Structural and Chemical Diversity and Complexity of Sulfur Minerals
Abstract
1. Introduction
2. Materials and Methods
2.1. Mineral Systems
2.2. Chemical and Structural Complexities
3. Results and Discussion
3.1. Distribution of S Minerals in Accordance with the Number of Species-Defining Elements
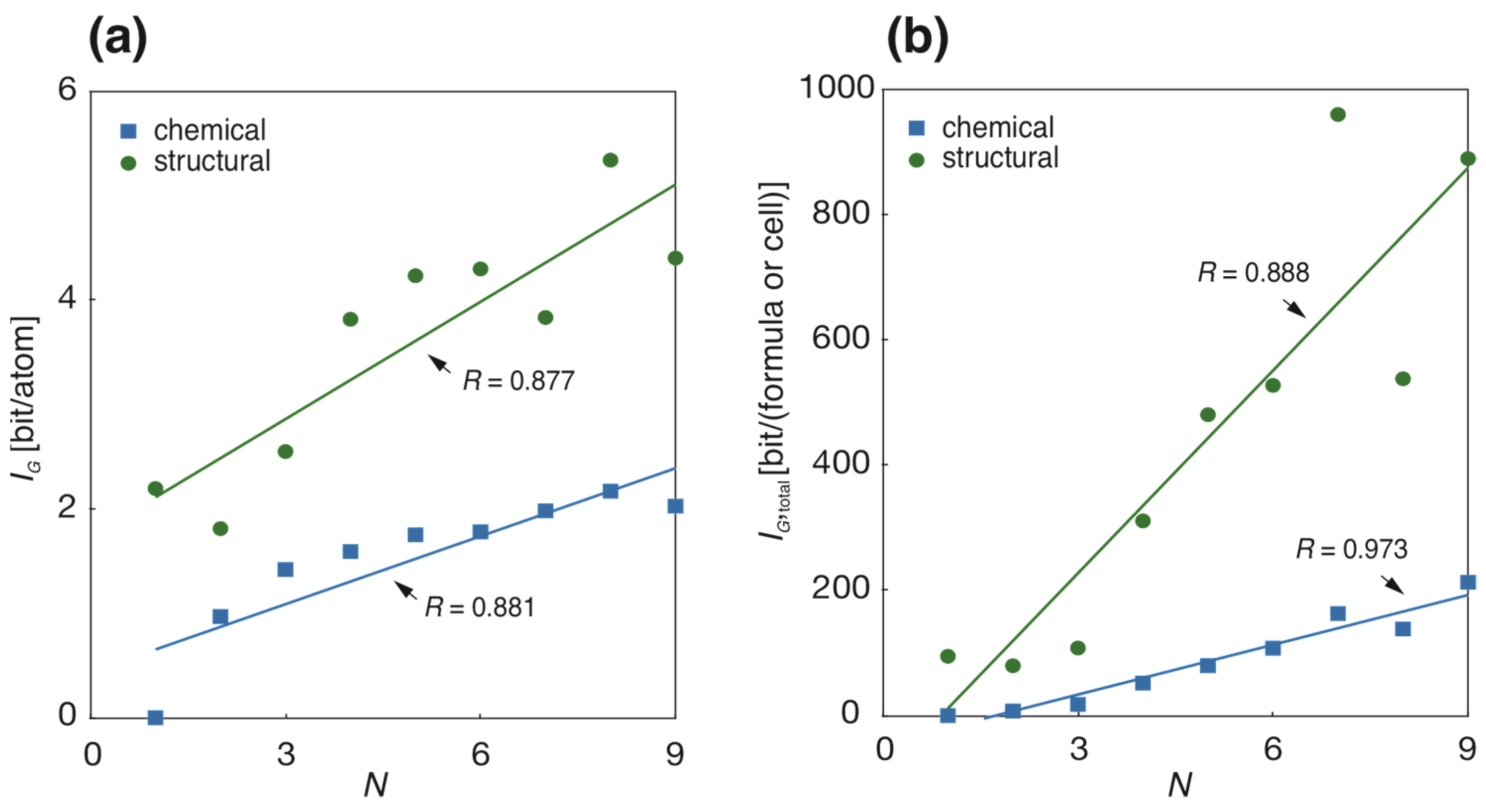
3.2. Chemical and Structural Complexities of Sulfur Minerals
3.3. Structural Complexity of Sulfur Minerals: Statistics and Mechanisms
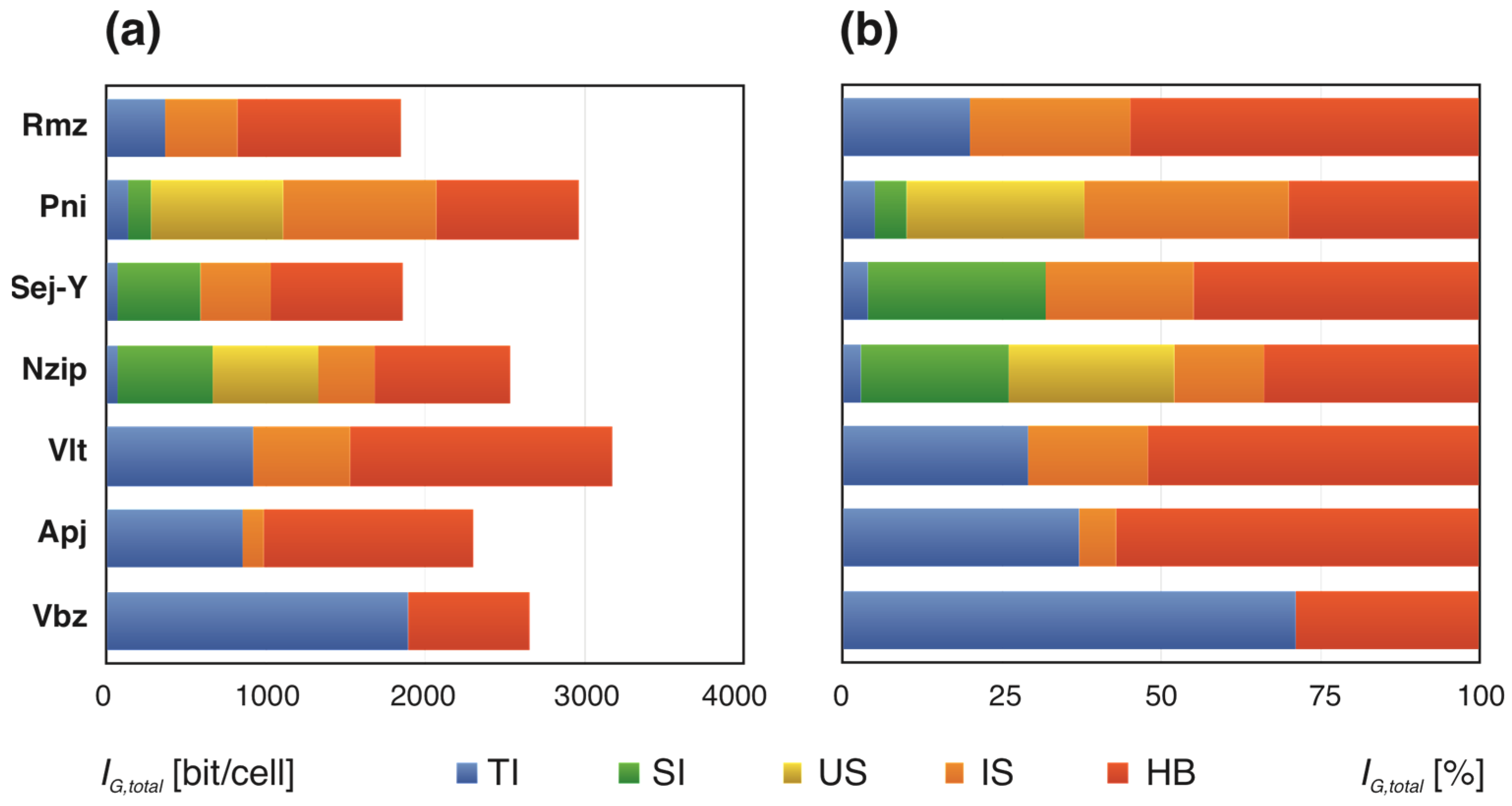
3.3.1. Sulfides and Sulfosalts
| Mineral Name | Chemical Formula | Space Group | v [atoms] | strIG [bit/atom] | strIG,total [bit/cell] | Ref. |
|---|---|---|---|---|---|---|
| Incomsartorite | Tl6Pb144As246S516 | P21/n | 924 | 7.852 | 7255.016 | [78] |
| Chovanite 8 Å | Pb15−2xSb14+2xS36Ox (x ~0.2) | P21/c | 524 | 7.033 | 3685.514 | [79,80] |
| Meerschautite | Ag12Pb84Sb90S224O | P21 | 414 | 7.693 | 3185.104 | [81] |
| Argentoliveingite | Ag3+xPb36−2xAs51+xS112 | P | 400 | 7.644 | 3057.542 | [82] |
| Parasterryite | Ag2Pb10Sb7As5S29 | P21/c | 424 | 6.728 | 2852.638 | [83] |
| Sterryite | CuAg3Pb19Sb22(As)2S56 | P21/n | 424 | 6.728 | 2852.638 | [83] |
| Djurleite | Cu31S16 | P21/c | 376 | 6.555 | 2464.525 | [70] |
| Hendekasartorite | Tl2Pb48As82S172 | P21/c | 304 | 6.248 | 1899.370 | [84] |
| Dalnegroite | Tl4Pb2As20S34 | P1 | 240 | 7.907 | 1897.654 | [85,86] |
| Cannizzarite | Pb8Bi10S33 | P21/m | 236 | 6.883 | 1624.304 | [87,88] |
3.3.2. Sulfates
| Mineral Name | Chemical Formula | Space Group | v [at.] | strIG [bit/atom] | strIG,total [bit/cell] | Ref. |
|---|---|---|---|---|---|---|
| Fantappièite | (Na82.5Ca33K16.5)[Al99Si99O396](SO4)33(H2O)6 | 821 | 7.245 | 5948.330 | [101] | |
| Sacrofanite | Na61K19Ca32[Si84Al84O336](SO4)26Cl2F6. 2H2O | 2c | 834 | 6.376 | 5317.353 | [102] |
| Voltaite | K2Fe5Fe4Al(SO4)12∙18H2O | c | 764 | 4.160 | 3177.944 | [103] |
| Putnisite | SrCa4Cr8(CO3)8(SO4)(OH)16·25H2O | Pnma | 488 | 6.078 | 2966.200 | [104] |
| Giuseppettite | Na42K16Ca6(Si48Al48O192)(SO4)10Cl2(H2O)5 | P31c | 430 | 6.333 | 2723.097 | [105] |
| Vonbezingite | Ca6Cu3(SO4)3(OH)12∙2H2O | P21/c | 400 | 6.644 | 2657.542 | [106] |
| Natrozippeite | Na5(UO2)8(SO4)4O5(OH)3∙12H2O | P21/n | 384 | 6.585 | 2528.626 | [107] |
| Apjohnite | MnAl2(SO4)4∙22H2O | P21/c | 356 | 6.476 | 2305.361 | [108] |
| Sejkoraite-(Y) | Y(UO2)4O3(SO4)2(OH)·13H2O | 264 | 7.044 | 1859.720 | [109] | |
| Ramazzoite | [Mg8Cu12(PO4)(CO3)4(OH)24] (HSO4)(SO4)2·56H2O | 3m | 400 | 4.620 | 1848.162 | [110] |
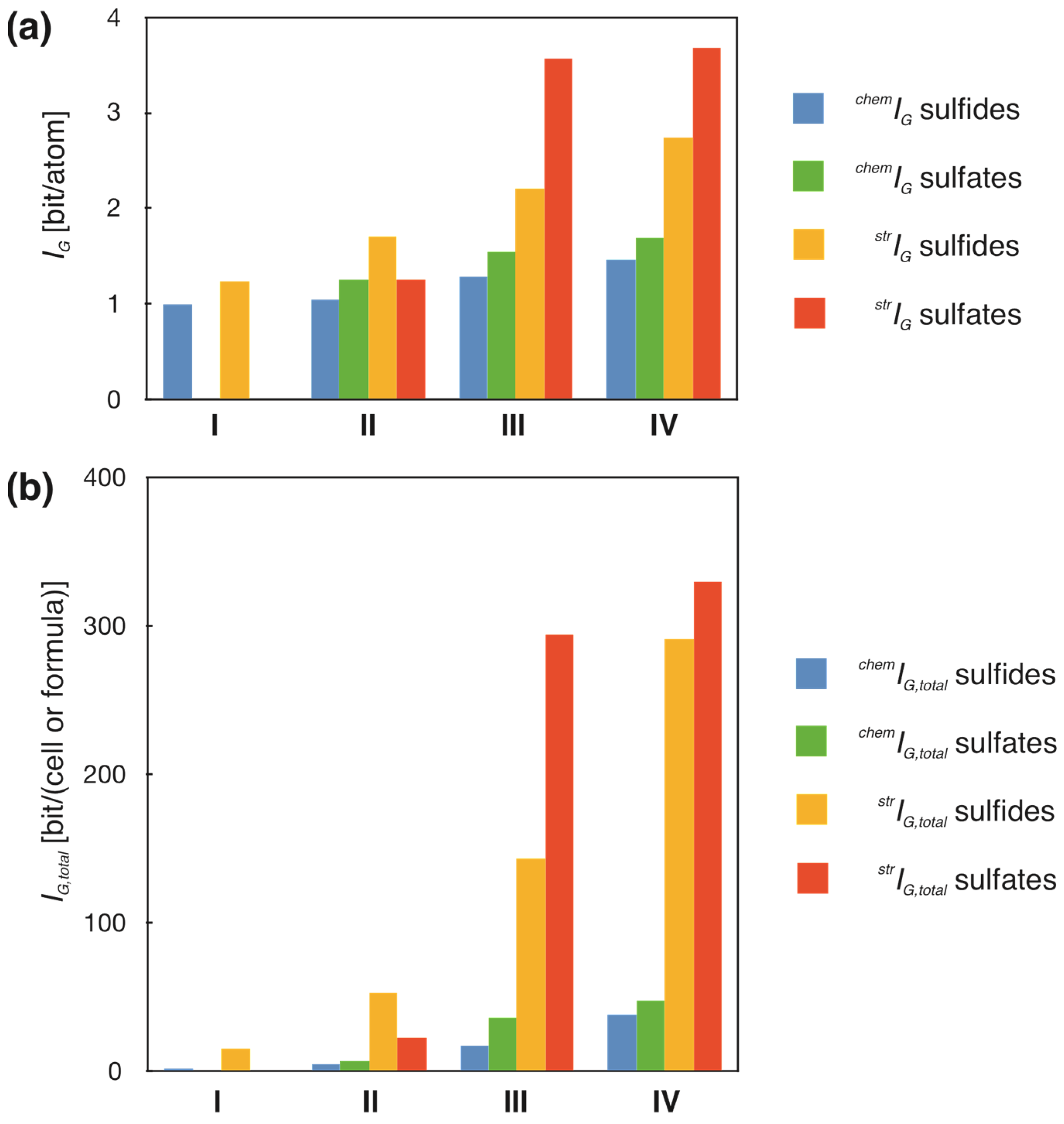
3.4. Evolution of the Chemical and Structural Complexity of S Minerals
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brimblecombe, P. 10.14—The Global Sulfur Cycle. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; pp. 559–591. ISBN 978-0-08-098300-4. [Google Scholar]
- Wedepohl, H.K. The Composition of the Continental Crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Vaughan, D.J. Sulfide Mineralogy and Geochemistry: Introduction and Overview. Rev. Miner. Geochem. 2006, 61, 1–5. [Google Scholar] [CrossRef]
- Gendrin, A.; Mangold, N.; Bibring, J.-P.; Langevin, Y.; Gondet, B.; Poulet, F.; Bonello, G.; Quantin, C.; Mustard, J.; Arvidson, R.; et al. Sulfates in Martian Layered Terrains: The OMEGA/Mars Express View. Science 2005, 307, 1587–1591. [Google Scholar] [CrossRef]
- Langevin, Y.; Poulet, F.; Bibring, J.-P.; Gondet, B. Sulfates in the North Polar Region of Mars Detected by OMEGA/Mars Express. Science 2005, 307, 1584–1586. [Google Scholar] [CrossRef]
- Wendt, L.; Gross, C.; Kneissl, T.; Sowe, M.; Combe, J.-P.; LeDeit, L.; McGuire, P.C.; Neukum, G. Sulfates and Iron Oxides in Ophir Chasma, Mars, Based on OMEGA and CRISM Observations. Icarus 2011, 213, 86–103. [Google Scholar] [CrossRef]
- Majzlan, J.; Alpers, C.N.; Koch, C.B.; McCleskey, R.B.; Myneni, S.C.B.; Neil, J.M. Vibrational, X-ray Absorption, and Mössbauer Spectra of Sulfate Minerals from the Weathered Massive Sulfide Deposit at Iron Mountain, California. Chem. Geol. 2011, 284, 296–305. [Google Scholar] [CrossRef]
- Wilson, S.; Bish, D.L. Stability of Mg-Sulfate Minerals in the Presence of Smectites: Possible Mineralogical Controls on H2O Cycling and Biomarker Preservation on Mars. Geochim. Cosmochim. Acta 2012, 96, 120–133. [Google Scholar] [CrossRef]
- Kong, W.G.; Wang, A.; Chou, I.-M. Experimental Determination of the Phase Boundary between Kornelite and Pentahydrated Ferric Sulfate at 0.1 MPa. Chem. Geol. 2011, 284, 333–338. [Google Scholar] [CrossRef]
- Norlund, K.L.I.; Baron, C.; Warren, L.A. Jarosite Formation by an AMD Sulphide-Oxidizing Environmental Enrichment: Implications for Biomarkers on Mars. Chem. Geol. 2010, 275, 235–242. [Google Scholar] [CrossRef]
- Marion, G.M.; Catling, D.C.; Kargel, J.S.; Crowley, J.K. Modeling Calcium Sulfate Chemistries with Applications to Mars. Icarus 2016, 278, 31–37. [Google Scholar] [CrossRef]
- Cao, H.; Chen, J.; Ling, Z. Laboratory Synthesis and Spectroscopic Studies of Hydrated Al-Sulfates Relevant to Mars. Icarus 2019, 333, 283–293. [Google Scholar] [CrossRef]
- Paramanick, S.; Rajesh, V.J.; Praveen, M.N.; Sajinkumar, K.S.; Bhattacharya, S. Spectral and Chemical Characterization of Copiapite and Rozenite from Padinjarathara in Wayanad, Southern India: Implications for Mars Exploration. Chem. Geol. 2021, 575, 120043. [Google Scholar] [CrossRef]
- Sheppard, R.Y.; Thorpe, M.T.; Fraeman, A.A.; Fox, V.K.; Milliken, R.E. Merging Perspectives on Secondary Minerals on Mars: A Review of Ancient Water-Rock Interactions in Gale Crater Inferred from Orbital and In-Situ Observations. Minerals 2021, 11, 986. [Google Scholar] [CrossRef]
- Suga, H.; Suzuki, K.; Usui, T.; Yamaguchi, A.; Sekizawa, O.; Nitta, K.; Takeichi, Y.; Ohigashi, T.; Takahashi, Y. A New Constraint on the Physicochemical Condition of Mars Surface during the Amazonian Epoch Based on Chemical Speciation for Secondary Minerals in Martian Nakhlites. Minerals 2021, 11, 514. [Google Scholar] [CrossRef]
- Szynkiewicz, A.; Bishop, J.L. Assessment of Sulfate Sources under Cold Conditions as a Geochemical Proxy for the Origin of Sulfates in the Circumpolar Dunes on Mars. Minerals 2021, 11, 507. [Google Scholar] [CrossRef]
- Grasby, S.E.; Percival, J.B.; Bilot, I.; Ardakani, O.H.; Smith, I.R.; Galloway, J.; Bringué, M.; McLoughlin-Coleman, T. Extensive Jarosite Deposits Formed through Auto-Combustion and Weathering of Pyritiferous Mudstone, Smoking Hills (Ingniryuat), Northwest Territories, Canadian Arctic—A Potential Mars Analogue. Chem. Geol. 2022, 587, 120634. [Google Scholar] [CrossRef]
- Wildner, M.; Zakharov, B.A.; Bogdanov, N.E.; Talla, D.; Boldyreva, E.V.; Miletich, R. Crystallography Relevant to Mars and Galilean Icy Moons: Crystal Behavior of Kieserite-Type Monohydrate Sulfates at Extraterrestrial Conditions down to 15 K. IUCrJ 2022, 9, 194–203. [Google Scholar] [CrossRef]
- Sheppard, R.Y.; Milliken, R.E.; Robertson, K.M. Presence of Clay Minerals Can Obscure Spectral Evidence of Mg Sulfates: Implications for Orbital Observations of Mars. Icarus 2022, 383, 115083. [Google Scholar] [CrossRef]
- Ju, E.; Shi, E.; Xin, Y.; Cao, H.; Liu, C.; Liu, P.; Chen, J.; Fu, X.; Ling, Z. Laboratory Synthesis, Spectroscopic Characteristics, and Conversion Relationships of Five Calcium Sulfate Double Salts Relevant to Mars. Icarus 2023, 401, 115610. [Google Scholar] [CrossRef]
- He, M.; Yan, C.; Li, J.; Suryawanshi, M.P.; Kim, J.; Green, M.A.; Hao, X. Kesterite Solar Cells: Insights into Current Strategies and Challenges. Adv. Sci. 2021, 8, 2004313. [Google Scholar] [CrossRef]
- Wang, A.; He, M.; Green, M.A.; Sun, K.; Hao, X. A Critical Review on the Progress of Kesterite Solar Cells: Current Strategies and Insights. Adv. Energy Mater. 2023, 13, 2203046. [Google Scholar] [CrossRef]
- Vaughan, D. (Ed.) Sulfide Mineralogy and Geochemistry. In Reviews in Mineralogy and Geochemistry; Mineralogical Society of America: Washington, DC, USA, 2006; Volume 61. [Google Scholar]
- Alpers, C.L.; Jambor, J.L.; Nordstron, D.K. (Eds.) Sulfate Minerals: Crystallography, Geochemistry, and Environmental Significance. In Reviews in Mineralogy and Geochemistry; Mineralogical Society of America: Washington, DC, USA, 2000; Volume 40. [Google Scholar]
- Makovicky, E. Crystal Structures of Sulfides and Other Chalcogenides. Rev. Mineral. Geochem. 2006, 61, 7–125. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Krivovichev, S.V.; Burns, P.C. The Crystal Chemistry of Sulfate Minerals. Rev. Mineral. Geochem. 2000, 40, 1–112. [Google Scholar] [CrossRef]
- Christy, A.G. Anomalous mineralogical diversity in the Periodic Table, and its causes. Miner. Mag. 2015, 79, 33–49. [Google Scholar] [CrossRef]
- Pasero, M. The IMA List of Minerals. 2023. Available online: http://cnmnc.units.it (accessed on 11 July 2023).
- Charykova, M.V.; Krivovichev, V.G. Mineral systems and the thermodynamics of selenites and selenates in the oxidation zone of sulfide ores—A review. Mineral. Petrol. 2017, 111, 121–134. [Google Scholar] [CrossRef]
- Krivovichev, V.G.; Charykova, M.V.; Vishnevsky, A.V. The thermodynamics of selenium minerals in near-surface environments. Minerals 2017, 7, 188. [Google Scholar] [CrossRef]
- Morrison, S.M.; Runyon, S.E.; Hazen, R.M. The Paleomineralogy of the Hadean Eon Revisited. Life 2018, 8, 64. [Google Scholar] [CrossRef]
- Hazen, R.M.; Morrison, S.M. An Evolutionary System of Mineralogy. Part I: Stellar Mineralogy (>13 to 4.6 Ga). Am. Miner. 2020, 105, 627–651. [Google Scholar] [CrossRef]
- Morrison, S.M.; Hazen, R.M. An Evolutionary System of Mineralogy. Part II: Interstellar and Solar Nebula Primary Condensation Mineralogy (>4.565 Ga). Am. Miner. 2020, 105, 1508–1535. [Google Scholar] [CrossRef]
- Hazen, R.M.; Morrison, S.M.; Prabhu, A. An Evolutionary System of Mineralogy. Part III: Primary Chondrule Mineralogy (4566 to 4561 Ma). Am. Miner. 2021, 106, 325–350. [Google Scholar] [CrossRef]
- Morrison, S.M.; Hazen, R.M. An Evolutionary System of Mineralogy, Part IV: Planetesimal Differentiation and Impact Mineralization (4566 to 4560 Ma). Am. Miner. 2021, 106, 730–761. [Google Scholar] [CrossRef]
- Hazen, R.M.; Morrison, S.M. An Evolutionary System of Mineralogy, Part V: Aqueous and Thermal Alteration of Planetesimals (Similar to 4565 to 4550 Ma). Am. Miner. 2021, 106, 1388–1419. [Google Scholar] [CrossRef]
- Morrison, S.M.; Prabhu, A.; Hazen, R.M. An Evolutionary System of Mineralogy, Part VI: Earth’s Earliest Hadean Crust (>4370 Ma). Am. Miner. 2023, 108, 42–58. [Google Scholar] [CrossRef]
- Hazen, R.M.; Morrison, S.M. On the Paragenetic Modes of Minerals: A Mineral Evolution Perspective. Am. Miner. 2022, 107, 1262–1287. [Google Scholar] [CrossRef]
- Hazen, R.M.; Morrison, S.M.; Krivovichev, S.V.; Downs, R.T. Lumping and Splitting: Toward a Classification of Mineral Natural Kinds. Am. Miner. 2022, 107, 1288–1301. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Topological Complexity of Crystal Structures: Quantitative Approach. Acta Crystallogr. 2012, A68, 393–398. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural Complexity of Minerals: Information Storage and Processing in the Mineral World. Miner. Mag. 2013, 77, 275–326. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Which Inorganic Structures Are the Most Complex? Angew. Chem. Int. Ed. 2014, 53, 654–661. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural Complexity and Configurational Entropy of Crystals. Acta Crystallogr. 2016, B72, 274–276. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Krivovichev, V.G.; Hazen, R.M.; Aksenov, S.M.; Avdontceva, M.S.; Banaru, A.M.; Gorelova, L.A.; Ismagilova, R.M.; Kornyakov, I.V.; Kuporev, I.V.; et al. Structural and Chemical Complexity of Minerals: An Update. Miner. Mag. 2022, 86, 183–204. [Google Scholar] [CrossRef]
- Hazen, R.M.; Downs, R.T.; Eleish, A.; Fox, P.; Gagne, O.C.; Golden, J.J.; Grew, E.S.; Hummer, D.R.; Hystad, G.; Krivovichev, S.V.; et al. Data-Driven Discovery in Mineralogy: Recent Advances in Data Resources, Analysis, and Visualization. Engineering 2019, 5, 397–405. [Google Scholar] [CrossRef]
- Prabhu, A.; Morrison, S.M.; Eleish, A.; Zhong, H.; Huang, F.; Golden, J.J.; Perry, S.N.; Hummer, D.R.; Ralph, J.; Runyon, S.E.; et al. Global Earth Mineral Inventory: A Data Legacy. Geosci. Data J. 2021, 8, 74–89. [Google Scholar] [CrossRef]
- Wang, C.; Hazen, R.M.; Cheng, Q.; Stephenson, M.H.; Zhou, C.; Fox, P.; Shen, S.; Oberhansli, R.; Hou, Z.; Ma, X.; et al. The Deep-Time Digital Earth Program: Data-Driven Discovery in Geosciences. Natl. Sci. Rev. 2021, 8, nwab027. [Google Scholar] [CrossRef]
- Grew, E.S.; Krivovichev, S.V.; Hazen, R.M.; Hystad, G. Evolution of Structural Complexity in Boron Minerals. Can. Miner. 2016, 54, 125–143. [Google Scholar] [CrossRef]
- Grew, E.S.; Hystad, G.; Hazen, R.M.; Krivovichev, S.V.; Gorelova, L.A. How Many Boron Minerals Occur in Earth’s Upper Crust? Am. Miner. 2017, 102, 1573–1587. [Google Scholar] [CrossRef]
- Krivovichev, V.G.; Krivovichev, S.V.; Charykova, M.V. Selenium Minerals: Structural and Chemical Diversity and Complexity. Minerals 2019, 9, 455. [Google Scholar] [CrossRef]
- Krivovichev, V.G.; Krivovichev, S.V.; Charykova, M.V. Tellurium Minerals: Structural and Chemical Diversity and Complexity. Minerals 2020, 10, 623. [Google Scholar] [CrossRef]
- Krivovichev, V.G.; Charykova, M.V. Classification of Mineral Systems; St. Petersburg University Press: Saint Petersburg, Russia, 2013; 196p. (In Russian) [Google Scholar]
- Krivovichev, V.G.; Charykova, M.V. Number of Minerals of Various Chemical elements: Statistics 2012 (a New Approach to an Old Problem). Zap. Ross. Mineral. Obs. 2013, 142, 36–42, (In Russian; English translation: Geol. Ore Depos. 2014, 56, 553–559). [Google Scholar] [CrossRef]
- Mandarino, J.A.; Nickel, E.H.; Cesbron, F. Rules of procedure of the Commission on New Minerals and Mineral Names, International Mineralogical Association. Can. Miner. 1984, 22, 367–368. [Google Scholar] [CrossRef]
- Nickel, E.H. The definition of a mineral. Can. Miner. 1995, 33, 689–690. [Google Scholar]
- Nickel, E.H.; Grice, J.D. The IMA Commission on New Minerals and Mineral Names: Procedures and Guidelines on Mineral Nomenclature. Can. Miner. 1998, 36, 91–926. [Google Scholar]
- Nickel, E.H.; Mandarino, J.A. Procedures involving the IMA Commission on New Minerals and Mineral Names and guidelines on mineral nomenclature. Am. Miner. 1987, 72, 1031–1042. [Google Scholar]
- Hawthorne, F.C. The use of end-member charge-arrangements in defining new mineral species and heterovalent substitutions in complex minerals. Can. Miner. 2002, 40, 699–710. [Google Scholar] [CrossRef]
- Hatert, F.; Burke, E.A.J. The IMA-CNMNC dominant-constituent rule revised and extended. Can. Miner. 2008, 46, 717–728. [Google Scholar] [CrossRef]
- Bulakh, A.G.; Zolotarev, A.A.; Krivovichev, V.G. Structures, Isomorphism, Formulae, Classification of Minerals; St. Petersburg University Press: Saint Petersburg, Russia, 2014; 133p. (In Russian) [Google Scholar]
- Krivovichev, V.G. Mineral Species; St. Petersburg University Press: Saint Petersburg, Russia, 2021; 601p. (In Russian) [Google Scholar]
- Krivovichev, V.G.; Charykova, M.V.; Krivovichev, S.V. The concept of mineral systems and its application to the study of mineral diversity and evolution. Eur. J. Mineral. 2018, 30, 219–230. [Google Scholar] [CrossRef]
- Pankova, Y.A.; Gorelova, L.A.; Krivovichev, S.V.; Pekov, I.V. The crystal structure of ginorite, Ca2[B14O20(OH)6](H2O)5, and the analysis of dimensional reduction and structural complexity in the CaO-B2O3-H2O system. Eur. J. Mineral. 2018, 30, 277–287. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Krivovichev, V.G.; Hazen, R.M. Structural and chemical complexity of minerals: Correlations and time evolution. Eur. J. Mineral. 2018, 30, 231–236. [Google Scholar] [CrossRef]
- Wall, F.J. Statistical Data Analysis Handbook; McGraw-Hill Inc.: New York, NY, USA, 1986; 546p. [Google Scholar]
- Krivovichev, S.V. Structural and Chemical Complexity of Minerals: The Information-Based Approach. In Celebrating the International Year of Mineralogy: Progress and Landmark Discoveries of the Last Decades; Bindi, L., Cruciani, G., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 101–129. ISBN 978-3-031-28805-0. [Google Scholar]
- Morimoto, N. Djurleite, a new copper sulfide mineral. Mineral. J. 1962, 3, 338–344. [Google Scholar] [CrossRef][Green Version]
- Szopa, K.; Krzykawski, T.; Banasik, K.; Król, P.; Skreczko, S.; Mounteanou, S.A.; Koziarska, M. EMPA, XRD, and Raman Characterization of Ag-Bearing Djurleite from the Lubin Mine, Lower Silesia, Poland. Minerals 2021, 11, 454. [Google Scholar] [CrossRef]
- Evans, H. Crystal structures of low chalcocite and djurleite. Z. Kristallogr. 1979, 150, 299–320. [Google Scholar] [CrossRef]
- Will, G.; Hinze, E.; Abdelrahman, A.R.M. Crystal Structure Analysis and Refinement of Digenite, Cu1.8S, in the Temperature Range 20 to 500 °C under Controlled Sulfur Partial Pressure. Eur. J. Miner. 2002, 14, 591–598. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, C.; Wen, B.; Zhang, L. Natural djurleite with refined composition Cu61.39S32 revealing disorder of some Cu sites. IUCrData 2022, 7, x220694. [Google Scholar] [CrossRef]
- Makovicky, E. The building principles and classification of bismuth-lead sulphosalts and related compounds. Fortschr. Miner. 1981, 59, 137–190. [Google Scholar]
- Makovicky, E. Modular crystal chemistry of sulphosalts and other complex sulphides. EMU Notes Miner. 1997, 1, 237–271. [Google Scholar]
- Makovicky, E. Modularity—Different types and approaches. EMU Notes Miner. 1997, 1, 315–343. [Google Scholar]
- Ferraris, G.; Makovicky, E.; Merlino, S. Crystallography of Modular Materials. In IUCr Monographs in Crystallography; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Makovicky, E. Modular Crystal Chemistry of Thallium Sulfosalts. Minerals 2018, 8, 478. [Google Scholar] [CrossRef]
- Topa, D.; Stoeger, B.; Makovicky, E.; Stanley, C. Incomsartorite, IMA 2016-035. CNMNC Newsletter No. 33, October 2016. Miner. Mag. 2016, 80, 1136. [Google Scholar]
- Topa, D.; Sejkora, J.; Makovicky, E.; Prsek, J.; Ozdin, D.; Putz, H.; Dittrich, H.; Karup-Moller, S. Chovanite, Pb15−2xSb14+2xS36Ox (x ~0.2), a New Sulphosalt Species from the Low Tatra Mountains, Western Carpathians, Slovakia. Eur. J. Miner. 2012, 24, 727–740. [Google Scholar] [CrossRef]
- Biagioni, C.; Moelo, Y. Lead-Antimony Sulfosalts from Tuscany (Italy). XIX. Crystal Chemistry of Chovanite from Two New Occurrences in the Apuan Alps and Its 8 Angstrom Crystal Structure. Miner. Mag. 2017, 81, 811–831. [Google Scholar] [CrossRef]
- Biagioni, C.; Moelo, Y.; Orlandi, P.; Stanley, C.J. Lead-Antimony Sulfosalts from Tuscany (Italy). XVII. Meerschautite, (Ag,Cu)5.5Pb42.4(Sb,As)45.1S112O0.8, a New Expanded Derivative of Owyheeite from the Pollone Mine, Valdicastello Carducci: Occurrence and Crystal Structure. Miner. Mag. 2016, 80, 675–690. [Google Scholar] [CrossRef]
- Topa, D.; Kolitsch, U.W.E.; Graeser, S.; Makovicky, E.; Stanley, C. Argentoliveingite, Ag3+xPb36−2xAs51+xS112 (0 <= x < 0.5), a New Homeotype of Liveingite from Lengenbach, Binntal, Switzerland, and the Crystal Chemistry of the Liveingite Group. Eur. J. Miner. 2019, 31, 1079–1097. [Google Scholar] [CrossRef]
- Moelo, Y.; Orlandi, P.; Guillot-Deudon, C.; Biagioni, C.; Paar, W.; Evain, M. Lead-antimony sulfosalts from tuscany (italy). XI. The new mineral species parasterryite, Ag4Pb20(Sb14.5As9.5)Σ24S58, and associated sterryite, Cu(Ag,Cu)3Pb-19(Sb,As)22(As-As)S56, from the Pollone mine, Tuscany, Italy. Can. Miner. 2011, 49, 623–638. [Google Scholar] [CrossRef]
- Topa, D.; Makovicky, E.; Stoeger, B.; Stanley, C. Heptasartorite, Tl7Pb22As55S108, Enneasartorite, Tl6Pb32As70S140 and Hendekasartorite, Tl2Pb48As82S172, Three Members of the Anion-Omission Series of “sartorites” from the Lengenbach Quarry at Binntal, Wallis, Switzerland. Eur. J. Miner. 2017, 29, 701–712. [Google Scholar] [CrossRef]
- Nestola, F.; Guastoni, A.; Bindi, L.; Secco, L. Dalnegroite, Tl5-xPb2x(As,Sb)21−xS34, a New Thallium Sulphosalt from Lengenbach Quarry, Binntal, Switzerland. Miner. Mag. 2009, 73, 1027–1032. [Google Scholar] [CrossRef]
- Bindi, L.; Nestola, F.; Guastoni, A.; Secco, L. The Crystal Structure of Dalnegroite, Tl5−xPb2x(As,Sb)21−xS34: A Masterpiece of Structural Complexity. Miner. Mag. 2010, 74, 999–1012. [Google Scholar] [CrossRef]
- Topa, D.; Makovicky, E.; Dittrich, H. The Crystal Structure of 7H:12Q Cannizzarite from Vulcano, Italy. Can. Miner. 2010, 48, 483–495. [Google Scholar] [CrossRef]
- Borisov, S.V.; Pervukhina, N.V.; Magarill, S.A.; Kuratieva, N.V.; Bryzgalov, I.A.; Mozgova, N.N.; Chaplygin, I.V. The Crystal Structure of (Cd,In)-Rich Cannizzarite from Kudriavy Volcano, Iturup Island, Kuriles, Russia. Can. Miner. 2012, 50, 387–395. [Google Scholar] [CrossRef]
- Berlepsch, P.; Armbruster, T.; Makovicky, E.; Topa, D. Another Step toward Understanding the True Nature of Sartorite: Determination and Refinement of a Ninefold Superstructure. Am. Miner. 2003, 88, 450–461. [Google Scholar] [CrossRef]
- Makovicky, E.; Topa, D.; Stoeger, B. The Crystal Structures of Heptasartorite, Tl7Pb22As55S108, and Enneasartorite, Tl6Pb32As70S140, Two Members of an Anion-Omission Series of Complex Sulfosalts from Lengenbach, the Swiss Alps, and Comparison with the Structures of As-Sb Sartorite Homologues. Eur. J. Miner. 2018, 30, 149–164. [Google Scholar] [CrossRef]
- Iitaka, Y.; Nowacki, W. A Refinement of the Pseudo Crystal Structure of Scleroclase PbAs2S4. Acta Crystallogr. 1961, 14, 1291–1292. [Google Scholar] [CrossRef]
- Robinson, S. Owyheeite. Am. Miner. 1949, 34, 398–402. [Google Scholar]
- Laufek, F.; Pazout, R.; Makovicky, E. Crystal Structure of Owyheeite, Ag1.5Pb4.43Sb6.07S14: Refinement from Powder Synchrotron X-Ray Diffraction. Eur. J. Miner. 2007, 19, 557–566. [Google Scholar] [CrossRef]
- Stöger, B.; Gob, C.; Topa, D. A Fresh View on the Structure and Twinning of Owyheeite, a Rod-Polytype and Twofold Superstructure. Acta Crystallogr. 2023, B79, 271–280. [Google Scholar] [CrossRef]
- Bindi, L.; Nespolo, M.; Krivovichev, S.V.; Chapuis, G.; Biagioni, C. Producing Highly Complicated Materials. Nature Does It Better. Rep. Progr. Phys. 2020, 83, 106501. [Google Scholar] [CrossRef]
- Biagioni, C.; Moëlo, Y.; Favreau, G.; Bourgoin, V.; Boulliard, J.-C. Structure of Pb-Rich Chabournéite from Jas Roux, France. Acta Crystallogr. 2015, B71, 81–88. [Google Scholar] [CrossRef]
- Matzat, E. Cannizzarite. Acta Crystallogr. 1979, B35, 133–136. [Google Scholar] [CrossRef]
- Bonaccorsi, E.; Merlino, S. Micro- and Mesoporous Mineral Phases. Rev. Miner. Geochem. 2005, 57, 241–290. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Aksenov, S.M.; Rastsvetaeva, R.K. Structural Chemistry, IR Spectroscopy, Properties, and Genesis of Natural and Synthetic Microporous Cancrinite- and Sodalite-Related Materials: A Review. Micropor. Mesopor. Mater. 2021, 323, 111098. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural and Topological Complexity of Zeolites: An Information-Theoretic Analysis. Micropor. Mesopor. Mater. 2013, 171, 223–229. [Google Scholar] [CrossRef]
- Cámara, F.; Bellatreccia, F.; Della Ventura, G.; Mottana, A.; Bindi, L.; Gunter, M.E.; Sebastiani, M. Fantappièite, a New Mineral of the Cancrinite-Sodalite Group with a 33-Layer Stacking Sequence: Occurrence and Crystal Structure. Am. Miner. 2010, 95, 472–480. [Google Scholar] [CrossRef]
- Bonaccorsi, E.; Ballirano, P.; Cámara, F. The Crystal Structure of Sacrofanite, the 74Å Phase of the Cancrinite–Sodalite Supergroup. Micropor. Mesopor. Mater. 2012, 147, 318–326. [Google Scholar] [CrossRef]
- Biagioni, C.; Mauro, D.; Pasero, M.; Bonaccorsi, E.; Lepore, G.O.; Zaccarini, F.; Skogby, H. Crystal-Chemistry of Sulfates from the Apuan Alps (Tuscany, Italy). VI. Tl-Bearing Alum-(K) and Voltaite from the Fornovolasco Mining Complex. Am. Miner. 2020, 105, 1088–1098. [Google Scholar] [CrossRef]
- Elliott, P.; Giester, G.; Rowe, R.; Pring, A. Putnisite, SrCa4Cr3+8(CO3)8SO4(OH)16·25H2O, a New Mineral from Western Australia: Description and Crystal Structure. Miner. Mag. 2014, 78, 131–144. [Google Scholar] [CrossRef]
- Bonaccorsi, E. The Crystal Structure of Giuseppettite, the 16-Layer Member of the Cancrinite–Sodalite Group. Micropor. Mesopor. Mater. 2004, 73, 129–136. [Google Scholar] [CrossRef]
- Dai, Y.; Harlow, G.E. Description and Crystal Structure of Vonbezingite, a New Ca-Cu-SO4-H2O Mineral from the Kalahari Manganese Field, South Africa. Am. Miner. 1992, 77, 1292–1300. [Google Scholar]
- Burns, P.C.; Deely, K.M.; Hayden, L.A. The crystal chemistry of the zippeite group. Can. Miner. 2003, 41, 687–706. [Google Scholar] [CrossRef]
- Menchetti, S.; Sabelli, C. The Halotrichite Group: The Crystal Structure of Apjohnite. Miner. Mag. 1976, 40, 599–608. [Google Scholar] [CrossRef]
- Plášil, J.; Dušek, M.; Novák, M.; Čejka, J.; Císařová, I.; Škoda, R. Sejkoraite-(Y), a New Member of the Zippeite Group Containing Trivalent Cations from Jáchymov (St. Joachimsthal), Czech Republic: Description and Crystal Structure Refinement. Am. Miner. 2011, 96, 983–991. [Google Scholar] [CrossRef]
- Kampf, A.R.; Rossman, G.R.; Ma, C.; Belmonte, D.; Biagioni, C.; Castellaro, F.; Chiappino, L. Ramazzoite, [Mg8Cu12(PO4)(CO3)4(OH)24(H2O)20][(H0.33SO4)3(H2O)36], the First Mineral with a Polyoxometalate Cation. Eur. J. Miner. 2018, 30, 827–834. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Polyoxometalate Clusters in Minerals: Review and Complexity Analysis. Acta Crystallogr. 2020, B76, 618–629. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Ladders of Information: What Contributes to the Structural Complexity of Inorganic Crystals. Z. Kristallogr. 2018, 233, 155–161. [Google Scholar] [CrossRef]
- Warr, L.N. IMA–CNMNC Approved Mineral Symbols. Miner. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Ertl, A.; Dyar, M.D.; Hughes, J.M.; Brandstaetter, F.; Gunter, M.E.; Prem, M.; Peterson, R.C. Pertlikite, a New Tetragonal Mg-Rich Member of the Voltaite Group from Madeni Zakh, Iran. Can. Miner. 2008, 46, 661–669. [Google Scholar] [CrossRef]
- Szakall, S.; Sajo, I.; Feher, B.; Bigi, S. Ammoniomagnesiovoltaite, a new voltaite-related mineral species from Pecs-Vasas, Hungary. Can. Miner. 2012, 50, 65–72. [Google Scholar] [CrossRef]
- Majzlan, J.; Schlicht, H.; Wierzbicka-Wieczorek, M.; Giester, G.; Poellmann, H.; Broemme, B.; Doyle, S.; Buth, G.; Koch, C.B. A Contribution to the Crystal Chemistry of the Voltaite Group: Solid Solutions, Mossbauer and Infrared Spectra, and Anomalous Anisotropy. Miner. Petrol. 2013, 107, 221–233. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Aksenov, S.M.; Rastsvetaeva, R.K.; Moehn, G.; Rusakov, V.S.; Pekov, I.V.; Scholz, R.; Eremina, T.A.; Belakovskiy, D.I.; Lorenz, J.A. Magnesiovoltaite, K2Mg5Fe3+5Al3(SO4)12·18H2O, a New Mineral from the Alcaparrosa Mine, Antofagasta Region, Chile. Eur. J. Miner. 2016, 28, 1005–1017. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Siidra, O.I.; Belakovsky, D.I.; Shilovskikh, V.V.; Nuzhdaev, A.A.; Ismagilova, R.M. Ammoniovoltaite, (NH4)2Fe2+5Fe3+3Al(SO4)12(H2O)18, a New Mineral from the Severo-Kambalny Geothermal Field, Kamchatka, Russia. Miner. Mag. 2018, 82, 1057–1077. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Ismagilova, R.M.; Zolotarev, A.A.; Krivovichev, S.V.; Shilovskikh, V.V.; Nuzhdaev, A.A.; Nazarova, M.A. The crystal structure of magnesian halotrichite, (Fe,Mg)Al2(SO4)4×22H2O: Hydrogen bonding, geometrical parameters and structural complexity. J. Geosci. 2023, 68, in press. [Google Scholar]
- Hazen, R.M.; Papineau, D.; Bleeker, W.; Downs, R.T.; Ferry, J.M.; McCoy, T.J.; Sverjensky, D.A.; Yang, H. Mineral evolution. Am. Miner. 2008, 93, 1693–1720. [Google Scholar] [CrossRef]
- Hazen, R.M. Paleomineralogy of the Hadean Eon: A preliminary species list. Am. J. Sci. 2013, 313, 807–843. [Google Scholar] [CrossRef]
- Hofmann, B.A.; Knill, M.D. Geochemistry and Genesis of the Lengenbach Pb-Zn-As-Tl-Ba-Mineralisation, Binn Valley, Switzerland. Miner. Dep. 1996, 31, 319–339. [Google Scholar] [CrossRef]
- De Rita, D.; Funiciello, R.; Rossi, U.; Sposato, A. Structure and Evolution of the Sacrofano-Baccano Caldera, Sabatini Volcanic Complex, Rome. J. Volcanol. Geotherm. Res. 1983, 17, 219–236. [Google Scholar] [CrossRef]
- Hong, Q.-J.; Ushakov, S.V.; Van de Walle, A.; Navrotsky, A. Melting Temperature Prediction Using a Graph Neural Network Model: From Ancient Minerals to New Materials. Proc. Natl. Acad. Sci. USA 2022, 119, e2209630119. [Google Scholar] [CrossRef]
- Hazen, R.M.; Morrison, S.M.; Prabhu, A.; Williams, J.R.; Wong, M.L.; Krivovichev, S.V.; Bermanec, M. On the Attributes of Mineral Paragenetic Modes. Can. J. Miner. Petrol. 2023, 61, 653–673. [Google Scholar] [CrossRef]
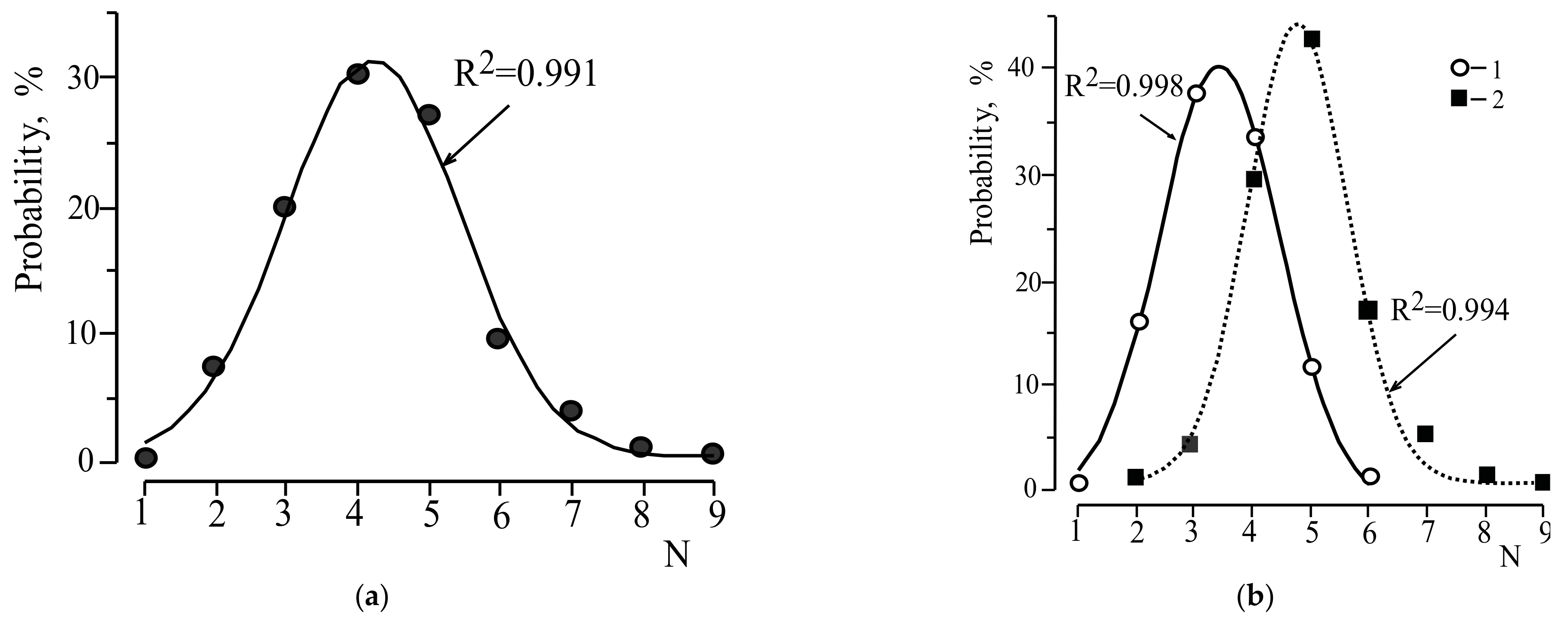
| N | Minerals without S–O Bonds (S2−, S0) | Minerals with S–O Bonds (S4+, S6+) | All S Minerals | |||
|---|---|---|---|---|---|---|
| mi | pi | mi | pi | mi | pi | |
| 1 | 2 | 0.38 | - | - | 2 | 0.18 |
| 2 | 84 | 15.88 | - | - | 84 | 7.51 |
| 3 | 199 | 37.62 | 22 | 4.14 | 221 | 19.77 |
| 4 | 177 | 33.46 | 160 | 29.38 | 337 | 30.14 |
| 5 | 61 | 11.53 | 242 | 42.94 | 303 | 27.10 |
| 6 | 6 | 1.13 | 103 | 17.14 | 109 | 9.75 |
| 7 | - | - | 46 | 5.08 | 46 | 4.11 |
| 8 | - | - | 13 | 1.13 | 13 | 1.17 |
| 9 | - | - | 3 | 0.19 | 3 | 0.27 |
| Total | 529 | 100.0 | 589 | 100.00 | 1118 | 100.0 |
| N | mi | chemIG | mi | strIG | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| [bit/atom] | [bit/f.u.] | [bit/atom] | [bit/cell] | |||||||
| 1 | 3 | 0 | 0 | 2.195 | 1.35 | 94.69 | 66.59 | |||
| 2 | 87 | 0.968 | 0.005 | 7.33 | 1.05 | 78 | 1.811 | 0.13 | 79.52 | 34.84 |
| 3 | 220 | 1.420 | 0.008 | 17.89 | 1.36 | 189 | 2.549 | 0.09 | 107.85 | 13.94 |
| 4 | 324 | 1.591 | 0.008 | 52.02 | 3.26 | 250 | 3.816 | 0.08 | 310.74 | 21.83 |
| 5 | 293 | 1.752 | 0.010 | 79.87 | 4.07 | 229 | 4.233 | 0.07 | 480.35 | 34.57 |
| 6 | 88 | 1.780 | 0.020 | 107.64 | 7.84 | 70 | 4.298 | 0.12 | 527.07 | 71.81 |
| 7 | 29 | 1.981 | 0.034 | 162.96 | 18.65 | 25 | 3.833 | 0.16 | 959.55 | 209.20 |
| 8 | 5 | 2.170 | 0.150 | 138.35 | 18.11 | 4 | 5.34 | 0.17 | 537.67 | 116.37 |
| 9 | 1 | 2.026 | 212.72 | 1 | 4.402 | 889.17 | ||||
| Complexity Parameters | Without S–O Bonds | With S–O Bonds | Student’s t-Test | |||||
|---|---|---|---|---|---|---|---|---|
| mi | mi | t | p | |||||
| chemIG [bit/atom] | 529 | 1.48 | 0.01 | 589 | 1.67 | 0.01 | 13.43 | <0.0001 |
| strIG [bit/atom] | 419 | 2.88 | 0.07 | 522 | 4.12 | 0.05 | 14.42 | <0.0001 |
| chemIG,total [bit/f.u.] | 529 | 39.98 | 2.63 | 589 | 75.24 | 3.03 | 8.79 | <0.0001 |
| strIG,total [bit/cell] | 419 | 197.64 | 15.6 | 522 | 460.99 | 25.46 | 8.82 | <0.0001 |
| Stages | mi | chemIG [bit/atom] | chemIG,total [bit/f.u.] | mi | strIG [bit/atom] | strIG,total [bit/cell] | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sulfides and sulfosalts | ||||||||||
| I | 3 | 1 | 0 | 2 | 0 | 3 | 1.24 | 0.24 | 15.17 | 13.17 |
| II | 10 | 1.04 | 0.05 | 5.27 | 1.83 | 10 | 1.70 | 0.42 | 52.63 | 41.67 |
| III | 77 | 1.28 | 0.04 | 17.27 | 4.17 | 77 | 2.21 | 0.16 | 142.99 | 44.87 |
| IV | 529 | 1.47 | 0.01 | 38.68 | 2.68 | 291 | 2.74 | 0.08 | 290.86 | 17.05 |
| Sulfates and sulfites | ||||||||||
| I | 0 | - | - | - | - | 0 | - | - | - | - |
| II | 1 | 1.25 | 1 | 7.51 | 1 | 1.25 | 22.530 | |||
| III | 20 | 1.54 | 0.05 | 36.73 | 5.26 | 20 | 3.57 | 0.31 | 294.47 | 60.13 |
| IV | 589 | 1.69 | 0.01 | 47.46 | 3.69 | 273 | 3.68 | 0.07 | 329.80 | 19.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krivovichev, V.G.; Krivovichev, S.V.; Starova, G.L. Structural and Chemical Diversity and Complexity of Sulfur Minerals. Minerals 2023, 13, 1069. https://doi.org/10.3390/min13081069
Krivovichev VG, Krivovichev SV, Starova GL. Structural and Chemical Diversity and Complexity of Sulfur Minerals. Minerals. 2023; 13(8):1069. https://doi.org/10.3390/min13081069
Chicago/Turabian StyleKrivovichev, Vladimir G., Sergey V. Krivovichev, and Galina L. Starova. 2023. "Structural and Chemical Diversity and Complexity of Sulfur Minerals" Minerals 13, no. 8: 1069. https://doi.org/10.3390/min13081069
APA StyleKrivovichev, V. G., Krivovichev, S. V., & Starova, G. L. (2023). Structural and Chemical Diversity and Complexity of Sulfur Minerals. Minerals, 13(8), 1069. https://doi.org/10.3390/min13081069







