Geochemical Characterization of Rock Samples from Selected Fiji Mine Sites to Evaluate On-Site Environmental Vulnerabilities
Abstract
1. Introduction
1.1. Background
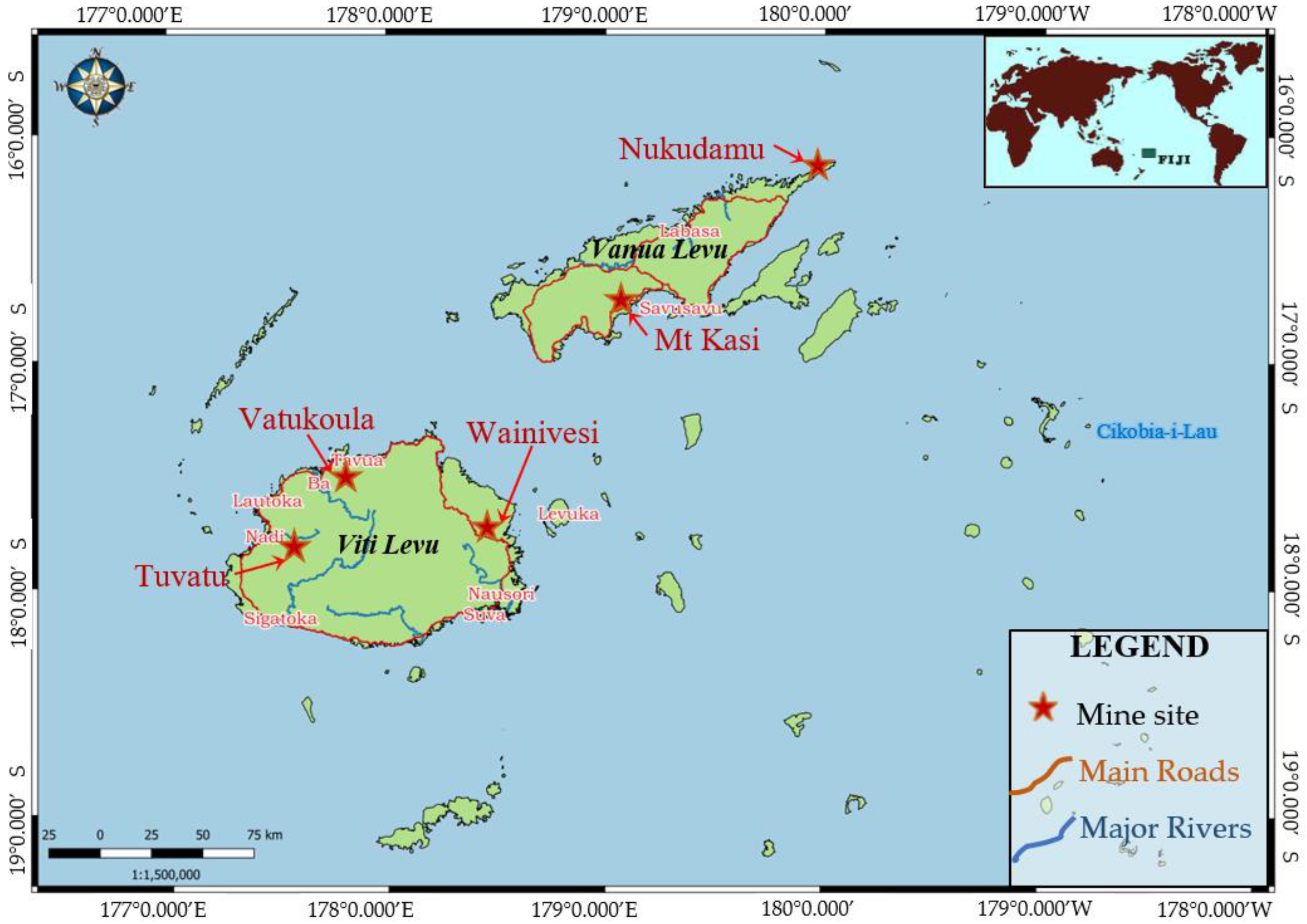
1.2. Study Sites
2. Materials and Methods
2.1. Sample Collection, Preparation, and Characterization
2.2. Batch Leaching Tests
2.3. Quality Assurance and Quality Control
2.4. Statistical Analysis and Thermodynamic Modeling
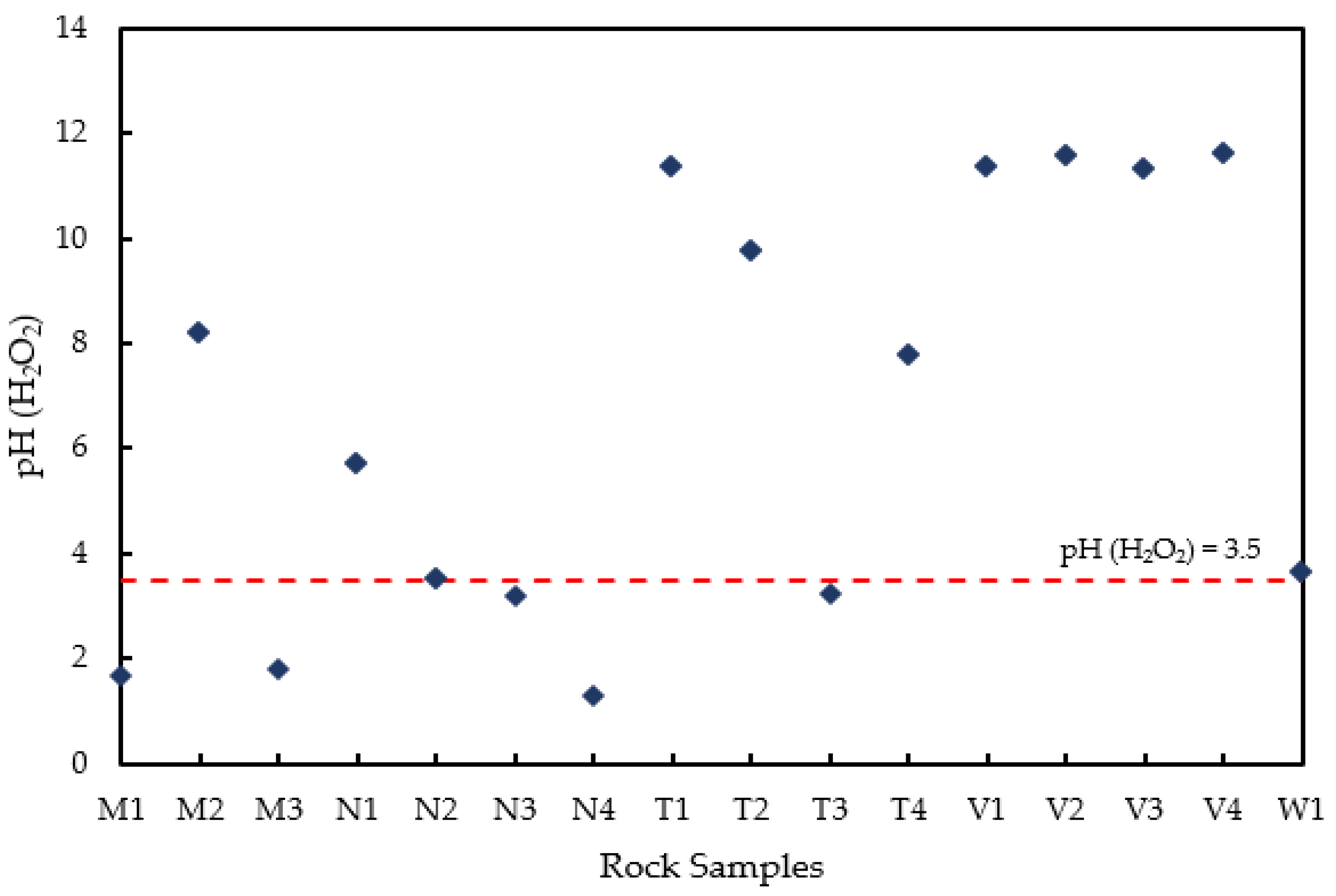
2.5. Acid–Base Accounting and pH Test with Hydrogen Peroxide
3. Results and Discussion
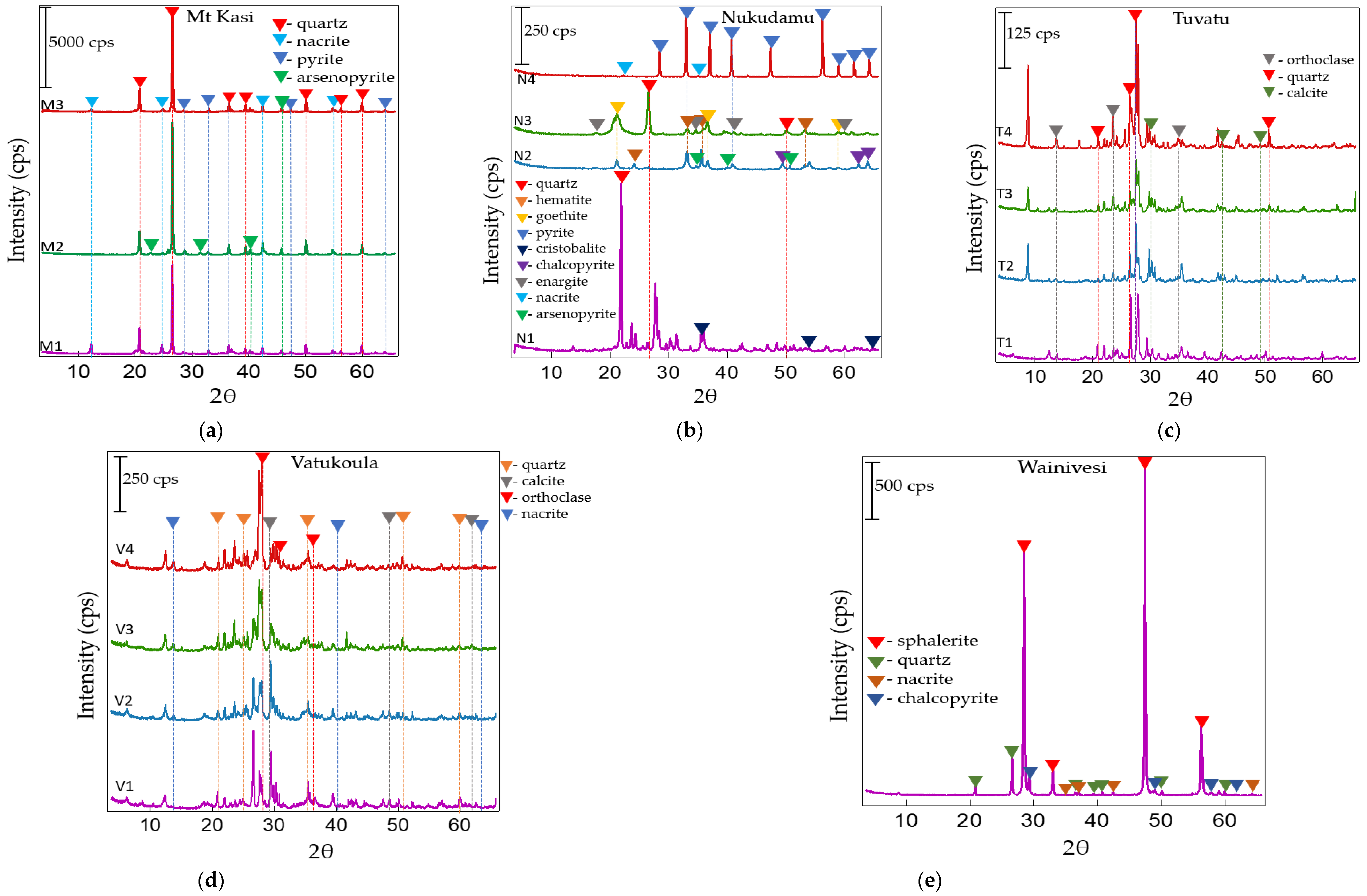
3.1. Mineralogical and Chemical Properties of the Rock Samples
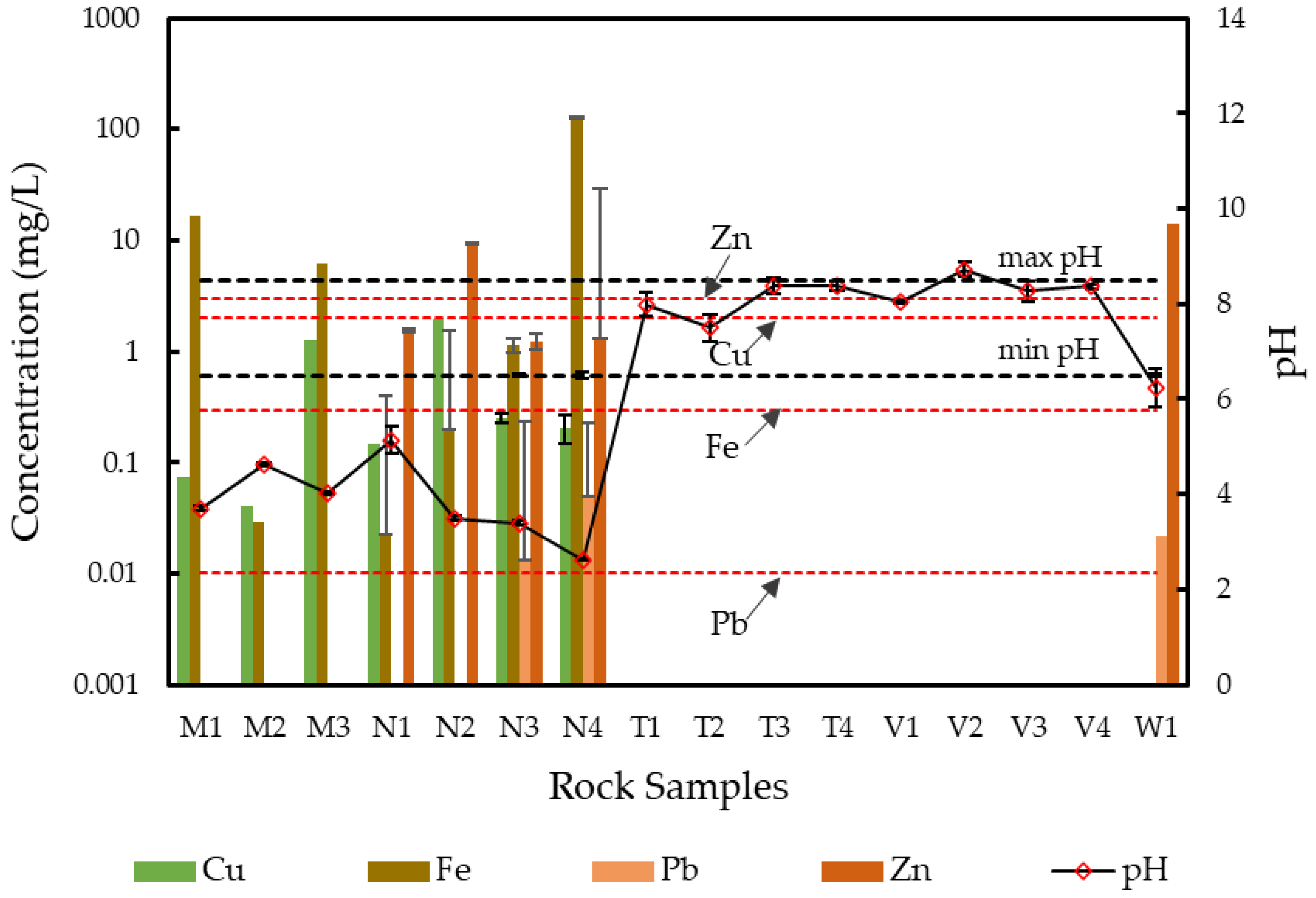
3.2. Leachate Characteristics of the Rock Samples—Release and Retention Mechanisms
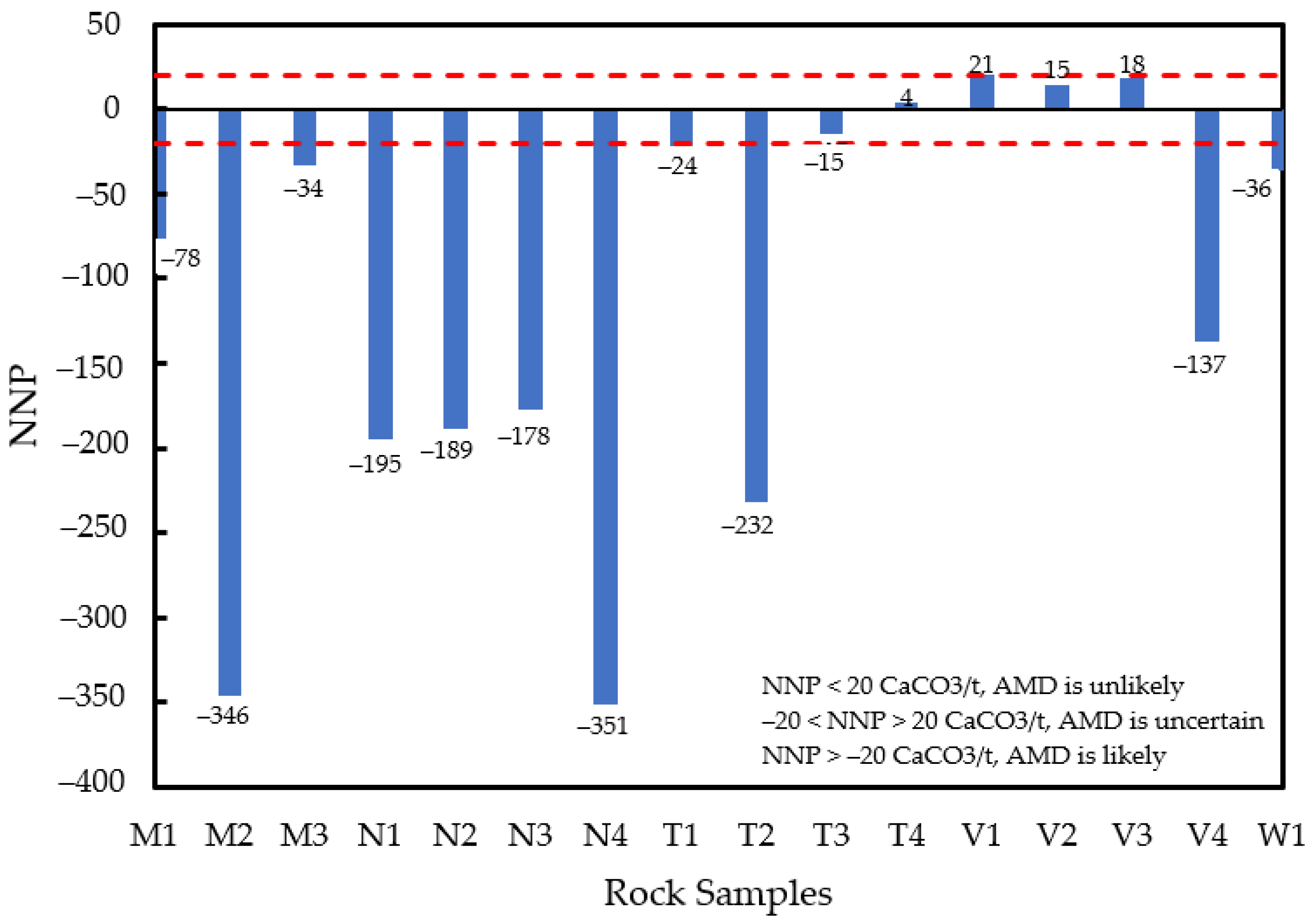
3.3. Relationship between the Rock Samples and Their Vulnerability in Generating Acid Mine Drainage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Q.; Wang, G.; Liang, X.; Qu, S.; Shi, Z.; Wang, X. Determination of mining-induced changes in hydrogeological parameters of overburden aquifer in a coalfield, northwest China: Approaches using the water level responses to earth tides. Geofluids 2021, 2021, 5516997. [Google Scholar] [CrossRef]
- McKinnon, E. The environmental effects of mining waste disposal at Lihir Gold Mine, Papua New Guinea. Rural Remote Health 2002, 1, 40–50. [Google Scholar]
- Banks, G.; Paull, D.; Mockler, S. The Social and Environmental Impact of Mining in Asia-Pacific: The Potential Contribution of a Remote-Sensing Approach; Resource Management in Asia-Pacific Working paper No. 60; The Australian National University: Canberra, Australia, 2005. [Google Scholar]
- Mudd, G.M.; Roche, C.; Northey, S.A.; Jowitt, S.M.; Gamato, G. Mining in Papua New Guinea: A complex story of trends, impacts and governance. Sci. Total Environ. 2020, 741, 140375. [Google Scholar] [CrossRef]
- Worlanyo, A.S.; Jiangfeng, L. Evaluating the environmental and economic impact of mining for post-mined land restoration and land-use: A review. J. Environ. Manag. 2021, 279, 111623. [Google Scholar] [CrossRef] [PubMed]
- Fashola, M.O.; Ngole-Jeme, V.M.; Babalola, O.O. Heavy metal pollution from gold mines: Environmental effects and bacterial strategies for resistance. Int. J Environ. Res. Public Health 2016, 13, 1047. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Saharan, M.R.; Gupta, K.K.; Jamal, A.; Sheoran, A.S. Management of acidic effluents from tailing dams in metalliferous mines. Mine Water Environ. 1995, 14, 85–93. [Google Scholar] [CrossRef]
- Akcil, A.; Soner, K. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Foli, G.; Apeah, O.B.; Amedjoe, C.G. Pre-mining water quality prediction from non-weathered sulphide ores along the Ashanti metallogenic belt in Ghana using Acid-Base accounting procedure. Am. J. Sci. Ind. Res. 2011, 2, 827–833. [Google Scholar] [CrossRef]
- Kumar, S.; Islam, A.R.M.T.; Islam, H.T.; Hasanuzzaman, M.; Ongoma, V.; Khan, R.; Mallick, J. Water resources pollution associated with risks of heavy metals from Vatukoula Goldmine region, Fiji. J. Environ. Manag. 2021, 293, 112868. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mosley, L. Trace metal levels in drinking water on Viti Levu, Fiji Islands. South Pac. J. Nat. Sci. 2003, 21, 31–34. [Google Scholar] [CrossRef]
- Kumar, S.; Islam, A.R.M.T.; Hasanuzzaman, M.; Salam, R.; Islam, M.S.; Khan, R.; Rahman, M.S.; Pal, S.C.; Ali, M.M.; Idris, A.M.; et al. Potentially toxic elemental contamination in Wainivesi River, Fiji impacted by gold-mining activities using chemometric tools and SOM analysis. Environ. Sci. Pollut. Res. 2022, 29, 42742–42767. [Google Scholar] [CrossRef]
- Ko, B.G.; Anderson, C.W.; Bolan, N.S.; Huh, K.Y.; Vogeler, I. Potential for the phytoremediation of arsenic-contaminated mine tailings in Fiji. Soil Res. 2008, 46, 493–501. [Google Scholar] [CrossRef]
- Matanitobua, V.; Ng, J.; Chiswell, B.; Harris, H.; Aalbersberg, W.; Noller, B. Arsenic adsorption and desorption processes downstream of a Fijian gold mine. In Arsenic in Geosphere and Human Diseases, Proceedings of the Arsenic 2010: Third International Congress on Arsenic in the Environment (As-2010), Tainan, Taiwan, 17–21 May 2010; CRC Press: London, UK, 2010; pp. 176–178. [Google Scholar]
- Matakarawa, S. Gold Mining and Acute Respiratory Infection in Children: A Retrospective Cohort Study in Vatukoula, Fiji. Master’s Thesis, University of Canterbury, Christchurch, New Zealand, 2018. [Google Scholar]
- Rabuku, A.T.W.; Abdul, Q.M. Natural radioactivity measurement of gold mine tailings in Vatukoula, Fiji Islands. Renew. Energy Environ. Sustain. 2020, 5, 10. [Google Scholar] [CrossRef]
- Mataki, M.; Koshy, K.C.; Lal, M. Baseline Climatology of Viti Levu (Fiji) and Current Climatic Trends. Pac. Sci. 2006, 60, 49–68. [Google Scholar] [CrossRef]
- Ongoma, V.; Rahman, M.A.; Ayugi, B.; Nisha, F.; Galvin, S.; Shilenje, Z.W.; Ogwang, B.A. Variability of diurnal temperature range over Pacific Island countries, a case study of Fiji. Meteorol. Atmos. Phys. 2021, 133, 85–95. [Google Scholar] [CrossRef]
- Colley, H.; Flint, D.J. Metallic Mineral Deposits of Fiji, No.4, Mineral Resources Department Memoir; Government of Fiji: Suva, Fiji, 1995; 196p.
- Turner, S.J. Fluid Inclusion, Alteration, and Ore Mineral Studies of an Epithermal, Mineralized Vein System: Mount Kasi, Vanua Levu, Fiji. Master’s Thesis, University of Auckland, Auckland, New Zealand, 1986. [Google Scholar]
- Colley, H.; Rice, C.M. A kuroko type ore deposit in Fiji. Econ. Geol. 1975, 70, 1373–1386. [Google Scholar] [CrossRef]
- Colley, H.; Rice, C.M. Kuroko-type deposits in Vanua Levu, Fiji. N. Z. J. Geol. Geophys. 1978, 21, 277–285. [Google Scholar] [CrossRef]
- Ashley, P.M.; Andrew, A.S. Petrographic, fluid inclusion and stable isotope investigation of a suite of samples from the Tuvatu prospect, Fiji. Commonwealth Sci. Indust. Res. Org. 1989, 50, 36. [Google Scholar]
- Feudigmann, P.; Taylor, I.; Woodward, A.; Lee, A.F.; Morgan, D.M. Lion One Metals Tuvatu Gold Project Preliminary Economic Assessment; National Instrument 43-101 Technical Report; Lion One: Nadi, Fiji, 2015. [Google Scholar]
- Ahmad, M.; Solomon, M.; Walsh, J.L. Mineralogical and geochemical studies of the Emperor gold telluride deposit, Fiji. Econ. Geol. 1987, 82, 345–370. [Google Scholar] [CrossRef]
- Anderson, W.B.; Eaton, P.C. Gold mineralisation at the Emperor mine, Vatukoula, Fiji. J. Geochem. Explor. 1990, 36, 267–296. [Google Scholar] [CrossRef]
- Eaton, P.C.; Setterfield, T.N. The relationship between epithermal and porphyry systems within the Tavua Caldera, Fiji. Econ. Geol. 1993, 88, 1053–1083. [Google Scholar] [CrossRef]
- White, N.C.; Leake, M.J.; McCaughey, S.N.; Parris, B.W. Epithermal gold deposits of the southwest Pacific. J. Geochem. Explor. 1995, 54, 87–136. [Google Scholar] [CrossRef]
- Houtz, R.E. Geology of North Tailevu, Viti Levu; Bulletin 1; Fiji Geological Survey: Viti Levu, Fiji, 1958; p. 19. [Google Scholar]
- JIS A 1226; Test Method for Ignition Loss of Soils. Japanese Standards Association: Tokyo, Japan, 2020; pp. 1–10.
- Marove, C.A.; Tangviroon, P.; Tabelin, C.B.; Igarashi, T. Leaching of hazardous elements from Mozambican coal and coal ash. J. Afr. Earth Sci. 2020, 168, 103861. [Google Scholar] [CrossRef]
- JLT-13; Departmental Notification No. 13 on Leaching Test Method for Landfill Wastes. Japanese Environmental Agency: Tokyo, Japan, 1973.
- Tabelin, C.B.; Hashimoto, A.; Igarashi, T.; Yoneda, T. Leaching of boron, arsenic, and selenium from sedimentary rocks: II. pH dependence, speciation, and mechanisms of release. Sci. Total Environ. 2014, 473, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Huyen, D.T.; Tabelin, C.B.; Thuan, H.M.; Dang, D.H.; Truong, P.T.; Vongphuthone, B.; Kobayashi, M.; Igarashi, T. The solid-phase partitioning of arsenic in unconsolidated sediments of the Mekong Delta, Vietnam and its modes of release under various conditions. Chemosphere 2019, 233, 512–523. [Google Scholar] [CrossRef]
- Khoeurn, K.; Sasaki, A.; Tomiyama, S.; Igarashi, T. Distribution of zinc, copper, and iron in the tailings dam of an abandoned mine in Shimokawa, Hokkaido, Japan. Mine Water Environ. 2019, 30, 119–129. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Silwamba, M.; Paglinawan, F.C.; Mondejar, A.J.S.; Duc, H.G.; Resabal, V.J.; Opiso, E.M.; Igarashi, T.; Tomiyama, S.; Ito, M.; et al. Solid-phase partitioning and release-retention mechanisms of copper, lead, zinc, and arsenic in soils impacted by artisanal and small-scale gold mining (ASGM) activities. Chemosphere 2020, 260, 127574. [Google Scholar] [CrossRef]
- Mufalo, W.; Tangviroon, P.; Igarashi, T.; Ito, M.; Sato, T.; Chirwa, M.; Nyambe, I.; Nakata, H.; Nakayama, S.; Ishizuka, M. Solid-phase partitioning and leaching behavior of Pb and Zn from playground soils in Kabwe, Zambia. Toxics 2021, 9, 248. [Google Scholar] [CrossRef]
- Gustafsson, J.P. Visual Minteq 3.1 User Guide; KTH, Department of land and Water Resources: Stockholm, Sweden, 2014; p. 550. [Google Scholar]
- Lawrence, K.A.; Wang, Y. Determination of neutralization potential in the prediction of acid rock drainage. In Proceedings of the 4th International Conference of Acid Rock Drainage, Vancouver, BC, Canada, 31 May–6 June 1997; pp. 451–464. [Google Scholar]
- Wang, L.; Li, Y.; Cui, X.; Wang, X.; Lu, A.; Wang, X.; Wang, C.; Gan, D. Weathering behavior and metal mobility of tailings under an extremely arid climate at Jinchuan Cu-Ni sulfide deposit, Western China. J. Geochem. Explor. 2017, 173, 1–12. [Google Scholar] [CrossRef]
- JGS 0271-2016; Test Method for Acidification Potential of Soils and Rock by Using Hydrogen Peroxide Solution. Japanese Geotechnical Society: Tokyo, Japan, 2016.
- Igarashi, T.; Oyama, T.; Saito, N. Experimental study on acidification potential of leachate from sedimentary rocks containing pyrite. J. Jpn. Soc. Eng. Geol. 2001, 42, 214–221. [Google Scholar] [CrossRef]
- Inui, T.; Katayama, M.; Katsumi, T.; Takai, A.; Kamon, M. Evaluating the long-term leaching characteristics of heavy metals in excavated rocks. J. Soc. Mater. Sci. Jpn. 2014, 63, 73–78. [Google Scholar] [CrossRef]
- Clarke, R.; Smith, D.; Naden, J. Source controls on: Regional geology and magmatic evolution of Fiji. Lithos 2022, 432–433, 106897. [Google Scholar] [CrossRef]
- Clarke, R.; Smith, D.J.; Naden, J.; Holwell, D.; Mann, S. Mineralogical constraints on the genesis of an alkalic-type epithermal Au-Te deposit: Tuvatu, Fiji. Ore Geol. Rev. 2023, 154, 105279. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Tabelin, C.B.; Igarashi, T.; Villacorte-Tabelin, M.; Park, I.; Opiso, E.M.; Ito, M.; Hiroyoshi, N. Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: A review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci. Total Environ. 2018, 645, 1522–1553. [Google Scholar] [CrossRef]
- Igarashi, T.; Herrera, P.S.; Uchiyama, H.; Miyamae, H.; Iyatomi, N.; Hashimoto, K.; Tabelin, C.B. The two-step neutralization ferrite-formation process for sustainable acid mine drainage treatment: Removal of copper, zinc, and arsenic and the influence of coexisting ions on ferritization. Sci. Total Environ. 2020, 715, 136877. [Google Scholar] [CrossRef]
- Bao, Y.; Guo, C.; Lu, G.; Yi, X.; Wang, H.; Dang, Z. Role of microbial activity in Fe (III) hydroxysulfate mineral transformations in an acid mine drainage-impacted site from the Dabaoshan Mine. Sci. Total Environ. 2018, 616, 647–657. [Google Scholar] [CrossRef]
- Kelderman, P.; Osman, A.A. Effect of redox potential on heavy metal binding forms in polluted canal sediments in Delft (The Netherlands). Water Res. 2007, 41, 4251–4261. [Google Scholar] [CrossRef]
- Popenda, A. Effect of redox potential on heavy metals and As behavior in dredged sediments. Desalination Water Treat. 2014, 52, 3918–3927. [Google Scholar] [CrossRef]
- Tangviroon, P.; Noto, K.; Igarashi, T.; Kawashima, T.; Ito, M.; Sato, T.; Mufalo, W.; Chirwa, M.; Nyambe, I.; Nakata, H.; et al. Immobilization of lead and zinc leached from mining residual materials in Kabwe, Zambia: Possibility of Chemical Immobilization by Dolomite, Calcined Dolomite, and Magnesium Oxide. Minerals 2020, 10, 763. [Google Scholar] [CrossRef]
- Khoshraftar, Z.; Hadiseh, M.; Ahad, G. An insight into the potential of dolomite powder as a sorbent in the elimination of heavy metals: A review. Case Stud. Chem. Environ. Eng. 2022, 7, 100276. [Google Scholar] [CrossRef]
- Mufalo, W.; Tangviroon, P.; Arima, T.; Igarashi, T.; Ito, M.; Noto, K.; Kawashima, T.; Nyambe, I.; Nakata, H.; Nakayama, S. Immobilization of Pb and Zn leached from mining residue materials in Kabwe, Zambia: Performance of calcined dolomite in column experiments. J. Geochem. Explor. 2023, 249, 107209. [Google Scholar] [CrossRef]
- Gu, X.; Evans, L.J. Surface complexation modelling of Cd (II), Cu (II), Ni (II), Pb (II), and Zn (II) adsorption onto kaolinite. Geochim. Cosmochim. Acta 2008, 72, 267–276. [Google Scholar] [CrossRef]
- Gunsinger, M.R.; Ptacek, C.J.; Blowes, D.W.; Jambor, J.L.; Moncur, M.C. Mechanisms controlling acid neutralization and metal mobility within a Ni-rich tailings impoundment. J. Appl. Geochem. 2006, 21, 1301–1321. [Google Scholar] [CrossRef]
- Brady, K.B.; Cravotta, C.A. Acid base accounting: An improved method of interpreting overburden chemistry to predict quality of coal mine drainage. In Proceedings of the 13th Annual West Virginia Surface Mine Drainage Task Force Symposium, Morgantown, WV, USA, 8–9 April 1992. [Google Scholar]
- Stewart, W.A.; Miller, S.D.; Smart, R. Advances in acid rock drainage (ARD) characterization of mine wastes. In Proceedings of the 7th International Conference on Acid Rock Drainage (ICARD), American Society of Mining and Reclamation (ASMR) 2006, St. Louis, MI, USA, 26–30 March 2006. [Google Scholar]
- Simate, G.S.; Ndlovu, S. Acid mine drainage: Challenges and opportunities. J. Environ. Chem. Eng. 2014, 2, 1785–1803. [Google Scholar] [CrossRef]
- Assawincharoenkij, T.; Hauzenberger, C.; Ettinger, K.; Sutthirat, C. Mineralogical and geochemical characterization of waste rocks from a gold mine in northeastern Thailand: Application for environmental impact protection. Environ. Sci. Pollut. Res. 2018, 25, 3488–3500. [Google Scholar] [CrossRef] [PubMed]

| Sample | SiO2 (wt%) | TiO2 (wt%) | Al2O3 (wt%) | Fe2O3 (wt%) | MnO (wt%) | MgO (wt%) | CaO (wt%) | Na2O (wt%) | K2O (wt%) | P2O5 (wt%) | As (mg/kg) | Cd (mg/kg) | Cu (mg/kg) | Pb (mg/kg) | Zn (mg/kg) | S (wt%) | TC (wt%) | IC (wt%) | LOI % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | 79.3 | 0.64 | 17.0 | 4.90 | <0.01 | <0.01 | 0.04 | 0.02 | 0.02 | 0.11 | 92 | 2 | 61 | 15 | 10 | 6.28 | 0.10 | <0.01 | 7.06 |
| M2 | 90.4 | 0.34 | 2.00 | 2.76 | <0.01 | <0.01 | 0.03 | 0.14 | 0.13 | 0.08 | 137 | 1 | 29 | 28 | 3 | 6.31 | 0.01 | <0.01 | 1.62 |
| M3 | 84.1 | 0.39 | 12.9 | 3.79 | <0.01 | 0.03 | 0.05 | 0.00 | 0.03 | 0.17 | 38 | <2 | 67 | 11 | 4 | 6.76 | <0.01 | <0.01 | 5.46 |
| N1 | 79.1 | 0.47 | 13.8 | 2.35 | <0.01 | 0.31 | 1.93 | 6.51 | 0.50 | <0.01 | 50 | 1 | 50 | 5 | 140 | 0.37 | 0.02 | <0.01 | 0.99 |
| N2 | 2.88 | 0.36 | 1.47 | 96.5 | 0.03 | <0.01 | 0.11 | <0.01 | 0.03 | <0.01 | 619 | <2 | 986 | 208 | 535 | 1.30 | 0.13 | <0.01 | 7.01 |
| N3 | 68.5 | 0.01 | 0.54 | 26.4 | 0.09 | <0.01 | 0.05 | <0.01 | 0.05 | 0.36 | 590 | <1 | 327 | 519 | 460 | 0.81 | 0.01 | <0.01 | 5.91 |
| N4 | 1.84 | 0.11 | 6.50 | 40.0 | 0.01 | <0.01 | 0.08 | 0.63 | 0.02 | 0.06 | 7 | <1 | 83 | 21 | 59 | 52.3 | 0.67 | <0.01 | 7.60 |
| T1 | 62.7 | 0.52 | 21.2 | 3.89 | 0.06 | 8.27 | 3.35 | 5.25 | 0.12 | 0.27 | <1 | <1 | 6 | <1 | 59 | 0.05 | 0.76 | 0.61 | 1.68 |
| T2 | 53.7 | 0.73 | 16.3 | 11.4 | 0.22 | 8.21 | 10.6 | 2.71 | 2.93 | 0.67 | 6 | <1 | 152 | 3 | 87 | 0.19 | 0.01 | <0.01 | 0.52 |
| T3 | 58.0 | 0.57 | 18.6 | 8.05 | 0.23 | 5.49 | 7.03 | 3.06 | 4.64 | 0.47 | 4 | <1 | 190 | 8 | 112 | 0.83 | 0.04 | 0.06 | 1.17 |
| T4 | 62.4 | 0.45 | 20.2 | 2.69 | 0.07 | 3.18 | 2.40 | 3.92 | 7.63 | 0.35 | 66 | 1 | 30 | 21 | 180 | 0.76 | 0.01 | 0.46 | 1.11 |
| V1 | 43.2 | 0.44 | 11.9 | 9.93 | 0.24 | 9.16 | 13.7 | 1.81 | 2.59 | 0.33 | 4 | <1 | 144 | 4 | 76 | 2.30 | 1.26 | 1.69 | 4.87 |
| V2 | 57.9 | 0.46 | 20.3 | 6.47 | 0.15 | 4.89 | 5.04 | 3.28 | 4.64 | 0.64 | 1 | 1 | 82 | 9 | 78 | 0.05 | 0.65 | 0.56 | 3.51 |
| V3 | 46.5 | 0.49 | 12.4 | 10.2 | 0.20 | 14.0 | 13.0 | 1.23 | 0.83 | 0.30 | 3 | <1 | 126 | 5 | 81 | 0.51 | 0.98 | 0.72 | 3.97 |
| V4 | 59.1 | 0.50 | 19.5 | 6.82 | 0.16 | 4.93 | 6.24 | 4.52 | 4.26 | 0.67 | 2 | <1 | 161 | 9 | 82 | 0.21 | 0.31 | 0.37 | 1.36 |
| W1 | 23.6 | 0.04 | 3.51 | 10.7 | <0.01 | 0.02 | <0.01 | <0.01 | 0.42 | 0.03 | 80 | 1600 | 28,700 | 507 | 358,000 | 70.7 | <0.01 | <0.01 | 4.41 |
| Variables | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| pH | −0.381 | 0.141 | −0.078 | −0.276 |
| EC | 0.308 | 0.393 | −0.082 | 0.103 |
| ORP | 0.362 | −0.171 | 0.181 | 0.310 |
| Al | 0.253 | −0.049 | 0.471 | −0.081 |
| Cu | 0.238 | −0.097 | 0.469 | −0.077 |
| Fe | 0.317 | 0.220 | −0.299 | 0.166 |
| Mg | −0.076 | 0.325 | 0.345 | −0.062 |
| Mn | 0.121 | −0.186 | 0.049 | 0.316 |
| Pb | 0.331 | 0.129 | −0.358 | −0.058 |
| Zn | 0.178 | −0.111 | 0.139 | −0.618 |
| Ca | −0.123 | 0.452 | 0.209 | −0.125 |
| K | −0.163 | 0.395 | 0.072 | 0.143 |
| Na | −0.297 | 0.052 | 0.047 | 0.290 |
| SO42− | 0.262 | 0.451 | 0.097 | 0.021 |
| Si | −0.216 | −0.008 | 0.306 | 0.410 |
| Eigenvalue | 5.00 | 3.03 | 2.42 | 1.30 |
| Variance% | 31.6 | 21.1 | 16.6 | 8.6 |
| Cumulative% | 31.6 | 52.7 | 69.2 | 77.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soro, A.T.; Mufalo, W.; Arima, T.; Tabelin, C.B.; Igarashi, T. Geochemical Characterization of Rock Samples from Selected Fiji Mine Sites to Evaluate On-Site Environmental Vulnerabilities. Minerals 2023, 13, 661. https://doi.org/10.3390/min13050661
Soro AT, Mufalo W, Arima T, Tabelin CB, Igarashi T. Geochemical Characterization of Rock Samples from Selected Fiji Mine Sites to Evaluate On-Site Environmental Vulnerabilities. Minerals. 2023; 13(5):661. https://doi.org/10.3390/min13050661
Chicago/Turabian StyleSoro, Apete Tuiyaro, Walubita Mufalo, Takahiko Arima, Carlito Baltazar Tabelin, and Toshifumi Igarashi. 2023. "Geochemical Characterization of Rock Samples from Selected Fiji Mine Sites to Evaluate On-Site Environmental Vulnerabilities" Minerals 13, no. 5: 661. https://doi.org/10.3390/min13050661
APA StyleSoro, A. T., Mufalo, W., Arima, T., Tabelin, C. B., & Igarashi, T. (2023). Geochemical Characterization of Rock Samples from Selected Fiji Mine Sites to Evaluate On-Site Environmental Vulnerabilities. Minerals, 13(5), 661. https://doi.org/10.3390/min13050661










