In Situ Trace Element and Fe-O Isotope Studies on Magnetite of the Iron-Oxide Ores from the Takab Region, North Western Iran: Implications for Ore Genesis
Abstract
1. Introduction
2. Materials and Methods
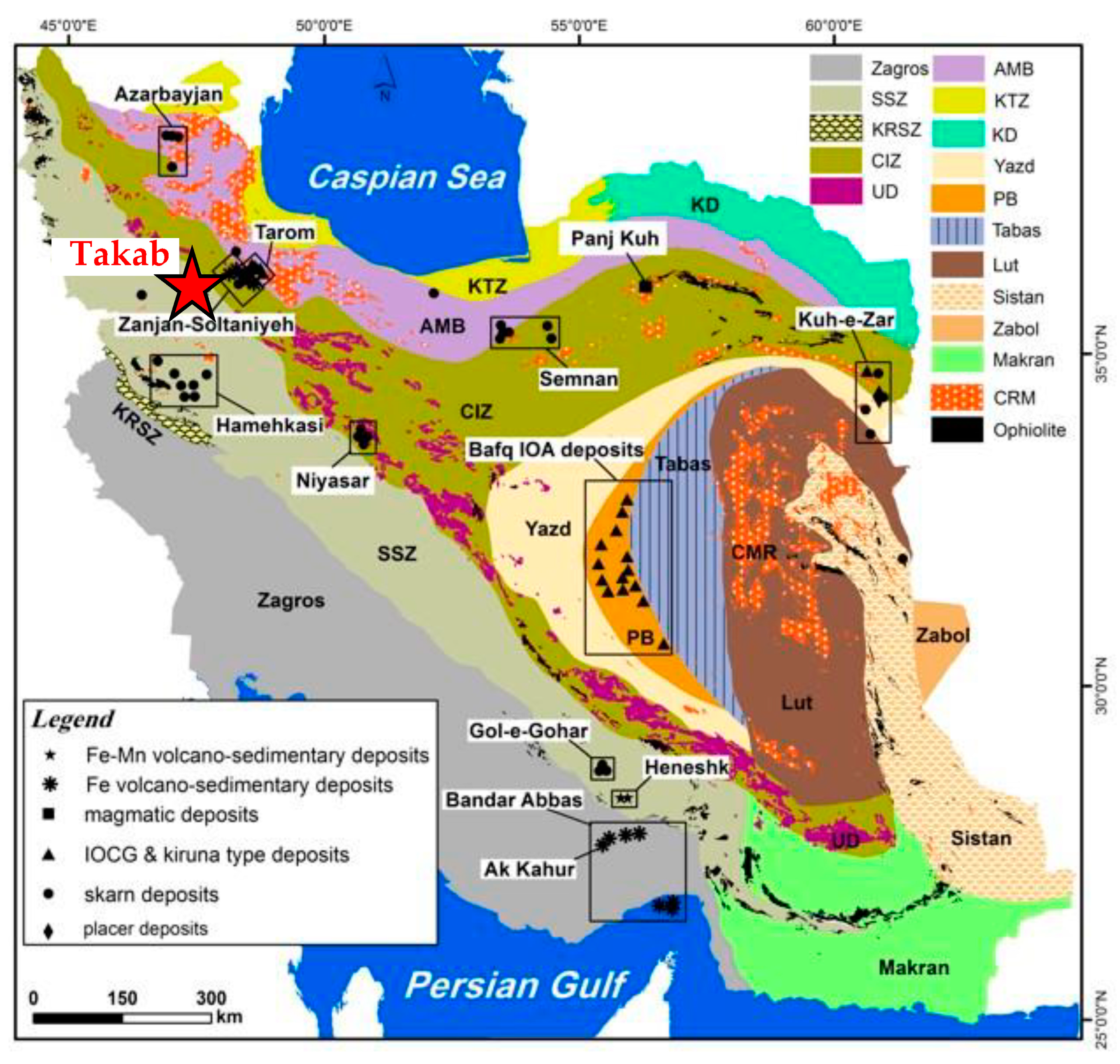
2.1. Geological Settings
2.2. Magnetite-Bearing Ore Types
2.3. Analytical Methods
2.3.1. ICP-MS Analysis
2.3.2. SEM and EMPA Analysis
2.3.3. SIMS Isotope Analysis
3. Results
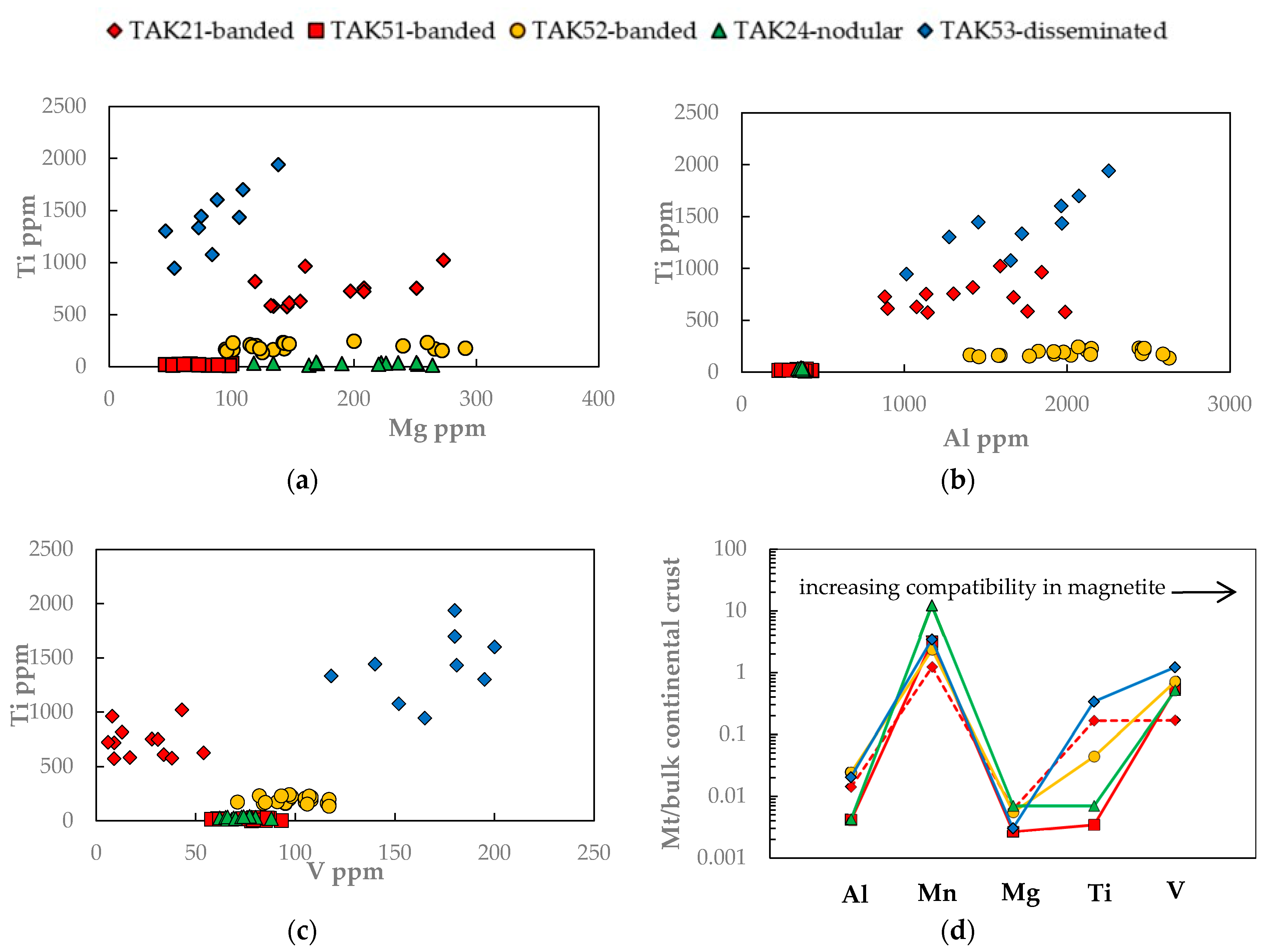
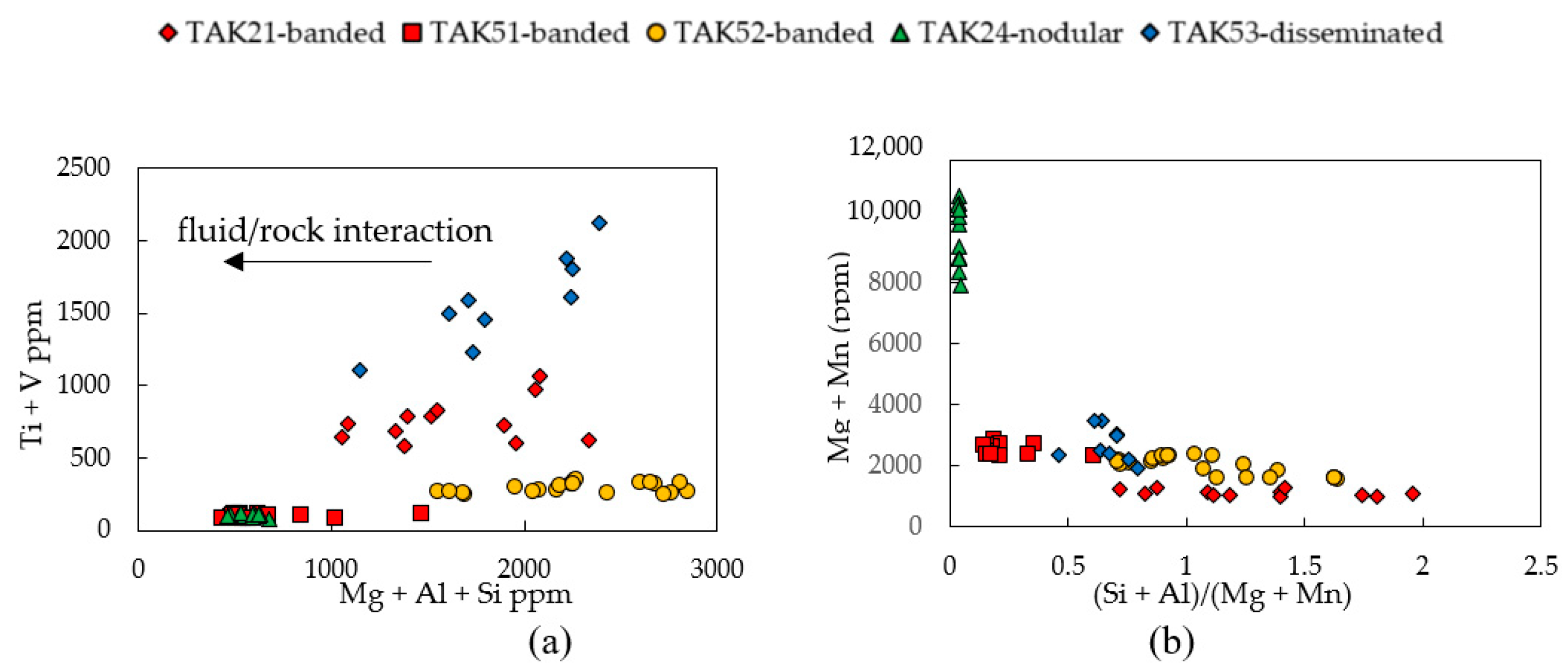
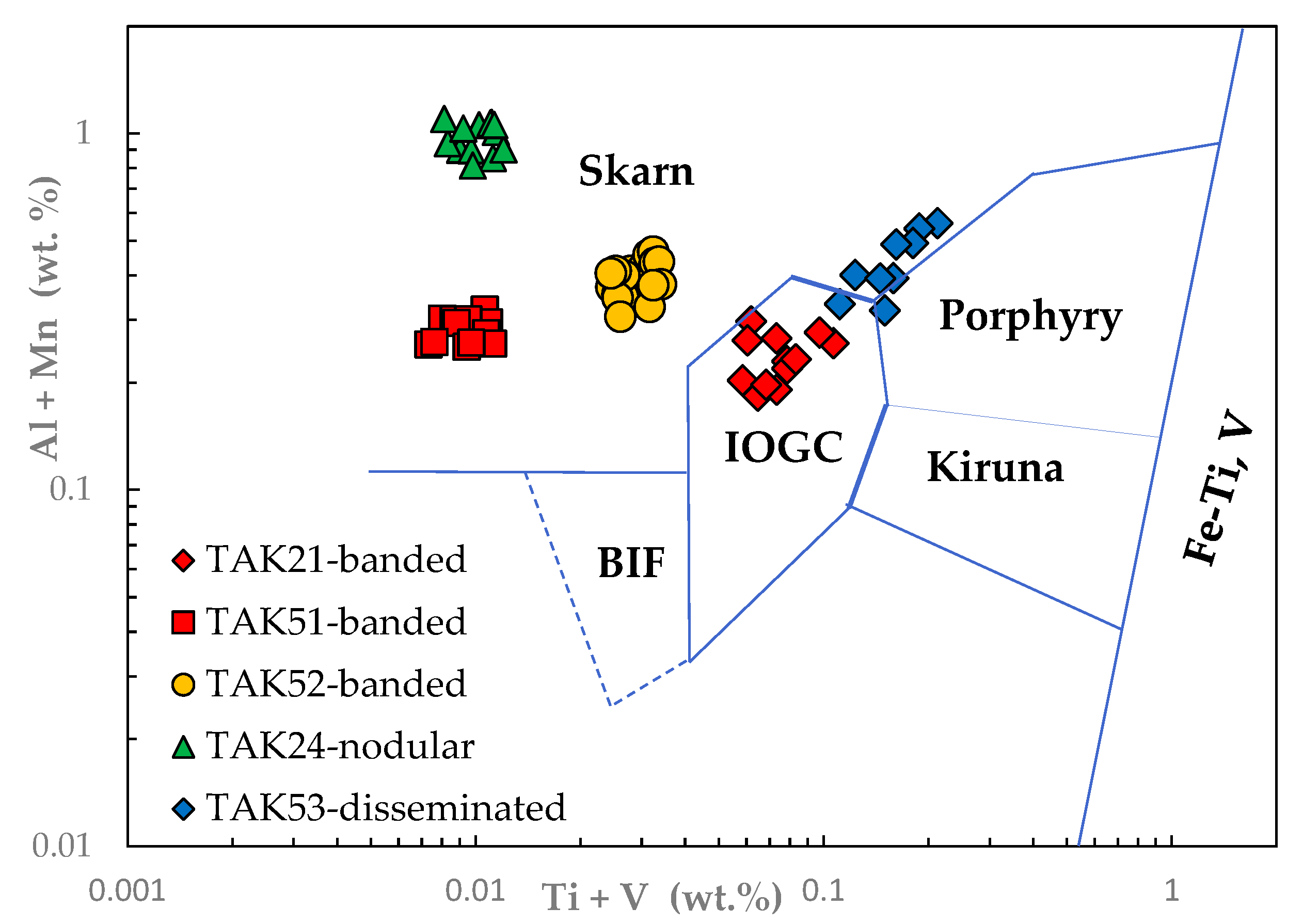
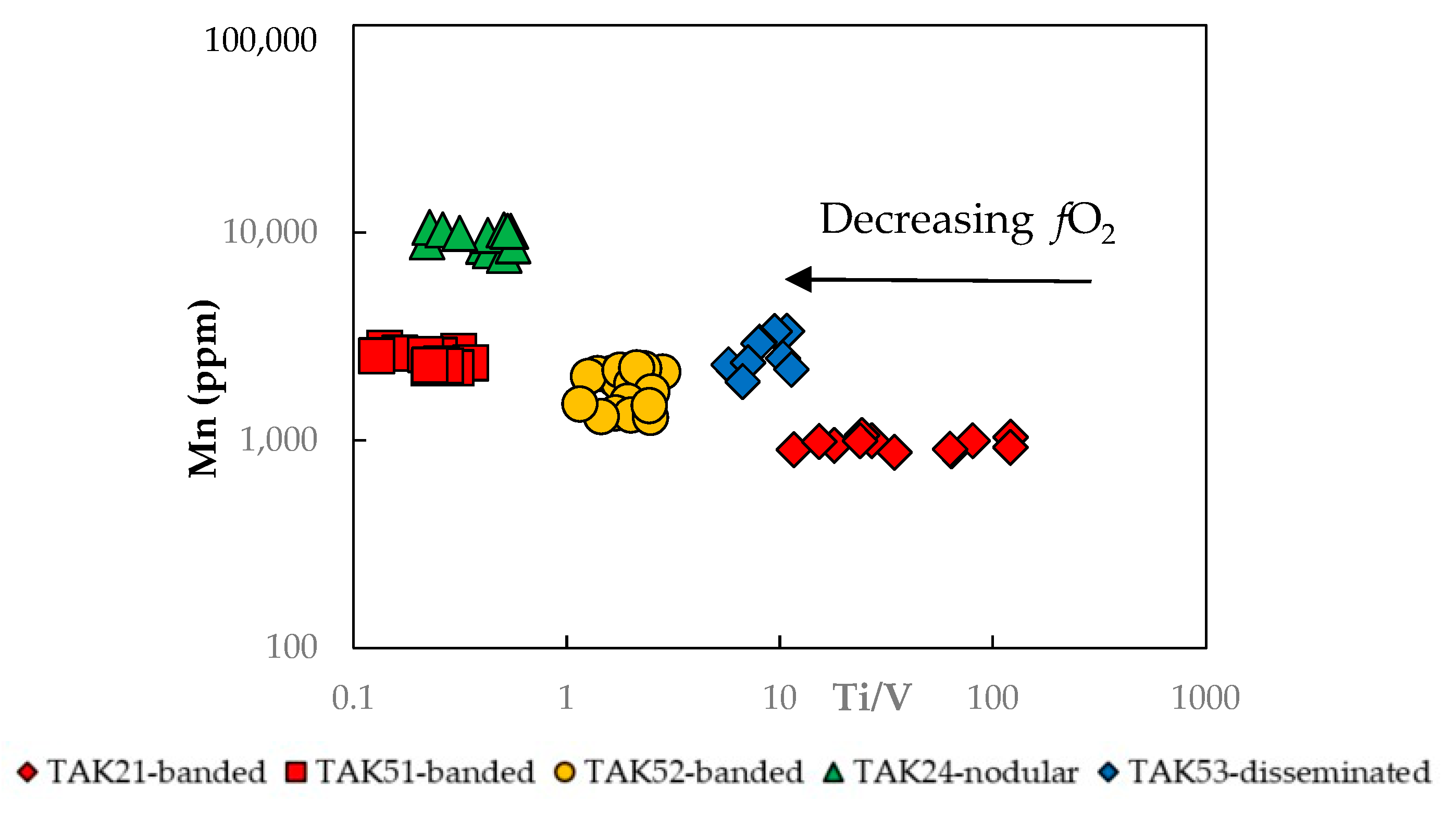
3.1. Magnetite Trace Elements
3.2. Rare Earth Element Compositions of Bulk Ore Sample
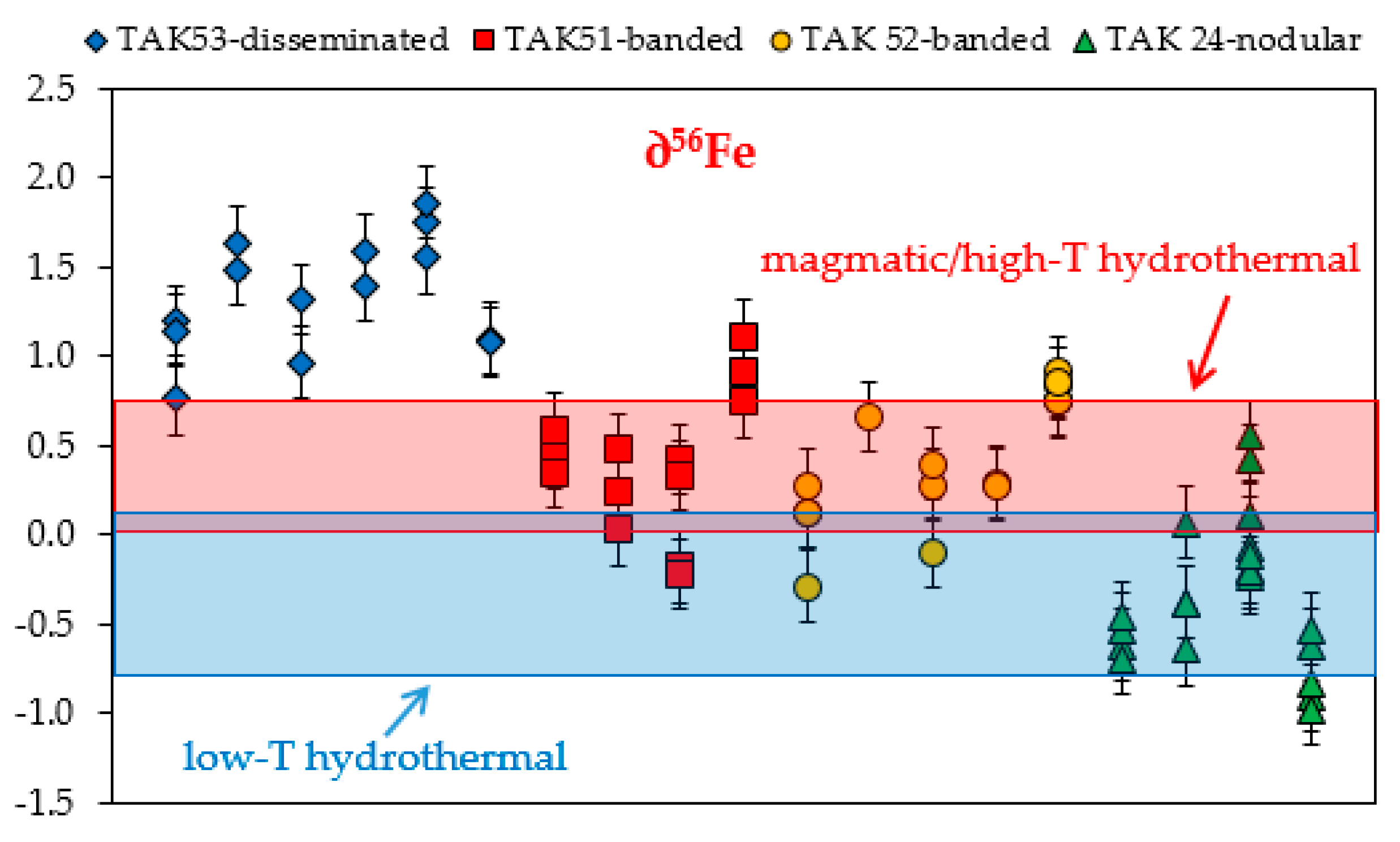
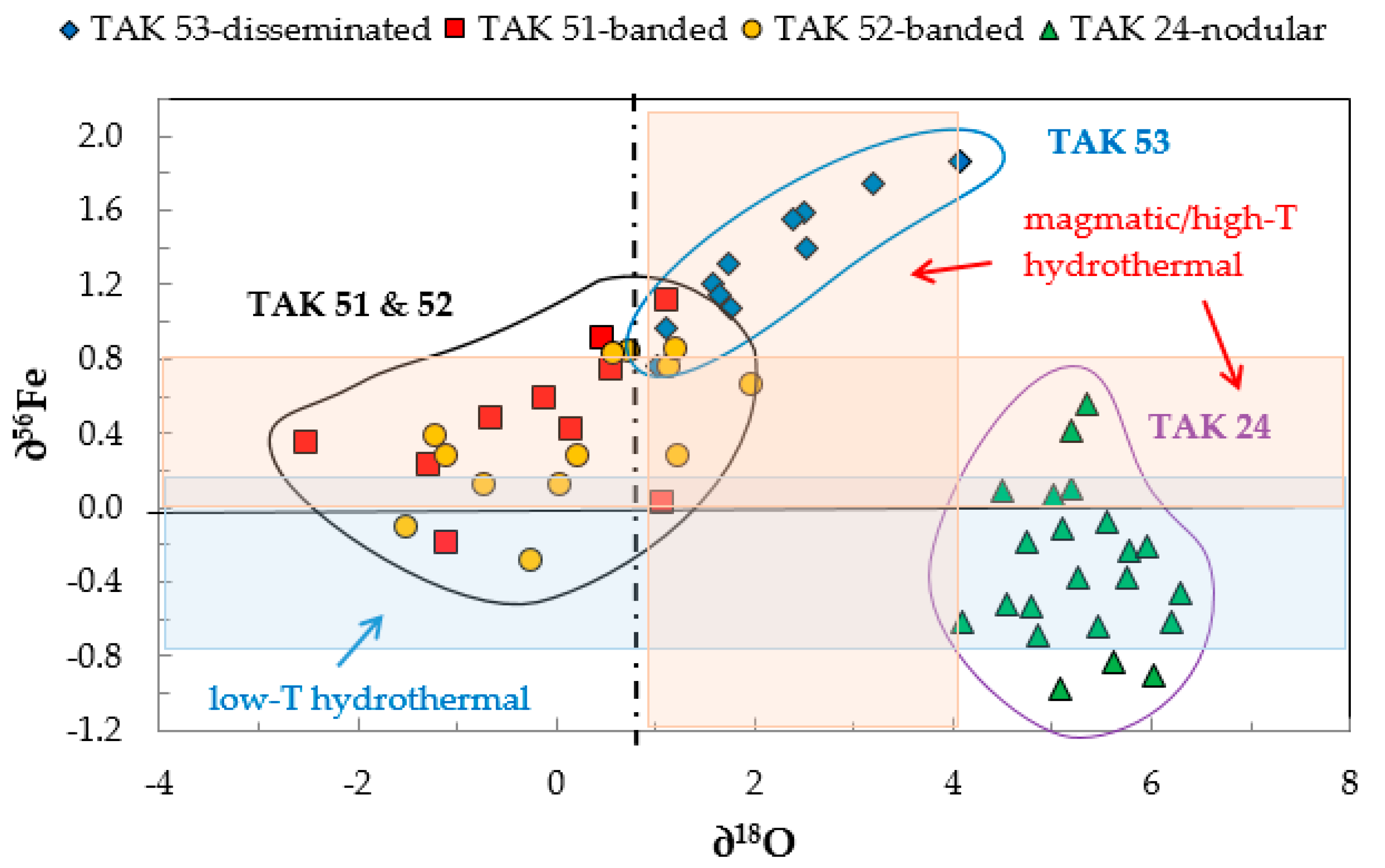
3.3. Fe Isotope Data
3.4. Oxygen Isotope Data
4. Discussion
4.1. Magnetite Composition and Ore-Forming Conditions
4.2. Magnetite Fe and O Isotope Signature
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dupuis, C.; Beaudouin, G. Discriminant diagrams for iron oxide trace element finger printing of mineral deposit types. Miner. Depos. 2011, 46, 319–335. [Google Scholar] [CrossRef]
- Childress, T.M.; Simon, A.C.; Day, W.C.; Lundstrom, C.C.; Bindeman, H.N. Iron and oxygen isotope signatures of the Pea Ridge and Pilot Knob magnetite-apatite deposits, Southeast Missouri, USA. Econ. Geol. 2016, 111, 2033–2044. [Google Scholar] [CrossRef]
- Dare, S.A.S.; Barnes, S.-J.; Beaudoin, G.; Méric, J.; Boutroy, E.; Potvin-Doucet, C. Trace elements in magnetite as petrogenetic indicators. Miner. Depos. 2014, 49, 785–796. [Google Scholar] [CrossRef]
- Nadoll, P.; Angerer, T.; Mauk, J.L.; French, D.; Walshe, J. The chemistry of hydrothermal magnetite: A review. Ore Geol. Rev. 2014, 16, 1–32. [Google Scholar] [CrossRef]
- Li, W.; Huberty, J.M.; Beard, B.L.; Kita, N.T.; Valley, J.W.; Johnson, C.M. Contrasting behavior of oxygen and iron isotopes in banded iron formations revealed by in situ isotopic analysis. Earth Planet. Sci. Lett. 2013, 50, 132–143. [Google Scholar] [CrossRef]
- Bilenker, L.D.; Simon, A.C.; Reich, M.; Lundstrom, C.C.; Gajos, N.; Bindeman, I.; Barra, F.; Munizaga, R. Fe–O stable isotope pairs elucidate a high-temperature origin of Chilean iron oxide-apatite deposits. Geochim. Cosmochim. Acta 2016, 177, 94–104. [Google Scholar] [CrossRef]
- Troll, V.R.; Weis, F.A.; Jonsson, E.; Andersson, U.B.; Majidi, S.A.; Högdahl, K.; Harris, C.; Millet, M.-A.; Chinnasamy, S.S.; Kooijman, E.; et al. Global Fe–O isotope correlation reveals magmatic origin of Kiruna-type apatite-iron-oxide ores. Nat. Commun. 2019, 10, 1712. [Google Scholar] [CrossRef]
- Zhou, Z.-J.; Tang, H.-S.; Chen, Y.-J.; Chen, Z.-L. Trace elements of magnetite and iron isotopes of the Zankan iron deposit, westernmost Kunlun, China: A case study of seafloor hydrothermal deposits. Ore Geol. Rev. 2017, 80, 1191–1205. [Google Scholar] [CrossRef]
- Foster, H.; Jafarzadeh, A. The Bafq mining district in Central Iran—A highly mineralized Infracambrian volcanic field. Econ. Geol. 1994, 89, 1697–1721. [Google Scholar] [CrossRef]
- Mazaheri, S.A.; Andrew, A.S.; Chenhall, B.E. Petrological Studies of Sangan Iron Ore Deposit; Center for Isotope Studies Research Report: Sydney, NSW, Australia, 1994; pp. 48–52. [Google Scholar]
- Daliran, F. Kiruna-Type Iron Oxide-Apatite Ores and Apatitites of the Bafq District, Iran, with an Emphasis on the REE Geochemistry of Their Apatites. In Hydrothermal Iron Oxide Copper Gold and Related Deposits. A Global Perspective; Porter, T.M., Ed.; PGC Publishing: Adelaide, SA, Australia, 2002; Volume 2, pp. 303–320. [Google Scholar]
- Daliran, F.; Stosch, H.G.; Williams, P.; Jamli, H.; Dorri, M.B. Lower Cambrian Iron Oxide-Apatite-REE (U) Deposits of the Bafq District, East-Central Iran. In Exploring for Iron Oxide Copper-Gold Deposits: Canada and Global Analogues; Corriveau, L., Mumin, H., Eds.; Geological Association of Canada Short Course Notes: St. John’s, NL, Canada, 2010; Volume 20, pp. 143–155. [Google Scholar]
- Nabatian, G.; Rastad, E.; Neubauer, F.; Honarmand, M.; Ghaderi, M. Iron and Fe-Mn mineralisation in Iran: Implications for Tethyan metallogeny. Aust. J. Earth Sci. 2015, 62, 211–241. [Google Scholar] [CrossRef]
- Nabatian, G.; Li, X.-H.; Honarmand, M.; Melgarejo, J.C. Geology, mineralogy and evolution of iron skarn deposits in the Zanjan district, NW Iran: Constraints from U-Pb dating, Hf and O isotope analyses of zircons and stable isotope geochemistry. Ore Geol. Rev. 2017, 84, 42–66. [Google Scholar] [CrossRef]
- Alavi, M. Sedimentary and structural characteristics of the Paleo-Tethys remnants in northeastern Iran. Geol. Soc. Am. Bull. 1991, 103, 983–992. [Google Scholar] [CrossRef]
- Jafari, A.; Karimpour, M.H.; Mazaheri, S.A.; Shafaroudi, A.M.; Ren, M. Geochemistry of metamorphic rocks and mineralization in the Gol-Gohar iron ore deposit (No. 1), Sirjan, SE Iran: Implications for Paleotectonic setting and ore genesis. J. Geochem. Explor. 2019, 205, 106330. [Google Scholar] [CrossRef]
- Berberian, M.; King, G.C.P. Towards a paleogeography and tectonic evolution of Iran. Can. J. Earth Sci. 1981, 18, 210–265. [Google Scholar] [CrossRef]
- Agard, P.; Omrani, J.; Jolivet, L.; Mouthereau, F. Convergence history across Zagros (Iran): Constraints from collisional and earlier deformation. Int. J. Earth Sci. 2005, 94, 401–419. [Google Scholar] [CrossRef]
- Sarjoughian, F.; Habibi, I.; Lentz, D.R.; Azizi, H.; Esna-Ashari, A. Magnetite compositions from the Baba Ali iron deposit in the Sanandaj-Sirjan zone, western Iran: Implications for ore genesis. Ore Geol. Rev. 2020, 126, 103728. [Google Scholar] [CrossRef]
- Nabatian, G.; Ghaderi, M.; Daliran, F.; Rashidnejad-Omran, N. Sorkhe-Dizaj iron oxide-apatite ore deposit in the Cenozoic Alborz-Azarbaijan magmatic belt, NW Iran. Resour. Geol. 2013, 63, 42–56. [Google Scholar] [CrossRef]
- Orberger, B.; Miska, S.; Tudryn, A.; Wagner, C.; Fialin, M.; Boudouma, O.; Derré, C.; Nabatian, G.; Hornamand, M.; Moussef, I.; et al. Iron-Oxide Mineralogy of Banded Iron Formations in the Takab Region, North Western Iran. In Proceedings of the 14th SGA Biannual Meeting, Québec, QC, Canada, 20–23 August 2017. [Google Scholar]
- Orberger, B.; Wagner, C.; Boudouma, O.; Villeneuve, J.; Deloule, E.; Fialin, M.; Rividi, N.; Nabatian, G.; Hornamand, M.; Moussef, I.; et al. Mineralogy and Fe-Isotopic Composition of Iron-Oxide Ore Takab Region, North Western Iran. In Proceedings of the 22nd International Mineralogical Association General Meeting, Melbourne, VIC, Australia, 13–17 August 2018. [Google Scholar]
- Orberger, B.; Wagner, C.; Boudouma, O.; Derré, C.; Fialin, M.; Rividi, N.; Villeneuve, J.; Deloule, E.; Nabatian, G.; Hornamand, M.; et al. Iron-Oxide Ores in the Takab Region, North Western Iran. In Proceedings of the 15th SGA Biannual Meeting, Glasgow, UK, 27–30 August 2019. [Google Scholar]
- Honarmand, M.; Nabatian, N.; Wagner, C.; Monsef, I.; Delpech, G.; Bayon, G.; Orberger, B. Late Ediacaran iron formation, NW Iran: Origin, depositional age and tectonic/climate significance. Precambrian Res. 2023; to be submitted. [Google Scholar]
- Moazzen, M.; Hajialioggli, R. Zircon SHRIMP Dating of Mafic Migmatites from NW Iran: Reporting the Oldest Rocks from the Iranian Crust. In Proceedings of the 5th Annual Meeting AOGS, Busan, Republic of Korea, 16–20 June 2008. [Google Scholar]
- Hassanzadeh, J.; Stockli, D.F.; Horton, B.K.; Axen, G.J.; Stockli, L.D.; Grove, M.; Schmitt, A.K.; Walker, J.D. U-Pb zircon geochronology of late Neoproterozoic-early Cambrian granitoids in Iran: Implications for paleogeography, magmatism, and exhumation history of Iranian basement. Tectonophysics 2008, 451, 71–96. [Google Scholar] [CrossRef]
- Marin-Carbonne, J.; Rollion-Bard, C.; Luais, B. In-situ measurements of iron isotopes by SIMS: MC-ICP-MS intercalibration and application to magnetite crystal from the Gunflint chert. Chem. Geol. 2011, 285, 50–61. [Google Scholar] [CrossRef]
- Valley, J.W.; Graham, C.M. Ion microprobe analysis of oxygen isotope ratios in granulite facies magnetites: Diffusive exchange as a guide to cooling history. Contrib. Mineral. Petrol. 1991, 109, 38–52. [Google Scholar] [CrossRef]
- Marin, J.; Chaussidon, M.; Robert, F. Microscale oxygen isotope variations in 1.9 Ga Guntflint cherts: Assessments of diagenesis effects and implications for oceanic paleotemperature reconstructions. Geochim. Cosmochim. Acta 2010, 74, 116–130. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. The Crust; Rudnick, R.L., Ed.; Elsevier Ltd.: Oxford, UK, 2005; pp. 1–64. [Google Scholar]
- McLennan, S.M. Rare earth elements in sedimentary rocks: Influence and of provenance and sedimentary processes. Rev. Mineral. 1989, 21, 169–200. [Google Scholar]
- Günther, T.; Klemd, R.; Zhang, X.; Horn, I.; Weyer, S. In-situ trace element and Fe-isotope studies on magnetite of the volcanic-hosted Zhibo and Chagangnuoer iron ore deposits in the Western Tianshan, NW China. Chem. Geol. 2017, 453, 11–127. [Google Scholar] [CrossRef]
- Taylor, H.P. The oxygen isotope geochemistry of igneous rocks. Contrib. Mineral. Petrol. 1968, 19, 1–71. [Google Scholar] [CrossRef]
- Jonsson, E.; Troll, V.R.; Högdahl, K.; Harris, C.; Weis, F.; Nilsson, K.P.; Skelton, A. Magmatic origin of giant “Kiruna-type” apatite-iron-oxide ores in Central Sweden. Sci. Rep. 2013, 3, 1644. [Google Scholar] [CrossRef]
- Hu, H.; Li, J.W.; Lentz, D.; Ren, Z.; Zhao, X.F.; Deng, X.D.; Hall, D. Dissolution-re precipitation process of magnetite from the Chengchao iron deposit: Insights into ore genesis and implication for in-situ chemical analysis of magnetite. Ore Geol. Rev. 2014, 57, 393–405. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, Y.; Zhou, T.; Xiao, X.; White, N.C.; Thompson, J.; Hong, H.; Zhang, L. Geochemical characteristics of magnetite in Longqiao skarn iron deposit in the Middle-Lower Yangtze Metallogenic Belt, Eastern China. Mineral. Depos. 2019, 54, 1229–1242. [Google Scholar] [CrossRef]
- Huang, X.W.; Beaudoin, G. Textures and chemical compositions of magnetite from iron oxide copper-gold (IOCG) and Kiruna-type iron oxide-apatite deposits and their implications for ore genesis and magnetite classification schemes. Econ. Geol. 2019, 114, 953–979. [Google Scholar] [CrossRef]
- Nielsen, R.L.; Forsythe, L.M.; Gallahan, W.E.; Fisk, M.R. Major- and trace-element magnetite-melt equilibria. Chem. Geol. 1994, 117, 167–191. [Google Scholar] [CrossRef]
- Toplis, M.J.; Caroll, M.R. An experimental study of oxygen fugacity on Fe-Ti oxide stability, phase relations, and mineral-melt equilibria in ferro-basaltic systems. J. Petrol. 1995, 36, 1137–1170. [Google Scholar] [CrossRef]
- Deditius, A.P.; Reich, M.; Simon, A.C.; Suvorova, A.; Knipping, J.; Roberts, M.P.; Rubanov, S.; Dodd, A.; Saunders, M. Nanogeochemistry of hydrothermal magnetite. Contrib. Mineral. Petrol. 2018, 173, 46. [Google Scholar] [CrossRef]
- Mathur, R. Personal Communication; Juniata College: Huntingdon, PA, USA, 2023. [Google Scholar]
- Emproto, C.; Mathur, R.; Simon, A.; Bindeman, I.; Godfrey, L.; Dhnaram, C.; Lisitsin, V. Integrated O, Fe, and Ti isotopic analysis elucidates multiple metal and fluid sources for magnetite from the Ernest Henry Iron oxide copper (IOCG) Deposit, Queensland, Australia. Ore Geol. Rev. 2022, 150, 105170. [Google Scholar] [CrossRef]
- Hill, P.S.; Schauble, E.A. Modeling the effects of bond environment on equilibrium iron isotope fractionation in ferric aquo-chloro complexes. Geochim. Cosmochim. Acta 2008, 72, 1938–1958. [Google Scholar] [CrossRef]
- Bilenker, L.D.; Simon, A.; Lundstrom, C.C.; Gajos, N.; Zajacz, Z. Experimental Constraints on Fe Isotope Fractionation in Fluid-melt-Oxide-Sulfide Assemblages. In Proceedings of the 23rd Goldschmidt Conference, Florence, Italy, 25–30 August 2013. [Google Scholar]
- Bilenker, L.D. Elucidating Igneous and Ore-Forming Processes by using Fe Isotopes through Experimental and Field-Based Methods. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, New Brunswick, NJ, USA, 2015. [Google Scholar]
- Tunnell, B.N.; Locmelis, M.; Seeger, C.; Moroni, M.; Dare, S.; Mathur, R.; Sullivan, B. The Sheperd Mountain iron ore deposit in Southeast Missouri, USA—An extension of the Pilot Knob magmatic-hydrothermal ore system: Evidence for iron ore chemistry. Ore Geol. Rev. 2022, 141, 104633. [Google Scholar] [CrossRef]
- Rodriguez-Mustafa, M.A.; Simon, A.C.; Del Real, I.; Thompson, J.F.H.; Bilenker, L.D.; Barra, F.; Bindeman, I.; Cadwell, D.A. Continuum from Iron Oxide Copper-Gold to Iron Oxide-Apatite Deposits: Evidence from Fe and O Stable Isotopes and Trace Element Chemistry of Magnetite. Econ. Geol. 2020, 115, 1443–1459. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, J.; Bagas, D.; Zhai, D.; Meng, G.; Verrall, M. New insights into the genesis of IOGC deposits: From a case study of the Yinachangd eposit in SW China. Ore Geol. Rev. 2020, 124, 103664. [Google Scholar] [CrossRef]
- Peters, S.T.M.; Alibabais, N.; Pack, A.; MsKibbin, S.J.; Raeisi, D.; Nayebi, N.; Torab, F.; Lehmann, B. Triple oxygen isotope variations in magnetite from iron-oxide deposits, central Iran, record magmatic fluid interaction with evaporite and carbonate host rocks. Geology 2019, 48, 211–215. [Google Scholar] [CrossRef]
- Frost, C.D.; Von Blankenburg, F.; Schoenberg, R.; Frost, B.R.; Swapp, S.M. Preservation of Fe heterogeneities during diagenesis and metamorphism of banded iron formation. Contrib. Mineral. Petrol. 2007, 153, 211–235. [Google Scholar] [CrossRef]

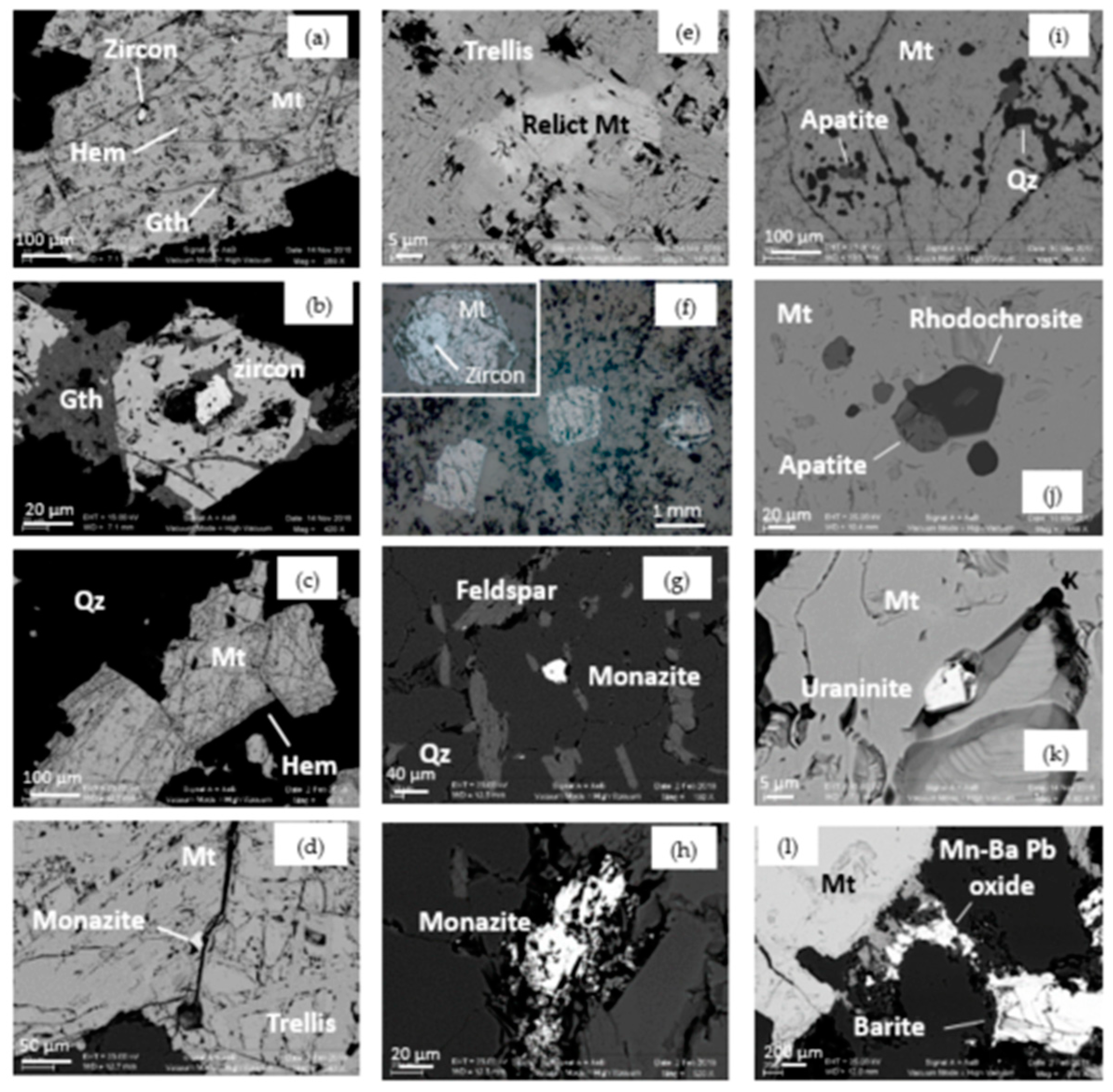



| Sample | Iron Ore Type | Magnetite | Fe-Oxide | Goethite |
|---|---|---|---|---|
| Hematite | ||||
| TAK21 | banded | x | late | surrounding & in veins in Mt |
| discontinuous | ||||
| TAK22 | banded | x | - | surrounding & in veins in Mt |
| discontinuous | ||||
| TAK23 | banded | trellis (martite) | late | interstitial to Mt |
| flow structure | with relic Mt | include minute hematite | ||
| TAK51 | banded | trellis (martite) | late | - |
| in large crystals | ||||
| TAK52 | banded | x | late in rim ± crack | - |
| abundant inclusions | ||||
| TAK24 | nodular | of apatite and Qz | - | - |
| in some crystals | ||||
| TAK53 | disseminated | x | late in rim ± crack | - |
| Sample | Uraninite | Frequent Accessories | Monazite | Zircon | Rare Accessories |
|---|---|---|---|---|---|
| Barite | |||||
| TAK21 | in goethite & Mt | x | - | in Mt | chalcopyrite |
| TAK22 | - | in matrix | - | in Mt & Qz | scheelite |
| TAK23 | - | - | x | in Mt | Mo-Se-(Pb) sulfide in Mt |
| TAK51 | - | in matrix | - | - | |
| TAK52 | x | very small (<1 µm) | small, fresh in Qz & Mt | in Mt | Mo sulfide in Qz |
| inclusions in feldspar | xenotime | ||||
| - | Mn-Ba-Pb oxides in Mt; PbS in Qz | ||||
| TAK24 | in Mt | in matrix | in Mt | inclusions of rhodochrosite | |
| and Cl-apatite in Mt | |||||
| TAK53 | very small inclusions | very small inclusions | abundant, altered | abundant | ilmenite, rutile, +Ti-Mt in altered Mt |
| in feldspar | in feldspar | small, fresh in Qz & Mt | in Mt & Qz |
| Sample | TAK 21 | TAK 51 | TAK 52 | TAK 24 | TAK 53 | |
|---|---|---|---|---|---|---|
| Ore Type | Banded | Banded | Banded | Nodular | Disseminated | |
| n Analyses | n = 12 | n = 12 | n = 21 | n = 12 | n = 9 | |
| Average | 178 | 76 | 158 | 201 | 86 | |
| Mg | SD | 47 | 20 | 66 | 47 | 29 |
| DL = 7 | Range | 145–251 | 46–100 | 95–291 | 118–264 | 46–138 |
| Average | 1390 | 353 | 2069 | 363 | 1704 | |
| Al | SD | 360 | 67 | 378 | 14 | 404 |
| DL = 6 | Range | 878–1986 | 227–431 | 1402–2588 | 342–386 | 1011–2254 |
| Average | 69 | 340 | 41 | 160 | ||
| Si | SD | 74 | 339 | 36 | nc | 89 |
| DL = 8 | Range | 9–218 | 0–1071 | 0–99 | 0–27 | 0–290 |
| Average | 18 | 18 | 19 | 3 | 16 | |
| Ca | SD | 18 | 9 | 23 | 4 | 6 |
| DL = 4 | Range | 9–218 | 10–39 | 0–71 | 0–13 | 6–24 |
| Average | 728 | 15 | 188 | 30 | 1420 | |
| Ti | SD | 142 | 9 | 32 | 9 | 305 |
| DL = 10 | Range | 575–1022 | 0–28 | 148–243 | 15–43 | 945–1940 |
| Average | 24 | 77 | 100 | 72 | 168 | |
| V | SD | 16 | 11 | 13 | 7 | 27 |
| DL = 9 | Range | 1–54 | 58–93 | 82–117 | 62–88 | 118–200 |
| Average | 962 | 2465 | 1838 | 9423 | 2658 | |
| Mn | SD | 58 | 190 | 344 | 942 | 516 |
| DL = 10 | Range | 893–1059 | 2240–2779 | 1293–2236 | 7809–10,609 | 1923–3360 |
| Average | nc | 10 | 12 | 9 | 15 | |
| Ni | SD | 0–17 | 20 | 10 | 11 | 10 |
| DL = 12 | Range | 23 | 0–70 | 0–30 | 0–33 | 4–37 |
| Average | 23 | 26 | 30 | 9 | 10 | |
| Cr | SD | 11 | 25 | 11 | 6 | 7 |
| DL = 8 | Range | 16–46 | 0–98 | 15–53 | 0–20 | 0–20 |
| Average | 17 | 6 | 16 | 5 | 14 | |
| Cu | SD | 15 | 12 | 12 | 8 | 11 |
| DL = 15 | Range | 0–49 | 0–40 | 0–40 | 0–22 | 0–27 |
| Element | TAK 21 | TAK 51 | TAK 52 | TAK 24 | TAK 53 | TAK 12 |
|---|---|---|---|---|---|---|
| Banded | Banded | Banded | Nodular | Disseminated | Calcschist | |
| La | 0.083 | 0.021 | 0.044 | 0.144 | 0.384 | 0.283 |
| Ce | 0.089 | 0.012 | 0.038 | 0.046 | 0.382 | 0.087 |
| Pr | 0.108 | 0.014 | 0.040 | 0.101 | 0.381 | 0.218 |
| Nd | 0.108 | 0.014 | 0.038 | 0.111 | 0.349 | 0.214 |
| Sm | 0.144 | 0.033 | 0.072 | 0.169 | 0.401 | 0.241 |
| Eu | 0.752 | 0.051 | 0.175 | 0.531 | 0.495 | 0.256 |
| Gd | 0.138 | 0.039 | 0.072 | 0.193 | 0.251 | 0.227 |
| Tb | 0.118 | 0.040 | 0.074 | 0.140 | 0.213 | 0.203 |
| Dy | 0.108 | 0.045 | 0.073 | 0.117 | 0.192 | 0.196 |
| Y | 2.51 | 1.09 | 2.49 | 0.046 | 5.19 | 7.00 |
| Ho | 0.109 | 0.044 | 0.072 | 0.101 | 0.191 | 0.200 |
| Er | 0.110 | 0.044 | 0.072 | 0.111 | 0.202 | 0.182 |
| Tm | 0.128 | 0.047 | 0.062 | 0.169 | 0.257 | 0.190 |
| Yb | 0.130 | 0.046 | 0.062 | 0.531 | 0.310 | 0.169 |
| Lu | 0.143 | 0.042 | 0.053 | 0.193 | 0.337 | 0.159 |
| Sample | Ore Type | ∂56Fe(‰) | 2σ | ∂18O(‰) | 2σ | |
|---|---|---|---|---|---|---|
| Average | 0.41 | 0.39 | –0.24 | 1.15 | ||
| TAK 51 | banded | Range | −0.22 to +1.12 | −2.52 to +1.10 | ||
| n analyses | 13 | 10 | ||||
| Average | 0.41 | 0.37 | 0.17 | 1.09 | ||
| TAK 52 | banded | Range | −0.28 to +0.92 | −1.52 to +1.95 | ||
| n analyses | 15 | 13 | ||||
| Average | –0.32 | 0.41 | 5.30 | 0.57 | ||
| TAK 24 | nodular | Range | −0.98 to +0.56 | +4.10 to +6.30 | ||
| n analyses | 22 | 23 | ||||
| Average | 1.35 | 0.32 | 2.15 | 0.87 | ||
| TAK 53 | disseminated | Range | +0.76 to +1.86 | +1.02 to +4.07 | ||
| n analyses | 14 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, C.; Villeneuve, J.; Boudouma, O.; Rividi, N.; Orberger, B.; Nabatian, G.; Honarmand, M.; Monsef, I. In Situ Trace Element and Fe-O Isotope Studies on Magnetite of the Iron-Oxide Ores from the Takab Region, North Western Iran: Implications for Ore Genesis. Minerals 2023, 13, 774. https://doi.org/10.3390/min13060774
Wagner C, Villeneuve J, Boudouma O, Rividi N, Orberger B, Nabatian G, Honarmand M, Monsef I. In Situ Trace Element and Fe-O Isotope Studies on Magnetite of the Iron-Oxide Ores from the Takab Region, North Western Iran: Implications for Ore Genesis. Minerals. 2023; 13(6):774. https://doi.org/10.3390/min13060774
Chicago/Turabian StyleWagner, Christiane, Johan Villeneuve, Omar Boudouma, Nicolas Rividi, Beate Orberger, Ghasem Nabatian, Maryam Honarmand, and Iman Monsef. 2023. "In Situ Trace Element and Fe-O Isotope Studies on Magnetite of the Iron-Oxide Ores from the Takab Region, North Western Iran: Implications for Ore Genesis" Minerals 13, no. 6: 774. https://doi.org/10.3390/min13060774
APA StyleWagner, C., Villeneuve, J., Boudouma, O., Rividi, N., Orberger, B., Nabatian, G., Honarmand, M., & Monsef, I. (2023). In Situ Trace Element and Fe-O Isotope Studies on Magnetite of the Iron-Oxide Ores from the Takab Region, North Western Iran: Implications for Ore Genesis. Minerals, 13(6), 774. https://doi.org/10.3390/min13060774






