Improved Bond Strength Performance of Geopolymer Mortars: Role of High Volume Ground Blast Furnace Slag, Fly Ash, and Palm Oil Fuel Ash Incorporation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Physicochemical and Mineral Characteristics of Constituents
2.2. Preparation of Mixes
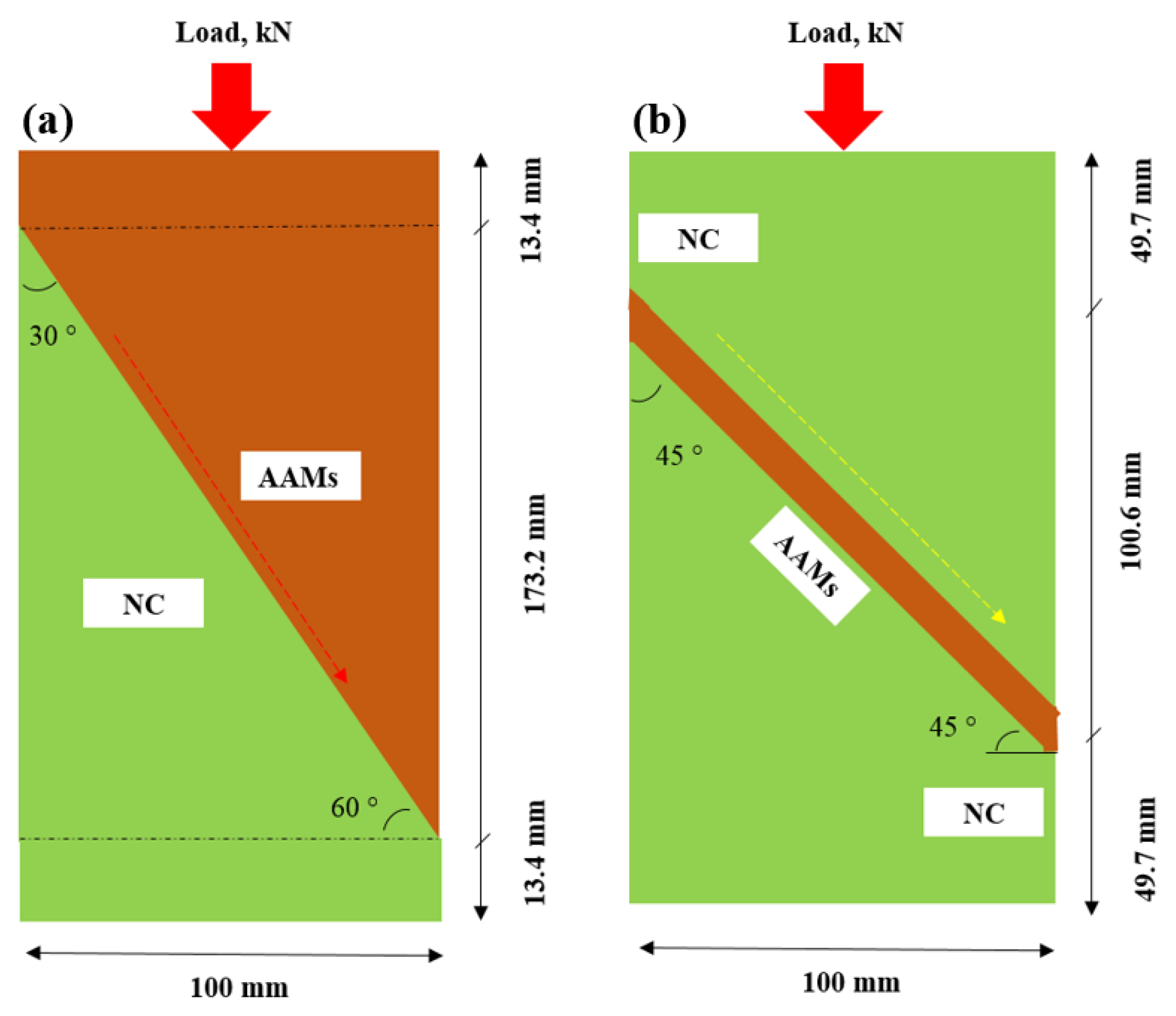
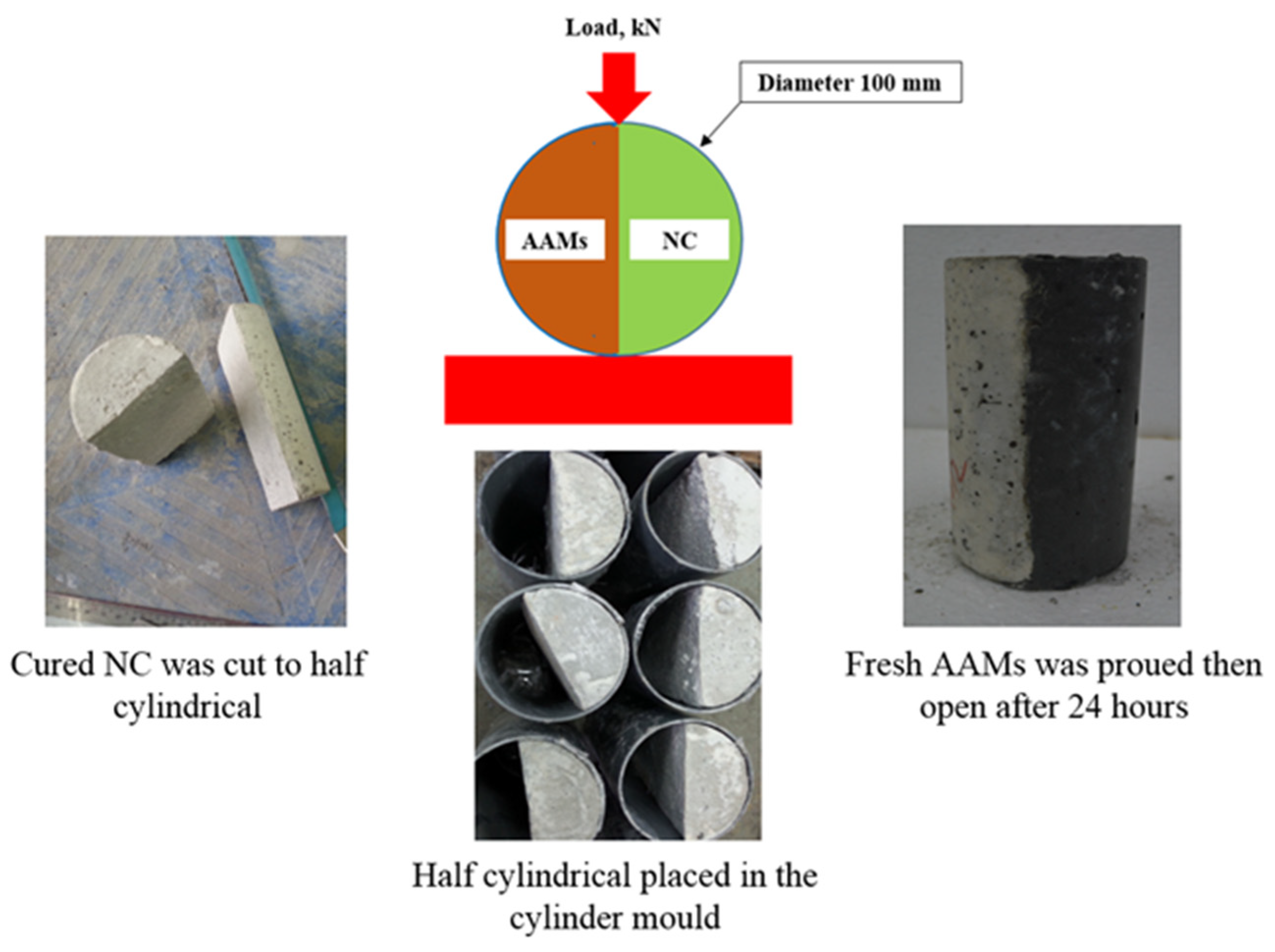
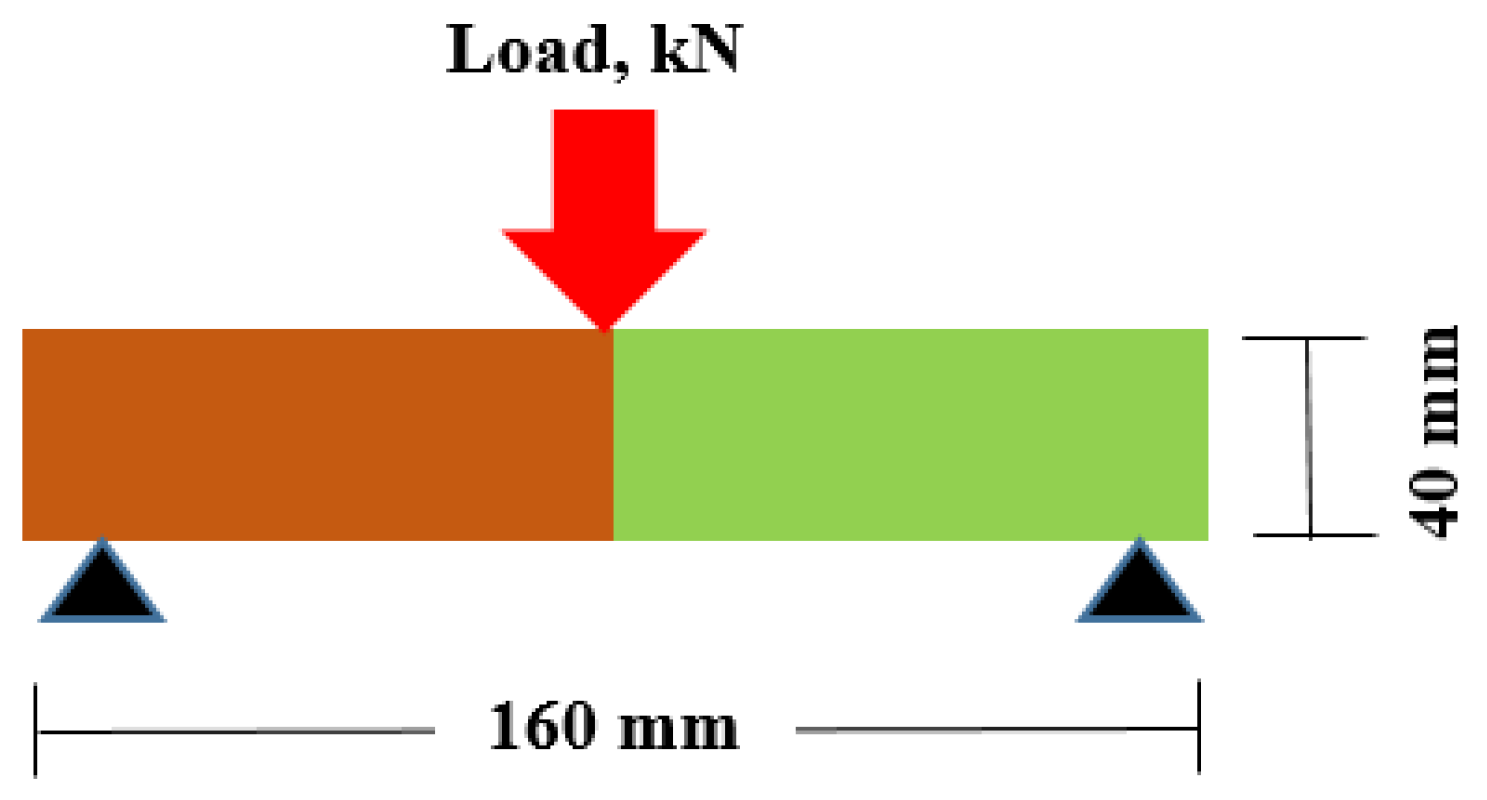
2.3. Testing Procedure
3. Results and Discussion
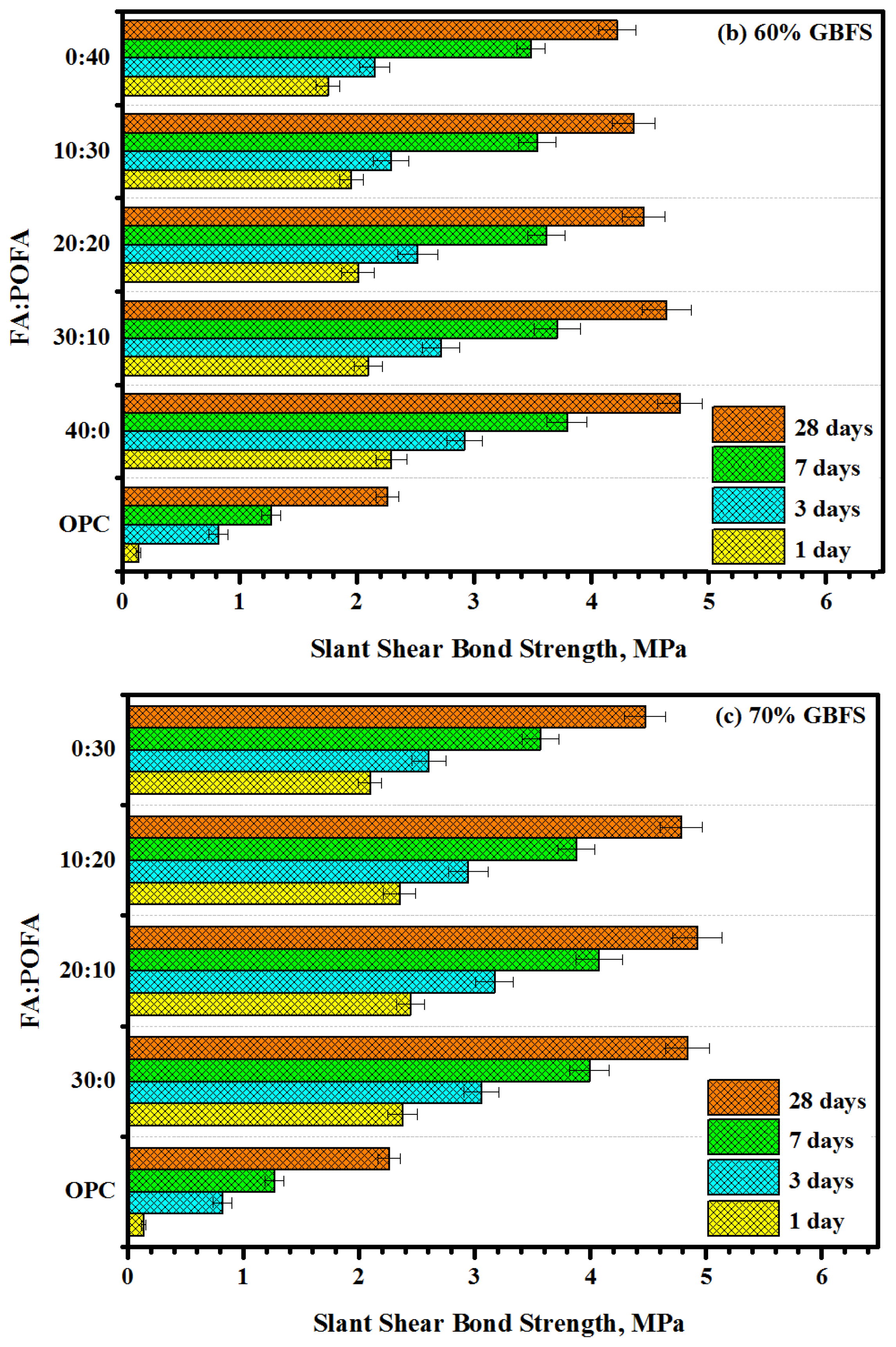
3.1. Slant Shear Bond Strength
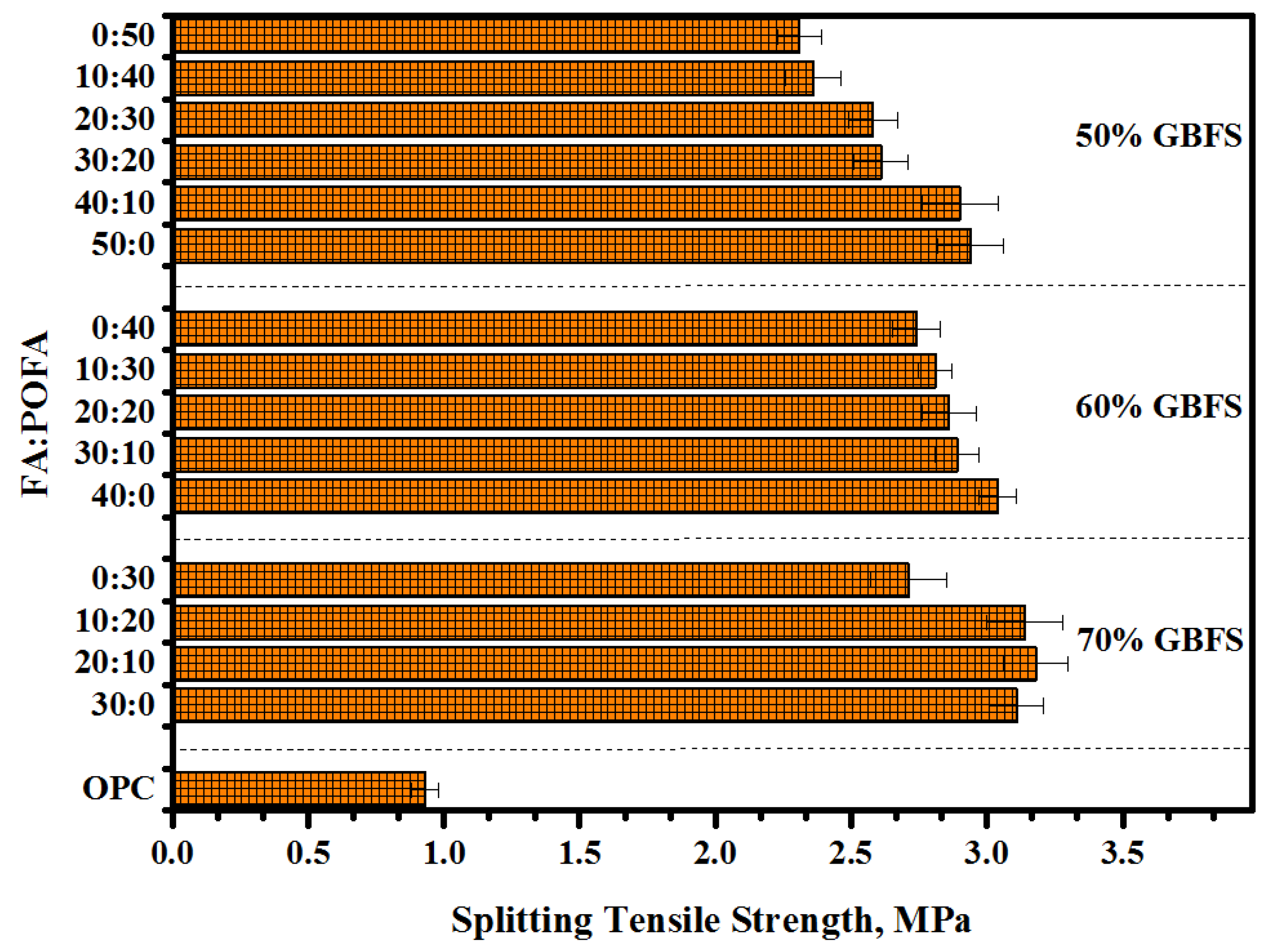
3.2. Splitting Tensile Strength (STS)
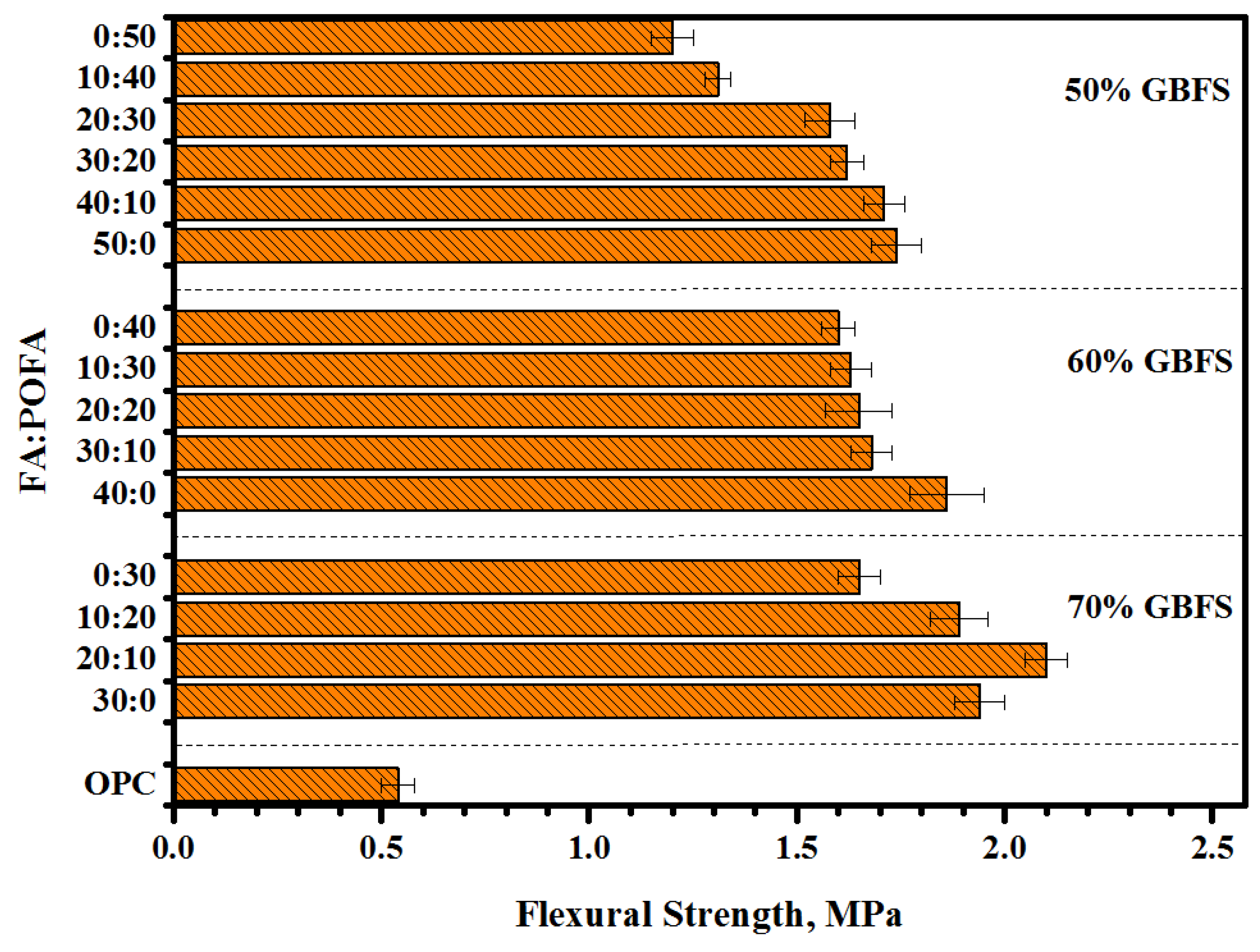
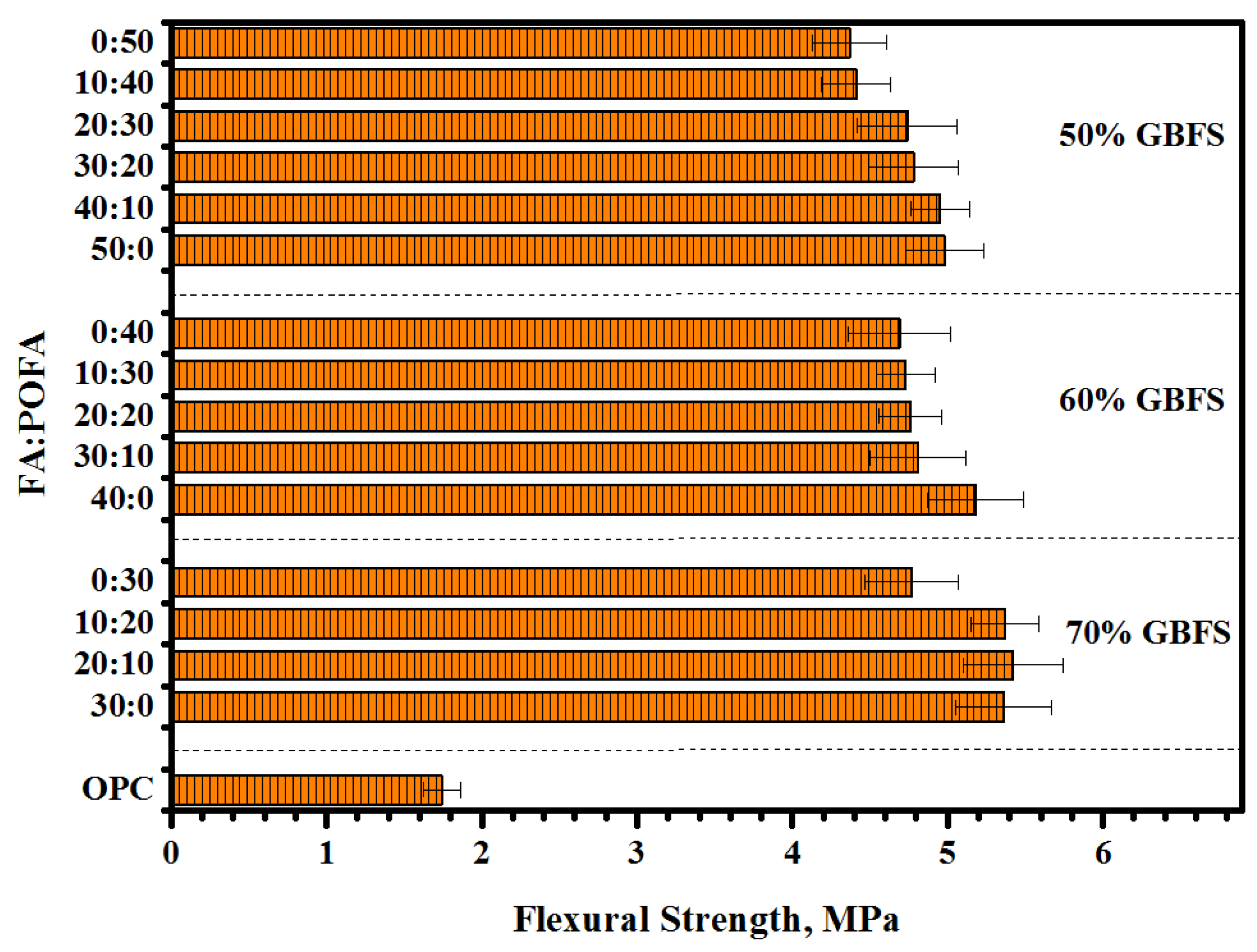
3.3. Flexural Strength (FS)
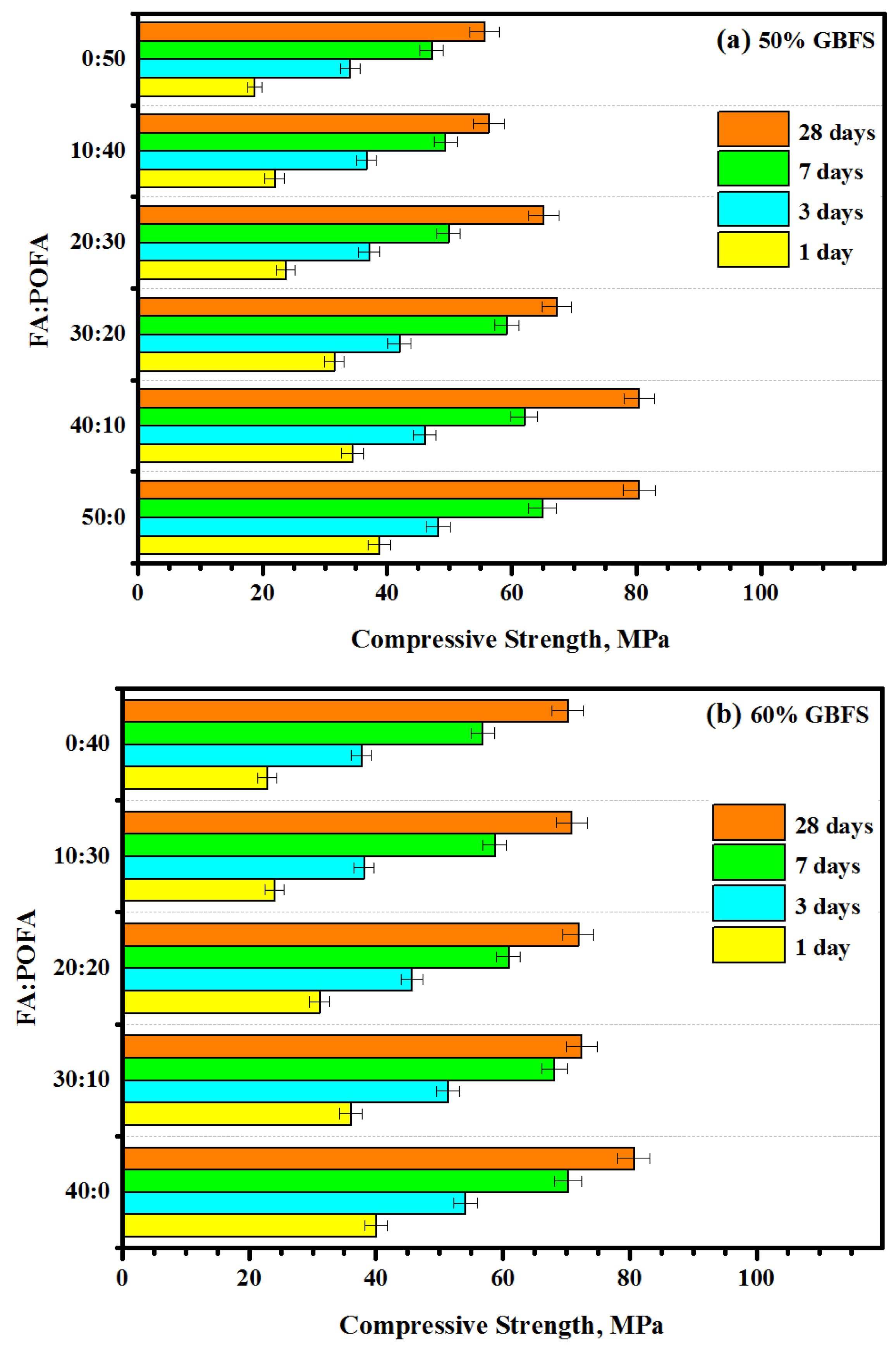
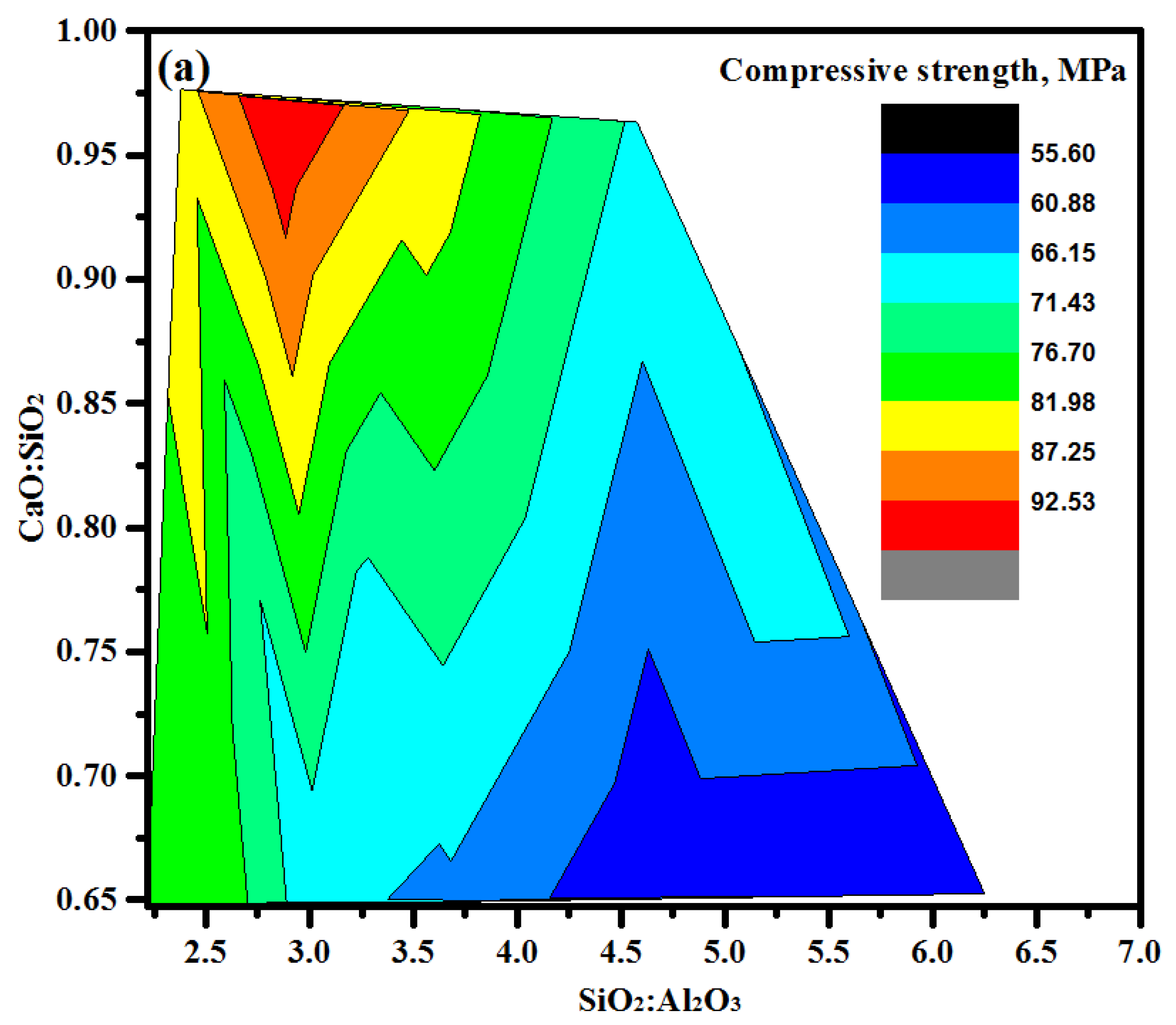
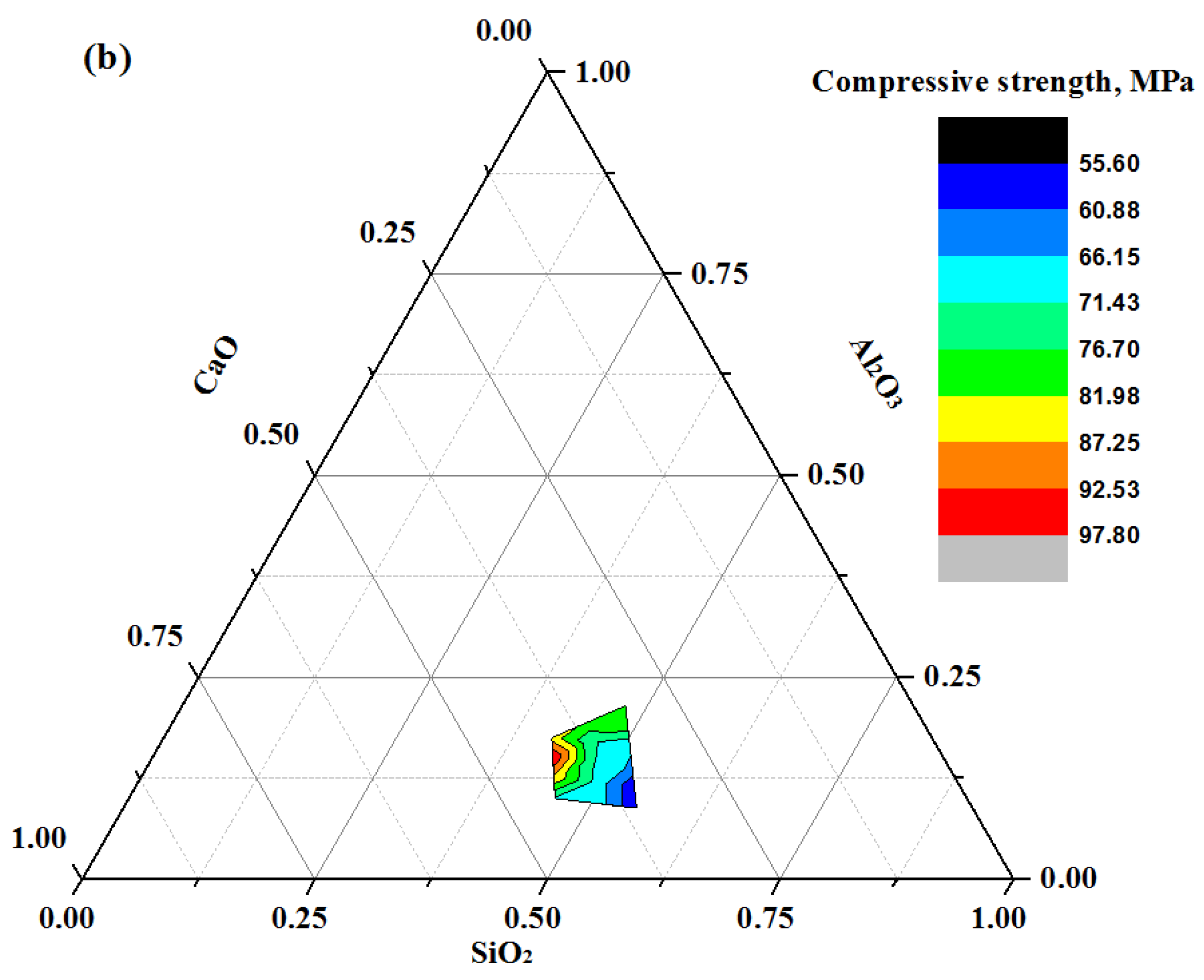
3.4. Compressive Strength (CS)
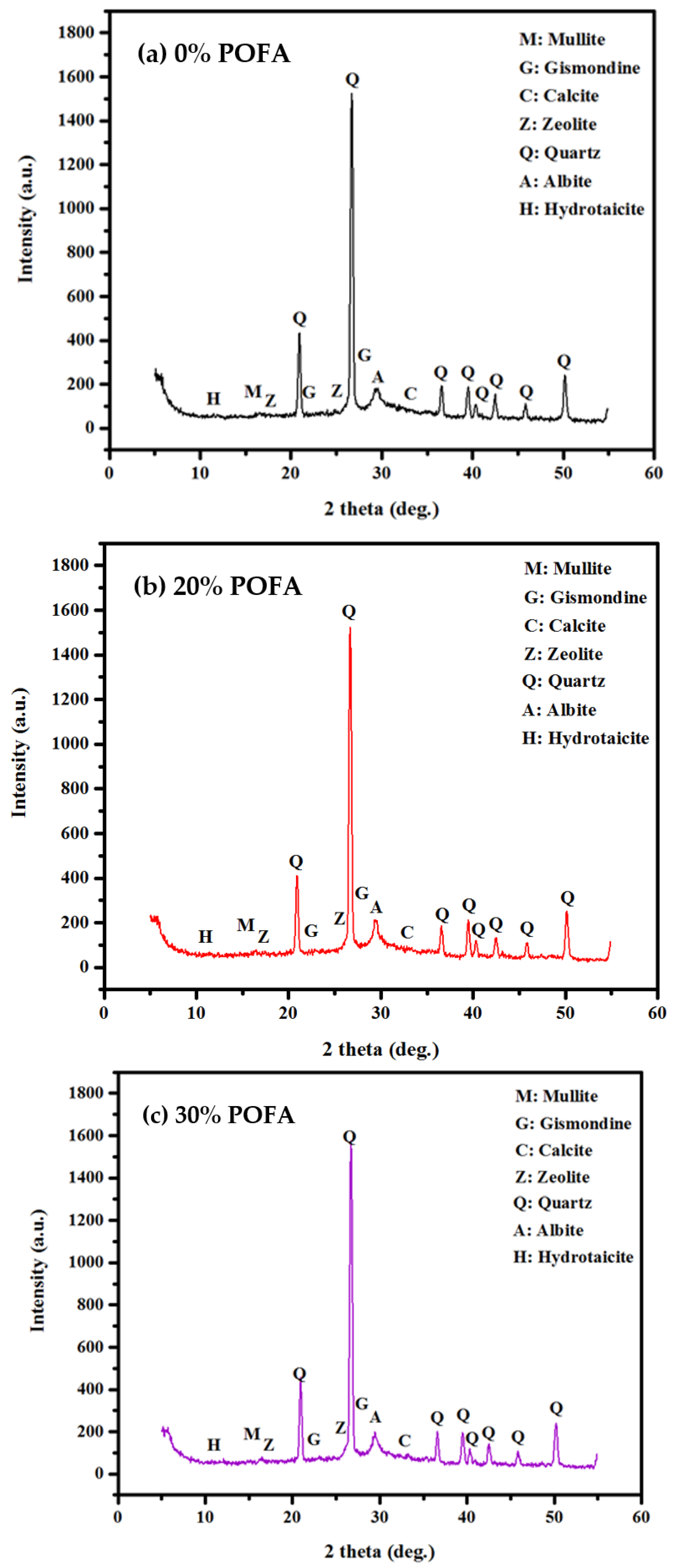
3.5. X-ray Diffraction Analysis
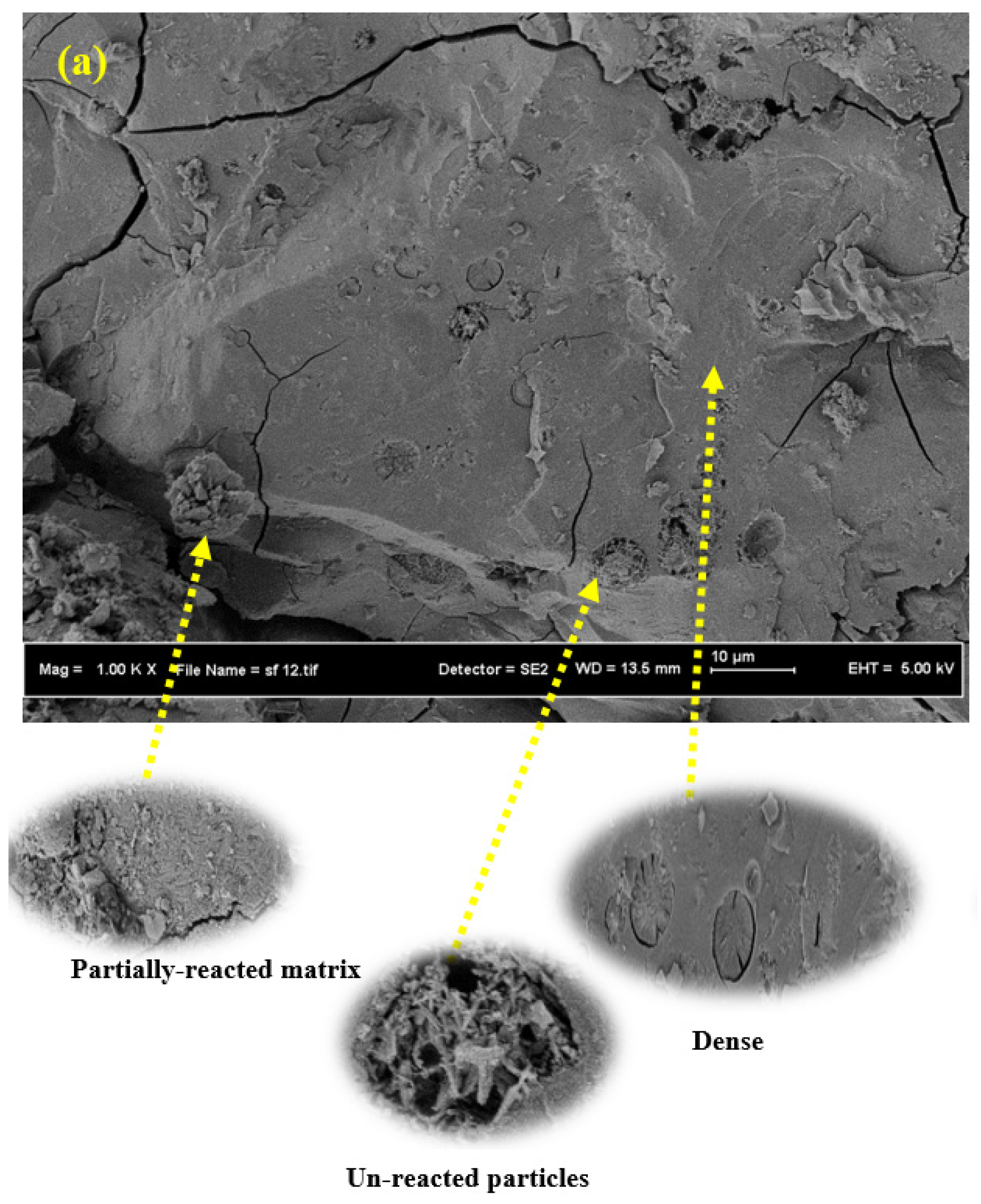
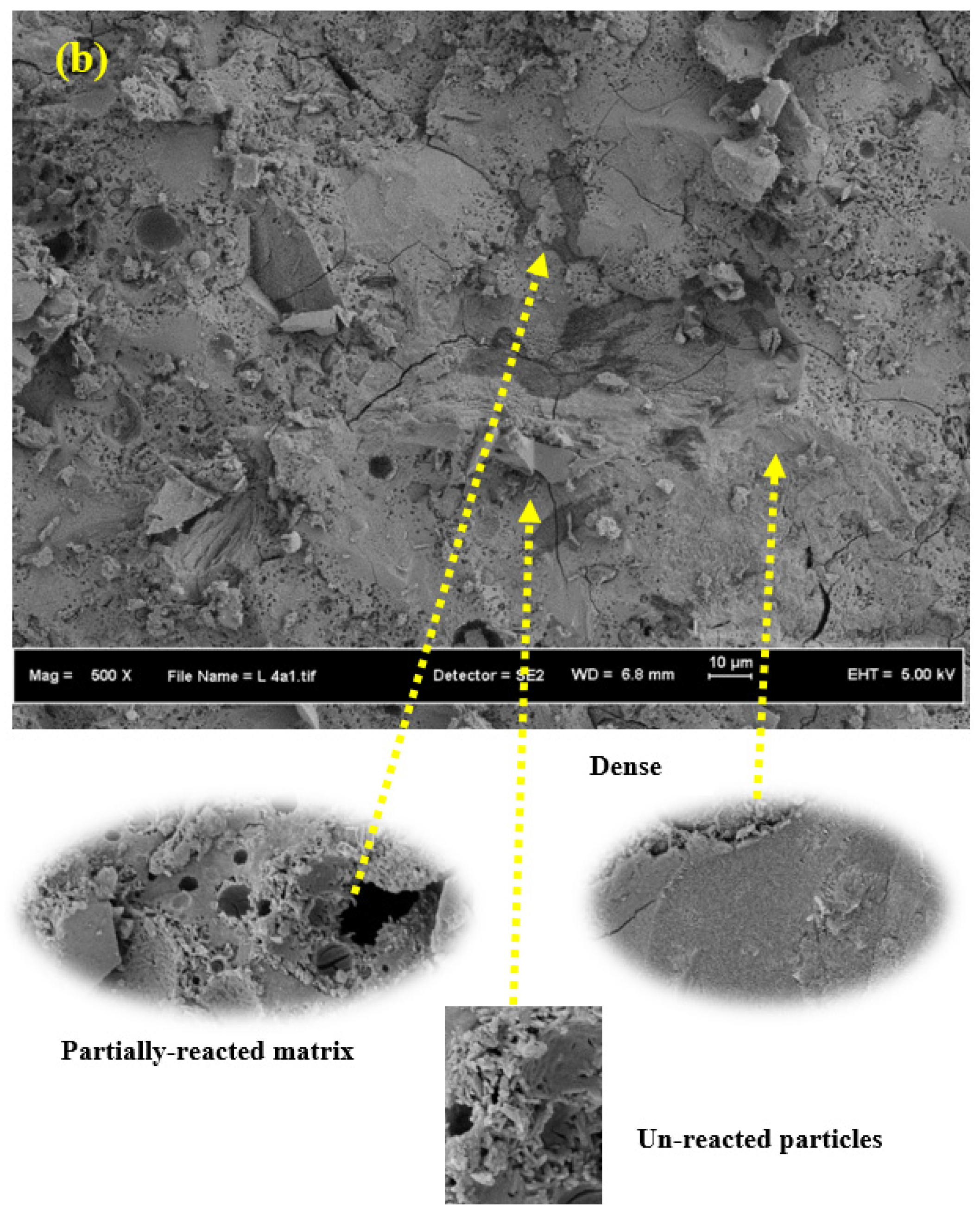
3.6. SEM Images
3.7. FTIR Analysis
4. Conclusions
- i.
- In the slant shear, splitting tensile, flexural strength, and bending stress tests, the geopolymers showed excellent performance in terms of bond strength between them and the concrete substrate.
- ii.
- Proposed mortars containing a high volume of GBFS (70%) presented the optimum value of bond strength. With increasing GBFS levels, more silicate was dissolved, thus improving the reaction rate to form more C-S-H gel.
- iii.
- Increasing the contents of POFA up to 50% in ternary-blended geopolymers reduced the bond strength more than 30%, as compared to other levels of POFA and FA contents.
- iv.
- Most geopolymer mixtures prepared with a high volume of FA presented an excellent bond strength which could be attributed to the low ratio of silica to aluminum as compared to POFA matrixes.
- v.
- The bond strength between geopolymers and the concrete substrate in critical condition (30° slant shear) presented excellent results.
- vi.
- The strength development of mortar mixes was influenced by the GBFS content, where the highest values of strength were recorded at its maximum level. At an early age, the high content (50%) of FA and POFA negatively effected the compressive strength values. The gain in compressive strength at an age of 28 days was negatively influenced with increasing contents of POFA. Most geopolymer specimens achieved a gain in the compressive strength between 81 and 94% at 28 days of curing age.
- vii.
- The results of bond strength including slant shear, splitting tensile, and flexural strength were observed to be directly proportional to the compressive strength.
- viii.
- The in-depth XRF, SEM, and FTIR analyses confirmed the achieved compressive strength improvement of geopolymers due to GBFS inclusion at low sodium hydroxide and sodium silicate molarity.
- ix.
- Based upon the mechanical test results data, a mixture containing 70% GBFS, 20% FA, and 10% of POFA is recommended as the optimum mix of repair materials.
- x.
- It is recommended to evaluate the effect of FA and POFA incorporating high volumes of GBFS on the proposed mortar’s durability performance. An evaluation of durability is the prerequisite for a broad range of applications in the construction sector.
- xi.
- In summary, there are possibilities to produce high performance geopolymer mortars using ternary blends of GBFS, FA, and POFA for several applications of repair in the construction industry.
5. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GPMs | geopolymer mortars |
| GPs | geopolymers |
| OPC | ordinary Portland cement |
| FA | fly ash |
| POFA | palm oil fuel ash |
| GBFS | ground blast furnace slag |
| ASs | aluminum silicates |
| NH | sodium hydroxide |
| NS | sodium silicate |
| NC | normal concrete |
| LOI | loss on ingnition |
| B | binders |
| A | aggregates |
| FS | flexural strength |
| STS | splitting tensile strength |
| CS | compressive strength |
| SSBS | slant shear bond strength |
References
- Huseien, G.F.; Mirza, J.; Ismail, M.; Ghoshal, S.K.; Hussein, A.A. Geopolymer mortars as sustainable repair material: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 80, 54–74. [Google Scholar] [CrossRef]
- Hamzah, H.K.; Huseien, G.F.; Asaad, M.A.; Georgescu, D.P.; Ghoshal, S.K.; Alrshoudi, F. Effect of waste glass bottles-derived nanopowder as slag replacement on mortars with alkali activation: Durability characteristics. Case Stud. Constr. Mater. 2021, 15, e00775. [Google Scholar] [CrossRef]
- Balaguru, P. Geopolymer for Protective Coating of Transportation Infrastructures; Dept. of Transportation: Trenton, NJ, USA, 1998.
- Zhang, Z.; Yao, X.; Wang, H. Potential application of geopolymers as protection coatings for marine concrete III. Field experiment. Appl. Clay Sci. 2012, 67, 57–60. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, X.; Zhu, H. Potential application of geopolymers as protection coatings for marine concrete: II. Microstructure and anticorrosion mechanism. Appl. Clay Sci. 2010, 49, 7–12. [Google Scholar] [CrossRef]
- Geissert, D.G.; Li, S.E.; Franz, G.C.; Stephens, J.E. Splitting prism test method to evaluate concrete-to-concrete bond strength. ACI Mater. J. 1999, 96, 359–366. [Google Scholar]
- Momayez, A.; Ehsani, M.R.; Ramezanianpour, A.A.; Rajaie, H. Comparison of methods for evaluating bond strength between concrete substrate and repair materials. Cem. Concr. Res. 2005, 35, 748–757. [Google Scholar] [CrossRef]
- Hardjito, D.; Wallah, S.E.; Sumajouw, D.M.J.; Rangan, B.V. Introducing fly ash-based geopolymer concrete: Manufacture and engineering properties. In Proceedings of the 30th Conference on our World in Concrete and Structures, Singapore, 23–24 August 2005. [Google Scholar]
- Nath, P.; Sarker, P.K. Use of OPC to improve setting and early strength properties of low calcium fly ash geopolymer concrete cured at room temperature. Cem. Concr. Compos. 2015, 55, 205–214. [Google Scholar] [CrossRef]
- Han, Q.; Wang, A.; Zhang, J. Research on the early fracture behavior of fly ash-based geopolymers modified by molybdenum tailings. J. Clean. Prod. 2022, 365, 132759. [Google Scholar] [CrossRef]
- Rafieizonooz, M.; Mirza, J.; Salim, M.R.; Hussin, M.W.; Khankhaje, E. Investigation of coal bottom ash and fly ash in concrete as replacement for sand and cement. Constr. Build. Mater. 2016, 116, 15–24. [Google Scholar] [CrossRef]
- Chinnu, S.; Minnu, S.N.; Bahurudeen, A.; Senthilkumar, R. Influence of palm oil fuel ash in concrete and a systematic comparison with widely accepted fly ash and slag: A step towards sustainable reuse of agro-waste ashes. Clean. Mater. 2022, 5, 100122. [Google Scholar] [CrossRef]
- Fernando, S.; Gunasekara, C.; Law, D.W.; Nasvi, M.C.M.; Setunge, S.; Dissanayake, R. Engineering properties of waste-based alkali activated concrete brick containing low calcium fly ash and rice husk ash: A comparison with traditional Portland cement concrete brick. J. Build. Eng. 2022, 46, 103810. [Google Scholar] [CrossRef]
- Perez, O.F.A.; Florez, D.R.; Vergara, L.M.Z.; Benavides, K.V.H. Innovative use of agro-waste cane bagasse ash and waste glass as cement replacement for green concrete. Cost analysis and carbon dioxide emissions. J. Clean. Prod. 2022, 379, 134822. [Google Scholar] [CrossRef]
- Huseien, G.F.; Hamzah, H.K.; Sam, A.R.M.; Khalid, N.H.A.; Shah, K.W.; Deogrescu, D.P.; Mirza, J. Alkali-activated mortars blended with glass bottle waste nano powder: Environmental benefit and sustainability. J. Clean. Prod. 2020, 243, 118636. [Google Scholar] [CrossRef]
- Mhaya, A.M.; Baharom, S.; Baghban, M.H.; Nehdi, M.L.; Faridmehr, I.; Huseien, G.F.; Algaifi, H.A.; Ismail, M. Systematic Experimental Assessment of POFA Concrete Incorporating Waste Tire Rubber Aggregate. Polymers 2022, 14, 2294. [Google Scholar] [CrossRef]
- Dobiszewska, M.; Bagcal, O.; Beycioğlu, A.; Goulias, D.; Köksal, F.; Płomiński, B.; Ürünveren, H. Utilization of rock dust as cement replacement in cement composites: An alternative approach to sustainable mortar and concrete productions. J. Build. Eng. 2023, 69, 106180. [Google Scholar] [CrossRef]
- Tunc, E.T. Recycling of marble waste: A review based on strength of concrete containing marble waste. J. Environ. Manag. 2019, 231, 86–97. [Google Scholar] [CrossRef]
- Attri, G.K.; Gupta, R.; Shrivastava, S. Sustainable precast concrete blocks incorporating recycled concrete aggregate, stone crusher, and silica dust. J. Clean. Prod. 2022, 362, 132354. [Google Scholar] [CrossRef]
- D’Angelo, G.; Fumo, M.; Merino, M.D.R.; Capasso, I.; Campanile, A.; Iucolano, F.; Caputo, D.; Liguori, B. Crushed Bricks: Demolition Waste as a Sustainable Raw Material for Geopolymers. Sustainability 2021, 13, 7572. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ismail, M.; Hussin, M.W. Influence of different curing temperatures and alkali activators on properties of GBFS geopolymer mortars containing fly ash and palm-oil fuel ash. Constr. Build. Mater. 2016, 125, 1229–1240. [Google Scholar] [CrossRef]
- Huseien, G.F.; Shah, K.W. Durability and life cycle evaluation of self-compacting concrete containing fly ash as GBFS replacement with alkali activation. Constr. Build. Mater. 2020, 235, 117458. [Google Scholar] [CrossRef]
- Awal, A.A.; Hussin, M.W. The effectiveness of palm oil fuel ash in preventing expansion due to alkali-silica reaction. Cem. Concr. Compos. 1997, 19, 367–372. [Google Scholar] [CrossRef]
- Alengaram, U.J.; Mahmud, H.; Jumaat, M. Enhancement and prediction of modulus of elasticity of palm kernel shell concrete. Mater. Des. 2011, 32, 2143–2148. [Google Scholar] [CrossRef]
- Ismail, M.; Yusuf, T.O.; Noruzman, A.H.; Hassan, I.O. Early strength characteristics of palm oil fuel ash and metakaolin blended geopolymer mortar. Adv. Mater. Res. 2013, 690, 1045–1048. [Google Scholar] [CrossRef]
- Kong, D.L.; Sanjayan, J.; Sagoe-Crentsil, K. Comparative performance of geopolymers made with metakaolin and fly ash after exposure to elevated temperatures. Cem. Concr. Res. 2007, 37, 1583–1589. [Google Scholar] [CrossRef]
- Awal, A.A.; Hussin, M.W. Effect of palm oil fuel ash in controlling heat of hydration of concrete. Procedia Eng. 2011, 14, 2650–2657. [Google Scholar] [CrossRef]
- Ranjbar, N.; Mehrali, M.; Alengaram, U.J.; Metselaar, H.S.C.; Jumaat, M.Z. Compressive strength and microstructural analysis of fly ash/palm oil fuel ash based geopolymer mortar under elevated temperatures. Constr. Build. Mater. 2014, 65, 114–121. [Google Scholar] [CrossRef]
- Ranjbar, N.; Mehrali, M.; Behnia, A.; Alengaram, U.J.; Jumaat, M.Z. Compressive strength and microstructural analysis of fly ash/palm oil fuel ash based geopolymer mortar. Mater. Des. 2014, 59, 532–539. [Google Scholar] [CrossRef]
- Mijarsh, M.; Johari, M.; Ahmad, Z. Compressive strength of treated palm oil fuel ash based geopolymer mortar containing calcium hydroxide, aluminum hydroxide and silica fume as mineral additives. Cem. Concr. Compos. 2015, 60, 65–81. [Google Scholar] [CrossRef]
- Ye, H.; Cartwright, C.; Rajabipour, F.; Radlińska, A. Understanding the drying shrinkage performance of alkali-activated slag mortars. Cem. Concr. Compos. 2017, 76, 13–24. [Google Scholar] [CrossRef]
- Prasittisopin, L.; Trejo, D. Effects of mixing time and revolution count on characteristics of blended cement containing rice husk ash. J. Mater. Civ. Eng. 2018, 30, 04017262. [Google Scholar] [CrossRef]
- Amer, I.; Kohail, M.; El-Feky, M.S.; Rashad, A.; Khalaf, M.A. Characterization of alkali-activated hybrid slag/cement concrete. Ain Shams Eng. J. 2021, 12, 135–144. [Google Scholar] [CrossRef]
- Aboulayt, A.; Souayfan, F.; Roziere, E.; Jaafri, R.; El Idrissi, A.C.; Moussa, R.; Justino, C.; Loukili, A. Alkali-activated grouts based on slag-fly ash mixtures: From early-age characterization to long-term phase composition. Constr. Build. Mater. 2020, 260, 120510. [Google Scholar] [CrossRef]
- Prasittisopin, L.; Trejo, D. Characterization of chemical treatment method for rice husk ash cementing materials. Spec. Publ. 2013, 294, 1–14. [Google Scholar]
- Phoo-ngernkham, T.; Sata, V.; Hanjitsuwan, S.; Ridtirud, C.; Hatanaka, S.; Chindaprasirt, P. High calcium fly ash geopolymer mortar containing Portland cement for use as repair material. Constr. Build. Mater. 2015, 98, 482–488. [Google Scholar] [CrossRef]
- Al-Majidi, M.H.; Lampropoulos, A.; Cundy, A.; Meikle, S. Development of geopolymer mortar under ambient temperature for in situ applications. Constr. Build. Mater. 2016, 120, 198–211. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.; Van Deventer, J. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D.E. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Mirza, J.; Durand, B.; Bhutta, A.R.; Tahir, M.M. Preferred test methods to select suitable surface repair materials in severe climates. Constr. Build. Mater. 2014, 50, 692–698. [Google Scholar] [CrossRef]
- Hu, S.; Wang, H.; Zhang, G.; Ding, Q. Bonding and abrasion resistance of geopolymeric repair material made with steel slag. Cem. Concr. Compos. 2008, 30, 239–244. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Adhesion characterization of tungsten mine waste geopolymeric binder. Influence of OPC concrete substrate surface treatment. Constr. Build. Mater. 2008, 22, 154–161. [Google Scholar] [CrossRef]
- Songpiriyakij, S.; Pulngern, T.; Pungpremtrakul, P.; Jaturapitakkul, C. Anchorage of steel bars in concrete by geopolymer paste. Mater. Des. 2011, 32, 3021–3028. [Google Scholar] [CrossRef]
- Suksiripattanapong, C.; Horpibulsuk, S.; Chanprasert, P.; Sukmak, P.; Arulrajah, A. Compressive strength development in fly ash geopolymer masonry units manufactured from water treatment sludge. Constr. Build. Mater. 2015, 82, 20–30. [Google Scholar] [CrossRef]
- Phoo-ngernkham, T.; Maegawa, A.; Mishima, N.; Hatanaka, S.; Chindaprasirt, P. Effects of sodium hydroxide and sodium silicate solutions on compressive and shear bond strengths of FA–GBFS geopolymer. Constr. Build. Mater. 2015, 91, 1–8. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Kodur, V.; Qi, S.L.; Wu, B. Characterizing the bond strength of geopolymers at ambient and elevated temperatures. Cem. Concr. Compos. 2015, 58, 40–49. [Google Scholar] [CrossRef]
- Hawa, A.; Tonnayopas, D.; Prachasaree, W.; Taneerananon, P. Development and performance evaluation of very high early strength geopolymer for rapid road repair. Adv. Mater. Sci. Eng. 2013, 2013, 764180. [Google Scholar] [CrossRef]
- Mhaya, A.M.; Shahidan, S.; Zuki, S.S.; Huseien, G.F.; Azmi, M.A.; Ismail, M.; Mirza, J. Durability and Acoustic Performance of Rubberized Concrete Containing POFA as Cement Replacement. Sustainability. 2022, 14, 15510. [Google Scholar] [CrossRef]
- Huseien, G.F.; Khamehchi, M.; Kubba, Z.; Benjeddou, O.; Mahmoodi, M.J. Freeze-thaw cycle and abrasion resistance of alkali-activated FA and POFA-based mortars: Role of high volume GBFS incorporation. Heliyon 2023, 9, e17672. [Google Scholar] [CrossRef]
- Huseien, G.F.; Shah, K.W. Performance evaluation of alkali-activated mortars containing industrial wastes as surface repair materials. J. Build. Eng. 2020, 30, 101234. [Google Scholar] [CrossRef]
- Buchwald, A.; Hilbig, H.; Kaps, C. Alkali-activated metakaolin-slag blends—Performance and structure in dependence of their composition. J. Mater. Sci. 2007, 42, 3024–3032. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Investigations on mix design of tungsten mine waste geopolymeric binder. Constr. Build. Mater. 2008, 22, 1939–1949. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Mehrotra, S. Influence of granulated blast furnace slag on the reaction, structure and properties of fly ash based geopolymer. J. Mater. Sci. 2010, 45, 607–615. [Google Scholar] [CrossRef]
- Shen, W.; Wang, Y.; Zhang, T.; Zhou, M.; Li, J.; Cui, X. Magnesia modification of alkali-activated slag fly ash cement. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2011, 26, 121–125. [Google Scholar] [CrossRef]
- Yu, R.; Spiesz, P.; Brouwers, H. Development of an eco-friendly Ultra-High Performance Concrete (UHPC) with efficient cement and mineral admixtures uses. Cem. Concr. Compos. 2015, 55, 383–394. [Google Scholar] [CrossRef]
- Nath, S.; Kumar, S. Influence of iron making slags on strength and microstructure of fly ash geopolymer. Constr. Build. Mater. 2013, 38, 924–930. [Google Scholar] [CrossRef]
- Khater, H. Effect of calcium on geopolymerization of aluminosilicate wastes. J. Mater. Civ. Eng. 2011, 24, 92–101. [Google Scholar] [CrossRef]
- Richardson, I.; Brough, A.R.; Groves, G.W.; Dobson, C.M. The characterization of hardened alkali-activated blast-furnace slag pastes and the nature of the calcium silicate hydrate (C-S-H) phase. Cem. Concr. Res. 1994, 24, 813–829. [Google Scholar] [CrossRef]
- Myers, R.J.; Bernal, S.A.; San Nicolas, R.; Provis, J.L. Generalized structural description of calcium–sodium aluminosilicate hydrate gels: The cross-linked substituted tobermorite model. Langmuir 2013, 29, 5294–5306. [Google Scholar] [CrossRef]
- Li, Z.; Liu, S. Influence of slag as additive on compressive strength of fly ash-based geopolymer. J. Mater. Civ. Eng. 2007, 19, 470–474. [Google Scholar] [CrossRef]
- Kabir, S.; Alengaram, U.J.; Jumaat, M.Z.; Sharmin, A.; Islam, A. Influence of molarity and chemical composition on the development of compressive strength in POFA based geopolymer mortar. Adv. Mater. Sci. Eng. 2015, 2015, 647071. [Google Scholar] [CrossRef]
- Islam, A.; Alengaram, U.J.; Jumaat, M.Z.; Bashar, I.I.; Kabir, S.A. Engineering properties and carbon footprint of ground granulated blast-furnace slag-palm oil fuel ash-based structural geopolymer concrete. Constr. Build. Mater. 2015, 101, 503–521. [Google Scholar] [CrossRef]
- Ariffin, M.; Hussin, M.W.; Rafique Bhutta, M.A. Mix design and compressive strength of geopolymer concrete containing blended ash from agro-industrial wastes. In Advanced Materials Research; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2011; Volume 339, pp. 452–457. [Google Scholar]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; De Silva, P.; Sagoe-Crentsil, K.; Hanjitsuwan, S. Effect of SiO2 and Al2O3 on the setting and hardening of high calcium fly ash-based geopolymer systems. J. Mater. Sci. 2012, 47, 4876–4883. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater. 2005, 86, 207–214. [Google Scholar] [CrossRef]
- Huseien, G.F.; Faridmehr, I.; Nehdi, M.L.; Abadel, A.A.; Aiken, T.A.; Ghoshal, S.K. Structure, morphology and compressive strength of Alkali-activated mortars containing waste bottle glass nanoparticles. Constr. Build. Mater. 2022, 342, 128005. [Google Scholar] [CrossRef]



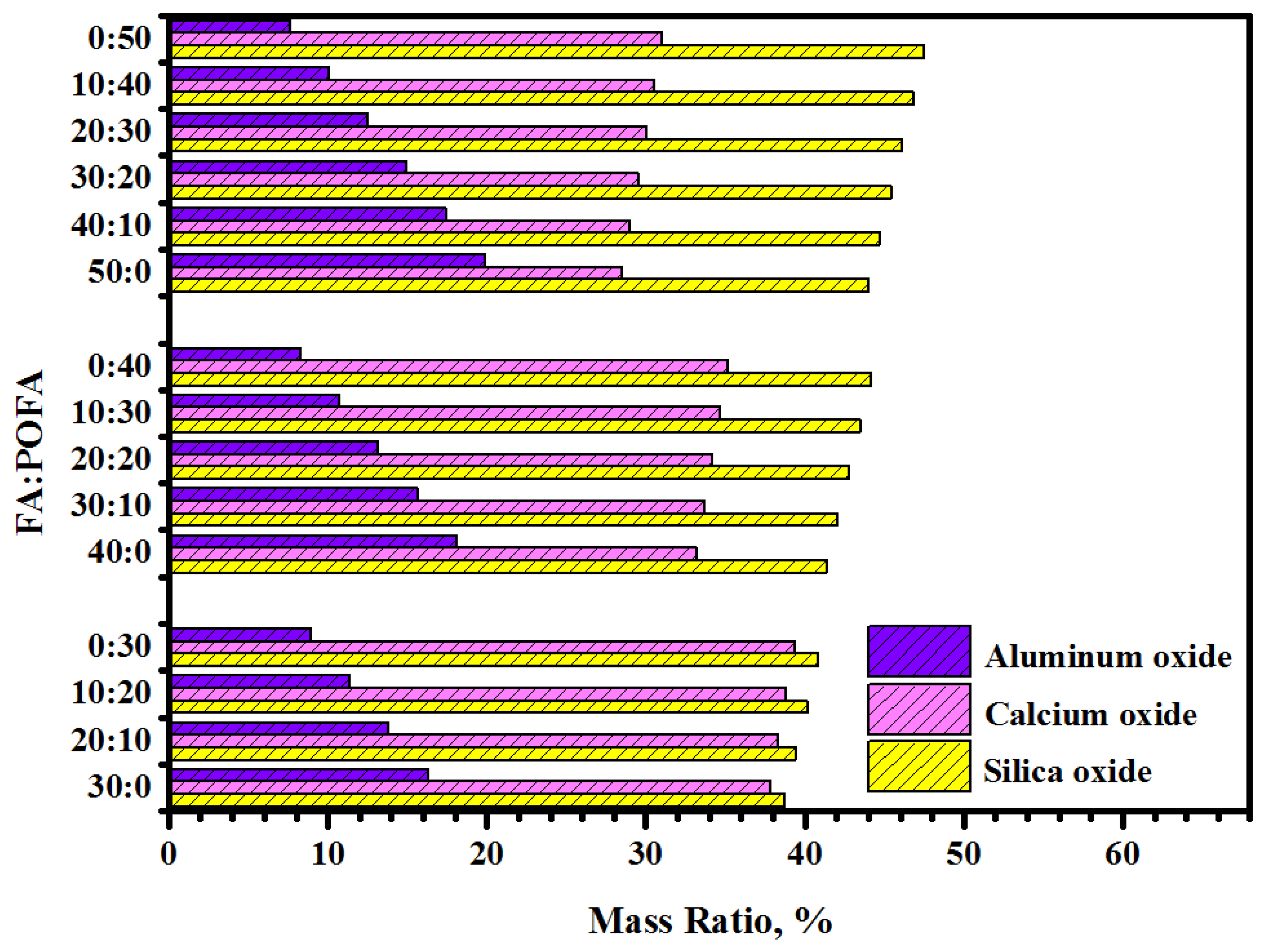





| Materials | Chemical Composition | Physical Properties | ||||
|---|---|---|---|---|---|---|
| Element | wt.% | Total | ||||
| POFA | Main elements | SiO2 | 64.3 | 96.9 | Color | Dark grey |
| CaO | 10.4 | Particle size (µm) | 8.2 | |||
| K2O | 8.8 | |||||
| MgO | 5.9 | Specific surface area (m2/g) | 23.1 | |||
| Al2O3 | 4.3 | |||||
| Fe2O3 | 3.2 | |||||
| Loss on ignition (LOI) | 1.8 | 1.8 | Specific gravity | 1.96 | ||
| Others (Na2O, SO3, etc.) | 1.3 | 1.3 | ||||
| FA | Main elements | SiO2 | 57.3 | 98.3 | Color | Grey |
| Al2O3 | 29.1 | Particle size (µm) | 10 | |||
| CaO | 5.3 | |||||
| Fe2O3 | 3.8 | Specific surface area (m2/g) | 18.2 | |||
| MgO | 1.6 | |||||
| K2O | 1.2 | |||||
| Loss on ignition (LOI) | 0.1 | 0.1 | Specific gravity | 2.2 | ||
| Others (Na2O, SO3, etc.) | 1.6 | 1.6 | ||||
| GBFS | Main elements | CaO | 51.8 | 99.3 | Color | Off-whit |
| SiO2 | 30.8 | Particle size (µm) | 12.8 | |||
| Al2O3 | 10.9 | |||||
| MgO | 4.6 | Specific surface area (m2/g) | 13.6 | |||
| Fe2O3 | 0.7 | |||||
| Na2O | 0.5 | |||||
| Loss on ignition (LOI) | 0.2 | 0.2 | Specific gravity | 2.89 | ||
| Others (K2O, SO3, etc.) | 0.5 | 0.5 | ||||
| Group | Mix | Alkali-Activated Binders and Ratios | |||||
|---|---|---|---|---|---|---|---|
| GBFS | FA | POFA | SiO2:Al2O3 | CaO:SiO2 | CaO:Al2O3 | ||
| A | S1 | 50 | 50 | 0 | 2.22 | 0.65 | 1.43 |
| S2 | 40 | 10 | 2.57 | 0.65 | 1.66 | ||
| S3 | 30 | 20 | 3.04 | 0.65 | 1.97 | ||
| S4 | 20 | 30 | 3.68 | 0.65 | 2.39 | ||
| S5 | 10 | 40 | 4.65 | 0.65 | 3.03 | ||
| S6 | 0 | 50 | 6.25 | 0.65 | 4.07 | ||
| B | S7 | 60 | 40 | 0 | 2.29 | 0.80 | 1.83 |
| S8 | 30 | 10 | 2.69 | 0.80 | 2.15 | ||
| S9 | 20 | 20 | 3.25 | 0.79 | 2.59 | ||
| S10 | 10 | 30 | 4.06 | 0.79 | 3.23 | ||
| S11 | 0 | 40 | 5.35 | 0.79 | 4.25 | ||
| C | S12 | 70 | 30 | 0 | 2.38 | 0.97 | 2.32 |
| S13 | 20 | 10 | 2.85 | 0.97 | 2.77 | ||
| S14 | 10 | 20 | 3.53 | 0.96 | 3.41 | ||
| S15 | 0 | 30 | 4.57 | 0.96 | 4.41 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huseien, G.F.; Hussein, Z.J.; Kubba, Z.; Mikhail Nikolaevich, B.; Mirza, J. Improved Bond Strength Performance of Geopolymer Mortars: Role of High Volume Ground Blast Furnace Slag, Fly Ash, and Palm Oil Fuel Ash Incorporation. Minerals 2023, 13, 1096. https://doi.org/10.3390/min13081096
Huseien GF, Hussein ZJ, Kubba Z, Mikhail Nikolaevich B, Mirza J. Improved Bond Strength Performance of Geopolymer Mortars: Role of High Volume Ground Blast Furnace Slag, Fly Ash, and Palm Oil Fuel Ash Incorporation. Minerals. 2023; 13(8):1096. https://doi.org/10.3390/min13081096
Chicago/Turabian StyleHuseien, Ghasan Fahim, Zahraa J. Hussein, Ziyad Kubba, Bryukhov Mikhail Nikolaevich, and Jahangir Mirza. 2023. "Improved Bond Strength Performance of Geopolymer Mortars: Role of High Volume Ground Blast Furnace Slag, Fly Ash, and Palm Oil Fuel Ash Incorporation" Minerals 13, no. 8: 1096. https://doi.org/10.3390/min13081096
APA StyleHuseien, G. F., Hussein, Z. J., Kubba, Z., Mikhail Nikolaevich, B., & Mirza, J. (2023). Improved Bond Strength Performance of Geopolymer Mortars: Role of High Volume Ground Blast Furnace Slag, Fly Ash, and Palm Oil Fuel Ash Incorporation. Minerals, 13(8), 1096. https://doi.org/10.3390/min13081096











