Polymineralic Inclusions in Loparite-(Ce) from the Lovozero Alkaline Massif (Kola Peninsula, Russia): Hydrothermal Association in Miniature
Abstract
1. Introduction
2. Geological Background
- (1)
- The layered complex (Figure 1a) with a thickness of 1700 m occupies 77% of the massif’s volume. This complex is called “layered” because it consists of a large number of subhorizontal layers (more commonly called “rhythms”) of alkaline rocks (Figure 1b). The top of each rhythm consists of lujavrite. This is a trachytoid meso- or melanocratic nepheline syenite, consisting mainly of nepheline, microcline-perthite, aegirine-(augite), and alkaline amphiboles. Down the cross section of the rhythm, the content of mafic minerals gradually decreases, and lujavrite passes into massive leucocratic nepheline syenite, called foyaite. Toward the bottom of the rhythm, the content of feldspar gradually decreases, and foyaite passes into an almost monomineral nepheline rock called urtite. In some rhythms, urtite may be absent. Whereas transitions between rocks within rhythms are gradual, contacts between rhythms are sharp. Pegmatites are often located at the contact between the rhythms. All rhythms of the layered complex are grouped into seven series (I–VII from top to bottom). In each series, the urtite layers are additionally indicated by Arabian numerals. Figure 1a shows only some of the urtite horizons, namely I-4, II-4, II-7, III-1, III-10, III-14, IV-1, and IV-2.
- (2)
- The eudialyte complex (18% of the massif’s volume), with a thickness of 100 to 800 m, overlaps the layered complex. The eudialyte complex is not layered and consists mainly of lujavrite enriched with minerals of the eudialyte group. A small part of the eudialyte complex is foyaite, as well as fine-grained/porphyritic nepheline syenites, which usually form small lenses and sheet-like bodies.
- (3)
- The rocks of the poikilitic complex form irregularly shaped bodies or lenses located among the rocks of both the eudialyte and the layered complexes (Figure 1a,c). The poikilitic complex (5% of the massif’s volume) consists of poikilitic and uneven-grained feldspathoid syenites. A main feature of these rocks is the presence of large (up to 10 cm in length) feldspar laths with numerous inclusions of feldspathoids (nepheline, sodalite, and vishnevite).
3. Materials and Methods
4. Results
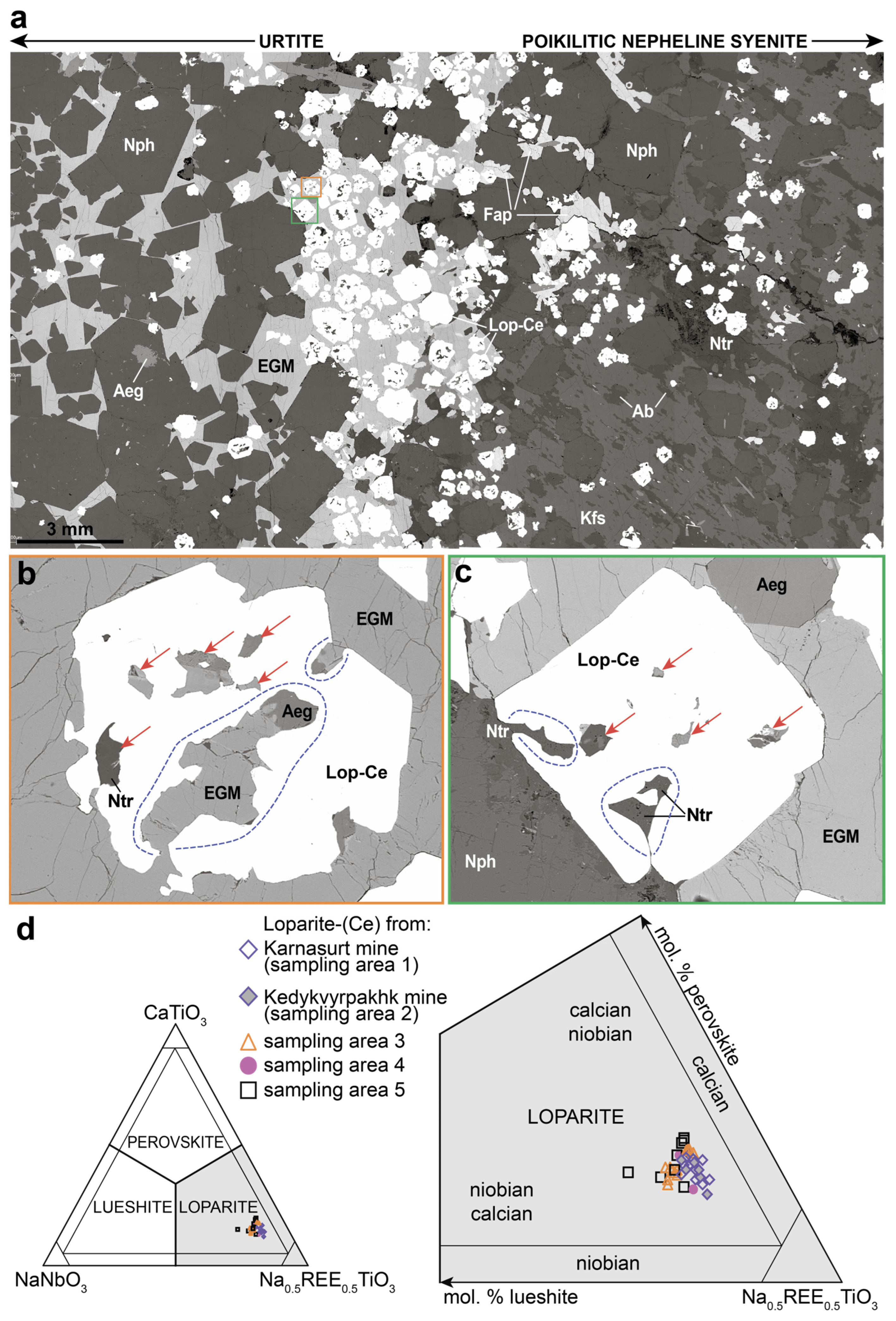
4.1. Loparite-(Ce) Morphology and Chemical Composition
4.1.1. Loparite-(Ce) Morphology in Contact Zones between Rhythms
4.1.2. Loparite-(Ce) Morphology in Contact Zone between Complexes
4.1.3. Loparite-(Ce) Chemical Composition
4.2. Inclusions in Loparite-(Ce)
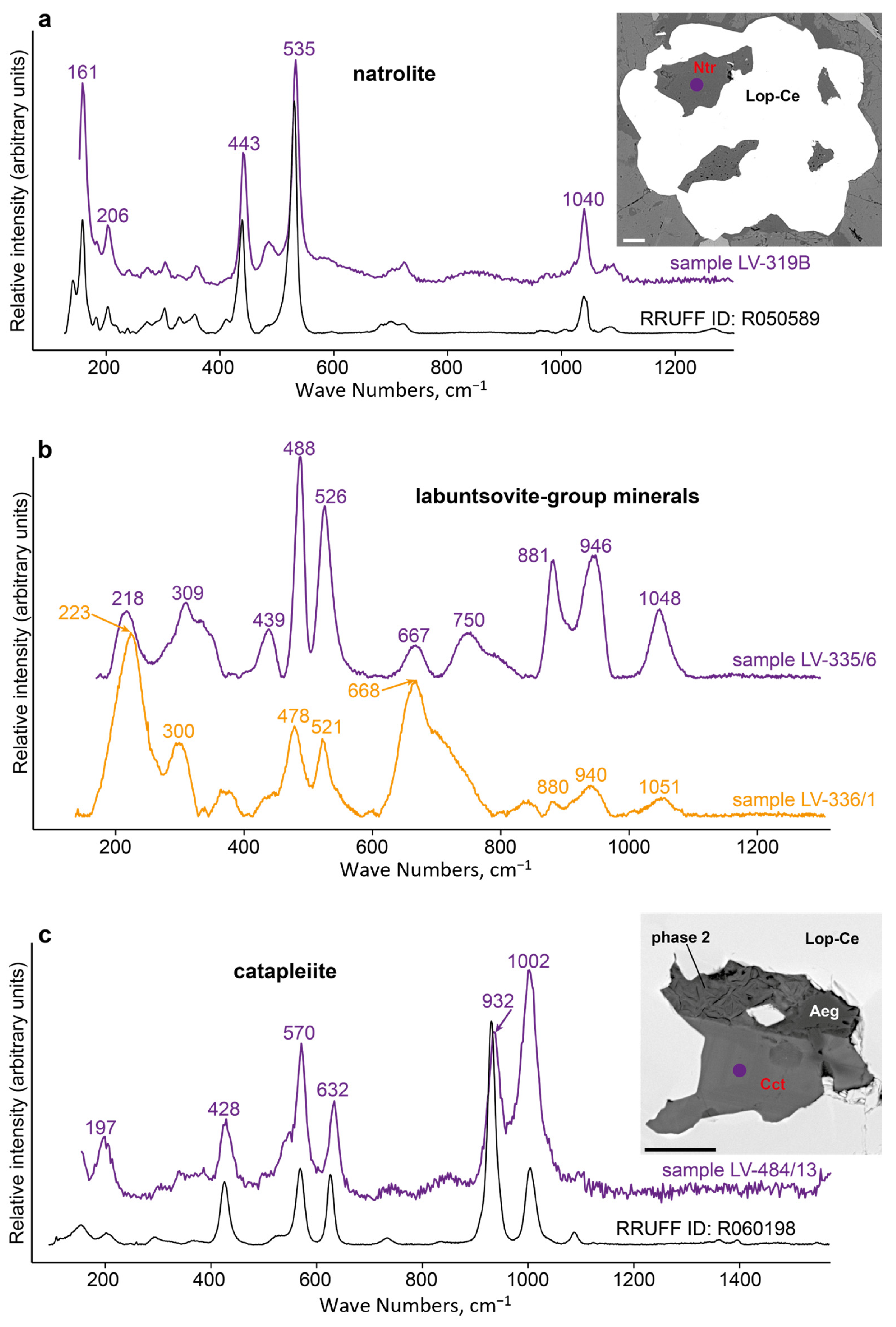
4.3. Minerals in Loparite-Hosted Inclusions
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kogarko, L.N.; Williams, C.T.; Woolley, A.R. Chemical Evolution and Petrogenetic Implications of Loparite in the Layered, Agpaitic Lovozero Complex, Kola Peninsula, Russia. Mineral. Petrol. 2002, 74, 1–24. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Chakhmouradian, A.R. Compositional Variation of Loparite from the Lovozero Alkaline Complex, Russia. Can. Mineral. 1996, 34, 977–990. [Google Scholar]
- Chakhmouradian, A.R.; Mitchell, R.H. Compositional Variation of Perovskite-Group Minerals from the Carbonatite Complexes of the Kola Alkaline Province, Russia. Can. Mineral. 1997, 35, 1293–1310. [Google Scholar]
- Platt, R.G. Perovskite, Loparite and Ba-Fe Hollandite from the Schryburt Lake Carbonatite Complex, Northwestern Ontario, Canada. Mineral. Mag. 1994, 58, 49–57. [Google Scholar] [CrossRef]
- Ramsay, W.; Hackman, V. Das Nephelinesyenitgebiet Auf Der Halbinsel Kola I. In Fennia; 1894; Volume 11, pp. 1–225. [Google Scholar]
- Kuznetsov, I.G. Loparite—A New Rare Earth Mineral from the Khibina Tundra. Izv. Geol. Kom. 1925, 44, 663–682. [Google Scholar]
- Mitchell, R.H.; Welch, M.D.; Chakhmouradian, A.R. Nomenclature of the Perovskite Supergroup: A Hierarchical System of Classification Based on Crystal Structure and Composition. Mineral. Mag. 2017, 81, 411–461. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Burns, P.C.; Chakhmouradian, A.R. The Crystal Structures of Loparite-(Ce). Can. Mineral. 2000, 38, 145–152. [Google Scholar] [CrossRef]
- Burns, P.C. CCD Area Detectors of X-rays Applied to the Analysis of Mineral Structures. Can. Mineral. 1998, 36, 847–853. [Google Scholar]
- Chakhmouradian, A.R.; Mitchell, R.H. New Data on Pyrochlore- and Perovskite-Group Minerals from the Lovozero Alkaline Complex, Russia. Eur. J. Mineral. 2002, 14, 821–836. [Google Scholar] [CrossRef]
- Pakhomovsky, Y.A.; Ivanyuk, G.Y.; Yakovenchuk, V.N. Loparite-(Ce) in Rocks of the Lovozero Layered Complex at Mt. Karnasurt and Mt. Kedykvyrpakhk. Geol. Ore Depos. 2014, 56, 685–698. [Google Scholar] [CrossRef]
- Kogarko, L.N. Peculiarities of the Formation of Loparite Ores: The Lovozero Rare Metal Deposit, East Fennoscandia. Dokl. Earth Sci. 2022, 505, 524–526. [Google Scholar] [CrossRef]
- Eliseev, N.A.; Nefedov, N.K. Loparite Deposits of Luyavrurt. In Productive Forces of Kola Peninsula; Fersman, A.E., Ed.; AN SSSR: Moscow, Russia, 1940. [Google Scholar]
- Ifantopulo, T.N.; Osokin, E.D. Accessory Loparite from a Stratified Alkaline Intrusion. In New Data on Mineralogy of Mineral Deposits from Alkaline Rocks; IMGRE Press: Moscow, Russia, 1979; pp. 20–28. [Google Scholar]
- Kramm, U.; Kogarko, L.N. Nd and Sr Isotope Signatures of the Khibina and Lovozero Agpaitic Centres, Kola Alkaline Province, Russia. Lithos 1994, 32, 225–242. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Wu, F.Y.; Yang, Y.H. In Situ U-Pb, Sr and Nd Isotopic Analysis of Loparite by LA-(MC)-ICP-MS. Chem. Geol. 2011, 280, 191–199. [Google Scholar] [CrossRef]
- Wu, F.Y.; Yang, Y.H.; Marks, M.A.W.; Liu, Z.C.; Zhou, Q.; Ge, W.C.; Yang, J.S.; Zhao, Z.F.; Mitchell, R.H.; Markl, G. In Situ U-Pb, Sr, Nd and Hf Isotopic Analysis of Eudialyte by LA-(MC)-ICP-MS. Chem. Geol. 2010, 273, 8–34. [Google Scholar] [CrossRef]
- Bussen, I.V.; Sakharov, A.S. Petrology of the Lovozero Alkaline Massif; Nauka: Leningrad, Russia, 1972. [Google Scholar]
- Gerasimovsky, V.I.; Volkov, V.P.; Kogarko, L.N.; Polyakov, A.I.; Saprykina, T.V.; Balashov, Y.A. Geochemistry of the Lovozero Alkaline Massif; Nauka: Moscow, Russia, 1966. [Google Scholar]
- Vlasov, K.A.; Kuzmenko, M.V.; Eskova, E.M. Lovozero Alkaline Massif; Academy of Sciences SSSR: Moskow, Russia, 1959. [Google Scholar]
- Saprykina, L.G.; Zhadritskii, V.L.; Panteleimonov, V.M.; Tereshkov, V.G. Report on Prospecting for Apatite within the Lovozero Alkaline Massif in 1974–76 and on the Search for Apatite Ores in the Rocks of the Eudialyte Complex of the Northeastern Part of the Lovozero Massif in 1975–77 (Murmansk Region); Revda, Russia, 1977. [Google Scholar]
- Arzamastsev, A.A. Unique Paleozoic Intrusions of the Kola Peninsula; Kola Science Centre Press House: Apatity, Russia, 1994. [Google Scholar]
- Kalashnikov, A.O.; Konopleva, N.G.; Pakhomovsky, Y.A.; Ivanyuk, G.Y. Rare Earth Deposits of the Murmansk Region, Russia—A Review. Econ. Geol. 2016, 111, 1529–1559. [Google Scholar] [CrossRef]
- Warr, L.N. IMA-CNMNC Approved Mineral Symbols. Mineral. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Johnsen, O.; Ferraris, G.; Gault, R.A.; Grice, J.D.; Kampf, A.R.; Pekov, I.V. The Nomenclature of Eudialyte-Group Minerals. Can. Mineral. 2003, 41, 785–794. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Pekov, I.V.; Khomyakov, A.P. Recommended Nomenclature for Labuntsovite-Group Minerals. Eur. J. Mineral. 2002, 14, 165–173. [Google Scholar] [CrossRef]
- Atencio, D.; Andrade, M.B.; Christy, A.G.; Gieré, R.; Kartashov, P.M. The Pyrochlore Supergroup of Minerals: Nomenclature. Can. Mineral. 2010, 48, 673–698. [Google Scholar] [CrossRef]
- Locock, A.J.; Mitchell, R.H. Perovskite Classification: An Excel Spreadsheet to Determine and Depict End-Member Proportions for the Perovskite- and Vapnikite-Subgroups of the Perovskite Supergroup. Comput. Geosci. 2018, 113, 106–114. [Google Scholar] [CrossRef]
- Pushcharovskii, D.Y.; Pekov, I.V.; Pasero, M.; Gobechiya, E.R.; Merlino, S.; Zubkova, N.V. Crystal Structure of Cation-Deficient Calciohilairite and Possible Mechanisms of Decationization in Mixed-Framework Minerals. Crystallogr. Rep. 2002, 47, 814–818. [Google Scholar] [CrossRef]
- Czaja, M.; Lisiecki, R.; Juroszek, R.; Krzykawski, T. Luminescence Properties of Tetrahedral Coordinated Mn2+; Genthelvite and Willemite Examples. Minerals 2021, 11, 1215. [Google Scholar] [CrossRef]
- Finch, A.A. Genthelvite and Willemite, Zinc Minerals Associated with Alkaline Magmatism from the Motzfeldt Centre, South Greenland. Mineral. Mag. 1990, 54, 407–412. [Google Scholar] [CrossRef]
- Zito, G.; Hanson, S.L. Genthelvite Overgrowths on Danalite Cores from a Pegmatite Miarolitic Cavity in Cheyenne Canyon, El Paso County, Colorado. Can. Mineral. 2017, 55, 195–206. [Google Scholar] [CrossRef]
- Welsch, B.; Hammer, J.; Baronnet, A.; Jacob, S.; Hellebrand, E.; Sinton, J. Clinopyroxene in Postshield Haleakala Ankaramite: 2. Texture, Compositional Zoning and Supersaturation in the Magma. Contrib. Mineral. Petrol. 2016, 171, 6. [Google Scholar] [CrossRef]
- Corrigan, G.M. Supercooling and the Crystallization of Plagioclase, Olivine, and Clinopyroxene from Basaltic Magmas. Mineral. Mag. 1982, 46, 31–42. [Google Scholar] [CrossRef]
- Lofgren, G.E.; Donaldson, C.H. Curved Branching Crystals and Differentiation in Comb-Layered Rocks. Contrib. Mineral. Petrol. 1975, 49, 309–319. [Google Scholar] [CrossRef]
- Vernon, R.H. A Practical Guide to Rock Microstructure; Cambridge University Press: Cambridge, UK, 2004; ISBN 052181443X. [Google Scholar]
- García-Ruiz, J.M.; Otálora, F. Crystal Growth in Geology: Patterns on the Rocks. In Handbook of Crystal Growth: Bulk Crystal Growth, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 2, pp. 1–43. ISBN 9780444633064. [Google Scholar]
- Sunagawa, I. Characteristics of Crystal Growth in Nature as Seen from the Morphology of Mineral Crystals. Bull. Mineral. 1981, 104, 81–87. [Google Scholar] [CrossRef]
- Friedrich, B.M.; Marques, J.C.; Olivo, G.R.; Frantz, J.C.; Joy, B.; Queiroz, W.J.A. Petrogenesis of the Massive Chromitite Layer from the Jacurici Complex, Brazil: Evidence from Inclusions in Chromite. Miner. Depos. 2020, 55, 1105–1126. [Google Scholar] [CrossRef]
- Prichard, H.M.; Barnes, S.J.; Godel, B.; Reddy, S.M.; Vukmanovic, Z.; Halfpenny, A.; Neary, C.R.; Fisher, P.C. The Structure and Origin of Nodular Chromite from the Troodos Ophiolite, Cyprus, Revealed Using High-Resolution X-ray Computed Tomography and Electron Backscatter Diffraction. Lithos 2015, 218–219, 87–98. [Google Scholar] [CrossRef]
- Raade, G.; Chukanov, N.V.; Kolitsch, U.; Möckel, S.; Zadov, A.E.; Pekov, I.V. Gjerdingenite-Mn from Norway—A New Mineral Species in the Labuntsovite Group: Descriptive Data and Crystal Structure. Eur. J. Mineral. 2004, 16, 979–987. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Pekov, I.V.; Golovina, N.I.; Zadov, A.E.; Nedel’ko, V.V. Kuzmenkoite, K2(Mn,Fe)(Ti,Nb)4[Si4O12]2(OH)4·5H2O, a New Mineral. Zap. Ross. Mineral. Obs. 1999, 128, 42–50. [Google Scholar]
- Pekov, I.V.; Chukanov, N.V.; Yamnova, N.A.; Zadov, A.E.; Tarassoff, P. Gjerdingenite-Na and Gjerdingenite-Ca, Two New Mineral Species of the Labuntsovite Group. Can. Mineral. 2007, 45, 529–539. [Google Scholar] [CrossRef]
- Pekov, I.V.; Chukanov, N.V.; Petersen, O.V.; Zadov, A.E.; Yamnova, N.A.; Kabalov, Y.K.; Schneider, J. Karupmollerite-Ca, (Na,Ca,K)2Ca(Nb,Ti)4 (Si4O12)2(O,OH)4·7H2O, a New Mineral of the Labuntsovite Group from the Ilimaussaq Alkaline Complex, South Greenland. Neues Jahrb. Für Mineral. Mon. 2002, 2002, 433–444. [Google Scholar] [CrossRef]
- Pekov, I.V.; Chukanov, N.V.; Ferraris, G.; Gula, A.; Pushcharovsky, D.Y.; Zadov, A.E. Tsepinite—Ca,(Ca,K,Na,□)2(Ti,Nb2(Si4O12)(OH,O)2·4H2O, a New Mineral of the Labuntsovite Group from the Khibiny Alkaline Massif, Kola Peninsula—Novel Disordered Sites in the Vuoriyarvite-Type Structure. Neues Jahrb. Fur Mineral. Mon. 2003, 2003, 461–480. [Google Scholar] [CrossRef]
- Sokolov, S.V. The Formation Conditions of Labuntsovite-Group Minerals in the Kovdor Massif, Kola Peninsula. Geol. Ore Depos. 2014, 56, 671–674. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Pekov, I.V.; Zadov, A.E.; Voloshin, A.V.; Subbotin, V.V.; Sorokhtina, N.V.; Rastsvetaeva, R.K.; Krivovichev, S.V. Minerals of the Labuntsovite Group; Nauka: Moscow, Russia, 2003. [Google Scholar]
- Pekov, I.V. Lovozero Massif: History, Pegmatites, Minerals; Ocean Pictures Ltd.: Moscow, Russia, 2002. [Google Scholar]
- Semenov, E.I. Mineralogy of the Lovozero Alkaline Massif; Nauka: Moscow, Russia, 1972. [Google Scholar]
- Borst, A.M.; Friis, H.; Andersen, T.; Nielsen, T.F.D.; Waight, T.E.; Smit, M.A. Zirconosilicates in the Kakortokites of the Ilímaussaq Complex, South Greenland: Implications for Fluid Evolution and HFSE-REE Mineralisation in 2 Agpaitic Systems. Mineral. Mag. 2016, 80, 5–30. [Google Scholar] [CrossRef]
- Heinrich, E.W.; Quon, S.H. Neptunite from Seal Lake, Labrador. Can. Mineral. 1963, 7, 650–654. [Google Scholar]
- Bussen, I.V. Mineralogy of Manganoneptunite. Zap. Vserossiyskogo Mineral. Oshchestva 1964, 94, 204–208. [Google Scholar]
- Burt, D.M. Stability of Genthelvite, Zn4(BeSiO4)3S: An Exercise in Chalcophilicity Using Exchange Operators. Am. Mineral. 1988, 73, 1384–1394. [Google Scholar]
- Eskova, E.M. Gentgelvite from Alkaline Pegmatites. Dokl. Acad. Sci. USSR 1957, 116, 348–351. [Google Scholar]
- Horvath, L.; Gault, R.A. The Mineralogy of Mont Saint-Hilaire, Quebec. Mineral. Rec. 1990, 21, 284–359. [Google Scholar]
- Kogarko, L.N. Problems of Genesis of Agpaitic Magmas; Nauka: Moskow, Russia, 1977. [Google Scholar]
- Khomyakov, A.P. Mineralogy of Hyperagpaitic Alkaline Rocks; Oxford Scientific Publications: Oxford, UK, 1995. [Google Scholar]
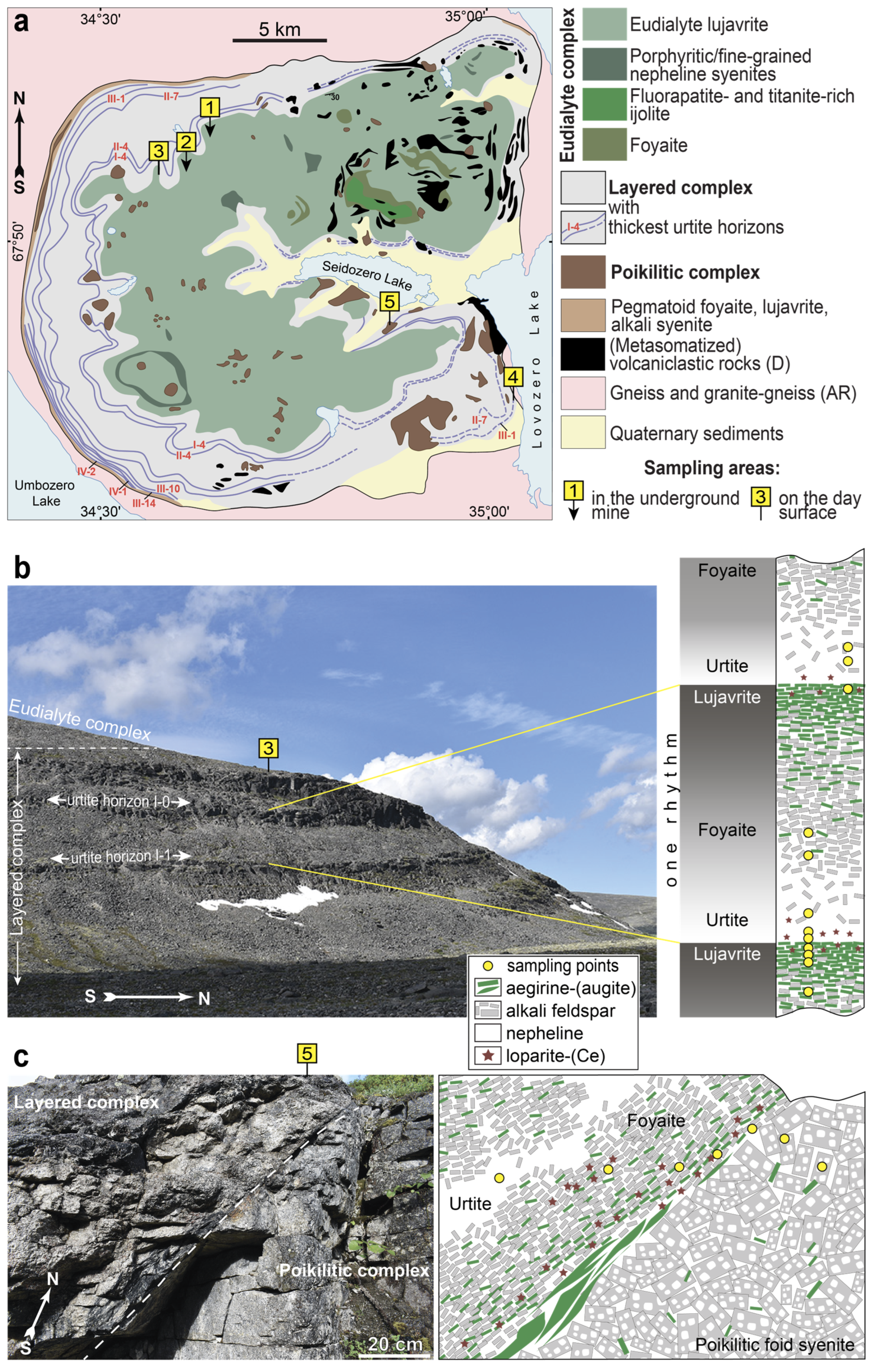








| Sampling Area (See Figure 1) | Location | Short Geological Description | List of Samples |
|---|---|---|---|
| Contact zones between rhythms | |||
| 1 | Karnasurt underground mine (the Lovozero loparite deposit) | Narrow (0.1–0.4 m) loparite-rich horizon at the contact with the I-4 urtite layer and underlying lujavrite | LV-III-4-1, LV-III-4-2, LV-III-4-4, LV-III-5-2, LV-III-5-3, LV-III-5-4, LV-III-5-5, LV-III-6-1, LV-III-6-2, LV-III-6-5 |
| 2 | Kedykvyrpakhk underground mine (the Lovozero loparite deposit) | Narrow (0.1–0.4 m) loparite-rich horizon at the contact with the II-4 urtite and underlying lujavrite | LV-IV-3-5, LV-IV-3-1, LV-IV-3-2, LV-IV-1-2, LV-I-7, LV-I-8, LV-IV-1-1 |
| 3 | Outcrop on the slope of Mt. Kedykvyrpakhk 67°52′35.3″ N 34°34′37.7″ E | local loparite-rich areas at the contacts with the I-0 and I-1 urtite and underlying lujavrite | LV-316/1, LV-319/1, LV-319B, LV-319D, LV-333, LV-335/6, LV-335A, LV-335B, LV-335C, LV-335D, LV-336/1, LV-336/2 |
| 4 | Outcrop on the slope of Mt. Punkaruaiv 67°44′39.1″ N 35°01′44.6″ E | local loparite-rich areas at the contacts with III-1 urtite and underlying foyaite | LV-454, LV-454/2, LV-454/3 |
| Contact zone between complexes | |||
| 5 | Outcrop on the slope of Mt. Ninchurt 67°47′27.7″ N 34°51′59.0″ E | local loparite-rich area at the contacts of the layered and poikilitic complexes | LV-484/2, LV-484/4, LV-484/5, LV-484/11, LV-484/12, LV-484/13 LV-487/1, LV-487/2 |
| Abbreviation | Mineral | Formula |
|---|---|---|
| Ab | albite | Na(AlSi3O8) |
| Aeg | aegirine | NaFe3+Si2O6 |
| Anl | analcime | Na(AlSi2O6)·H2O |
| Arf | arfvedsonite | NaNa2(Fe2+4Fe3+)Si8O22(OH)2 |
| Blmp | barytolamprophyllite | (BaK)Ti2Na3Ti(Si2O7)2O2(OH)2 |
| Bri-Ce | britholite-(Ce) | (Ce,Ca)5(SiO4)3(OH) |
| By | barylite | Be2Ba(Si2O7) |
| Ctp | catapleiite | Na2Zr(Si3O9)·2H2O |
| EGM | eudialyte-group mineral | N15M16M23M3M4Z3[Si24O73]O′4X2; N = Na, Ca, K, Sr, REE, Ba, Mn, H3O+; M1 = Ca, Mn, REE, Na, Sr, Fe; M2 = Fe, Mn, Na, Zr, Ta, Ti, K, Ba, H3O+; M3,4 = Si, Nb, Ti, W, Na; Z = Zr, Ti, Nb; O′ = O, OH−, H2O; X = H2O, Cl, F, OH−,CO32−, SO42− [25] |
| Fap | fluorapatite | Ca5(PO4)3F |
| Flr | fluorite | CaF2 |
| Ghv | genthelvite | Be3Zn4(SiO4)3S |
| Gon | gonnardite | (Na,Ca)2(Si,Al)5O10·3H2O |
| Kfs | K-feldspar | KAlSi3O8 |
| Lmp | lamprophyllite | (SrNa)Ti2Na3Ti(Si2O7)2O2(OH)2 |
| LGM | labuntsovite-supergroup mineral | [26] |
| Lom | lomonosovite | Na6Na2Ti2Na2Ti2(Si2O7)2(PO4)2O4 |
| Lop-Ce | loparite-(Ce) | (Na,Ce,Sr)(Ce,Th)(Ti,Nb)2O6 |
| Lrz | lorenzenite | Na2Ti2(Si2O6)O3 |
| Marf | magnesio-arfvedsonite | NaNa2(Mg4Fe3+)Si8O22(OH)2 |
| Mnnpt | manganoneptunite | KNa2LiMn2+2Ti2Si8O24 |
| Nph | nepheline | Na3K(Al4Si4O16) |
| Npt | neptunite | KNa2LiFe2+2Ti2Si8O24 |
| Ntr | natrolite | Na2(Si3Al2)O10·2H2O |
| Pcl | pyrochlore-group mineral | A2−mB2X6−wY1−n; A = Na, Ca, Sr, Pb, Sn, Sb, Y, □; B = Ta, Nb, Ti, Sb, W; X = O; Y = □, H2O, OH−, O, F [27] |
| Rha-Ce | rhabdophane-(Ce) | Ce(PO4)·H2O |
| Sdl | sodalite | Na4(Si3Al3)O12Cl |
| Vgd | vinogradovite | Na4Ti4(Si2O6)2[(Si,Al)4O10]O4·(H2O,Na,K)3 |
| Sampling Area | 1 | 2 | 3 | 3 | 4 | 4 | 5 | 5 |
|---|---|---|---|---|---|---|---|---|
| Sample | LV-IV-3-5 | LV-III-6-2 | LV-335B | LV-336/1 | LV-454 | LV-454/3 | LV-484/13 | LV-484/11 |
| Nb2O5, wt. % | 6.92 | 6.99 | 6.72 | 7.20 | 8.40 | 7.88 | 7.02 | 8.86 |
| Ta2O5 | 0.70 | 0.63 | 0.57 | 0.20 | 0.80 | 0.63 | 0.66 | 0.82 |
| TiO2 | 39.35 | 40.84 | 41.90 | 42.74 | 40.74 | 42.03 | 43.22 | 40.82 |
| ThO2 | 0.27 | 0.47 | 0.85 | 0.93 | 0.65 | 0.84 | 0.77 | 0.82 |
| Fe2O3 | 0.32 | 0.42 | 0.24 | 0.21 | 0.40 | 0.19 | 0.19 | 0.22 |
| La2O3 | 9.25 | 9.15 | 8.90 | 8.76 | 9.29 | 8.54 | 8.32 | 8.98 |
| Ce2O3 | 17.52 | 17.45 | 18.07 | 17.68 | 19.42 | 18.11 | 17.46 | 18.37 |
| Pr2O3 | 1.73 | 1.52 | 1.14 | 1.33 | 1.21 | 0.83 | 0.94 | 1.19 |
| Nd2O3 | 4.04 | 4.05 | 4.47 | 4.51 | 3.76 | 3.77 | 3.85 | 3.78 |
| CaO | 4.59 | 4.81 | 5.38 | 5.47 | 3.93 | 5.24 | 5.61 | 3.93 |
| SrO | 3.65 | 3.95 | 3.31 | 2.93 | 1.78 | 3.05 | 3.71 | 3.30 |
| Na2O | 9.04 | 8.82 | 8.16 | 7.95 | 8.62 | 8.09 | 7.95 | 8.31 |
| K2O | bdl | bdl | 0.05 | bdl | 0.03 | 0.04 | 0.05 | 0.04 |
| Total | 97.35 | 99.08 | 99.76 | 99.90 | 99.03 | 99.22 | 99.75 | 99.44 |
| Formulae based on O = 3 pfu | ||||||||
| Nb | 0.09 | 0.09 | 0.09 | 0.09 | 0.11 | 0.10 | 0.09 | 0.11 |
| Ta | 0.01 | - | - | - | 0.01 | - | 0.01 | 0.01 |
| Ti | 0.87 | 0.89 | 0.90 | 0.91 | 0.88 | 0.90 | 0.91 | 0.88 |
| Th | - | - | 0.01 | 0.01 | - | 0.01 | - | 0.01 |
| Fe3+ | 0.01 | 0.01 | 0.01 | - | 0.01 | - | - | 0.01 |
| La | 0.10 | 0.10 | 0.09 | 0.09 | 0.10 | 0.09 | 0.09 | 0.10 |
| Ce | 0.19 | 0.18 | 0.19 | 0.18 | 0.20 | 0.19 | 0.18 | 0.19 |
| Pr | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Nd | 0.04 | 0.04 | 0.05 | 0.05 | 0.04 | 0.04 | 0.04 | 0.04 |
| Ca | 0.14 | 0.15 | 0.16 | 0.17 | 0.12 | 0.16 | 0.17 | 0.12 |
| Sr | 0.06 | 0.07 | 0.05 | 0.05 | 0.03 | 0.05 | 0.06 | 0.05 |
| Na | 0.52 | 0.49 | 0.45 | 0.44 | 0.48 | 0.45 | 0.43 | 0.46 |
| Mol. % end members | ||||||||
| loparite | 72 | 69 | 69 | 68 | 72 | 67 | 65 | 69 |
| perovskite | 11 | 14 | 17 | 17 | 12 | 16 | 18 | 12 |
| lueshite | 10 | 10 | 9 | 9 | 12 | 11 | 10 | 12 |
| tausonite | 6 | 7 | 6 | 5 | 3 | 5 | 6 | 6 |
| ThTi2O6 | - | - | - | 1 | 1 | 1 | 1 | 1 |
| Mineral/Group of Minerals | Contact Zone between Complexes | Contact Zones between Rhythms | |||
|---|---|---|---|---|---|
| In Inclusions | In Rock | In Inclusions | In Rock | ||
| minerals typical of rocks bearing loparite-(Ce) grains (groundmass minerals) | natrolite | ● | ● | ● | ● |
| analcime | ● | ● | ● | ● | |
| aegirine | ● | ● | ● | ● | |
| magnesio-arfvedsonite | ● | ● | ● | ● | |
| alkali feldspar | ● | ● | ● | ● | |
| albite | ● | ● | ● | ● | |
| fluorapatite | ● | ● | ● | ● | |
| sodalite | ● | ● | ● | ● | |
| rhabdophane-(Ce) | ● | ● | ● | ● | |
| barytolamprophyllite | ● | ● | ● | ● | |
| pyrochlore-group minerals | ● | ● | ● | ● | |
| bastnäsite-(Ce) | ● | ● | ● | ● | |
| minerals that were not found in the rock outside loparite-(Ce) grains | labuntsovite-group minerals | ● | ● | ||
| lorenzenite | ● | ● | |||
| catapleiite | ● | ● | |||
| vinogradovite | ● | ||||
| manganoneptunite | ● | ||||
| barylite | ● | ||||
| genthelvite | ● | ||||
| britholite-(Ce) | ● | ||||
| barite | ● | ||||
| fluorite | ● | ||||
| neptunite | ● | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailova, J.A.; Pakhomovsky, Y.A.; Selivanova, E.A.; Kompanchenko, A.A. Polymineralic Inclusions in Loparite-(Ce) from the Lovozero Alkaline Massif (Kola Peninsula, Russia): Hydrothermal Association in Miniature. Minerals 2023, 13, 715. https://doi.org/10.3390/min13060715
Mikhailova JA, Pakhomovsky YA, Selivanova EA, Kompanchenko AA. Polymineralic Inclusions in Loparite-(Ce) from the Lovozero Alkaline Massif (Kola Peninsula, Russia): Hydrothermal Association in Miniature. Minerals. 2023; 13(6):715. https://doi.org/10.3390/min13060715
Chicago/Turabian StyleMikhailova, Julia A., Yakov A. Pakhomovsky, Ekaterina A. Selivanova, and Alena A. Kompanchenko. 2023. "Polymineralic Inclusions in Loparite-(Ce) from the Lovozero Alkaline Massif (Kola Peninsula, Russia): Hydrothermal Association in Miniature" Minerals 13, no. 6: 715. https://doi.org/10.3390/min13060715
APA StyleMikhailova, J. A., Pakhomovsky, Y. A., Selivanova, E. A., & Kompanchenko, A. A. (2023). Polymineralic Inclusions in Loparite-(Ce) from the Lovozero Alkaline Massif (Kola Peninsula, Russia): Hydrothermal Association in Miniature. Minerals, 13(6), 715. https://doi.org/10.3390/min13060715






