The Influence of Surface Heterogeneity of Fluorite on the Adsorption of Alkyl Sulfonates
Abstract
1. Introduction
2. Materials and Methods
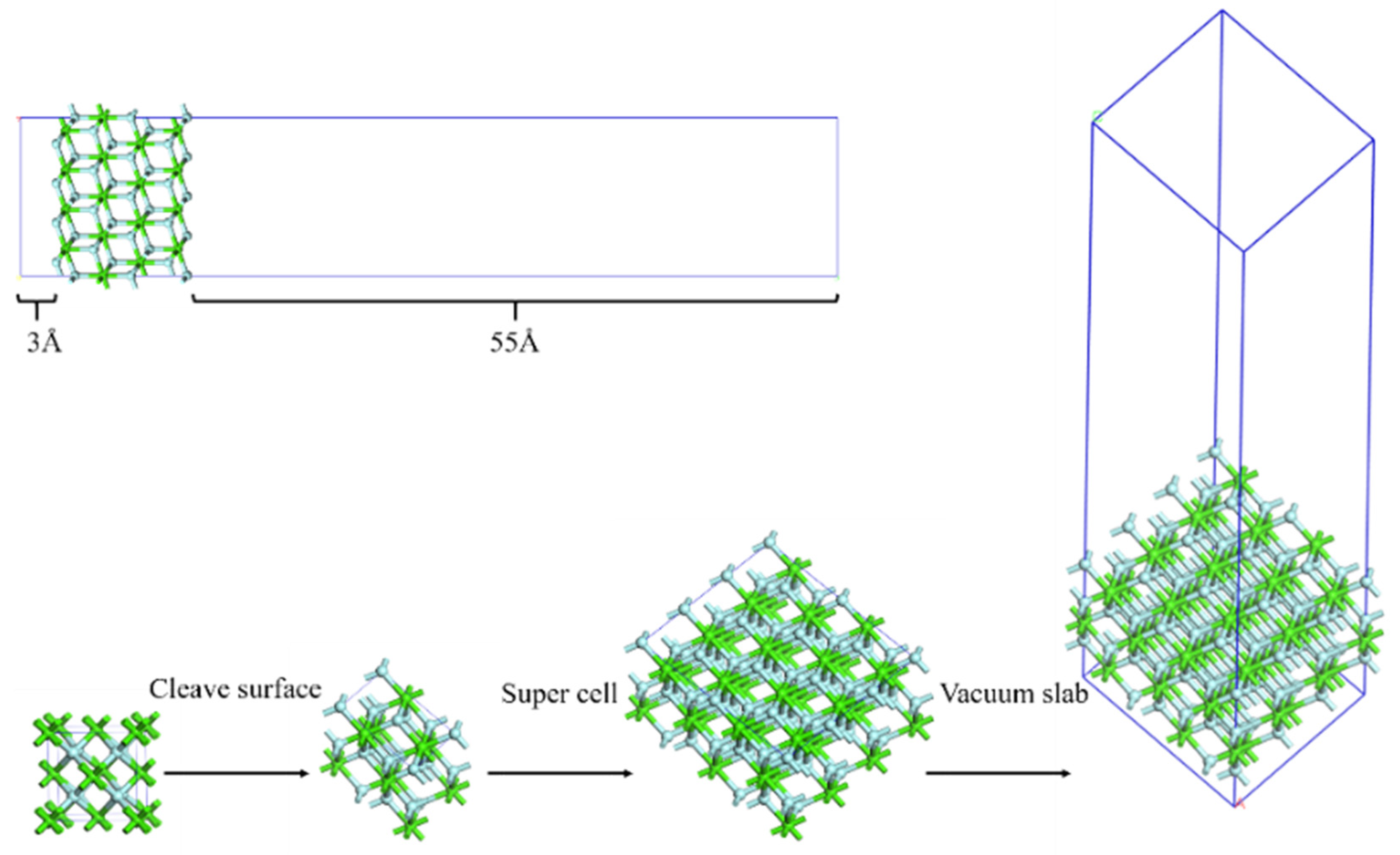
2.1. Model and Parameters
2.2. Adsorption Energy Calculation
3. Results
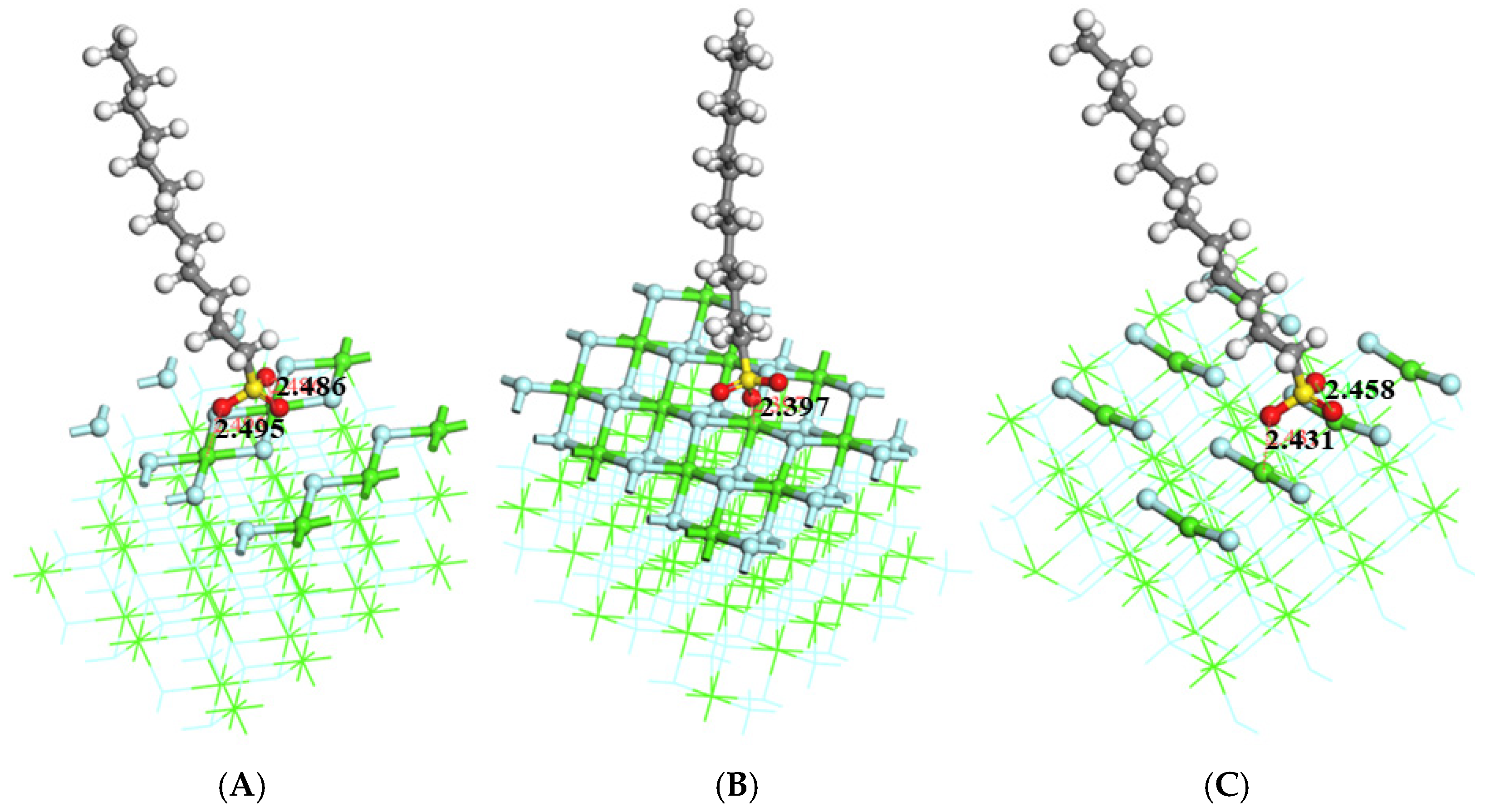
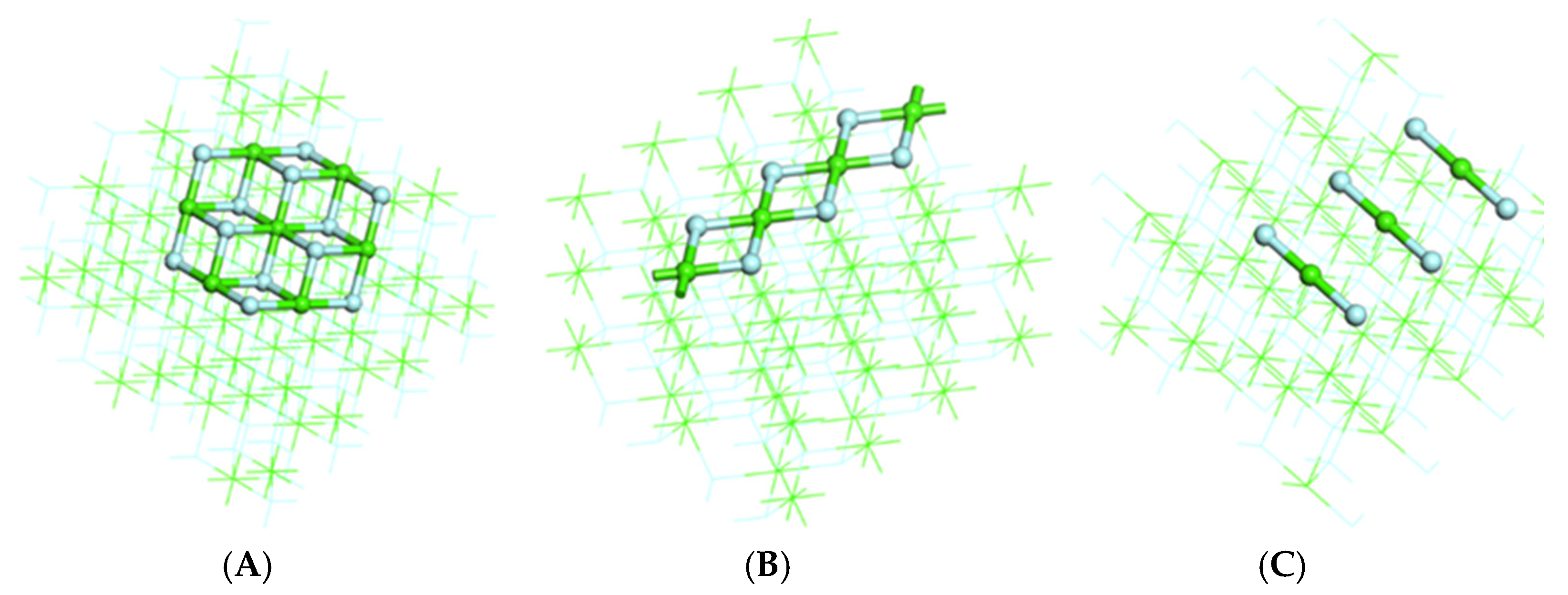
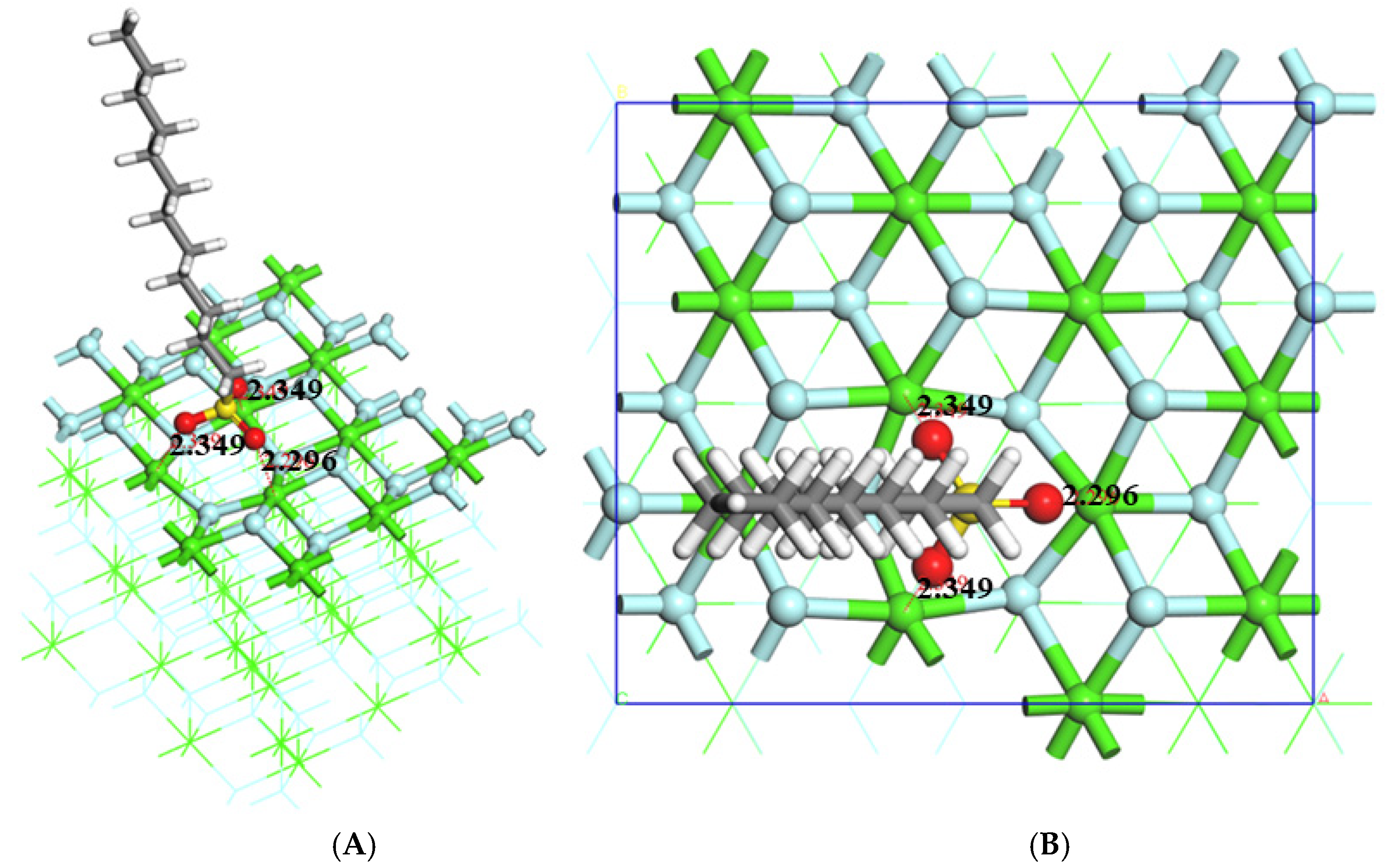
3.1. Structural Analysis
3.2. Comparison of Adsorption Energy and Bond Length
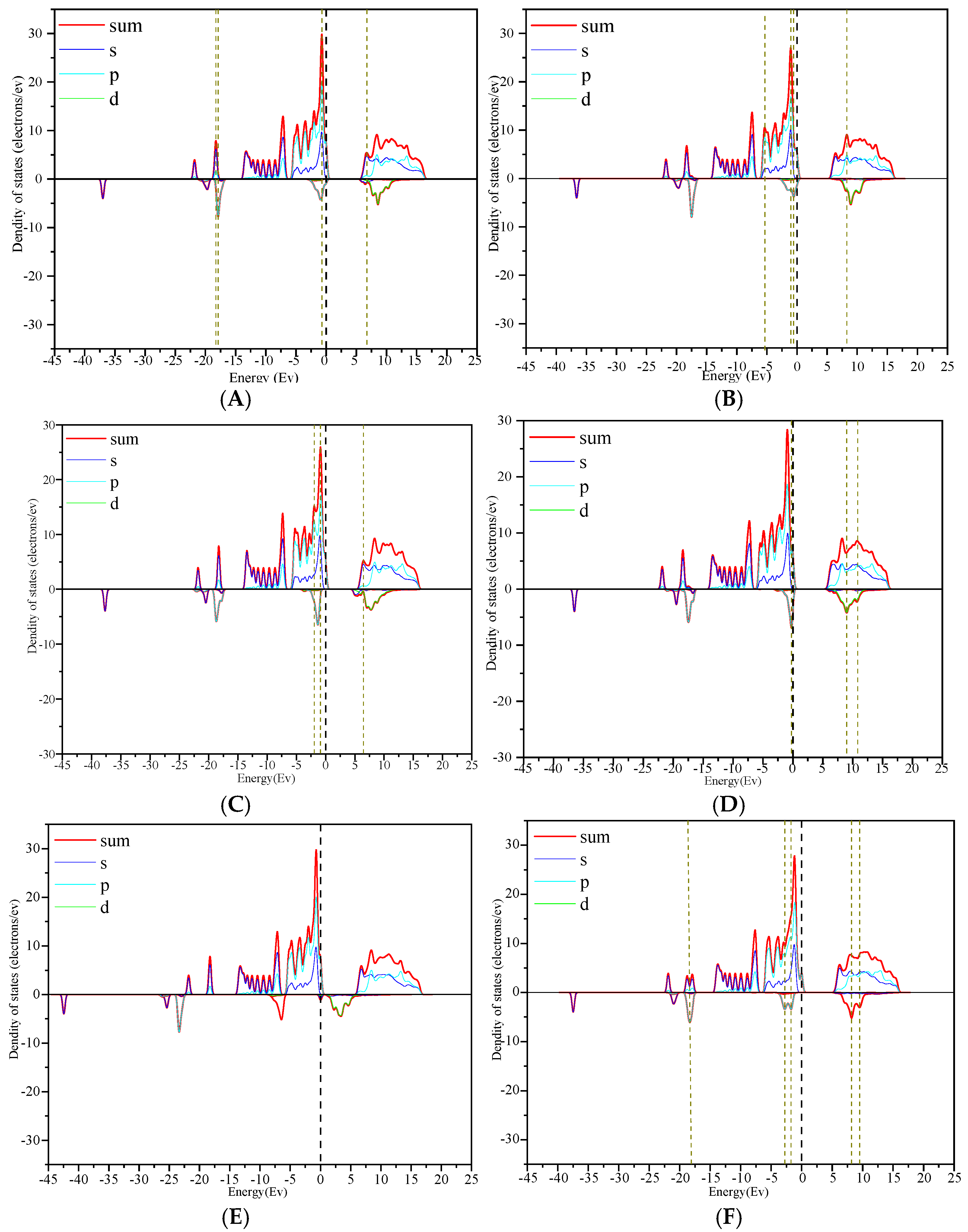
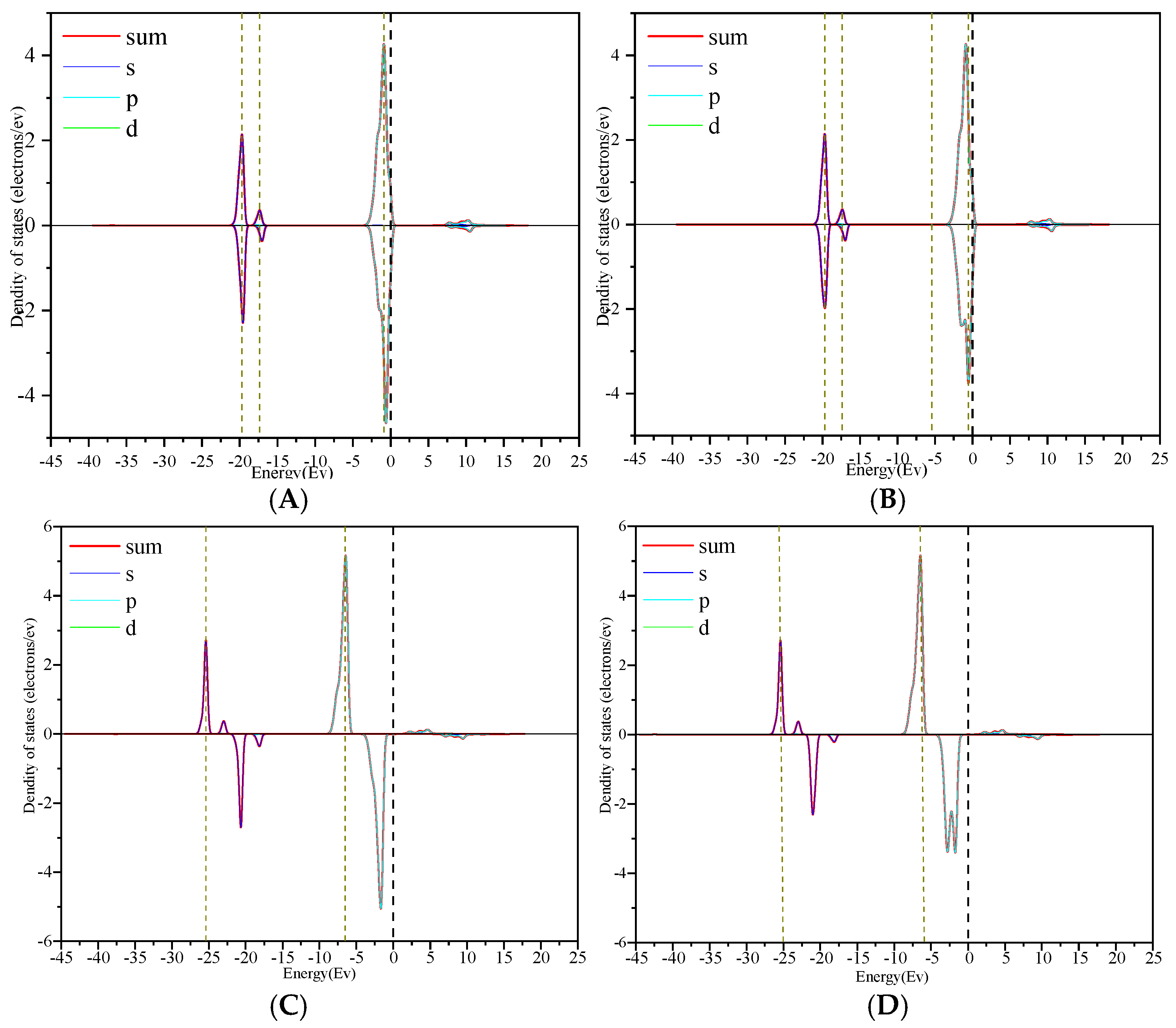
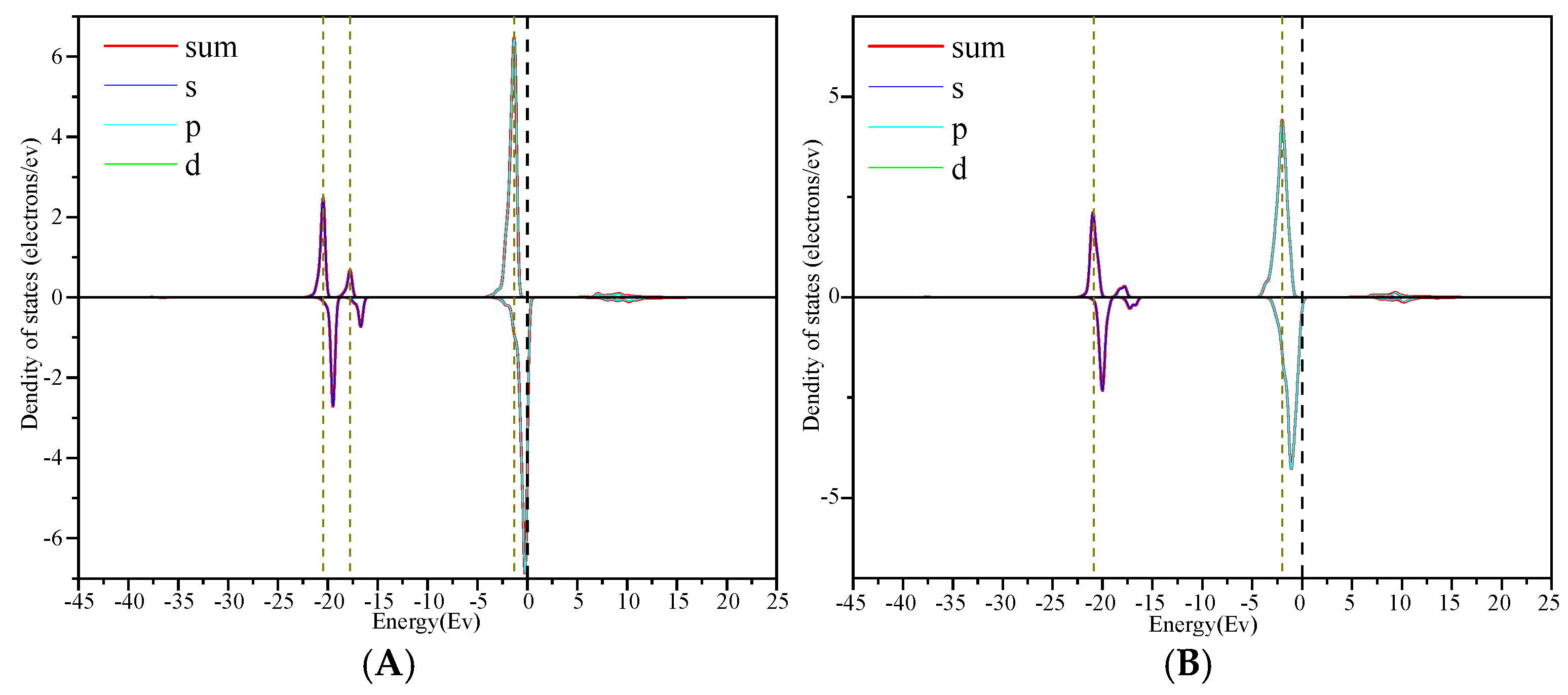
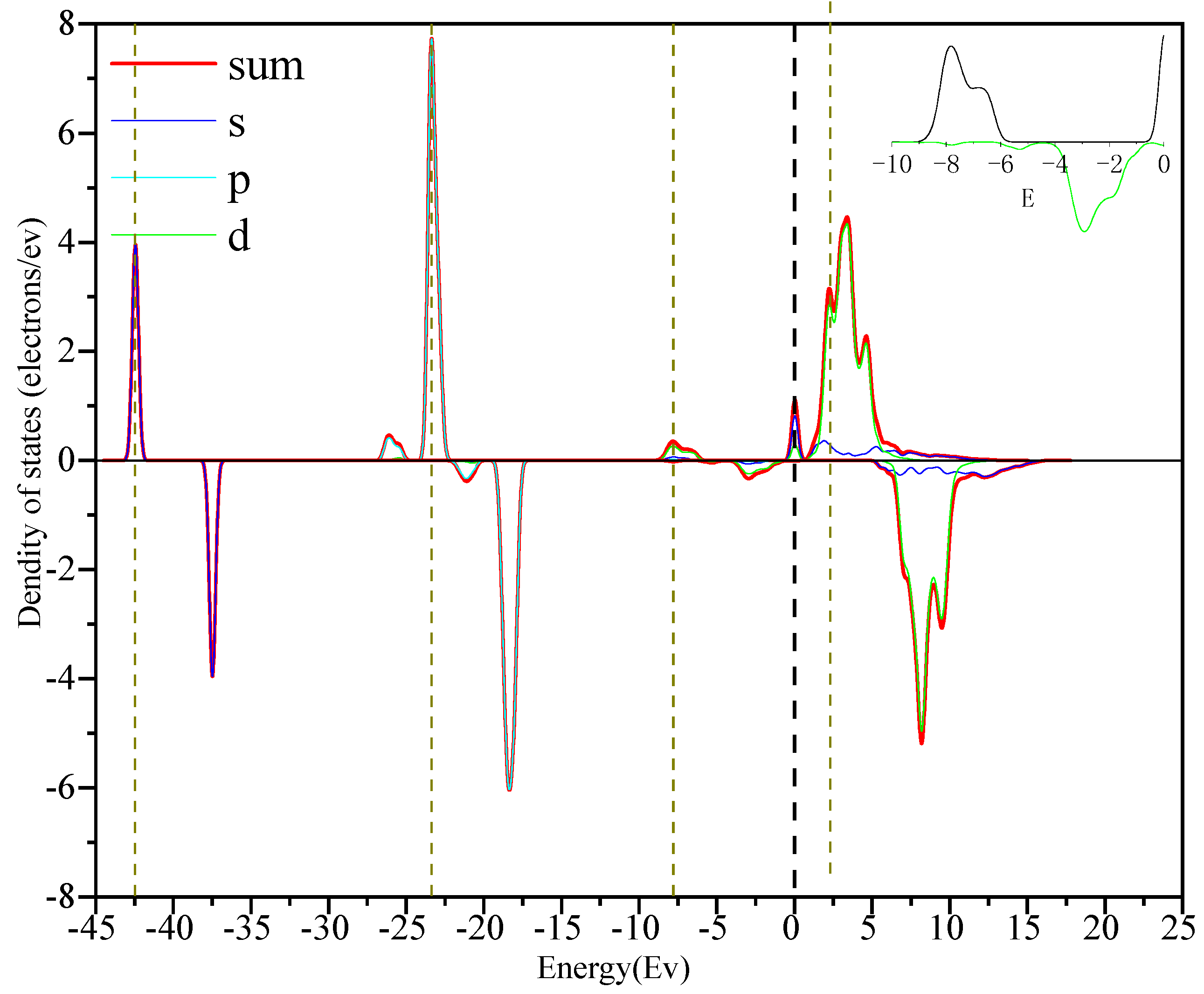
3.3. Density of State Analysis
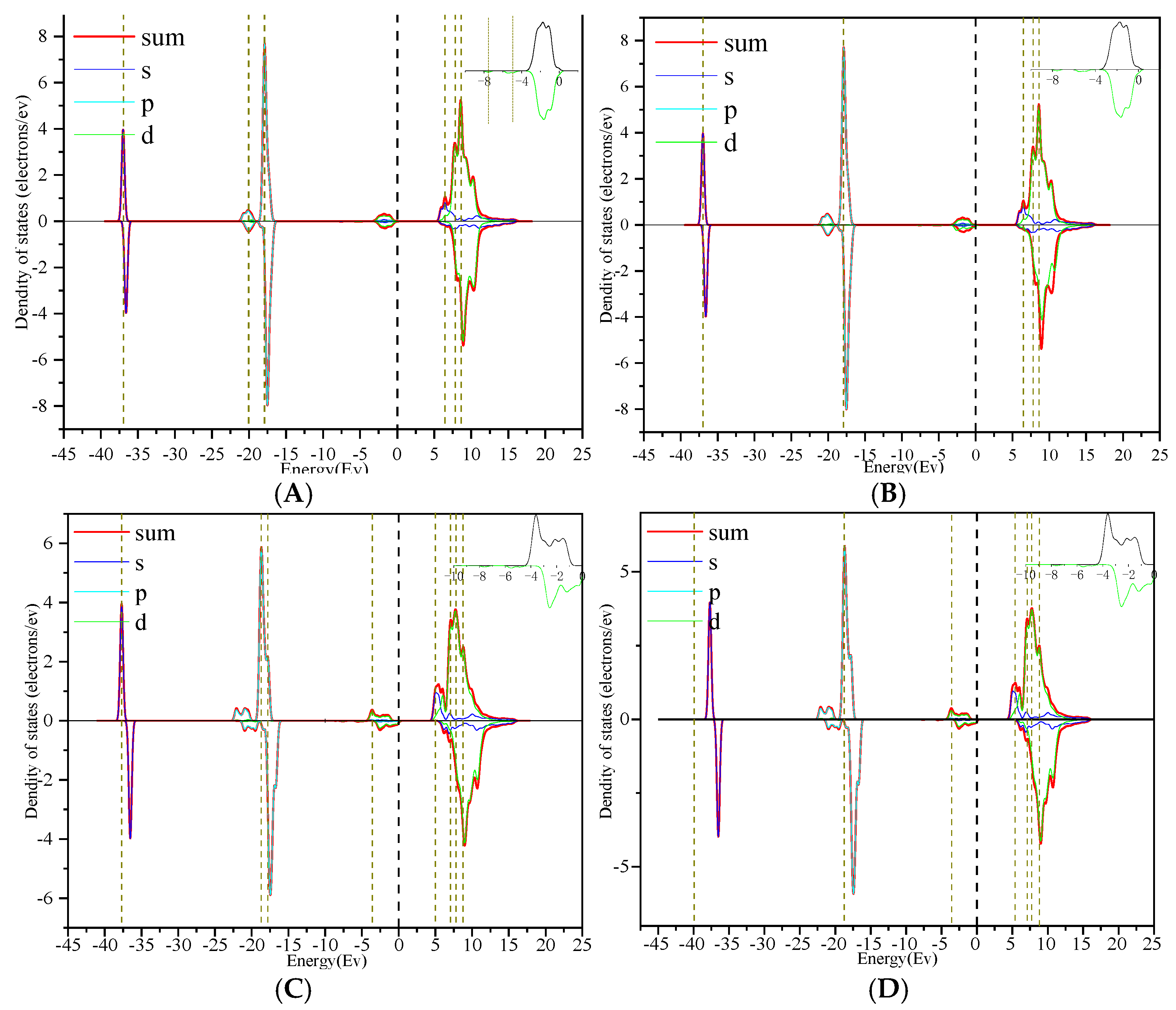
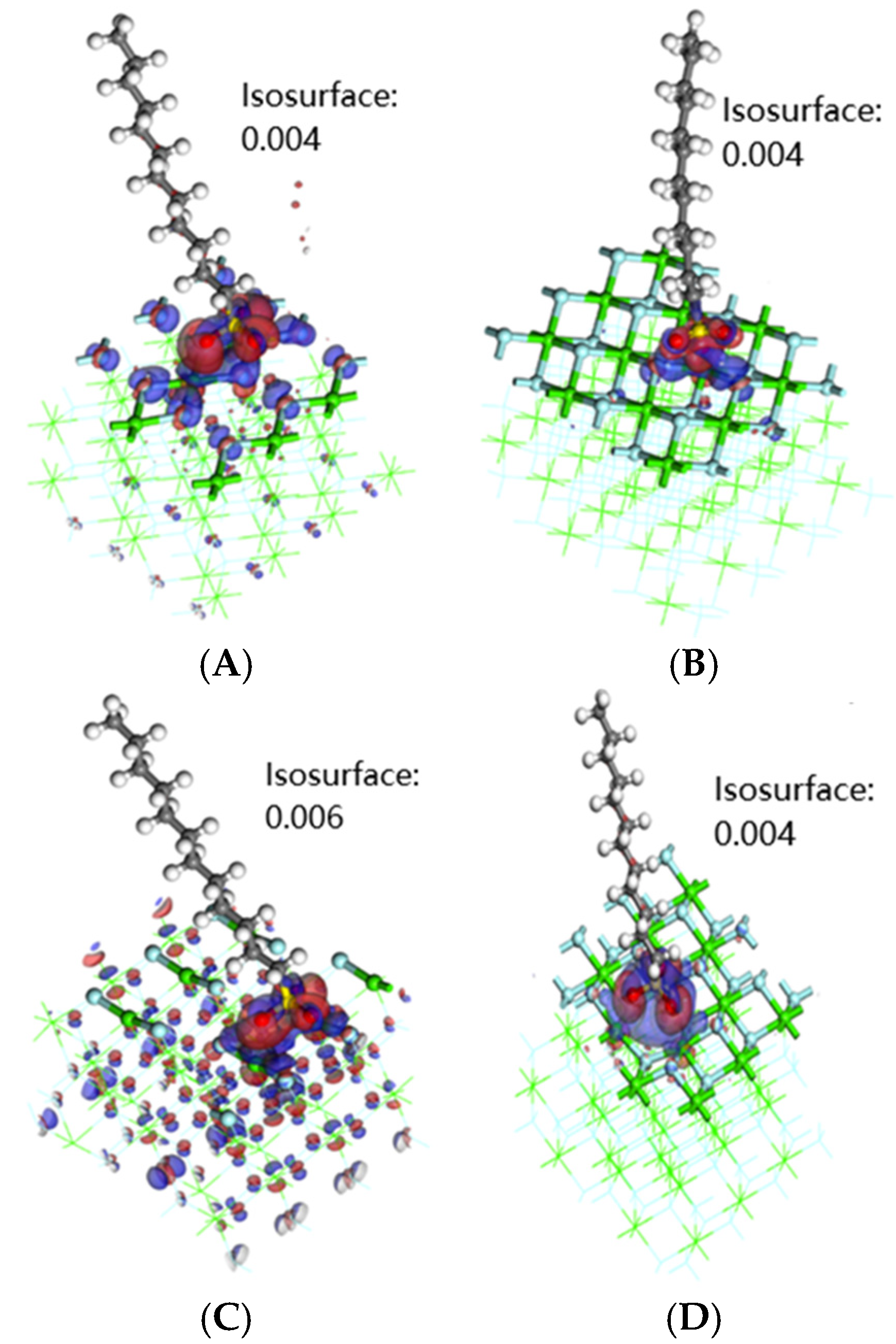
3.4. Electron Difference
4. Conclusions
- (1)
- The stable adsorption configuration of sodium dodecyl sulfonate on the (1 1 1) surface was in single coordination mode, while the stable adsorption configuration with edges was in bridge mode, and the stable action configuration with (1 1 1) _vacancy was in tridentate mode. The interaction between sodium dodecyl sulfonate and several simulated surfaces and (1 1 1) _vacancy was in the order of (1 1 1) _vacancy > (3 1 1) > (1 1 1) > (1 1 0).
- (2)
- Through the analysis of the density of states and differential charges on the surfaces of edges and vacancy, the 2p orbit of fluorine had an obvious effect on the collector, and the electron was lost in the reaction process, which hinders the adsorption. The 3d orbits of calcium ions react with the anti-bonding orbits of collectors above the Fermi level, and then broaden to the low energy level, and resonate with their corresponding bonding orbits. Calcium gained some electrons in this process, which was beneficial to adsorption.
- (3)
- By comparing the adsorption energies and average bond lengths between different surfaces, the adsorption intensity of alkyl sulfonate on fluorite was found to be directly proportional to the action intensity of the collector on the 3d orbits of calcium ions on the surface and vacancy.
- (4)
- The existence of edges required a high collector concentration to achieve saturated monolayer adsorption. So, increasing the surface vacancy of fluorite is an effective method to improve the adsorption of sulfonate on fluorite surfaces. By using acid or alkali to etch the surface of fluorite, it may be possible to increase the vacancies on the fluorite surface, which would lead to improved flotation results.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, L.; Liu, J.; Zhu, Y.; Gong, G.; Han, Y. Mechanism of HCA and CEPPA in flotation separation of cassiterite and fluorite. Miner. Eng. 2022, 187, 107773. [Google Scholar] [CrossRef]
- Qian, Y.; Qiu, X.; Shen, T.; Huai, Y.; Chen, B.; Wang, Z. Effect of Calcium Ion on the Flotation of Fluorite and Calcite Using Sodium Oleate as Collector and Tannic Acid as Depressant. Minerals 2022, 12, 996. [Google Scholar] [CrossRef]
- Wang, R.; Han, H.; Sun, W.; Nguyen, A.; Sun, W.; Wei, Z. Hydrophobic behavior of fluorite surface in strongly alkaline solution and its application in flotation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125661. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, Z.; Gao, H.; Qian, Y.; Zheng, R. Effect of modified starch on separation of fluorite from barite using sodium oleate. Physicochem. Probl. Miner. Process. 2018, 54, 228–237. [Google Scholar]
- Nikita, V.; Roman, Y.; Marina, F.; Igor, V.; Anatoly, N.; Vasily, D.; Dmitry, A. Crystal Chemistry, Isomorphism, and Thermal Conversions of Extra-Framework Components in Sodalite-Group Minerals. Minerals 2022, 12, 887. [Google Scholar]
- Gao, Z.; Fan, R.; Ralston, J.; Sun, W.; Hu, Y. Surface broken bonds: An efficient way to assess the surface behavior of fluorite. Miner. Eng. 2019, 130, 15–23. [Google Scholar]
- Zheng, R.; Ren, Z.; Gao, H.; Chen, Z.; Qian, Y.; Li, Y. Effects of crystal chemistry on sodium oleate adsorption on fluorite surface investigated by molecular dynamics simulation. Miner. Eng. 2018, 124, 77–85. [Google Scholar] [CrossRef]
- Li, C.; Gao, Z. Tune surface physicochemical property of fluorite particles by regulating the exposure degree of crystal surfaces. Miner. Eng. 2018, 128, 123–132. [Google Scholar] [CrossRef]
- Foucaud, Y.; Lebegue, S.; Filippov, L.; Filippova, I.V.; Badawi, M. Molecular Insight into Fatty Acid Adsorption on Bare and Hydrated (1 1 1) Fluorite Surface. J. Phys. Chem. B 2018, 122, 12403–12410. [Google Scholar] [CrossRef]
- Motzer, C.; Reichling, M. Morphological classification and quantitative analysis of etch pits. J. Appl. Phys. 2010, 108, 113523. [Google Scholar] [CrossRef]
- Pieper, H.; Barth, C.; Reichling, M. Characterization of atomic step structures on CaF2 (1 1 1) by their electric potential. Appl. Phys. Lett. 2012, 101, 051601. [Google Scholar] [CrossRef]
- Puchin, V.; Huisinga, M.; Reichling, M.; Puchina, A. Theoretical modelling of steps on the CaF2 (1 1 1) surface. J. Phys. Condens. Matter. 2001, 13, 2081–2094. [Google Scholar] [CrossRef]
- Kobayashi, N.; Itakura, S.; Asakawa, H.; Fukuma, H. Atomic-Scale Processes at the Fluorite−Water Interface Visualized by Frequency Modulation Atomic Force Microscopy. J. Phys. Chem. C 2013, 117, 24388–24396. [Google Scholar] [CrossRef][Green Version]
- de Leeuw, N.H.; Parker, S.C.; Rao, K.H. Modeling the Competitive Adsorption of Water and Methanoic Acid on Calcite and Fluorite Surfaces. Langmuir 1998, 20, 5900–5906. [Google Scholar] [CrossRef]
- Dabringhaus, H.; Wandelt, K. Theoretical study of the adsorption of a CaF2 molecule at the (1 1 1) surface of CaF2 I. Equilibrium adsorption positions. Surf. Sci. 2003, 526, 257–272. [Google Scholar] [CrossRef]
- Xu, B.; Wu, J.; Dong, Z.; Tao, J.; Qian, L.; Yang, Y. Flotation performance, structure–activity relationship and adsorption mechanism of a newly-synthesized collector for copper sulfide minerals in Gacun polymetallic ore. Appl. Surf. Sci. 2021, 551, 149420. [Google Scholar] [CrossRef]
- Li, M.; Sun, B.; Ao, Z.; An, T.; Wang, G. Atomic-scale identification of influencing factors of sodium dendrite growth on different current collectors. J. Mater. Chem. A 2020, 8, 10199–10205. [Google Scholar] [CrossRef]
- Weinberg, W.; Merrill, R. Crystal field surface orbit—Bond-energy bond- order (CFSO-BEBO) model for chemisorption: Application to hydrogen adsorption on a platinum (1 1 1) surface. Surf. Sci. 1972, 33, 493–515. [Google Scholar] [CrossRef]
- Zhu, H.; Deng, H.; Chen, C. Flotation separation of andalusite from quartz using sodium petroleum sulfonate as collector. Trans. Nonferrous Met. Soc. China 2015, 25, 1279–1285. [Google Scholar] [CrossRef]
- Jin, J.; Gao, H.; Ren, Z.; Chen, Z. The Flotation of Kyanite and Sillimanite with Sodium Oleate as the Collector. Minerals 2016, 6, 90. [Google Scholar] [CrossRef]
- Ren, Z.; Shen, Y.; Gao, H.; Chen, H.; Liu, C.; Chen, Z. Comparison of Sodium Oleate and Sodium Petroleum Sulfonate for Low-Temperature Flotation of Fluorite and the Collecting Mechanisms. Min. Met. Explor. 2021, 38, 2527–2536. [Google Scholar] [CrossRef]
- Clark, S.; Segall, M.; Pickard, C.; Hasnip, P.; Probert, M.; Refson, K.; Payne, M. First principles methods using CASTEP. Z. Krist. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Tkatchenko, A.; Scheffler, M. Accurate Molecular Van Der Waals Interactions from Ground-State Electron Density and Free-Atom Reference Data. J. Phys. Rev. Lett. 2009, 102, 073005. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Han, Y. Gene Properties of Barite (0 0 1) Surface Based on CASTEP Simulation. Met. Mine 2020, 49, 94–98. [Google Scholar]
- Chen, Z.; Ren, Z.; Gao, H.; Lu, J.; Jin, J.; Min, F. The Effects of Calcium Ions on the Flotation of Sillimanite Using Dodecylammonium Chloride. Minerals 2017, 7, 28. [Google Scholar] [CrossRef]
- Tankaria, H.; Sugimoto, S.; Yamashita, N. A regularized limited memory BFGS method for large-scale unconstrained optimization and its efficient implementations. Comput. Optim. Appl. 2022, 82, 61–68. [Google Scholar] [CrossRef]
- Segall, M.; Lindan, P.; Probert, M.; Pickard, C.; Hasnip, P.; Clark, S.; Payne, M. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter. 2002, 14, 2717. [Google Scholar] [CrossRef]
- Gao, Z.; Li, C.; Sun, W.; Hu, Y. Anisotropic surface properties of calcite: A consideration of surface broken bonds. Colloids Surf. A. Physicochem. Eng. Asp. 2017, 520, 53–61. [Google Scholar] [CrossRef]
- Marinalis, K.; Shergold, H. The mechanism of fatty acid adsorption in the presence of fluorite, calcite and barite. Int. J. Miner. Process. 1985, 14, 161–176. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, Z.; Gao, H.; Zheng, R.; Jin, Y.; Niu, G. Flotation studies of fluorite and barite with sodium petroleum sulfonate and sodium hexametaphosphate. J. Mater. Res. Technol. 2019, 8, 1267–1273. [Google Scholar] [CrossRef]



| Surface | Bond Length (Å) | Average Bond Length (Å) | Surface Energy (eV) | ||
|---|---|---|---|---|---|
| (1 1 1) | 2.397 | 2.397 | −84,052.59 | ||
| (1 1 0) | 2.495 | 2.486 | 2.492 | −112,076.89 | |
| (3 1 1) | 2.431 | 2.458 | 2.445 | −84,041.33 | |
| (1 1 1) _vacancy | - | - | - | 2.331 | −111,437.02 |
| Surface | C12S (eV) | Surface (eV) | C12S + Surface (eV) | Adsorption Energy (eV) |
|---|---|---|---|---|
| (1 1 1) | −3914.47 | −84,035.9 | −87,951.26 | −0.89 |
| (1 1 0) | −3913.21 | −112,054.92 | −115,969.59 | −1.46 |
| (3 1 1) | −3914.55 | −84,024.3 | −87,940.39 | −1.54 |
| (1 1 1) _vacancy | −3914.54 | −111,388.99 | −115,310.28 | −6.75 |
| Surface | Adsorption Energy (eV) | Average Bond Length (Å) | Bonding Atoms |
|---|---|---|---|
| (1 1 1) | −0.89 | 2.492 | 2 |
| (1 1 0) | −1.46 | 2.397 | 1 |
| (3 1 1) | −1.54 | 2.445 | 2 |
| (1 1 1) _vacancy | −6.75 | 2.331 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Wang, Z.; Ren, Z.; Zheng, R.; Gao, H.; Chen, Z. The Influence of Surface Heterogeneity of Fluorite on the Adsorption of Alkyl Sulfonates. Minerals 2023, 13, 1005. https://doi.org/10.3390/min13081005
He Y, Wang Z, Ren Z, Zheng R, Gao H, Chen Z. The Influence of Surface Heterogeneity of Fluorite on the Adsorption of Alkyl Sulfonates. Minerals. 2023; 13(8):1005. https://doi.org/10.3390/min13081005
Chicago/Turabian StyleHe, Yuhao, Zengzi Wang, Zijie Ren, Renji Zheng, Huimin Gao, and Zhijie Chen. 2023. "The Influence of Surface Heterogeneity of Fluorite on the Adsorption of Alkyl Sulfonates" Minerals 13, no. 8: 1005. https://doi.org/10.3390/min13081005
APA StyleHe, Y., Wang, Z., Ren, Z., Zheng, R., Gao, H., & Chen, Z. (2023). The Influence of Surface Heterogeneity of Fluorite on the Adsorption of Alkyl Sulfonates. Minerals, 13(8), 1005. https://doi.org/10.3390/min13081005








