Experimental Verification for the Graphitization of Inertinite
Abstract
1. Introduction
2. Samples and Methods
2.1. Samples
- (1)
- The sample was crushed and sieved through a 200-mesh (75 μm) standard sieve.
- (2)
- Approximately 15 g of coal powder (proportional to the increase in sample weight) was placed in a plastic beaker and mixed with 80 mL of HCl solution (36% mass fraction).
- (3)
- The mixture was stirred for 4 h at a constant temperature of 60 °C in a water bath.
- (4)
- The HCl solution was then filtered out, and 80 mL of HF solution (40%) was added to the coal sample.
- (5)
- The same water bath and acid washing process were repeated.
- (1)
- Take a 10 g sample and place it in a crucible, then put it into an induction furnace for graphitization.
- (2)
- Vacuum is applied to remove gas from graphitization furnace and then maintain an argon gas atmosphere throughout the process.
- (3)
- Increase the temperature with controlled ventilation. The pressure inside the furnace is maintained at 20–30 KPa above standard atmospheric pressure.
- (4)
- The temperature is raised from room temperature to 1000 °C at a rate of 5 °C/min. The sample is held at 1000 °C for 60 min.
- (5)
- The temperature ramp rate is then changed to 10 °C/min until reaching the target temperature point. The sample is held at the target temperature for 90 min.
- (6)
- Afterward, the furnace is allowed to naturally cool down.
- (7)
- The graphitization temperature range starts at 1800 °C, with intervals of 300 °C, and the maximum temperature point is set at 3000 °C. A total of five treatment temperature points are used.
2.2. X-ray Diffraction
2.3. Raman Spectroscopy
2.4. Transmission Electron Microscopy
3. Results
3.1. X-ray Diffraction
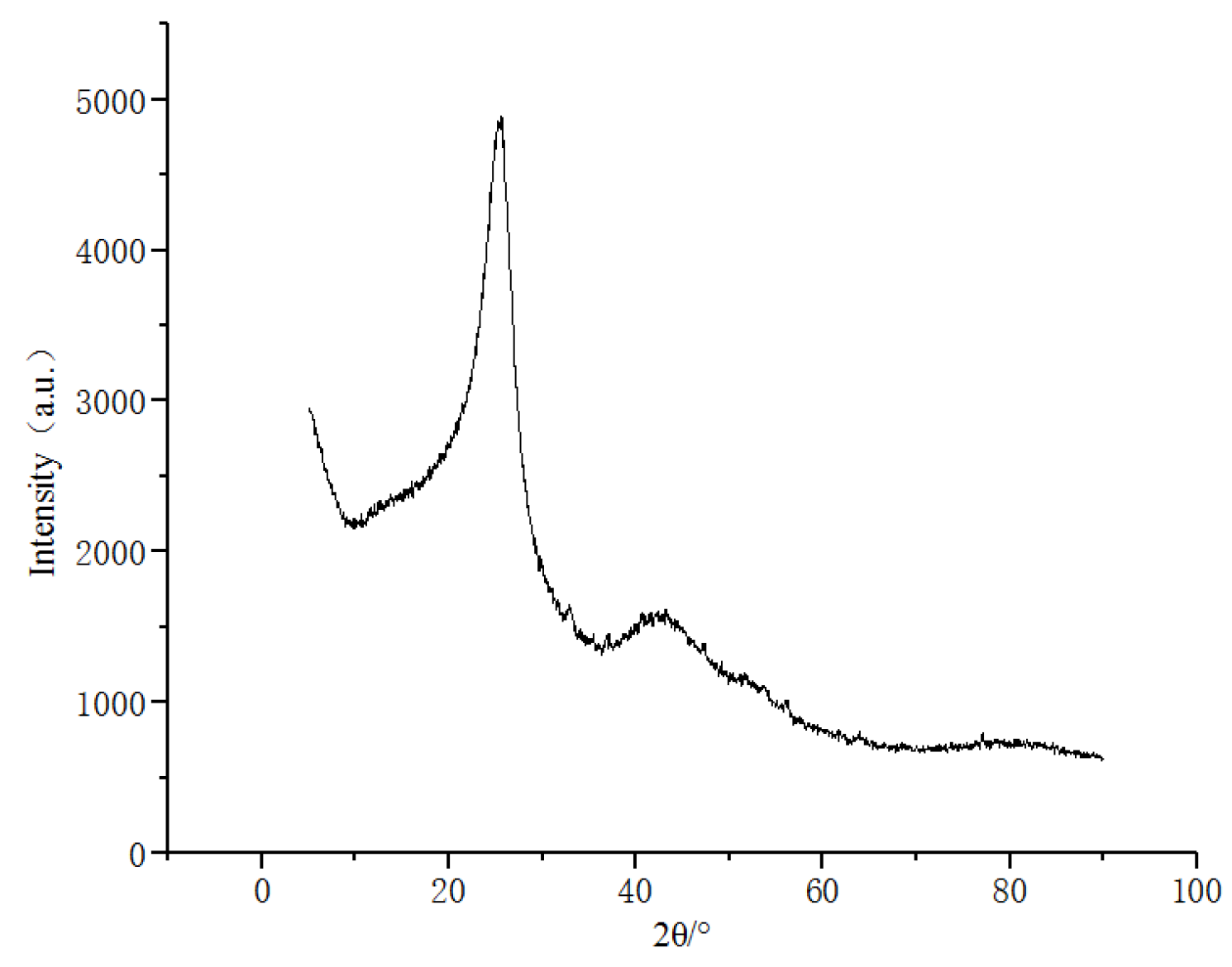
3.1.1. Precursors
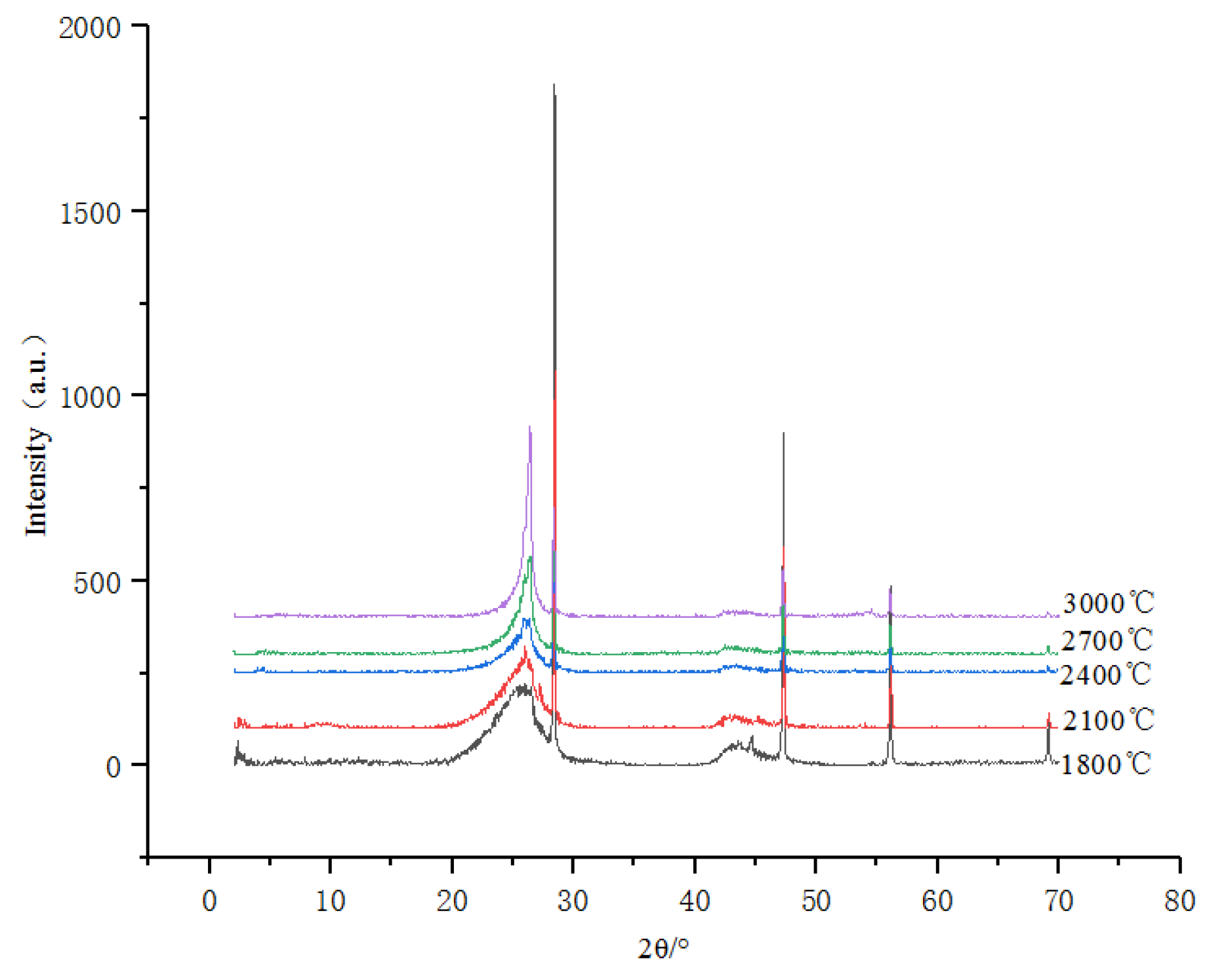
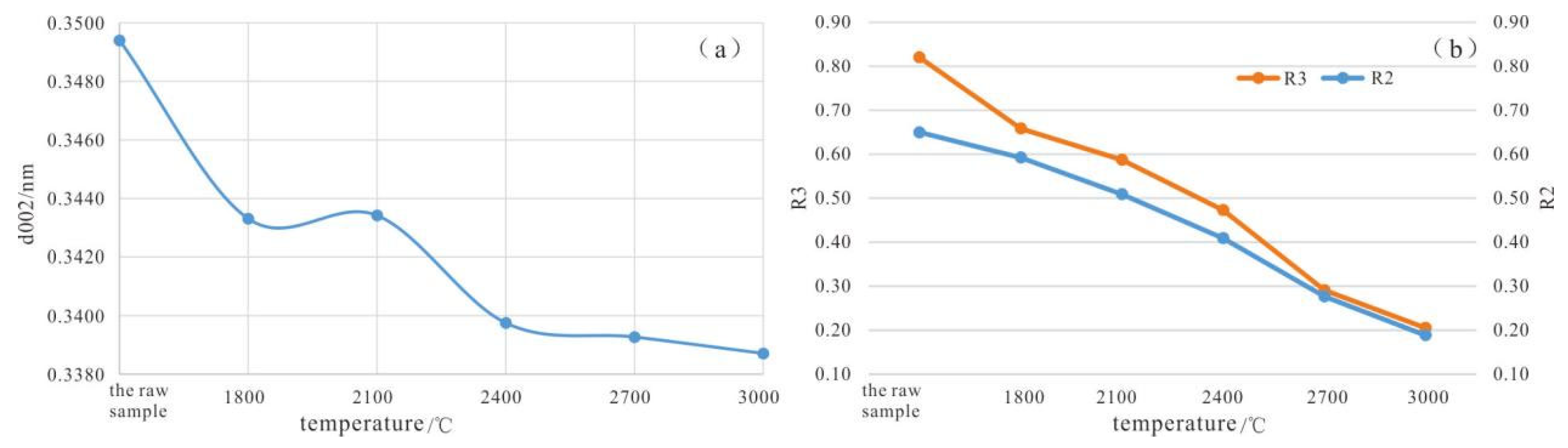
3.1.2. High-Temperature Thermal Simulation Experiments (HTT)
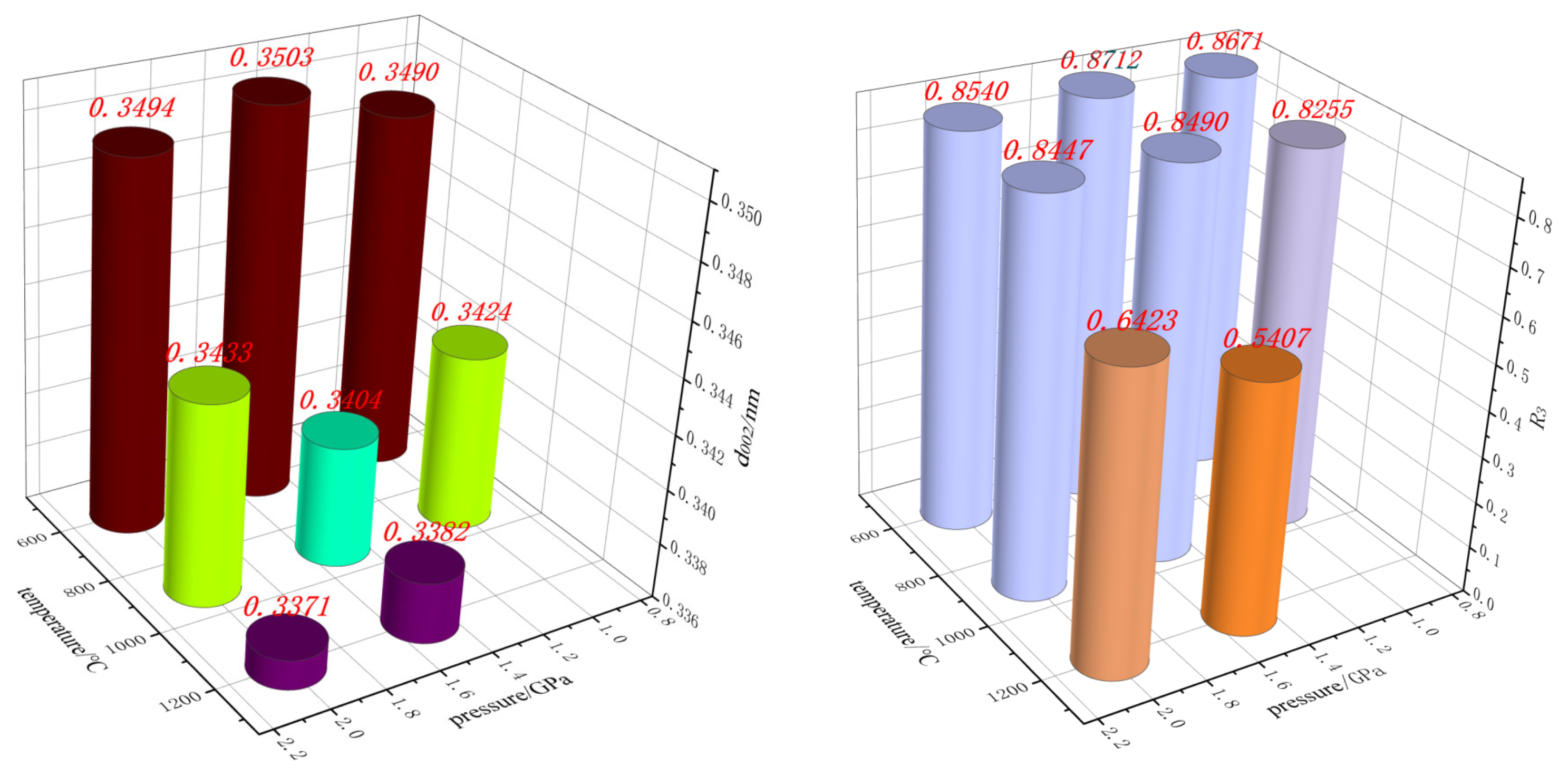
3.1.3. High-Temperature and High-Pressure Simulation Experiments (HTHP)
3.2. Raman Spectroscopy
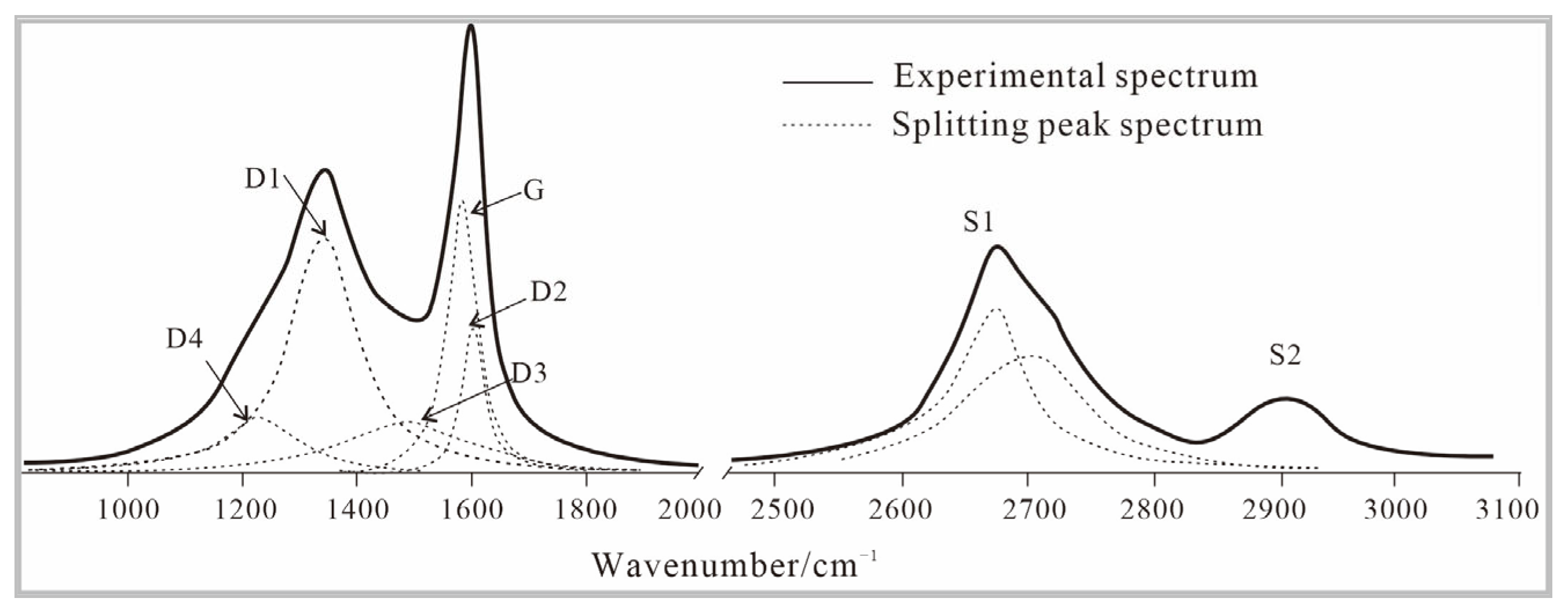
3.2.1. Precursors
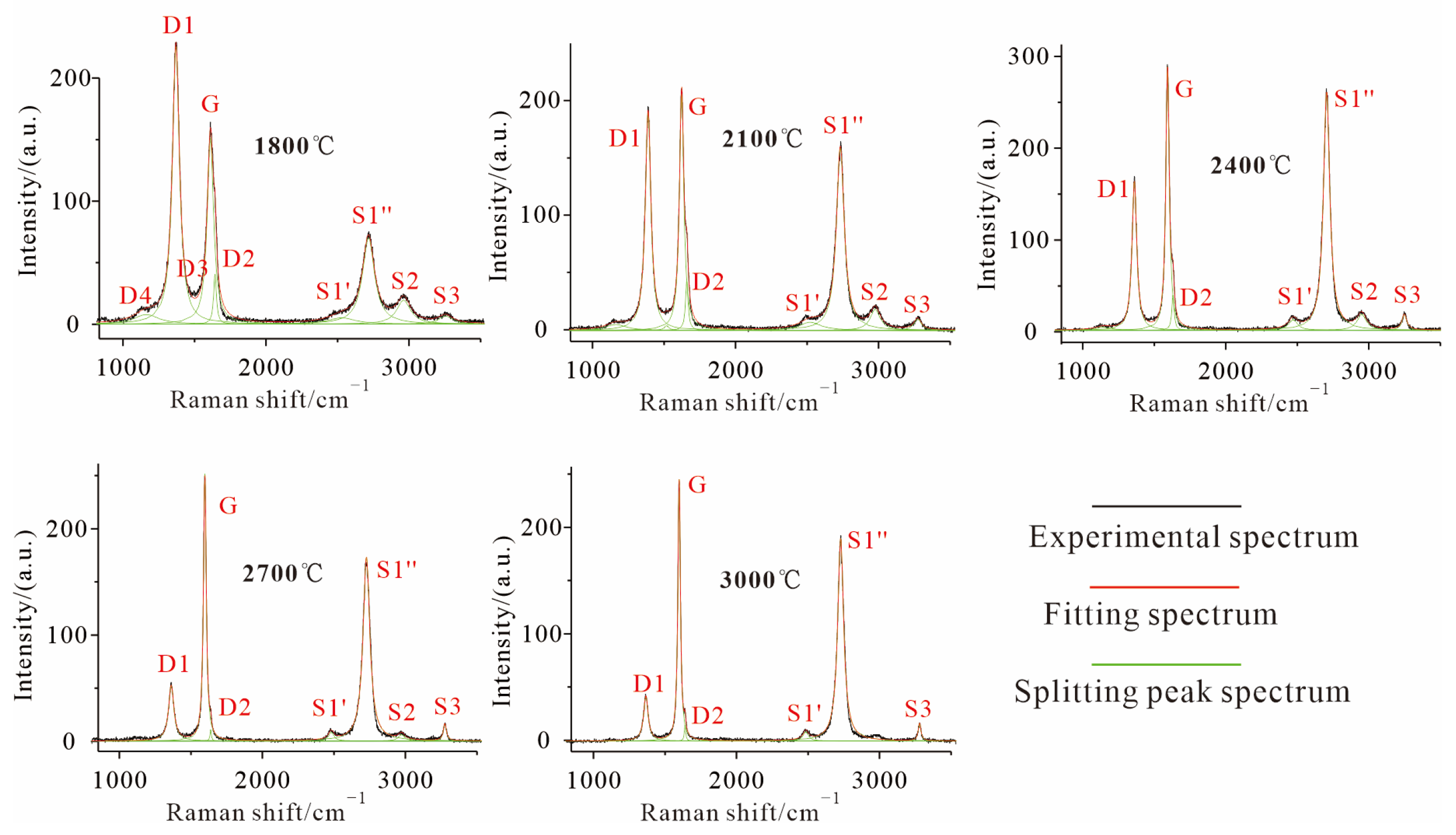
3.2.2. High-Temperature Thermal Simulation (HTT)
3.2.3. High-Temperature and High-Pressure Simulation Experiments (HTHP)
3.3. Transmission Electron Microscopy
3.3.1. Precursors
3.3.2. High-Temperature Thermal Treatment (HTT)
3.3.3. High-Temperature and High-Pressure Simulation Experiment (HTHP)
4. Discussion
4.1. Threshold Conditions for Graphitization of Inertinite
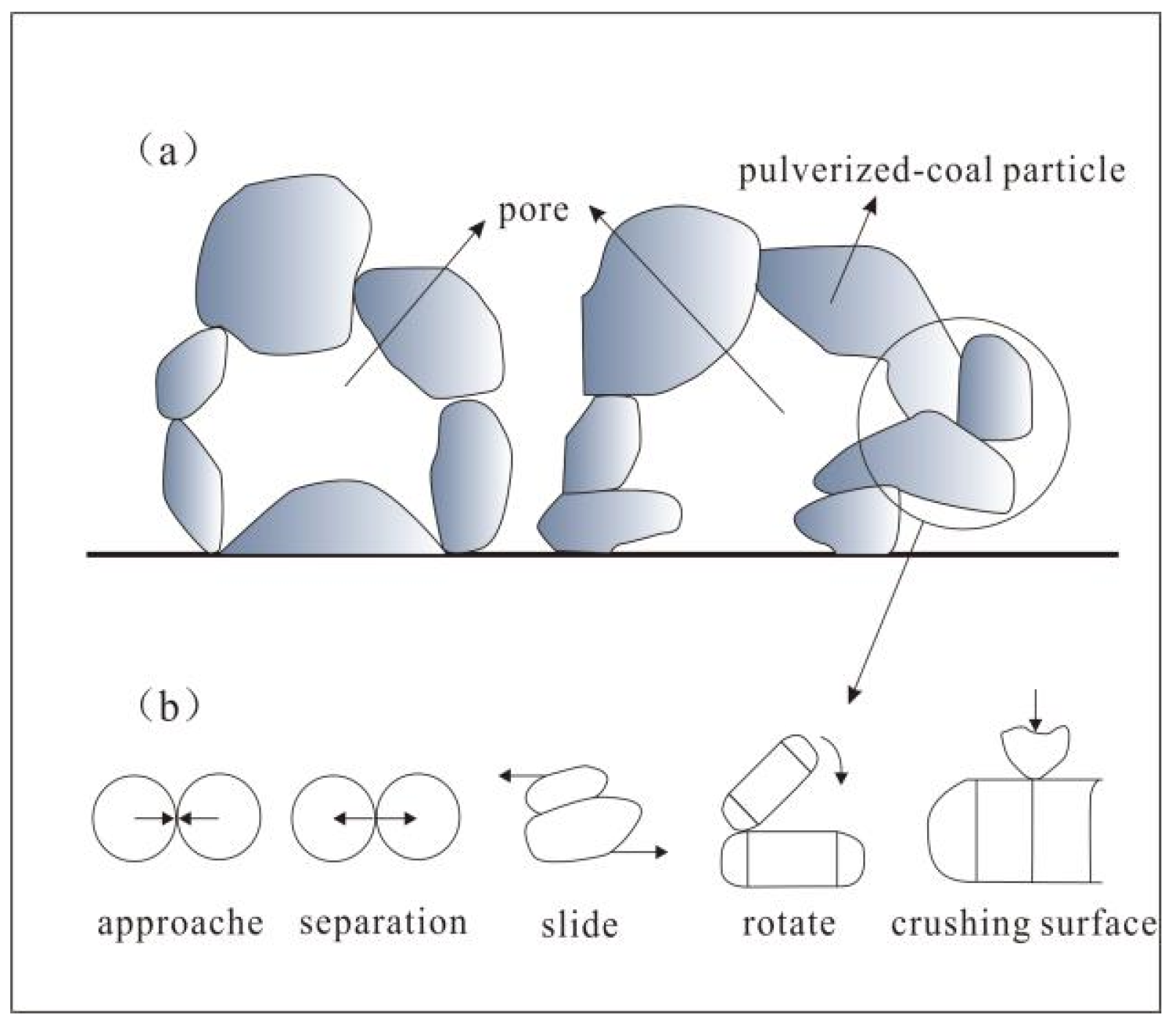
4.2. Catalytic Effect of Structural Stress on Graphitization
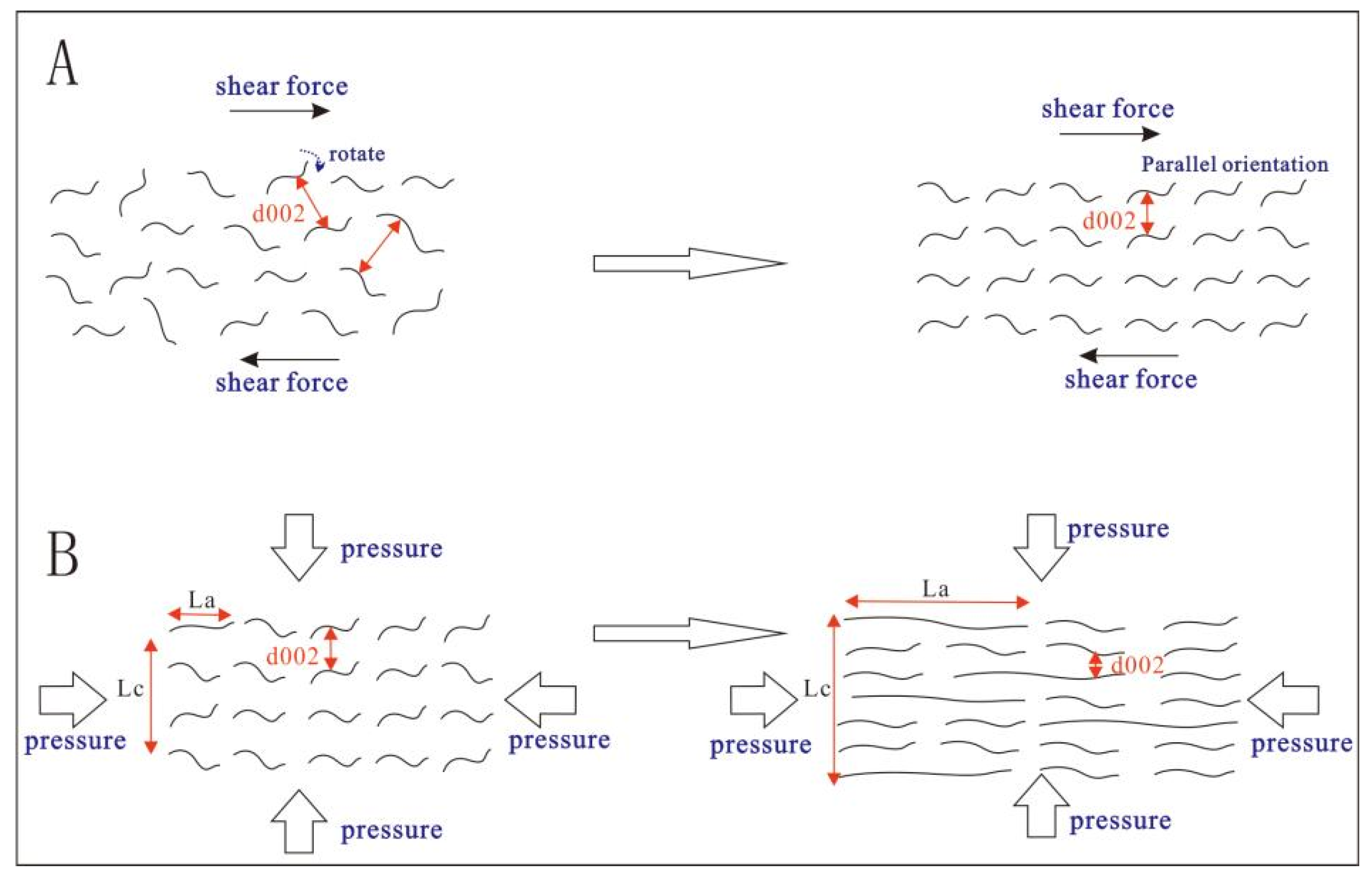
4.3. The Mechanism of Stress in the Graphitization Process
5. Conclusions
- (1)
- Through HTT and HTHP simulation experiments, it has been demonstrated that the inertinite in coal can undergo graphitization, but there exists a structural transformation threshold. In the HTT simulation experiments, the inertinite remains in an “inert” state (with d002 stagnant before reaching 0.3420 nm) within the temperature range of 1800 °C to 2100 °C. However, beyond 2100 °C, the inertinite undergoes transformation.
- (2)
- The HTHP experiments indicate that at a temperature of 600 °C, the variation in pressure does not lead to any significant structural changes. However, at 900 °C/1.5 GPa, the d002 value decreases to below 0.3420 nm. There exists an optimal pressure for the development of the graphite lattice.
- (3)
- Force has a significant promoting effect on the construction of the three-dimensional lattice, while its impact on the elimination of lattice defects is relatively minor. Compared to the high-temperature treatment at 3000 °C (atmospheric pressure), the sample obtained at 1200 °C/1.5 GPa exhibits a smaller d002 value but a higher degree of defects (as indicated by the higher D peaks), albeit lower than that of the pure high-temperature treatment at 1800 °C.
- (4)
- A potential mechanism by which force promotes graphitization is analyzed and summarized. It involves the internal structure of the sample responding to external forces through different modes (migration or reconstruction) to release and eliminate shear and pressure effects, thereby promoting the rotation and orientation of aromatic layers and reducing the interlayer spacing (d002).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marqués, M.; Suárez-Ruiz, I.; Flores, D.; Guedes, A.; Rodrigues, S. Correlation between optical, chemical and micro-structural parameters of high-rank coals and graphite. Int. J. Coal Geol. 2009, 77, 377–382. [Google Scholar] [CrossRef]
- Cao, D.; Zhang, H.; Dong, Y.; Yang, C. Nanoscale Microscopic Features and Evolution Sequence of Coal-Based Graphite. J. Nanosci. Nanotechnol. 2017, 17, 6276–6283. [Google Scholar] [CrossRef]
- Rao, J.; Zhang, P.; He, S.; Li, Z.; Ma, H.; Shen, Z.; Miao, S. A review on the utilization of natural graphite and graphite-based materials. Sci. Sin. Technol. 2017, 47, 13–31. [Google Scholar] [CrossRef]
- Cao, D.Y.; Zhang, H.; Dong, Y.J.; Wu, G.; Ning, S.; Mo, J.; Li, X. Research status and key directions of geological study on coal-based graphite. Earth Sci. Front. 2017, 24, 317–327. (In Chinese) [Google Scholar]
- Franklin, R.E. The structure of graphitic carbon. Acta Crystallogr. 1951, 4, 253–261. [Google Scholar] [CrossRef]
- Bonijoly, M.; Oberlin, M.; Oberlin, A. A possible mechanism for natural graphite formation. Int. J. Coal Geol. 1982, 1, 283–312. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.J.; Zhang, H.; Cao, D.Y. Factors influencing coal graphitization and their experimental verification. J. Mineral. Sci. 2018, 3, 9–19. (In Chinese) [Google Scholar]
- Nyathi, M.S.; Clifford, C.B.; Schobert, H.H. Characterization of graphitic materials prepared from different rank Pennsylvania anthracites. Fuel 2013, 114, 244–250. [Google Scholar] [CrossRef]
- Beyssac, O.; Rouzaud, J.N.; Goffe, B.; Brunet, F.; Chopin, C. Graphitization in a high-pressure, low-temperature metamorphic gradient: A Raman microspectroscopy and HRTEM study. Contrib. Mineral. Petrol. 2002, 143, 19–31. [Google Scholar] [CrossRef]
- Diessel, C.F.K.; Brothers, R.N.; Black, P.M. Coalification and graphitization in high-pressure schists in New Caledonia. Contrib. Mineral. Petrol. 1978, 68, 63–78. [Google Scholar] [CrossRef]
- Rodrigues, S.; Suárez-Ruiz, I.; Marques, M.; Flores, D. Catalytic role of mineral matter in structural transformation of anthracites during high temperature treatment. Int. J. Coal Geol. 2012, 93, 49–55. [Google Scholar] [CrossRef]
- Li, J.; Qin, Y.; Chen, Y.; Luo, Q.; Deng, R.; Guo, S.; Chen, Q. Differential graphitization of organic matter in coal: Some new understandings from reflectance evolution of meta-anthracite macerals. Int. J. Coal Geol. 2021, 240, 103747. [Google Scholar] [CrossRef]
- Dai, S.F.; Bechtel, A.; Eble, C.F.; Flores, R.M.; French, D.; Graham, I.T.; Madison, M.; Hood, M.M.; Hower, J.C.; Korasidis, V.A.; et al. Recognition of peat depositional environments in coal: A review. Int. J. Coal Geol. 2020, 219, 103383. [Google Scholar] [CrossRef]
- Niekerk, D.V.; Jonathan, P.; Mathews, J.P. Molecular representations of Permian-aged vitrinite-rich and inertinite-rich South African coals. Fuel 2010, 89, 73–82. [Google Scholar] [CrossRef]
- Li, J.; Feng, J.; Wen-Ying, L.I. Thermochemical Reaction Representation of Shenfu Dongshen Inertinite and Vitrinite. Acta Phys. Chim. Sin. 2009, 25, 1311–1319. [Google Scholar]
- Wilks, K.R.; Mastalerz, M.; Ross, J.V.; Bustin, R.M. The effect of experimental deformation on the graphitization of Pennsylvania anthracite. Int. J. Coal Geol. 1993, 24, 347–369. [Google Scholar] [CrossRef]
- Bustin, R.M.; Ross, J.V.; Rouzaud, J.N. Mechanisms of graphite formation from kerogen: Experimental evidence. Int. J. Coal Geol. 1995, 28, 1–36. [Google Scholar] [CrossRef]
- Pappano, P.J. A Mechanism of Pennsylvania Anthracite Graphitization Involving Carbide Formation and Decomposition; The Pennsylvania State University: State College, PA, USA, 2003. [Google Scholar]
- Atria, J.V.; Rusinko, F.; Schobert, H.H. Structural Ordering of Pennsylvania Anthracites on Heat Treatment to 2000–2900 °C. Energy Fuels 2002, 16, 1343–1347. [Google Scholar] [CrossRef]
- Pappano, P.J.; Schobert, H.H. Effect of Natural Mineral Inclusions on the Graphitizability of a Pennsylvania Anthracite. Energy Fuels 2009, 23, 422–428. [Google Scholar] [CrossRef]
- He, Q.; Tang, J.J.; Wang, F.; Liu, X. An assembly of six-face top-pressure machines suitable for extreme high-temperature conditions. Chin. J. High Press. Phys. 2014, 28, 145–151. (In Chinese) [Google Scholar]
- Jiang, B.; Qin, Y. Experimental study on coal deformation under high temperature and high pressure. J. China Coal Soc. 1997, 22, 82–86. (In Chinese) [Google Scholar]
- Jiang, B.; Qin, Y.; Jin, F.L. Deformation characteristics of coal macerals under high temperature and high pressure. Geol. Sci. 1998, 21, 17–24. (In Chinese) [Google Scholar]
- Zhao, F.H. Composition and Structure of Coal—Transmission Electron Microscopy (TEM) and X-ray Diffraction (XRD) Study on Coal Structure; China University of Mining and Technology: Beijing, China, 1994. (In Chinese) [Google Scholar]
- Beny-Bassez, C.; Rouzaud, J.N. Characterization of carbonaceous materials by correlated electron and optical microscopy and Raman microspectroscopy. Scanning Electron Microsc. 1985, 1985, 119–132. [Google Scholar]
- Beyssac, O.; Goffé, B.; Chopin, C.; Rouzaud, J.N. Raman spectra of carbonaceous material in metasediments: A new geothermometer. J. Metamorph. Geol. 2002, 20, 859–871. [Google Scholar] [CrossRef]
- Beyssac, O.; Goffé, B.; Petitet, J.P.; Froigneux, E.; Moreau, M.; Rouzaud, J.N. On the characterization of disordered and heterogeneous carbonaceous materials by Raman spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2003, 59, 2267–2276. [Google Scholar] [CrossRef]
- Rantitsch, G.; Lämmerer, W.; Fisslthaler, E.; Mitsche, S.; Kaltenböck, H. On the discrimination of semi-graphite and graphite by Raman spectroscopy. Int. J. Coal Geol. 2016, 159, 48–56. [Google Scholar] [CrossRef]
- Harris, L. Quantitative X-ray diffraction analysis and its application to various coals. Carbon 2001, 39, 1821–1833. [Google Scholar]
- Su, X.B.; Si, Q.; Song, J.X. Raman spectroscopic characteristics of coal. J. China Coal Soc. 2016, 41, 1197–1202. (In Chinese) [Google Scholar]
- Oberlin, A. Carbonization and graphitization. Carbon 1984, 22, 521–541. [Google Scholar] [CrossRef]
- Zhou, H. Study on Microcrystal Structure and Pore Structure Characteristics of Organic Microscopic Components in Coal; Henan Polytechnic University: Zhengzhou, China, 2018. (In Chinese) [Google Scholar]
- Chen, Y.X.; Du, J.G.; Liu, H. Distribution of boundary stress of mineral particles under high temperature and high static pressure. J. Cent. South Univ. 2010, 41, 286–292. (In Chinese) [Google Scholar]
- Ciz, R.; Siggins, A.F.; Gurevich, B.; Dvorkin, J. Influence of microheterogeneity on effective stress law for elastic properties of rocks. Geophysics 2007, 73, 7–14. [Google Scholar] [CrossRef]
- Liu, L.M.; Wu, Y.Z. Chemical behavior of crystalline minerals under shear stress and its geological significance. Geol. Explor. 1996, 31, 26–31. (In Chinese) [Google Scholar]
- Feng, Z.C.; Zhao, D.; Wang, J.F. Stress distribution characteristics of rocks under static water pressure. In Proceedings of the 11th National Conference on Rock Mechanics and Engineering, Wuhan, China, 19–21 November 2011; Chinese Society for Rock Mechanics and Engineering: Beijing, China, 2011. (In Chinese). [Google Scholar]
- Sun, J. In-Situ Transmission Electron Microscopy Study on Structural Evolution of Materials under External Fields; Southeast University: Nanjing, China, 2016. (In Chinese) [Google Scholar]
- Wang, M.B. Study on Powder Compaction; Jilin University: Changchun, China, 2007. (In Chinese) [Google Scholar]




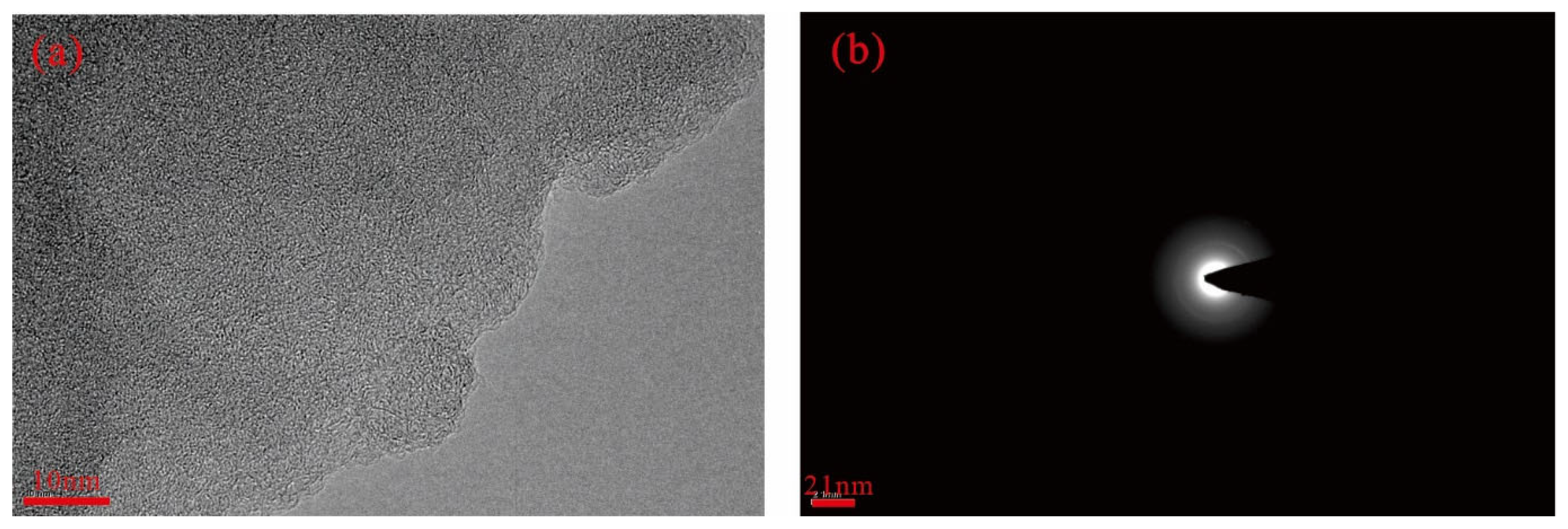
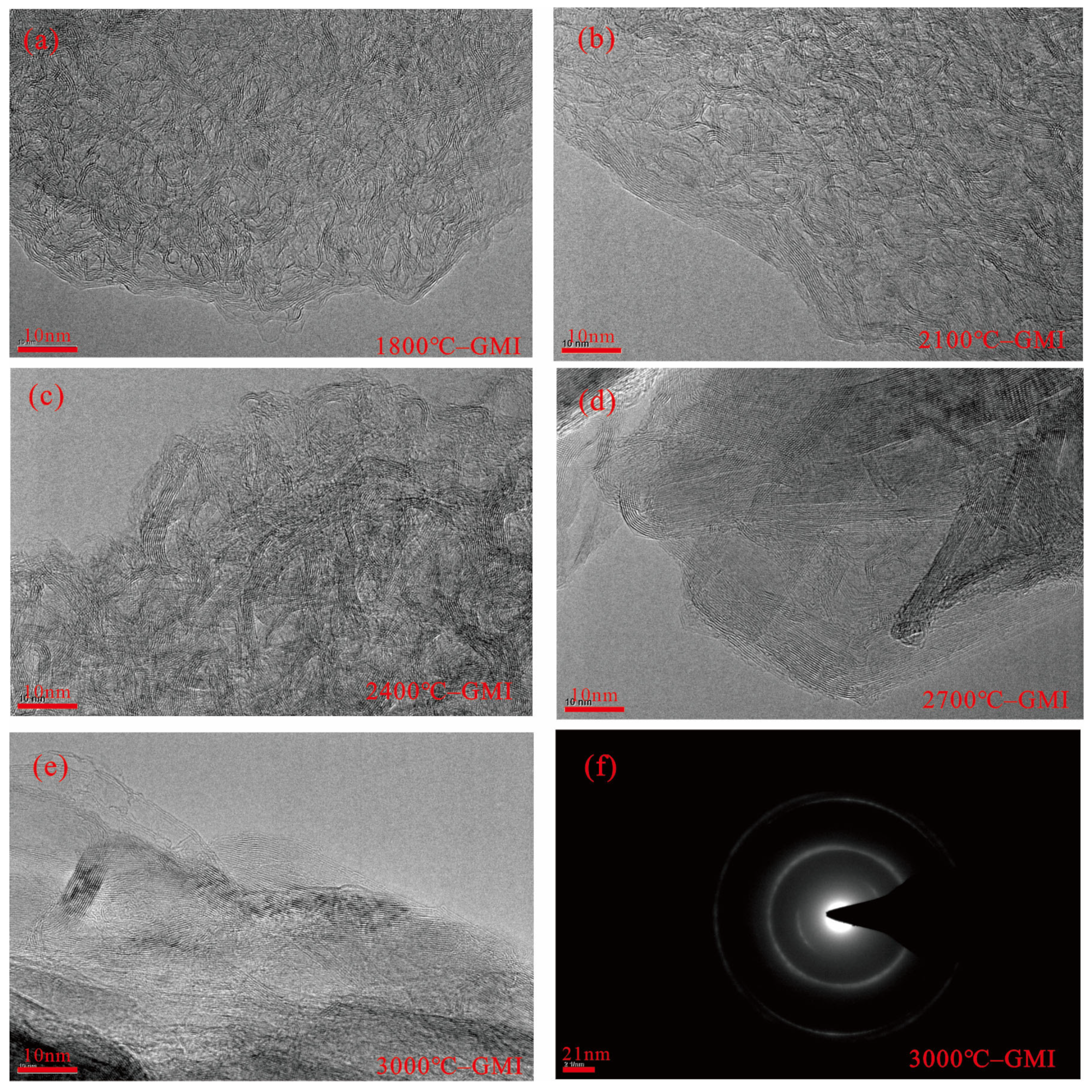

| Sample | Maceral | Industrial Analysis | Elemental Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitrinite | Inertinite | Liptinite | Mad | Ad | Vdaf | FCd | St | Odaf | Cdaf | Hdaf | Ndaf | |
| GMI | 8.4% | 91.6% | 0.0% | 0.5% | 7.3% | 16.0% | 81.2% | 0.5% | 3.2% | 91.9% | 3.5% | 0.8% |
| Processing Temperature | 2θ002/° | Lc/nm | La/nm | d002/nm |
|---|---|---|---|---|
| 1800 °C | 25.93 | 3.18 | 10.76 | 0.3433 |
| 2100 °C | 25.92 | 3.43 | 12.06 | 0.3434 |
| 2400 °C | 26.21 | 7.57 | 17.88 | 0.3398 |
| 2700 °C | 26.25 | 9.12 | 17.98 | 0.3393 |
| 3000 °C | 26.29 | 17.99 | 19.84 | 0.3387 |
| Processing Temperature/°C | Processing Pressure/Gpa | 2θ002/° | Lc/nm | La/nm | d002/nm |
|---|---|---|---|---|---|
| 600 | 1.0 | 25.499 | 1.62 | 3.95 | 0.3490 |
| 1.5 | 25.405 | 1.88 | 1.60 | 0.3503 | |
| 2.0 | 25.483 | 1.43 | 2.70 | 0.3494 | |
| 900 | 1.0 | 26.000 | 2.72 | 9.74 | 0.3424 |
| 1.5 | 26.157 | 3.11 | 10.57 | 0.3404 | |
| 2.0 | 25.932 | 2.30 | 6.23 | 0.3433 | |
| 1200 | 1.5 | 26.330 | 17.68 | 60.79 | 0.3382 |
| 2.0 | 26.421 | 20.73 | 42.33 | 0.3371 |
| Processing Temperature/°C | 1800 | 2100 | 2400 | 2700 | 3000 |
|---|---|---|---|---|---|
| R2 | 0.592 | 0.509 | 0.409 | 0.277 | 0.189 |
| R3 | 0.658 | 0.587 | 0.473 | 0.291 | 0.205 |
| Processing Temperature/°C | 600 | 900 | 1200 | |||||
|---|---|---|---|---|---|---|---|---|
| Processing pressure/Gpa | 1.0 | 1.5 | 2.0 | 1.0 | 1.5 | 2.0 | 1.5 | 2.0 |
| R2 | 0.712 | 0.692 | 0.689 | 0.745 | 0.763 | 0.760 | 0.472 | 0.527 |
| R3 | 0.867 | 0.871 | 0.854 | 0.826 | 0.849 | 0.845 | 0.541 | 0.642 |
| Experimental Conditions | Sample ID | XRD Parameters | Raman | |||||
|---|---|---|---|---|---|---|---|---|
| Temperature/°C | Ressure/Gpa | d002/nm | Lc/nm | La/nm | R1 | R2 | R3 | |
| 1800 | Atmospheric | 1800 °C–GMI | 0.3433 | 3.18 | 10.76 | 1.52 | 0.589 | 0.663 |
| 3000 | 3000 °C–GMI | 0.3387 | 17.99 | 19.84 | 0.13 | 0.19 | 0.21 | |
| 1200 | 1.5 | 1200 °C–1.5 GPa–GMI–7 | 0.3382 | 17.68 | 60.79 | 0.6 | 0.47 | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Cao, D.; Chen, G.; Bi, Z.; Chen, Q. Experimental Verification for the Graphitization of Inertinite. Minerals 2023, 13, 888. https://doi.org/10.3390/min13070888
Liu Z, Cao D, Chen G, Bi Z, Chen Q. Experimental Verification for the Graphitization of Inertinite. Minerals. 2023; 13(7):888. https://doi.org/10.3390/min13070888
Chicago/Turabian StyleLiu, Zhifei, Daiyong Cao, Gaojian Chen, Zhongwei Bi, and Qingtong Chen. 2023. "Experimental Verification for the Graphitization of Inertinite" Minerals 13, no. 7: 888. https://doi.org/10.3390/min13070888
APA StyleLiu, Z., Cao, D., Chen, G., Bi, Z., & Chen, Q. (2023). Experimental Verification for the Graphitization of Inertinite. Minerals, 13(7), 888. https://doi.org/10.3390/min13070888






