Fluid Inclusions and REE Geochemistry of White and Purple Fluorite: Implications for Physico-Chemical Conditions of Mineralization; an Example from the Pinavand F Deposit, Central Iran
Abstract
1. Introduction
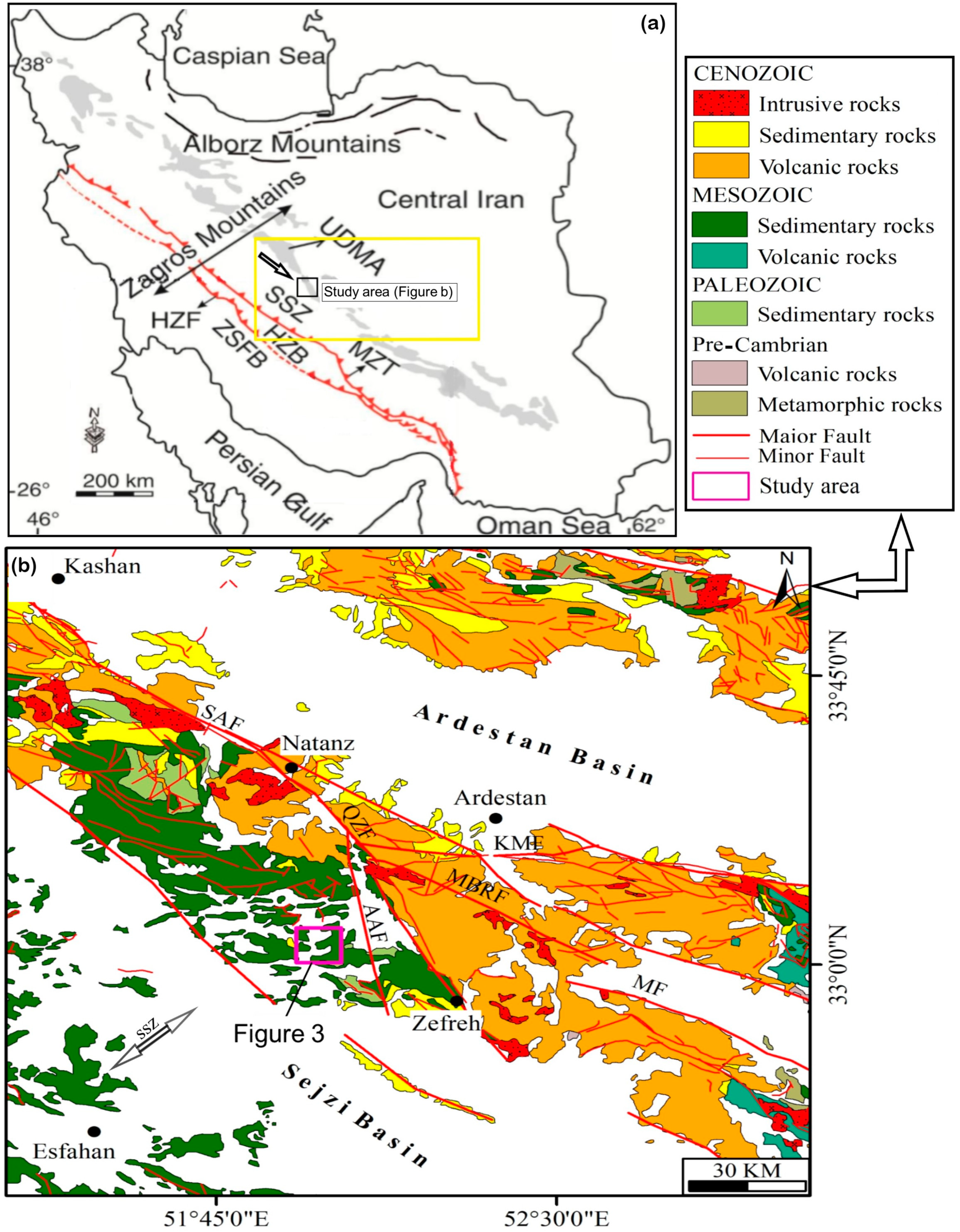
2. Regional Geological Setting
3. Geology of the Pinavand Area
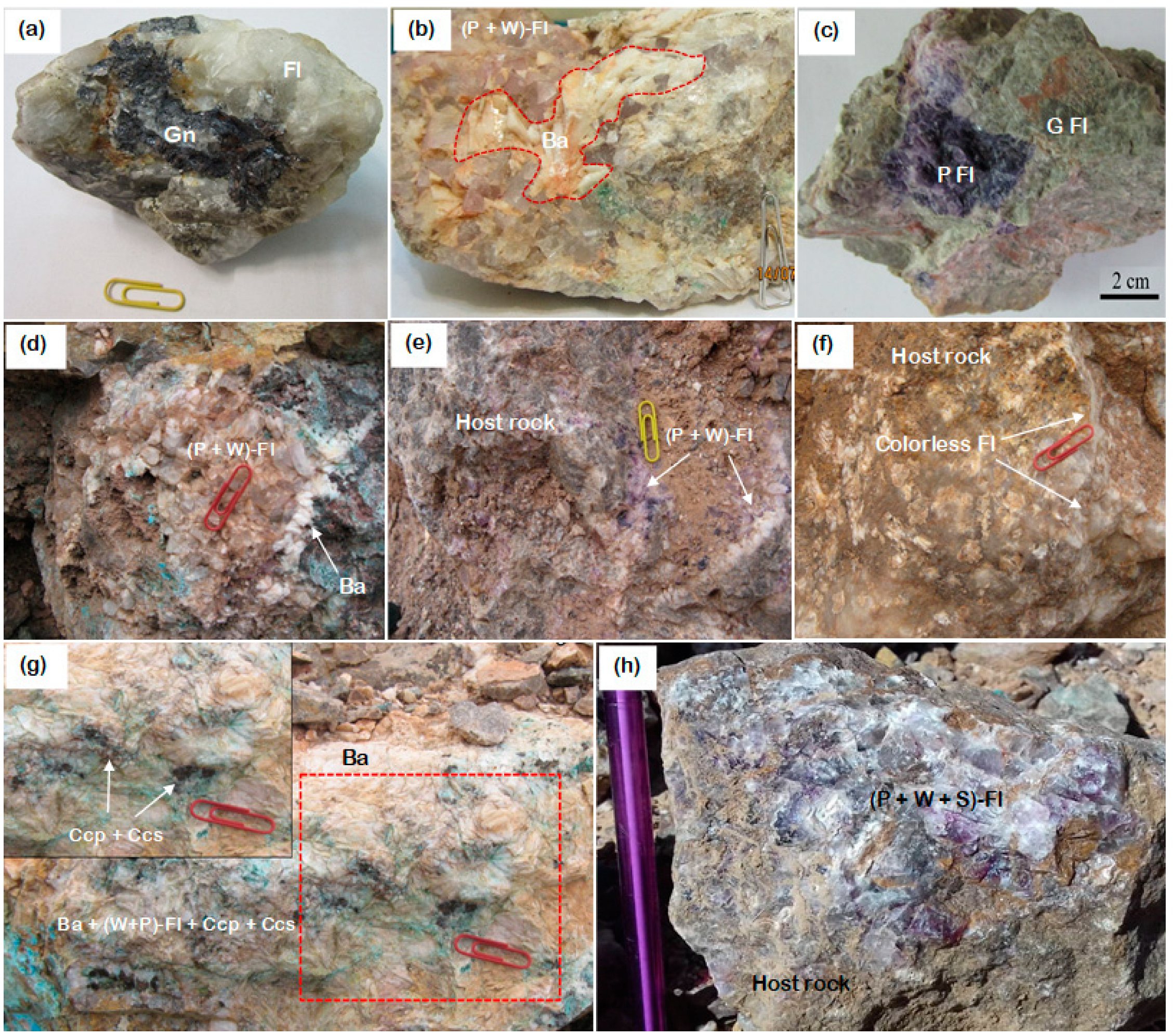
Mineralization and Textural Relationship
4. Sampling and Analytical Methods
5. Results
5.1. Geochemistry
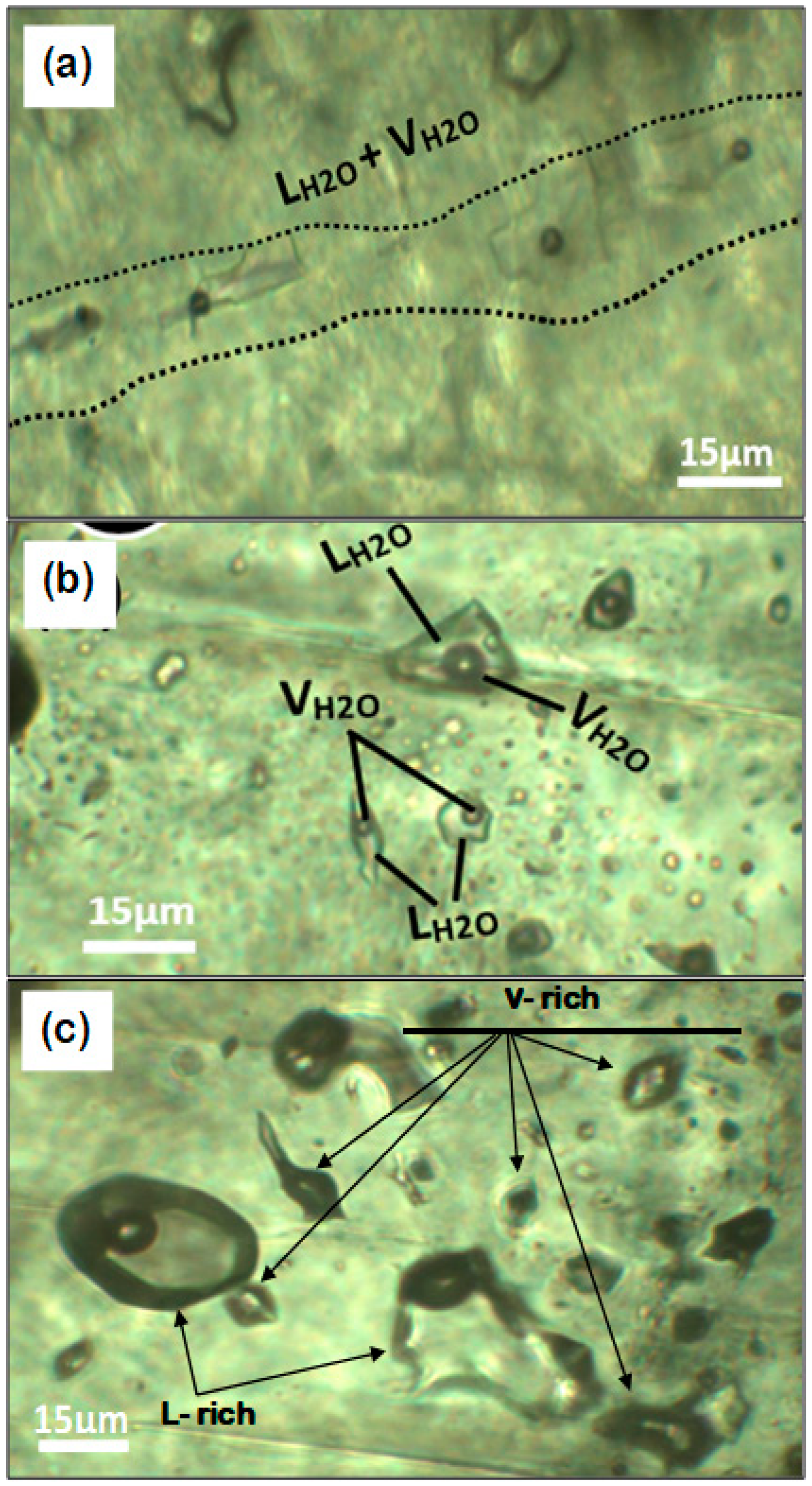
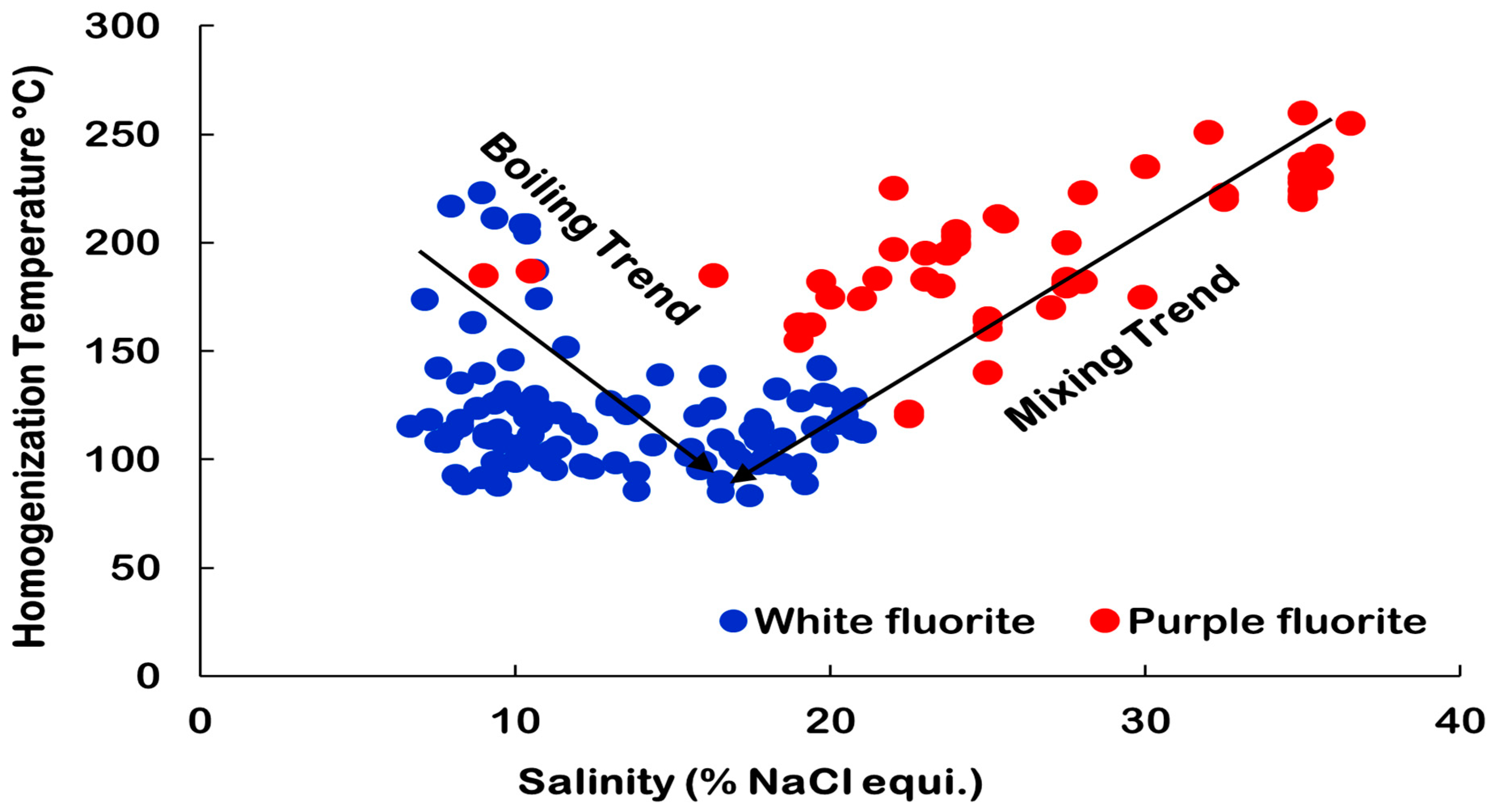
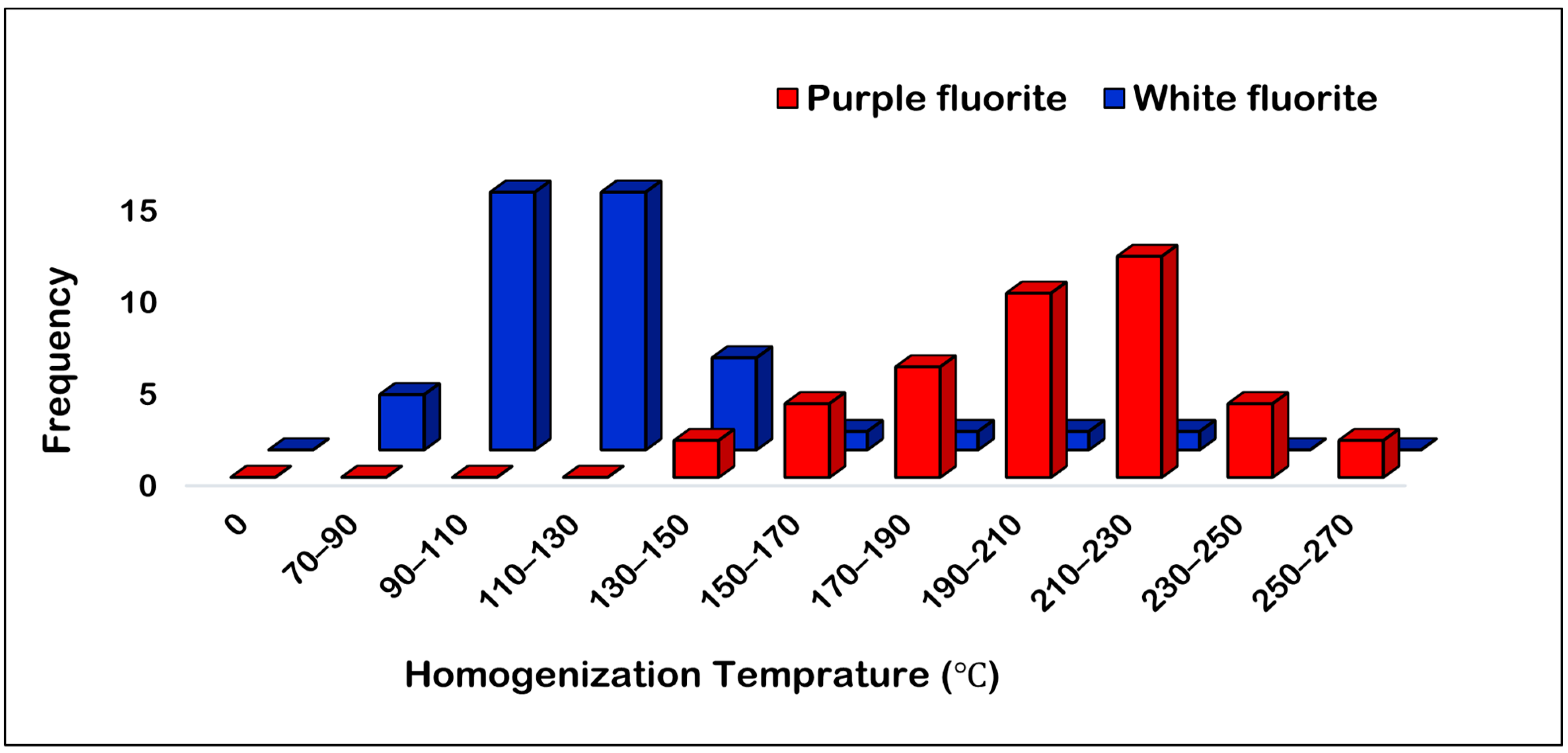
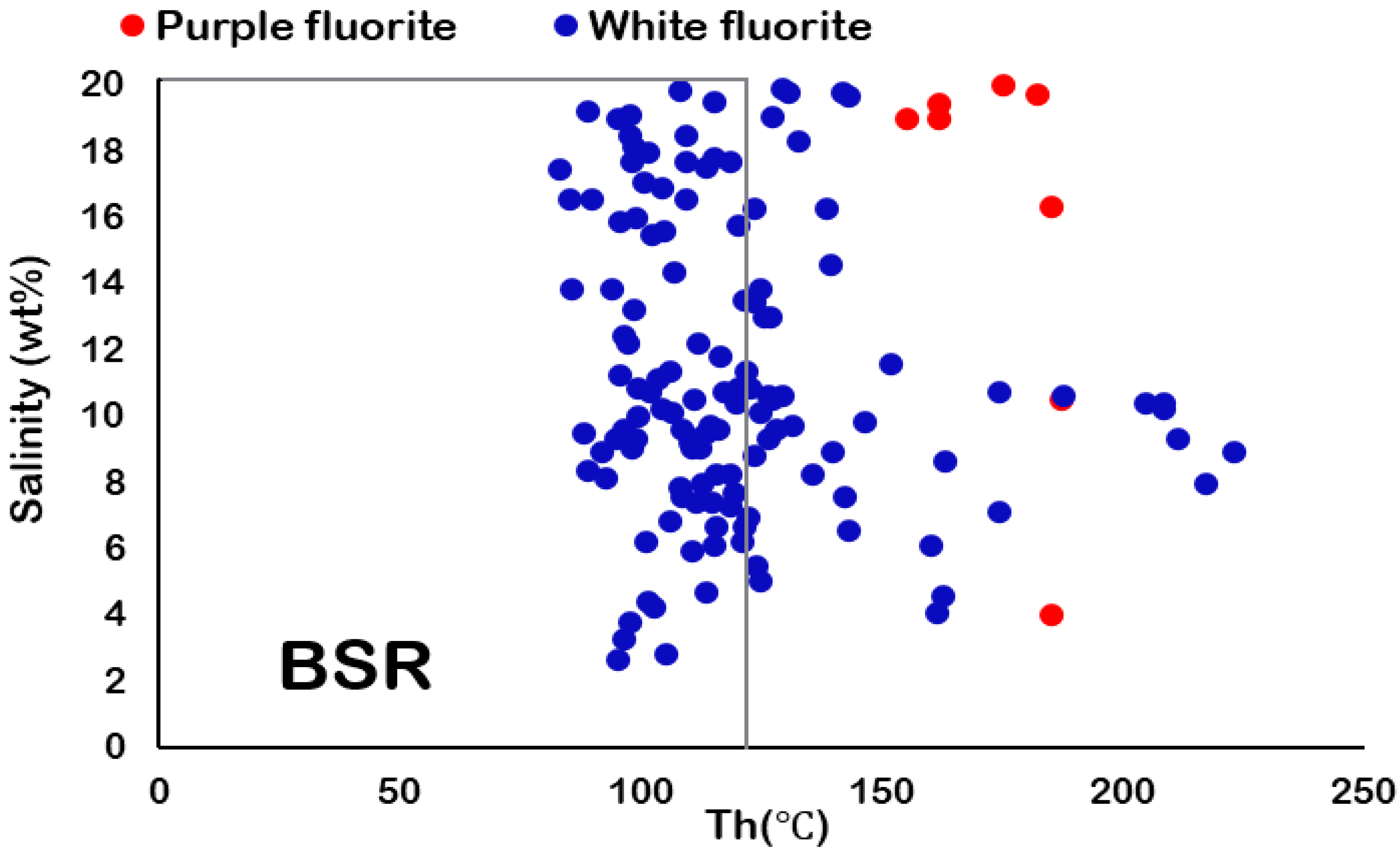
5.2. Fluid Inclusion Microthermometry
6. Discussion
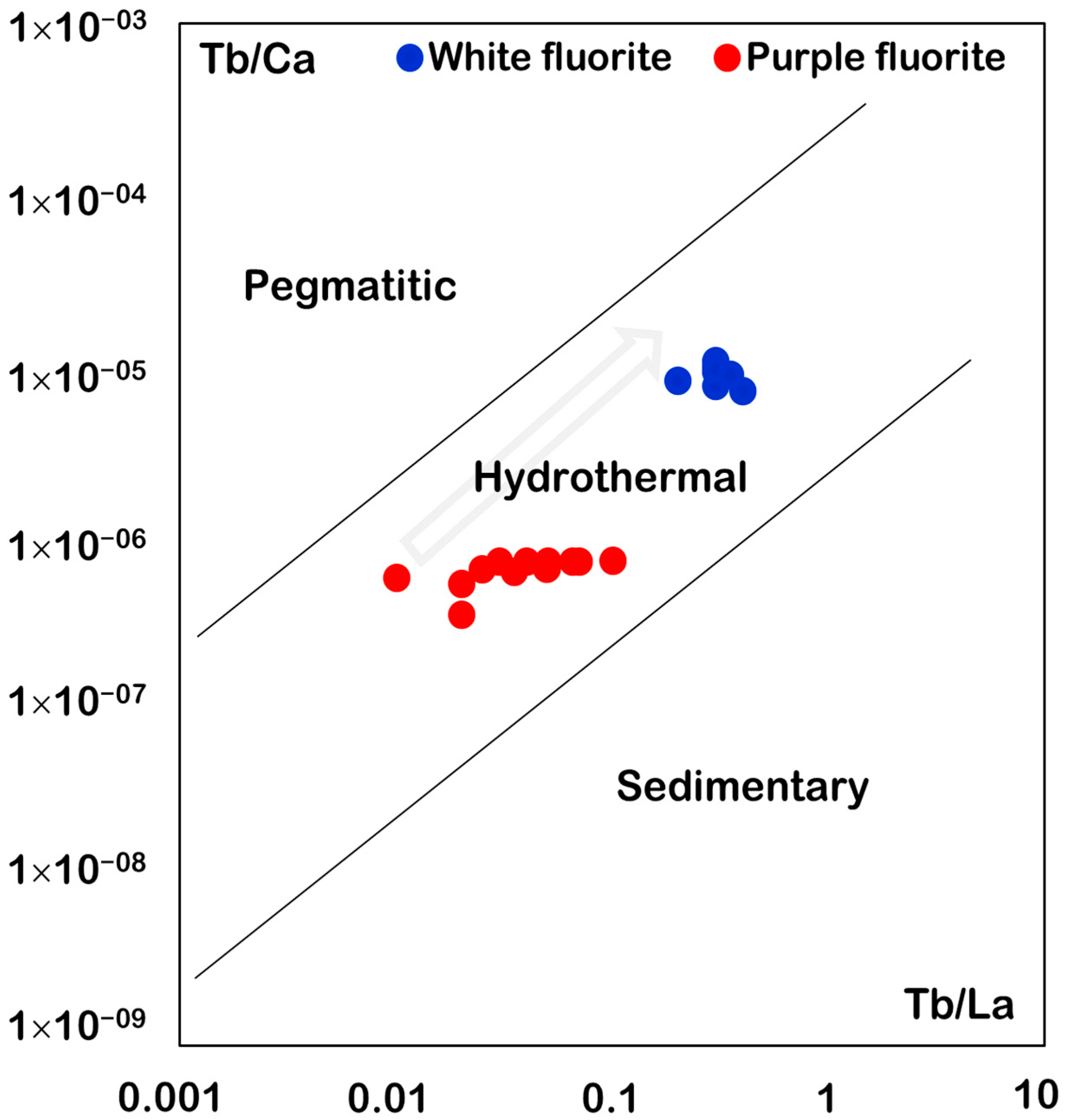
6.1. Tb/Ca and Tb/La Ratios
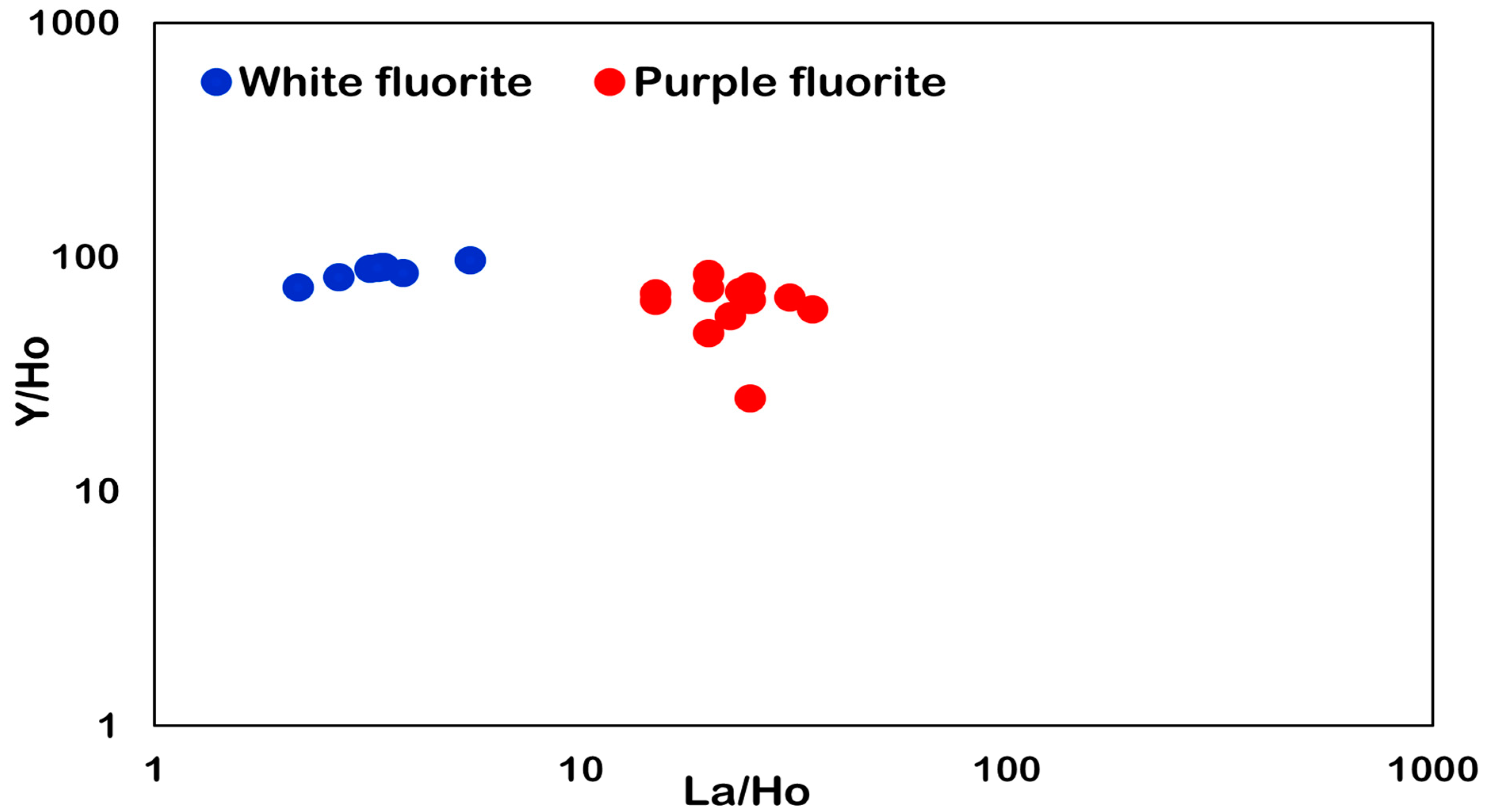
6.2. Y–Ho Fractionation
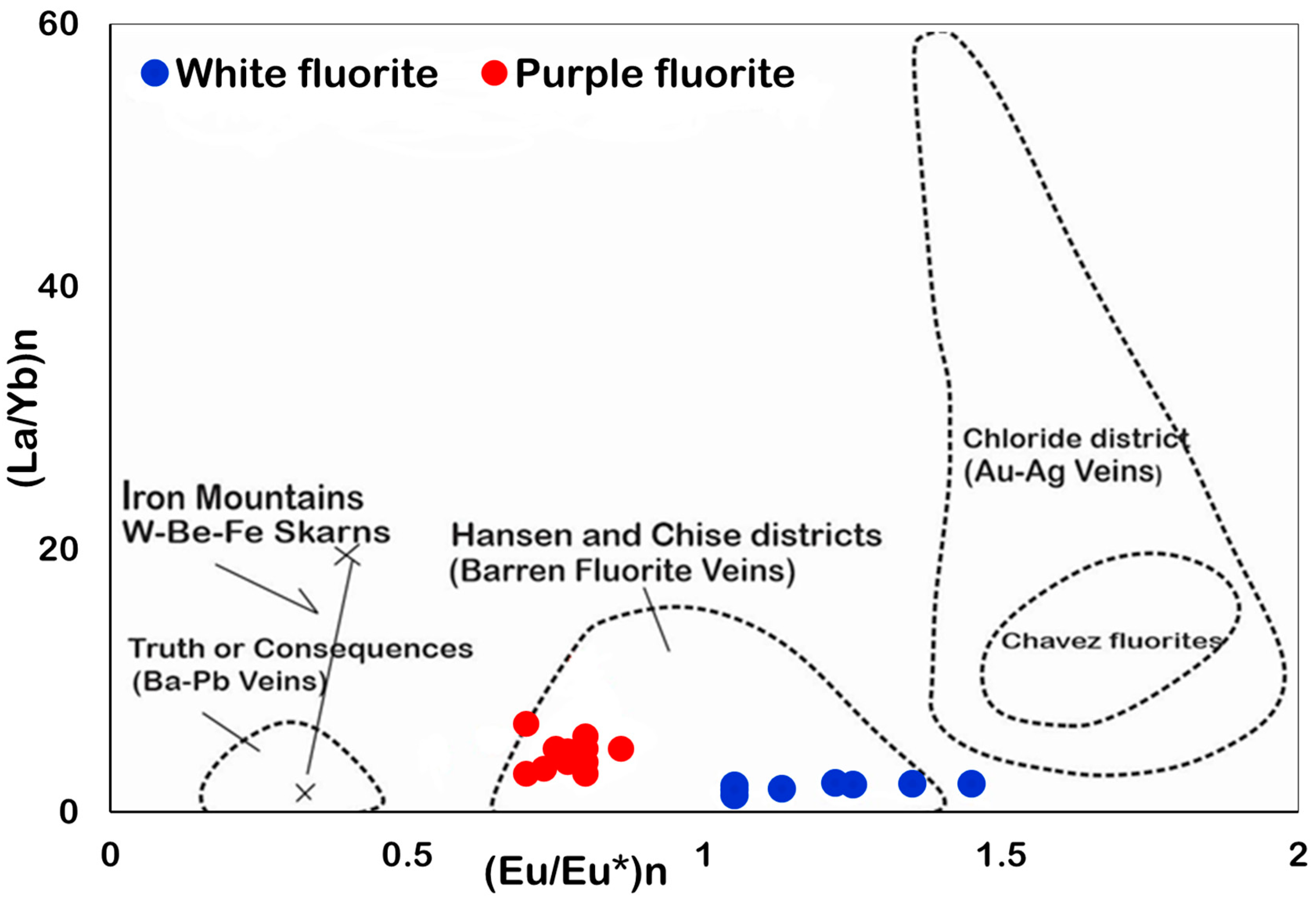
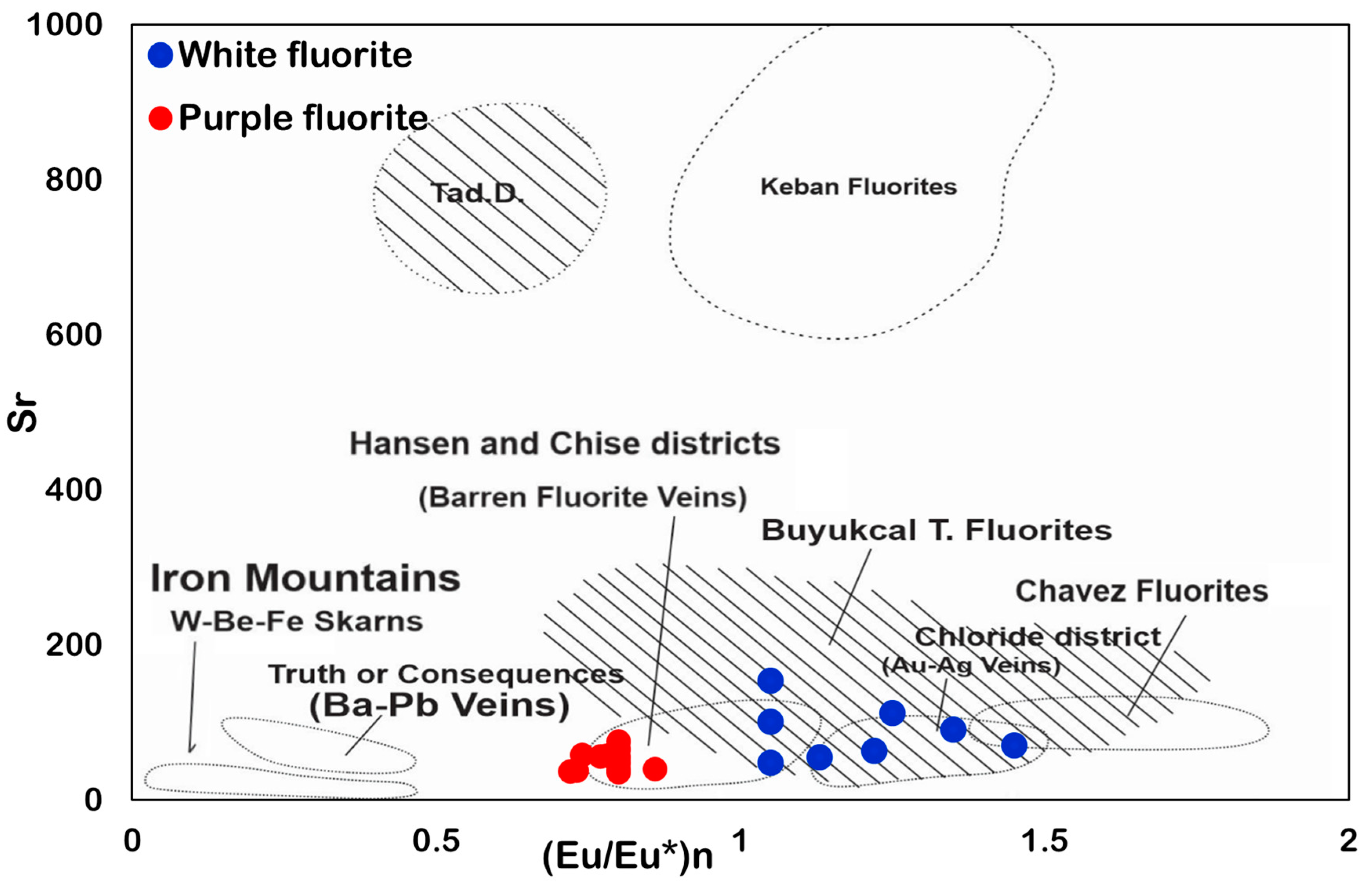
6.3. Eu and Ce Anomalies
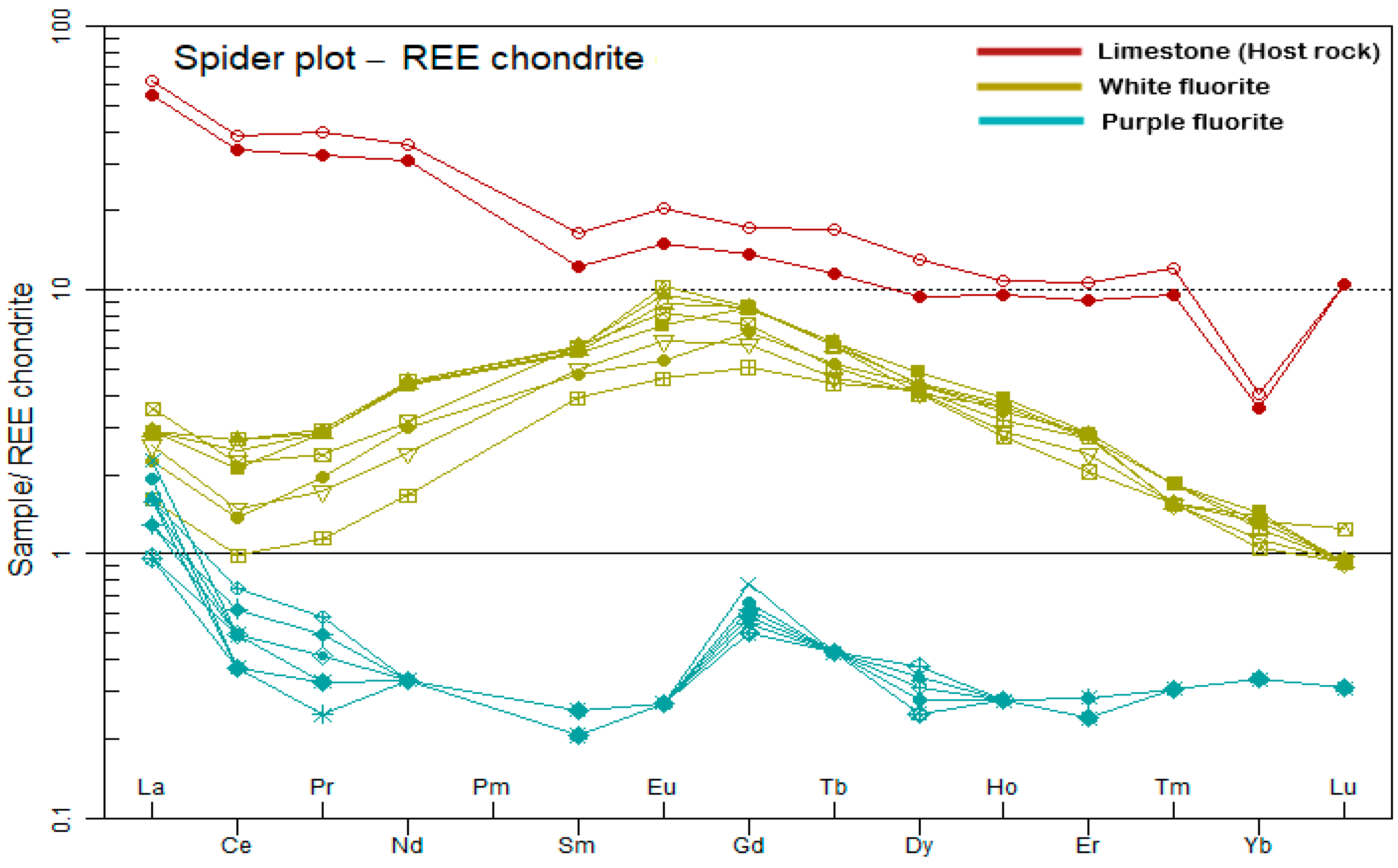
6.4. REE Enrichment
6.5. Evolution of REEs
6.6. Fluorite-Host Rock Relationship
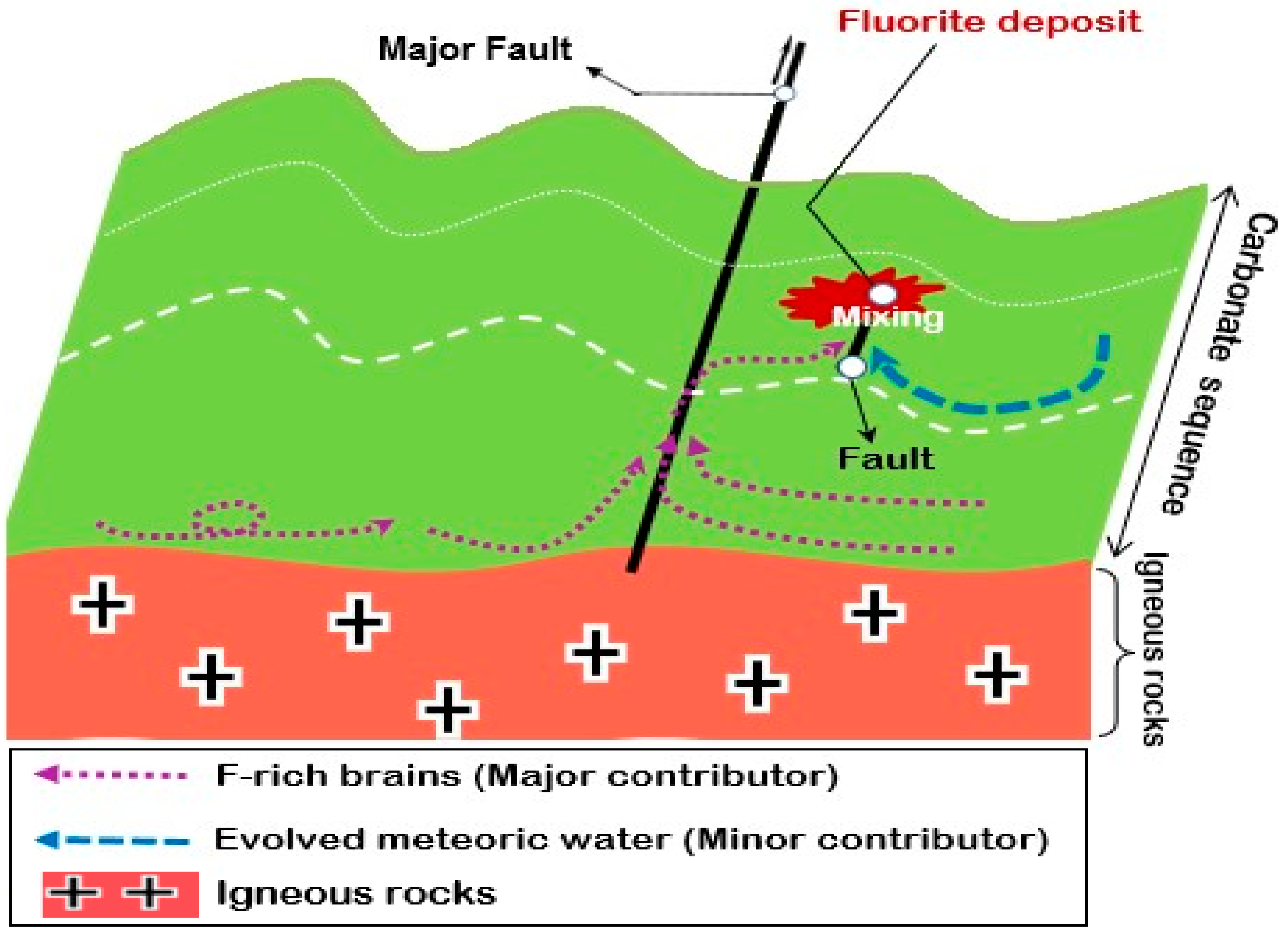
6.7. Source of REE and Hydrothermal Fluid
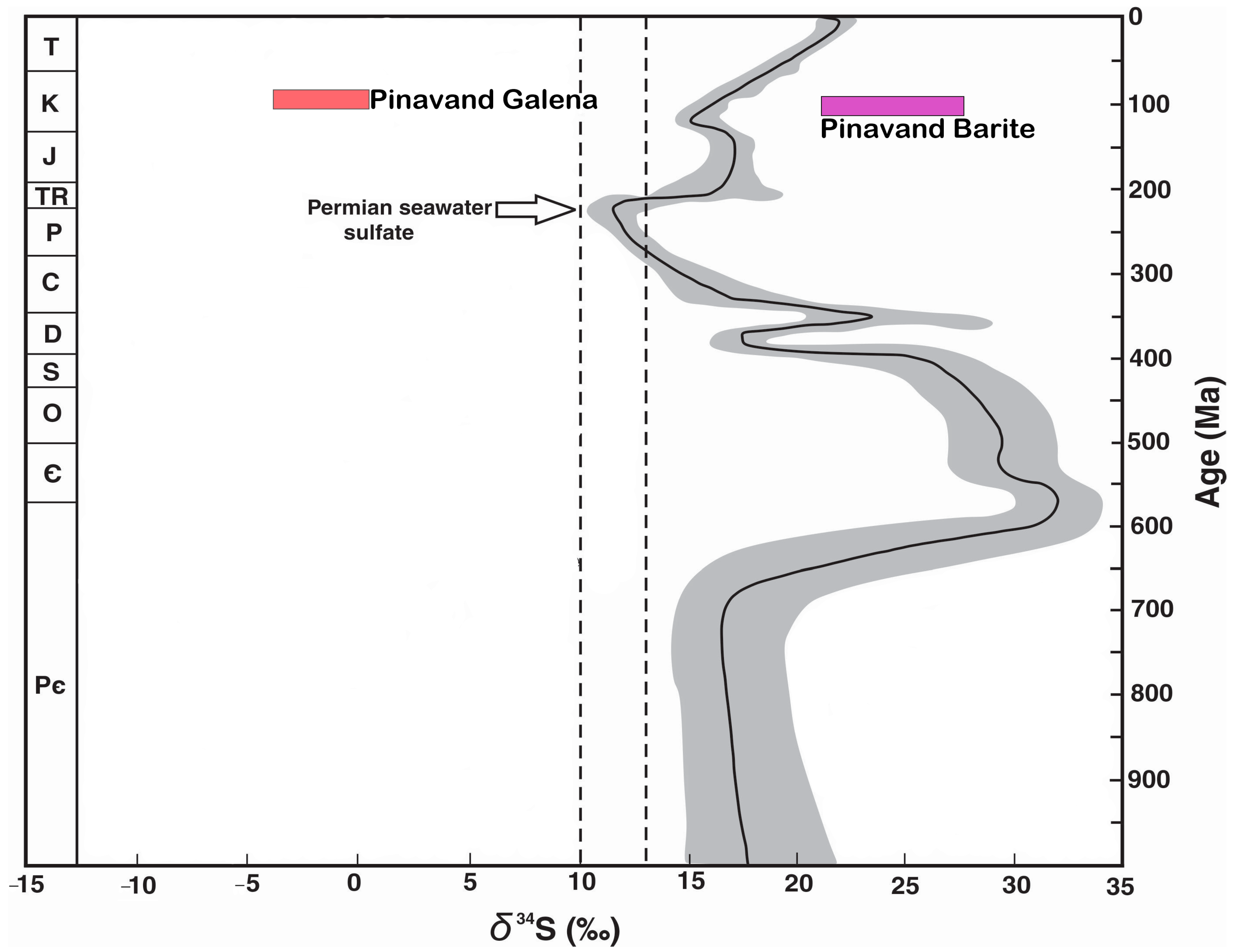
6.8. Source(s) of Sulfur
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eppinger, R.G.; Closs, L.G. Variation of trace elements and rare earth elements in fluorite; a possible tool for exploration. Econ. Geol. Bull. Soc. 1990, 85, 1896–1907. [Google Scholar] [CrossRef]
- Hill, G.T.; Campbell, A.R.; Kyle, P.R. Geochemistry of southwestern New Mexico fluorite occurrences implications for precious metals exploration in fluorite-bearing systems. J. Geochem. Explor. 2000, 68, 1–20. [Google Scholar] [CrossRef]
- Chen, Y.J.; Li, N. Nature of ore-fluids of intracontinental intrusion-related hypothermal deposits and its difference from those in island arcs. Acta Petrol. Sin. 2009, 25, 2477–2508. [Google Scholar]
- Bau, M.; Dulski, P. Comparative study of yttrium and rare-earth element behaviours in fluorine-rich hydrothermal fluids. Contrib. Mineral. Petrol. 1995, 119, 213–223. [Google Scholar] [CrossRef]
- Schwinn, G.; Markl, G. REE systematics in hydrothermal fluorite. Chem. Geol. 2005, 216, 225–248. [Google Scholar] [CrossRef]
- Loges, A.; Wagner, T.; Barth, M.; Bau, M.; Göb, S.; Markl, G. Negative Ce anomalies in Mn oxides: The role of Ce4+ mobility during water–mineral interaction. Geochim. Cosmochim. Acta 2012, 86, 296–317. [Google Scholar] [CrossRef]
- Göb, S.; Loges, A.; Nolde, N.; Bau, M.; Jacob, D.E.; Markl, G. Major and trace element compositions (including REE) of mineral, thermal, mine and surface waters in SW Germany and implications for water–rock interaction. Appl. Geochem. 2013, 33, 127–152. [Google Scholar] [CrossRef]
- Kraemer, D.; Viehmann, S.; Banks, D.; Sumoondur, A.D.; Koeberl, C.; Bau, M. Regional variations in fluid formation and metal sources in MVT mineralization in the Pennine Orefield, UK: Implications from rare earth element and yttrium distribution, Sr-Nd isotopes and fluid inclusion compositions of hydrothermal vein fluorites. Ore Geol. Rev. 2019, 107, 960–972. [Google Scholar] [CrossRef]
- Bilal, B.; Langer, P. Complex formation of trace elements in geochemical systems: Stability constants of fluorocomplexes of the lanthanides in a fluorite bearing model system up to 200 °C and 1000 bar. Inorganica Chim. Acta 1987, 140, 297–298. [Google Scholar] [CrossRef]
- Bau, M.; Romer, R.L.; Lüders, V.; Dulski, P. Tracing element sources of hydrothermal mineral deposits: REE and Y distribution and Sr-Nd-Pb isotopes in fluorite from MVT deposits in the Pennine Orefield, England. Miner. Depos. 2003, 38, 992–1008. [Google Scholar] [CrossRef]
- Möller, P.; Maus, H.; Gundlach, H. Die Entwicklung von Flußspatmineralisationen im Bereich des Schwarzwaldes. Jahresh. Geol. Landesamtes Baden-Württ. 1982, 24, 35–70. [Google Scholar]
- Castorina, F.; Masi, U.; Padalino, G.; Palomba, M. Trace-element and Sr–Nd isotopic evidence for the origin of the Sardinian fluorite mineralization (Italy). Appl. Geochem. 2008, 23, 2906–2921. [Google Scholar] [CrossRef]
- Sánchez, V.; Cardellach, E.; Corbella, M.; Vindel, E.; Martín-Crespo, T.; Boyce, A.J. Variability in fluid sources in the fluorite deposits from Asturias (N Spain): Further evidences from REE, radiogenic (Sr, Sm, Nd) and stable (S, C, O) isotope data. Ore Geol. Rev. 2010, 37, 87–100. [Google Scholar] [CrossRef]
- Sasmaz, A.; Kryuchenko, N.; Zhovinsky, E.; Suyarko, V.; Konakci, N.; Akgul, B. Major, trace and rare earth element (REE) geochemistry of different colored fluorites in the Bobrynets region, Ukraine. Ore Geol. Rev. 2018, 102, 338–350. [Google Scholar] [CrossRef]
- Darvishzadeh, A. Geology of Iran; Neda: Tehran, Iran, 1991; 901p.
- Rajabzadeh, M.A. A fluid inclusion study of a large MVT barite–fluorite deposit: Komshecheh, Central Iran. Iran. J. Sci. Technol. 2007, 31, 73–87. [Google Scholar]
- Rajabi, A.; Rastad, E.; Canet, C. Metallogeny of Permian–Triassic carbonate-hosted Zn–Pb and F deposits of Iran: A review for future mineral exploration. J. Geol. Soc. Aust. 2013, 60, 197–216. [Google Scholar] [CrossRef]
- Qishlaqi, A. Geochemistry and Genesis of Pinavand Fluorite Mines. Master’s Thesis, Shiraz University, Shiraz, Iran, 2002; 242p. [Google Scholar]
- Qishlaqi, A.; Moore, F. Recognition of Pinavand fluorite mines occurrence based on geochemistry and REE data. Iran. J. Crystallogr. Mineral. 2006, 2, 325–338. (In Farsi) [Google Scholar]
- Shafaeizadeh, E. Mineralogical and Fluid Inclusion Studies of Fluorite and Barites in the Pinavand Area, Isfahan Province. Master’s Thesis, Isfahan University, Isfahan, Iran, 2012; 110p. [Google Scholar]
- Ghasemi, A.; Talbot, C. A new tectonic scenario for the Sanandaj–Sirjan Zone (Iran). J. Asian Earth Sci. 2006, 26, 683–693. [Google Scholar] [CrossRef]
- Stocklin, J. Structural history and tectonics of Iran: A review. AAPG Bull. 1968, 52, 1229–1258. [Google Scholar]
- Berberian, M.; King, G.C.P. Towards a paleogeography and tectonic evolution of Iran. Can. J. Earth Sci. 1981, 18, 210–265. [Google Scholar] [CrossRef]
- Alavi, M. Structures of the Zagros fold-thrust belt in Iran. Am. J. Sci. 2007, 307, 1064–1095. [Google Scholar] [CrossRef]
- Alavi, M. Regional stratigraphy of the Zagros fold-thrust belt of Iran and its proforeland evolution. Am. J. Sci. 2004, 304, 1–20. [Google Scholar] [CrossRef]
- Agard, P.; Omrani, J.; Jolivet, L.; Whitechurch, H.; Vrielynck, B.; Spakman, W.; Monié, P.; Meyer, B.; Wortel, R. Zagros orogeny: A subduction-dominated process. Geol. Mag. 2011, 148, 692–725. [Google Scholar] [CrossRef]
- Aghanabati, A. Geology of Iran; Geological Survey of Iran: Tehran, Iran, 2004; pp. 1–582.
- Berberian, F.; Berberian, M. Tectono–Plutonic Episodes in Iran. Geol. Surv. Iran Rep. 1981, 52, 566–593. [Google Scholar]
- Alaminia, Z.; Tadayon, M.; Finger, F.; Lentz, D.; Waitzinger, M. Analysis of the infiltrative metasomatic relationships controlling skarn mineralization at the Abbas-Abad Fe-Cu Deposit, Isfahan, north Zefreh Fault, Central Iran. Ore Geol. Rev. 2020, 117, 103321. [Google Scholar] [CrossRef]
- Beygi, S.; Nadimi, A.; Safaei, H. Tectonic history of seismogenic fault structures in Central Iran. J. Geosci. 2016, 61, 127–144. [Google Scholar] [CrossRef]
- Aghanabati, A. Explanatory Text of the Bakhtaran Quadrangle Map 1: 250,000; Geological Survey of Iran: Tehran, Iran, 1990; pp. 115–132.
- Sengör, A.M.C. A new model for the Late Paleozoic–Mesozoic tectonic evolution of Iran and implications for Oman. In The Geology and Tectonics of the Oman Region; Special Publication; Robertson, A.H.F., Searle, M.P., Ries, A.C., Eds.; Geological Society: London, UK, 1990; Volume 49, pp. 797–831. [Google Scholar]
- Mohajjel, M.; Fergusson, C.L.; Sahandi, M.R. Cretaceous–Tertiary convergence and continental collision, Sanandaj–Sirjan zone, western Iran. J. Asian Earth Sci. 2003, 21, 397–412. [Google Scholar] [CrossRef]
- Alavi, M. Tectonics of the zagros orogenic belt of iran: New data and interpretations. Tectonophysics 1994, 229, 211–238. [Google Scholar] [CrossRef]
- Zahedi, M.; Amidi, M. The Geological Map of Kashan Scale 1: 250,000; Geological Survey of Iran: Tehran, Iran, 1991.
- Radfar, J.; Amini Chehragh, M.R.; Emani, M.H. Geological Map of the Ardestan Scale 1: 100,000; Geological Survey of Iran: Tehran, Iran, 1999.
- Radfar, J.; Kohansal, R.; Zolfaghari, S. Geological Map of the Kuhpayeh Scale 1: 100,000; Geological Survey of Iran: Tehran, Iran, 2002.
- Deer, W.A.; Howie, R.A.; Zussman, J. Rock-Formig Minerals Book; Prentice Hall: Hoboken, NJ, USA, 1998. [Google Scholar]
- Heinrich, E.W.M. Microscopic Petrography; Mcgraw-Hill Book Company, Inc.: New York, NY, USA, 1956. [Google Scholar]
- Moncada, D.; Mutchler, S.; Nieto, A.; Reynolds, T.J.; Rimstidt, J.D.; Bodnar, R.J. Mineral textures and fluid inclusion petrography of the epithermal Ag–Au deposits at Guanajuato, Mexico: Application to exploration. J. Geochem. Explor. 2012, 114, 20–35. [Google Scholar] [CrossRef]
- Bodnar, R. Revised equation and table for determining the freezing point depression of H2O-Nacl solutions. Geochim. Cosmochim. Acta 1993, 57, 683–684. [Google Scholar] [CrossRef]
- Dill, H.G.; Weber, B. Variation of color, structure and morphology of fluorite and the origin of the hydrothermal F-Ba deposits at Nabburg-Wölsendorf, SE Germany. Neues Jahrb. Mineral.-Abh. 2010, 187, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Dill, H.; Hansen, B.; Weber, B. REE contents, REE minerals and Sm/Nd isotopes of granite- and unconformity-related fluorite mineralization at the western edge of the Bohemian Massif: With special reference to the Nabburg-Wölsendorf District, SE Germany. Ore Geol. Rev. 2011, 40, 132–148. [Google Scholar] [CrossRef]
- Roedder, E. Fluid inclusions. Miner. Soc. Am. Rev. Miner. 1984, 12, 644. [Google Scholar]
- Roedder, E. The Composition of Fluid Inclusions; USGS Professional Paper; USGS: Reston, VA, USA, 1972; 164p.
- Moncada, D.; Baker, D.; Bodnar, R.J. Mineralogical, petrographic and fluid inclusion evidence for the link between boiling and epithermal Ag-Au mineralization in the La Luz area, Guanajuato Mining District, México. Ore Geol. Rev. 2017, 89, 143–170. [Google Scholar] [CrossRef]
- Goldstein, R.H.; Reynolds, T.J. Systematics of fluid inclusions in diagenetic minerals. Soc. Sediment. Geol. Short Course 1994, 31, 199. [Google Scholar]
- Bodnar, R.J.; Reynolds, T.J.; Kuehn, C.A. Fluid-inclusion systematics in epithermal systems. Rev. Econ. Geol. 1985, 2, 73–97. [Google Scholar]
- Möller, P.; Morteani, G. On the chemical fractionation of REE during the formation of Ca- minerals and its application to problems of the genesis of ore deposits. In The Significance of Trace Elements in Solving Petro-Genetic Problems; Augustithis, S., Ed.; Theophrastis Publishers: Athens, Greece, 1983; pp. 747–791. [Google Scholar]
- Möller, P.; Parekh, P.P.; Schneider, H.-J. The application of Tb/Ca-Tb/La abundance ratios to problems of fluorspar genesis. Miner. Depos. 1976, 11, 111–116. [Google Scholar] [CrossRef]
- Ekambaram, V.; Brookins, D.G.; Rosenberg, P.E.; Emanuel, K.M. REE geochemistry of fluorite-carbonate deposits in Western Montana, USA. Chem. Geol. 1986, 54, 319–331. [Google Scholar] [CrossRef]
- Constantopoulos, J. Fluid inclusion and REE geochemistry of fluorite from southcentral Idaho. Econ. Geol. 1988, 83, 626–636. [Google Scholar] [CrossRef]
- Sasmaz, A.; Yavuz, F.; Sagiroglu, A.; Akgul, B. Geochemical patterns of the Akdagmadeni (Yozgat, Central Turkey) fluorite deposits and implications. J. Asian Earth Sci. 2005, 24, 469–479. [Google Scholar] [CrossRef]
- Chesley, J.T.; Halliday, A.N.; Scrivener, R.C. Samarium-Neodymium Direct Dating of Fluorite Mineralization. Science 1991, 252, 949–951. [Google Scholar] [CrossRef] [PubMed]
- Möller, P.; Holzbecher, E. Eu anomalies in hydrothermal fluids and minerals. A combined thermochemical and dynamic phenomenon. Freib. Forsch. 1998, 475, 73–84. [Google Scholar]
- Bau, M.; Dulski, P. Distribution of yttrium and rare-earth elements in the Penge and Kuruman iron-formations, Transvaal Supergroup, South Africa. Precambrian Res. 1996, 79, 37–55. [Google Scholar] [CrossRef]
- Cherniak, D.; Zhang, X.; Wayne, N.; Watson, E. Sr, Y, and REE diffusion in fluorite. Chem. Geol. 2001, 181, 99–111. [Google Scholar] [CrossRef]
- Anders, E.; Grevesse, N. Abundances of the elements: Meteoritic and solar. Geochim. Cosmochim. Acta 1989, 53, 197–214. [Google Scholar] [CrossRef]
- Deng, X.-H.; Chen, Y.-J.; Yao, J.-M.; Bagas, L.; Tang, H.-S. Fluorite REE-Y (REY) geochemistry of the ca. 850Ma Tumen molybdenite–fluorite deposit, eastern Qinling, China: Constraints on ore genesis. Ore Geol. Rev. 2014, 63, 532–543. [Google Scholar] [CrossRef]
- Bau, M.; Möller, P. Rare-earth element fractionation in metamorphogenic hydrothermal calcite, magnesite and siderite. Miner. Petrol. 1992, 45, 231–246. [Google Scholar] [CrossRef]
- Chen, Y.J.; Zhao, Y.C. Geochemical characteristics and evolution of REE in the Early Precambrian sediments: Evidences from the southern margin of the North China craton. Episodes 1997, 20, 109–116. [Google Scholar]
- Ehya, F. Variation of mineralizing fluids and fractionation of REE during the emplacement of the vein-type fluorite deposit at Bozijan, Markazi Province, Iran. J. Geochem. Explor. 2012, 112, 93–106. [Google Scholar] [CrossRef]
- Schönenberger, J.; Köhler, J.; Markl, G. REE systematics of fluorides, calcite and siderite in peralkaline plutonic rocks from the Gardar Province, South Greenland. Chem. Geol. 2008, 247, 16–35. [Google Scholar] [CrossRef]
- Haas, J.R.; Shock, E.L.; Sassani, D.C. Rare earth in hydrothermal systems: Estimates of standard partial molal thermodynamic of aqueous complexes of rare earth elements at high pressures and temperatures. Geochim. Cosmochim. Acta 1995, 59, 4329–4350. [Google Scholar] [CrossRef]
- Deng, X.H.; Chen, Y.J.; Santosh, M.; Yao, J.M. Re–Os geochronology, fluid inclusions and genesis of the 0.85 Ga Tumen molybdenite–fluorite deposit in Eastern Qinling, China: Implications for pre-Mesozoic Mo-enrichment and tectonic setting. Geol. J. 2013, 48, 484–497. [Google Scholar] [CrossRef]
- Michard, A. Rare earth element systematics in hydrothermal fluids. Geochim. Cosmochim. Acta 1989, 53, 745–750. [Google Scholar] [CrossRef]
- Dai, A.B. Coordination Chemistry; Science Press: Beijing, China, 1987; 870p. (In Chinese) [Google Scholar]
- Gramaccioli, C.M.; Diella, V.; Demartin, F. The role of fluoride complexes in REE geochemistry and the importance of 4f electrons: Some examples in minerals. Eur. J. Miner. 1999, 11, 983–992. [Google Scholar] [CrossRef]
- Phillips, G.N.; Evans, K.A. Role of CO2 in the formation of gold deposits. Nature 2004, 429, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Boynton, W.V. Geochemistry of the rare earth elements: Meteorite studies. In REE Geochemistry; Henderson, P., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; pp. 63–114. [Google Scholar]
- Wendlandt, R.F.; Harrison, W.J. Rare earth partitioning between immiscible carbonate and silicate liquids and CO2 vapor: Results and implications for the formation of light rare earth-enriched rocks. Contrib. Miner. Pet. 1979, 69, 409–419. [Google Scholar] [CrossRef]
- Fontbote, L.; Gorzawski, H. Genesis of the mississippi valley-type Zn-Pb deposit of San Vicente, central Peru; geologic and isotopic (Sr, O, C, S, Pb) evidence. Econ. Geol. 1990, 85, 1402–1437. [Google Scholar] [CrossRef]
- Ghazban, F.; McNutt, R.H.; Schwarcz, H.P. Genesis of sediment-hosted Zn-Pb-Ba deposits in the Irankuh District, Esfahan area, west-central Iran. Econ. Geol. 1994, 89, 1262–1278. [Google Scholar] [CrossRef]
- Plumlee, G.S.; Goldhaber, M.B.; Rowan, E.L. The potential role of magmatic gases in the genesis of Illinois-Kentucky fluorspar deposits; implications from chemical reaction path modeling. Econ. Geol. 1995, 90, 999–1011. [Google Scholar] [CrossRef]
- Camprubí, A.; González-Partida, E.; Richard, A.; Boiron, M.-C.; González-Ruiz, L.E.; Aguilar-Ramírez, C.F.; Fuentes-Guzmán, E.; González-Ruiz, D.; Legouix, C. MVT-Like Fluorite Deposits and Oligocene Magmatic-Hydrothermal Fluorite–Be–U–Mo–P–V Overprints in Northern Coahuila, Mexico. Minerals 2019, 9, 58. [Google Scholar] [CrossRef]
- González-Partida, E.; Camprubí, A. Evolution of mineralizing fluids in the Zn-Pb-Cu(-Ag±Au) skarn and epithermal deposits of the world-class San Martín district, Zacatecas, Mexico. J. Geochem. Explor. 2006, 89, 138–142. [Google Scholar] [CrossRef]
- Levresse, G.; González-Partida, E.; Tritlla, J.; Camprubí, A.; Cienfuegos-Alvarado, E.; Morales-Puente, P. Fluid origin of the world-class, carbonate-hosted Las Cuevas fluorite deposit (San Luis Potosí, Mexico). J. Geochem. Explor. 2003, 78–79, 537–543. [Google Scholar] [CrossRef]
- Ruiz, J.; Kesler, S.E.; Jones, L.M.; Sutter, J.F. Geology and geochemistry of the Las Cuevas fluorite deposit, San Luis Potosi, Mexico. Econ. Geol. 1980, 75, 1200–1209. [Google Scholar] [CrossRef]
- Albinson, T.; Norman, D.I.; Cole, D.; Chomiak, B. Controls on the formation of low-sulfidation epithermal deposits in Mexico: Constraints from fluid inclusions and stable isotope data. Soc. Econ. Geol. Spec. 2001, 8, 1–32. [Google Scholar]
- Camprubí, A. Tectonic and metallogenic history of Mexico. In Tectonics, Metallogeny, and Discovery: The North American Cordillera and Similar Accretionary Settings; Colpron, M., Bissig, T., Rusk, B.G., Thompson, J.F.H., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2013; Volume 17, pp. 201–243. [Google Scholar]
- Albinson, T.; Rubio, M.A. Mineralogic and thermal structure of the Zuloaga vein, San Martín de Bolaños District, Jalisco, Mexico. Soc. Econ. Geol. Spec. 2001, 8, 115–132. [Google Scholar]
- González-Sánchez, F.; Puente-Solís, R.; González-Partida, E.; Camprubí, A. Estratigrafía del Noreste de México y su relación con los yacimientos estratoligados de fluorita, barita, celestina y Zn-Pb. Bol. Soc. Geol. Mex. 2007, 59, 43–62. [Google Scholar] [CrossRef]
- González-Partida, E.; Camprubí, A.; Canet, C.; González-Sánchez, F. Fisicoquímica de salmueras e hidrocarburos en cuencas petroleras y en depósitos minerales tipo Mississippi Valley asociados. Part II: Ejemplos de la Cuenca de Sabinas y la Cuenca del Sureste, México. Bol. Soc. Geol. Mex. 2008, 60, 23–42. [Google Scholar] [CrossRef]
- González-Sánchez, F.; Camprubí, A.; González-Partida, E.; Puente-Solís, R.; Canet, C.; Centeno-García, E.; Atudorei, V. Regional stratigraphy and distribution of epigenetic stratabound clestine, fluorine, barite and Pb-Zn deposits in the MVT province of northeastern Mexico. Miner. Depos. 2009, 44, 343–361. [Google Scholar] [CrossRef]
- González-Partida, E.; Birkle, P.; Torres-Alvarado, I. Evolution of the hydrothermal system at Los Azufres, Mexico, based on petrologic, fluid inclusion and isotopic data. J. Volcanol. Geotherm. Res. 2000, 104, 277–296. [Google Scholar] [CrossRef]
- Levresse, G.; Tritlla, J.; Villareal, J.; Gonzalez-Partida, E. The “El Pilote” fluorite skarn: A crucial deposit in the understanding and interpretation of the origin and mobilization of F from northern Mexico deposits. J. Geochem. Explor. 2006, 89, 205–209. [Google Scholar] [CrossRef]
- Levresse, G.; Tritlla, J.; Solorio-Munguía, J.G.; Valencia, V.; Linares, P.J.P. Fluid inclusions and U/Pb dating of the El Pilote Fluorite skarn occurrence: Metallogenic implications. Comptes Rendus Geosci. 2011, 343, 342–350. [Google Scholar] [CrossRef]
- McAnulty, W.N.; Sewell, C.R.; Rasberry, J.M. Geology and Fluorspar Ore Deposits in Cerro Aguachile, Pico Etereo District, Coahuila, Mexico; The Dow Chemical Company: Midland, MI, USA, 1960; unpublished report. [Google Scholar]
- McAnulty, W.N.; Sewell, C.R.; Atkinson, D.R.; Rasberry, J.M. Aguachile Beryllium-Bearing Fluorspar District, Coahuila, Mexico. Geol. Soc. Am. Bull. 1963, 74, 735–744. [Google Scholar] [CrossRef]
- Claypool, G.E.; Holser, W.T.; Kaplan, I.R.; Sakai, H.; Zak, I. The age curves of sulfur and oxygen isotopes in marine sulfate and their mutual interpretation. Chem. Geol. 1980, 28, 199–260. [Google Scholar] [CrossRef]
- Basuki, N.I.; Taylor, B.E.; Spooner, E.T.C. Sulfur Isotope Evidence for Thermochemical Reduction of Dissolved Sulfate in Mississippi Valley-Type Zinc-Lead Mineralization, Bongara Area, Northern Peru. Econ. Geol. 2008, 103, 783–799. [Google Scholar] [CrossRef]
- Hofes, J. Stable Isotope Geochemistry, 5th ed.; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Hoefs, J. Stable Isotope Geochemistry, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1980; p. 208. [Google Scholar]
- Orr, W.L. Changes in sulfur content and isotopic ratios of sulfur during petroleum maturation study of Big Horn basin Palaeozoic oils. Am. Assoc. Pet. Geol. Bull. 1974, 58, 2295–2318. [Google Scholar]
- Krouse, H.R.; Viau, C.A.; Eliuk, L.S.; Ueda, A.; Halas, S. Chemical and isotopic evidence of thermochemical sulphate reduction by light hydrocarbon gases in deep carbonate reservoirs. Nature 1988, 333, 415–419. [Google Scholar] [CrossRef]
- Ohmoto, H.; Goldhaber, M.B. Sulfur and carbon isotopes. In Geochemistry of Hydrothermal Ore Deposits, 3rd ed.; Barnes, H.L., Ed.; Wiley and Sons: Hoboken, NJ, USA, 1997; pp. 517–611. [Google Scholar]
- Ehrlich, H.L.; Newman, D.K. Geomicrobiology; CRC Press: Boca Raton, FL, USA, 2009; p. 628. [Google Scholar]
- Kucha, H.; Schroll, E.; Raith, J.G.; Halas, S. Microbial sphalerite formation in carbonate-hosted Zn-Pb ores, Bleiberg, Austria: Micro- to nanotextural and sulfur isotope evidence. Econ. Geol. 2010, 105, 1005–1023. [Google Scholar] [CrossRef]
- Druschel, G.K.; Labrenz, M.; Thomsen-Ebert, T.; Fowle, D.A.; Banfield, J.F. Geochemical Modeling of ZnS in Biofilms: An Example of Ore Depositional Processes. Econ. Geol. 2002, 97, 1319–1329. [Google Scholar] [CrossRef]
- Abidi, R.; Slim-Shimi, N.; Marignac, C.; Somarin, A.K.; Renac, C.; Deloule, E.; Hatira, N.; Gasquet, D. The microbial controls on the deposition of Pb-Zn minerals in carbonate-hosted Tunisian ore deposits. Resour. Geol. 2022, 72, e12287. [Google Scholar] [CrossRef]





| Sample No. | Detection | WF-01 | WF-02 | WF-05 | WF-07 | WF-06 | WF-12 | WF-13 | WF-20 | PF-03 | PF-04 | PF-08 | PF-09 | PF-10 | PF-11 | PF-15 | PF-14 | PF-16 | PF-17 | PF-18 | PF-19 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colors | Limits | White | White | White | White | White | White | White | White | Purple | Purple | Purple | Purple | Purple | Purple | Purple | Purple | Purple | Purple | Purple | Purple |
| SiO2 | wt% | 0.06 | 0.6 | 0.71 | 0.07 | 0.57 | 0.39 | 0.33 | 0.35 | 0.75 | 0.62 | 2.04 | 0.47 | 2.8 | 1.94 | 1.25 | 0.68 | 2.37 | 0.97 | 1.8 | 2 |
| Al2O3 | wt% | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.03 | <0.01 | <0.01 | 0.02 | <0.02 | <0.01 | <0.02 | <0.02 | <0.02 | <0.02 |
| Fe2O3 | wt% | <0.04 | 0.13 | <0.04 | <0.04 | <0.04 | <0.04 | 0.13 | 0.07 | 0.06 | 0.11 | 0.13 | 0.24 | 0.15 | 0.05 | 0.18 | 0.08 | 0.1 | 0.14 | 0.11 | 0.1 |
| MgO | wt% | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.01 | <0.01 | <0.01 | 0.02 | 0.01 | <0.01 | 0.02 | 0.01 | <0.01 | 0.01 |
| Na2O | wt% | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.03 |
| K2O | wt% | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.01 | <0.01 | <0.01 | 0.02 | 0.01 | <0.01 | 0.02 | <0.01 | <0.01 | 0.02 |
| Cr2O3 | wt% | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | 0.003 | 0.003 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | 0.003 | <0.002 | <0.002 | 0.002 | <0.002 |
| F | wt% | 27.78 | 27.68 | 25.77 | 28.61 | 26.2 | 27.2 | 27.7 | 28.5 | 24.84 | 29.11 | 23.64 | 26.66 | 29.26 | 24.79 | 25.13 | 27 | 27.01 | 26.1 | 27 | 24.5 |
| Ba | 1 ppm | 37 | 61 | 67 | 6727 | 1650 | 3397 | 49 | 3420 | 8 | 15 | 99 | 57 | 415 | 74 | 78 | 11.5 | 233.5 | 45.2 | 210.5 | 85.8 |
| Co | 0.2 ppm | 3.9 | 11.4 | 2.6 | 5.1 | 3.3 | 3.85 | 7.65 | 8.3 | 14.2 | 13.8 | 9.1 | 6.6 | 4.5 | 8.9 | 7.8 | 14 | 6.7 | 11 | 9.4 | 9 |
| Mo | 0.1 ppm | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | I.S. | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Pb | 0.1 ppm | 42 | 10.1 | 20.2 | 33 | 24 | 26.6 | 26.05 | 22 | 11.6 | I.S. | 48.4 | 5.8 | 128.4 | 28.8 | 27.1 | 11.6 | 78.6 | 20.1 | 75.2 | 39.5 |
| Ni | 0.1 ppm | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 1.1 | I.S. | 0.2 | 0.1 | 1.5 | <0.1 | 0.1 | 1.1 | 1.5 | 0.7 | 1.2 | 0.2 |
| Au | 0.5 ppm | 1.1 | <0.5 | <0.5 | 1.6 | 0.9 | 1.6 | 1.1 | 1.6 | 3.4 | I.S. | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | 3.4 | <0.5 | <0.5 | 2.2 | <0.5 |
| Th | 0.2 ppm | 0.8 | 0.9 | 1 | 1 | 0.9 | 1 | 0.8 | 1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Sample No. | WF-01 | WF-02 | WF-05 | WF-07 | WF-06 | WF-12 | WF-13 | WF-20 | PF-03 | PF-04 | PF-08 | PF-09 | PF-10 | PF-11 | PF-14 | PF-15 | PF-16 | PF-17 | PF-18 | PF-19 | LSt-16 | LSt-17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colors | White | White | White | White | White | White | White | White | Purple | Purple | Purple | Purple | Purple | Purple | Purple | Purple | Purple | Purple | Purple | Purple | Limestone | Limestone |
| Ca% | 51.18 | 50.91 | 51.98 | 50.56 | 51.63 | 51.27 | 51.04 | 50.74 | 52.61 | 50.09 | 51.36 | 51.03 | 50.53 | 50.47 | 51.62 | 51.2 | 50.71 | 51.32 | 52 | 51.2 | 17.83 | 17.26 |
| La | 1.1 | 0.5 | 0.9 | 0.9 | 0.9 | 0.9 | 0.8 | 0.7 | 0.4 | 0.3 | 0.7 | 0.5 | 0.5 | 0.3 | 0.3 | 0.6 | 0.4 | 0.5 | 0.4 | 0.5 | 19.3 | 17.11 |
| Ce | 1.8 | 0.8 | 2.2 | 1.7 | 2.2 | 2 | 1.2 | 1.1 | 0.5 | 0.3 | 0.4 | 0.3 | 0.6 | 0.3 | 0.4 | 0.3 | 0.4 | 0.4 | 0.5 | 0.3 | 31.34 | 27.72 |
| Pr | 0.29 | 0.14 | 0.36 | 0.35 | 0.35 | 0.35 | 0.21 | 0.24 | 0.06 | 0.03 | 0.04 | 0.04 | 0.07 | 0.04 | 0.05 | 0.04 | 0.05 | 0.04 | 0.6 | 0.04 | 4.86 | 3.98 |
| Nd | 1.9 | 1 | 2.7 | 2.6 | 2.7 | 2.65 | 1.45 | 1.8 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 21.3 | 18.65 |
| Sm | 1.19 | 0.76 | 1.18 | 1.13 | 1.2 | 1.15 | 0.98 | 0.94 | 0.04 | 0.05 | 0.04 | 0.04 | 0.05 | 0.05 | 0.04 | 0.04 | 0.05 | 0.05 | 0.05 | 0.04 | 3.2 | 2.4 |
| Eu | 0.6 | 0.34 | 0.76 | 0.54 | 0.7 | 0.65 | 0.47 | 0.4 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 1.5 | 1.1 |
| Gd | 1.92 | 1.32 | 2.22 | 2.23 | 2.2 | 2.22 | 1.62 | 1.8 | 0.16 | 0.14 | 0.2 | 0.13 | 0.15 | 0.13 | 0.15 | 0.17 | 0.14 | 0.16 | 0.15 | 0.16 | 4.45 | 3.52 |
| Tb | 0.24 | 0.21 | 0.29 | 0.3 | 0.3 | 0.29 | 0.22 | 0.25 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.8 | 0.55 |
| Dy | 1.3 | 1.34 | 1.3 | 1.57 | 1.4 | 1.43 | 1.32 | 1.4 | 0.08 | 0.08 | 0.11 | 0.08 | 0.1 | 0.12 | 0.08 | 0.09 | 0.11 | 0.08 | 0.09 | 0.11 | 4.21 | 3.02 |
| Ho | 0.2 | 0.23 | 0.26 | 0.28 | 0.26 | 0.27 | 0.21 | 0.25 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.78 | 0.69 |
| Er | 0.43 | 0.58 | 0.58 | 0.6 | 0.58 | 0.59 | 0.5 | 0.6 | 0.06 | 0.05 | 0.06 | 0.06 | 0.05 | 0.05 | 0.05 | 0.06 | 0.05 | 0.06 | 0.05 | 0.5 | 2.26 | 1.91 |
| Tm | 0.05 | 0.06 | 0.05 | 0.06 | 0.05 | 0.05 | 0.05 | 0.06 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.39 | 0.31 |
| Yb | 0.22 | 0.26 | 0.28 | 0.3 | 0.28 | 0.29 | 0.24 | 0.28 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.84 | 0.75 |
| Lu | 0.03 | 0.03 | 0.04 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.34 | 0.34 |
| Y | 19.4 | 17.1 | 23.7 | 25.1 | 24.05 | 24.4 | 18.25 | 21.1 | 1.7 | 1.3 | 1.2 | 1.5 | 0.5 | 1.4 | 1.5 | 1.4 | 0.9 | 1.42 | 1.1 | 1.3 | ||
| ∑REE | 11.3 | 7.4 | 13.2 | 12.6 | 13.05 | 12.9 | 9.3 | 10 | 2.1 | 1.2 | 1.8 | 1.2 | 2 | 1.3 | 1.6 | 1.5 | 1.7 | 1.6 | 2.05 | 1.5 | 95.57 | 82.05 |
| (La/Yb) n | 2.23 | 1.3 | 2.17 | 2.02 | 2.14 | 2.09 | 1.76 | 1.7 | 3.8 | 2.9 | 6.7 | 4.8 | 4.8 | 2.9 | 3.3 | 5.75 | 3.8 | 4.6 | 4.3 | 4.8 | 14.12 | 14.02 |
| (Tb/Yb) n | 4.85 | 3.6 | 4.61 | 4.45 | 4.6 | 4.53 | 4.23 | 4.02 | 1.7 | 1.8 | 1.7 | 1.6 | 1.7 | 1.8 | 1.75 | 1.7 | 1.7 | 1.6 | 1.6 | 1.8 | 0.42 | 0.33 |
| (Tb/La) n | 1.44 | 2.76 | 2.12 | 2.2 | 2.14 | 2.16 | 2.1 | 2.5 | 0.32 | 0.44 | 0.19 | 0.26 | 0.26 | 0.44 | 0.4 | 0.23 | 0.35 | 0.31 | 0.29 | 0.31 | 0.03 | 0.02 |
| La/Ho | 5.5 | 2.17 | 3.46 | 3.21 | 3.4 | 3.33 | 3.83 | 2.7 | 20 | 15 | 35 | 25 | 25 | 15 | 20 | 31 | 20 | 23.8 | 22.4 | 25 | 24.74 | 24.79 |
| Ce/Ce* | 0.77 | 0.38 | 0.85 | 0.73 | 0.85 | 0.85 | 0.66 | 0.64 | 0.7 | 0.76 | 0.57 | 0.51 | 0.77 | 0.66 | 0.73 | 0.54 | 0.71 | 0.63 | 0.72 | 0.61 | 0.75 | 0.78 |
| Eu/Eu* | 1.22 | 1.05 | 1.45 | 1.05 | 1.35 | 1.25 | 1.13 | 1.05 | 0.77 | 0.74 | 0.73 | 0.86 | 0.8 | 0.8 | 0.78 | 0.8 | 0.8 | 0.78 | 0.8 | 0.75 | 1.21 | 1.15 |
| Y/Y* | 3.26 | 2.64 | 3.5 | 3.25 | 3.43 | 3.37 | 2.95 | 2.8 | 2.66 | 2.78 | 2.19 | 3.21 | 1.15 | 2.44 | 2.7 | 2.8 | 1.8 | 2.7 | 1.9 | 2.3 | ||
| Y/Ho | 97 | 74.35 | 91.15 | 89.64 | 90.8 | 90.39 | 85.67 | 82.1 | 85 | 65 | 60 | 75 | 25 | 70 | 73.7 | 67.5 | 47.5 | 71.25 | 56 | 65.5 |
| Sample No. | Host Mineral | Number of Measurements | Th (°C) | Tmice (°C) | Salinity (wt% NaCl eq.) | Density (g/cm3) | Size (µm) | Data Sources |
|---|---|---|---|---|---|---|---|---|
| P6 | White Fluorite | 14 | 95.6 to 187 | −5.7 to −11.9 | 8.8 to 15.9 | 0.96 to 1.07 | 6.7 to 26.3 | This Study |
| P7 | White Fluorite | 16 | 95.3 to 223.1 | −1.6 to −6.9 | 2.6 to 10.3 | 0.91 to 1.01 | 6.4 to 15.4 | |
| P42 | White Fluorite | 10 | 83.3 to 141.7 | −12.3 to −17.3 | 16.2 to 20.4 | 1.05 to 1.10 | 4.2 to 20.5 | |
| Purple Fluorite | 40 | 130 to 270 | 2.5 to 36 | 5–20 | [18] | |||
| Fluorite (white and Purple) | 19 | 75 to 189 | −0.2 to −14.8 | 0.3 to 18.6 | 0.96 to 1.09 | 5 to 20 | [20] |
| Sample No. | Mineral | δ34S (‰ VCDT) |
|---|---|---|
| PS1 | Galena | −3.7 |
| PS2 | Galena | −0.3 |
| PS3 | Galena | −0.2 |
| PS4 | Galena | −0.6 |
| PB2 | Barite | 21.1 |
| PB3 | Barite | 25.4 |
| Average PS | Galena | −1.2 |
| Average PB | Barite | 23.25 |
| Deposit | Type | Ore | Model of Formation | T (°C) | Salinity (wt% NaCl equiv.) | Comments | References |
|---|---|---|---|---|---|---|---|
| Las Cuevas & Río Verde, San Luis Potosí | Skarn-related | Fluorite | Contact metamorphism and retrograde hydrothermal fluids from F-rich volcanic rocks. | 60 to 130 | ~0 | [76] | |
| MVT | Diluted basinal brines reacted with host limestones. | 60 to 110 | 0 to 0.2 | [77] | |||
| MVT-like | Diluted basinal brines reacted with F-rich modified meteoric water and host limestones. | 49 to 177 | 0 to 1.9 (mostly 0.2) | [75] | |||
| Bolaños & San Martín de Bolaños, Jalisco | Epithermal | Polymetallic, rich in fluorite | Essentially Ag-rich intermediate sulfide deposits with stages of mineralization very rich in fluorite, deposited through boiling or conductive cooling. | 150 to 340 | 0 to 16 | [78,79] | |
| Several small deposits in Central Mexico | Tin rhyolites | Sn | Fumarolic deposits associated with extremely differentiated F-rich rhyolites and rhyodacites. | n.a. | n.a. | [80,81] | |
| Buenavista, Coahuila | MVT | Fluorite | Dense F-rich basinal brines that reacted with platform and reef carbonates. | 50 to 155 | 5.7 to 18.1 | [75,82,83,84] | |
| El Pilote, Coahuila | Skarn | Fluorite | Shallow hypabyssal rocks associated with hydrothermal fluids that dissolved pre-existing MVT-like fluorite mantos, and fluorite re-precipitated around the skarn. | 78 to 423 | 5 to 34 | 24.5 to 29.1 wt% CaCl2 fluids | [75,85,86,87] |
| Aguachile and Cuatro Palmas, Coahuila | Shallow hydrothermal | Fluorite, Be, U, Mo, etc. | Fluids largely exsolved from cooling hypabyssal alkaline to calc-alkaline rocks that reacted with host carbonate rocks. | 70 to 180 | 0.9 to 8.8 | Fluids generally below 4 wt% NaCl equivalents | [75,88,89] |
| Pinavand district, Iran | Hydrothermal | Fluorite | Reaction of the hydrothermal solution. with host limestone | 75 to 270 | 0.3 to 36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaedi, F.; Taghipour, B.; Somarin, A.K.; Fazli, S. Fluid Inclusions and REE Geochemistry of White and Purple Fluorite: Implications for Physico-Chemical Conditions of Mineralization; an Example from the Pinavand F Deposit, Central Iran. Minerals 2023, 13, 836. https://doi.org/10.3390/min13070836
Ghaedi F, Taghipour B, Somarin AK, Fazli S. Fluid Inclusions and REE Geochemistry of White and Purple Fluorite: Implications for Physico-Chemical Conditions of Mineralization; an Example from the Pinavand F Deposit, Central Iran. Minerals. 2023; 13(7):836. https://doi.org/10.3390/min13070836
Chicago/Turabian StyleGhaedi, Fatemeh, Batoul Taghipour, Alireza K. Somarin, and Samaneh Fazli. 2023. "Fluid Inclusions and REE Geochemistry of White and Purple Fluorite: Implications for Physico-Chemical Conditions of Mineralization; an Example from the Pinavand F Deposit, Central Iran" Minerals 13, no. 7: 836. https://doi.org/10.3390/min13070836
APA StyleGhaedi, F., Taghipour, B., Somarin, A. K., & Fazli, S. (2023). Fluid Inclusions and REE Geochemistry of White and Purple Fluorite: Implications for Physico-Chemical Conditions of Mineralization; an Example from the Pinavand F Deposit, Central Iran. Minerals, 13(7), 836. https://doi.org/10.3390/min13070836







