Phosphate Petrochronology of the Belcina REE Mineralization (Ditrău Alkaline Massif, Romania)
Abstract
1. Introduction


2. Geological Background
Overview
- ₋
- Ditrău suite, comprising syenites to monzo-syenites (equivalent to the ‘Ditrău syenite’ of [5]) dated at 230.7 ± 0.2 Ma;
- ₋
- ₋
3. Analytical Methods
3.1. Optical Microscopy
3.2. Backscattered Electron Imaging (BEI)
3.3. EMP Mineral Analyses
3.4. EMP (U+Th)-Total Pb Dating
4. Results
4.1. Sampling and Petrography
4.2. Phosphate Morphology and Zonation
4.2.1. Monazite-(Ce)
- ₋
- Type 1 monazite-(Ce) (mnz1) is rock-forming making up ca. 20 vol-% of the rock. It either forms xenomorphic to hypidiomorphic aggregated mineral clusters of ca. 1 cm size or independent 100 µm–500 µm sized crystals. It also is found as (relict) cores in type 2 monazite-(Ce) (Figure 4a,b,d and Figure 5a).
- ₋
- ₋
- Type 3 monazite-(Ce) (mnz3) is mostly found as inclusions in apatite or sometimes bordering apatite, tens of microns in size, and randomly oriented (Figure 4b,c and Figure 5a). It is xenomorphic to hypidiomorphic in shape. Grain boundaries of mnz3 with the host apatite are either straight and crystallographically oriented or lobate.
4.2.2. Xenotime-(Y)
- ₋
- Type 1 xenotime-(Y) (xnt1) is only present as distinct domains in the cores of type 2 xenotime-(Y) (Figure 6a). It can only be identified by mineral chemical means and not texturally (see below).
- ₋
- Type 2 xenotime-(Y) (xnt2) forms a characteristic mineral assemblage with mnz1 within which it forms skeletal inclusions of >100 µm size (Figure 4a,d and Figure 6a). Xnt2 is never found as independent crystals. Grain boundaries of the xenotime-(Y) with the host monazite-(Ce) are either straight and crystallographically oriented or lobate. Xnt2 does not show any preferred orientation within the host but is often related to fractures in mnz1.
- ₋
- Type 3 xenotime-(Y) (xnt3), is tens of microns in size, randomly oriented, mainly found as inclusions in apatite, seldomly in xnt2 (Figure 4b,c and Figure 6a). It is xenomorphic to hypidiomorphic in shape. Grain boundaries of xnt3 with the host apatite are either straight and crystallographically oriented or lobate. In appearance, xnt3 is identical to mnz3.
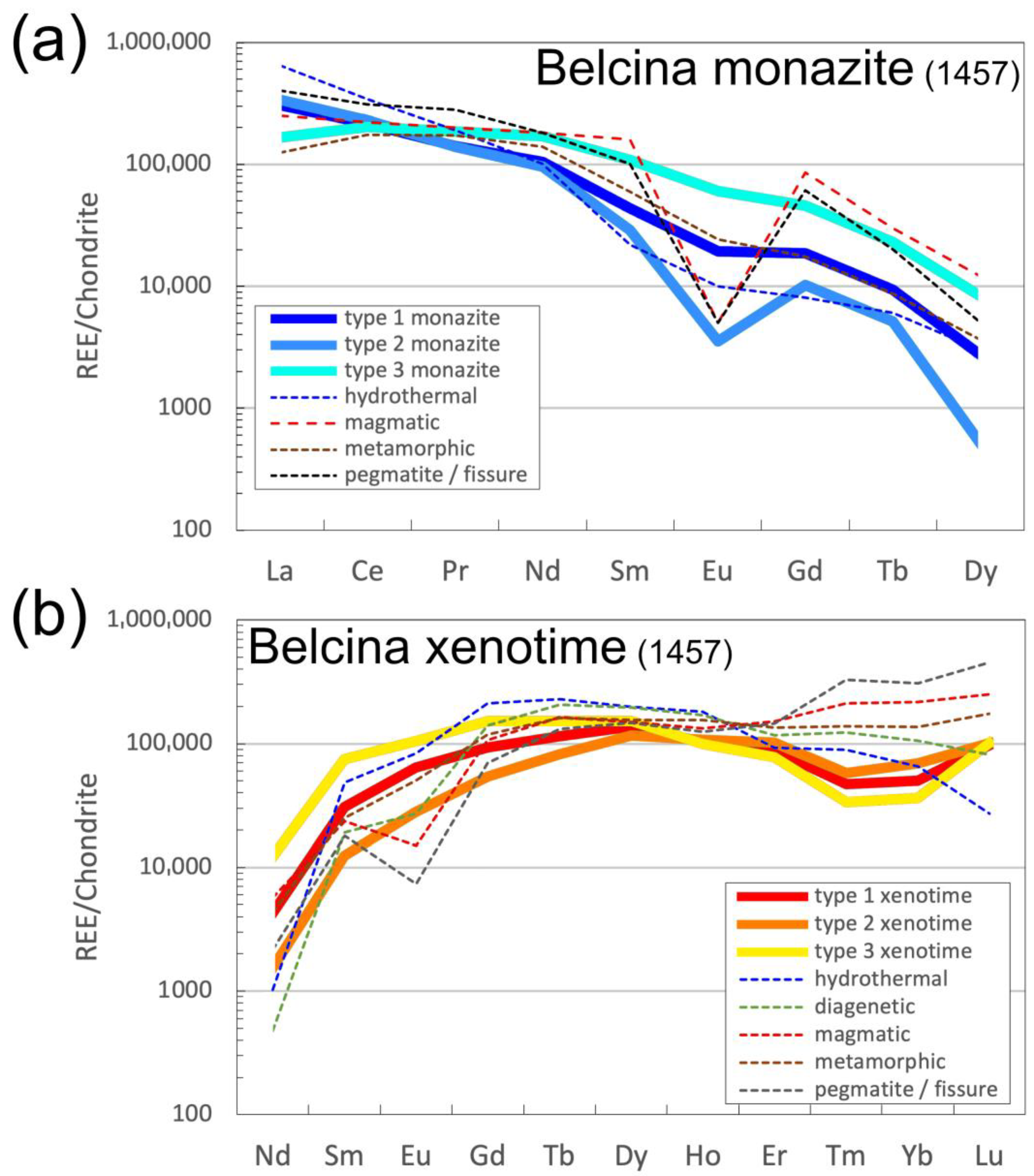
4.3. Phosphate Mineral Chemistry
4.3.1. Monazite-(Ce)
- ₋
- Trend 1 shows a relative actinide enrichment at constant xenotime-(Y) component;
- ₋
- Trend 2 shows enrichment in the xenotime-(Y) component at constants actinides.
4.3.2. Xenotime-(Y)
4.4. EMP (U+Th)-Total Pb Ages
4.4.1. Monazite-(Ce)
4.4.2. Xenotime-(Y)
5. Discussion
5.1. Phosphate Textures and Chemistry
5.1.1. Common Features
5.1.2. Monazite-(Ce) Chemistry
5.1.3. Xenotime-(Y) Chemistry
- ₋
- Yb(pg) = (Ho2O3 + Er2O3 + Yb2O3 + Lu2O3 + Nd2O3): All Dim1 positive and Dim2 negative with the exception of Nd which in Dim1 negative. Lu is not significant.
- ₋
- Gd(pg) = (Gd2O3 + Tb2O3 + Dy2O3 + Eu2O3 + Sm2O3): All Dim1 negative and Dim2 positive with the exception of Sm which is negative in Dim2.
5.1.4. Overall Interpretation
- (a)
- xnt1 + xnt2 + mnz1
- (b)
- mnz2 + plagioclase
- (c)
- xnt3 + mnz3 + apatite + Fe-dolomite
5.2. Phosphate Ages
5.3. Geological Implications
6. Conclusions
- (a)
- xnt1 + xnt2 + mnz1
- (b)
- mnz2 + plagioclase
- (c)
- xnt3 + mnz3 + apatite + Fe-dolomite
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pál-Molnár, E.; Kiri, L.; Lukács, R.; Dunkl, I.; Batki, A.; Szemerédi, M.; Almási, E.; Sogrik, E.; Harangi, H. Timing of magmatism of the Ditrău Alkaline Massif, Romania—A review based on new U–Pb and K/Ar data. Cent. Eur. Geol. 2021, 64, 18–37. [Google Scholar] [CrossRef]
- Klötzli, U.; Burda, J.; Li, Q.L.; Liu, Y.; Jakab, G.; Ionescu, L.; Tibuleac, P. Petrochronological evidence for a three-stage magmatic evolution of nepheline syenites from the Ditrău Alkaline Massif, Romania. Minerals 2022, 12, 657. [Google Scholar] [CrossRef]
- Hîrtopanu, P.; Andersen, J.C.; Fairhurst, R. Nb, Ta, REE (Y), Ti, Zr, Th, U and Te rare element minerals within the Ditrău alkaline intrusive complex, eastern Carpathians, Romania. In Mineralogy of Székelyland, Eastern Transylvania, Romania; Csik County Nature and Conservation Society: Miercurea Ciuc, Romania, 2010; pp. 89–128. [Google Scholar]
- Hîrtopanu, P.; Andersen, J.C.; Fairhurst, R. Thorite, thorogummite and xenotime-(Y) occurrence in Ditrău alkaline intrusive massif, East Carpathians. Proc. Rom. Acad. Sci. Ser. B 2013, 15, 111–132. [Google Scholar]
- Honour, V.C.; Goodenough, K.M.; Shaw, R.A.; Gabudianu, I.; Hîrtopanu, P. REE mineralisation within the Ditrău Alkaline Complex, Romania: Interplay of magmatic and hydrothermal processes. Lithos 2018, 314, 360–381. [Google Scholar] [CrossRef]
- Jakab, G. Geneza Masivului Alcalin Ditrau; Editura Mark House: Gheorgheni, Romania, 2017; pp. 1–166. [Google Scholar]
- Jakab, G. Geologia Masivului alcalin de la Ditrău; Editura Mark House: Gheorgheni, Romania, 1998; pp. 1–95. [Google Scholar]
- Hîrtopanu, P. New Minerals and Mineral Varieties for Romania. Vergiliu 2018, 1–395. [Google Scholar]
- Săbău, G. HFSE and REE vein mineralization associated to the Ditrău Alkaline Massif. In Proceedings of the XXXII International Conference on Alkaline Magmatism of the Earth and Related Strategic Metal Deposits, Apatity, Russia, 7–14 August 2015; pp. 1–2. Available online: http://alkaline.web.ru/2015 (accessed on 2 May 2023).
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. In The Crust. Vol 3. Treaties of Geochemistry; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1–64. [Google Scholar]
- Goodenough, K.M.; Schilling, J.; Jonsson, E.; Kalvig, P.; Charles, N.; Tuduri, J.; Deady, E.A.; Sadeghi, M.; Schiellerup, H.; Müller, A.; et al. Europe’s rare earth element resource potential: An overview of REE metallogenetic provinces and their geodynamic setting. Ore Geol. Rev. 2016, 72, 838–856. [Google Scholar] [CrossRef]
- Bea, F. Controls on the trace element composition of crustal melts. Trans. R. Soc. Edinb. Earth Sci. 1996, 87, 33–41. [Google Scholar]
- Gratz, R.; Heinrich, W. Monazite-xenotime thermobarometry: Experimental calibration of the miscibility gap in the binary system CePO4-YPO4. Am. Mineral. 1997, 82, 772–780. [Google Scholar] [CrossRef]
- Spear, F.S.; Pyle, J.M. Apatite, monazite, and xenotime in metamorphic rocks. In Phosphates: Geochemical, Geobiological, and Materials Importance; Kohn, M.J., Rakovan, J., Hughes, J.M., Eds.; Mineralogical Society of America: Washington DC, USA, 2002; volume 48, pp. 293–335. [Google Scholar]
- Janots, E.; Engi, M.; Berger, A.; Allaz, J.; Schwarz, J.O.; Spandler, C. Prograde metamorphic sequence of REE minerals in pelitic rocks of the Central Alps: Implications for allanite-monazite-xenotime phase relations from 250 to 610 degrees C. J. Metamorph. Geol. 2008, 26, 509–526. [Google Scholar] [CrossRef]
- Berger, A.; Rosenberg, C.; Schaltegger, U. Stability and isotopic dating of monazite and allanite in partially molten rocks: Examples from the Central Alps. Swiss J. Geosci. 2009, 102, 15–29. [Google Scholar] [CrossRef]
- Harlov, D.E.; Wirth, R.; Hetherington, C.J. Fluid-mediated partial alteration in monazite; the role of coupled dissolution-reprecipitation in element redistribution and mass transfer. Contrib. Mineral. Petrol. 2011, 162, 329–348. [Google Scholar] [CrossRef]
- Ondrejka, M.; Putiš, M.; Uher, P.; Schmiedt, I.; Pukančík, L.; Konečný, P. Fluid-driven destabilization of REE-bearing accessory minerals in the granitic orthogneisses of North Veporic basement (Western Carpathians, Slovakia). Mineral. Petrol. 2016, 110, 561–580. [Google Scholar] [CrossRef]
- Engi, M. Petrochronology Based on REE-Minerals: Monazite, Allanite, Xenotime, Apatite. Rev. Mineral. Geochem. 2017, 83, 365–418. [Google Scholar] [CrossRef]
- Ódri, Á.; Harris, C.; Le Roux, P. The role of crustal contamination in the petrogenesis of nepheline syenite to granite magmas in the Ditrău Complex, Romania: Evidence from O-, Nd-, Sr- and Pb-isotopes. Contrib. Mineral. Petrol. 2020, 175, 100. [Google Scholar] [CrossRef]
- Höck, V.; Ionescu, C.; Balintoni, I.; Koller, F. The Eastern Carpathians “ophiolites” (Romania): Remnants of a Triassic ocean. Lithos 2009, 108, 151–171. [Google Scholar] [CrossRef]
- Balintoni, I.; Balica, C.; Ducea, M.N.; Hann, H.P. Peri-Gondwanan terranes in the Romanian Carpathians: A review of their spatial distribution, origin, provenance, and evolution. Geosci. Front. 2014, 5, 395–411. [Google Scholar] [CrossRef]
- Pál-Molnár, E.; Batki, A.; Almási, E.; Kiss, B.; Upton, B.G.; Markl, G.; Odling, N.; Harangi, S. Origin of mafic and ultramafic cumulates from the Ditrău Alkaline Massif, Romania. Lithos 2015, 239, 1–18. [Google Scholar] [CrossRef]
- Morogan, V.; Upton, B.G.J.; Fitton, J.G. The petrology of the Ditrău alkaline complex, Eastern Carpathians. Mineral. Petrology. 2000, 69, 227–265. [Google Scholar] [CrossRef]
- Fall, A.; Bodnar, R.J.; Szabó, C.; Pál-Molnár, E. Fluid evolution in the nepheline syenites of the Ditrău Alkaline Massif, Transylvania, Romania. Lithos 2007, 95, 331–345. [Google Scholar] [CrossRef]
- Batki, A.; Pál-Molnár, E.; Dobosi, G.; Skelton, A. Petrogenetic significance of ocellar camptonite dykes in the Ditrău Alkaline Massif, Romania. Lithos 2014, 200, 181–196. [Google Scholar] [CrossRef]
- Batki, A.; Pál-Molnár, E.; Jankovics, M.É.; Kerr, A.C.; Kiss, B.; Markl, G.; Heincz, A.; Harangi, S. Insights into the evolution of an alkaline magmatic system: An in situ trace element study of clinopyroxenes from the Ditrău Alkaline Massif, Romania. Lithos 2018, 300, 51–71. [Google Scholar] [CrossRef]
- Pană, D.; Balintoni, I.; Heaman, L.M. Precise U-Pb zircon dating of the syenite phase from the Ditrău Alkaline Igneous Complex. Stud. Univ. Babes-Bolyai Geologia. 2000, 45, 79–89. [Google Scholar]
- Pyle, J.M.; Spear, F.S.; Wark, D.A. Electron microprobe analysis of REE in apatite, monazite and xenotime: Protocols and pitfalls. In Phosphates: Geochemical, Geobiological, and Materials Importance; Kohn, M.J., Rakovan, J., Hughes, J.M., Eds.; Mineralogical Society of America: Washington DC, USA, 2002; Volume 48, pp. 337–362. [Google Scholar]
- Montel, J.M.; Foret, S.; Veschambre, M.; Nicollet, C.; Provost, A. Electron microprobe dating of monazite. Chem. Geol. 1996, 131, 37–53. [Google Scholar] [CrossRef]
- Cocherie, A.; Albarède, F. An improved U-Th-Pb age calculation for electron microprobe dating of monazite. Geochim. Cosmochim. Acta 2001, 65, 4509–4522. [Google Scholar] [CrossRef]
- Suzuki, K.; Adachi, M. Middle Precambrian detrital monazite and zircon from the Hida Gneiss on Oki-Dogo Island, Japan; their origin and implications for the correlation of basement gneiss of Southwest Japan and Korea. Tectonophysics 1994, 235, 277–292. Available online: http://search.proquest.com/docview/50210704?accountid=14682 (accessed on 2 May 2023). [CrossRef]
- Vermeesch, P. IsoplotR: A free and open toolbox for geochronology. Geosci. Front. 2018, 9, 1479–1493. [Google Scholar] [CrossRef]
- Dahl, P.S.; Hamilton, M.A.; Jercinovic, M.J.; Terry, M.P.; Williams, M.L.; Frei, R. Comparative isotopic and chemical geochronometry of monazite, with implications for U-Th-Pb dating by electron microprobe; an example from metamorphic rocks of the eastern Wyoming Craton (U.S.A.). Am. Mineral. 2005, 90, 619–638. [Google Scholar] [CrossRef]
- Klötzli, U.; Klötzli-Chowanetz, E.; Košler, J. Characterization of a new laser ablation xenotime U-Pb age standard. Geochim. Cosmochim. Acta 2010, 74, A524. [Google Scholar]
- Konečný, P.; Kusiak, M.A.; Dunkley, D.J. Improving U-Th-Pb electron microprobe dating using monazite age references. Chem. Geol. 2018, 484, 22–35. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Repina, S.A. Fractionation of REE in the xenotime and florencite paragenetic association from Au-REE mineral occurrences of the Nether-Polar Urals. Geochem. Int. 2011, 49, 868–887. [Google Scholar] [CrossRef]
- Suzuki, K.; Adachi, M.; Kajizuka, I. Electron microprobe observations of Pb diffusion in metamorphosed detrital monazites. Earth Planet. Sci. Lett. 1994, 128, 391–405. [Google Scholar] [CrossRef]
- Harlov, D.E.; Förster, H.J. Fluid-induced nucleation of (Y+REE)-phosphate minerals within apatite: Nature and experiment. Part II. Fluorapatite. Am. Mineral. 2003, 88, 1209–1229. [Google Scholar] [CrossRef]
- Harlov, D.E.; Prochazka, V.; Foerster, H.J.; Matejka, D. Origin of monazite-xenotime-zircon-fluorapatite assemblages in the peraluminous Melechov granite massif, Czech Republic. Mineral. Petrol. 2008, 94, 9–26. [Google Scholar] [CrossRef]
- Alves, F.E.A.; Neumann, R.; Ávila, C.A.; Faulstich, F.R.L. Monazite-(Ce) and xenotime-(Y) micro-inclusions in fluorapatite of the pegmatites from the Volta Grande mine, Minas Gerais state, southeast Brazil, as witnesses of the dissolution–reprecipitation process. Mineral. Mag. 2019, 83, 595–606. [Google Scholar] [CrossRef]
- Lipin, B.R.; McKay, G.A. Geochemistry and Mineralogy of Rare Earth Elements. In Reviews in Mineralogy; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 1989; Volume 21. [Google Scholar]
- Chen, W.; Liu, H.Y.; Lu, J.; Jiang, S.Y.; Simonetti, A.; Xu, C.; Zhang, W. The formation of the ore-bearing dolomite marble from the giant Bayan Obo REE-Nb-Fe deposit, Inner Mongolia: Insights from micron-scale geochemical data. Miner. Depos. 2020, 55, 131–146. [Google Scholar] [CrossRef]
- Irber, W. The lanthanide tetrad effect and its correlation with K/Rb, Eu/Eu*, Sr/Eu, Y/Ho, and Zr/Hf of evolving peraluminous granite suites. Geochim. Cosmochim. Acta 1999, 63, 489–508. [Google Scholar] [CrossRef]
- Duc-Tin, Q.; Keppler, H. Monazite and xenotime solubility in granitic melts and the origin of the lanthanide tetrad effect. Contrib. Mineral. Petrol. 2015, 169. [Google Scholar] [CrossRef]
- Anenburg, M.; Williams, M.J. Quantifying the Tetrad Effect, Shape Components, and Ce–Eu–Gd Anomalies in Rare Earth Element Patterns. Math. Geosci. 2022, 54, 47–70. [Google Scholar] [CrossRef]
- Tramm, F.; Wirth, R.; Budzyń, B.; Sláma, J.; Schreiber, A. LA-ICP-MS and TEM constraints on the magmatic and post-magmatic processes recorded by the zircon-xenotime intergrowth in pegmatite (Piława Górna, Góry Sowie Block, SW Poland). Lithos 2021, 404, 106480. [Google Scholar] [CrossRef]
- Daniel, C.G.; Pyle, J.M. Monazite-xenotime thermochronometry and Al2SiO5 reaction textures in the Picuris range, northern New Mexico, USA: New evidence for a 1450-1400 Ma orogenic event. J. Petrol. 2006, 47, 97–118. [Google Scholar] [CrossRef]
- Daniel, C.G.; Pyle, J.M. Integrating monazite and xenotime thermochronology to determine the timing and nature of the Al2SiO5 triple-point metamorphism in the Picuris mountains, New Mexico, USA. Geochim. Cosmochim. Acta 2010, 74, A206. [Google Scholar]
- Suzuki, K.; Adachi, M.; Kato, T.; Yogo, S. CHIME dating method and its application to the analysis of evolutional history of orogenic belts. Geochemistry 1999, 33, 1–22. [Google Scholar]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klötzli, U.; Burda, J.; Tibuleac, P. Phosphate Petrochronology of the Belcina REE Mineralization (Ditrău Alkaline Massif, Romania). Minerals 2023, 13, 739. https://doi.org/10.3390/min13060739
Klötzli U, Burda J, Tibuleac P. Phosphate Petrochronology of the Belcina REE Mineralization (Ditrău Alkaline Massif, Romania). Minerals. 2023; 13(6):739. https://doi.org/10.3390/min13060739
Chicago/Turabian StyleKlötzli, Urs, Jolanta Burda, and Paul Tibuleac. 2023. "Phosphate Petrochronology of the Belcina REE Mineralization (Ditrău Alkaline Massif, Romania)" Minerals 13, no. 6: 739. https://doi.org/10.3390/min13060739
APA StyleKlötzli, U., Burda, J., & Tibuleac, P. (2023). Phosphate Petrochronology of the Belcina REE Mineralization (Ditrău Alkaline Massif, Romania). Minerals, 13(6), 739. https://doi.org/10.3390/min13060739







