Biotite Geochemistry and Its Implication for the Difference in Mineralization in the Xiongcun Porphyry Cu–Au Ore District, Tibet
Abstract
1. Introduction
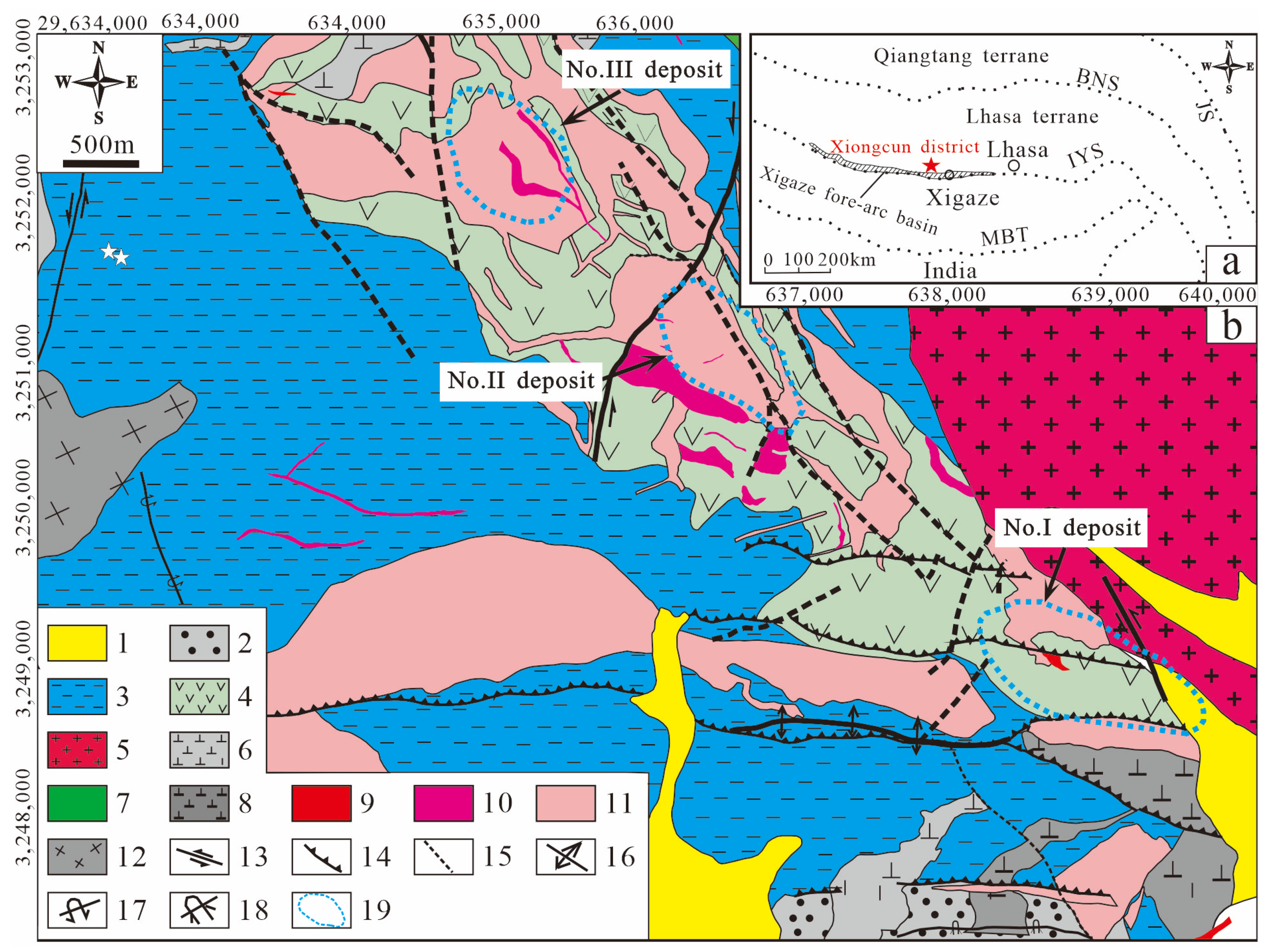
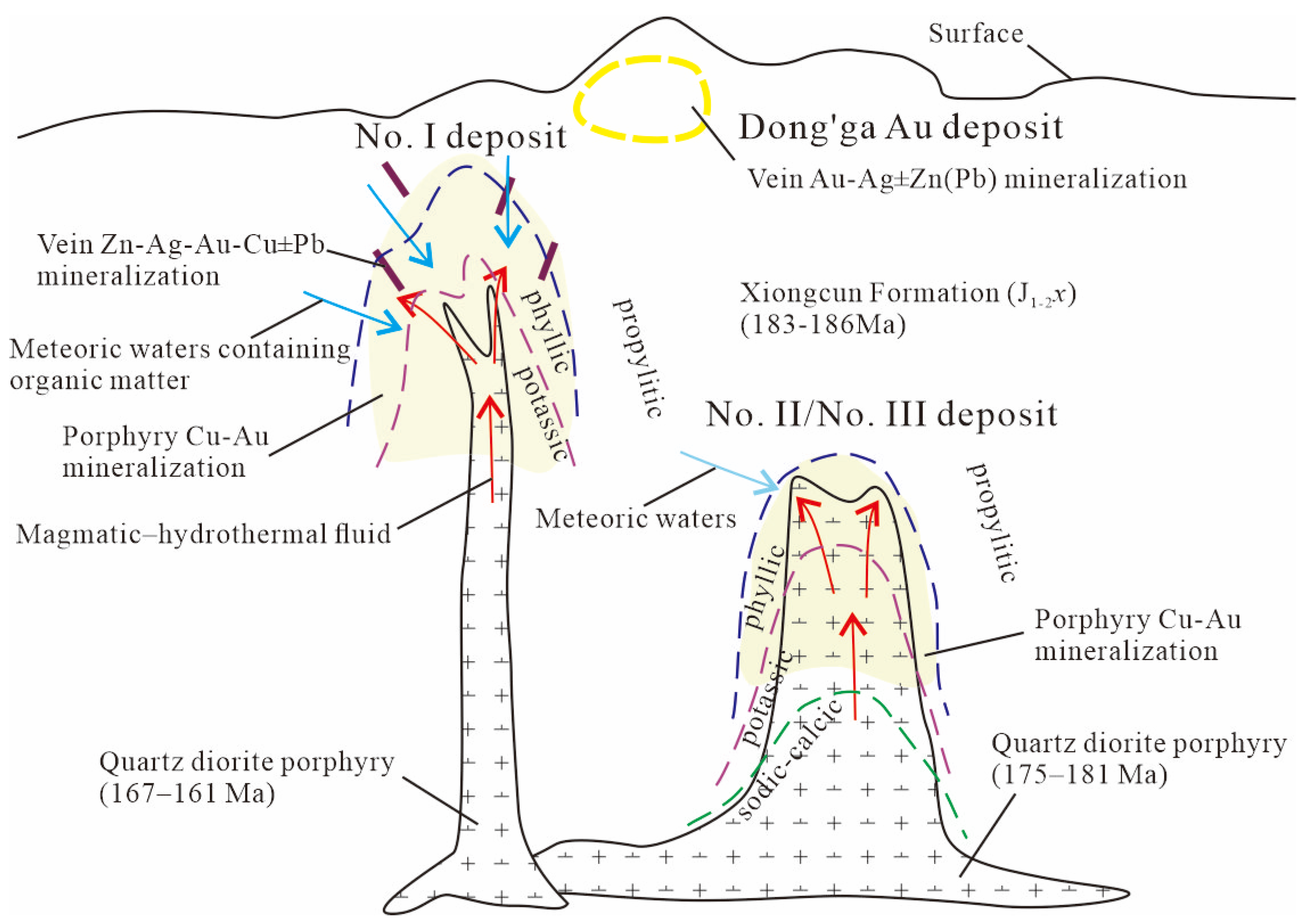
2. Deposit Geology
2.1. No. I Deposit
2.2. No. II Deposit
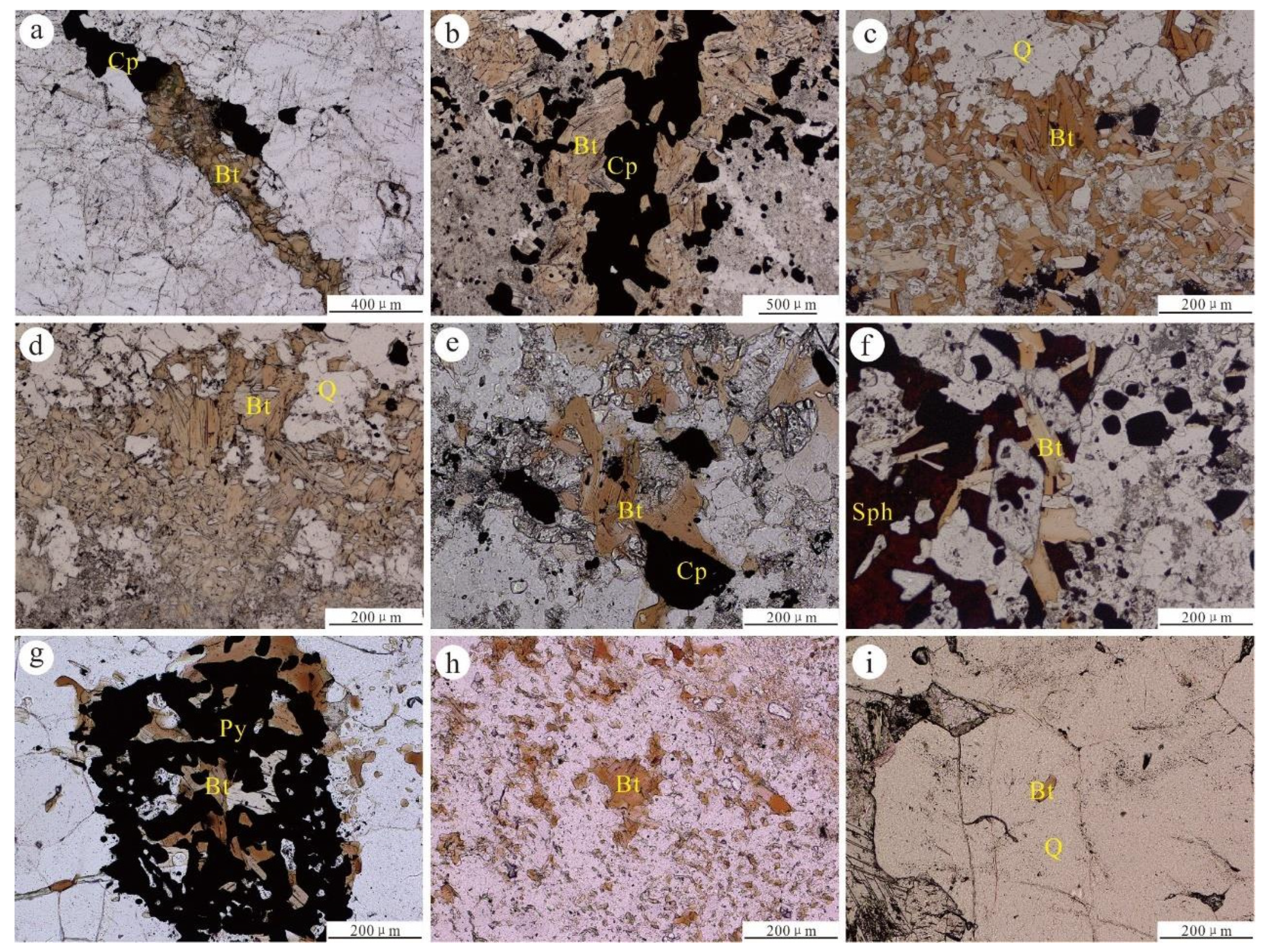
3. Hydrothermal Biotite Types
4. Samples and Methods
5. Results
5.1. Major Element Compositions and Volatiles
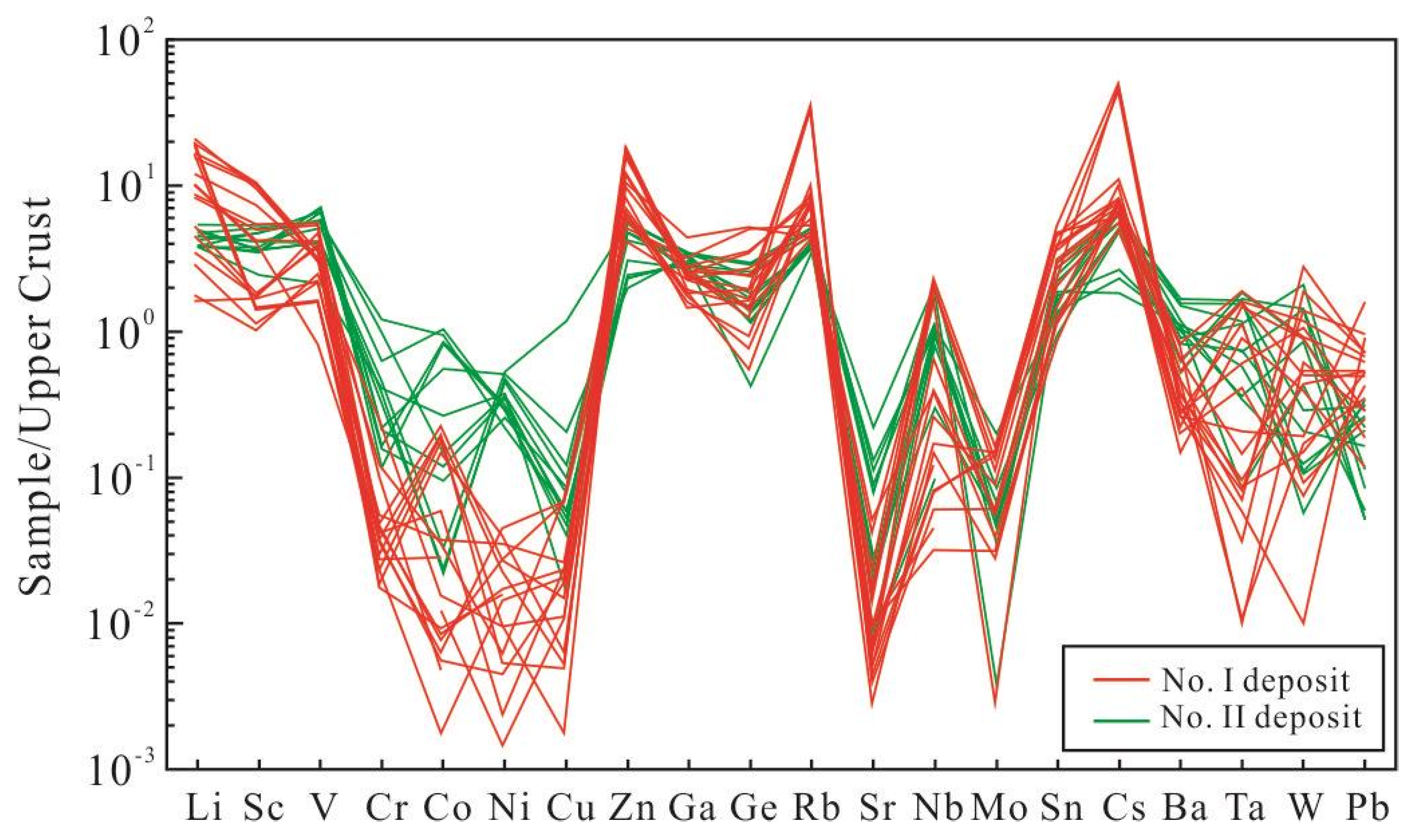
5.2. Trace Element Compositions
6. Discussion
6.1. Biotite-Forming Conditions
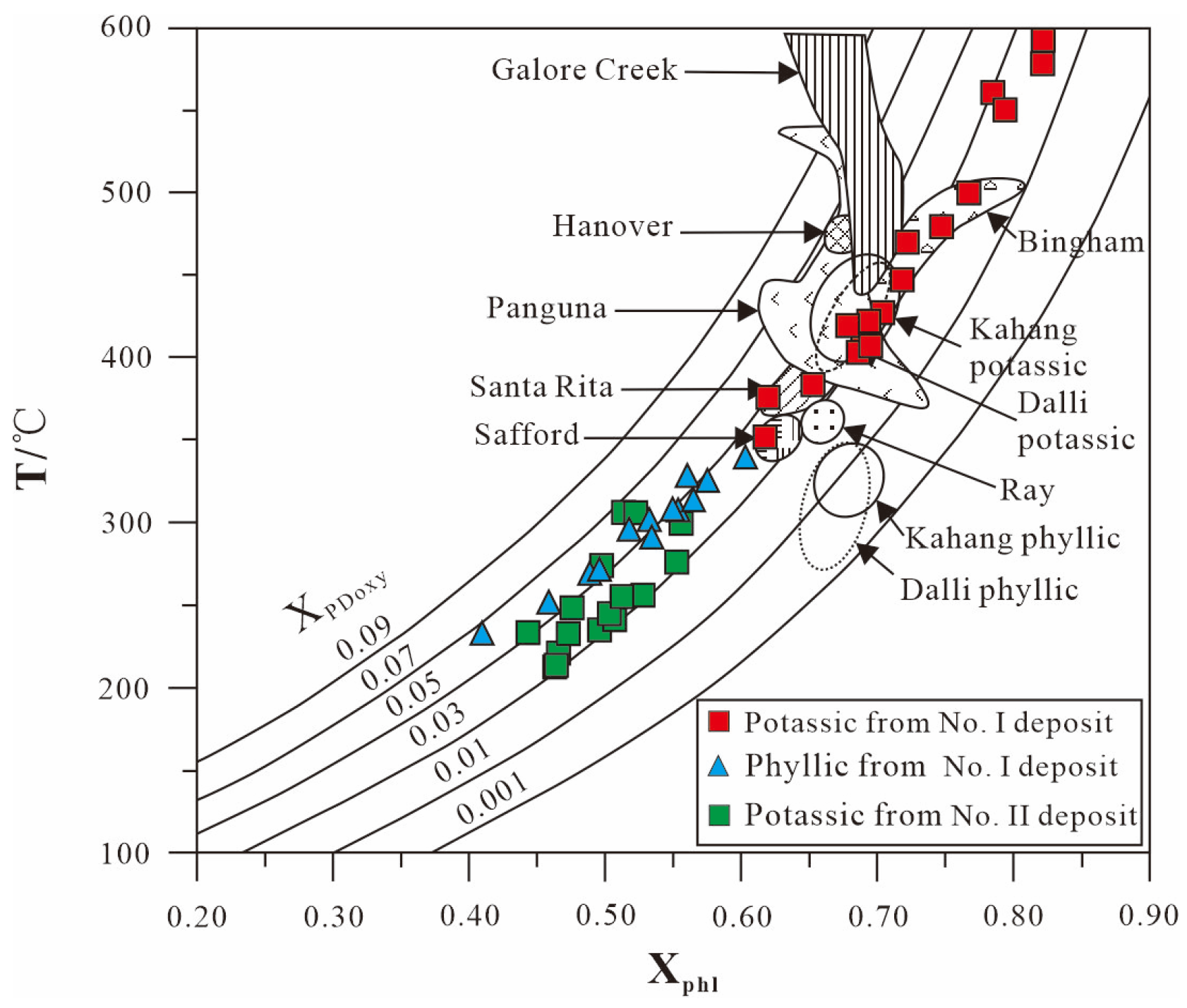
6.1.1. Temperature
6.1.2. Oxygen Fugacity
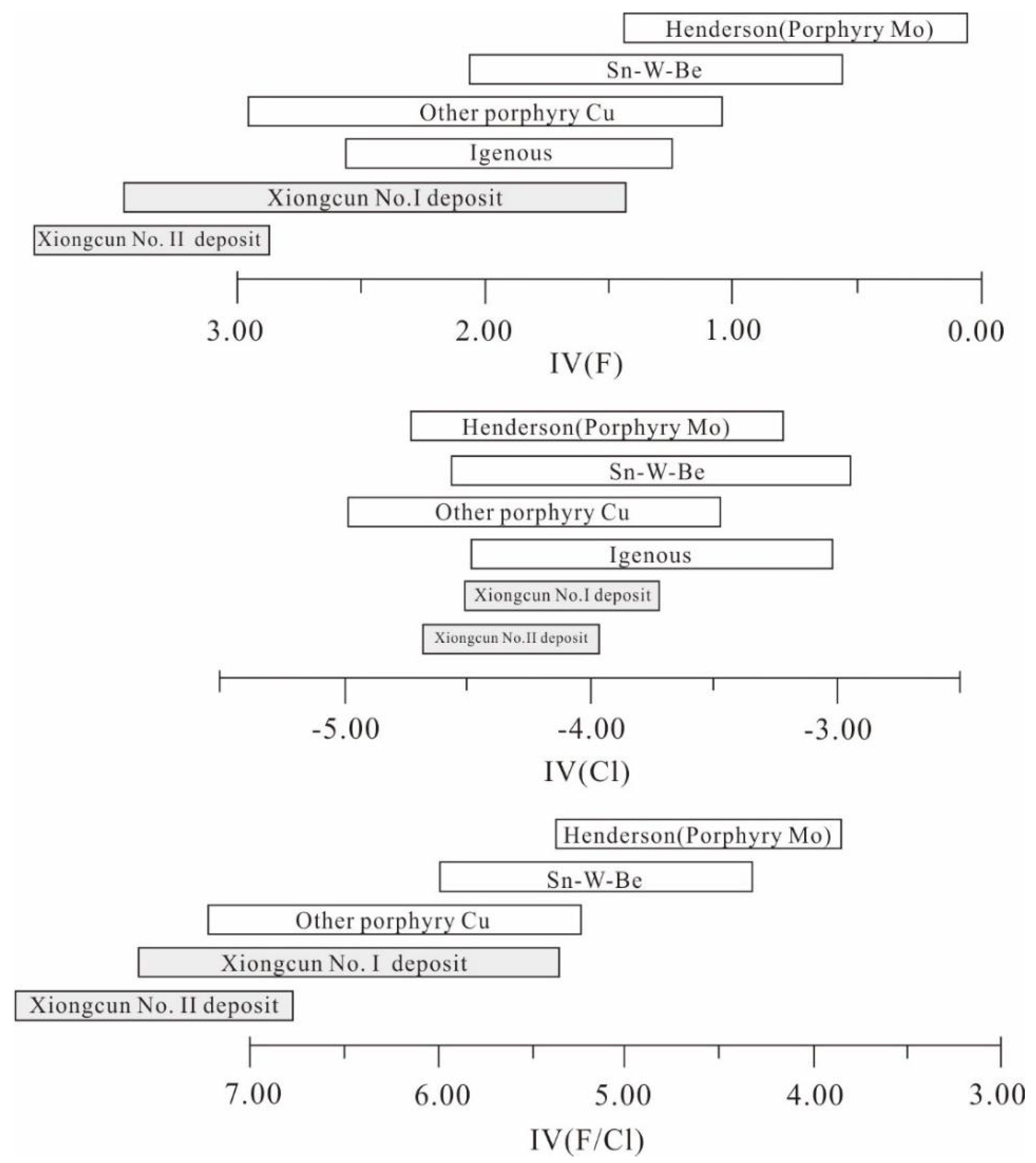
6.1.3. Halogen Fugacity
6.2. Metallogenic Implication of Biotite
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hou, Z.Q.; Cook, N.J. Metallogenesis of the Tibetan collisional orogeny: A review and introduction to the special issue. Ore Geol. Rev. 2009, 36, 2–24. [Google Scholar] [CrossRef]
- Tang, J.X.; Wang, Q.; Yang, H.H.; Gao, X.; Zhang, Z.B.; Zou, B. Mineralization, exploration and resource potential of porphyry-skarn-epithermal copper polymetallic deposits in Tibet. Acta Geosci. Sin. 2017, 38, 571–613, (In Chinese with English abstract). [Google Scholar]
- Lin, B.; Tang, J.X.; Chen, Y.C.; Song, Y.; Hall, G.; Wang, Q.; Yang, C.; Fang, X.; Duan, J.L.; Yang, H.H.; et al. Geochronology and genesis of the tiegelongnan porphyry Cu(Au) deposit in Tibet: Evidence from U-Pb, Re-Os Dating and Hf, S, and H-O isotopes. Resour. Geol. 2017, 67, 1–21. [Google Scholar] [CrossRef]
- Lin, B.; Chen, Y.C.; Tang, J.X.; Wang, Q.; Song, Y.; Yang, C.; Wang, W.L.; He, W.; Zhang, L.J. 40Ar/39Ar and Rb-Sr ages of the Tiegelongnan porphyry Cu-(Au) deposit in the Bangong Co-Nujiang Metallogenic Belt of Tibet, China: Implication for generation of super-large deposit. Acta Geol. Sin. (Engl. Ed.) 2017, 91, 602–616. [Google Scholar] [CrossRef]
- Lang, H.; Wang, X.H.; Deng, Y.L.; Tang, J.X.; Xie, F.W.; Zhou, Y.; Huang, Y.; Li, Z.; Yin, Q.; Jiang, K. Early Jurassic volcanic rocks in the Xiongcun district, southern Lhasa subterrane, Tibet: Implications for the tectono-magmatic events associated with the early evolution of the Neo-Tethys Ocean. Lithos 2019, 340–341, 166–180. [Google Scholar] [CrossRef]
- Lang, X.H.; Deng, Y.L.; Wang, X.H.; Tang, J.X.; Yin, Q.; Xie, F.W.; Yang, Z.Y.; Li, Z.; He, Q.; Li, L.; et al. Geochronology and geochemistry of volcanic rocks of the Bima Formation, southern Lhasa subterrane, Tibet: Implications for early Neo-Tethyan sunduction. Gondwana Res. 2020, 80, 335–349. [Google Scholar] [CrossRef]
- Xie, F.W.; Tang, J.X.; Chen, Y.C.; Lang, X.H. Apatite and zircon geochemistry of Jurassic porphyries in the Xiongcun district, southern Gangdese porphyry copper belt: Implications for petrogenesis and mineralization. Ore Geol. Rev. 2018, 96, 98–114. [Google Scholar] [CrossRef]
- Tang, J.X.; Lang, X.H.; Xie, F.W.; Gao, Y.M.; Li, Z.J.; Huang, Y.; Ding, F.; Yang, H.H.; Zhang, L.; Wang, Q.; et al. Geological characteristics and genesis of the Jurassic No. I porphyry Cu–Au deposit in the Xiongcun district, Gangdese porphyry copper belt, Tibet. Ore Geol. Rev. 2015, 70, 438–456. [Google Scholar] [CrossRef]
- Lang, X.H.; Guo, W.B.; Wang, X.H.; Deng, Y.L.; Yang, Z.Y.; Xie, F.W.; Li, Z.; Zhang, Z.; Jiang, K. Petrogenesis and tectonic implications of the ore-bearing porphyries in the Xiongcun district: Constraints from the geochronology and geochemistry. Acta Petrol. Sin. 2019, 35, 2105–2123, (In Chinese with English abstract). [Google Scholar]
- Selby, D.; Nesbitt, B.E. Chemical composition of biotite from the Casino porphyry Cu–Au–Mo mineralization, Yukon, Canada: Evaluation of magmatic and hydrothermal fluid chemistry. Chem. Geol. 2000, 171, 77–93. [Google Scholar] [CrossRef]
- Coulson, I.M.; Dipple, G.M.; Raudsepp, M. Evolution of HF and HCl activity in magmatic volatiles of the gold-mineralized Emerald Lake pluton, Yukon Territory, Canada. Miner. Depos. 2001, 36, 594–606. [Google Scholar] [CrossRef]
- Tang, P.; Tang, J.X.; Lin, B.; Wang, L.Q.; Zheng, W.B.; Leng, Q.F.; Gao, X.; Zhang, Z.B.; Tang, X.Q. Mineral chemistry of magmatic and hydrothermal biotites from the Bangpu porphyry Mo (Cu) deposit, Tibet. Ore Geol. Rev. 2019, 115, 103122. [Google Scholar] [CrossRef]
- Tang, P.; Chen, Y.C.; Tang, J.X.; Wang, Y.; Zheng, W.B.; Leng, Q.F.; Lin, B.; Wu, C.N. Advances in research of mineral chemistry of magmatic and hydrothermal biotites. Acta Geol. Sin. (Engl. Ed.) 2019, 93, 1947–1966. [Google Scholar] [CrossRef]
- Tang, P.; Tang, J.X.; Wang, Y.; Lin, B.; Leng, Q.F.; Zhang, Q.Z.; He, L.; Zhang, Z.B.; Sun, M.; Wu, C.N.; et al. Genesis of the Lakang’e porphyry Mo (Cu) deposit, Tibet: Constraints from geochemistry, geochronology, Sr-Nd-Pb-Hf isotopes, zircon and apatite. Lithos 2021, 380–381, 1–38. [Google Scholar] [CrossRef]
- Sun, Z.J.; Fang, W.X.; Lu, J.; Guo, Y.Q.; Song, L.H. Mineral Geochemiscal Characteristics and Indication Significance of Biotite—Rutile in the Gabbro Intrusions in the Yinmin Iron-Copper District, Yunnan. Acta Geol. Sin. (Engl. Ed.) 2017, 91 (Suppl. S1), 224–226. [Google Scholar] [CrossRef]
- Lin, B.; Tang, J.X.; Chen, Y.C.; Michael, B.; Song, Y.; Yang, H.H.; Wang, Q.; He, W.; Liu, Z.B. Geology and geochronology of Naruo large porphyry–breccia Cu deposit in the Duolong district, Tibet. Gondwana Res. 2018, 66, 168–182. [Google Scholar] [CrossRef]
- Feng, Y.Z.; Deng, C.Z.; Xiao, B.; Gong, L.; Yin, R.S.; Sun, D.Y. Biotite geochemistry and its implication on the temporal and spatial difference of Cu and Mo mineralization at the Xiaokele porphyry Cu-Mo deposit, NE China. Ore Geol. Rev. 2021, 139, 104508. [Google Scholar] [CrossRef]
- Munoz, J.L. Calculation of HF and HCl fugacities from biotite compositions: Revised equations. Geol. Soc. Am. Abstr. Programs 1992, 24, A221. [Google Scholar]
- Abdel-Rahman, A.M. Nature of biotites from alkaline, calc-alkaline, and peraluminous magmas. J. Petrol. 1994, 35, 525–541. [Google Scholar] [CrossRef]
- Webster, J.D. The exsolution of magmatic hydrosaline chloride liquids. Chem. Geol. 2004, 210, 33–48. [Google Scholar] [CrossRef]
- Siahcheshm, K.; Calagari, A.A.; Abedini, A.; Lentz, D.R. Halogen signatures of biotites from the Maher-Abad porphyry copper deposit, Iran: Characterization of volatiles in syn-to post-magmatic hydrothermal fluids. Int. Geol. Rev. 2012, 54, 1353–1368. [Google Scholar] [CrossRef]
- Ayati, F.; Yavuz, F.; Noghreyan, M.; Haroni, H.A.; Yavuz, R. Chemical characteristics and composition of hydrothermal biotite from the Dalli porphyry copper prospect, Arak, central province of Iran. Mineral. Petrol. 2008, 94, 107–122. [Google Scholar] [CrossRef]
- Boomeri, M.; Nakashima, K.; Lentz, D.R. The Sarcheshmeh porphyry copper deposit, Kerman, Iran: A mineralogical analysis of the igneous rocks and alteration zones including halogen element systematics related to Cu mineralization processes. Ore Geol. Rev. 2010, 38, 367–381. [Google Scholar] [CrossRef]
- Boomeri, M.; Nakashima, K.; Lentz, D.R. The Miduk porphyry Cu deposit, Kerman, Iran: A geochemical analysis of the potassic alteration including halogen element systematics related to Cu mineralization processes. J. Geochem. Explor. 2009, 103, 17–29. [Google Scholar] [CrossRef]
- Afshooni, S.Z.; Mirnejad, H.; Esmaeily, D.; Haroni, H.A. Mineral chemistry of hydrothermal biotite from the Kahang porphyry copper deposit (NE Isfahan), Central Province of Iran. Ore Geol. Rev. 2013, 54, 214–232. [Google Scholar] [CrossRef]
- Parsapoor, A.; Khalili, M.; Tepley, F.; Maghami, M. Mineral chemistry and isotopic composition of magmatic, reequilibrated and hydrothermal biotites from Darreh-Zar porphyry copper deposit, Kerman (Southeast of Iran). Ore Geol. Rev. 2015, 66, 200–218. [Google Scholar] [CrossRef]
- Fedele, L.; Lustrino, M.; Melluso, L.; Morra, V.; Zanetti, A.; Vannucci, R. Trace-element partitioning between plagioclase, alkali feldspar, Ti-magnetite, biotite, apatite, and evolved potassic liquids from Campi Flegrei (Southern Italy). Am. Mineral. 2015, 100, 233–249. [Google Scholar] [CrossRef]
- Zhang, W.; Lentz, D.R.; Thorne, K.G.; McFarlane, C. Geochemical characteristics of biotite from felsic intrusive rocks around the Sisson Brook W-Mo-Cu deposit, west-central New Brunswick: An indicator of halogen and oxygen fugacity of magmatic systems. Ore Geol. Rev. 2016, 77, 82–96. [Google Scholar] [CrossRef]
- Sun, K.; Chen, B.; Deng, J. Biotite in highly evolved granites from the Shimensi W-Cu-Mo polymetallic ore deposit, China: Insights into magma source and evolution. Lithos 2019, 350–351, 105245. [Google Scholar] [CrossRef]
- Li, J.X.; Fan, W.M.; Zhang, L.Y.; Ding, L.; Yue, Y.H.; Xie, J.; Cai, F.L.; Quan, Q.Y.; Sein, K. Biotite geochemistry deciphers magma evolution of Sn-bearing granite, southern Myanmar. Ore Geol. Rev. 2020, 121, 103565. [Google Scholar] [CrossRef]
- Yu, K.L.; Li, G.M.; Zhao, J.X.; Evans, N.J.; Li, J.X.; Jiang, G.W.; Zou, X.Y.; Qin, K.Z.; Guo, H. Biotite composition as a tracer of fluid evolution and mineralization center: A case study at the Qulong porphyry Cu-Mo deposit, Tibet. Miner. Depos. 2022, 57, 1047–1069. [Google Scholar] [CrossRef]
- Azadbakht, Z.; Lentz, D.R.; McFarlane, C.R.M.; Whalen, J.B. Using magmatic biotite chemistry to differentiate barren and mineralized Silurian-Devonian granitoids of New Brunswick, Canada. Contrib. Mineral. Petrol. 2020, 175, 69. [Google Scholar] [CrossRef]
- Lang, X.H.; Tang, J.X.; Li, Z.J.; Huang, Y.; Ding, F.; Yang, H.H.; Xie, F.W.; Zhang, L.; Wang, Q.; Zhou, Y. U-Pb and Re–Os geochronological evidence for the Jurassic porphyry metallogenic event of the Xiongcun district in the Gangdese porphyry copper belt, southern Tibet, PRC. J. Asian Earth Sci. 2014, 79, 608–622. [Google Scholar] [CrossRef]
- Tang, J.X.; Chen, Y.C.; Wang, D.H.; Wang, C.H.; Xu, Y.P.; Qu, W.J.; Huang, W.; Huang, Y. Re–Os Dating Molybdenite from the Sharang porphyry molybdenum deposit in Gongbógyamda county, Tibet and its geological significance. Acta Geol. Sin. 2009, 83, 698–704, (In Chinese with English abstract). [Google Scholar]
- Wang, L.Q.; Tang, J.X.; Deng, J.; Kang, H.R.; Lin, B.; Cheng, W.B.; Li, Z.; Zhang, Z. The Longmala and Mengya’a skarn Pb–Zn deposits, Gangdese region, Tibet: Evidence from U–Pb and Re–Os geochronology for formation during early India–Asia collision. Int. Geol. Rev. 2015, 57, 1825–1842. [Google Scholar] [CrossRef]
- Tang, P.; Tang, J.X.; Wang, Y.; Lin, B.; Zheng, W.B.; Leng, Q.F.; Zhang, Z.B.; Yang, Y.; Wu, C.N.; Qi, J.; et al. Zircon U-Pb geochronology, geochemistry, S-Pb-Hf isotopic compositions, and mineral chemistry of the Xin’gaguo skarn Pb-Zn deposit, Tibet, China. Geol. J. 2020, 55, 4790–4809. [Google Scholar] [CrossRef]
- Yang, Z.M.; Hou, Z.Q.; White, N.C.; Chang, Z.S.; Li, Z.Q.; Song, Y. Geology of the post-collisional porphyry copper–molybdenum deposit at Qulong, Tibet. Ore Geol. Rev. 2009, 36, 133–159. [Google Scholar] [CrossRef]
- Zheng, W.B.; Tang, J.X.; Zhong, K.H.; Ying, L.J.; Leng, Q.F.; Ding, S.; Lin, B. Geology of the Jiama porphyry copper–polymetallic system, Lhasa Region, China. Ore Geol. Rev. 2016, 74, 151–169. [Google Scholar] [CrossRef]
- Lang, X.H.; Tang, J.X.; Xie, F.W.; Li, Z.J.; Huang, Y.; Ding, F.; Yang, H.H.; Zhou, Y.; Wang, Q. Geochronology and geochemistry of the southern porphyry in the Xiongcun district, Tibet and its geological implications. Geotecton. Metallog. 2014, 38, 609–620, (In Chinese with English abstract). [Google Scholar]
- Lang, X.H.; Wang, X.H.; Tang, J.X.; Deng, Y.L.; Cui, Z.W.; Yin, Q.; Xie, F.W. Composition and age of Jurassic diabase dikes in the Xiongcun porphyry copper–gold district, southern margin of the Lhasa terrane, Tibet, China: Petrogenesis and tectonic setting. Geol. J. 2017, 2017, 1–21. [Google Scholar] [CrossRef]
- Lang, X.H.; Tang, J.X.; Yin, Q.; Cui, Z.W.; Huang, Y.; Zhang, J.S.; Gao, Y.M.; Li, Z.J.; Ding, F.; Xie, F.W.; et al. Geochemistry and genesis of Eocene lamprophyres in the Xiongcun porphyry copper–gold district, southern margin of the Lhasa terrane, Tibet, China. Geol. Mag. 2017, 51, 123–142. [Google Scholar] [CrossRef]
- Lang, X.H.; Deng, Y.L.; Wang, X.H.; Tang, J.X.; Xie, F.W.; Yang, Z.Y.; Yin, Q.; Jiang, K. Reduced fluids in porphyry copper-gold systems reflect the occurrence of the wall-thermogenic process: An example from the No.1 deposit in the Xiongcun district, Tibet, China. Ore Geol. Rev. 2020, 118, 103212. [Google Scholar] [CrossRef]
- Lang, X.H.; Deng, Y.L.; Wang, X.H.; Tang, J.X.; Xie, F.W.; Yang, Z.Y.; Yin, Q.; Jiang, K. Hydrothermal evolution and ore precipitation of the No. 2 porphyry Cu–Au deposit in the Xiongcun district, Tibet: Evidence from cathodoluminescence, fluid inclusions, and isotopes. Ore Geol. Rev. 2019, 114, 103141. [Google Scholar] [CrossRef]
- Liu, Y.S.; Hu, Z.C.; Gao, S.; Günther, D.; Xu, J.; Gao, C.G.; Chen, H.H. In situ analysis of major and trace elements of anhydrous minerals by LA–ICP–MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- Tischendorf, G.; Gottesmann, B.; Förster, H.J.; Trumbull, R.B. On Li-bearing micas: Estimating Li from electron microprobe analyses and an improved diagram for graphical representation. Miner. Mag. 1997, 61, 809–834. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the continental crust. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Pergamon: Oxford, UK, 2003; pp. 1–64. [Google Scholar]
- Wones, D.R.; Eugster, H.P. Stability of biotiteexperiment theory and application. Am. Mineral. 1965, 50, 1228. [Google Scholar]
- Beane, R.E. Biotite stability in the porphyry copper environment. Econonic Geol. 1974, 69, 241–256. [Google Scholar] [CrossRef]
- Ford, J.H. A chemical study of alteration at the Panguna porphyry copper deposit, Bougainville, Papua New Guinea. Econ. Geol. 1978, 73, 703–720. [Google Scholar] [CrossRef]
- Lanier, G.; Raab, W.J.; Folsom, R.B.; Cone, S. Alteration of equigranular monzonite, Bingham mining district, Utah. Econ. Geol. 1978, 73, 1270–1286. [Google Scholar] [CrossRef]
- Grabezkev, A.I.; Vigorova, V.G.; Chashukhina, V.A. Behavior of fluorine during crystallization of granites (in connection with validation of the criteria of granite specialization). Geochem. Int. 1979, 16, 23–33. [Google Scholar]
- Speer, J.A. Micas in igneous rocks. Rev. Mineral. Geochem. 1984, 13, 299–356. [Google Scholar]
- Munoz, J.L. F-OH and Cl-OH exchange in micas with applications to hydrothermal ore deposits. Rev. Mineral. Geochem. 1984, 13, 469–493. [Google Scholar]
- Van Middelaar, W.T.; Keith, J.D. Mica chemistry as an indicator of oxygen and halogen fugacities in the CanTung and other W-related granitoids in the North American Cordillera. Geol. Soc. Am. Spec. Pap. 1990, 246, 205–220. [Google Scholar]
- Yavuz, F. Evaluating micas in petrologic and metallogenic aspect: I–definitions and structure of the computer program MICA. Comput. Geosci. 2003, 29, 1215–1228. [Google Scholar] [CrossRef]
- Loferski, P.J.; Ayuso, R.A. Petrography and mineral chemistry of the composite Deboullie pluton, northern Maine, USA: Implications for the genesis of Cu–Mo mineralization. Chem. Geol. 1995, 123, 89–105. [Google Scholar] [CrossRef]
- Zhu, C.; Sverjensky, D.A. Partitioning of F-Cl-OH between minerals and hydrothermal fluids. Geochim. Cosmochim. Acta 1991, 55, 1837–1858. [Google Scholar] [CrossRef]
- Zhu, C.; Sverjensky, D.A. F-Cl-OH partitioning between biotite and apatite. Geochim. Cosmochim. Acta 1992, 56, 3435–3467. [Google Scholar] [CrossRef]
- Rowins, S.M. Reduced porphyry copper–gold deposits: A new variation on an old theme. Geology 2000, 28, 491–494. [Google Scholar] [CrossRef]
- Sillitoe, R.H. Porphyry copper systems. Econ. Geol. 2010, 105, 3–41. [Google Scholar] [CrossRef]
- Hedenquist, J.; Lowenstern, J. The role of magmas in the formation of hydrothermal ore deposits. Nature 1994, 370, 519–527. [Google Scholar] [CrossRef]
- Pirajno, F. A review of the geology and geodynamic evolution of the Palaeoproterozoic Earaheedy Basin, Western Australia. Earth Sci. Rev. 2009, 94, 39–77. [Google Scholar] [CrossRef]
- Candela, P.A.; Holland, H.D. The partitioning of copper and molybdenum between silicate melts and aqueous fluids. Geochim. Cosmochim. Acta 1984, 48, 373–380. [Google Scholar] [CrossRef]
- Keppler, H.; Wyllie, P.J. Partitioning of Cu, Sn, Mo, W, U, and Th between melt and aqueous fluid in the systems haplogranite–H2O–HCl and haplogranite–H2O–HF. Contrib. Mineral. Petrol. 1991, 109, 139–150. [Google Scholar] [CrossRef]
- Ruaya, J.R. Estimation of instability constants of metal chloride complexes in hydrothermal solutions up to 300 °C. Geochim. Cosmochim. Acta 1988, 52, 1983–1996. [Google Scholar] [CrossRef]
- Xiao, Z.; Gammons, C.H.; Williams-Jones, A.E. Experimental study of copper (I) chloride complexing in hydrothermal solutions at 40 to 300 °C and saturated vapor pressure. Geochim. Cosmochim. Acta 1998, 62, 2949–2964. [Google Scholar] [CrossRef]
- Bai, T.B.; van Groos, A.F.K. The distribution of Na, K, Rb, Sr, Al, Ge, Cu, W, Mo, La, and Ce between granitic melts and coexisting aqueous fluids. Geochim. Cosmochim. Acta 1999, 63, 1117–1131. [Google Scholar] [CrossRef]
- Gammons, C.H.; Williams-Jones, A.E. Chemical mobility of gold in the porphyry-epithermal environment. Econ. Geol. 1997, 92, 45–59. [Google Scholar] [CrossRef]
- Muller, D.; Groves, D.I. Potassic Igneous Rocks and Associated Gold-Copper Mineralization; Springer: Berlin/Heidelberg, Germany, 2000; p. 252. [Google Scholar]
- Groves, D.I.; Goldfarb, R.J.; Robert, F.; Hart, C.J.R. Gold deposits in metamorphic belts: Overview of current understanding, outstanding problems, future research, exploration significance. Econ. Geol. 2003, 98, 1–29. [Google Scholar]
- Idrus, A.; Kolb, J.; Meyer, F.M. Chemical composition of rockforming minerals in copper-gold-bearing tonalite porphyries at the Batu Hijau Deposit, Sumbawa Island, Indonesia: Implications for crystallization conditions and fluorine-chlorine fugacity. Resour. Geol. 2007, 57, 102–113. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, P.; Tang, J.; Lang, X.; Lin, B.; Xie, F.; Sun, M.; Li, F.; Qi, J.; Cui, H.; Wang, M.; et al. Biotite Geochemistry and Its Implication for the Difference in Mineralization in the Xiongcun Porphyry Cu–Au Ore District, Tibet. Minerals 2023, 13, 876. https://doi.org/10.3390/min13070876
Tang P, Tang J, Lang X, Lin B, Xie F, Sun M, Li F, Qi J, Cui H, Wang M, et al. Biotite Geochemistry and Its Implication for the Difference in Mineralization in the Xiongcun Porphyry Cu–Au Ore District, Tibet. Minerals. 2023; 13(7):876. https://doi.org/10.3390/min13070876
Chicago/Turabian StyleTang, Pan, Juxing Tang, Xinghai Lang, Bin Lin, Fuwei Xie, Miao Sun, Faqiao Li, Jing Qi, Hao Cui, Mengdie Wang, and et al. 2023. "Biotite Geochemistry and Its Implication for the Difference in Mineralization in the Xiongcun Porphyry Cu–Au Ore District, Tibet" Minerals 13, no. 7: 876. https://doi.org/10.3390/min13070876
APA StyleTang, P., Tang, J., Lang, X., Lin, B., Xie, F., Sun, M., Li, F., Qi, J., Cui, H., Wang, M., Xiong, Y., & Tao, G. (2023). Biotite Geochemistry and Its Implication for the Difference in Mineralization in the Xiongcun Porphyry Cu–Au Ore District, Tibet. Minerals, 13(7), 876. https://doi.org/10.3390/min13070876







