Characters and Metallogenetic Significance of Organic Matter in Coal from the Daying Sandstone-Hosted Uranium Deposit in the Northern Ordos Basin, China
Abstract
:1. Introduction
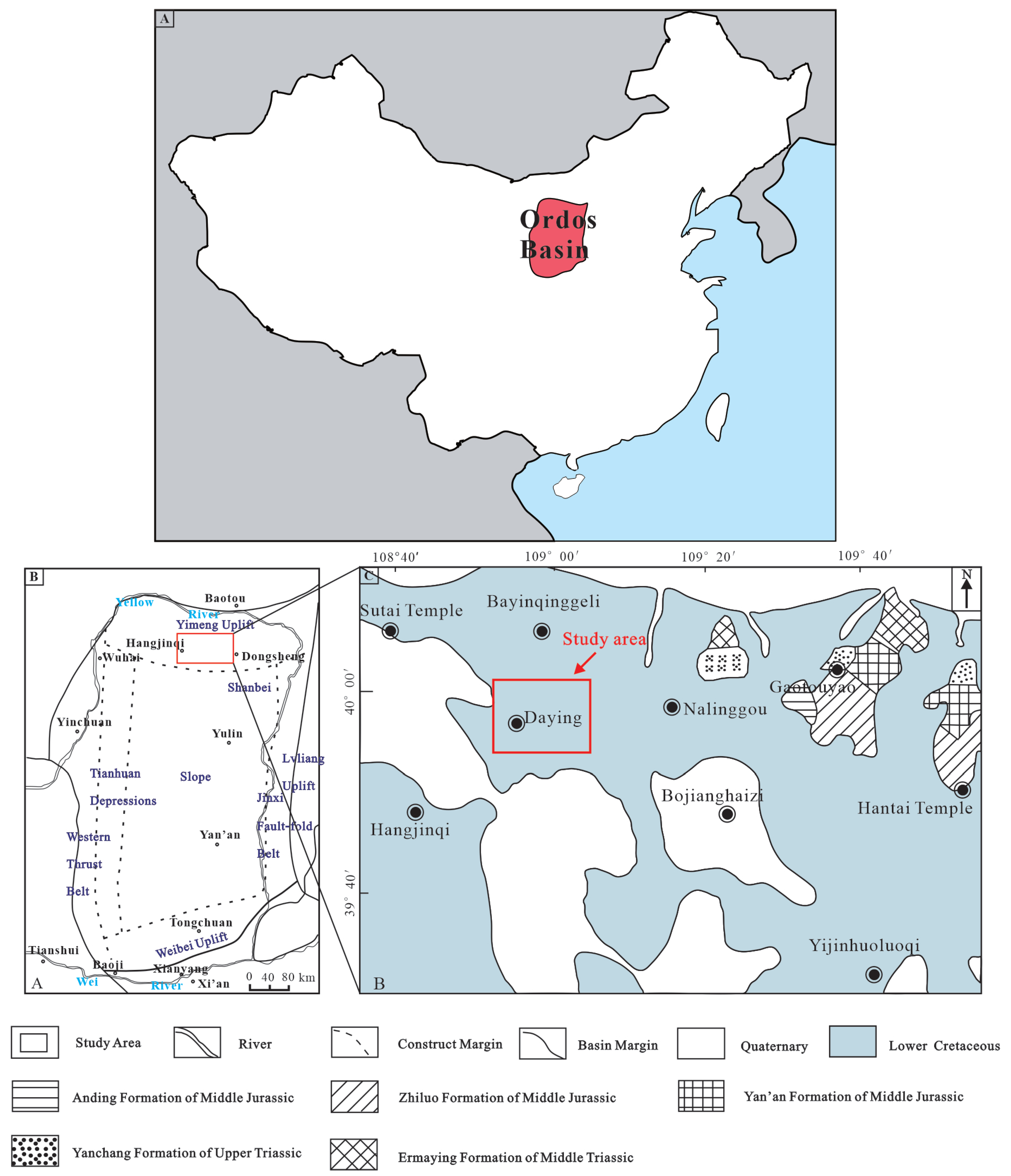
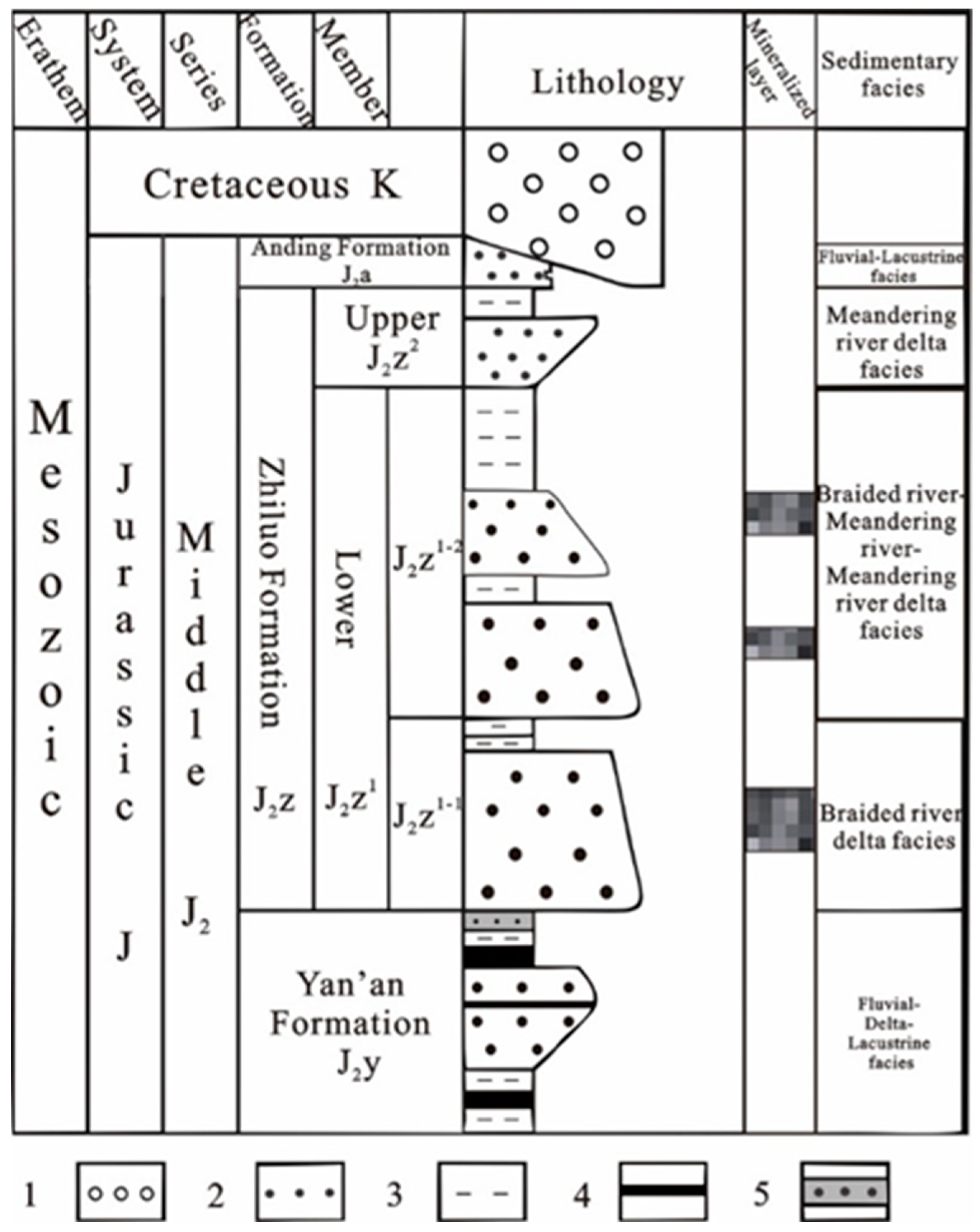
2. Geological Setting
3. Samples and Methodology
3.1. Samples
3.2. Methodology
4. Results
4.1. Geological Characters of Coal Organic Matters
4.1.1. Correlation of Uranium to Organic Carbon Contents
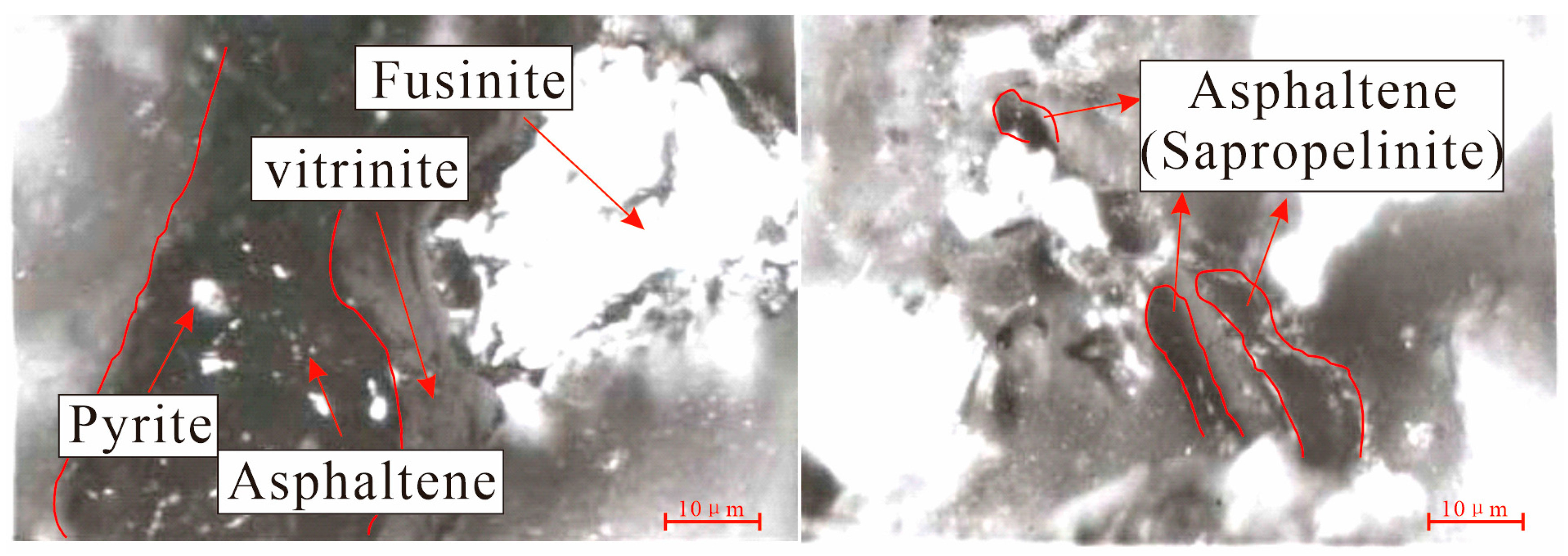
4.1.2. Organic Macerals
4.1.3. Maturity of Organic Matter
4.2. Geochemical Characters of Coal Organic Matter
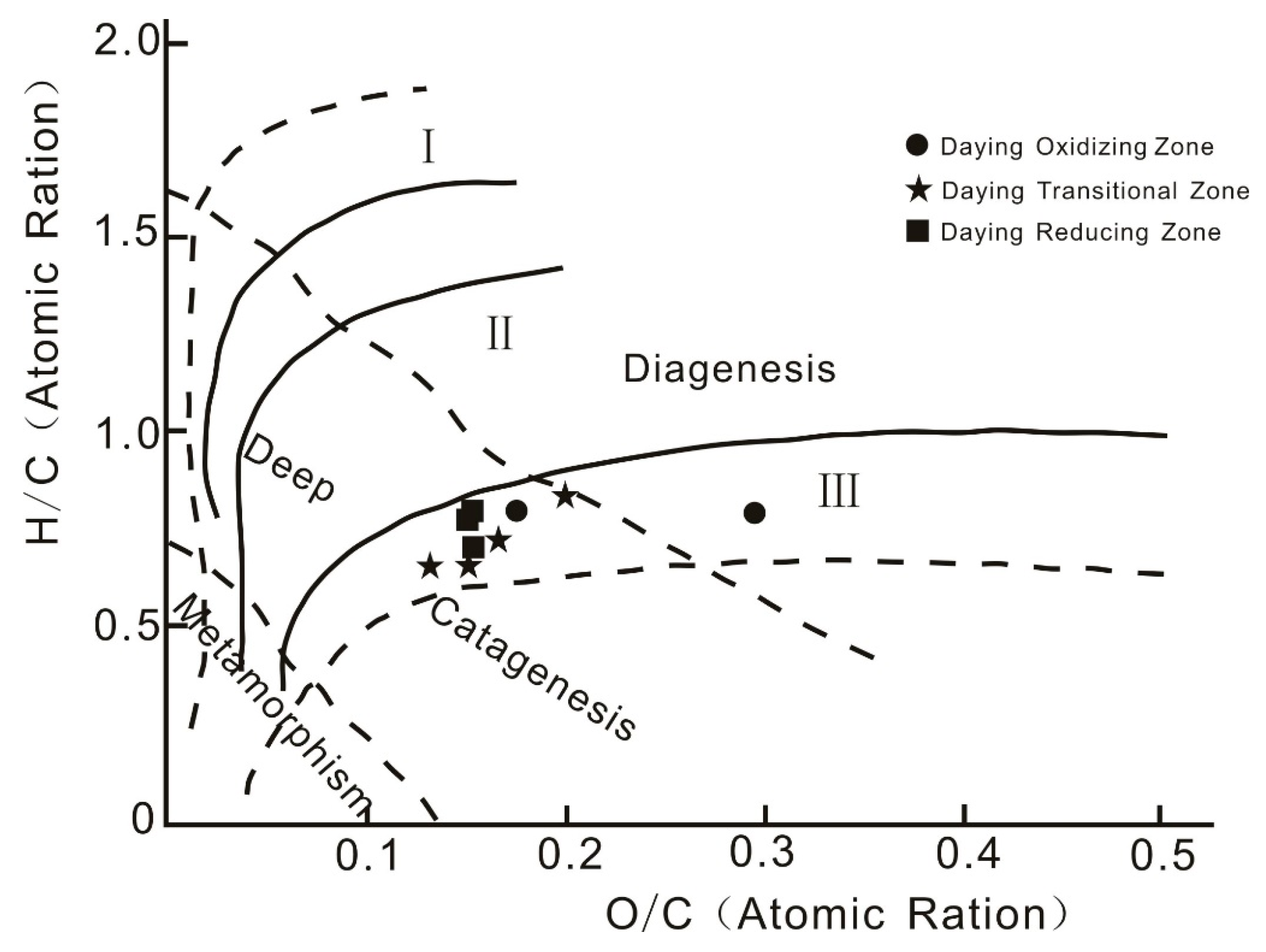
4.2.1. Kerogen Types—Carbon Isotopes
4.2.2. Kerogen Chemistry
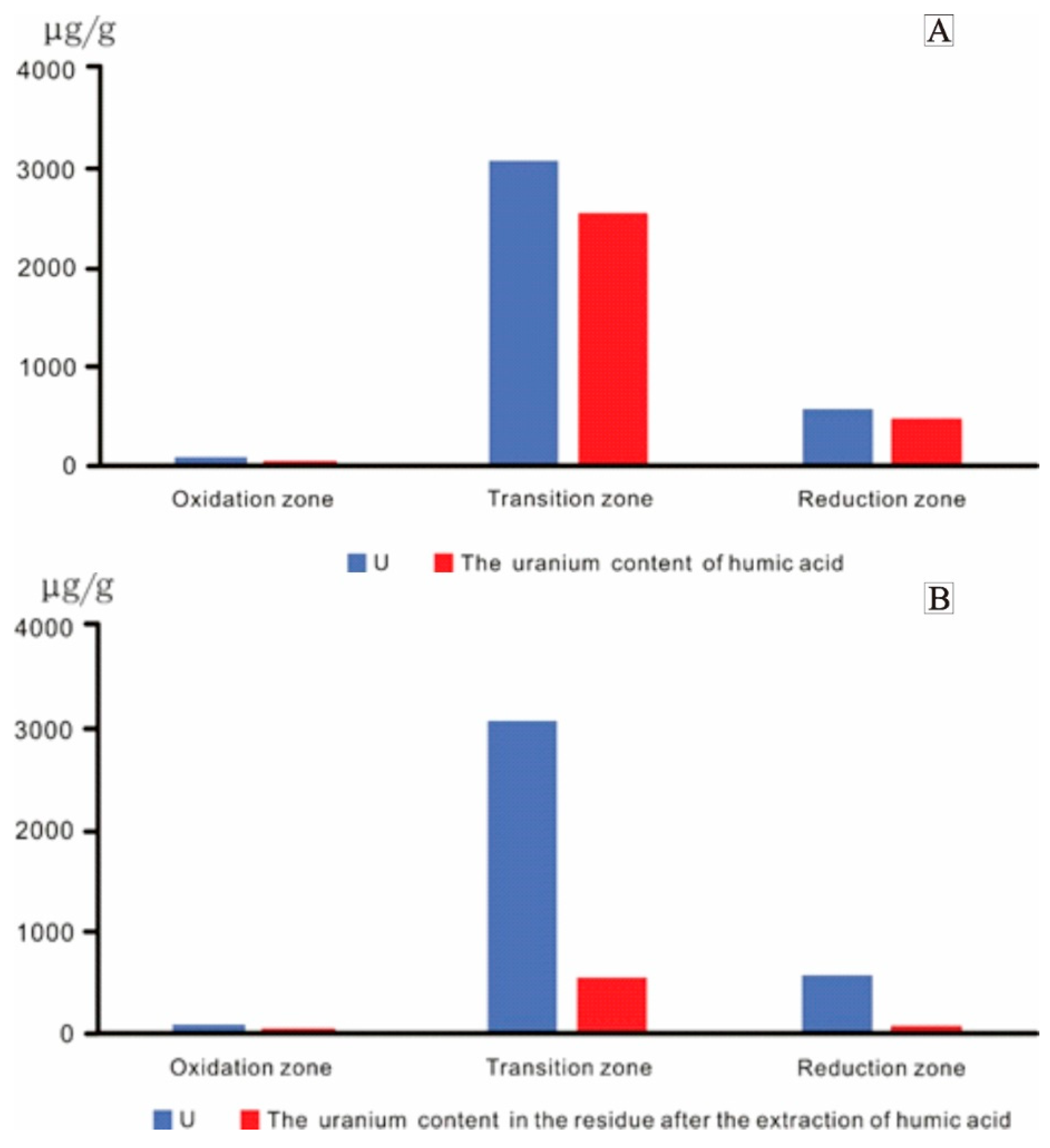
4.3. Analysis of Humic Acid and Uranium Content
5. Discussion
5.1. Immature Organic Matter
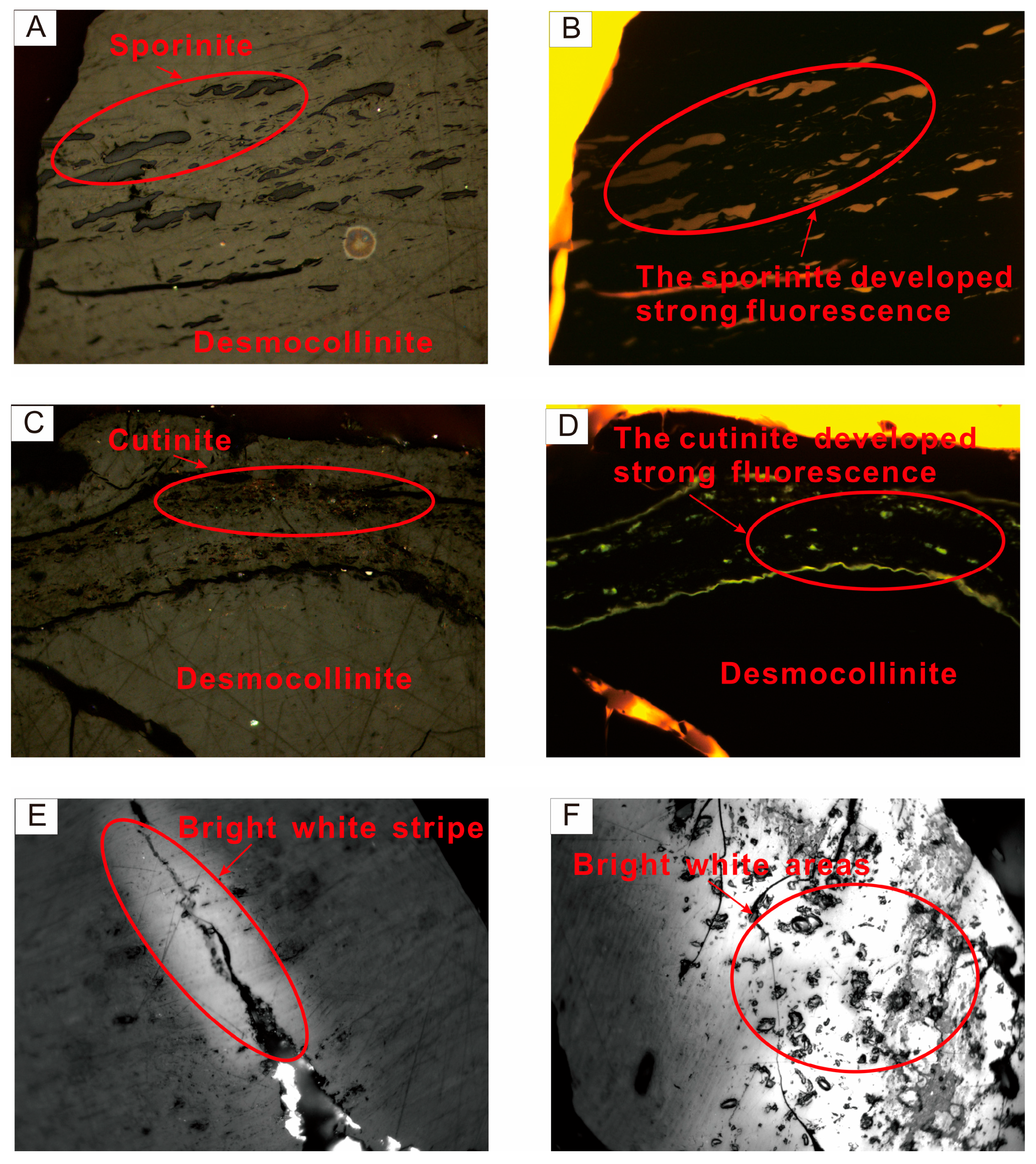
5.2. Relationship between Strong Fluorescence Properties and Uranium Mineralization
5.3. Role of Organic Matter during Uranium Mineralization
6. Conclusions
- (1)
- The ore of the Daying uranium mine usually contains lots of coal organic matter which is positively correlated to uranium enrichment. The primary maceral of coal organic matter is mostly vitrinite, reflecting the characters of humic coal. And the organic matter is in the immature stage, which is equivalent to immature lignite or the lignite-long-flame coal stage, due to an Ro < 0.5%.
- (2)
- The organic matter in coal at Daying is dominated by type Ⅲ (humic type) kerogen. This kind of organic matter has a strong adsorption capacity for uranium and acts as a reductant in uranium enrichment and mineralization. The humic acid in the coal organic matter plays an important role in uranium mineralization, as evidenced by humic substance extraction and separation experiments. In the transition zone, uranium was precipitated in the form of uranium humate, and the organic carbon content in the transition zone increased. Kerogen chemistry shows that coal organic matter is sourced from terrestrial higher plants and its sedimentation occurred during the deep burial epigenetic stage, which was strongly influenced by epigenesis, fluids, and natural gas.
- (3)
- The vitrinite reflectance (Ro) of the coal is relatively higher in the transition zone (mineralized zone) than that in the reduction and oxidation zones. The phenomenon can be attributed to the catalytic influence of uranium mineralization on organic matter, leading to a marginally greater degree of organic matter maturation compared to other zones. Furthermore, it can serve as a definitive indication of mineral exploration.
- (4)
- In the transition zone (mineralized zone), the microspore and cutinite of the organic matter exhibit strong fluorescence (hydrocarbon shows) and bright white bands in the organic maceral components. These can also be utilized for the purpose of mineral exploration. These phenomena are associated with the radioactive properties of uranium and the impact of hydrocarbons. The phenomenon of fluorescent localized sapropelinite can be attributed to the localized enrichment of uranium within the organic matter, resulting in enhanced maturation of the organic matter and early generation of hydrocarbon production.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.Y.; Zhao, H.G.; Gui, X.J.; Yue, L.P.; Zhao, J.F.; Wang, J.Q. Space-Time Coordinate of the Evolution and Reformation and Mineralization Response in Ordos Basin. Acta Geol. Sin. 2006, 5, 617–638, (In Chinese with English abstract). [Google Scholar]
- Jin, R.S.; Miao, P.S.; Sima, X.Z.; Feng, X.X.; Tang, C.; Zhu, Q.; Li, G.Y. Discussion on the classification about the uranium deposit. Geol. Surv. Res. 2014, 37, 1–5. [Google Scholar]
- Charles, S. The roles of organic matter in the formation of uranium deposit in sedimentary rock. Ore Geol. Rev. 1996, 11, 54–55. [Google Scholar]
- Shanbhag, P.M. Binding of uranyl by humic acid. Nucl. Chem. 1981, 43, 41–54. [Google Scholar] [CrossRef]
- Wood, S.A. The role of humic substances in the transport and fixation of metals of economic interest (Au, Pt, U, V). Ore Geol. Rev. 1996, 11, 2–6. [Google Scholar] [CrossRef]
- Wu, B.L.; Liu, C.Y.; Wang, J.Q. Basical Characters of Fluid Geologic process of Interlayer Oxidation Zone Standstone-hosted Uranium Deposit. Sci. China Ser. D Earth Sci. 2007, 50, 185–194. [Google Scholar] [CrossRef]
- Wu, B.L.; Xu, G.W.; Liu, C.Y.; Qiu, X.W.; Wang, J.Q.; Hu, L.; Zhang, B.H. Alteration Effects of Hydrocarbon Dissipation in the Dongsheng Uranium Deposit, The Ordos Basin-Explanation for Green Alteration and Bleaching Phenomenon. Energy Explor. Explor. 2009, 27, 181–199. [Google Scholar]
- Xiang, W.D.; Chen, Z.B.; Chen, Z.Y.; Yin, J.S. Discussion on the mineralization of organic matter and epigenetic sandstone-hosted uranium deposit—a case study of Shihongtan area in the Tuha basin. Uranium Geol. 2000, 16, 65–114. [Google Scholar]
- Xiang, W.D.; Yin, J.S. The mechanism of humic acid in the mineralization process of sandstone-type uranium deposit. World Nucl. Geol. 2006, 23, 73–77. [Google Scholar]
- Xiang, W.D.; Fang, X.Y.; Li, T.G.; Chen, X.L.; Pang, Y.Q.; Cheng, H.H. Metallogenic characters and metallogenic model of Dongsheng uranium deposit in northeastern the Ordos Basin. Uranium Geol. 2006, 22, 257–265. [Google Scholar]
- Qiao, H.M.; Song, Z. Study on the interaction of microorganism and organic matter in uranium mineralization process of Shihongtan uranium deposit in the Tuha Basin. Ore Geol. Rev. 2015, 61, 229–236. [Google Scholar]
- Sun, Y. The organic geochemical zonation of sandstone uranium deposit and its relationship with uranium mineralization—A case study of the Zaohuohao uranium deposit in Inner Mongolia. Uranium Geol. 2016, 32, 129–136. [Google Scholar]
- Zhang, L.; Wu, B.L.; Liu, C.Y.; Lei, K.Y.; Hou, H.Q.; Sun, L.; Cun, X.N.; Wang, J.Q. Province Source Analysis of Zhiluo Formation of Sandstone-hosted Uranium Deposit in North the Ordos Basin and Its Uranium Mineralization Significance. Acta Geol. Sin. 2016, 90, 3441–3453. [Google Scholar]
- Zhu, Q.; Yu, R.A.; Li, J.G.; Sima, X.Z.; Si, Q.H.; Li, G.Y.; Wen, S.B.; Liu, X.X.; Wang, S.B. Pairs of Reducing Media in the Tarangaole Area in the Northeastern Part of the Ordos Basin Control of Sandstone-Type Uranium Deposits. Coal Geol. Explor. 2018, 46, 11–18. [Google Scholar]
- Jiao, Y.Q.; Wu, L.Q.; Rong, H. The Dual Reducing Medium Model of Sandstone-Type Uranium Deposits and its Joint Ore-Controlling Mechanism: A Discussion on the Daying and Qianjiadian Uranium Deposits. J. Earth Sci. 2018, 43, 459–474. [Google Scholar]
- Zhu, Q.; Yu, R.A.; Li, G.Y.; Wen, S.B.; Li, J.G.; Si, Q.H.; Guo, H. Associated Minerals of Sandstone-Type Uranium Deposits in the Northeast of Ordos Basin and Their Geological Significance. Geotecton. Metallog. 2021, 45, 327–344. [Google Scholar]
- Landais, P. Organic geochemistry of sedimentary uranium ore deposit. Ore Geol. Rev. 1996, 11, 33–51. [Google Scholar] [CrossRef]
- Yi, C.; Gao, H.W.; Li, X.D.; Zhang, K.; Chen, X.L.; Li, J.X. Exploration on the significance of constant element indication for sandstone type uranium deposits of the Zhiluo Formation in northeastern Ordos Basin. Miner. Depos. Geol. 2015, 34, 801–813. [Google Scholar]
- Yang, D.Z.; Xia, B. An Experiment Study on the Role of Organic Materials in Ore-forming of Sandstone-Type Uranium Deposit—A Case Study of the Shihongtan Deposit, Turpan-Hami Basin. Ore Geol. Rev. 2004, 50, 218–222. [Google Scholar]
- Wang, G.R. Oxidization and Migration of Organic Matter in Mineralization of Interlayer Seeping Uranium. Xinjiang Geol. 2002, 20, 134–136. [Google Scholar]
- Zhang, W.H. Experimental Study on the Coordination Role of Humic Acid in Uranium Mineralization Process. Master’s Thesis, Northwest University, Xi’an, China, 2006. [Google Scholar]
- Cai, Y.G. Experimental Simulation of the Role of Coal-Gas-Oil in Uranium Mineralization. Master’s Thesis, Northwest University, Xi’an, China, 2008. [Google Scholar]
- He, Z.X.; Fu, J.H.; Xi, S.L.; Fu, S.T.; Bao, H.P. Geological characters of the reservoir in gas field in the Sulige. J. Oil 2003, 24, 6–12. [Google Scholar]
- Quan, Z.G. Classification of interstratigraphic oxide zone boundaries and evaluation of uranium mineralization potential. Geol. Miner. Search Ser. 2003, 4, 225–228. [Google Scholar]
- Yang, R.C.; Han, Z.Z.; Fan, A.P. Sedimentary microphase and stratigraphic stratigraphy of sandstone uranium deposits in the Dongsheng area of Ordos Basin. J. Stratigr. 2007, 31, 261–266. [Google Scholar]
- Miao, A.S.; Jiao, Y.Q.; Chang, B.C.; Wu, L.Q.; Rong, H.; Liu, Z.B. Fine dissection of the ancient interstratified oxide zone of the Dongsheng uranium deposit in northeastern Ordos Basin. Geol. Sci. Technol. Inf. 2010, 29, 55–61. [Google Scholar]
- Zhang, J.D.; Li, Z.Y.; Xu, G.Z. Significant Progress and Breakthroughs in Uranium Exploration in China—Examples of Newly Discovered and Proven Uranium Deposits since the Entrance of the New Century, 2nd ed.; Geology Press: Beijing, China, 2015; ISBN 9787116091641. [Google Scholar]
- Han, X.Z.; Zhang, Z.L.; Yao, C.L.; Li, X.D.; Li, S.X.; Miao, A.S. Study on uranium mineralization model in the northeast of the Ordos Basin. Ore Depos. Geol. 2008, 27, 415–421. [Google Scholar]
- Jin, R.S.; Miao, P.S.; Sima, X.Z. Structure Styles of Mesozoic-Cenozoic U-bearing Rock Series in Northern China. Acta Geol. Sin. (Engl. Ed.) 2016, 90, 2104–2116. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Wang, S.Y.; Jin, R.S.; Li, J.G.; Ao, C.; Teng, X.M. Global Miocene tectonics and regional sandstone-style uranium mineralization. Ore Geol. Rev. 2019, 106, 238–250. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Wang, S.Y.; Li, Y.; Ao, C.; Li, J.G.; Li, H.; Zhang, T. Late Cretaceous–Cenozoic thermochronology in the southern Songliao Basin, NE China: New insights from apatite and zircon fission track analysis. J. Asian Earth Sci. 2018, 160, 95–106. [Google Scholar] [CrossRef]
- Xia, Y.L.; Lin, J.R.; Liu, H.B.; Fan, G.; Hou, Y.G. Chronology of uranium mineralization in the main uranium basin in north China. Uranium Geol. 2003, 3, 129–160. [Google Scholar]
- Chen, F.Z. Paleo hydrogeological conditions and prospect of uranium mineralization in northern the Ordos Basin. Uranium Geol. 2002, 18, 287–294. [Google Scholar]
- Sun, Y.; Li, Z.Y.; Xiao, X.J.; Hou, G.W. Discuss the relationship between oil and gas trap and uranium mineralization in northern the Ordos Basin. Uranium Geol. 2004, 20, 337–343. [Google Scholar]
- Jin, R.S.; Cheng, Y.H.; Yang, J.; Ao, C.; Li, J.G.; Li, Y.F.; Zhou, X.X. Classification and correlation of Jurassic uranium-bearing series in the Junggar Basin. Acta Geol. Sin. 2016, 90, 3293–3309. [Google Scholar]
- Wu, B.L.; Wang, J.Q.; Liu, C.Y.; Wang, F.Y. Geochemical characters of natural gas geology in the formation of Dongsheng sandstone type uranium ore. Pet. Nat. Gas Geol. 2006, 2, 225–232. [Google Scholar]
- Li, Z.Y.; Fang, X.F.; Chen, A.P.; Ou, G.X.; Xiao, X.J.; Sun, Y.; Liu, C.Y.; Wang, Y. Genesis of gray-green sandstone in sandstone type uranium target layer in northern Ordos Basin. Chin. Sci. (Ser. D Earth Sci.) 2007, S1, 139–146. [Google Scholar]
- EJ/T550-2000; Determination of Uranium in Soil and Rock Samples by Laser-Induced Fluorometry. Commission of Science, Technology and Industry for National Defence of the People’s Republic of China: Beijing, China, 2000.
- DZ/T0279.27-2016; Determination of Organic Carbon Contents by Potassium Dichromate Volumetric Method. Ministry of Land and Resources of the People’s Republic of China: Beijing, China, 2016.
- GB/T6948-2008; Method of Determining Microscopically the Reflectance of Vitrinite in Coal. Standardization Administration of China: Beijing, China, 2008.
- Zhuang, X.G.; Yang, S.K.; Zeng, R.S. Characters of Trace Elements in Several Major Coal Producing Areas in China. Geotech. Inf. 1999, 18, 63–66. [Google Scholar]
- The Organic Geochemistry and Sedimentology Laboratory in the Institute of Geochemistry of the Chinese Academy of Sciences. Organic Geochemistry, 1st ed.; Science Press: Beijing, China, 1982; ISBN CN13031.3168. [Google Scholar]
- Mao, G.Z.; Liu, C.Y.; Zhang, D.D.; Qiu, X.W.; Wang, J.Q.; Liu, B.Q.; Liu, J.J.; Qu, S.D.; Deng, Y.; Wang, F.F.; et al. Experimental study on the role of uranium in the evolution of hydrocarbon generation in type III hydrocarbon rocks. China Sci. Earth Sci. 2014, 44, 1740–1750. [Google Scholar]
- Mao, G.Z.; Liu, C.Y.; Zhang, D.D.; Qiu, X.W.; Wang, J.Q.; Liu, B.Q.; Liu, J.J.; Qu, S.D.; Zhang, S.; Deng, Y.; et al. Influence of uranium on the hydrocarbon evolution of (type II) low-maturity hydrocarbon source rocks. J. Geol. 2012, 86, 1833–1840. [Google Scholar]
- Mao, G.Z.; Liu, C.Y.; Liu, B.Q.; Zhang, D.D.; Qiu, X.W.; Wang, J.Q. Influence of uranium on the evolution of hydrocarbon generation in type Ⅰ low-maturity hydrocarbon source rocks. J. China Univ. Pet. (Nat. Sci. Ed.) 2012, 36, 172–181. [Google Scholar]
- Wu, B.L. Geology and Mineralization of Sandstone Type Uranium Ore in the Middle Cenozoic Basin of Northwest China. Ph.D. Thesis, Northwest University, Xi’an, China, 2005. [Google Scholar]
- Yang, D.Z. Organic Geochemistry of Uranium in the Southwestern Jurassic of the Tuha Basin; Beijing Institute of Geology of Nuclear Industry: Beijing, China, 2002. [Google Scholar]
- Li, S.F.; Zhang, Y. Formation mechanism of uranium minerals at sandstone-type uranium deposits. Uranium Geol. 2004, 20, 81–84. [Google Scholar]


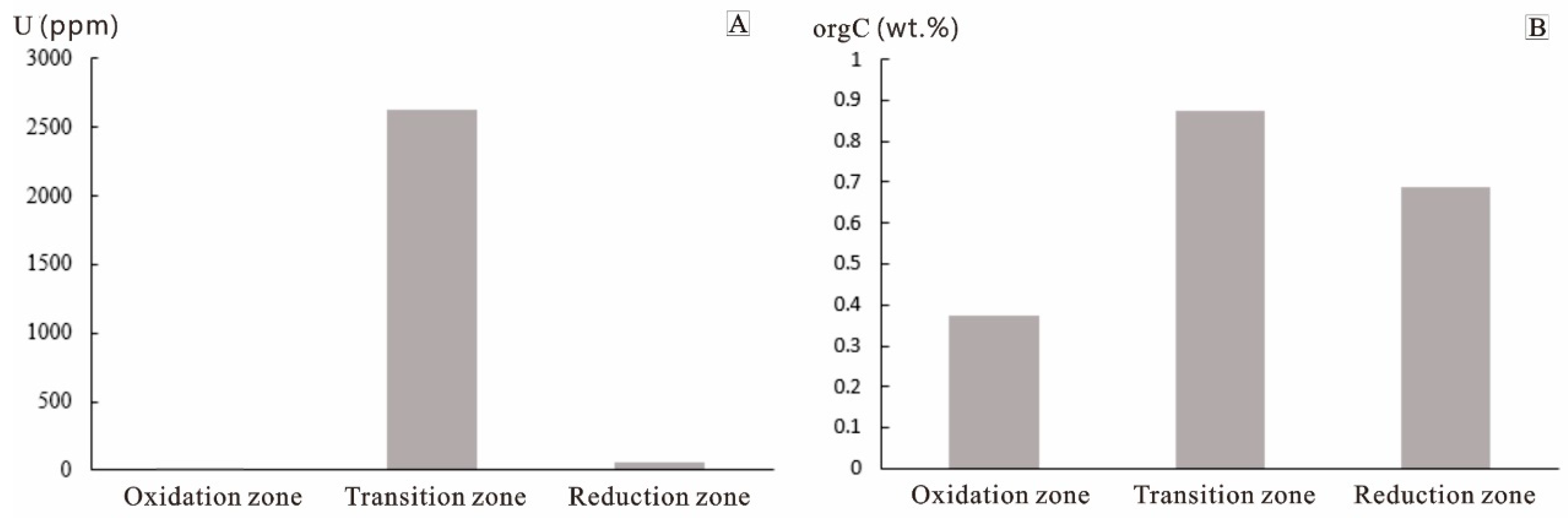
| Sample | Zoning | Lithologic Description | U ppm | orgC wt.% |
|---|---|---|---|---|
| ZKD112-96(3) | Oxidation zone | Brown-red middle-fine sandstone | 5.54 | 0.05 |
| ZKD112-96(4) | Brown-red middle-fine sandstone | 11.2 | 0.06 | |
| D63-16 | Gray-white middle-fine sandstone with carbonaceous strips | 6.75 | 1.01 | |
| Average | 7.83 | 0.37 | ||
| ZKD96-47(2) | Transition zone | Gray-white medium sandstone with carbonaceous strips | 3640 | 2.55 |
| D32-63-4 | Gray medium sandstone contains a small amount of carbonaceous debris | 3590 | 1.10 | |
| ZKD80-39-2 | Gray medium sandstone contains a small amount of carbonaceous debris | 1680 | 0.48 | |
| ZKD96-47-1 | Gray-white medium sandstone | 1290 | 0.35 | |
| 2014DY-27 | Gray siltstone sandstone contains a small amount of carbonaceous debris | 2101 | 0.75 | |
| ZKD128-81-6 | Light gray medium sandstone | 683 | 0.28 | |
| 2014DY-31 | Gray medium sandstone contains a small amount of carbonaceous debris | 2315 | 0.81 | |
| ZKD208-15-1 | Gray-green medium sandstone contains carbonaceous strips. | 828 | 0.32 | |
| ZKD96-31-1 | Light grayish-green coarse sandstone contains carbonaceous detritus | 1700 | 0.57 | |
| ZKD112-47-2 | Grayish-white coarse sandstone contains carbonaceous detritus. | 8480 | 1.53 | |
| Average | 3647.6 | 0.88 | ||
| ZKD144-71(8) | Reduction zone | Grayish-white medium sandstone | 25.1 | 0.10 |
| D127-55-1 | Grayish-white middle-coarse sandstone contains carbonaceous strips | 96.3 | 1.28 | |
| Average | 60.7 | 0.69 |
| Sample | Zoning | Statistical Parameter | Organic Matter = 100% | Maceral Description | |||
|---|---|---|---|---|---|---|---|
| Vitrinite | Inertinite | Exinite | Total | ||||
| D63-16 | Oxidation zone | Number of measuring point | 446 | 16 | 2 | 464 | It is mainly telocollinite, pure and homogeneous, with a small amount of telinite. The fusinite, the microspore of the shell, and a small amount of asphaltene can be found. The fluorescence of asphaltene is very weak, and the illumination of the degree of metamorphism is very low. |
| Percentage/% | 96.1 | 3.5 | 0.4 | 100.0 | |||
| ZKD95-16-1 | Number of measuring point | 145 | 4 | 1 | 150 | It is mainly telocollinite, which is transformed from the telinite, and the second is the telinite, which contains a small amount of the vitrinite. The semifusinite body develops and contains pyrite crystals, and the fluorescence of pyrite crystals is weak. It is indicated that pyrite crystals contain a small amount of bituminous bodies. | |
| Percentage/% | 96.7 | 2.7 | 0.6 | 100.0 | |||
| ZKD96-31-1 | Transition zone | Number of measuring point | 266 | 11 | 3 | 280 | It is mainly telocollinite, homogeneous and pure; the second is telinite. The development of the fusinite of inertinite. The sporinite of exinite with strong fluorescence. |
| Percentage/% | 95.0 | 3.9 | 1.1 | 100.0 | |||
| D32-63-4 | Number of measuring point | 87 | 12 | 1 | 100 | It is mainly telocollinite and has a small amount of telinite, which contains small microsporophy of exinite and fusinite of inertinite. | |
| Percentage/% | 87 | 12 | 1 | 100.0 | |||
| ZKD208-23-2 | Number of measuring point | 121 | 7 | 0 | 128 | It is mainly homogeneous vitrinite, pure and homogenous, in which pyrite crystals are developed, and pyrite has a weak fluorescence. The fusinite of inertinite and inertoderinite was developed and the exinite was not developed. | |
| Percentage/% | 94.5 | 5.5 | 0.0 | 100.0 | |||
| D127-55-1 | Reduction zone | Number of measuring point | 186 | 0 | 2 | 188 | It is mainly homogeneous vitrinite, and a small amount of desmocollinite and telinite are developed. The cutinite of exinite and microsporinite was distributed in the desmocollinite, in which inertinite was not developed. |
| Percentage/% | 98.9 | 0.0 | 1.1 | 100.0 | |||
| D127-55-3 | Number of measuring point | 173 | 0 | 1 | 174 | It is mainly telocollinite, which contains pyrite crystals, and the pyrite contains the weak fluorescence of organic matter. We can see the desmocollinite and a small amount of telinite. The cutinite of exinite and microsporinite were distributed in the desmocollinite. | |
| Percentage/% | 99.4 | 0.0 | 0.6 | 100.0 | |||
| Vitrinite Reflectance (Ro) | The Maturity of Organic Matter |
|---|---|
| Ro < 0.5% | Immature stage |
| Ro = 0.5%–1.0% | Low-mature stage |
| Ro = 1.0%–1.35% | Medium-mature stage |
| Ro = 1.35%–2.0% | High-mature stage |
| Ro > 2.0% | Over-mature stage |
| Sample | Lithologic Description | Zoning | Buried depth/m | Ro (%) | Standard Deviation | Maturity |
|---|---|---|---|---|---|---|
| D63-16 | Gray-white middle-fine sandstone with carbonaceous strips. | Oxidation zone | 630.5 | 0.38 | 0.034 | Immature stage (lignite stage) |
| ZKD95-16-1 | Light grayish-green sandstone with a lenticular carbonaceous strip. | Transition zone | 606.00 | 0.39 | 0.033 | |
| ZKD96-31-1 | The grayish-green coarse sandstone contains carbonaceous detritus. | 669.46 | 0.40 | 0.030 | ||
| D32-63-4 | Gray medium sandstone contains a small amount of carbonaceous debris. | 685.00 | 0.43 | 0.032 | ||
| ZKD208-23-2 | Light grayish-green medium sandstone with a lenticular carbonaceous strip. | 626.74 | 0.49 | 0.038 | ||
| D127-55-1 | Grayish-white middle-coarse sandstone contains carbonaceous strips. | Reduction zone | 594.10 | 0.35 | 0.029 | |
| D127-55-3 | The grayish-green medium sandstone contains carbonaceous strip. | 626.35 | 0.37 | 0.018 |
| The Content of δ13C | Organic Matter Type |
|---|---|
| δ13C < −28‰ | Type I (sapropel) |
| δ13C = −28–−26‰ | Type Ⅱ1 (humic-sapropel) |
| δ13C = −26–−24‰ or −24.5‰ | Type Ⅱ2 (sapropelic-humic type) |
| δ13C > −24‰ or −24.5‰ | Type Ⅲ (humic type) |
| Sample | Lithologic Description | Zoning | Depth/m | Kerogen δ13C‰ (PDB) |
|---|---|---|---|---|
| ZKD112-96(3) | Brown-red middle-fine sandstone | Oxidation zone | 691.67 | −23.7 |
| D63-16 | Gray-white middle-fine sandstone with carbonaceous strips | 630.50 | −23.2 | |
| ZKD208-23-2 | Gray-green sandstone contains a lenticular carbonaceous strip | Transition zone | 626.74 | −22.7 |
| D32-63-4 | Gray medium sandstone contains a small amount of carbonaceous debris | 685.00 | −21.5 | |
| ZKD95-16-1 | Gray-green sandstone contains a lenticular carbonaceous strip | 606.00 | −22.5 | |
| ZKD208-15-1 | Gray-green medium sandstone contains carbonaceous strips | 625.00 | −19.2 | |
| ZKD144-71(3) | Gray-green siltstone contains carbonaceous clastic and pyrite nodules | Reduction zone | 614.47 | −23.1 |
| D127-55-1 | Grayish-white middle-coarse sandstone contains carbonaceous strips | 614.24 | −23.6 | |
| D127-55-3 | Gray-green middle-fine sandstone with carbonaceous strips | 626.74 | −24.2 |
| Sample | Lithologic Description | Test Project | |||||
|---|---|---|---|---|---|---|---|
| N% | C% | H% | O% | H/C | O/C | ||
| ZKD112-96(3) | Brown-red middle-fine sandstone | 0.23 | 59.26 | 3.95 | 23.54 | 0.79 | 0.29 |
| D63-16 | Gray-white middle-fine sandstone with carbonaceous strips | 0.85 | 64.32 | 4.18 | 15.43 | 0.78 | 0.18 |
| ZKD208-23-2 | Gray-green sandstone contains a lenticular carbonaceous strip | 0.90 | 67.84 | 3.82 | 12.51 | 0.67 | 0.14 |
| D32-63-4 | Gray medium sandstone contains a small amount of carbonaceous debris | 0.14 | 53.28 | 3.18 | 12.65 | 0.72 | 0.18 |
| ZKD95-16-1 | Gray-green sandstone contains a lenticular carbonaceous strip | 0.66 | 48.53 | 3.33 | 12.70 | 0.82 | 0.20 |
| ZKD208-15-1 | Gray-green medium sandstone contains carbonaceous strips | 0.59 | 56.20 | 3.13 | 11.53 | 0.67 | 0.15 |
| ZKD144-71(3) | Gray-green siltstone contains carbonaceous clastic and pyrite nodules | 1.03 | 66.48 | 4.74 | 14.01 | 0.85 | 0.16 |
| D127-55-1 | Grayish-white middle-coarse sandstone contains carbonaceous strips | 0.80 | 64.16 | 4.34 | 13.04 | 0.81 | 0.15 |
| D127-55-3 | Gray-green middle-fine sandstone with carbonaceous strips | 0.81 | 63.22 | 4.39 | 13.90 | 0.83 | 0.16 |
| Sample | Lithologic Description | Zoning | Humic Acid (%) | U (μg/g) | The Uranium Content in the Residue after the Extraction of Humic Acid (μg/g) | The Uranium Content of Humic Acid (μg/g) |
|---|---|---|---|---|---|---|
| D63-16 | Gray-white middle-fine sandstone with carbon strips | Oxidation zone | 0.05 | 25.6 | 11.7 | 13.9 |
| D112-96(4) | Brown-red middle-fine sandstone | 0.27 | 137.5 | 51.7 | 85.8 | |
| D96-31(1) | Gray-green coarse sandstone contains carbonaceous debris | Transition zone | 0.10 | 1231 | 480 | 751 |
| D112-47(2) | Gray-white coarse sandstone contains carbonaceous debris | 0.02 | 3933 | 943 | 2990 | |
| D32-63-4 | Gray medium sandstone contains a small amount of carbonaceous debris | 0.83 | 5030 | 595 | 4435 | |
| ZKD95-16-1 | Gray-green sandstone contains a lenticular carbon strip | 5.54 | 5085 | 672 | 4413 | |
| ZKD208-15-1 | Gray-green medium sandstone contains carbon strips | 1.13 | 96.2 | 20 | 76.2 | |
| ZKD144-71(3) | Gray-green siltstone contains carbonaceous clastic and pyrite nodules | Reduction zone | 0.02 | 17.5 | 6.17 | 11.33 |
| ZKD127-55-1 | Grayish-white middle-coarse sandstone contains carbonaceous strips | 2.07 | 1127 | 112 | 1015 |
| No. | Percentage (Organic Matter = 100%) | ||||
|---|---|---|---|---|---|
| Vitrinite | Inertinite | Exinite | Sapropelinite | Description of Organic Components | |
| Wd04-5 | 94.7 | 2.1 | 0.0 | 3.2 | Mainly telocollinite, with a small amount of desmocollinite and semifusinite. The symbiosis between asphaltene and pyrite can be found. |
| Wd04-95 | 95.1 | 0.0 | 0.5 | 4.4 | Mainly telocollinite and desmocollinite. Fusinite can be found with sporophyte and resinite. Abundant asphaltene is developed in fine striations in symbiosis with pyrite and clay or distributed in desmocollinite. |
| Wd04-103 | 84.7 | 8.5 | 3.4 | 3.4 | Mainly telocollinite and desmocollinite with abundant telinite, semifusinite, and fusinite. The exinite is cutinite and sporophyte. And asphaltene can be found. |
| Wd04-108 | 87.3 | 10.2 | 1.5 | 1.0 | Mainly telocollinite and desmocollinite, with abundant structural vitrinite, semifusinite, and fusinite. The exinite is cutinite and sporophyte. And asphaltene can be found. |
| Wd04-109 | 91.7 | 1.0 | 3.1 | 4.2 | Mainly non-telocollinite with pyrite and abundant endogenous fissures. Fusinite can be found with sporophyte, cutinite, and sporophyte. Telocollinite is dark gray and may adsorb asphaltene. |
| Wd04-111 | 98.6 | 0.0 | 0.7 | 0.7 | Mainly telocollinite with an amorphous flocculent distribution; a few desmocollinite and telinite with sporophyte and cutinite. Semifusinite and inertodetrinite can be seen, with asphaltene distribution. |
| Wd04-129 | 93.7 | 0.0 | 1.6 | 4.8 | Mainly telocollinite with abundant endogenous fissures; a few desmocollinite and telinite with sporophyte, cutinite, and asphaltene. Telocollinite is dark gray and may adsorb asphaltene. |
| Wd04-138 | 93.5 | 1.1 | 2.2 | 3.2 | Mainly telinite with abundant endogenous fissures, and structureless vitrinite is less frequent. Inertodetrinite, secretinite, and semifusinite fragments can be found, and asphaltene is present. |
| Average | 92.4 | 2.9 | 1.6 | 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Wu, B.; Luo, J.; Yang, S.; Wang, M.; Liu, M.; Lin, Z.; Zhang, X.; Zhang, L.; Wang, J.; et al. Characters and Metallogenetic Significance of Organic Matter in Coal from the Daying Sandstone-Hosted Uranium Deposit in the Northern Ordos Basin, China. Minerals 2023, 13, 1002. https://doi.org/10.3390/min13081002
Li Q, Wu B, Luo J, Yang S, Wang M, Liu M, Lin Z, Zhang X, Zhang L, Wang J, et al. Characters and Metallogenetic Significance of Organic Matter in Coal from the Daying Sandstone-Hosted Uranium Deposit in the Northern Ordos Basin, China. Minerals. 2023; 13(8):1002. https://doi.org/10.3390/min13081002
Chicago/Turabian StyleLi, Qi, Bailin Wu, Jingjing Luo, Songlin Yang, Miao Wang, Mingyi Liu, Zhouyang Lin, Xiaorui Zhang, Long Zhang, Jiangqiang Wang, and et al. 2023. "Characters and Metallogenetic Significance of Organic Matter in Coal from the Daying Sandstone-Hosted Uranium Deposit in the Northern Ordos Basin, China" Minerals 13, no. 8: 1002. https://doi.org/10.3390/min13081002
APA StyleLi, Q., Wu, B., Luo, J., Yang, S., Wang, M., Liu, M., Lin, Z., Zhang, X., Zhang, L., Wang, J., & Yang, M. (2023). Characters and Metallogenetic Significance of Organic Matter in Coal from the Daying Sandstone-Hosted Uranium Deposit in the Northern Ordos Basin, China. Minerals, 13(8), 1002. https://doi.org/10.3390/min13081002






