Using Manganese Oxidizing Fungi to Recover Metals from Electronic Waste
Abstract
1. Introduction
2. Materials and Methods
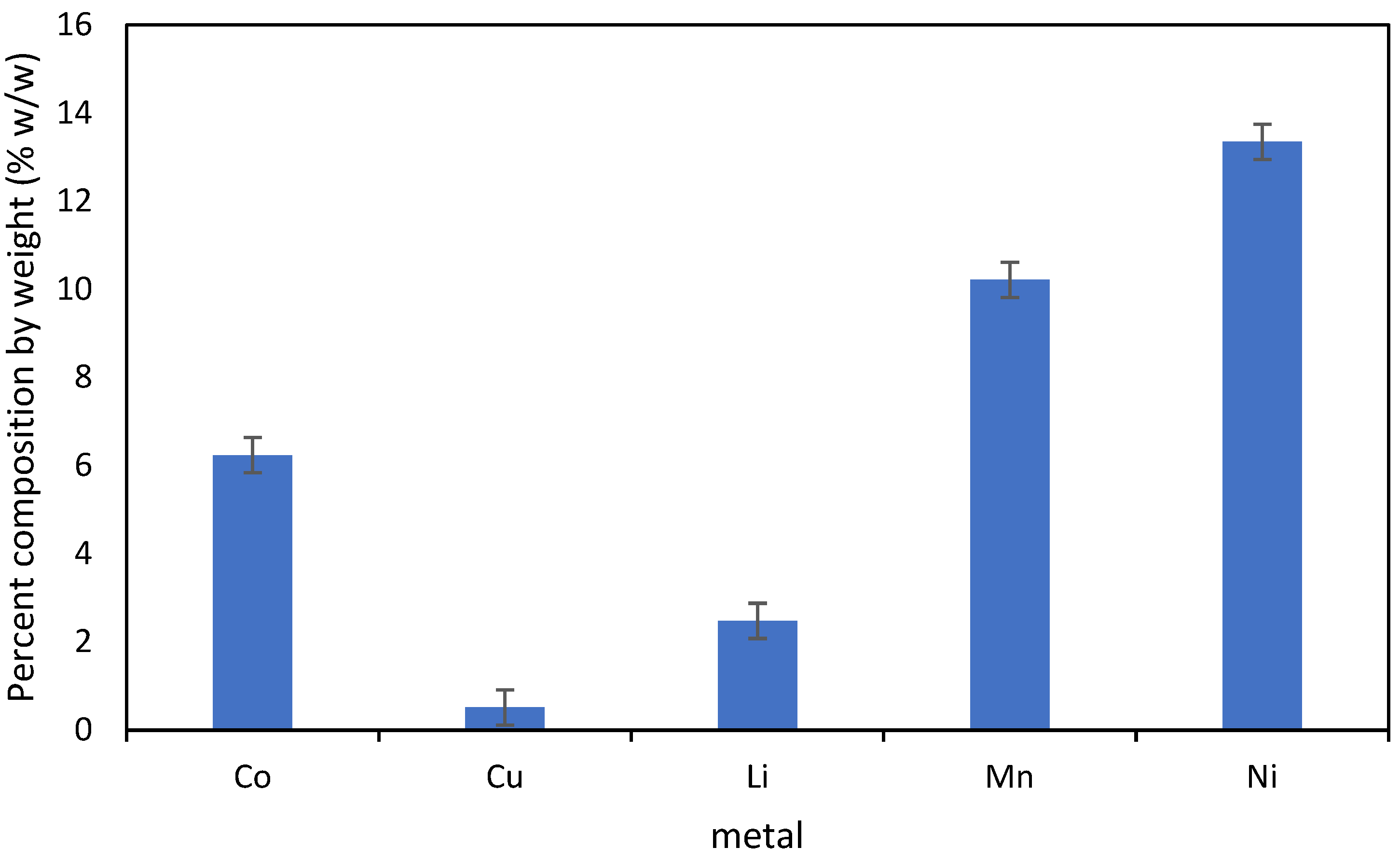
2.1. E-Waste
2.2. Fungal Cultures
2.3. Incubation Experiment and Extractions
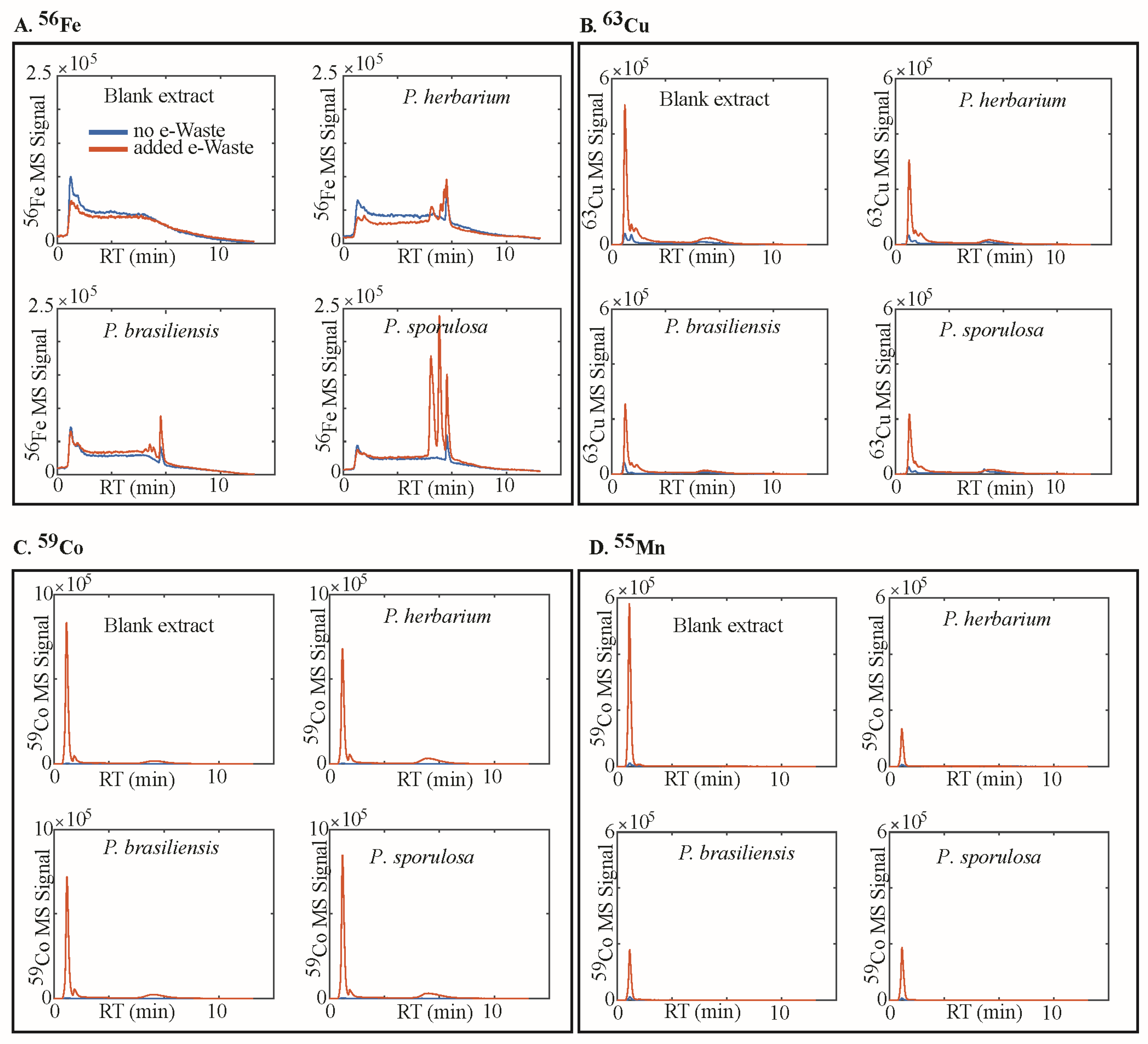
2.4. Analysis of Metal Speciation by Liquid Chromatography–Inductively Coupled Plasma-Mass Spectrometry (LC-ICP-MS)
2.5. Siderophore Analysis by Colorimetric Assays and Liquid Chromatography–Electrospray Ionization-Mass Spectrometry (LC-ESI-MS)
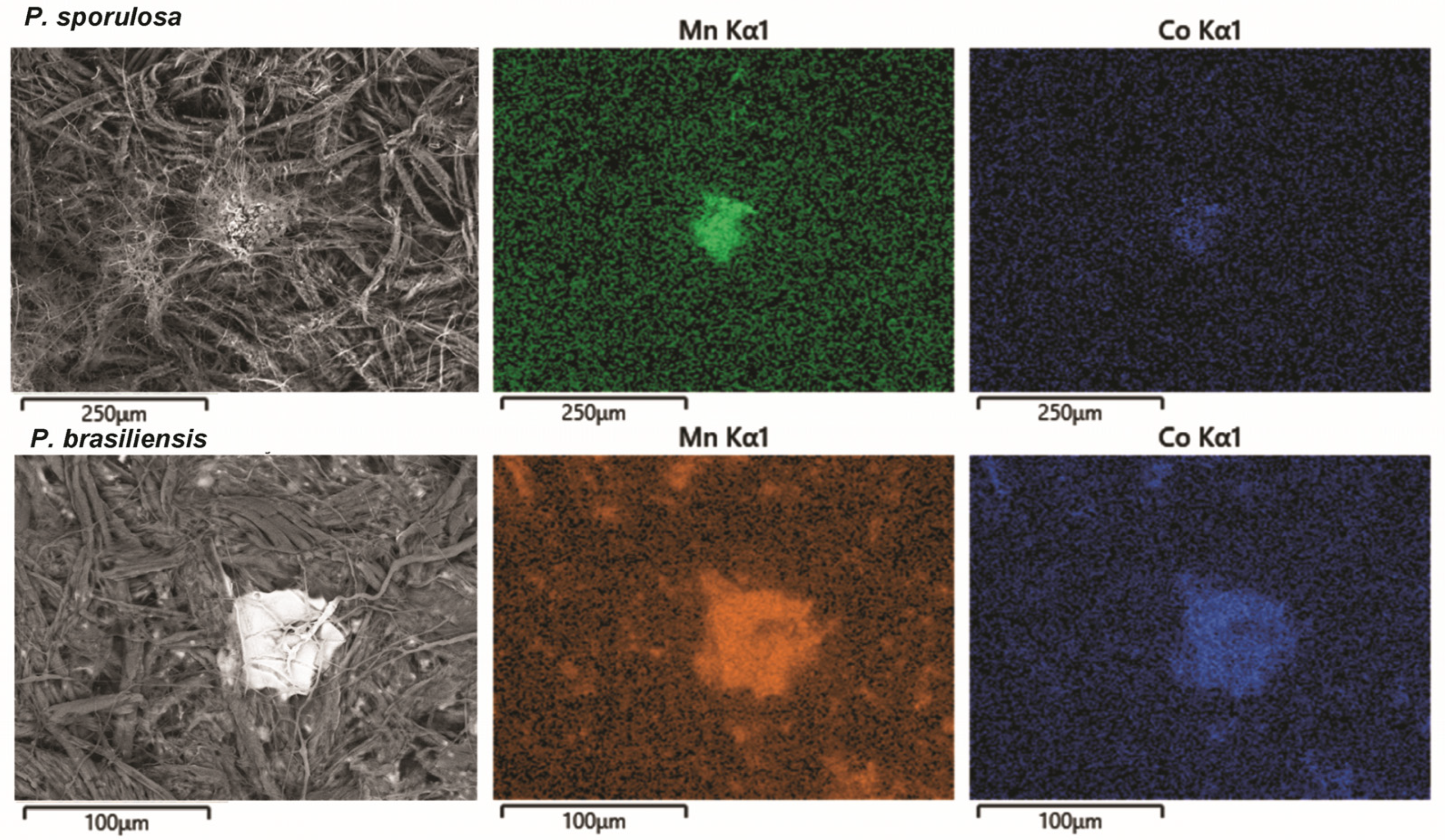
2.6. Scanning Electron Microscopy with Energy Dispersive X-ray Spectrometry
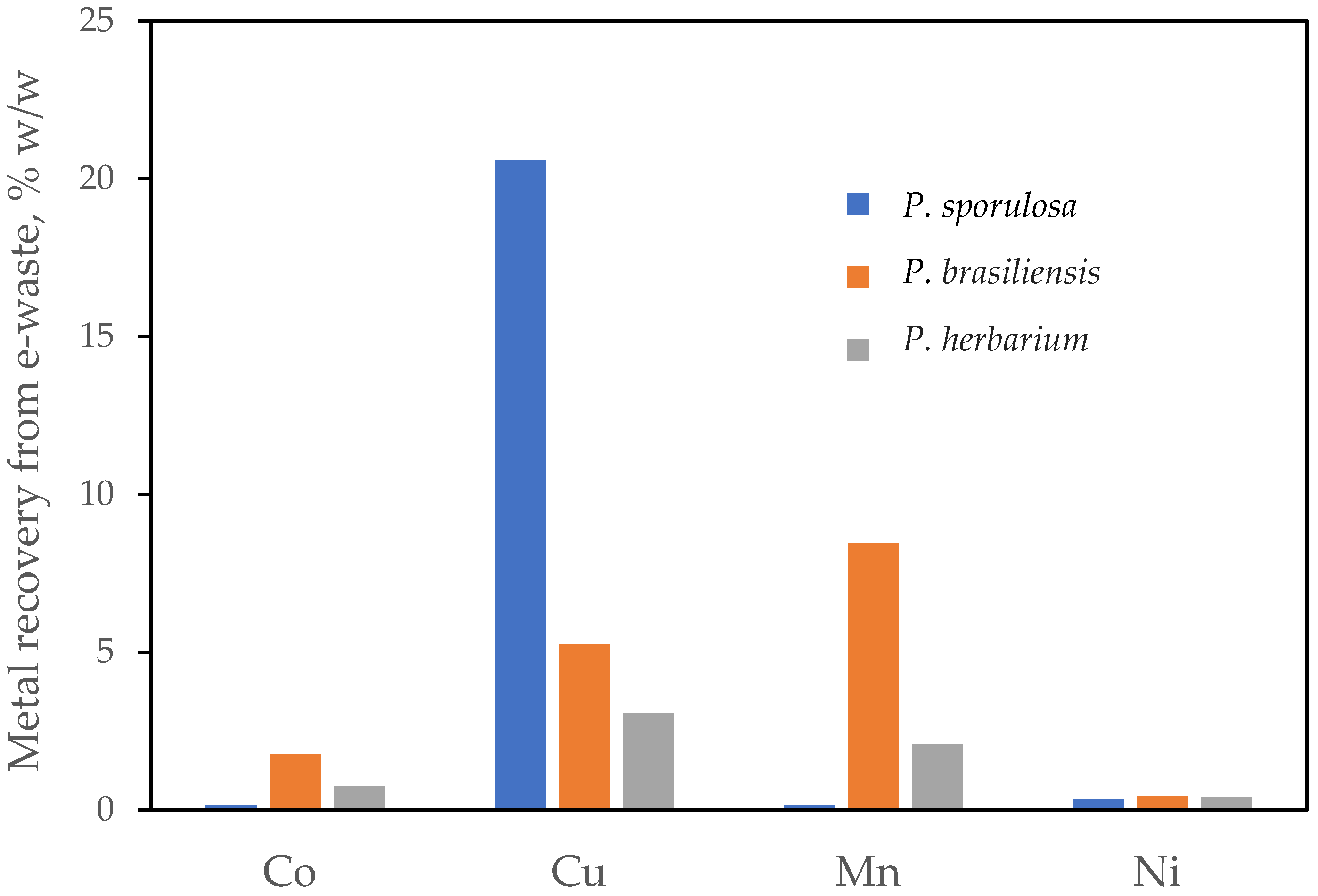
3. Results
4. Discussion
4.1. Siderophores, Metal Speciation in Media, and the Mechanism of Dissolution
4.2. Fungal-Associated Metal Speciation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Economic Forum and Platform for Accelerating the Circular Economy (PACE). A New Circular Vision for Electronics: Time for a Global Reboot; World Economic Forum: Cologny, Switzerland, 2019. [Google Scholar]
- Forti, V.; Balde, C.P.; Kuehr, R.; Bel, G. The Global E-Waste Monitor 2020: Quantities, Flows and the Circular Economy Potential; United Nations University/United Nations Institute for Training and Research, International Telecommunication Union, and International Solid Waste Association: Bonn, Germany; Geneva, Switzerland; Rotterdam, The Netherlands, 2020. [Google Scholar]
- Shittu, O.S.; Williams, I.D.; Shaw, P.J. Global E-waste management: Can WEEE make a difference? A review of e-waste trends, legislation, contemporary issues and future challenges. Waste Manag. 2021, 120, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Andeobu, L.; Wibowo, S.; Grandhi, S. An assessment of e-waste generation and environmental management of selected countries in Africa, Europe and North America: A systematic review. Sci. Total Environ. 2021, 792, 148078. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, G.; Jadhao, P.R.; Pant, K.K.; Nigam, K.D.P. Novel technologies and conventional processes for recovery of metals from waste electrical and electronic equipment: Challenges & opportunities—A review. J. Environ. Chem. Eng. 2018, 6, 1288–1304. [Google Scholar] [CrossRef]
- Zhang, K.; Schnoor, J.L.; Zeng, E.Y. E-Waste Recycling: Where Does It Go from Here? Environ. Sci. Technol. 2012, 46, 10861–10867. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Garzon, J.; Hubau, A.; Joulian, C.; Guezennec, A.-G. Bioleaching of E-Waste: Influence of Printed Circuit Boards on the Activity of Acidophilic Iron-Oxidizing Bacteria. Front. Microbiol. 2021, 12, 669738. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Priyanka, B.; Senthil Kumar, P.; Karishma, S.; Jeevanantham, S.; Indraganti, S. A review on recent advancements in recovery of valuable and toxic metals from e-waste using bioleaching approach. Chemosphere 2022, 287, 132230. [Google Scholar] [CrossRef]
- Adetunji, A.I.; Oberholster, P.J.; Erasmus, M. Bioleaching of Metals from E-Waste Using Microorganisms: A Review. Minerals 2023, 13, 828. [Google Scholar] [CrossRef]
- Meyer, V.; Basenko, E.Y.; Benz, J.P.; Braus, G.H.; Caddick, M.X.; Csukai, M.; de Vries, R.P.; Endy, D.; Frisvad, J.C.; Gunde-Cimerman, N.; et al. Growing a circular economy with fungal biotechnology: A white paper. Fungal Biol. Biotechnol. 2020, 7, 5. [Google Scholar] [CrossRef]
- Kraemer, S.M.; Butler, A.; Borer, P.; Cervini-Silva, J. Siderophores and the Dissolution of Iron-bearing Minerals in Marine Systems. In Molecular Geomicrobiology; Banfield, J.F., Cervini-Silva, J., Nealson, K.H., Eds.; Mineralogical Society of America: Chantilly, VA, USA, 2005; Volume 59, pp. 53–84. [Google Scholar]
- Kraemer, S.M.; Crowley, D.E.; Kretzschmar, R. Geochemical aspects of phytosiderophore-promoted iron acquisition by plants. Adv. Agron. 2006, 91, 1–46. [Google Scholar] [CrossRef]
- Crumbliss, A.L.; Harrington, J.M. Iron Sequestration by small molecules: Thermodynamic and kinetic studies of natural siderophores and synthetic model Compounds. Adv. Inorg. Chem. 2009, 61, 179–250. [Google Scholar]
- Harrington, J.; Duckworth, O.; Haselwandter, K. The fate of siderophores: Antagonistic environmental interactions in exudate-mediated micronutrient uptake. BioMetals 2015, 28, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.L.; Verity, R.; Webb, S.M. Biogenic manganese oxides: Properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef]
- Goldberg, E.D. Marine Geochemistry 1. Chemical Scavengers of the Sea. J. Geol. 1954, 62, 249–265. [Google Scholar] [CrossRef]
- Duckworth, O.W.; Bargar, J.R.; Sposito, G. Sorption of ferric iron from ferrioxamine B to synthetic and biogenic layer type manganese oxides. Geochim. Cosmochim. Acta 2008, 72, 3371–3380. [Google Scholar] [CrossRef]
- Gardner, T.G.; Rivera, N.; Anderws, M.Y.; Santelli, C.; Duckworth, O.W. Diversity and Abundance of Microbial Community in NC Superfund Site Remediation System Biofilm, and Their Role in Mn Oxidation. In Proceedings of the ASA, CSSA and SSSA International Annual Meetings, Minneapolis, MN, USA, 15–18 November 2015. [Google Scholar]
- Droz, B.; Dumas, N.; Duckworth, O.W.; Peña, J. A Comparison of the Sorption Reactivity of Bacteriogenic and Mycogenic Mn Oxide Nanoparticles. Environ. Sci. Technol. 2015, 49, 4200–4208. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, O.W.; Rivera, N.A.; Gardner, T.G.; Andrews, M.Y.; Santelli, C.M.; Polizzotto, M.L. Morphology, structure, and metal binding mechanisms of biogenic manganese oxides in a superfund site treatment system. Environ. Sci. Process. Impacts 2017, 19, 50–58. [Google Scholar] [CrossRef]
- Park, Y.; Liu, S.; Gardner, T.; Johnson, D.; Keeler, A.; Ortiz, N.; Rabah, G.; Ford, E. Biohybrid nanofibers containing manganese oxide–forming fungi for heavy metal removal from water. J. Eng. Fibers Fabr. 2020, 15, 1558925019898954. [Google Scholar] [CrossRef]
- Peña, J.; Kwon, K.D.; Refson, K.; Bargar, J.R.; Sposito, G. Mechanisms of nickel sorption by a bacteriogenic birnessite. Geochim. Cosmochim. Acta 2010, 74, 3076–3089. [Google Scholar] [CrossRef]
- Andrews, M.Y.; Santelli, C.M.; Duckworth, O.W. Layer plate CAS assay for the quantitation of siderophore production and determination of exudation patterns for fungi. J. Microbiol. Methods 2016, 121, 41–43. [Google Scholar] [CrossRef]
- Andrews, M.Y.; Santelli, C.M.; Duckworth, O.W. Digital image quantification of siderophores on agar plates. Data Brief 2016, 6, 890–898. [Google Scholar] [CrossRef][Green Version]
- Smith, A.W. 6.13 Iron Starvation and Siderophore-Mediated iron Transport. In Methods in Microbiology; Williams, P., Ketley, J., Salmond, G., Eds.; Academic Press: Cambridge, MA, USA, 1998; Volume 27, pp. 331–342. [Google Scholar]
- Arnow, L.E. Colorimetric Determination of the Components of 3,4-Dihydroxyphenylalaninetyrosine Mixtures. J. Biol. Chem. 1937, 118, 531–537. [Google Scholar] [CrossRef]
- Baars, O.; Morel, F.M.M.; Perlman, D.H. ChelomEx: Isotope-Assisted Discovery of Metal Chelates in Complex Media Using High-Resolution LC-MS. Anal. Chem. 2014, 86, 11298–11305. [Google Scholar] [CrossRef] [PubMed]
- Baars, O.; Perlman, D.H. Small Molecule LC-MS/MS Fragmentation Data Analysis and Application to Siderophore Identification. In Applications from Engineering with MATLAB Concepts; Jan, V., Ed.; IntechOpen: Rijeka, Croatia, 2016; Chapter 9. [Google Scholar]
- Whitaker, A.H.; Austin, R.E.; Holden, K.L.; Jones, J.L.; Michel, F.M.; Peak, D.; Thompson, A.; Duckworth, O.W. The structure of natural biogenic iron (oxyhydr)oxides formed in circumneutral pH environments. Geochim. Cosmochim. Acta 2021, 308, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Keller-Schierlein, W.; Deér, A. Stoffwechselprodukte von Mikroorganismen. 44. Mitteilung. Zur Konstitution von Ferrichrysin und Ferricrocin. Helv. Chim. Acta 1963, 46, 1907–1920. [Google Scholar] [CrossRef]
- Schwecke, T.; Göttling, K.; Durek, P.; Dueñas, I.; Käufer, N.F.; Zock-Emmenthal, S.; Staub, E.; Neuhof, T.; Dieckmann, R.; von Döhren, H. Nonribosomal Peptide Synthesis in Schizosaccharomyces pombe and the Architectures of Ferrichrome-Type Siderophore Synthetases in Fungi. ChemBioChem 2006, 7, 612–622. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Bertrand, S.; Larcher, G.; Landreau, A.; Richomme, P.; Duval, O.; Bouchara, J.-P. Hydroxamate siderophores of Scedosporium apiospermum. BioMetals 2009, 22, 1019–1029. [Google Scholar] [CrossRef]
- Renshaw, J.C.; Robson, G.D.; Trinci, A.P.J.; Wiebe, M.G.; Livens, F.R.; Collison, D.; Taylor, R.J. Fungal siderophores: Structures, functions and applications. Mycol. Res. 2002, 106, 1123–1142. [Google Scholar] [CrossRef]
- Winkelmann, G. Ecology of siderophores with special reference to the fungi. BioMetals 2007, 20, 379–392. [Google Scholar] [CrossRef]
- Hider, R.C.; Kong, X. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010, 27, 637–657. [Google Scholar] [CrossRef]
- Kraemer, S.; Duckworth, O.; Harrington, J.; Schenkeveld, W.C. Metallophores and Trace Metal Biogeochemistry. Aquat. Geochem. 2015, 21, 159–195. [Google Scholar] [CrossRef]
- Martínez, J.L.; Delgado-Iribarren, A.; Baquero, F. Mechanisms of iron acquisition and bacterial virulence. FEMS Microbiol. Rev. 1990, 6, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Schaible, U.E.; Kaufmann, S.H.E. Iron and microbial infection. Nat. Rev. Microbiol. 2004, 2, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Eisendle, M.; Schrettl, M.; Kragl, C.; Müller, D.; Illmer, P.; Haas, H. The Intracellular Siderophore Ferricrocin Is Involved in Iron Storage, Oxidative-Stress Resistance, Germination, and Sexual Development in Aspergillus nidulans. Eukaryot. Cell 2006, 5, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Wallner, A.; Blatzer, M.; Schrettl, M.; Sarg, B.; Lindner, H.; Haas, H. Ferricrocin, a Siderophore Involved in Intra- and Transcellular Iron Distribution in Aspergillus fumigatus. Appl. Environ. Microbiol. 2009, 75, 4194–4196. [Google Scholar] [CrossRef] [PubMed]
- Eisendle, M.; Oberegger, H.; Buttinger, R.; Illmer, P.; Haas, H. Biosynthesis and uptake of siderophores is controlled by the PacC-mediated ambient-pH Regulatory system in Aspergillus nidulans. Eukaryot. Cell 2004, 3, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Haas, H. Molecular genetics of fungal siderophore biosynthesis and uptake: The role of siderophores in iron uptake and storage. Appl. Microbiol. Biotechnol. 2003, 62, 316–330. [Google Scholar] [CrossRef]
- Braud, A.; Hoegy, F.; Jezequel, K.; Lebeau, T.; Schalk, I.J. New insights into the metal specificity of the Pseudomonas aeruginosa pyoverdine–iron uptake pathway. Environ. Microbiol. 2009, 11, 1079–1091. [Google Scholar] [CrossRef]
- Nair, A.; Juwarkar, A.A.; Singh, S.K. Production and Characterization of Siderophores and its Application in Arsenic Removal from Contaminated Soil. Water Air Soil Pollut. 2007, 180, 199–212. [Google Scholar] [CrossRef]
- Teitzel, G.M.; Geddie, A.; De Long, S.K.; Kirisits, M.J.; Whiteley, M.; Parsek, M.R. Survival and growth in the presence of elevated copper: Transcriptional profiling of copper-stressed Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 7242–7256. [Google Scholar] [CrossRef]
- Illmer, P.; Buttinger, R. Interactions between iron availability, aluminium toxicity and fungal siderophores. Biometals 2006, 19, 367–377. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Nolan, E.M. Beyond iron: Non-classical biological functions of bacterial siderophores. Dalton Trans. 2015, 44, 6320–6339. [Google Scholar] [CrossRef] [PubMed]
- Money, N.P. Biomechanics of Invasive Hyphal Growth. In Biology of the Fungal Cell; Howard, R.J., Gow, N.A.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 3–17. [Google Scholar]
- Gadd, G.M. Geomycology: Biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol. Res. 2007, 111, 3–49. [Google Scholar] [CrossRef] [PubMed]
- Andrino, A.; Guggenberger, G.; Kernchen, S.; Mikutta, R.; Sauheitl, L.; Boy, J. Production of Organic Acids by Arbuscular Mycorrhizal Fungi and Their Contribution in the Mobilization of Phosphorus Bound to Iron Oxides. Front. Plant Sci. 2021, 12, 661842. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Fungal Production of Citric and Oxalic Acid: Importance in Metal Speciation, Physiology and Biogeochemical Processes. In Advances in Microbial Physiology; Poole, R.K., Ed.; Academic Press: Cambridge, MA, USA, 1999; Volume 41, pp. 47–92. [Google Scholar]
- Martin, S.T. Precipitation and dissolution of iron and manganese oxides. In Environmental Catalysis; Grassian, V.H., Ed.; Marcel-Dekker, CRC Press: Boca Raton, FL, USA, 2005; pp. 61–81. [Google Scholar]
- Gadd, G.M. Interactions of fungi with toxic metals. New Phytol. 1993, 124, 25–60. [Google Scholar] [CrossRef]
- Santelli, C.M.; Webb, S.M.; Dohnalkova, A.C.; Hansel, C.M. Diversity of Mn oxides produced by Mn(II)-oxidizing fungi. Geochim. Cosmochim. Acta 2011, 75, 2762–2776. [Google Scholar] [CrossRef]
- Burns, R.G. The uptake of cobalt into ferromanganese nodules, soils, and synthetic manganese (IV) oxides. Geochim. Cosmochim. Acta 1976, 40, 95–102. [Google Scholar] [CrossRef]
- Murray, J.W. The interaction of cobalt with hydrous manganese dioxide. Geochim. Cosmochim. Acta 1975, 39, 635–647. [Google Scholar] [CrossRef]
- Simanova, A.A.; Peña, J. Time-Resolved Investigation of Cobalt Oxidation by Mn(III)-Rich δ-MnO2 Using Quick X-ray Absorption Spectroscopy. Environ. Sci. Technol. 2015, 49, 10867–10876. [Google Scholar] [CrossRef]
- Manceau, A.; Nagy, K.L.; Marcus, M.A.; Lanson, M.; Geoffroy, N.; Jacquet, T.; Kirpichtchikova, T. Formation of metallic copper nanoparticles at the soil-root interface. Environ. Sci. Technol. 2008, 42, 1766–1772. [Google Scholar] [CrossRef]
- Verma, A.; Shalu; Singh, A.; Bishnoi, N.R.; Gupta, A. Biosorption of Cu (II) using free and immobilized biomass of Penicillium citrinum. Ecol. Eng. 2013, 61, 486–490. [Google Scholar] [CrossRef]
- Bahaloo-Horeh, N.; Mousavi, S.M. Enhanced recovery of valuable metals from spent lithium-ion batteries through optimization of organic acids produced by Aspergillus niger. Waste Manag. 2017, 60, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Bahaloo-Horeh, N.; Mousavi, S.M.; Baniasadi, M. Use of adapted metal tolerant Aspergillus niger to enhance bioleaching efficiency of valuable metals from spent lithium-ion mobile phone batteries. J. Clean. Prod. 2018, 197, 1546–1557. [Google Scholar] [CrossRef]
- Heydarian, A.; Mousavi, S.M.; Vakilchap, F.; Baniasadi, M. Application of a mixed culture of adapted acidophilic bacteria in two-step bioleaching of spent lithium-ion laptop batteries. J. Power Sources 2018, 378, 19–30. [Google Scholar] [CrossRef]
- Desmarais, M.; Pirade, F.; Zhang, J.; Rene, E.R. Biohydrometallurgical processes for the recovery of precious and base metals from waste electrical and electronic equipments: Current trends and perspectives. Bioresour. Technol. Rep. 2020, 11, 100526. [Google Scholar] [CrossRef]
- Power, D.A.; Zimbro, M.J. Difco & BBL Manual: Manual of Microbiological Culture Media; Difco Laboratories, Division of Becton Dickinson and Co.: Sparks, MD, USA, 2003. [Google Scholar]
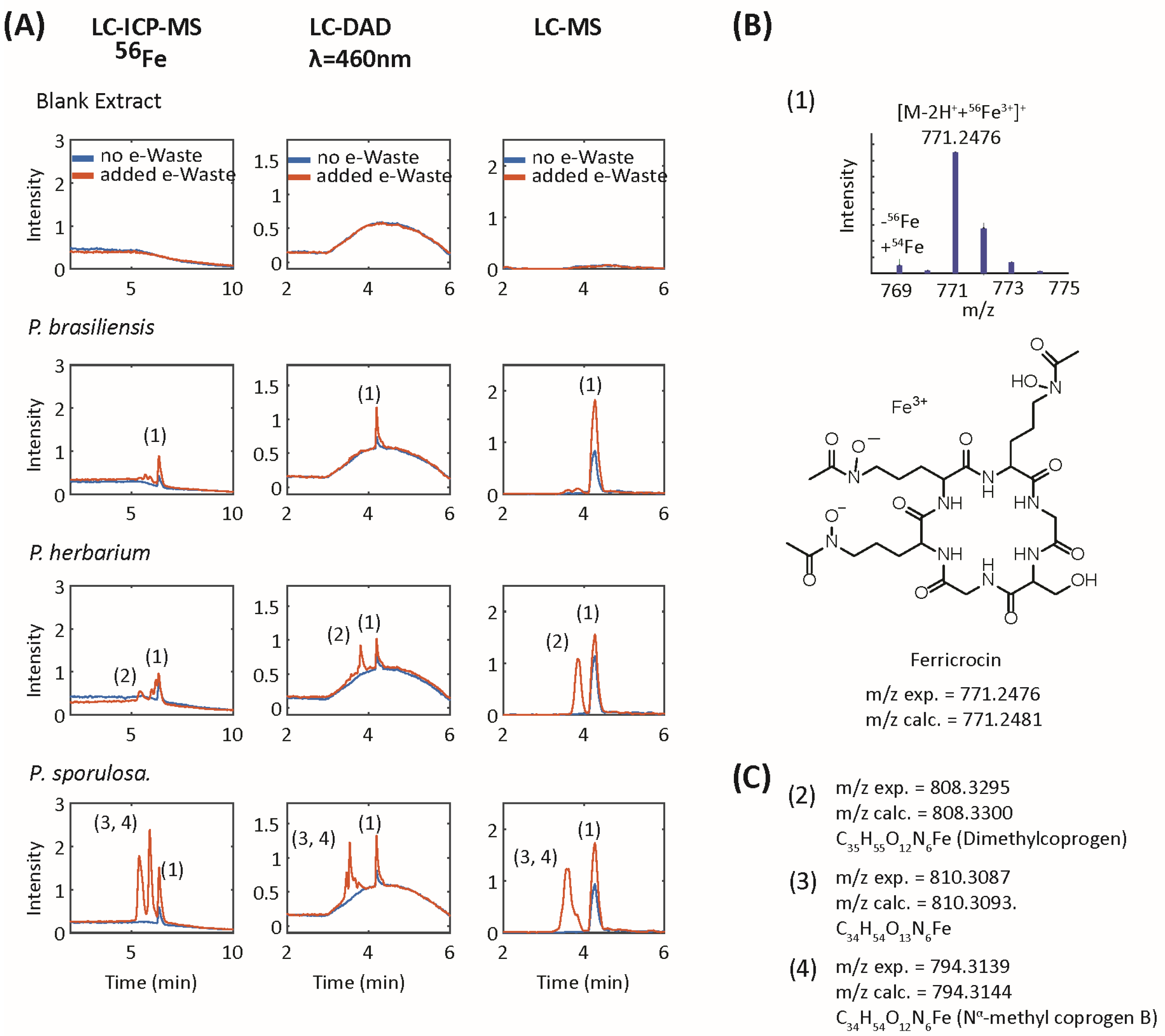
| # | m/z exp. | m/z calc. | Δppm | Formula | RT (min) | Identity | MS/MS Fragments (Intensity) | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 771.2478 | 771.2481 | 0.4 | C28H45O13N9Fe | 6.4 | Ferricrocin | 398.088 (100); 455.110 (85); 581.15 (74); 599.164 (73); 370.098 (63); 553.157 (45) | GNPS Library Spectrum CCMSLIB00005436133 [30,31,32] |
| 2 | 808.3295 | 808.33 | 0.6 | C35H55O12N6Fe | 7.9 | coprogen-type (dimethylcoprogen) | 538.171 (100) | [33] |
| 3 | 810.3087 | 810.3098 | 1.4 | C34H54O13N6Fe | 5.4 | coprogen-type (hydroxy-2-N-methylcoprogen) | 538.171 (100) | [33] |
| 4 | 794.3139 | 794.3144 | 0.6 | C34H54O12N6Fe | 5.9 | coprogen-type (2-N-methylcoprogen) | 538.171 (100) | [33] |
| Culture | Siderophore | Concentration (µM) |
|---|---|---|
| P. sporulosa + e-waste | 3 | 67.5 |
| 4 | 60.4 | |
| 1 | 31.9 | |
| P. sporulosa − e-waste | 1 | 10.8 |
| P. brasiliensis + e-waste | 1 | 18.2 |
| P. brasiliensis − e-waste | 1 | 7.1 |
| P. herbarium + e-waste | 2 1 | 7.9 34.6 |
| P. herbarium − e-waste | 1 | 13.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doydora, S.A.; Baars, O.; Cubeta, M.A.; Duckworth, O.W. Using Manganese Oxidizing Fungi to Recover Metals from Electronic Waste. Minerals 2024, 14, 111. https://doi.org/10.3390/min14010111
Doydora SA, Baars O, Cubeta MA, Duckworth OW. Using Manganese Oxidizing Fungi to Recover Metals from Electronic Waste. Minerals. 2024; 14(1):111. https://doi.org/10.3390/min14010111
Chicago/Turabian StyleDoydora, Sarah A., Oliver Baars, Marc A. Cubeta, and Owen W. Duckworth. 2024. "Using Manganese Oxidizing Fungi to Recover Metals from Electronic Waste" Minerals 14, no. 1: 111. https://doi.org/10.3390/min14010111
APA StyleDoydora, S. A., Baars, O., Cubeta, M. A., & Duckworth, O. W. (2024). Using Manganese Oxidizing Fungi to Recover Metals from Electronic Waste. Minerals, 14(1), 111. https://doi.org/10.3390/min14010111






