Geochronology and Geochemistry of Cretaceous Adakitic Rocks of the Dongguashan Cu Deposit in the Lower Yangtze River Belt: Insights into Petrogenesis and Mineralization
Abstract
1. Introduction
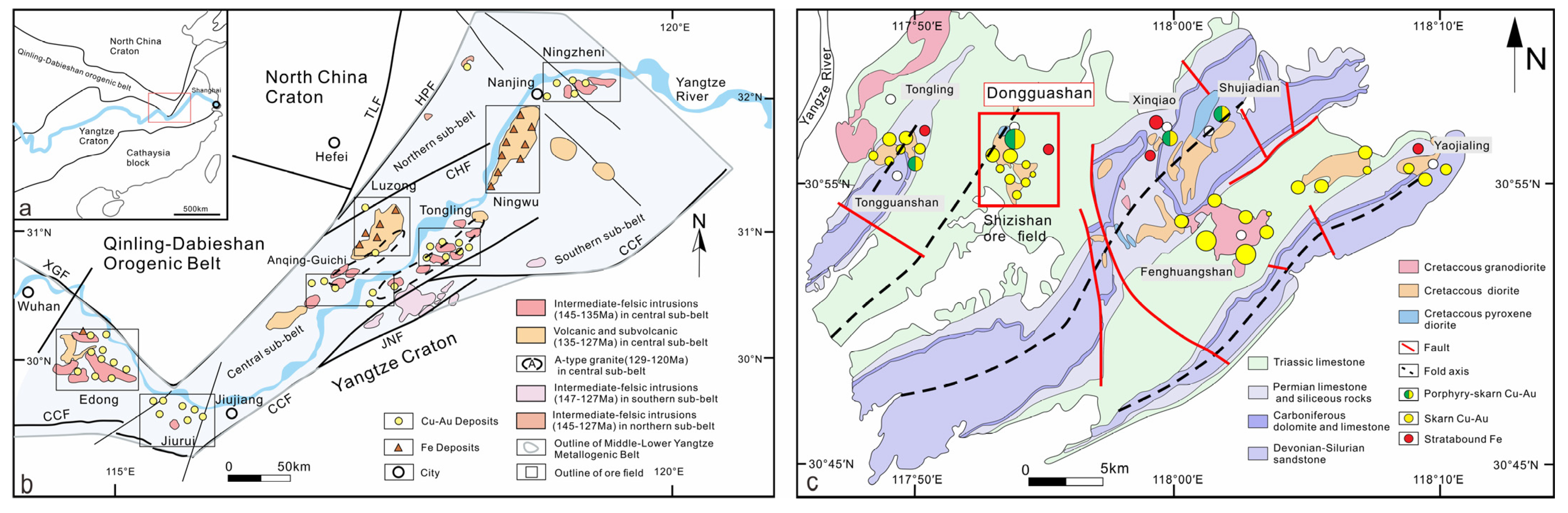
2. Geological Background
3. Analytical Methods
3.1. In Situ SIMS Zircon U–Pb and Oxygen Isotope Analysis
3.2. Whole-Rock Major and Trace Elements and Sr–Nd Isotopes
3.3. Apatite Major Elements Analysis
4. Results
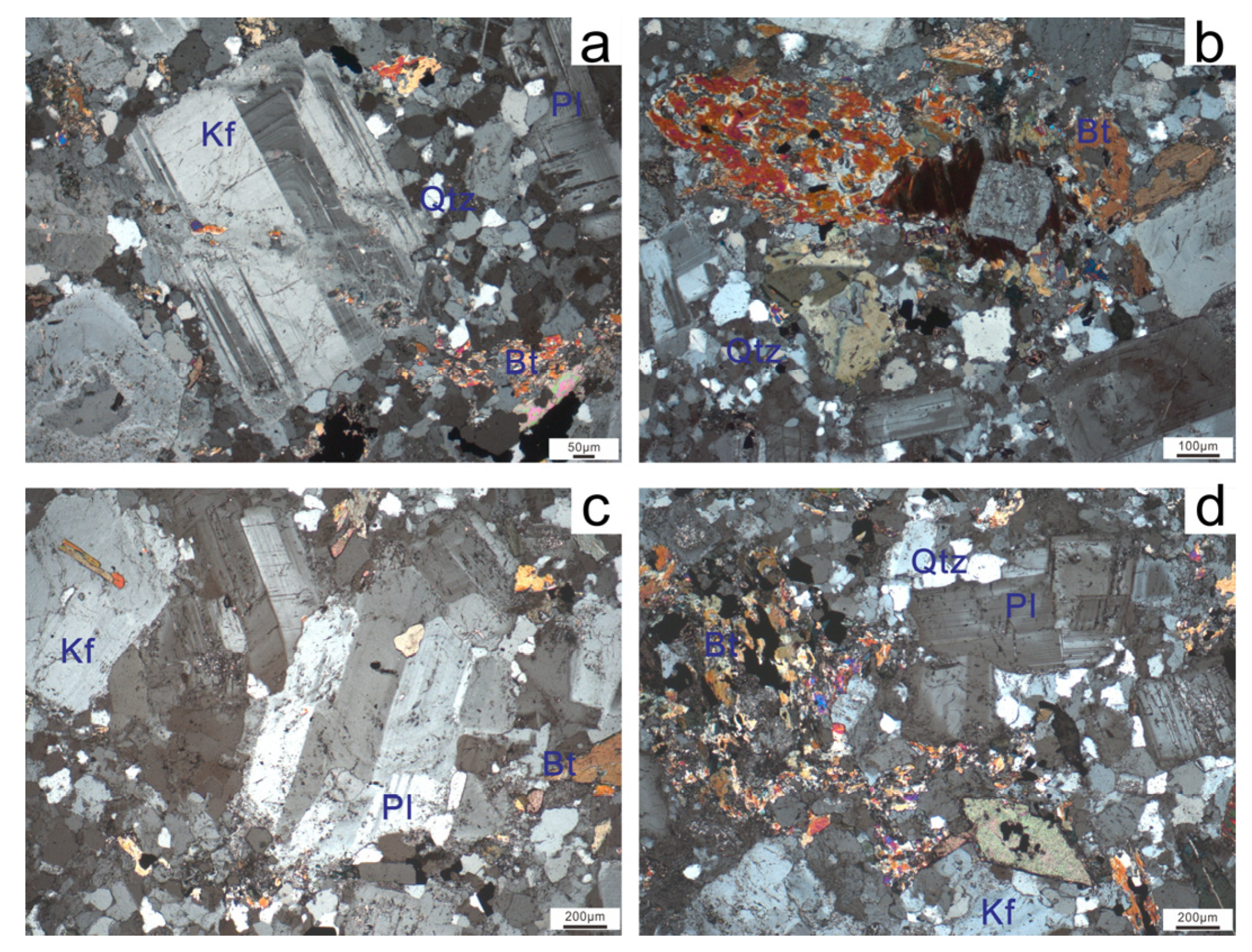
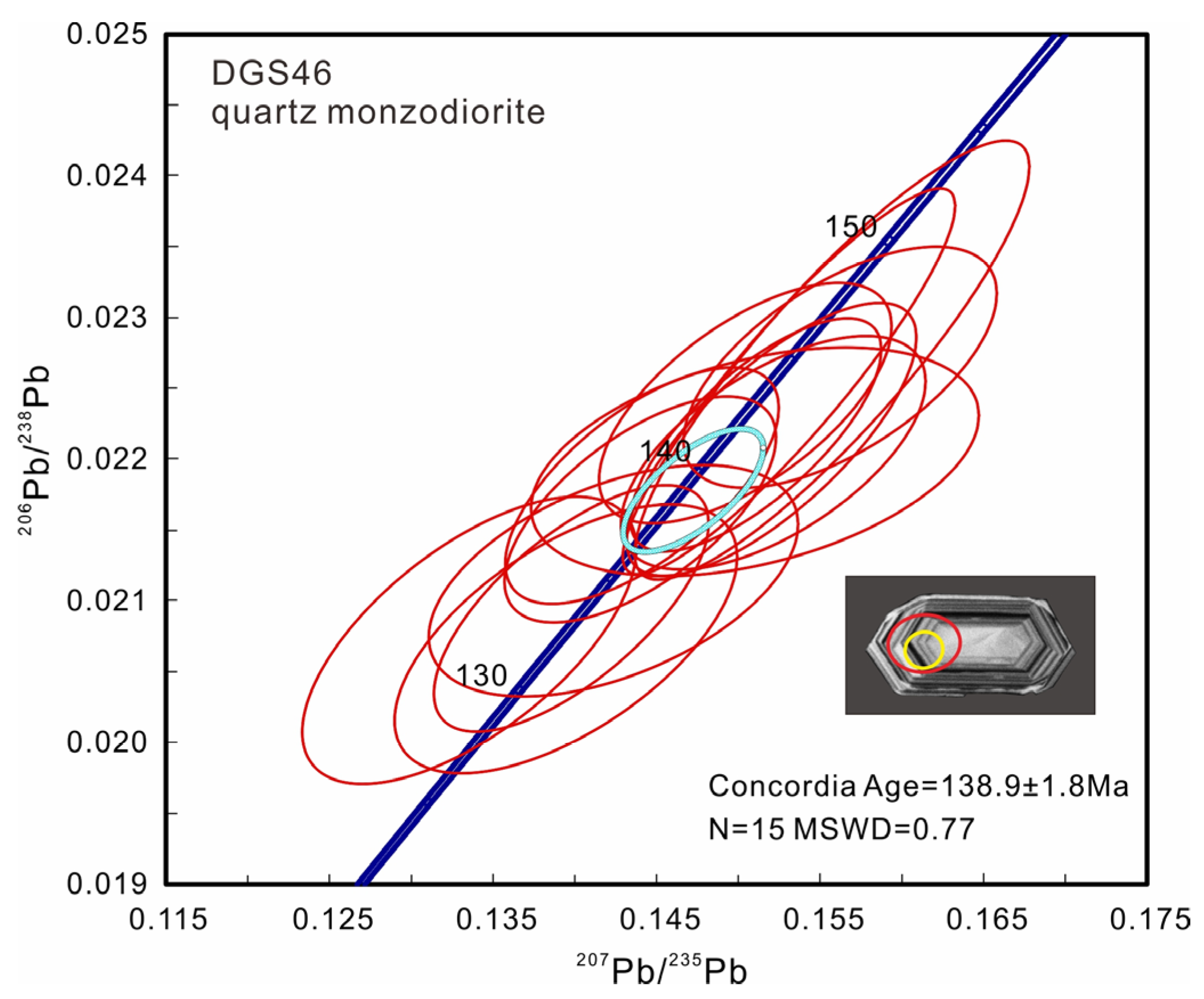
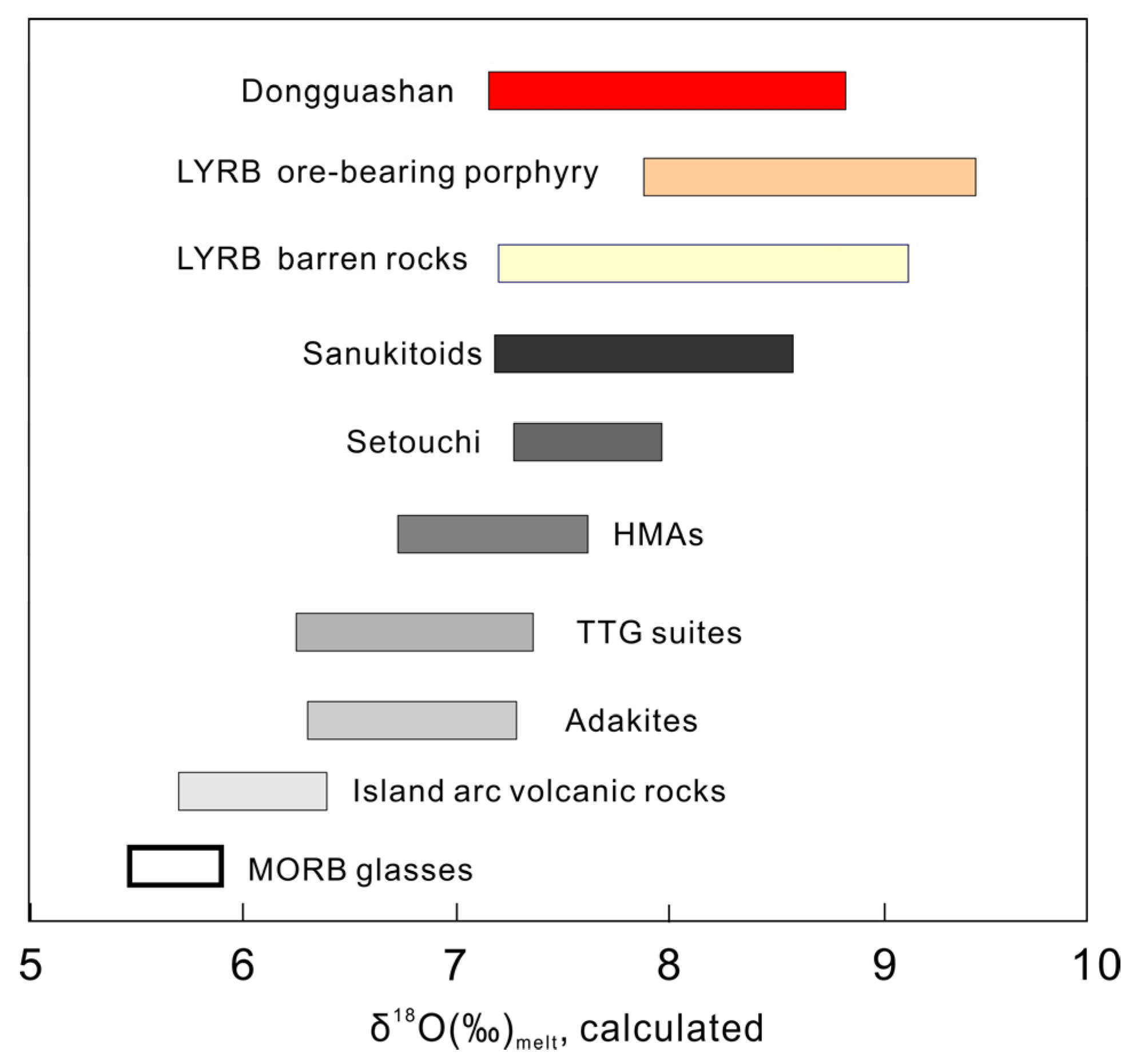
4.1. Zircon U-Pb Age and O Isotopic Compositions
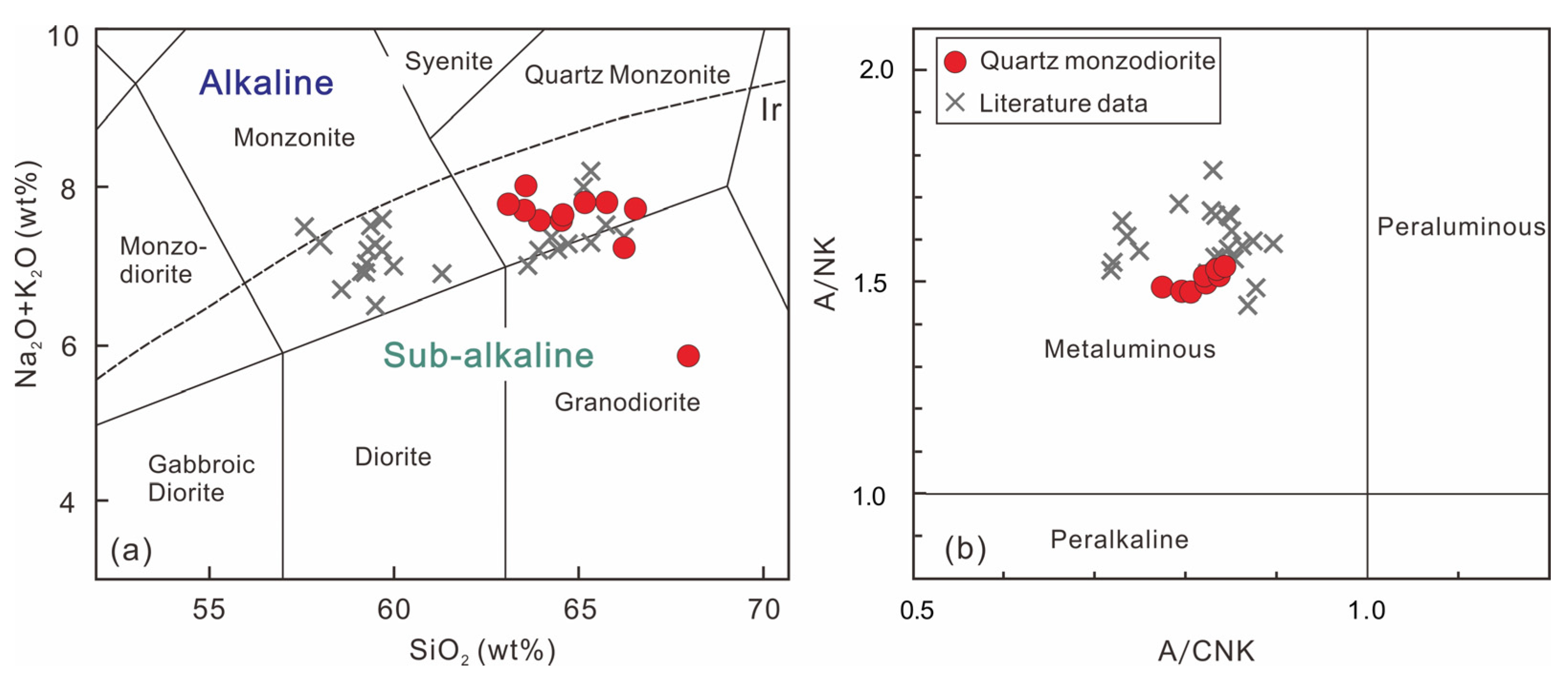
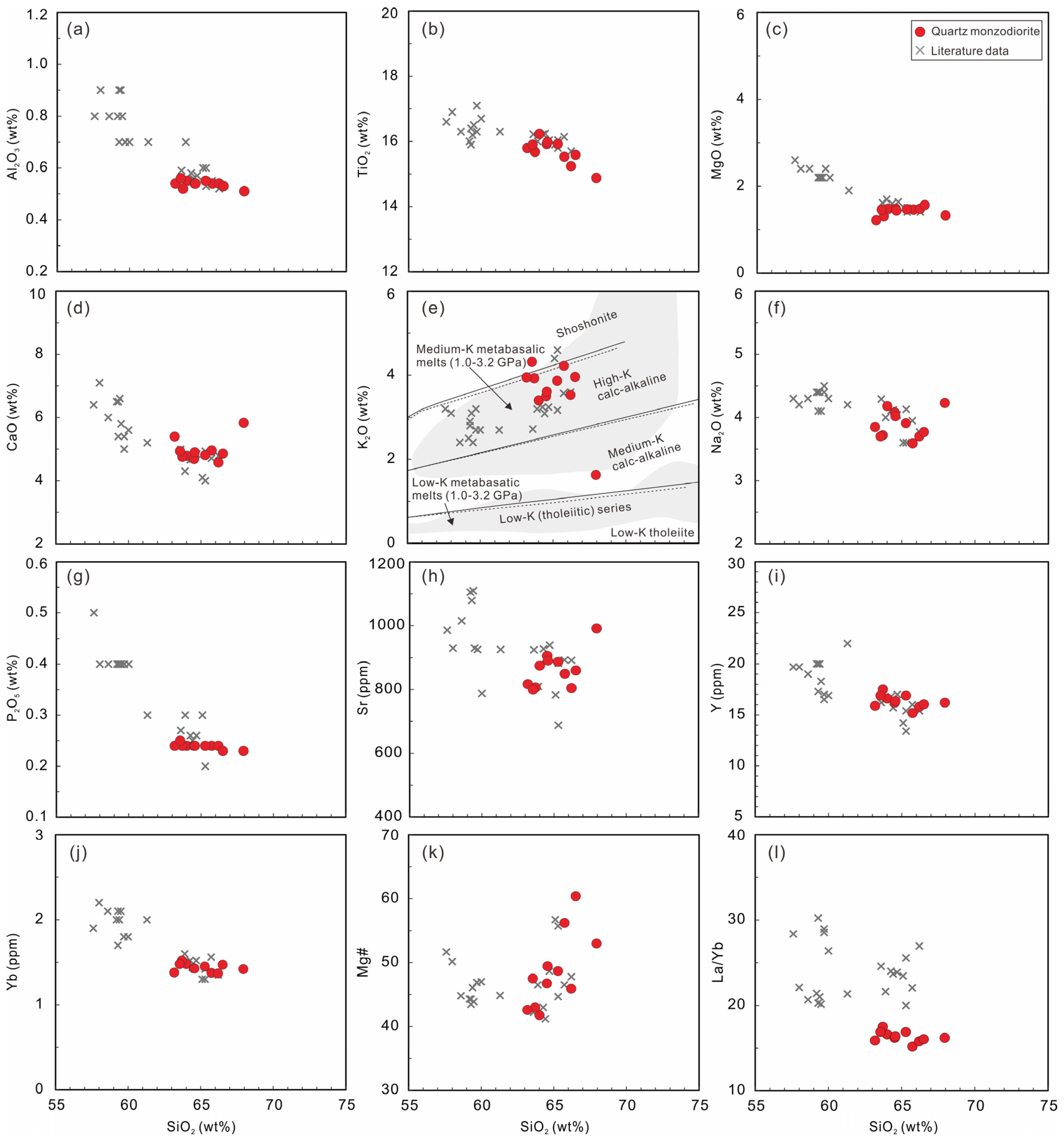
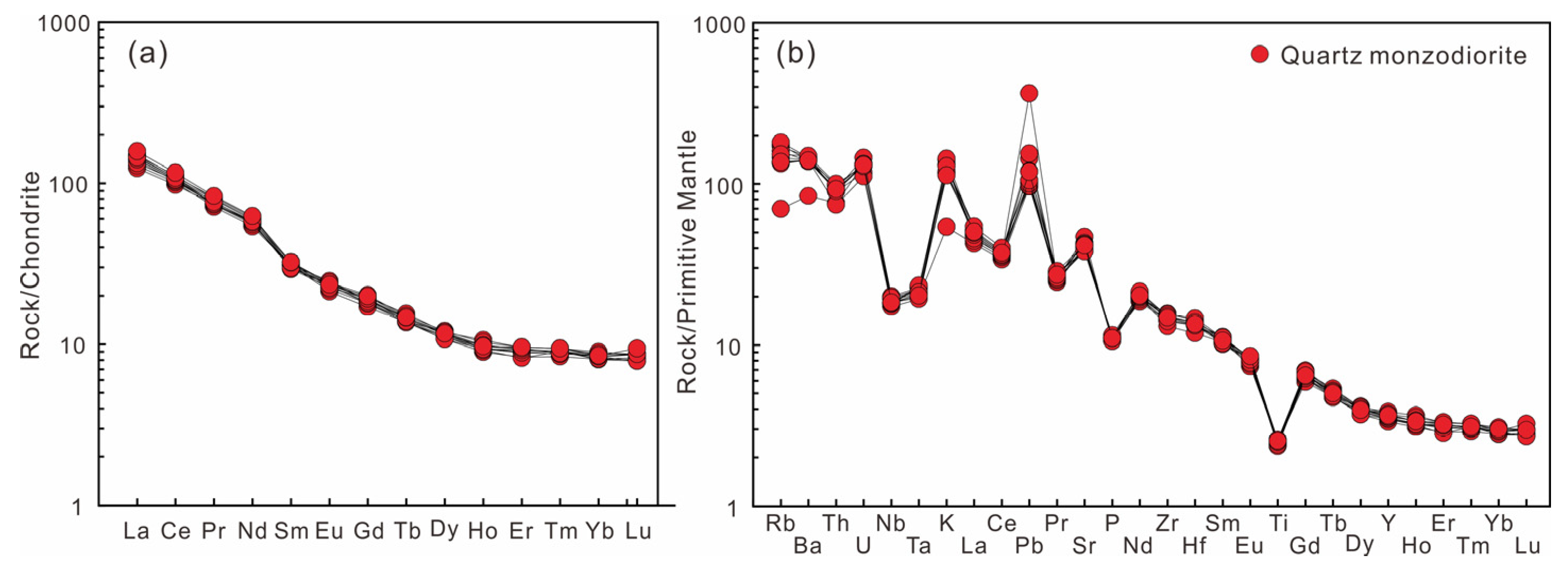
4.2. Whole-Rock Major and Trace Elements and Sr-Nd Isotope
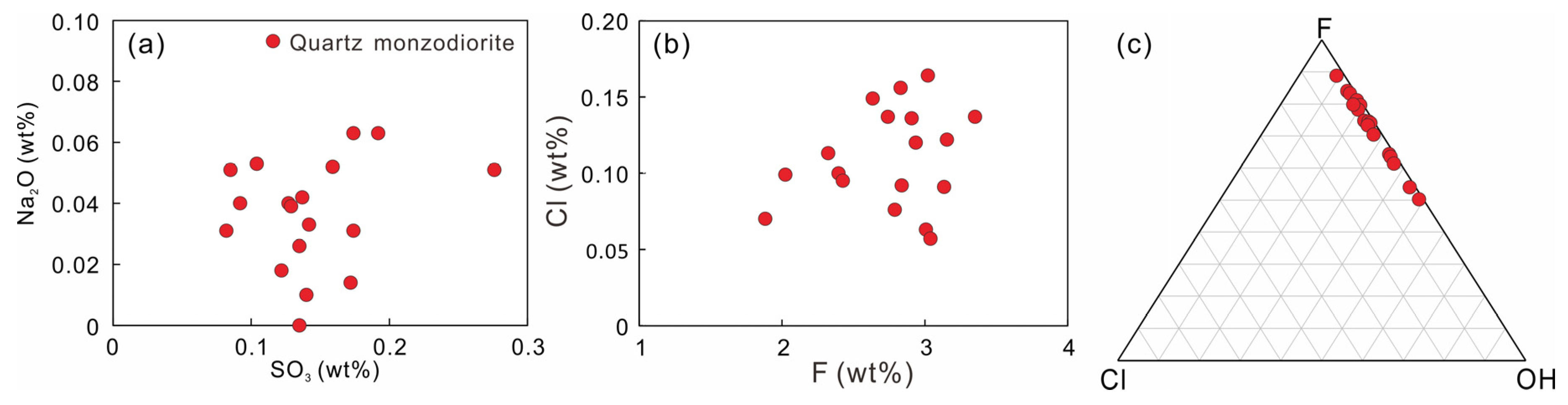
4.3. Apatite Geochemistry
5. Discussion
5.1. Petrogenesis of the DGS Quartz Monzodiorite

5.2. Temporal Relationship between the Adakitic Rocks and Cu polymetallic Mineralization in the DGS Deposit
5.3. Metallogenic Implications for the Cretaceous Adakites in the LYRB
6. Conclusions
- (1)
- The DGS intrusion mainly consists of quartz monzodiorite. The whole-rock compositions show geochemical features of adakite, characterized by high Sr and low Y and Yb concentrations and, consequently, high Sr/Y and (La/Yb)N ratios. According to the comprehensive geochemical data, the adakites are most probably produced by the partial melting of a subducted altered oceanic crust and possibly incorporated sediments.
- (2)
- Highly precise and accurate SIMS in situ zircon U–Pb age suggests that the adakites crystallized at 138.9 ± 1.8 Ma, which is coeval with the Cu mineralization in the DGS deposit, Tongling ore cluster, and implies a close temporal relationship between the adakites and Cu polymetallic mineralization in the LYRB.
- (3)
- The high Cu content, high oxygen fugacity, and volatile contents of the DGS adakite imply an oxidized and volatile enriched environment, which is conducive to the formation of large-scale Cu polymetallic mineralization.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, Y.F.; Liu, X.P.; Wu, Y.C. The Copper-Iron Belt of the Lower and Middle Districts of the Yangtze River; Geological Publishing House: Beijing, China, 1991; pp. 1–379. (In Chinese) [Google Scholar]
- Pan, Y.M.; Dong, P. The Lower Changjiang (Yangzi/Yangtze River) metallogenic belt, east central China: Intrusion- and wall rock-hosted Cu-Fe-Au, Mo, Zn, Pb, Ag deposits. Ore Geol. Rev. 1999, 15, 177–242. [Google Scholar] [CrossRef]
- Zhai, Y.S.; Xiong, Y.L.; Yao, S.Z.; Lin, X.D. Metallogeny of copper and iron deposits in the Eastern Yangtze Craton, east-central China. Ore Geol. Rev. 1996, 11, 229–248. [Google Scholar] [CrossRef]
- Mao, J.W.; Xie, G.Q.; Duan, C.; Pirajno, F.; Ishiyama, D.; Chen, Y.C. A tectono-genetic model for porphyry-skarn-stratabound Cu-Au-Mo-Fe and magnetite-apatite deposits along the Middle-Lower Yangtze River valley, Eastern China. Ore Geol. Rev. 2011, 43, 294–314. [Google Scholar] [CrossRef]
- Zhou, T.F.; Wang, S.W.; Fan, Y.; Yuan, F.; Zhang, D.Y.; White, N.C. A review of the intracontinental porphyry deposits in the Middle-Lower Yangtze River Valley metallogenic belt, Eastern China. Ore Geol. Rev. 2015, 65, 433–456. [Google Scholar] [CrossRef]
- Ling, M.X.; Wang, F.Y.; Ding, X.; Hu, Y.H.; Zhou, J.B.; Zartman, R.E.; Yang, X.Y.; Sun, W.D. Cretaceous ridge subduction along the lower Yangtze River Belt, Eastern China. Econ. Geol. 2009, 104, 303–321. [Google Scholar] [CrossRef]
- Xie, J.C.; Wang, Y.; Li, Q.Z.; Yan, J.; Sun, W.D. Petrogenesis and metallogenic implications of Late Mesozoic intrusive rocks in the Tongling region, eastern China: A case study and perspective review. Int. Geol. Rev. 2018, 60, 1361–1380. [Google Scholar] [CrossRef]
- Xie, J.C.; Tang, D.W.; Xia, D.M.; Wang, Y.; Li, Q.Z.; Yan, J.; Yang, X.Y.; Sun, W.D. Geochronological and geochemical constraints on the formation of Chizhou Cu-Mo polymetallic deposits, middle and lower Yangtze metallogenic belt, eastern China. Ore Geol. Rev. 2019, 109, 322–347. [Google Scholar] [CrossRef]
- Tang, Y.C.; Wu, Y.C.; Chu, G.Z.; Xing, F.M.; Wang, Y.M.; Cao, F.Y.; Chang, Y.F. Geology of Copper-Gold Polymetallic Deposits in the Along-Changjiang Area of Anhui Province; Geological Publishing House: Beijing, China, 1998; pp. 1–351. (In Chinese) [Google Scholar]
- Zhao, Y.M.; Zhang, Y.N.; Bi, C.S. Geology of gold-bearing skarn deposits in the middle and lower Yangtze River Valley and adjacent regions. Ore Geol. Rev. 1999, 14, 227–249. [Google Scholar] [CrossRef]
- Wang, S.W.; Zhou, T.F.; Yuan, F.; Fan, Y.; Zhang, L.J.; Song, Y.L. Petrogenesis of Dongguashan skarn-porphyry Cu-Au deposit related intrusion in the Tongling district, eastern China: Geochronological, mineralogical, geochemical and Hf isotopic evidence. Ore Geol. Rev. 2015, 64, 53–70. [Google Scholar] [CrossRef]
- Mao, J.W.; Wang, Y.T.; Lehmann, B.; Yu, J.J.; Du, A.D.; Mei, Y.X.; Li, Y.F.; Zang, W.S.; Stein, H.J.; Zhou, T.F. Molybdenite Re-Os and albite 40Ar/39Ar dating of Cu-Au-Mo and magnetite porphyry systems in the Yangtze River valley and metallogenic implications. Ore Geol. Rev. 2006, 29, 307–324. [Google Scholar] [CrossRef]
- Liu, S.A.; Li, S.G.; He, Y.S.; Huang, F. Geochemical contrasts between early Cretaceous ore-bearing and ore barren high-Mg adakites in central-eastern China: Implications for petrogenesis and Cu-Au mineralization. Geochim. Cosmochim. Acta 2010, 74, 7160–7178. [Google Scholar] [CrossRef]
- Yan, J.; Liu, J.M.; Li, Q.Z.; Xing, G.F.; Liu, X.Q.; Xie, J.C.; Chu, X.Q.; Chen, Z.F. In situ zircon Hf-O isotopic analyses of late Mesozoic magmatic rocks in the Lower Yangtze River Belt, central eastern China: Implications for petrogenesis and geodynamic evolution. Lithos 2015, 227, 57–76. [Google Scholar] [CrossRef]
- Xie, J.C.; Wang, Y.; Li, Q.Z.; Liu, J.M.; Yan, J.; Sun, W.D. Early Cretaceous adakitic rocks in the Anqing region, southeastern China: Constraints on petrogenesis and metallogenic significance. Int. Geol. Rev. 2017, 60, 1362672. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Ling, M.X.; Wu, K.; Zhang, Z.K.; Sui, Q.L.; Sun, W.D.; Xia, X.P. Insights into the origin of coexisting A1– And A2–Type granites: Implications from zircon Hf-O isotopes of the Huayuangong intrusion in the Lower Yangtze River Belt, eastern China. Lithos 2018, 318–319, 230–243. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Deng, J.H.; Luo, J.C.; Zhang, L.P.; Luo, Z.B.; Yan, H.B.; Sun, W.D. Petrogenesis of Early Cretaceous adakites in Tongguanshan Cu–Au polymetallic deposit, Tongling region, Eastern China. Ore Geol. Rev. 2020, 126, 103717. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Zhang, Z.Z.; Luo, J.C.; Wei, L.M.; Jiang, K.N. Two-stage, U-mineralization of A-type granites from the Huangmeijian complex, eastern China. Solid Earth Sci. 2023, 8, 12–24. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Jiang, S.Y.; Hu, J.; Gu, L.X.; Li, J. Geochronology, geochemistry, and mineralization of the granodiorite porphyry hosting the Matou Cu–Mo (±W) deposit, Lower Yangtze River metallogenic belt, eastern China. J. Asian Earth Sci. 2014, 79, 623–640. [Google Scholar] [CrossRef]
- Ling, M.X.; Wang, F.Y.; Ding, X.; Zhou, J.B.; Sun, W.D. Different origins of adakites from the Dabie Mountains and the Lower Yangtze River belt in eastern China, Geochemical constraints. Int. Geol. Rev. 2011, 53, 727–740. [Google Scholar] [CrossRef]
- Sun, W.D.; Ling, M.X.; Yang, X.Y. Ridge subduction and porphyry copper gold mineralization: An overview. Sci. China Earth Sci. 2010, 53, 475–484. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Wang, Y.L. Preliminary study on the components of the lower crust in east China Plateau during Yanshan Period: Constraints on Sr and Nd isotopic compositions of adakite-like rocks. Acta Petrol. Sin. 2001, 17, 505–513, (In Chinese with English Abstract). [Google Scholar]
- Wang, Q.; Zhao, Z.H.; Xu, J.F.; Li, X.H.; Xiong, X.L.; Bao, Z.W. Petrogenesis of the Mesozoic intrusive rocks in the Tongling area, Anhui Province, China and their constraint on geodynamic process. Sci. China. Ser. D Earth Sci. 2003, 46, 801–815. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, J.F.; Zhao, Z.H.; Xiong, X.L.; Bao, Z.W.; Li, C.F.; Bai, Z.H. Petrogenesis of Cretaceous adakitic and shoshonitic igneous rocks in the Luzong area, Anhui Province (eastern China), Implications for geodynamics and Cu-Au mineralization. Lithos 2006, 89, 424–446. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, J.F.; Ji, G.Y. A perspective on the geotectonic of early Cretaceous adakite-like rocks in the lower reaches of Yangtze River and its significance for copper-gold mineralization. Acta Petrol. Sin. 2004, 20, 297–314, (In Chinese with English Abstract). [Google Scholar]
- Deng, J.F.; Wu, Z.X. Lithospheric thinning event in the Lower Yangtze craton and Cu-Fe metallogenic belt in the middle and Lower Yangtze River reaches. Geol. Anhui 2001, 11, 86–91, (In Chinese with English Abstract). [Google Scholar]
- Qin, X.L. Studies on Sulfi-Metal Oxide Inclusions from Mesozoic Intrusions and Their Rock xenoliths in Tongling, Anhui Province. Ph.D. Thesis, China University of Geosciences, Beijing, China, 2007; pp. 1–170, (In Chinese with English Abstract). [Google Scholar]
- Yang, X.N.; Xu, Z.W.; Lu, X.C.; Jiang, S.Y.; Ling, H.F.; Liu, L.G.; Chen, D.Y. Porphyry and skarn Au-Cu deposits in the Shizishan orefield, Tongling, East China, U-Pb dating and in-situ Hf isotope analysis of zircons and petrogenesis of associated granitoids. Ore Geol. Rev. 2011, 43, 182–193. [Google Scholar] [CrossRef]
- Guo, W.M.; Lu, J.J.; Jiang, S.Y.; Zhang, R.Q.; Zhao, Z.J. Chronology, Hf isotopes, geochemistry, and petrogenesis of the magmatic rocks in the Shizishan ore field of Tongling, Anhui Province. Sci. China Earth Sci. 2013, 8, 1268–1286, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Di, Y.; Zhao, H.; Zhang, Y.; Zhao, J.; Yang, L. Petrographic evidences for magma mixing in the granitoids from Tongling area, Anhui Province. Beijing Geol. 2003, 15, 12–17, (In Chinese with English Abstract). [Google Scholar]
- Wu, C.L.; Chen, S.Y.; Shi, R.D.; Hao, M.Y. Origin and features of the Mesozoic intermediate-acid intrusive in the Tongling area, Anhui, China. Acta Geosci. Sin. 2003, 24, 41–48, (In Chinese with English Abstract). [Google Scholar]
- Wu, C.L.; Dong, S.W.; Gou, H.P.; Guo, X.Y.; Gao, Q.M.; Liu, L.G.; Chen, Q.L.; Lei, M.; Wooden, J.L.; Mazadab, F.K.; et al. Zircon SHRIMP U-Pb dating of intermediate-acid intrusive rocks from Shizishan, Tongling and magmatism. Acta Petrol. Sin. 2008, 24, 1801–1812, (In Chinese with English Abstract). [Google Scholar]
- Xu, J.H.; Xie, Y.L.; Yang, Z.S.; Meng, Y.F.; Zeng, P.S. Trace elements in fluid inclusions of submarine exhalation-sedimentation system in Tongling metallogenic province. Miner. Depos. 2004, 23, 344–352, (In Chinese with English Abstract). [Google Scholar]
- Du, Y.; Deng, J.; Cao, Y.; Li, D. Petrology and geochemistry of Silurian–Triassic sedimentary rocks in Tongling ore-cluster region of Eastern China: Their roles in the genesis of large stratabound skarn ore deposits. Ore Geol. Rev. 2015, 67, 255–263. [Google Scholar] [CrossRef]
- Valley, J.W.; Lackey, J.S.; Cavosie, A.J.; Clechenko, C.C.; Spicuzza, M.J.; Basei, M.A.S.; Bindeman, I.N.; Ferreira, V.P.; Sial, A.N.; King, E.M.; et al. 4.4 billion years of crustal maturation: Oxygen isotope ratios of magmatic zircon. Contrib. Mineral. Petrol. 2005, 150, 561–580. [Google Scholar] [CrossRef]
- Valley, J.W.; Kinny, P.D.; Schulze, D.J.; Spicuzza, M.J. Zircon megacrysts from kimberlite: Oxygen isotope variability among mantle melts. Contrib. Mineral. Petrol. 1998, 133, 1–11. [Google Scholar] [CrossRef]
- Cavosie, A.J.; Kita, N.T.; Valley, J.W. Primitive oxygen-isotope ratio recorded in magmatic zircon from the Mid-Atlantic Ridge. Am. Mineral. 2009, 94, 926–934. [Google Scholar] [CrossRef]
- Harlov, D.E. Apatite: A fingerprint for metasomatic processes. Elements 2015, 11, 171–176. [Google Scholar] [CrossRef]
- Pan, L.C.; Hu, R.Z.; Wang, X.S.; Bi, X.W.; Zhu, J.J.; Li, C. Apatite trace element and halogen compositions as petrogenetic-metallogenic indicators: Examples from four granite plutons in the Sanjiang region, SW China. Lithos 2016, 254, 118–130. [Google Scholar] [CrossRef]
- Pan, L.C.; Hu, R.Z.; Bi, X.W.; Wang, Y.; Yan, J. Evaluating magmatic fertility of Paleo-Tethyan granitoids in eastern Tibet using apatite chemical composition and Nd isotope. Ore Geol. Rev. 2020, 127, 103757. [Google Scholar] [CrossRef]
- Qu, P.; Li, N.B.; Niu, H.C.; Yang, W.B.; Shan, Q.; Zhang, Z. Zircon and apatite as tools to monitor the evolution of fractionated I-type granites from the central Great Xing’an Range, NE China. Lithos 2019, 348–349, 105207. [Google Scholar] [CrossRef]
- Qu, P.; Li, N.B.; Niu, H.C.; Shan, Q.; Weng, Q.; Zhao, X. Difference in the nature of ore-forming magma between the Mesozoic porphyry Cu-Mo and Mo deposits in NE China: Records from apatite and zircon geochemistry. Ore Geol. Rev. 2021, 135, 104218. [Google Scholar] [CrossRef]
- Qu, P.; Yang, W.B.; Niu, H.C.; Li, N.B.; Wu, D. Apatite fingerprints on the magmatic-hydrothermal evolution of the Daheishan giant porphyry Mo deposit, NE China. Geol. Soc. Am. Bull. 2022, 134, 1863–1876. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, G.Y.; Xiang, L.; Zhang, R.Q.; Zhang, L.P.; Sun, W.D. Combined mica and apatite chemical compositions to trace magmatic-hydrothermal evolution of fertile granites in the Dachang Sn-polymetallic district, South China. Ore Geol. Rev. 2022, 151, 105168. [Google Scholar] [CrossRef]
- Liu, Z.F.; Shao, Y.J.; Zhang, Y.; Wang, C. Geochemistry and geochronology of the Qingshanjiao granites: Implications for genesis of the Dongguashan Cu-(Au) ore deposit in the Tongling ore district, Eastern China. Ore Geol. Rev. 2018, 99, 42–57. [Google Scholar] [CrossRef]
- Liu, Z.F.; Shao, Y.J.; Wang, C.; Liu, Q.Q. Genesis of the Dongguashan skarn Cu-(Au) deposit in Tongling, Eastern China: Evidence from fluid inclusions and H-O-S-Pb isotopes. Ore Geol. Rev. 2019, 104, 462–476. [Google Scholar] [CrossRef]
- Zhang, J.D.; Liu, L.; Yu, Z.Q.; Yang, X.Y.; Liu, Z.F.; Li, H. Petrogenesis and metallogeny of the Dongguashan Cu-Au deposit in the Tongling ore-cluster region, the Lower Yangtze River Metallogenic Belt: Constraints from geochemistry and geochronology. Geochemistry 2021, 81, 125822. [Google Scholar] [CrossRef]
- Zhou, T.F.; Fan, Y.; Feng, Y. Advances on petrogensis and metallogeny study of the mineralization belt of the middle and lower reaches of the Yangtze River Area. Acta Petrol. Sin. 2008, 24, 1665–1678, (In Chinese with English Abstract). [Google Scholar]
- Zhai, Y.S.; Yao, S.Z.; Lin, X.D.; Zhou, X.N.; Wan, T.F.; Jin, F.Q.; Zhou, Z.G. Fe–Cu (Au) Metallogeny of the Middle-Lower Changjiang Region; Geological Publishing House: Beijing, China, 1992; p. 235. [Google Scholar]
- Chen, C.J.; Chen, B.; Li, Z.; Wang, Z.Q. Important role of magma mixing in generating the Mesozoic monzodioritic-granodioritic intrusions related to Cu mineralization, Tongling, East China: Evidence from petrological and in situ Sr-Hf isotopic data. Lithos 2016, 248–251, 80–93. [Google Scholar] [CrossRef]
- Chu, G.Z. Metallogenic System of Shizishan Cu-Au Ore-Field in Tongling Area and ITS prospecting significances. Ph.D. Thesis, China University of Geosciences, Beijing, China, 2003; pp. 1–100, (In Chinese with English Abstract). [Google Scholar]
- Sláma, J.; Košler, J.; Condon, D.J.; Crowley, J.L.; Gerdes, A.; Hanchar, J.M.; Horstwood, M.S.A.; Morris, G.A.; Nasdala, L.; Norberg, N.; et al. Plešovice zircon—A new natural reference material for U-Pb and Hf isotopic microanalysis. Chem. Geol. 2008, 249, 1–35. [Google Scholar] [CrossRef]
- Nasdala, L.; Hofmeister, W.; Norberg, N.; Mattinson, J.M.; Corfu, F.; Dörr, W.; Kamo, S.L.; Kennedy, A.K.; Kronz, A.; Reiners, P.W.; et al. Zircon M257—A homogeneous natural reference material for the ion microprobe U-Pb analysis of zircon. Geostand. Geoanal. Res. 2008, 32, 47–265. [Google Scholar] [CrossRef]
- Li, X.H.; Liu, Y.; Li, Q.L.; Guo, C.H.; Chamberlain, K.R. Precise determination of Phanerozoic zircon Pb/Pb age by multicollector SIMS without external standardization. Geochem. Geophys. Geosyst. 2009, 10, Q04010. [Google Scholar]
- Stacey, J.T.; Kramers, J.D. Approximation of terrestrial lead isotope evolution by a two-stage model. Earth Planet. Sci. Lett. 1975, 26, 207–221. [Google Scholar] [CrossRef]
- Ludwig, K.R. Users’ Manual for Isoplot 3.70: A Geochronological Toolkit for Mircrosoft Excel; Berkeley Geochronology Center Special Publication No. 4; Berkeley Geochronology Center: Berkeley, CA, USA, 2008. [Google Scholar]
- Tang, G.Q.; Li, X.H.; Li, Q.L.; Liu, Y.; Ling, X.X.; Yin, Q.Z. Deciphering the physical mechanism of the topography effect for oxygen isotope measurements using a Cameca IMS-1280 SIMS. J. Anal. At. Spectrom. 2015, 30, 950–956. [Google Scholar] [CrossRef]
- Li, X.H.; Long, W.G.; Li, Q.L. Penglai zircon megacrysts: A potential new working reference material for microbeam determination of Hf–O isotopes and U-Pb age. Geostand. Geoanal. Res. 2010, 34, 117–134. [Google Scholar] [CrossRef]
- Liu, Y.S.; Hu, Z.C.; Gao, S.; Gunther, D.; Xu, J.; Gao, C.G.; Chen, H.H. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- Li, X.H.; Liu, D.Y.; Sun, M.; Li, W.X.; Liang, X.R.; Liu, Y. Precise Sm–Nd and U-Pb isotopic dating of the super-giant Shizhuyuan polymetallic deposit and its host granite, Southeast China. Geol. Mag. 2004, 141, 225–231. [Google Scholar] [CrossRef]
- Tanaka, T.; Togashi, S.; Kamioka, H.; Amakawa, H.; Kagami, H.; Hamamoto, T.; Yuhara, M.; Orihashi, Y.; Yoneda, S.; Shimizu, H.; et al. JNdi–1: A neodymium isotopic reference in consistency with LaJolla neodymium. Chem. Geol. 2000, 168, 279–281. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.Y.; Deng, J.H.; Liu, S.S.; Sun, C.; Hou, Q.; Wang, L.J. A comprehensive overview on the origin of intrusive rocks and polymetallic mineralization in the Tongling ore-cluster region, lower Yangtze River Metallogenic Belt: Geological and geochemical constraints. Ore Geol. Rev. 2021, 141, 104625. [Google Scholar] [CrossRef]
- Middlemost, E.A.K. Naming materials in the magma/igneous rock system. Earth Sci. Rev. 1994, 37, 215–224. [Google Scholar] [CrossRef]
- Sajona, F.G.; Maury, R.C.; Bellon, H.; Cotten, J.; Defant, M.J.; Pubellier, M. Initiation of subduction and the generation of slab melts in western and eastern Mindanao, Philippines. Geology 1993, 21, 1007–1010. [Google Scholar] [CrossRef]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Pearce, J.A.; Harris, N.B.; Tindle, A.G. Trace element discrimination diagrams for the tectonic interpretation of granitic rocks. J. Petrol. 1984, 25, 956–983. [Google Scholar] [CrossRef]
- Hofmann, A.; Bolhar, R.; Dirks, P.; Jelsma, H. The geochemistry of Archaean shales derived from a Mafic volcanic sequence, Belingwe greenstone belt, Zimbabwe: Provenance, source area unroofing and submarine versus subaerial weathering. Geochim. Cosmochim. Acta 2003, 67, 421–440. [Google Scholar] [CrossRef]
- Plank, T.; Langmuir, C.H. The chemical composition of subducting sediment and its consequences for the crust and mantle. Chem. Geol. 1998, 145, 325–394. [Google Scholar] [CrossRef]
- Chen, J.F.; Yan, J.; Xie, Z.; Xu, X.; Xing, F.M. Nd and Sr isotopic compositions of igneous rocks from the Lower Yangtze Region in Eastern China: Constrains on sources. Phys. Chem. Earth (A) 2001, 26, 719–731. [Google Scholar] [CrossRef]
- Yan, J.; Chen, J.F.; Xu, X.S. Geochemistry of Cretaceous mafic rocks from the Lower Yangtze region, eastern China: Characteristics and evolution of the lithospheric mantle. J. Asian Earth Sci. 2008, 33, 177–193. [Google Scholar] [CrossRef]
- Ames, L.; Zhou, G.Z.; Xiong, B.C. Geochronology and isotopic character of ultrahigh-pressure metamorphism with implications for collision of the Sino-Korean and Yangtze cratons, central China. Tectonics 1996, 15, 472–489. [Google Scholar] [CrossRef]
- Webster, J.D.; Piccoli, P.M. Magmatic apatite: A powerful, yet deceptive, mineral. Elements 2015, 11, 177–182. [Google Scholar] [CrossRef]
- Pan, Y.; Fleet, M.E. Compositions of the apatite-group minerals: Substitution mechanisms and controlling factors. Rev. Mineral. Geochem. 2002, 48, 13–49. [Google Scholar] [CrossRef]
- Piccoli, P.M.; Candela, P.A. Apatite in igneous systems. Rev. Mineral. Geochem. 2002, 48, 255–292. [Google Scholar] [CrossRef]
- Wang, H.R.; Cai, K.D.; Sun, M.; Xia, X.P.; Lai, C.K.; Li, P.F.; Wan, B.; Zhang, Z.Y. Apatite as a magma redox indicator and its application in metallogenic research. Lithos 2022, 422, 106749. [Google Scholar] [CrossRef]
- Pang, K.N.; Li, C.S.; Zhou, M.F.; Ripley, E.M. Abundant Fe–Ti oxide inclusions in olivine from the Panzhihua and Hongge layered intrusions, SW China: Evidence for early saturation of Fe–Ti oxides in ferrobasaltic magma. Contrib. Mineral. Petrol. 2008, 156, 307–321. [Google Scholar] [CrossRef]
- Hoskin, P.W.O.; Kinny, P.D.; Wyborn, D.; Chappell, B.W. Identifying accessory mineral saturation during differentiation in granitoid magmas: An integrated approach. J. Petrol. 2000, 41, 1365–1396. [Google Scholar] [CrossRef]
- Drummond, M.S.; Defant, M.J. A model for trondhjemite–tonalite–dacite genesis and crustal growth via slab melting: Archaean to modern comparisons. J. Geophys. Res. 1990, 95, 21503–21521. [Google Scholar] [CrossRef]
- Martin, H. The adakitic magmas: Modern analogues of Archaean granitoids. Lithos 1999, 46, 411–429. [Google Scholar] [CrossRef]
- Moyen, J.F. High Sr/Y and La/Yb ratios: The meaning of the “adakitic signature”. Lithos 2009, 112, 556–574. [Google Scholar] [CrossRef]
- Zhang, C.C.; Sun, W.D.; Wang, J.T.; Zhang, L.P.; Sun, S.J.; Wu, K. Oxygen fugacity and porphyry mineralization: A zircon perspective of Dexing porphyry Cu deposit, China. Geochim. Cosmochim. Acta 2017, 206, 343–363. [Google Scholar] [CrossRef]
- Deng, J.H.; Yang, X.Y.; Qi, H.S.; Zhang, Z.F.; Mastoi, A.S.; Berador, A.E.G.; Sun, W.D. Early cretaceous adakite from the atlas porphyry Cu-Au deposit in Cebu Island, Central Philippines: Partial melting of subducted oceanic crust. Ore Geol. Rev. 2019, 110, 102937. [Google Scholar] [CrossRef]
- Deng, J.H.; Yang, X.Y.; Zhang, L.P.; Duan, L.A.; Mastoi, A.S.; Liu, H. An overview on the origin of adakites/adakitic rocks and related porphyry Cu-Au mineralization, Northern Luzon, Philippines. Ore Geol. Rev. 2020, 124, 103610. [Google Scholar] [CrossRef]
- Hernández-Uribe, D.; Hernández-Montenegro, J.D.; Cone, K.A.; Palin, R.M. Oceanic slab-top melting during subduction: Implications for trace-element recycling and adakite petrogenesis. Geology 2020, 48, 216–220. [Google Scholar] [CrossRef]
- Petford, N.; Atherton, M. Na-rich partial melts from newly underplated basaltic crust: The Cordillera Blanca Batholith, Peru. J. Petrol. 1996, 37, 1491–1521. [Google Scholar] [CrossRef]
- Guo, Z.F.; Wilson, M.; Liu, J.Q. Post-collisional adakites in south Tibet: Products of partial melting of subduction-modified lower crust. Lithos 2007, 96, 205–224. [Google Scholar] [CrossRef]
- He, Y.S.; Li, S.G.; Hoefs, J.; Huang, F.; Liu, S.A.; Hou, Z.H. Post-collisional granitoids from the Dabie orogen: New evidence for partial melting of a thickened continental crust. Geochim. Cosmochim. Acta 2011, 75, 3815–3838. [Google Scholar] [CrossRef]
- Li, N.B.; Niu, H.C.; Yang, W.B.; Lai, C.K.; Zhao, Z.H. Orogenic root delamination induced by eclogitization of thickened lower crust in the Chinese Western Tianshan: Constraints from adakites. J. Geophys. Res. Solid Earth 2019, 124, 11089–11104. [Google Scholar] [CrossRef]
- Castillo, P.R.; Janney, P.E.; Solidum, R. Petrology and geochemistry of Camiguin Island, southern Philippines: Insights into the source of adakite and other lavas in a complex arc tectonic setting. Contrib. Mineral. Petrol. 1999, 134, 33–51. [Google Scholar] [CrossRef]
- Richards, J.P.; Kerrich, R. Special paper: Adakite-like rocks: Their diverse origins and questionable role in metallogenesis. Econ. Geol. 2007, 102, 537–576. [Google Scholar] [CrossRef]
- Ma, Q.; Zheng, J.P.; Xu, Y.G.; Griffin, W.L.; Zhang, R.S. Are continental “adakites” derived from thickened or foundered lower crust? Earth Planet. Sci. Lett. 2015, 419, 125–133. [Google Scholar] [CrossRef]
- Bourdon, E.; Eissen, J.P.; Monzier, M.; Robin, C.; Martin, H. Adakite-like lavas from Antisana volcano (Ecuador): Evidence from slab melt metasomatism beneath the Andean Northern volcanic zone. J. Petrol. 2002, 43, 99–217. [Google Scholar] [CrossRef]
- Wang, J.Z.; Li, J.W.; Zhao, X.F.; Qian, Z.Z.; Ma, C.Q. Genesis of the Chaoshan gold deposit and its host intrusion, Tongling area: Constraints from 40Ar/39Ar ages and elemental and Sr-Nd-O-C-S isotope geochemistry. Acta Petrol. Sin. 2008, 24, 1875–1888, (In Chinese with English Abstract). [Google Scholar]
- Li, X.H.; Li, Z.X.; Li, W.X.; Wang, X.C.; Gao, Y. Revisiting the “C-type adakites” of the Lower Yangtze River Belt, central eastern China: In-situ zircon Hf–O isotope and geochemical constraints. Chem. Geol. 2013, 345, 1–15. [Google Scholar] [CrossRef]
- Ge, X.Y.; Li, X.H.; Chen, Z.G. The geochemical characteristics and petrogenesis of Mesozoic high Sr-low Y type intermediate-acid igneous rocks in the east China: Constraint for the thickness of the east China. Chin. Sci. Bull. 2002, 47, 474–480, (In Chinese with English abstract). [Google Scholar]
- Xiong, X.L.; Keppler, H.; Audétat, A.; Ni, H.; Sun, W.; Li, Y. Partitioning of Nb and Ta between rutile and felsic melt and the fractionation of Nb/Ta during partial melting of hydrous metabasalt. Geochim. Cosmochim. Acta 2011, 75, 1673–1692. [Google Scholar] [CrossRef]
- Sun, W.D.; Hu, Y.H.; Kamenetsky, V.S.; Eggins, S.M.; Chen, M.; Arculus, R.J. Constancy of Nb/U in the mantle revisited. Geochim. Cosmochim. Acta 2008, 72, 3542–3549. [Google Scholar] [CrossRef]
- Sun, W.D.; Ling, M.X.; Chung, S.L.; Ding, X.; Yang, X.Y.; Liang, H.Y.; Fan, W.M.; Goldfarb, R.; Yin, Q.Z. Geochemical constraints on adakites of different origins and copper mineralization. J. Geol. 2012, 120, 105–120. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the continental crust. Treat. Geochem. 2003, 3, 1–64. [Google Scholar]
- Foley, S.F.; Barth, M.G.; Jenner, G.A. Rutile/melt partition coefficients for trace elements and an assessment of the influence of rutile on the trace element characteristics of subduction zone magmas. Geochim. Cosmochim. Acta 2000, 64, 933–938. [Google Scholar] [CrossRef]
- Schmidt, M.; Dardon, A.; Chazot, G.; Vannucci, R. The dependence of Nb and Ta rutile–melt partitioning on melt composition and Nb/Ta fractionation during subduction processes. Earth Planet. Sci. Lett. 2004, 226, 415–432. [Google Scholar] [CrossRef]
- Ding, X.; Lundstrom, C.; Huang, F.; Li, J.; Zhang, Z.M.; Sun, X.M.; Liang, J.L.; Sun, W.D. Natural and experimental constraints on formation of the continental crust based on niobium–tantalum fractionation. Int. Geol. Rev. 2009, 51, 473–501. [Google Scholar] [CrossRef]
- John, T.; Klemd, R.; Klemme, S.; Pf¨ander, J.A.; Hoffmann, J.E.; Gao, J. Nb–Ta fractionation by partial melting at the titanite–rutile transition. Contrib. Mineral. Petrol. 2011, 161, 35–45. [Google Scholar] [CrossRef]
- Bindeman, I.N.; Eiler, J.M.; Yogodzinski, G.M.; Tatsumi, Y.; Stern, C.R.; Grove, T.L.; Portnyagin, M.; Hoernle, K.; Danyushevsky, L.V. Oxygen isotope evidence for slab melting in modern and ancient subduction zones. Earth Planet. Sci. Lett. 2005, 235, 480–496. [Google Scholar] [CrossRef]
- Xu, Z.W.; Lu, X.C.; Ling, H.F.; Lu, J.J.; Jiang, S.Y.; Nie, G.P.; Huang, S.S.; Hua, M. Study on metallogenic mechanism and time of hydrothermal superimposed transformation of Dongguashan stratified copper Deposit in Anhui Province. Acta. Geol. Sin. 2005, 3, 372, (In Chinese with English Abstract). [Google Scholar]
- Lu, S.M. The Magmatism and Fluid Mineralization of Shizishan Copper-Gold Orefield of Tongling, Anhui Province. Ph.D. Thesis, Hefei University of Technology, Hefei, China, 2007; pp. 1–100, (In Chinese with English abstract). [Google Scholar]
- Chen, K.; Shao, Y.J.; Zhang, J.K.; Zhang, Y.; Tan, H.J.; Zhang, Y.C.; Liu, Z.F. Garnet U-Pb geochronology and geochemistry reveal deposit types and fluid evolution: An example from the Dongguashan Cu-Au deposit, eastern China. Ore Geol. Rev. 2022, 145, 104883. [Google Scholar] [CrossRef]
- Xiao, X.; Zhou, T.F.; White, N.C.; Zhang, L.J.; Fan, Y.; Chen, X.F. Multiple generations of titanites and their geochemical characteristics record the magmatic hydrothermal processes and timing of the Dongguashan porphyry-skarn Cu-Au system, Tongling district, Eastern China. Miner. Depos. 2021, 56, 363–380. [Google Scholar]
- Xu, X.C.; Lu, S.M.; Xie, Q.Q.; Bai, L. SHRIMP Zircon U-Pb Dating for the Magmatic Rocks in Shizishan Ore-field of Tongling, Anhui Province, and Its Geological Implications. Acta Geol. Sin. 2008, 82, 500–510, (In Chinese with English Abstract). [Google Scholar]
- Sun, W.D.; Bennett, V.C.; Eggins, S.M.; Arculus, R.J.; Perfit, M.R. Rhenium systematics in submarine MORB and back-arc basin glasses: Laser ablation ICP-MS results. Chem. Geol. 2003, 196, 259–281. [Google Scholar]
- Thiéblemont, D.; Stein, G.; Lescuyer, J.L. Epithermal and porphyry deposits: The adakite connection. C. R. L’acad. Sci. Paris 1997, 325, 103–109. [Google Scholar]
- Oyarzún, R.; Márquez, A.; Lillo, J.; López, I.; Rivera, S. Giant vs small porphyry copper deposits of Cenozoic age in northern Chile: Adakitic vs normal calc-alkaline magmatism. Miner. Depos. 2001, 36, 794–798. [Google Scholar] [CrossRef]
- Ballard, J.R.; Palin, M.J.; Campbell, I.H. Relative oxidation states of magmas inferred from Ce(IV)/Ce(III) in zircon: Application to porphyry copper deposits of northern Chile. Contrib. Mineral. Petrol. 2002, 144, 347–364. [Google Scholar] [CrossRef]
- Sun, W.D.; Huang, R.F.; Li, H.; Hu, Y.B.; Zhang, C.C.; Sun, S.J.; Zhang, L.P.; Ding, X.; Li, C.Y.; Zartman, R.E.; et al. Porphyry deposits and oxidized magmas. Ore Geol. Rev. 2015, 65, 97–131. [Google Scholar]
- Sun, W.D.; Wang, J.T.; Zhang, L.P.; Zhang, C.C.; Li, H.; Ling, M.X.; Ding, X.; Li, C.Y.; Liang, H.Y. The formation of porphyry copper deposits. Acta Geochim. 2017, 36, 9–15. [Google Scholar]
- Nadeau, O.; Willians-Jones, A.E.; Stix, J. Sulphide magma as a source of metals in arcrelated magmatic hydrothermal ore fluids. Nat. Geosci. 2010, 3, 501–505. [Google Scholar] [CrossRef]
- Brounce, M.; Boyce, J.; McCubbin, F.M.; Humphreys, J.; Reppart, J.; Stolper, E.; Eiler, J. The oxidation state of sulfur in lunar apatite. Am. Mineral. 2019, 104, 307–312. [Google Scholar]
- Chu, M.F.; Wang, K.L.; Griffin, W.L.; Chung, S.L.; O’Reilly, S.Y.; Pearson, N.J.; Iizuka, Y. Apatite composition: Tracing petrogenetic processes in Transhimalayan granitoids. J. Petrol. 2009, 50, 1829–1855. [Google Scholar]
- Miles, A.J.; Graham, C.M.; Hawkesworth, C.J.; Gillespie, M.R.; Hinton, R.W.; Bromiley, G.D. Apatite: A new redox proxy for silicic magmas? Geochim. Cosmochim. Acta 2014, 132, 101–119. [Google Scholar]
- Sha, L.K.; Chappell, B.W. Apatite chemical composition, determined by electron microprobe and laser-ablation inductively coupled plasma mass spectrometry, as a probe into granite petrogenesis. Geochim. Cosmochim. Acta 1999, 63, 3861–3881. [Google Scholar]
- Konecke, B.A.; Fiege, A.; Simon, A.C.; Linsler, S.; Holtz, F. An experimental calibration of a sulfur-in-apatite oxybarometer for mafic systems. Geochim. Cosmochim. Acta 2019, 265, 242–258. [Google Scholar]
- Sadove, G.; Konecke, B.A.; Fiege, A.; Simon, A.C. Structurally bound S2+, S1+, S4+, S6+ in terrestrial apatite: The redox evolution of hydrothermal fluids at the Phillips mine, New York, USA. Ore Geol. Rev. 2019, 107, 1084–1096. [Google Scholar]
- Ding, T.; Ma, D.S.; Lu, J.J.; Zhang, R.Q. Apatite in granitoids related to polymetallic mineral deposits in southeastern Hunan province, Shi-Hang zone, China: Implications for petrogenesis and metallogenesis. Ore Geol. Rev. 2015, 69, 104–117. [Google Scholar]
- Qian, L.; Wang, Y.; Xie, J.C.; Sun, W.D. The Late Mesozoic granodiorite and polymetallic mineralization in southern Anhui Province, China: A perspective from apatite geochemistry. Solid Earth Sci. 2019, 4, 178–189. [Google Scholar]
- Mathez, E.A.; Webster, J.D. Partitioning behavior of chlorine and fluorine in the system apatite-silicate melt-fluid. Geochim. Cosmochim. Acta 2005, 69, 1275–1286. [Google Scholar] [CrossRef]
- McCubbin, F.M.; Kaaden, K.E.V.; Tart`ese, R.; Boyce, J.W.; Mikhail, S.; Whitson, E.S.; Bell, A.S.; Anand, M.; Franchi, I.A.; Wang, J. Experimental investigation of F, Cl, and OH partitioning between apatite and Fe-rich basaltic melt at 1.0–1.2 GPa and 950–1000 °C. Am. Mineral. 2015, 100, 1790–1802. [Google Scholar] [CrossRef]
- Boyce, J.W.; Hervig, R.L. Apatite as a monitor of late-stage magmatic processes at Volcán Irazú, Costa Rica. Contrib. Mineral. Petrol. 2009, 157, 135–145. [Google Scholar]
- Boyce, J.W.; Liu, Y.; Rossman, G.R.; Guan, Y.B.; Eiler, J.M.; Stolper, E.M.; Taylor, L.A. Lunar apatite with terrestrial volatile abundances. Nature 2010, 466, 466–470. [Google Scholar]
- McCubbin, F.M.; Hauri, E.H.; Elardo, S.M.; Vander Kaaden, K.E.; Wang, J.; Shearer, C.K. Hydrous melting of the martian mantle produced both depleted and enriched shergottites. Geology 2012, 40, 683–686. [Google Scholar]
- Tartése, R.; Anand, M.; Barnes, J.J.; Starkey, N.A.; Franchi, I.A.; Sano, Y. The abundance, distribution, and isotopic composition of hydrogen in the Moon as revealed by basaltic lunar samples: Implications for the volatile inventory of the Moon. Geochim. Cosmochim. Acta 2013, 122, 58–74. [Google Scholar]
- Dingwell, D.B.; Hess, K.U.; Romano, C. Extremely fluid behavior of hydrous peralkaline rhyolites. Earth Planet. Sci. Lett. 1998, 158, 31–38. [Google Scholar]
- Filiberto, J.; Treiman, A.H. The effect of chlorine on the liquidus of basalt: First results and implications for basalt genesis on Mars and Earth. Chem. Geol. 2009, 263, 60–68. [Google Scholar]
- Bai, T.B.; Koster Van Groos, A.F. The distribution of Na, K, Rb, Sr, Al, Ge, Cu, W, Mo, La, and Ce between granitic melts and coexisting aqueous fluids. Geochim. Cosmochim. Acta 1999, 63, 1117–1131. [Google Scholar]
- Hezarkhani, A.; Williams-Jones, A.E.; Gammons, C.H. Factors controlling copper solubility and chalcopyrite deposition in the Sungun porphyry copper deposit, Iran. Miner. Depos. 1999, 34, 770–783. [Google Scholar] [CrossRef]
- Archibald, S.M.; Migdisov, A.A.; Williams-Jones, A.E. The stability of Au-chloride complexes in water vapor at elevated temperatures and pressures. Geochim. Cosmochim. Acta 2001, 65, 4413–4423. [Google Scholar] [CrossRef]
- Lassiter, J.C.; Hauri, E.H.; Nikogosian, I.K.; Barsczus, H.G. Chlorine–potassium variations in melt inclusions from Raivavae and Rapa, Austral Islands: Constraints on chlorine recycling in the mantle and evidence for brine-induced melting of oceanic crust. Earth Planet. Sci. Lett. 2002, 202, 525–540. [Google Scholar]
- Blevin, P.L.; Chappell, B.W. The role of magma sources, oxidation states and fractionation in determining the granite metallogeny of eastern Australia. Earth Environ. Sci. Trans. R. Soc. Edinb. 1992, 83, 305–316. [Google Scholar]
- Stroncik, N.A.; Haase, K.M. Chlorine in oceanic intraplate basalts: Constraints on mantle sources and recycling processes. Geology 2004, 32, 945–948. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Jiang, X.; Guo, J.; Jiang, K. Geochronology and Geochemistry of Cretaceous Adakitic Rocks of the Dongguashan Cu Deposit in the Lower Yangtze River Belt: Insights into Petrogenesis and Mineralization. Minerals 2023, 13, 953. https://doi.org/10.3390/min13070953
Zhang Z, Jiang X, Guo J, Jiang K. Geochronology and Geochemistry of Cretaceous Adakitic Rocks of the Dongguashan Cu Deposit in the Lower Yangtze River Belt: Insights into Petrogenesis and Mineralization. Minerals. 2023; 13(7):953. https://doi.org/10.3390/min13070953
Chicago/Turabian StyleZhang, Zanzan, Xiaoyan Jiang, Jia Guo, and Kenan Jiang. 2023. "Geochronology and Geochemistry of Cretaceous Adakitic Rocks of the Dongguashan Cu Deposit in the Lower Yangtze River Belt: Insights into Petrogenesis and Mineralization" Minerals 13, no. 7: 953. https://doi.org/10.3390/min13070953
APA StyleZhang, Z., Jiang, X., Guo, J., & Jiang, K. (2023). Geochronology and Geochemistry of Cretaceous Adakitic Rocks of the Dongguashan Cu Deposit in the Lower Yangtze River Belt: Insights into Petrogenesis and Mineralization. Minerals, 13(7), 953. https://doi.org/10.3390/min13070953




