmochim. Acta 2009, 73, 4004–4022. [Google Scholar] [CrossRef]
- O’Loughlin, E.J.; Gorski, C.A.; Scherer, M.M. Effects of phosphate on secondary mineral formation during the bioreduction of akaganeite (β-FeOOH): Green rust versus framboidal magnetite. Curr. Inorg. Chem. 2015, 5, 214–224. [Google Scholar] [CrossRef]
- O’Loughlin, E.J.; Gorski, C.A.; Flynn, T.M.; Scherer, M.M. Electron donor utilization and secondary mineral formation during the bioreduction of lepidocrocite by Shewanella putrefaciens CN32. Minerals 2019, 9, 434. [Google Scholar] [CrossRef]
- O’Loughlin, E.J.; Boyanov, M.I.; Gorski, C.A.; Scherer, M.M.; Kemner, K.M. Effects of Fe(III) oxide mineralogy and phosphate on Fe(II) secondary mineral formation during microbial iron reduction. Minerals 2021, 11, 149. [Google Scholar] [CrossRef]
- Zhang, Y.; O’Loughlin, E.J.; Park, S.Y.; Kwon, M.J. Effects of Fe(III) (hydr)oxide mineralogy on the development of microbial communities originating from soil, surface water, groundwater, and aerosols. Sci. Total Environ. 2023, 905, 166993. [Google Scholar] [CrossRef] [PubMed]
- Behrends, T.; Van Cappellen, P. Transformation of hematite into magnetite during dissimilatory iron reduction-conditions and mechanisms. Geomicrobiol. J. 2007, 24, 403–416. [Google Scholar] [CrossRef]
- Boyanov, M.I.; O’Loughlin, E.J.; Kemner, K.M. Iron phase transformations resulting from the respiration of Shewanella putrefaciens on a mixed mineral phase. J. Phys. Conf. Ser. 2009, 190, 1–4. [Google Scholar] [CrossRef]
- Dong, Y.; Sanford, R.A.; Boyanov, M.I.; Flynn, T.M.; O’Loughlin, E.J.; Kemner, K.M.; George, S.; Fouke, K.E.; Li, S.; Huang, D.; et al. Controls on iron reduction and biomineralization over broad environmental conditions as suggested by the Firmicutes Orenia metallireducens strain Z6. Environ. Sci. Technol. 2020, 54, 10128–10140. [Google Scholar] [CrossRef]
- Glasauer, S.; Weidler, P.G.; Langley, S.; Beveridge, T.J. Controls on Fe reduction and mineral formation by a subsurface bacterium. Geochim. Cosmochim. Acta 2003, 67, 1277–1288. [Google Scholar] [CrossRef]
- Kukkadapu, R.K.; Zachara, J.M.; Fredrickson, J.K.; Kennedy, D.W.; Dohnalkova, A.C.; Mccready, D.E. Ferrous hydroxy carbonate is a stable transformation product of biogenic magnetite. Am. Mineral. 2005, 90, 510–515. [Google Scholar] [CrossRef][Green Version]
- Shelobolina, E.; Konishi, H.; Xu, H.; Benzine, J.; Xiong, M.Y.; Wu, T.; Blothe, M.; Roden, E. Isolation of phyllosilicate-iron redox cycling microorganisms from an illite-smectite rich hydromorphic soil. Front. Microbiol. 2012, 3, 134. [Google Scholar] [CrossRef]
- Wang, G.; Teng, Z.; Zhao, X.; Luo, W.; Liang, J.; Guo, Y.; Ji, X.; Hu, W.; Li, M. AQDS-mediated dissimilatory reduction of iron (hydr)oxides induces the formation of large grain vivianite: A new insight for phosphorus pollution control in sediment. J. Clean. Prod. 2023, 419, 138217. [Google Scholar] [CrossRef]
- Myers, C.R.; Nealson, K.H. Microbial reduction of manganese oxides: Interactions with iron and sulfur. Geochim. Cosmochim. Acta 1988, 52, 2727–2732. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrence and Uses, 2nd ed.; Wiley-VCH: New York, NY, USA, 2003; p. 664. [Google Scholar]
- Parker, C.; Wolf, J.; Auler, A.; Barton, H.; Senko, J. Microbial Reducibility of Fe(III) Phases Associated with the Genesis of Iron Ore Caves in the Iron Quadrangle, Minas Gerais, Brazil. Minerals 2013, 3, 395–411. [Google Scholar] [CrossRef]
- Maitte, B.; Jorand, F.P.A.; Grgic, D.; Abdelmoula, M.; Carteret, C. Remineralization of ferrous carbonate from bioreduction of natural goethite in the Lorraine iron ore (Minette) by Shewanella putrefaciens. Chem. Geol. 2015, 412, 48–58. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Campbell, A.N.; Tfaily, M.M.; Lin, Y.; Kukkadapu, R.K.; Silver, W.L.; Nico, P.S.; Pett-Ridge, J. Redox Fluctuations Control the Coupled Cycling of Iron and Carbon in Tropical Forest Soils. Environ. Sci. Technol. 2018, 52, 14129–14139. [Google Scholar] [CrossRef] [PubMed]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2000; p. 188. [Google Scholar]
- Cortea, I.M.; Ghervase, L.; Rădvan, R.; Serițan, G. Assessment of Easily Accessible Spectroscopic Techniques Coupled with Multivariate Analysis for the Qualitative Characterization and Differentiation of Earth Pigments of Various Provenance. Minerals 2022, 12, 755. [Google Scholar] [CrossRef]
- Mastrotheodoros, G.P.; Beltsios, K.G. Pigments-Iron-based red, yellow, and brown ochres. Archaeol. Anthropol. Sci. 2022, 14, 35. [Google Scholar] [CrossRef]
- Bousserrhine, N.; Gasser, U.G.; Jeanroy, E.; Berthelin, J. Bacterial and chemical reductive dissolution of Mn-, Co-, Cr-, and Al-substituted goethites. Geomicrobiol. J. 1999, 16, 245–258. [Google Scholar] [CrossRef]
- Kukkadapu, R.K.; Zachara, J.M.; Smith, S.C.; Fredrickson, J.K.; Liu, C. Dissimilatory bacterial reduction of Al-substituted goethite in subsurface sediments. Geochim. Cosmochim. Acta 2001, 65, 2913–2924. [Google Scholar] [CrossRef]
- O’Loughlin, E.J.; Larese-Casanova, P.; Scherer, M.M.; Cook, R.E. Green rust formation from the bioreduction of γ-FeOOH (lepidocrocite): Comparison of several Shewanella species. Geomicrobiol. J. 2007, 24, 211–230. [Google Scholar] [CrossRef]
- Stookey, L.L. Ferrozine-A new spectrophotometric reagent for iron. ANalytical Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef]
- Nicola, M.; Mastrippolito, C.; Masic, A. Iron oxide-based pigmants and their use in history. In Iron Oxides: From Nature to Applications; Faivre, D., Ed.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2016; pp. 545–565. [Google Scholar]
- Bikiaris, D.; Daniilia, S.; Sotiropoulou, S.; Katsimbiri, O.; Pavlidou, E.; Moutsatsou, A.P.; Chryssoulakis, Y. Ochre-differentiation through micro-Raman and micro-FTIR spectroscopies: Application on wall paintings at Meteora and Mount Athos, Greece. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2000, 56, 3–18. [Google Scholar] [CrossRef]
- Juliá, C.G.; Bonafé, C.P. The use of natural earths in picture: Study and differentiation by thermal analysis. Thermochim. Acta 2004, 413, 185–192. [Google Scholar] [CrossRef]
- Genestar, C.; Pons, C. Earth pigments in painting: Characterisation and differentiation by means of FTIR spectroscopy and SEM-EDS microanalysis. Anal. Bioanal. Chem. 2005, 382, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Elias, M.; Chartier, C.; Prévot, G.; Garay, H.; Vignaud, C. The colour of ochres explained by their composition. Mater. Sci. Eng. 2006, 127, 70–80. [Google Scholar] [CrossRef]
- Hansel, C.M.; Benner, S.G.; Nico, P.; Fendorf, S. Structural constraints of ferric (hydr)oxides on dissimilatory iron reduction. Geochim. Cosmochim. Acta 2004, 68, 3217–3229. [Google Scholar] [CrossRef]
- Bonneville, S.; Behrends, T.; Van Cappellen, P. Solubility and dissimilatory reduction kinetics of iron(III) oxyhydroxides: A linear free energy relationship. Geochim. Cosmochim. Acta 2009, 73, 5273–5282. [Google Scholar] [CrossRef]
- Lovley, D.R.; Coates, J.D.; Blunt-Harris, E.L.; Phillips, E.J.P.; Woodward, J.C. Humic-substances as electron acceptors for microbial respiration. Nature 1996, 382, 445–448. [Google Scholar] [CrossRef]
- Nevin, K.P.; Lovley, D.R. Potential for nonenzymatic reduction of Fe(III) via electron shuttling in subsurface sediments. Environ. Sci. Technol. 2000, 34, 2472–2478. [Google Scholar] [CrossRef]
- Bond, D.R.; Lovley, D.R. Reduction of Fe(III) oxide by methanogens in the presence and absence of extracellular quinones. Environ. Microbiol. 2002, 4, 115–124. [Google Scholar] [CrossRef]
- Straub, K.L.; Schink, B. Evaluation of electron-shuttling compounds in microbial ferric iron reduction. FEMS Microbiol. Lett. 2003, 220, 229–233. [Google Scholar] [CrossRef]
- Zegeye, A.; Ruby, C.; Jorand, F. Kinetic and thermodynamic analysis during dissimilatory γ-FeOOH reduction: Formation of green rust 1 and magnetite. Geomicrobiol. J. 2007, 24, 51–64. [Google Scholar] [CrossRef]
- Coker, V.S.; Bell, A.M.T.; Pearce, C.I.; Pattrick, R.A.D.; van der Laan, G.; Lloyd, J.R. Time-resolved synchrotron powder X-ray diffraction study of magnetite formation by the Fe(III)-reducing bacterium Geobacter sulfurreducens. Am. Mineral. 2008, 93, 540–547. [Google Scholar] [CrossRef]
- O’Loughlin, E.J. Effects of electron transfer mediators on the biodegradation of lepidocrocite (γ-FeOOH) by Shewanella putrefaciens CN32. Environ. Sci. Technol. 2008, 42, 6876–6882. [Google Scholar] [CrossRef]
- Jung, J.; Bae, S.; Lee, W. Indirect contact of bio-transformation of lepidocrocite: Role of electron transfer mediator. Sustain. Environ. Res. 2012, 23, 193–198. [Google Scholar]
- Bae, S.; Lee, W. Biotransformation of lepidocrocite in the presence of quinones and flavins. Geochim. Cosmochim. Acta 2013, 114, 144–155. [Google Scholar] [CrossRef]
- Tratnyek, P.G.; Macalady, D.L. Abiotic reductions of nitro aromatic pesticides in anaerobic laboratory systems. J. Agric. Food Chem. 1989, 37, 248–254. [Google Scholar] [CrossRef]
- Cooper, D.C.; Picardal, F.; Rivera, J.; Talbot, C. Zinc immobilization and magnetite formation via ferric oxide reduction by Shewanella putrefaciens 200. Environ. Sci. Technol. 2000, 34, 100–106. [Google Scholar] [CrossRef]
- Liu, C.; Kota, S.; Zachara, J.M.; Fredrickson, J.K.; Brinkman, C.K. Kinetic analysis of the bacterial reduction of goethite. Environ. Sci. Technol. 2001, 35, 2482–2490. [Google Scholar] [CrossRef]
- Liu, D.; Wang, H.; Dong, H.; Qiu, X.; Dong, X.; Cravotta, C.A., III. Mineral transformations associated with goethite reduction by Methanosarcina barkeri. Chem. Geol. 2011, 288, 53–60. [Google Scholar] [CrossRef]
- Yan, B.; Wrenn, B.A.; Basak, S.; Biswas, P.; Giammar, D.E. Microbial reduction of Fe(III) in hematite nanoparticles by Geobacter sulfurreducens. Environ. Sci. Technol. 2008, 42, 6526–6531. [Google Scholar] [CrossRef]
- Luo, H.-W.; Zhang, X.; Chen, J.-J.; Yu, H.-Q.; Sheng, G.-P. Probing the biotransformation of hematite nanoparticles and magnetite formation mediated by Shewanella oneidensis MR-1 at the molecular scale. Environ. Sci. Nano 2017, 4, 2395–2404. [Google Scholar] [CrossRef]
- Dominik, P.; Pohl, H.N.; Bousserrhine, N.; Berthelin, J.; Kaupenjohann, M. Limitations to the reductive dissolution of Al-substituted goethites by Clostridium butyricum. Soil Biol. Biochem. 2002, 34, 1147–1155. [Google Scholar] [CrossRef]
- Ekstrom, E.B.; Learman, D.R.; Madden, A.S.; Hansel, C.M. Contrasting effects of Al substitution on microbial reduction of Fe(III) (hydr)oxides. Geochim. Cosmochim. Acta 2010, 74, 7086–7099. [Google Scholar] [CrossRef]
- O’Loughlin, E.J.; Boyanov, M.I.; Flynn, T.M.; Gorski, C.; Hofmann, S.M.; McCormick, M.L.; Scherer, M.M.; Kemner, K.M. Effects of bound phosphate on the bioreduction of lepidocrocite (γ-FeOOH) and maghemite (γ-Fe2O3) and formation of secondary minerals. Environ. Sci. Technol. 2013, 47, 9157–9166. [Google Scholar] [CrossRef] [PubMed]

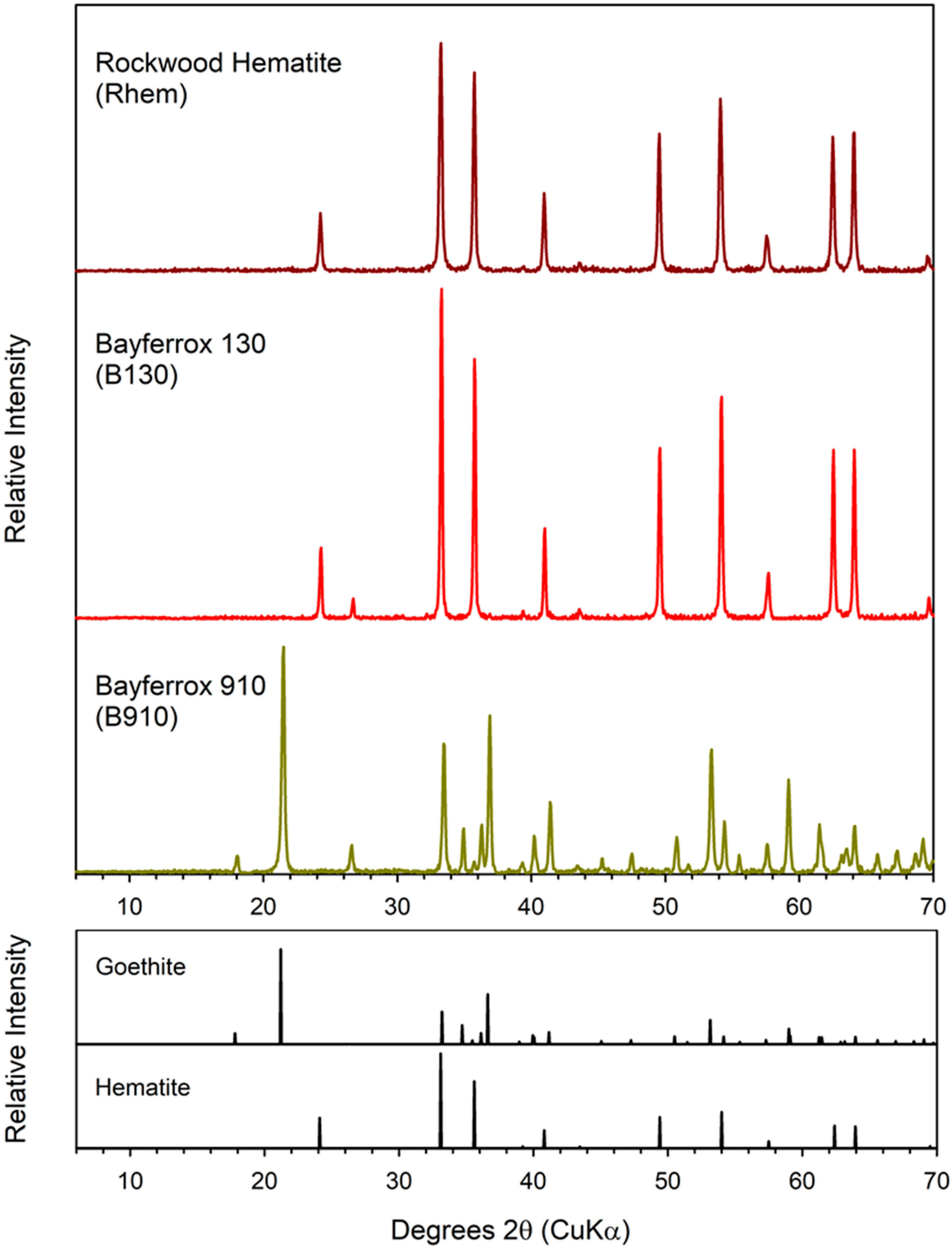
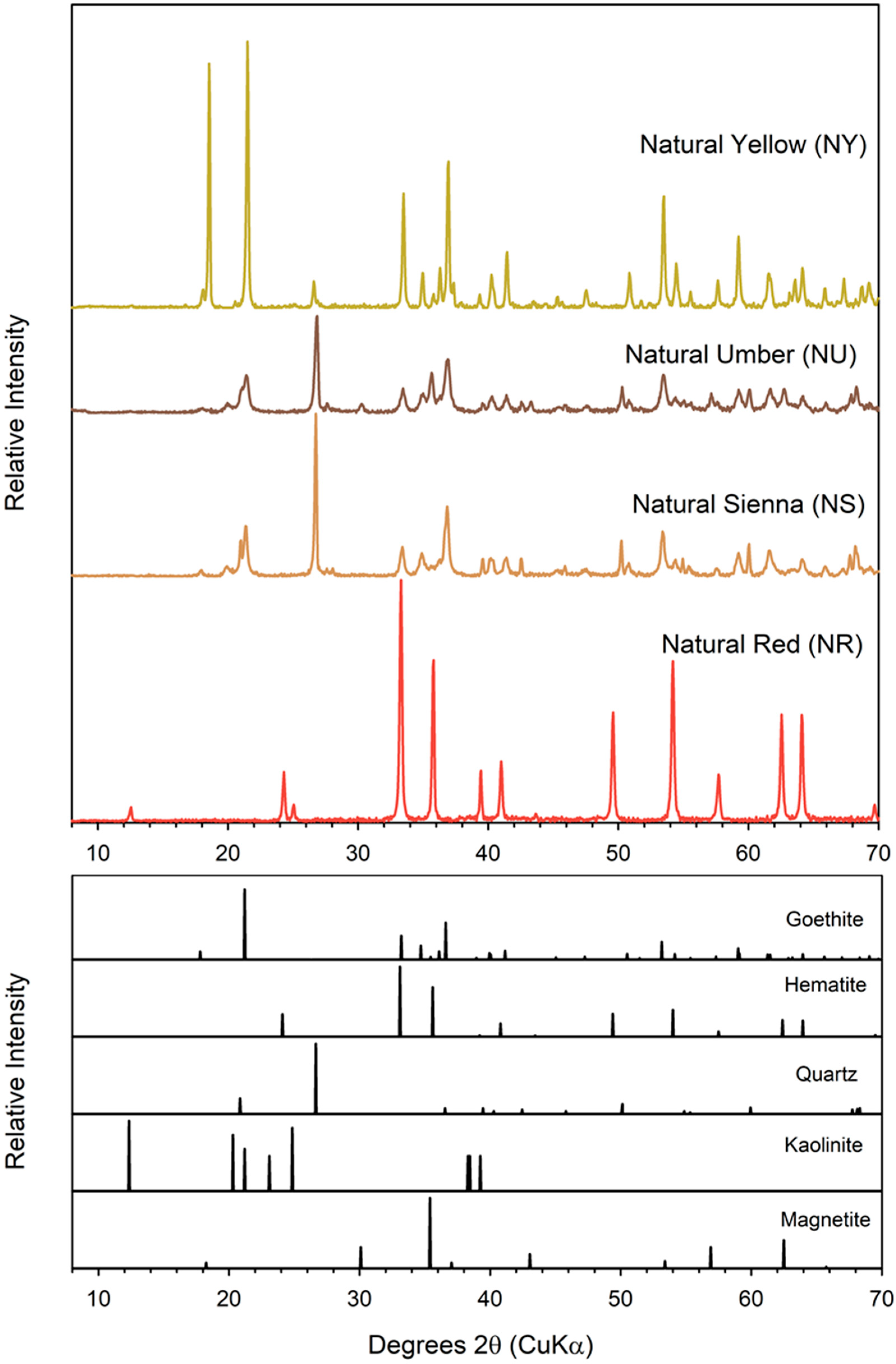




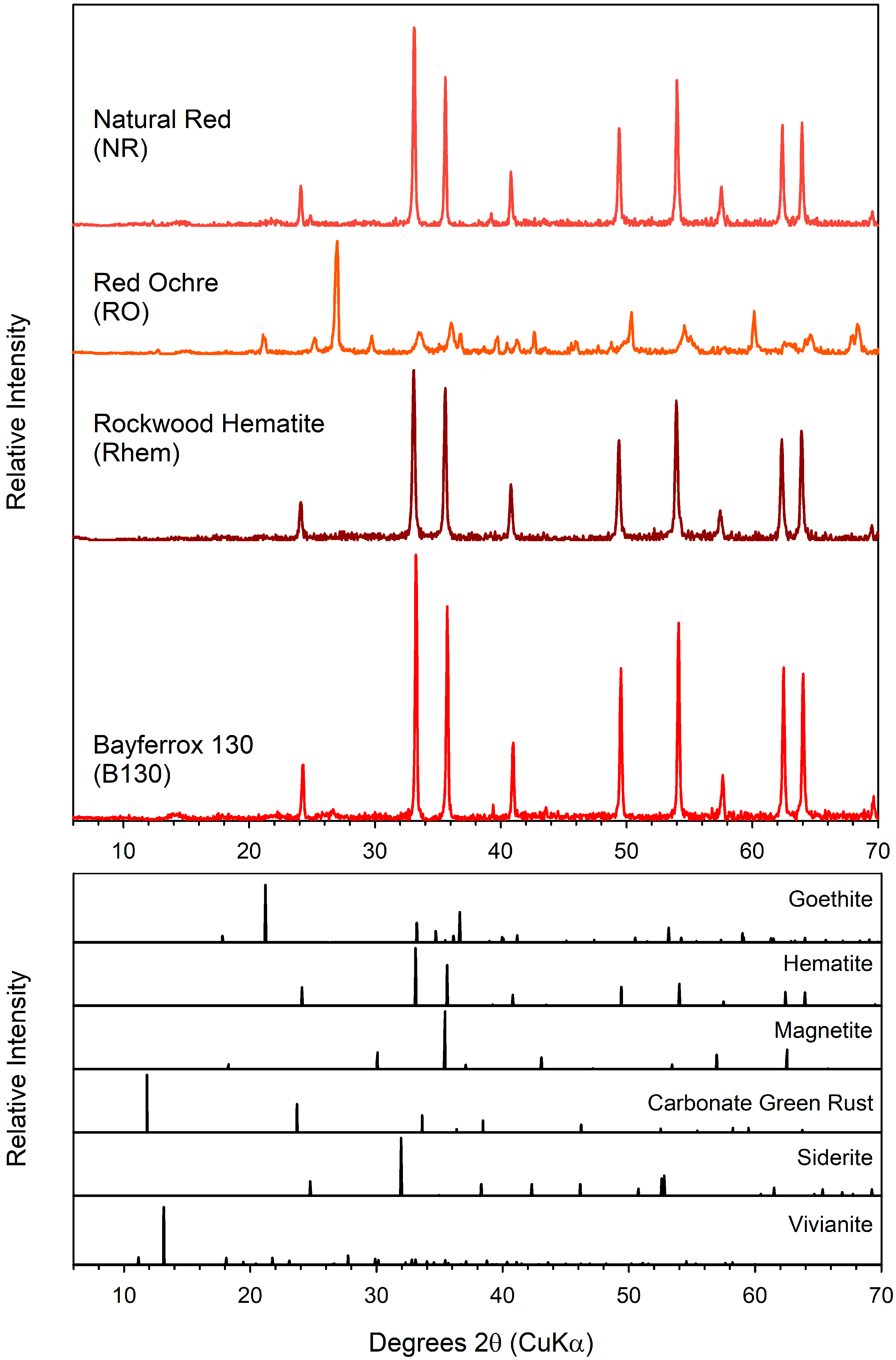
| Material | Source | Iron Oxide | Other Crystalline Components | Fe wt % | AQDS | Fe(II)tot a mM | Fraction of Total Fe(III) Reduced | Fe(II)tot Production During Bioreduction b mM d−1 (r2) | |
|---|---|---|---|---|---|---|---|---|---|
| Bayferrox 910 (B910) | Lanx | synthetic | goethite | none | 62.6 | – | 1.40 ± 0.46 | 1.8% | 0.12 ± 0.03 (0.942) |
| + | 2.64 ± 0.05 | 3.3% | 0.20 ± 0.04 (0.970) | ||||||
| Bayferrox 130 (B130) | Lanx | synthetic | hematite | none | 69.9 | – | 1.08 ± 0.22 | 1.4% | 0.43 ± 0.0 (1) |
| + | 1.88 ± 0.62 | 2.4% | 0.54 ± 0.11 (0.958) | ||||||
| Rockwood Hematite (Rhem) | Rockwood | synthetic | hematite | none | 69.9 | – | 2.65 ± 0.59 | 3.3% | 0.13 ± 0.01 (0.986) |
| + | 4.33 ± 0.42 | 5.4% | 0.80 ± 0.25 (0.908) | ||||||
| Dark Ochre (DO) | Kremer Pigments | natural | goethite | quartz/kaolinite | 31.3 | + | 13.27 ± 0.05 | 16.6% | 4.18 ± 1.07 (0.859) |
| French Ochre JALS (FOJ) | Kremer Pigments | natural | goethite | quartz/kaolinite | 12.7 | + | 5.58 ± 1.98 | 7.0% | 0.21 ± 0.01 (0.975) |
| Red Ochre (RO) | The Earth Pigments Co. | natural | goethite/hematite | quartz/kaolinite | 15.2 | + | 8.79 ± 0.33 | 11.0% | 3.43 ± 0.0 (1) |
| Natural Red (NR) | The Earth Pigments Co. | natural | hematite | kaolinite | 56.3 | + | 2.36 ± 0.60 | 3.0% | 0.18 ± 0.09 (0.788) |
| Natural Sienna (NS) | The Earth Pigments Co. | natural | goethite | quartz/kaolinite | 22.1 | + | 20.57 ± 0.58 | 25.7% | 5.65 ± 1.40 (0.870) |
| Natural Umber (NU) | The Earth Pigments Co. | natural | goethite | quartz | 23.6 | + | 21.72 ± 0.34 | 27.1% | 10.5 ± 0.0 (1) |
| Natural Yellow (NY) | The Earth Pigments Co. | natural | goethite | quartz | 45.9 | + | 2.16 ± 0.81 | 2.7% | 0.19 ± 0.04 (0.933) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Loughlin, E.J. Microbial Reduction of Geogenic and Synthetic Goethite and Hematite. Minerals 2024, 14, 1086. https://doi.org/10.3390/min14111086
O’Loughlin EJ. Microbial Reduction of Geogenic and Synthetic Goethite and Hematite. Minerals. 2024; 14(11):1086. https://doi.org/10.3390/min14111086
Chicago/Turabian StyleO’Loughlin, Edward J. 2024. "Microbial Reduction of Geogenic and Synthetic Goethite and Hematite" Minerals 14, no. 11: 1086. https://doi.org/10.3390/min14111086
APA StyleO’Loughlin, E. J. (2024). Microbial Reduction of Geogenic and Synthetic Goethite and Hematite. Minerals, 14(11), 1086. https://doi.org/10.3390/min14111086






