Tomographic Imaging of Bauxite Grains Leached Using Hydrochloric Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Mineralogical Characterization of Raw Materials
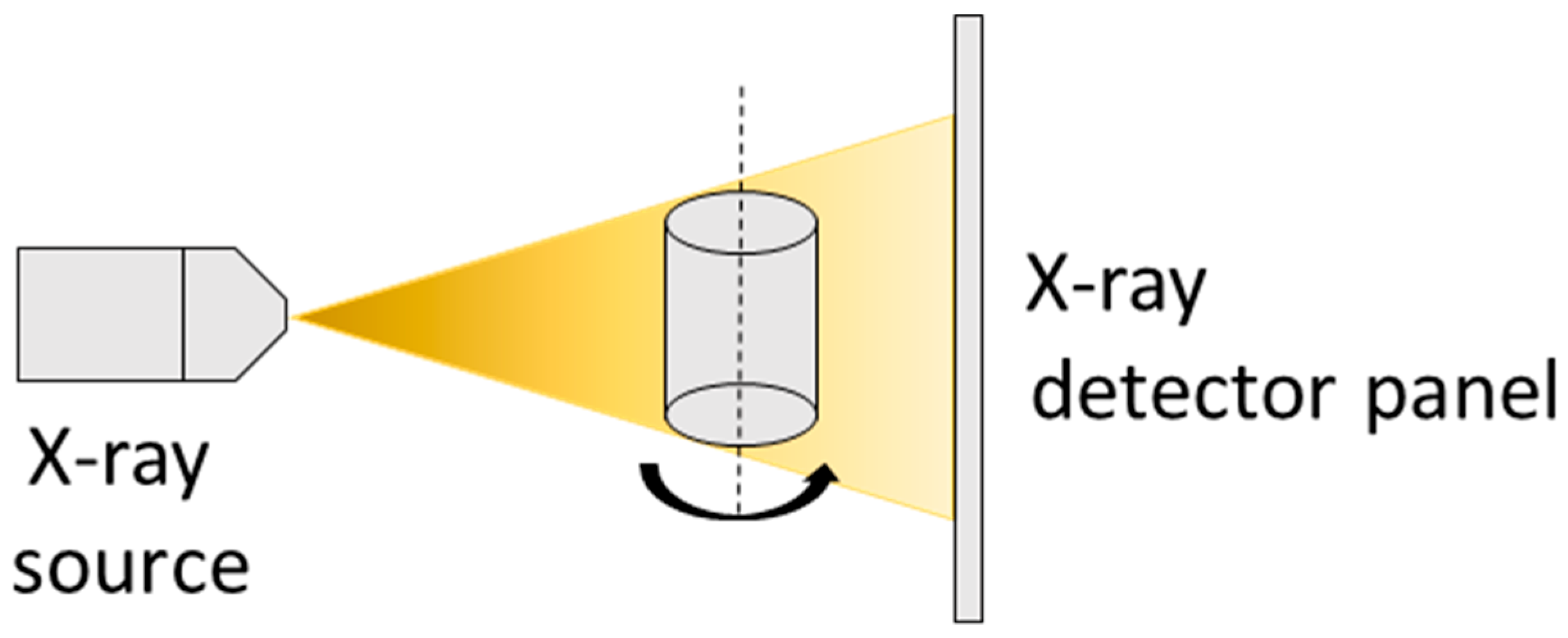
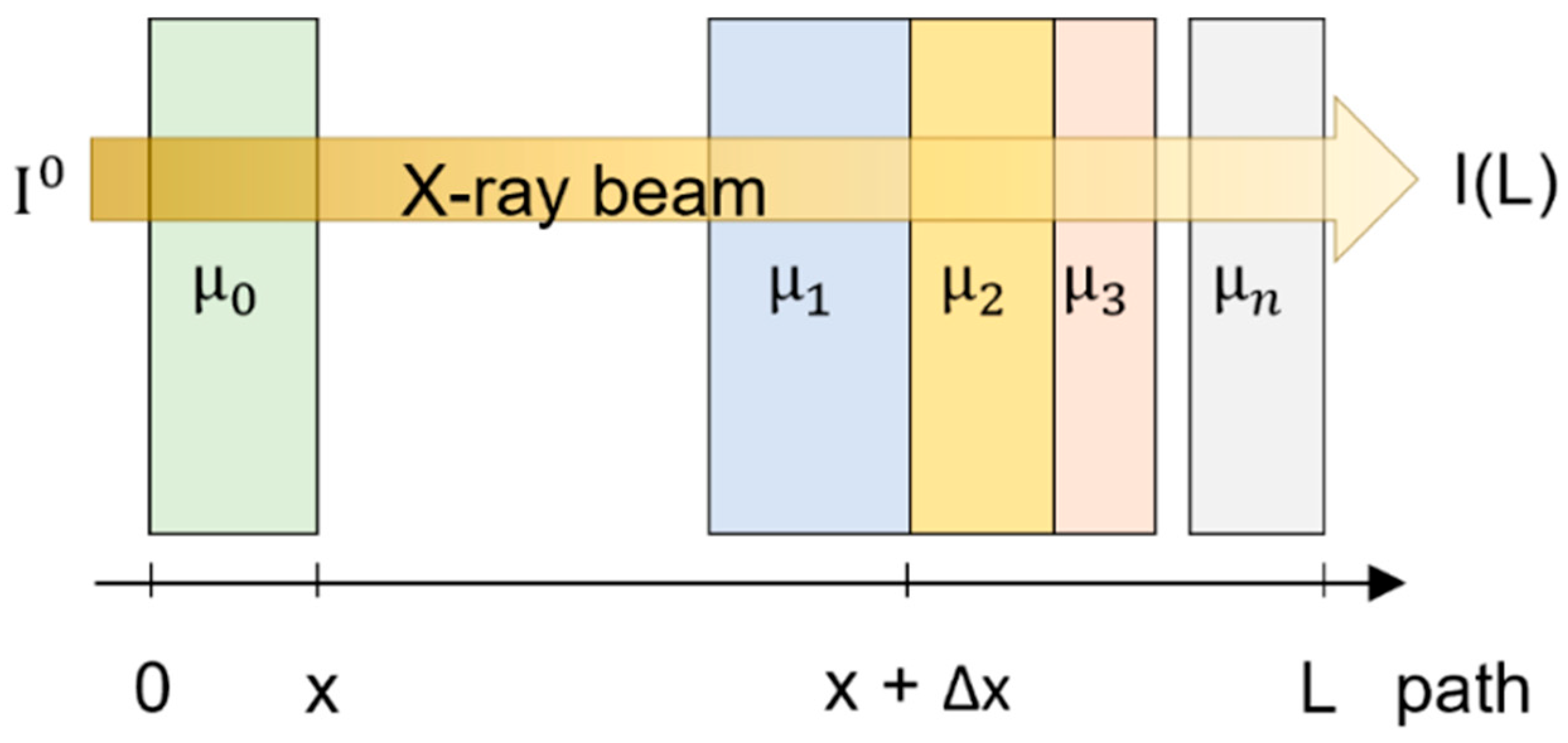
2.2. XRT Examination
2.3. Leaching of Bauxites
3. Results and Discussion
3.1. Chemical and Mineralogical Characterization of Raw Material Qualities
3.1.1. XRF Results
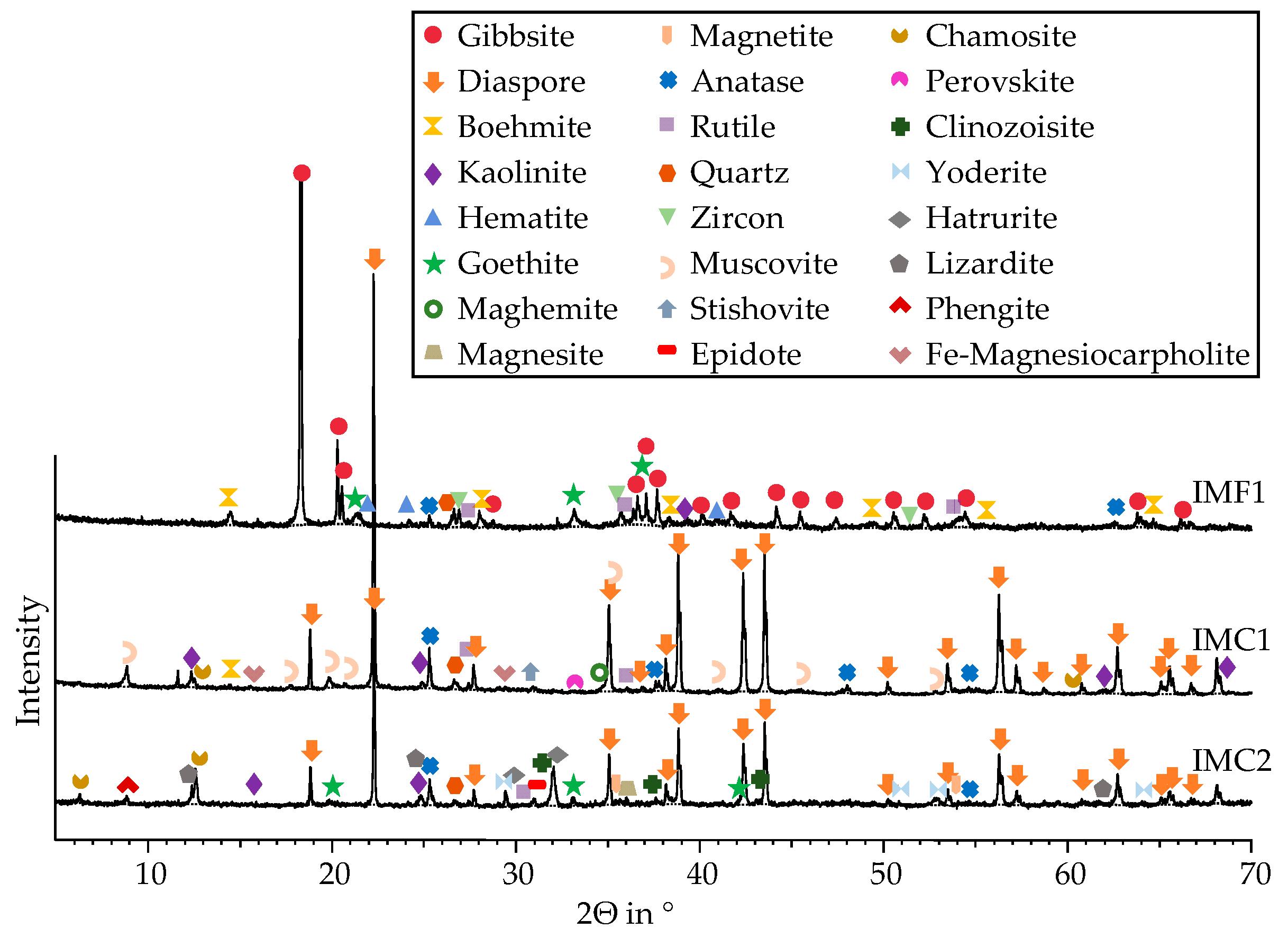
3.1.2. XRD Results
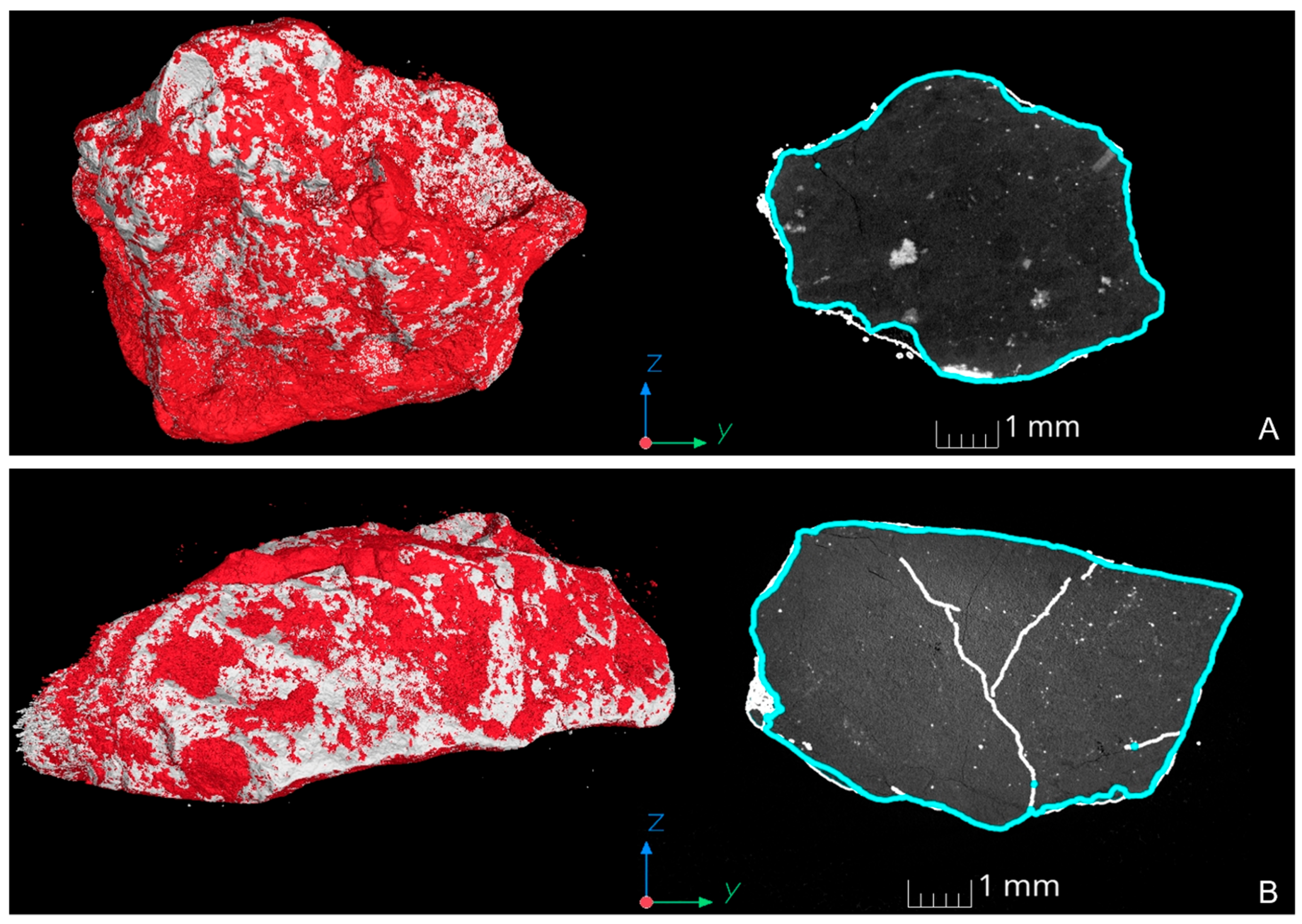
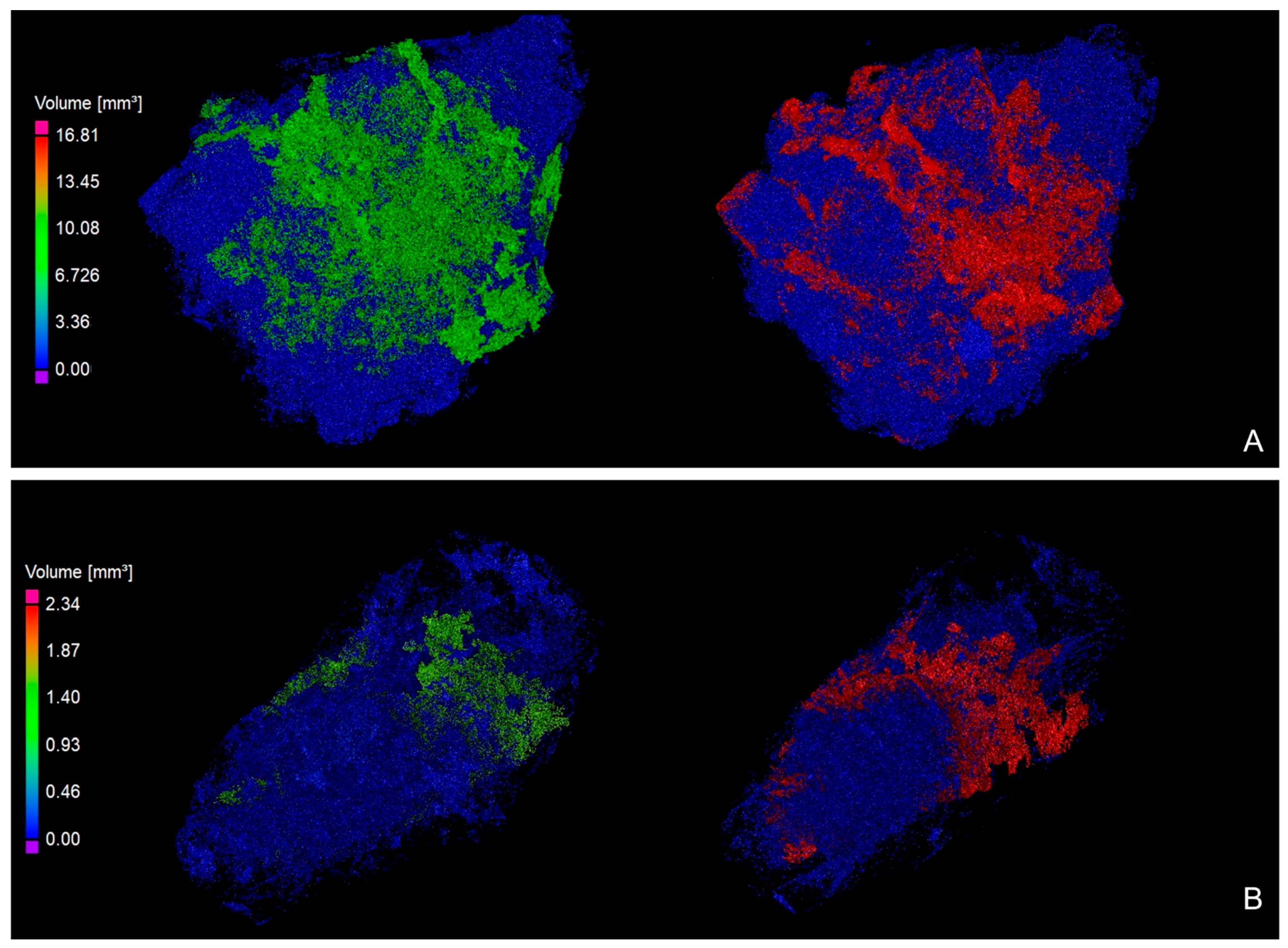
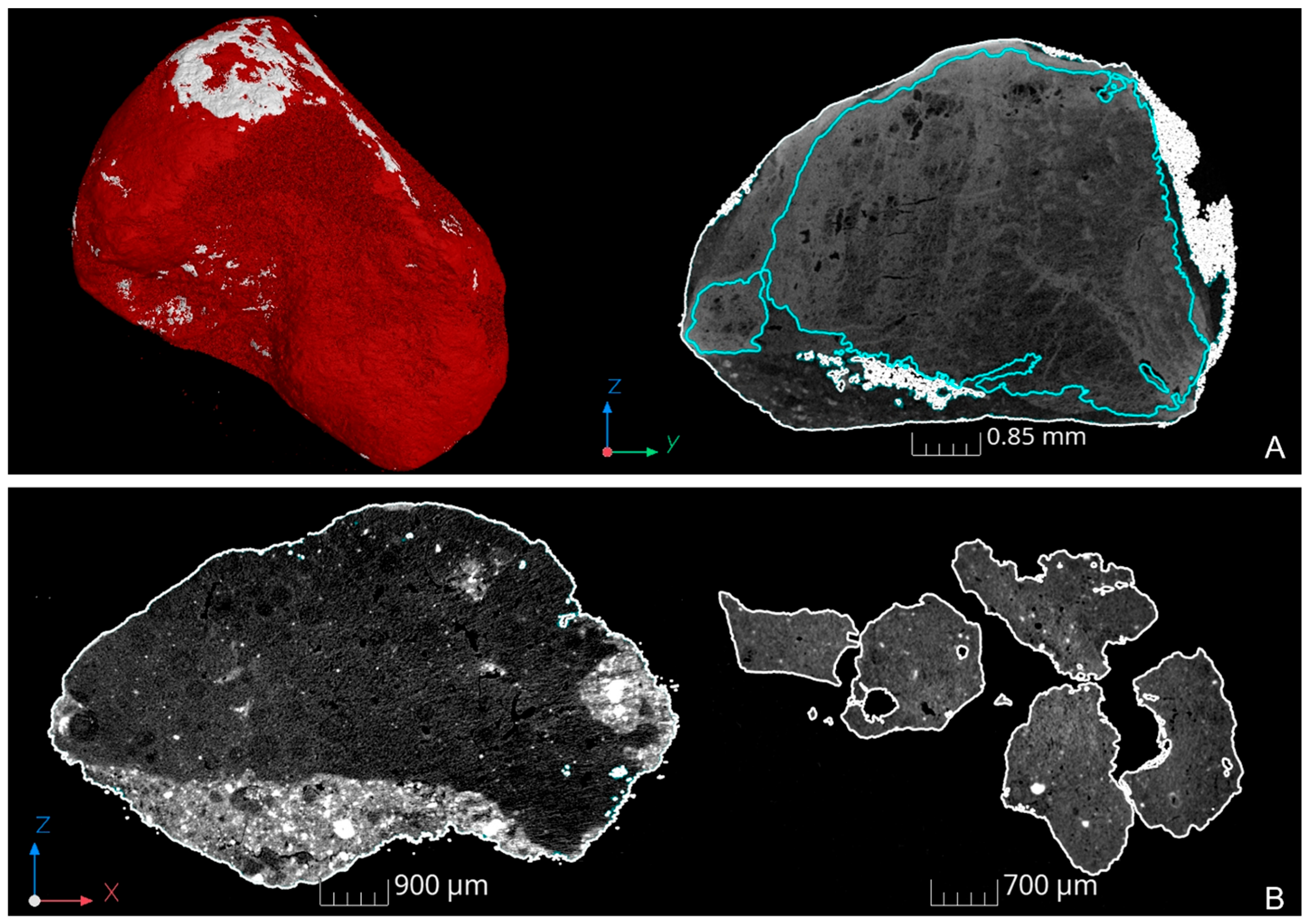
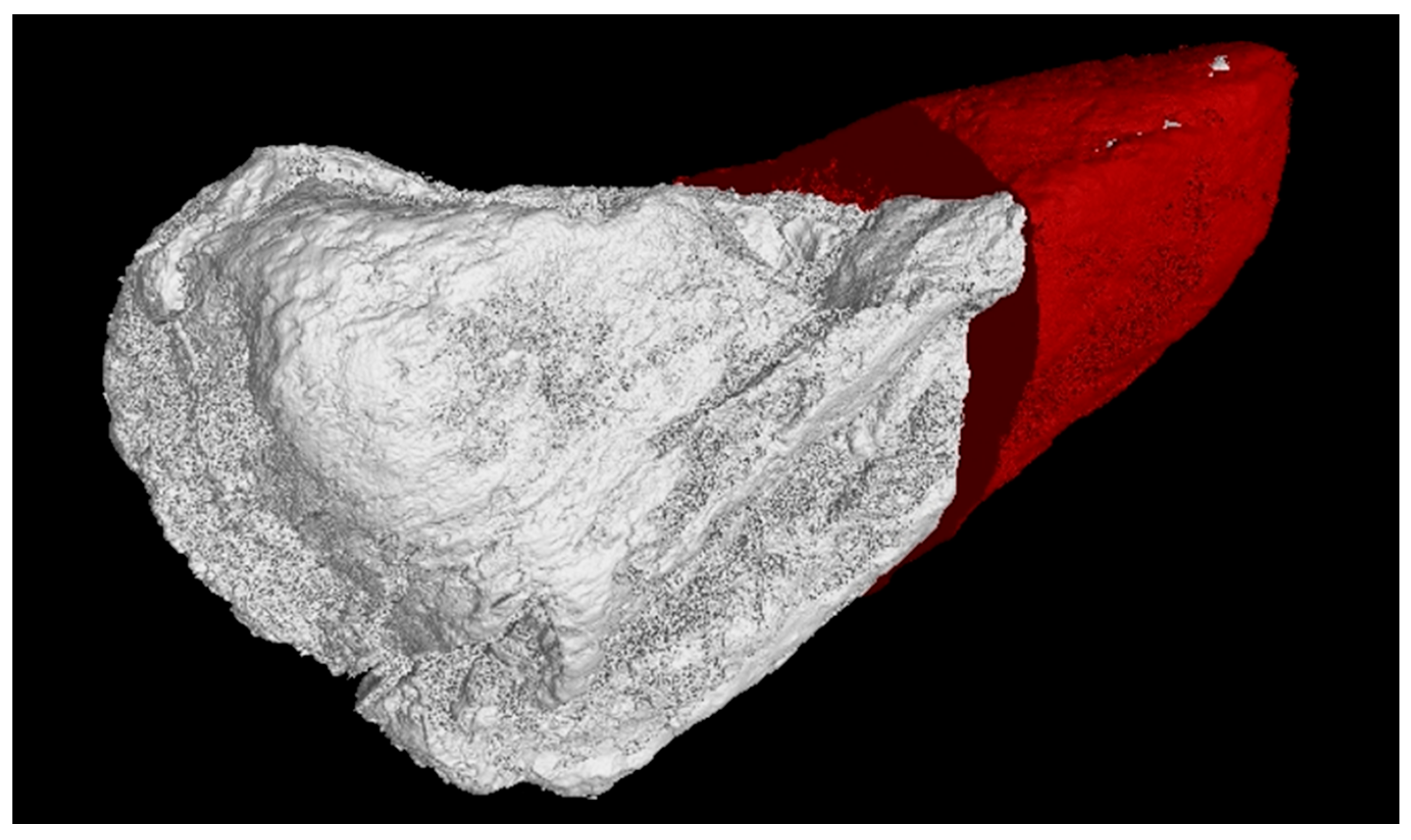
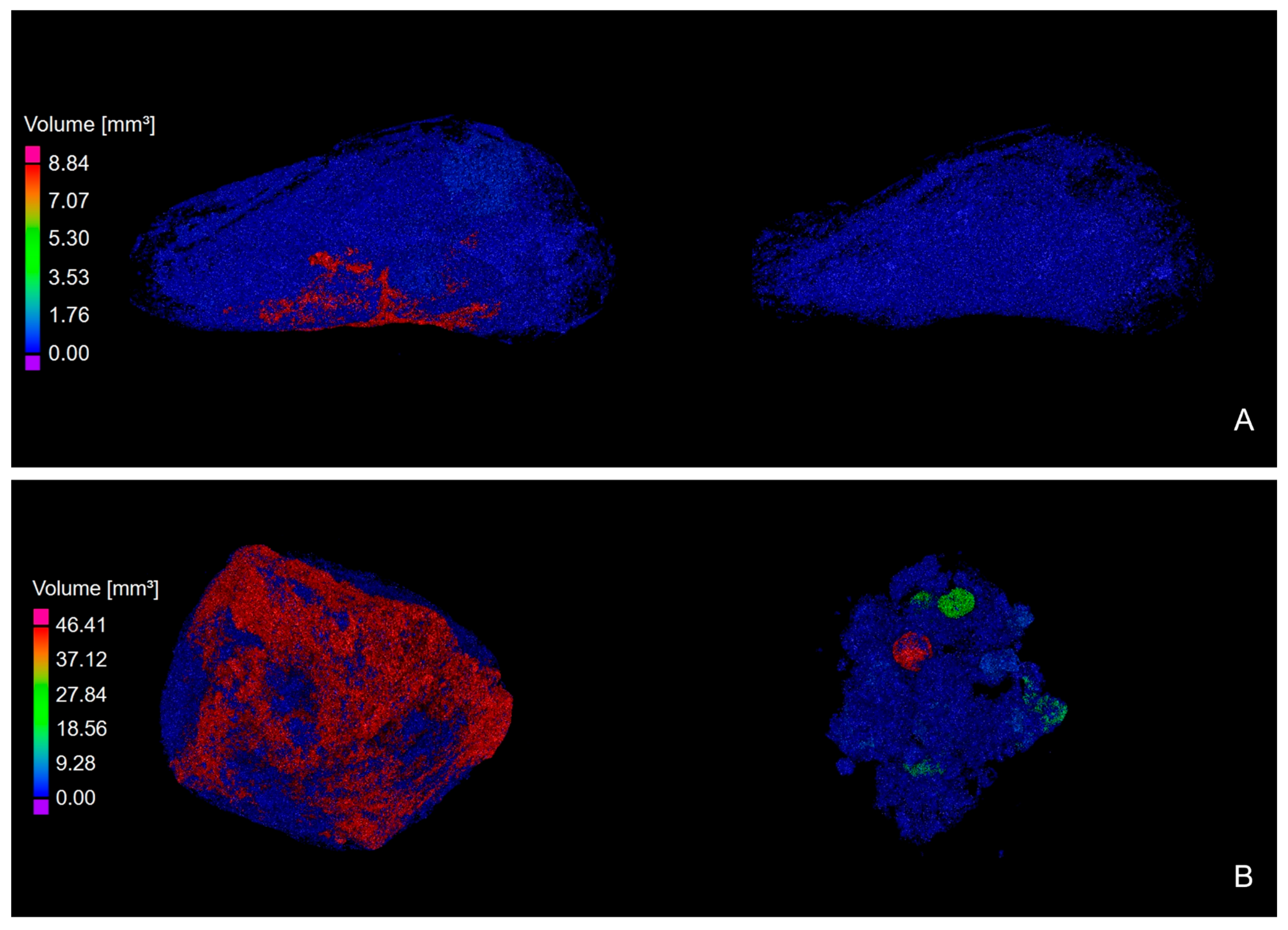
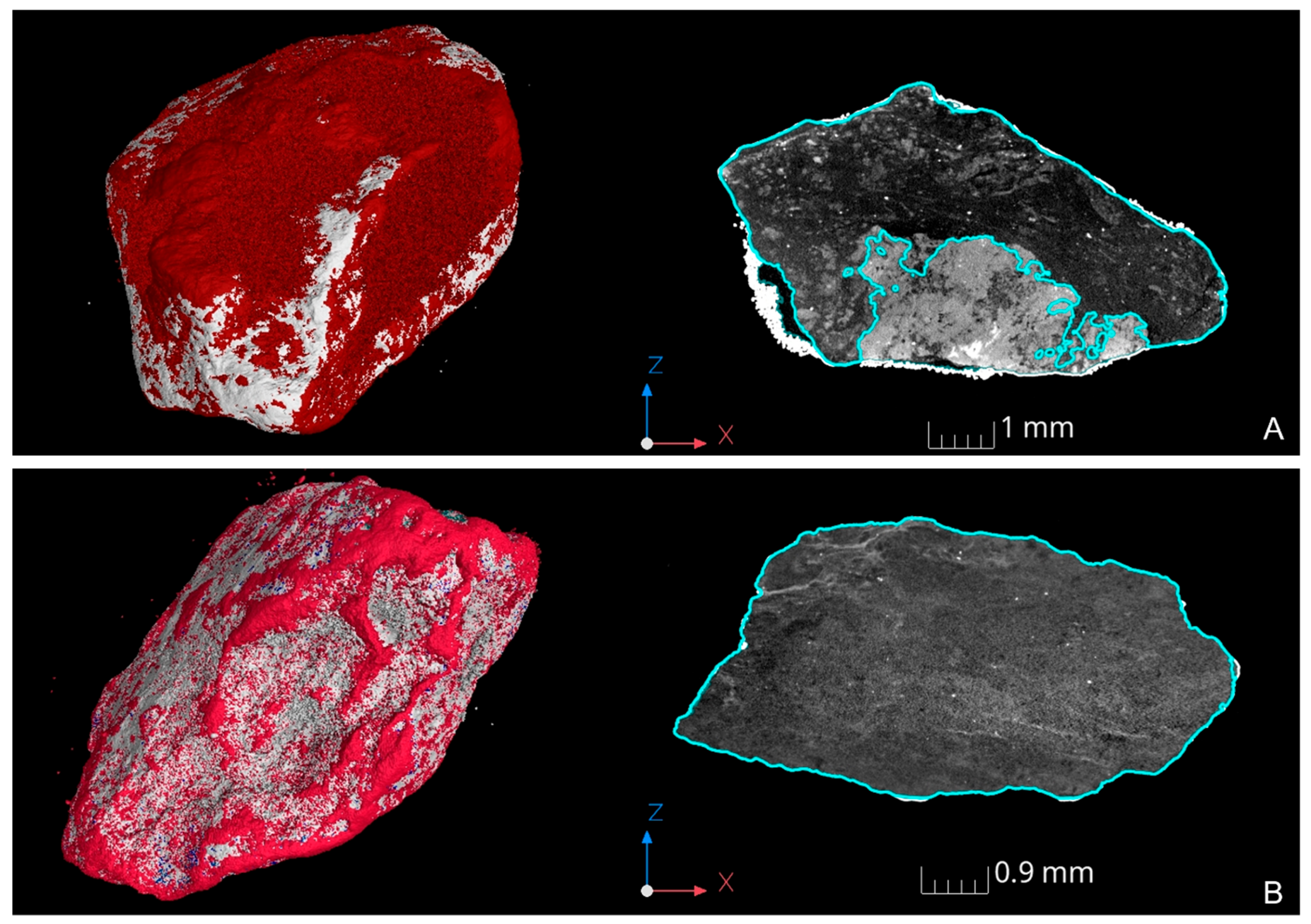
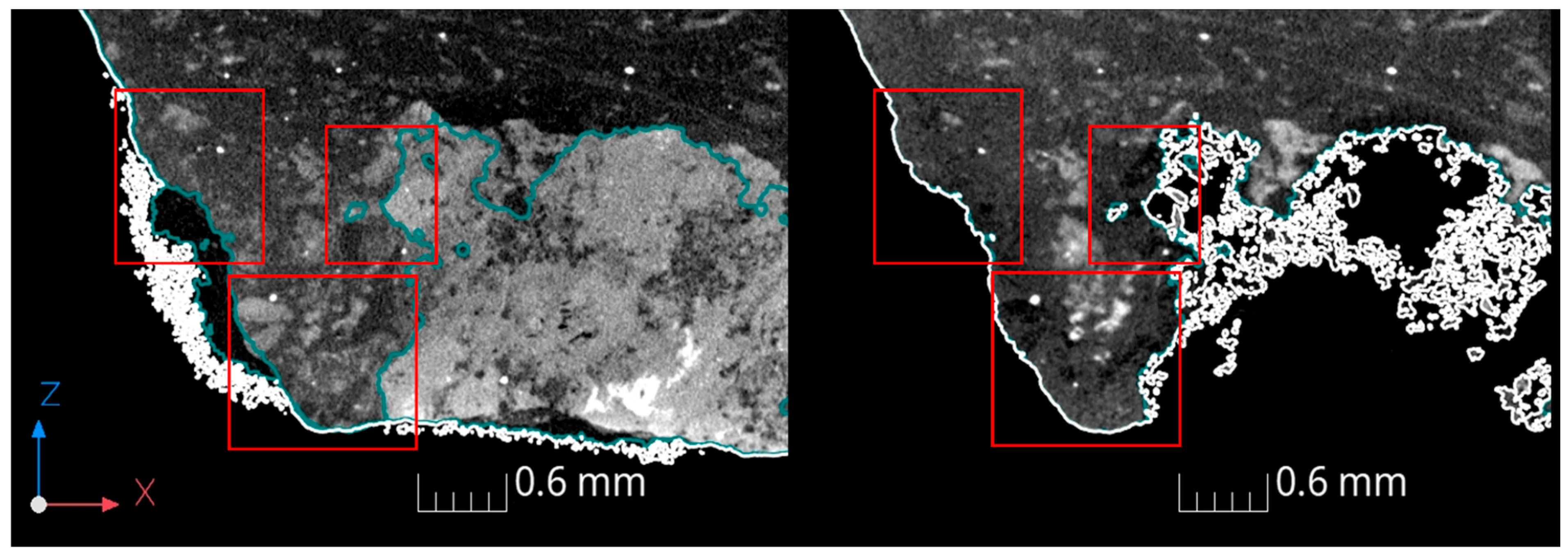
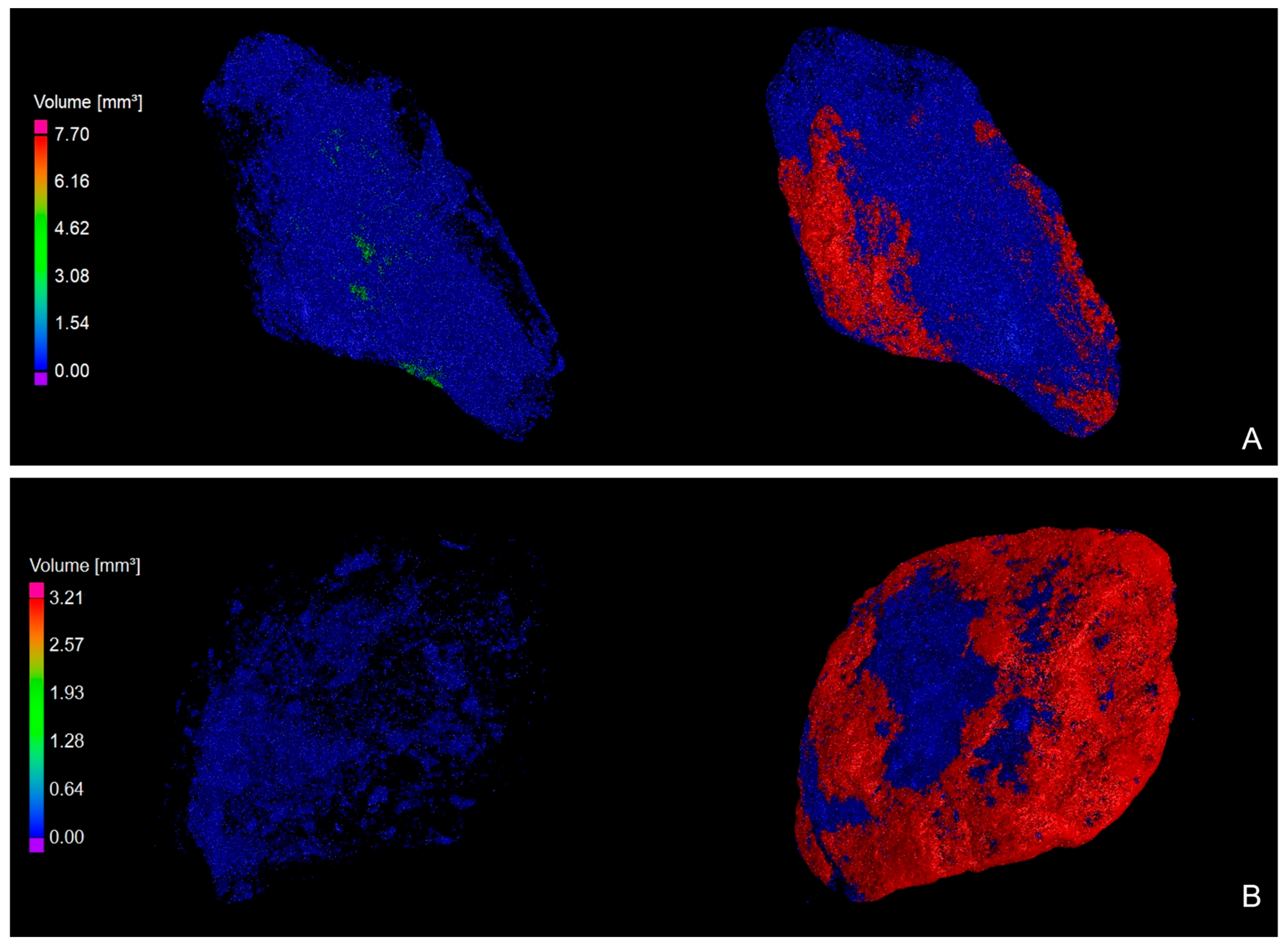
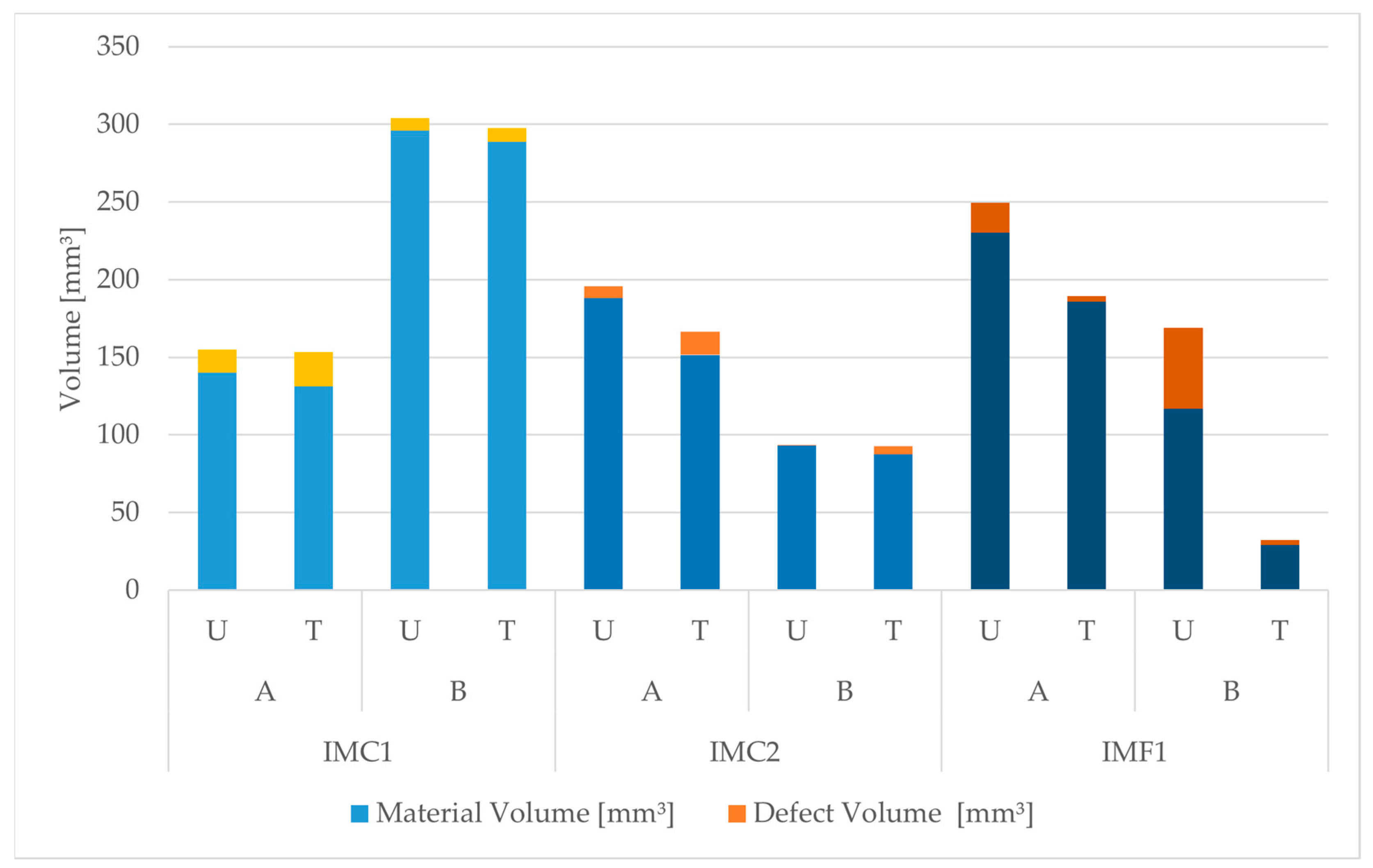
3.2. XRT Examination
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Commission. Study on the EU’s List of Critical Raw Materials (2020): Factsheets on Critical Raw Materials; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar] [CrossRef]
- Bárdossy, G. Karst Bauxites: Bauxite Deposits on Carbonate Rocks; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands; Oxford, UK; New York, NY, USA, 1982. [Google Scholar]
- Bárdossy, G.; Aleva, G.J.J. Lateritic Bauxites; Elsevier: Amsterdam, The Netherlands; Oxford, UK; New York, NY, USA; Tokyo, Japan, 1990. [Google Scholar]
- Niggli, P.; Niggli, E. Gesteine und Minerallagerstätten: Zweiter Band: Exogene Gesteine und Minerallagerstätten; Birkhäuser: Basel, Switzerland, 1952. [Google Scholar]
- Arnold, B. Von Rubinen und Implantaten: Aluminiumoxid und Seine Vielfältige Welt; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Sukla, L.B.; Pattanaik, A.; Pradhan, D. Advances in Beneficiation of Low-Grade Bauxite. In Light Metals 2019; Chesonis, C., Ed.; Springer: Cham, Switzerland, 2019; pp. 3–10. [Google Scholar]
- Meyer, F.M. Availability of Bauxite Reserves. Nat. Resour. Res. 2004, 13, 161–172. [Google Scholar] [CrossRef]
- Schönwelski, W. Possible Solutions for the global shortage of refractory bauxite. Refract. Worldforum 2009, 1, 17–19. [Google Scholar]
- Schulle, W. Feuerfeste Werkstoffe: Feuerfestkeramik. Eigenschaften, Prüftechnische Beurteilung, Werkstofftypen, 1. Auflage; Deutscher Verlag für Grundstoffindustrie: Leipzig, Germany, 1990. [Google Scholar]
- Routschka, G.; Wuthnow, H. Praxishandbuch Feuerfeste Werkstoffe: Aufbau—Eigenschaften—Prüfung, 5. Auflage; Vulkan-Verlag: Essen, Germany, 2011. [Google Scholar]
- Kuys, K.; Ralston, J.; Smart, R.; Sobieraj, S.; Wood, R.; Turner, P.S. Surface characterisation, iron removal and enrichment of bauxite ultrafines. Miner. Eng. 1990, 3, 421–435. [Google Scholar] [CrossRef]
- Rao, R.B.; Besra, L.; Reddy, B.R.; Banerjee, G.N. The Effect of Pretreatment on Magnetic Separation of Ferruginous Minerals in Bauxite. Magn. Electr. Sep. 1997, 8, 115–123. [Google Scholar] [CrossRef]
- Valeev, D.; Pankratov, D.; Shoppert, A.; Sokolov, A.; Kasikov, A.; Mikhailova, A.; Salazar-Concha, C.; Rodionov, I. Mechanism and kinetics of iron extraction from high silica boehmite–kaolinite bauxite by hydrochloric acid leaching. Trans. Nonferrous Met. Soc. China 2021, 31, 3128–3149. [Google Scholar] [CrossRef]
- Swain, R.; Rao, R.B. Kinetic study on leaching of iron in Partially Laterised Khondalite rocks for ceramic industrial applications. Int. J. Miner. Process. 2012, 112–113, 77–83. [Google Scholar] [CrossRef]
- Reddy, B.; Mishra, S.; Banerjee, G. Kinetics of leaching of a gibbsitic bauxite with hydrochloric acid. Hydrometallurgy 1999, 51, 131–138. [Google Scholar] [CrossRef]
- Gülfen, G.; Gülfen, M.; Aydın, A.O. Dissolution kinetics of iron from diasporic bauxite in hydrochloric acid solution. Indian J. Chem. Technol. 2006, 13, 386–390. [Google Scholar]
- Dissanayake, D.; Mantilaka, M.; de Silva, R.T.; de Silva, K.; Pitawala, H. Laterite and its potential as an alternative-bauxite. Clean. Mater. 2021, 1, 100016. [Google Scholar] [CrossRef]
- Cui, L.; Guo, Y.; Wang, X.; Du, Z.; Cheng, F. Dissolution kinetics of aluminum and iron from coal mining waste by hydrochloric acid. Chin. J. Chem. Eng. 2015, 23, 590–596. [Google Scholar] [CrossRef]
- Stein, A.; Sax, A.; Quirmbach, P. Iron leaching from nonrefractory grade bauxite: Individual process optimization and prediction by using DOE. Int. J. Ceram. Eng. Sci. 2022, 4, 112–118. [Google Scholar] [CrossRef]
- Afar, Z.I. Determination of semi empirical kinetic model for dissolution of bauxite ore with sulfuric acid: Parametric cumulative effect on the Arrhenius parameters. Chem. Eng. J. 2008, 141, 233–241. [Google Scholar] [CrossRef]
- Li, Z.; Cao, Y.; Han, G.; Fan, G.; Huang, Y. Research on Impurity Removal of Low Grade Bauxite. In Light Metals 2018; Martin, O., Ed.; Springer: Cham, Switzerland, 2018; pp. 23–27. [Google Scholar]
- Salehi, S.; Noaparast, M.; Shafaei, S.Z.; Amini, A.; Heidarnia, A. Iron leaching from bauxite ore in hydrochloric acid using response surface methodology. J. Min. Environ. 2015, 1, 103–108. [Google Scholar] [CrossRef]
- Buzug, T.M. Einführung in Die Computertomographie: Mathematisch-Physikalische Grundlagen der Bildrekonstruktion, Softcover Reprint of the Original, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Grangeat, P. Tomography; ISTE Ltd. and John Wiley & Sons Inc: London, UK, 2009. [Google Scholar]
- Carmignato, S.; Dewulf, W.; Leach, R. Industrial X-ray Computed Tomography; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Baruchel, J.; Buffiere, J.-Y.; Maire, E.; Merle, P.; Peix, G. X-ray Tomography in Material Science: Workshop on the Application of X-Ray Tomography in Material Science, Held Oct. 1999 in Villeurbanne; Hermes Science: Paris, France, 2000. [Google Scholar]
- Phillips, D.H.; Lannutti, J.J. Measuring physical density with X-ray computed tomography. NDT E Int. 1997, 30, 339–350. [Google Scholar] [CrossRef]
- Romans, L.E. Computed Tomography for Technologists: A Comprehensive Text; Wolters Kluwer/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Villarraga-Gómez, H.; Herazo, E.L.; Smith, S.T. X-ray computed tomography: From medical imaging to dimensional metrology. Precis. Eng. 2019, 60, 544–569. [Google Scholar] [CrossRef]
- Kruth, J.P.; Bartscher, M.; Carmignato, S.; Schmitt, R.; de Chiffre, L.; Weckenmann, A. Computed tomography for dimensional metrology. CIRP Ann. 2011, 60, 821–842. [Google Scholar] [CrossRef]
- Spiro, C.L.; Holmes, D.S.; Lobos, J.; Maylotte, D.H. Use of x-ray computed tomography to examine microbial desulfurization of lump coal. Energy Fuels 1987, 1, 76–79. [Google Scholar] [CrossRef]
- Lin, Q.; Neethling, S.J.; Courtois, L.; Dobson, K.J.; Lee, P.D. Multi-scale quantification of leaching performance using X-ray tomography. Hydrometallurgy 2016, 164, 265–277. [Google Scholar] [CrossRef]
- DIN EN ISO 12677:2011; Chemische Analyse von feuerfesten Erzeugnissen durch Röntgenfluoreszenz-Analyse (RFA)—Schmelzaufschluss-Verfahren. International Organization for Standardization: Geneva, Switzerland, 2011.
- Demtröder, W. Experimentalphysik 4: Kern-, Teilchen- und Astrophysik, 5. Auflage; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Slotwinski, J.A.; Garboczi, E.J.; Hebenstreit, K.M. Porosity Measurements and Analysis for Metal Additive Manufacturing Process Control. J. Res. Natl. Inst. Stand. Technol. 2014, 119, 494–528. [Google Scholar] [CrossRef] [PubMed]
- Wits, W.W.; Carmignato, S.; Zanini, F.; Vaneker, T.H. Porosity testing methods for the quality assessment of selective laser melted parts. CIRP Ann. 2016, 65, 201–204. [Google Scholar] [CrossRef]
| Component | Mass Fraction in IMF1 in % | Mass Fraction in IMC1 in % | Mass Fraction in IMC2 in % |
|---|---|---|---|
| Al2O3 | 46.34 | 70.16 | 52.48 |
| SiO2 | 2.28 | 8.21 | 4.70 |
| Fe2O3 | 23.69 | 2.49 | 21.63 |
| TiO2 | 2.90 | 3.45 | 2.80 |
| CaO | 0.06 | 0.42 | 1.15 |
| MgO | 0.02 | 0.36 | 1.11 |
| K2O | 0.05 | 1.09 | 0.33 |
| Loss on ignition (1025 °C) | 24.56 | 13.76 | 15.71 |
| Component | Al | Si | Fe | Ti |
|---|---|---|---|---|
| Atomic number Z | 13 | 14 | 26 | 22 |
| Intrinsic density ϱ in g/cm3 | 2.70 | 2.33 | 7.86 | 4.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razavi, A.; Stein, A.; Quirmbach, P. Tomographic Imaging of Bauxite Grains Leached Using Hydrochloric Acid. Minerals 2023, 13, 884. https://doi.org/10.3390/min13070884
Razavi A, Stein A, Quirmbach P. Tomographic Imaging of Bauxite Grains Leached Using Hydrochloric Acid. Minerals. 2023; 13(7):884. https://doi.org/10.3390/min13070884
Chicago/Turabian StyleRazavi, Anita, Alena Stein, and Peter Quirmbach. 2023. "Tomographic Imaging of Bauxite Grains Leached Using Hydrochloric Acid" Minerals 13, no. 7: 884. https://doi.org/10.3390/min13070884
APA StyleRazavi, A., Stein, A., & Quirmbach, P. (2023). Tomographic Imaging of Bauxite Grains Leached Using Hydrochloric Acid. Minerals, 13(7), 884. https://doi.org/10.3390/min13070884






