Abstract
Interpretation of stable isotope (C and O) composition of lacustrine carbonates requires in-depth knowledge about the interplay between the abiotic and biotic processes in sedimentary environments. The present study, focused on Mg-carbonates from a well-characterized alkaline and ephemeral lake, gives new insight into the behavior of the stable isotopes during the seasonal precipitation of a variety of carbonates. Dolomite and Mg-calcite precipitate intracellularly within Spirogyra during spring and show lighter isotopic signatures (δ13C aver. −4.10‰ and δ18O aver. −0.75‰, VPDB) than a second association of carbonates, such as hydromagnesite, northupite and traces of magnesite among other sodium-bearing carbonates (δ13C aver., −1.34‰ and δ18O aver. 4.52‰, VPDB). The latter precipitate in association with degraded microbial mats as the lake desiccates during summer. Covariant trends between carbonate δ13C and δ18O reflect isotope enrichment related to evapoconcentration. The seasonal cycling of inorganic carbon among carbonate minerals, microbial biomass, lake water and pore water was also analyzed, revealing variations of δ13C within a range of −12.40‰ to −0.43‰. The more depleted 13C derives from the decay of the microbial mats. The less negative values are distinctive of the bulk carbonates forming crusts in summer. Intracellular calcite and dolomite have δ13C and δ18O values (VPDB) ranging, from −5.45‰ to −3.07‰ and −2.48‰ to 1.58‰, respectively, that are intermediate between those two endmembers. These intracellular carbonates are enriched in 13C by 5‰ with respect to dissolved inorganic carbon (δ13C in the range of −11.79‰ to −6.87‰, VPDB) due to the vital effect of photosynthesis. The crust of carbonates deposited as the lake desiccates dissolve interannually. Alternatively, dolomite and Mg-calcite as well as their isotopic compositions persist during synsedimentary diagenesis, confirming that carbonate biominerals provide isotopic signatures related to the environmental conditions of formation with potential of preservation in the rock record.
1. Introduction
Dolomite deposits are common in ancient lacustrine successions, and these carbonates have often formed during deposition [1,2,3,4,5,6]. In modern ephemeral environments, dolomite [CaMg(CO3)2] precipitation is favored by high-carbonate alkalinities and high Mg/Ca ratio conditions and may be or not enhanced by evaporation processes [7,8,9,10]. It is widely accepted that benthic and, to a lesser extent, planktonic microorganisms can catalyze the nucleation of dolomite in alkaline lakes [9,11,12,13,14,15,16]. Multiple field and laboratory studies have demonstrated that the precipitation of dolomite at low-temperature occurs in specific metabolic pathways, including sulfate reduction [11,17,18,19], sulfide oxidation [20], aerobic respiration [21], methanogenesis [22,23], methane oxidation [24], oxygenic [25,26] and anoxygenic [27] photosynthesis, although the mineralization of microbial mats is commonly a result of the interplay between different metabolisms [28].
Recently, ref. [10] provided a new mechanism of mineralization of dolomite and magnesian calcite by intracellular accumulation within the filamentous algae Spirogyra (Chlorophyta). This finding links the precipitation of dolomite and Mg-calcite [(Ca1−x Mgx)CO3] to the photosynthetic removal of CO2 from H2O by algae during springtime. In shallow alkaline environments, carbonate mineral precipitation (mostly hydromagnesite, Mg5 (CO3)4(OH)2·4H2O) is caused by the degradation and dehydration of the microbial substrate as the lake desiccates [9]. Alternatively, it is suggested that magnesite [MgCO3] precipitates directly from the solution or forms through the dissolution-re-precipitation after hydromagnesite [29]. In the geological record, the mechanisms of formation of carbonates are inferred using several chemical and sedimentary signatures. Among others, carbon and oxygen stable isotopes of lacustrine carbonates have been long used in paleoenvironmental reconstruction [2,3,5,6,16,30,31,32,33]. However, updating the interpretation of the isotopic signal of ancient Mg-carbonates has proven difficult because the effects of microbial metabolisms under known environmental conditions on the isotopic values of Mg-carbonates have been poorly constrained in modern lakes. The highly alkaline water bodies of the Coca-Olmedo wetland (Central Spain), with a pH higher than 9, are one of the few modern environments where dolomite and calcite precipitate in association with magnesite and hydromagnesite, among other carbonates [9,15,34,35]. Overall, alkaline lakes play a role in the global carbon cycle, through CO2 sequestration, that at high pH is rapidly transformed into HCO3− and CO3− [36], as well as by the extensive precipitation of Mg-carbonates [9]. In the seasonal Lake Caballo Alba, one of the water basins of the Coca-Olmedo wetland, the Mg-carbonates precipitate via distinct mechanisms as the lake desiccates, resulting in the deposition of biominerals and crusts. To the best of our knowledge, Caballo Alba is the only lake where the intracellular accumulation of magnesium carbonates by the alga Spirogyra and its involvement in changing the chemistry of the water was reported [10], which offers a unique opportunity to analyze the isotopic signal of this process. The aim of this work was to study the impact of these formative conditions in the stable isotope compositions of the carbonate minerals from a broad perspective, including the inorganic carbon of the solutions and the extracellular polymeric substance reservoirs. The use of the isotopes as environmental indicators in the geological record of microbial Mg-carbonates is also discussed by contrasting the isotopic compositions of dolomite and calcite deposited on the surface lake sediment with those preserved in buried sediment layers.
Geological Setting and Hydrochemistry
Lake Caballo Alba is located at an altitude of 768 m in a closed wetland (41°14′38.6″ N/4°36′22.9″ W) in the south-western area of the Duero Depression (Central Spain) (Figure 1). The water basin overlies Cenozoic successions of the Duero Basin. The lake, with a maximum area of 0.17 km2, is a shallow basin up to 0.5 m deep [37]. The formation and morphology of Caballo Alba were controlled by NNW-SSE faults developed in the substrate. These fractures provide pathways of groundwater to the lake that represent the main water source [37]. Low contributions of water derive from a small and highly intermittent creek. The wetland has a continental climate, with low annual precipitation, usually averaging 400 to 500 mm. The annual temperatures range between −10 °C in January and 38 °C in July [15,34]. The lake waters are brackish to saline (1 a 9 g·L−1), and their composition ranges from Na+-Cl−(SO42−)-(HCO3−), Na+-HCO3− and Na+-Cl− [15]. Notably, the Mg/Ca ratio varies seasonally from 5.5 to 267.7.
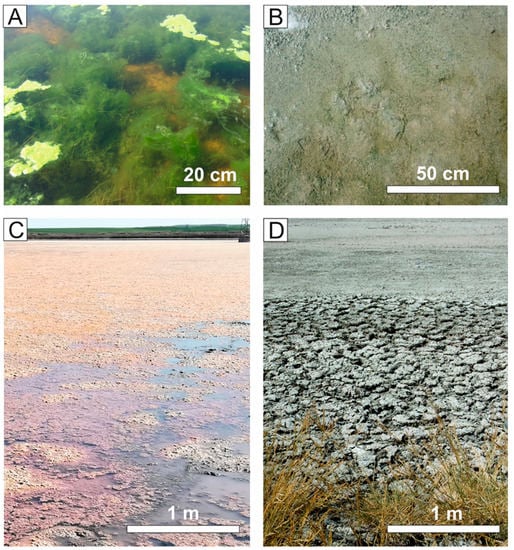
Figure 1.
Seasonal sedimentary variations in Lake Caballo Alba. (A) Floating microbial mats containing intracellular dolomite and Mg-calcite (April 2018). (B) View of benthic microbial mats (December 2018). (C) Extensive and subaerially exposed microbial mats (March 2019). (D) Desiccation cracks formed in the subarially exposed microbial mats (July 2020).
In spring, benthic microbial mats thrive and expand across the lake. Eventually the mats float as the oxygen bubbles produced by photosynthetic microbes rise to the surface (Figure 1). Floating mats comprise the filamentous alga Spirogyra, Diatoms and Cyanobacteria as main producers. Dolomite and magnesian calcite form intracellularly within Spirogyra coinciding with low values of dissolved bicarbonate and carbonate in the water [10]. From June to October, corresponding to the lower precipitation period of the year, the surficial lake water dries and transforms into a playa. A whitish mineral crust precipitates on the playa floor. The crust, besides calcite and dolomite, contains a variety of authigenic carbonates (nesquehonite, northupite, natron and trona) among other minerals [10,15,34].
2. Materials and Methods
2.1. Analyzed Samples
Analyses of stable isotopes were carried out in different reservoirs: carbonate minerals, surficial lake water, pore water and extracellular polymeric substances (EPSs) secreted by the microbial populations (Table 1, Tables S1 and S3). The analyses of carbonates were performed on twenty-five samples of sediment containing calcite and dolomite collected from benthic and floating mats during 2017 and 2021 (Figure 1A–C). Dolomite was isolated from calcite in five of these samples by selective dissolution of calcite in contact with 1 M of solution of acetic acid and sodium acetate during 24 h [38]. Dissolution of calcite was confirmed by XRD analysis. Five samples comprising complex assemblages of carbonates were collected from desiccated mats during the dry season (Figure 1D). Five carbonate samples were extracted from a lake sediment core (14 cm long). C and O stable isotopes were analyzed on five non-filtrated water samples, four corresponding to surficial waters and one to porewater (Figure 1B). For comparison with these, an additional filtrated sample was analyzed. The inorganic carbon and oxygen of EPS of benthic and floating microbial mats was also analyzed in purified and non-purified samples (Table S3).

Table 1.
Carbonate content and isotopic signatures of wet and dry surficial sediments and a core. Abbreviations: hydromagnesite (Hmg), northupite (Ntp), gregoryite (Na-Cb), tychite (Tyc), calcite (Cc), dolomite (Dol) and magnesite (Mgs).
2.2. Carbon and Oxygen Isotope Analyses
Carbon and oxygen isotope analyses were performed in continuous flow mode following the procedure described in [39] using an isotope ratio mass spectrometer (IRMS) Thermo Scientific MAT-253 mass spectrometer coupled with a Kiel IV device specific to carbonate micro-sample analyses. These analyses were performed in the ISOLAB-1 analytical unit at the Complutense University of Madrid, Spain. No pre-treatment to remove the organic was performed due to the very low organic content of the majority of lacustrine samples [40]. Depending on the carbonate content (in a ratio of 100 µg for samples with abundances of 50% carbonate), a homogenized fine fraction of sediment sample powder was weighed into borosilicate glass vials and oven-dried at 104 °C overnight. Samples were run with supersaturated phosphoric acid at 70 °C for 12 min for mixtures of calcite and dolomite and 22 min for more complex assemblages together with international standards NBS-18 and NBS-19 (IAEA) and Carrara Marble as internal standard for 1 h. All results are reported in the traditional δ notation in parts per thousand (‰) relative to PDB (δ13C, δ18O) and SMOW (δ18O) (Table 1). The external standard deviation for oxygen is ˂0.08‰ and for carbon ˂0.04‰. The isotopic composition of isolated dolomite and details regarding sample preparation and measurement are reported in [41].
Lake water, pore water and microbial extracellular polymeric substances were analyzed in a Thermo Finningan Gasbench II interfaced with a Thermo Finningan Delta mass spectrometer in the Stable Isotope Laboratory of Earth Science Department at the Vrije Universiteit Amsterdam, The Netherlands. δ18O analyses of water samples were carried out on borosilicate vials of 12 mL where samples and standards were as follows: DNS3, ALW, KEILA (calibrated using the VSMOW) and KONA (control standard) were transferred and flushed with 0.2% CO2 in helium. After 24 h of equilibration, the oxygen isotope ratio was then measured. The standard deviation of the δ18O value of KONA is <0.1‰. For the determination of DIC δ13C in water samples, the alkalinity of the sample was measured in order to calculate the exact amount of sample transferred into 12 mL exetainer vials. The vials were closed with a pierceable cap and flushed with helium. Samples were acidified with a drop of 100% phosphoric acid. During the measurement, two carbonate standards (NBS19 and LSVEC) were used for the calibration of δ13C values. A solution of sodium bicarbonate was used as a control standard. For these analyses, the standard deviation is <0.15‰. For the DIC concentration, two more concentrations of sodium bicarbonate were prepared and used for the calibration of [DIC] versus amplitude of the CO2 peaks.
For comparison between water δ18O isotopic values (VSMOW) and those obtained from carbonates and EPS samples, conversion of δ18O VPDB values into δ18O VSMOW was performed following this equation: δ18O(VSMOW) = ((1.03092)×(δ18O VPDB)) + 30.92 [42].
2.3. Scanning Electron Microscopy
Scanning electron microscopy (SEM) of freshly fractured surficial sediment samples were air-dried and coated with Au. Imaging and microanalyses were performed using a FEI INSPECT microscope (FEI Company, Hillsboro, OR, USA) working at low vacuum mode and equipped with an Oxford Instruments energy dispersive X-ray spectroscopy (EDX) detector. Electron microphotographs and EDX analyses were carried out in the Service of Non-destructive Techniques at National Natural Sciences Museum, Madrid (Spain).
2.4. Molecular Diversity
2.4.1. DNA Extraction, Library Preparation and Sequencing
DNA extraction, library preparation and sequencing were performed at the Genomics Unit of the Complutense University of Madrid, Spain. Taxonomic analyses of the microbiota were based on assembled 16S rRNA transcripts. The DNA was extracted from 0.2 g of the floating and benthic mat samples using the Power Biofilm DNA Isolation Kit (MO BIO Laboratories, Qiagen, Germantown, MD, USA). The total DNA was quantified with a Qubit 2.0 fluorometer (Life Technologies, Carlsbad, CA, USA). Until further processed, samples were stored at −20 °C.
The sequences were prepared following the Illumina “16S rRNA genes sequencing library protocol (Illumina, San Diego, CA, USA)”. The hypervariable V3-V4 regions of the 16S rRNA gene were amplified using primers (314 F and 805R sequences) and Illumina-specific adaptors. Illumina-sequencing adapters and duela-index barcodes were added to the amplicons. The NEB Next Library Quantification kit was used to quantify libraries and pooled equally.
DNA libraries were paired-end sequenced with the use of the 600 cycle MiSeq Reagent Kit v3 following the protocol recommended by the manufacturer on an Illumina MiSeq system.
2.4.2. Analysis of 16S rRNA Sequencing Data
MiSeq Control software was used to demultiplex sequences, and illumine adaptors were trimmed The obtained FASTQ files of the V3-V4 16 rRNA hypervariable regions were merged, filtered and length-trimmed (default parameters) for taxonomy classification within the CLG Genomics Workbench—Microbial Genomic Module (Qiagen). Similarity of 97% or higher homology among sequences was considered as a cluster. Finally, Greengenes database v13.5 was compared with a representative sequence for each OTU. These analyses were carried out at Genomics Unit of the Complutense University of Madrid, Spain.
2.5. EPS Extraction and Purification
The EPS extraction protocol was based on previously described extractions of microbial mat samples [43,44]. A total of 400 g of microbial mat mass was homogenized with deionized water (1:1 water and EPS) and filtered using 45 µm nitrocellulose. The filtrate was centrifuged for 10 min at 2500 RPM and 4 °C. The precipitate was recovered by centrifugation (6000 RPM for 10 min) in an Eppendorf 5810R centrifuge and, finally, freeze-dried in a Telstar Cryodos device.
For EPS dialysis, 5 g of freeze-dried powdered EPS was dissolved in 40 mL deionized water. In order to remove carbonates, 1 mL of 1M HCl was added while stirring. The rehydrated EPS was placed into a dialysis bag (10 kDa) and dialyzed three times for 24 h against 1 mM of EDTA and four times with deionized water (>18 MΩ) at 4 °C.
3. Results
3.1. Microbial Mat Communities
The 16S rRNA-based analyses of floating and benthic microbial mats were dominated by Bacteria. At the phylum level, the bacterial diversity remained similar between the two kinds of microbial mats. The bacterial groups included Proteobacteria (up to 41%), Bacteroidetes (up to 29%), Firmicutes (up to 20%) and Cyanobacteria (up to 16%), which constituted up to 88% of the total prokaryotic diversity (Figure 2). Less abundant phyla (<10%) included Verrucomicrobia, Actinobacteria, Planctomycetes, Chloroflexi and others.
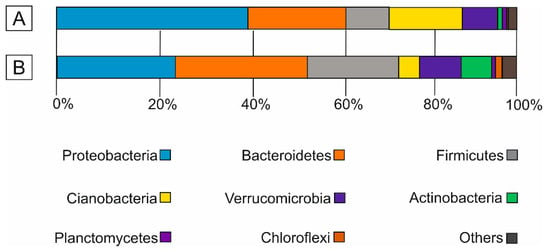
Figure 2.
Bar graphs showing the prokaryotic diversity at the phylum level of (A) floating (sample Ccd-12) and (B) benthic microbial mats (sample Ccd-11).
The results of bacterial diversity in the two microbial mats revealed a typical distribution at the phylum level. Invariably, the number of Cyanobacteria decreased in the submerged benthic microbial mats (Figure 2B) in relation to the exposed to air-floating microbial mats (Figure 2A). In contrast, the abundance of Firmicutes increased in the benthic microbial mats (Figure 2B). Proteobacteria were the dominant phylum in floating microbial mats, followed by Bacteroidetes. By contrast, the benthic microbial mats were highly enriched in Bacteroidetes (28.5%).
3.2. Mineral Associations
The analyses of surficial sediments through the years 2017–2021 showed seasonal variations of the mineral composition. During the dry period, which usually lasts from June to October, the dry sediments of the bed were mainly composed of carbonates (up to >45% of the total, Table 1, Figure 3). Among these carbonates, some magnesium minerals were abundant. Northupite [Na3Mg(CO3)2Cl] and hydromagnesite represented more than 30% of the bulk mineralogy. Less abundant were other Na-carbonates such as gregoryite [(Na2,K2,Ca)CO3] and tychite [Na6Mg2(CO3)4(SO4)] identified in amounts <10%. Additionally, trace amounts of magnesite were detected. Along with carbonates, silicates (<40% of the total), native sulfur (<10% of the total) and halides (<10% of the total) were typical of the crusts. In contrast, from November to June, the lakebed was covered by water or at least damp, decreasing the carbonates content to up to 30% in the wet sediments. In this period, carbonate minerals were mainly magnesium-enriched calcite and dolomite. Calcite persistently had higher abundance than dolomite. The ratio of the two carbonates remained relatively constant up to 14 cm in depth as reflected the mineralogy of a sediment core (Table 1).
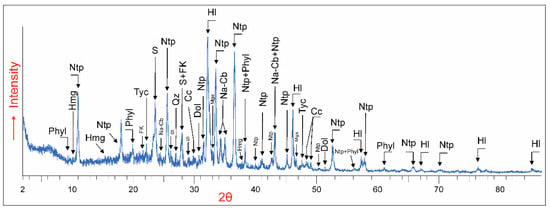
Figure 3.
X-ray diffractogram of sample Cm-4 showing the mineralogical composition of a representative sample taken from the upper part of the sediments. Patterns used for identification from the PDF2 database were hydromagnesite (Hmg) = 00-025-0513, northupite (Ntp) = 01-074-1843, mica clays (Phyl) = 01-082-0576, potassium feldspar (FK) = 01-084-0710, native sulfur (S) = 00-008-0247, halite (Hl) = 01-075-0306, gregoryite (Na-Cb) = 00-025-0815, tychite (Tyc) = 00-019-1212, calcite (Cc) = 01-083-1762, quartz (Qz) = 01-079-1910, dolomite (Dol) = 01-074-1687 and magnesite (Mgs) = 01-086-2344.
The SEM–EDX analyses of floating mats (Figure 1A) revealed that dolomite and Mg-calcite mainly occurred as inclusions in the filaments of the green algae Spirogyra (Figure 4 and Figure 5). The transparent sheaths of the algae enclosed the intracellular inclusions (Figure 4A). As these sheaths degraded, the minerals were released into the extracellular environment, where they formed complex organomineral associations. Scarce dolomite crystals were attached to the frustules of diatoms (Figure 4B and Figure 5(4)). Commonly, small barite (BaSO4) crystals were found to be associated with the carbonates and the algae remains (Figure 4A). Both calcite and dolomite precipitates contained variable proportions of Mg and Si (Figure 5).
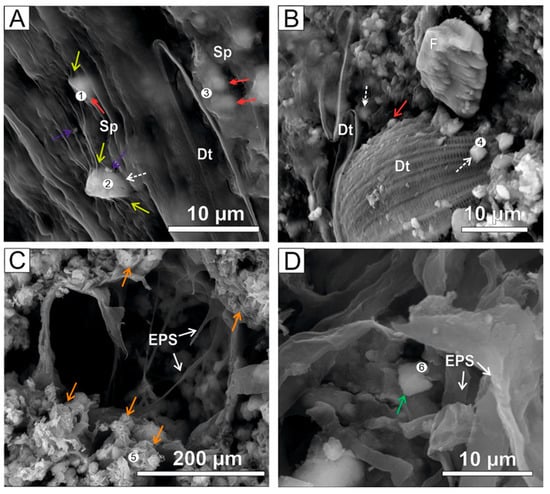
Figure 4.
SEM microphotographs of authigenic carbonates associated with Spirogyra sheaths (Sp, yellow arrows), diatoms (Dt) and EPS in Lake Caballo Alba. (A) Intracellular crystals of calcite (red arrows), dolomite (white arrows) and barite (purple arrows) are embedded by collapsed Spirogyra (sample Ccd-8). (B) Isolated calcite and dolomite crystals associated with decayed diatoms and algae (sample Ccd-8). (C) Globular aggregates of hydromagnesite (orange arrows) replacing decaying EPS (sample Cm-1). (D) Idiomorphic crystal of Northupite (green arrow) resting on decayed EPS (sample Cm-4). Note: The results of the selected EDS analyses (numbering points 1–6) are shown in Figure 5.
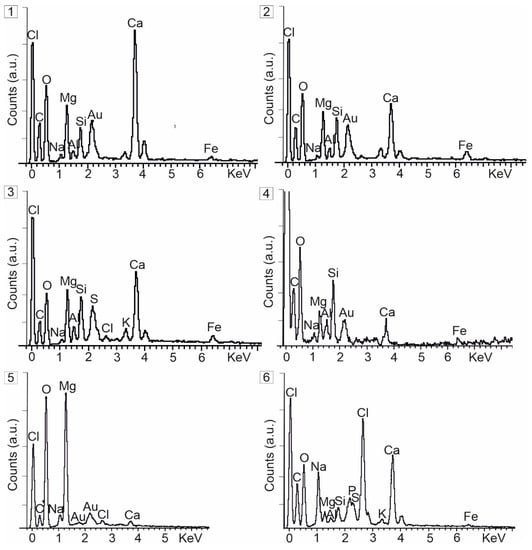
Figure 5.
EDS analysis results of numbering points (1–6) in Figure 4. (1) Mg-calcite (sample Ccd-8). (2) Dolomite (sample Ccd-8). (3) Mg-calcite (sample Ccd-8). (4) Dolomite and opal (sample Ccd-8). (5) Hydromagnesite (sample Cm-1). (6) Northupite (sample Cm-4).
In the dried sediment crusts, hydromagnesite precipitated as the biomass (EPS) was gradually degraded (Figure 4C and Figure 5(5)). Platelet-like hydromagnesite crystals appeared as slightly to highly tight aggregates that showed rosette and random morphologies (Figure 4C). Besides the hydrous Mg-carbonates, cubic and isolated northupite crystals rested on the EPS that contained abundant S and P (Figure 4D and Figure 5(6)).
3.3. Stable Isotopic Composition
Dissolved inorganic carbon (DIC) concentrations and the isotopic composition of the analyzed reservoirs showed remarkable variations along the studied period (see Table 2 and Figure 6).

Table 2.
Isotopic signatures of purified and non-purified extracellular polymeric substances (EPSs), and pore (Cw-4) and surface waters. * Purified EPS samples/Filtered water samples.
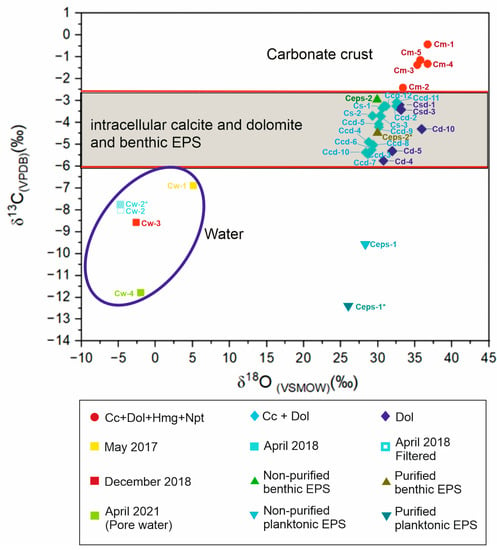
The concentration of DIC in the lake water (Table 2) varied greatly from 10,930 to 99,100 mM during spring, coincident with the lowest and highest values of salinity recorded in the study period at a pH higher than 9 (Table S2). Between these two extremes, intermediate values of DIC were recorded in winter lake water and in sediment pore waters. The δ13C of DIC (VPDB) ranged between −11.79‰ (pore water) and −6.87‰ (evapoconcentrated solutions). The δ13C DIC of water averaged −8.6‰ that was in the range of values obtained in the extracellular polymeric substances (EPSs) produced by phytoplankton in spring (Figure 6). Notably, the δ18O values of lake waters were always lighter than those recorded in other reservoirs. The lightest δ18O value of water (−4.72‰, VSMOW) was associated with high water levels and highly diluted conditions in the lake (Figure 1A). During the wet to dry transition and more concentrated conditions, the isotopic composition of lake water was enriched in δ18O (up to 5.08‰, VSMOW).
In the mineral pool, the two mineral assemblages differentiated upon mineralogical and petrographic characteristics (Table 1) were also identified by their distinct isotopic composition (Figure 6). The first assemblage comprised calcite and dolomite (Cc+Dol). The second group contained the bulk carbonate minerals forming surficial crusts in summer composed of calcite, dolomite, hydromagnesite, northupite (Cc+Dol+Hmg+Npt) and trace mineral phases (Table 1). Thus, the bulk isotopic composition was influenced by the proportions of Cc+Dol, but this content remained relatively constant in the samples (Table 1).
The δ18OCc+Dol (VSMOW) values ranged from 28.36 to 32.55‰, and δ13CCc+Dol (VPDB) values ranged from −5.45 to −3.07‰. δ18OCc+Dol (VSMOW) showed a positive covariance with δ13CCc+Dol (VPDB), resulting in a correlation coefficient of 0.73. The isotopic composition of isolated dolomite was characterized by both lower δ13C (−5.75 to −3.2‰, VPDB) and higher δ18O (30.76 to 35.91‰, VSMOW) values than the samples composed of dolomite and calcite (Figure 6).
δ18O and δ13C values of the association Cc+Dol+Hmg+Npt had higher average values of δ18O (35.58‰, VSMOW) and δ13C (−1.37‰, VPDB) than the association of Cc+Dol. This complex mineral assemblage was characterized by an increasing trend in δ18O and δ13C, defined by a positive correlation coefficient of 0.79.
The isotopic composition of inorganic carbon fixed to the EPS varied seasonally and between non-purified and purified samples, being up to −2.84‰ lighter in the latter. Distinctively, the purified EPS produced by floating microbial communities in spring yielded lower δ13C (−12.4‰, VPDB) and δ18O (−26.01‰, VSMOW) than the EPS obtained from benthic communities in December (δ13C −4.48‰, VPDB and δ18O 29.98‰, VSMOW). In December, the δ18O values remained constant in purified and non-purified EPS. By contrast, the δ18O value of the non-purified EPS of April was higher (28.30‰, VSMOW) than in the purified (26.01‰, VSMOW).
4. Discussion
The present study on Mg-carbonates deposited in a well-characterized alkaline lake helps to decipher the behavior of stable isotopes of carbonates during their precipitation as intracellular accumulations in algae and in desiccated microbial mats as well as during synsedimentary diagenesis. The seasonal cycling of inorganic carbon in the water and in the EPS fractions is also described in a range of environmental conditions, including dry (summer) and wet (winter and spring); see Figure 7.
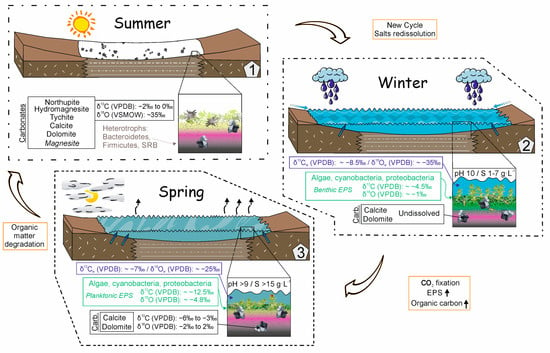
Figure 7.
Model of seasonal cycling of inorganic carbon in the water, minerals and in EPS fractions in Lake Caballo Alba.
As typically occurs in highly alkaline lakes, lake waters in Caballo Alba are rich in DIC, with up to 99,100 µM in spring, mainly due to the transformation of CO2 into bicarbonate (HCO3−) and carbonate (CO32−) at pH higher than 9 [36]. The δ13C isotopic values of DIC in the water column are in a narrow range from −8.56 to −6.91‰, VPDB, related to diluted and concentrated waters, respectively. The relative abundance of green algae and Cyanobacteria, among other microbial producers such as Gammaproteobacteria, coupled with high pH (from 9 to 10.4) and high rates of dissolved oxygen (from 35.7 to 122.8%), confirm a higher rate of photosynthesis in relation to respiration and decomposition, which are less relevant in the lake water during spring. The photosynthesis and evaporation contribute to 13C enrichment of the DIC after uptaking the lighter isotope, 12C, into the biomass [45,46]. The δ18O (VSMOW) values of concentrated lake waters show enrichments of 9.8‰ with respect to diluted waters. These shifts are within a range of enrichment between 5 and 15‰, commonly caused by evaporative processes in lakes [32,47]. Added to this, the positive correlation between δ13C and δ18O DIC values supports that the lake water becomes enriched in the heavy isotope (18O) upon evaporation, as typically occurs in closed basins [48].
In the pore waters of the benthic microbial mats developed during spring, the pore water δ13C DIC value (−11.7‰) is close to the δ13C value of purified phytoplanktonic EPS (−12.4‰) and more negative than values measured in the surface water (−6.87 to −11.79‰, VPDB). These light 13C values reflect a relative increase in CO2 contributed by the degradation of 13C-depleted organic matter [45,49]; those exceeding the 13C-enriched inputs that are supplied by the associated photosynthetic microorganisms. This interpretation is consistent with high concentrations of DIC (43,801 µM) due to organic matter decomposition and the dominance of heterotrophic microorganisms in the benthic mats. The Bacteroidetes phylum, which is capable of degrading a broad range of carbohydrates [50], is the most abundant among these organisms. The high abundance of Firmicutes also suggests a main role in the transformation of complex organic components. Firmicutes are also predominant in the mats of the lake Las Eras, located in the vicinity, and are suggested to play a role in the reductive phase of the sulfur cycle [9,35]. Overall, results show a significant impact of the heterotrophic activity on the isotopic composition of DIC of pore water that differs greatly from the DIC of overlying water.
The extracellular polymeric substances (EPSs) are produced by Cyanobacteria, green algae and many bacteria [51] that are part of the microbial communities of Caballo Alba. The EPSs comprise the cell walls of microorganisms and the mucilaginous matrix surrounding them. The primary producers are highly productive in spring, increasing the production of exopolysaccharides and CO2 uptake (manifested but relatively low concentrations of DIC (10,930 µM)). Dissolved inorganic carbon may also be removed from the solution and bound to the negatively charged functional groups of EPS [44]. The functional groups, such as carboxyl, amide and hydroxyl groups present in the EPS and associated with phytoplankton during spring blooms [10]. The isotopic composition of the inorganic carbon fixed to the EPS of phytoplankton shows a shift with the δ13C DIC value within −4.4‰, being the deviation higher in the purified (−12.4‰) than in the non-purified EPS (−9.5‰). Compositional differences between the non-purified and the purified EPS, which only retain the strongly bound ions [10], appear to play a role in their distinct isotopic signatures. It is inferred that in purified phytoplanktonic EPS, the inorganic carbon is strongly attached to the remaining ammonium (NH4+) groups at pH > 10 or to reluctant metals by cationic bridging [10,44]. This pool of inorganic carbon, depleted in 13C and 18O, could be sourced by the degradation of 13C-depleted organic matter in restricted environments. In the non-purified EPS, however, the inorganic carbon appears as poorly bound anionic carbonate groups that show negligible isotopic differences with DIC, suggesting that this second pool is derived mostly from the lake water.
In December, decomposers are dominant with respect to the producers (i.e., manifested by highly negative values of ORP, and low rates of dissolved oxygen, in the overlying water, Table S2). The isotopic composition of inorganic carbon linked to the EPS associated with benthic microbial community was expected to be sourced from the oxidation of organic matter and, thereby, depleted in 13C and 18O. By contrast, it is enriched in 18O and 13C, up to 4.7‰ relative to that of the DIC of the overlying waters, suggesting a mix of sources that produce intermediate compositions. The δ18O and δ13C values of benthic EPS inorganic carbon are closer to the mineral fraction. Remarkably, hydromagnesite and the Na-bearing carbonates, the mineral phases that are more enriched in 13C and 18O, show high solubility [52] and readily dissolve in winter. Accordingly, the dissolution of these carbonates releases isotopically enriched CO2 that eventually fixes to the EPS as anionic carbonate groups. As previously discussed, the lower isotopic records (δ13C −12.4‰, VPDB; δ18O 26.01‰, VSMOW) of the purified phytoplanktonic EPS suggest that the functional groups tend to retain depleted inorganic carbon.
In the mineral fraction, carbonates are distinctly separated into two groups by stable isotopes, confirming that their deposition occurs under different physicochemical conditions in an interval of a few months. The first group consists of a mixture of dolomite and Mg-calcite. Petrographic observations confirm that these carbonates deposited intracellularly within Spirogyra during spring blooms, as previously suggested [10]. The intracellular accumulations of Mg-calcite and dolomite result in carbonate δ13C values (aver. −4.10‰) enriched in 13C within 5‰ of the bulk measured δ13C DIC values (aver. −8.6‰). This deviation is in good agreement with shifts in the range from 2 to 5‰ caused by photosynthetic activity [45,46] and supports the photosynthetic origin proposed by [10] on basis of geochemical, cytochemical, mineralogical and petrological evidence. It is well known that photosynthesis creates alkalinity that enhances the precipitation of carbonates [53]. As several physicochemical parameters strongly impact the rates of photosynthesis, intracellular carbonates may provide isotopic signatures related to the environmental conditions of formation. The isotopic composition of these inclusions, even in those grown during the same season, varies within a range, where the higher values reflect DIC progressively enriched in 13C and 18O due to the effect of evaporation and degasification [32]. The positive covariation in δ18O and δ13C recorded for the carbonates is a typical effect of kinetic fractionation during evaporation in terminal lakes [48,54]. Intracellular calcite and dolomite are produced by living algae, and our results, in agreement with [10], show that these minerals have distinctive mineralogical features as well as representative isotopic and chemical composition. As these intracellular carbonates are the result of a cytoplasmatic uptake of elements, they can be defined as biominerals, according to [53], although the algae, affected by sifting environmental conditions, do not have full control over the crystal growth [10]. Overall, dolomite crystals have lighter δ13C (aver. −4.40‰, VPDB) but higher δ18O values (aver. 32.92‰, VSMOW) than the mixtures where dolomite coexists with calcite (δ13C aver. −4.10‰, VPDB and δ18O aver. 30.15‰, VSMOW). The different δ18O isotopic values between cogenetic dolomite and calcite formed within Spirogyra cells can be explained in terms of mineralogy [55], which implies that dolomite is about 3‰ to 6‰ heavier than coprecipitated calcite, and dismiss the effect of environmental conditions. Interestingly, the isotopic differences remain unaltered in sediments below the lake floor up to a depth of 14 cm. The isotopic composition of calcite and dolomite, which are the only minerals persisting after deposition, is stable during synsedimentary diagenesis. Thus, it is important to consider the oxygen and carbon isotope fractionation between dolomite and calcite when the stable isotopes of primary carbonate mixtures are used in paleoenvironmental reconstructions.
The carbonate crusts formed in summer embrace a suite of authigenic minerals that are isotopically discriminable by having higher contents in 13C (aver. −1.34‰, VPDB) and in 18O (aver. 35.58‰, VSMOW) than the association of calcite with dolomite (δ13C aver. −4.10‰, VPDB and δ18O aver. 30.15‰, VSMOW) and by showing strong covariance between δ18O and δ13C (0.79). These increments are compatible with brine evaporation and the coeval uptake of the heavier isotope 13C with increasing Mg content in the carbonate [56,57]. Spontaneous precipitation of hydromagnesite from the solutions is not observed, despite the waters being supersaturated with respect to this carbonate [10]. Instead, direct observations as well as microscopic examinations support that hydromagnesite precipitation is specifically associated with the degradation of the EPS. The decay of the organic matrix may release CO32− and Mg2+ ions, among others, which bind in high quantities to the EPS [10], inducing the formation of carbonates by impacting the saturation index [53]. Ref. [9] reported similar occurrences of hydromagnesite in the nearby lake Las Eras and concluded that its precipitation is assisted by heterotrophic bacteria that became entombed as the EPS dehydrates. The cellular lysis produces biogenic carbon depleted in 13C that is expected to be incorporated in the precipitating minerals. Accordingly, the isotopic signal of hydromagnesite-bearing sediments reflects the contribution of organic and inorganic carbon consistent with the decay of EPS subjected to evaporation. Along the same lines, [58] in Salda Lake (Turkey) and [59,60] in the Atlin playas (Canada) observed hydromagnesite-rich deposits associated with benthic microbial mats. In those modern alkaline environments, hydromagnesite is characterized by δ13C > 0‰ and δ18O values in the range of the hydromagnesite-bearing associations of the Caballo Alba. These signals have been inferred to register the impact of evaporation and CO2 degassing during the precipitation of the carbonate crust. In this sense, hydromagnesite can be defined as a biologically induced carbonate [53] that records the biotic and abiotic interactions, that is, interactions between heterotrophic bacteria (mainly Firmicutes and Bacteroidetes) and the environment (strong evaporation and degassing).
Results give evidence that modern lacustrine Mg-carbonates can record the mechanisms of formation and the environmental variations in their isotopic compositions in accordance with previous works [13,16,57,61,62]. The stable isotopes of dolomite and calcite, which are the minerals preserved in buried layers, do not record early diagenetic overprinting, providing valuable proxies of conditions for ancient analogue environments. In this sense, the isotopic composition of microbial dolomite in Miocene lacustrine successions of the Madrid (aver., δ13C −4.6‰ and δ18O −1.7‰, VPDB) and Duero Basins (aver., δ13C −4.83‰ and δ18O −0.3‰, VPDB) reported by [63,64], Las Minas Basin [3] and modern coastal lacustrine dolomite [13] fall within the range of Caballo Alba dolomite and Mg-calcite assemblages (aver., δ13C −4.10‰ and δ18O −0.75‰, VPDB).
5. Conclusions
Isotopic monitoring of biotic carbonates precipitating in modern lakes provides crucial information to understand carbonate isotopes in the rock record. This study gives new insight into the seasonal cycling of inorganic carbon in the different mineral phases, water and EPS reservoirs in a highly alkaline lake (Caballo Alba) based on carbonate stable isotope data. This lake is a highly productive seasonal lake, where primary producers such as green algae and Cyanobacteria increase CO2 uptake and stimulate the intracellular precipitation of Mg-calcite and dolomite within Spirogyra cells. These carbonates are enriched in 13C within 5‰ (δ13C aver. −4.10‰, VPDB) in relation to ambient DIC (aver. −8.6‰). A second group of carbonates (hydromagnesite, magnesite northupite and some other Na-bearing minerals) precipitates forming crusts with the contribution of organic and inorganic carbon, as a result of the decay of EPS subjected to desiccation. This carbonate assemblage with sulphate minerals is richer in 13C (δ13C aver. −1.34‰, VPDB) and in 18O (δ18O aver. 4.52‰, VPDB) than Mg-calcite and dolomite (δ13C aver. −4.10‰, VPDB and δ18O aver. −0.75‰, VPDB). This difference reflects a higher influence of evaporation, CO2 degassing, and coeval uptake of 13C with increasing Mg content in the carbonate. Dolomite and calcite preserved in buried layers do not record early diagenetic overprinting, providing valuable proxies of conditions for ancient analogue environments on Earth and Mars, where Mg-carbonates are common.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/min13050617/s1, Table S1: List of studied samples including the name (Sample ID = Sample identification) and the type of sample; Table S2: Variation of the physicochemical values in the surface waters of the Lake Caballo Alba (taken from Del Buey and Sanz-Montero, 2022): temperature (T), pH, dissolved oxygen (DO), salinity (S) and oxidation-reduction potential (ORP); Table S3: Type of analysis carried out on each sample. XRD: X-ray diffraction, SEM: Scanning Electron Microscopy, DNA: Genomic Analyses, SI: Stable Isotopes, HC: Hydrogeochemistry.
Author Contributions
Conceptualization, M.E.S.-M. and P.d.B.; methodology, M.E.S.-M., P.d.B., Ó.C. and M.S.-R.; writing—original draft preparation, M.E.S.-M. and P.d.B.; writing—review and editing, M.E.S.-M., P.d.B., Ó.C. and M.S.-R.; project administration and funding acquisition, M.E.S.-M. All authors have read and agreed to the published version of the manuscript.
Funding
PID2021-123735OB-C22 Project (Spanish Ministry of Science and Innovation, AEI and ERDF A Way of Making Europe). Research Group UCM-910404. Postdoctoral Grant (Margarita Salas CT18/22). OCENW.KLEIN.037 grant (Dutch Research Council, NWO).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Aitor Anton and Rodolfo Pozuelo (Complutense University of Madrid) and Suzan Verdegaal-Warmerdam (Vrije Universiteit Amsterdam) are greatly acknowledged for stable isotope analyses of the carbonate minerals, EPS and water samples. Comments about inorganic carbon in purified EPS provided by Olivier Braissant (University of Basel) have been very useful. The editor and anonymous reviewers are acknowledged for evaluating this manuscript and improving its quality.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Last, W.M. Lacustrine dolomite-an overview of modern, Holocene, and Pleistocene occurrences. Earth Sci. Rev. 1990, 27, 221–263. [Google Scholar] [CrossRef]
- García del Cura, M.Á.; Calvo, J.P.; Ordóñez, S.; Jones, B.F.; Cañaveras, J.C. Petrographic and geochemical evidence for the formation of primary, bacterially induced lacustrine dolomite: La Roda “white earth” (Pliocene, central Spain). Sedimentology 2001, 48, 897–915. [Google Scholar] [CrossRef]
- Pineda, V.; Gibert, L.; Carranza, A.; Soria, J.M.; Sánchez-Román, M. Interevaporitic deposits of Las Minas Gypsum Unit (Eastern Betic Cordillera): A record of Late Tortonian marine incursions and dolomite precipitation in Las Minas evaporitic Basin (SE Spain). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 564, 110171. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.; Fang, Y.; Ma, J.; Shen, B.; Huang, F.; Zhou, T.; Wang, J.; Zhang, W. Origin and palaeoenvironmental indications of Eocene to Oligocene primary lacustrine dolomite, Northern Tianshan Mountains, NW China. J. Asian Earth Sci. 2020, 198, 104135. [Google Scholar] [CrossRef]
- Wen, Y.; Sánchez-Román, M.; Li, Y.; Wang, C.; Gao, Y.; Han, Z. Nucleation and stabilization of Eocene dolomite in evaporative lacustrine deposits from central Tibetan plateau. Sedimentology 2020, 67, 3333–3354. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, X.; Han, Z.; Gao, X.; Meng, R.; Wang, Q.; Tucker, M.E.; Li, M.; Sánchez-Román, M. Lacustrine-evaporitic microbial dolomite from a Plio-Pleistocene succession recovered by the SG-1 borehole in the Qaidam Basin, NE Tibetan Plateau. Chem. Geol. 2023, 622, 121376. [Google Scholar] [CrossRef]
- Warren, J. Dolomite: Occurrence, evolution and economically important associations. Earth. Sci. Rev. 2000, 52, 1–81. [Google Scholar] [CrossRef]
- Luzón, A.; Mayayo, M.J.; Pérez, A. Stable isotope characterization of co-existing carbonates from the Holocene Gallocanta Lake (NE Spain): Palaeolimnological implications. Int. J. Earth. Sci. 2009, 98, 1129–1150. [Google Scholar] [CrossRef]
- Sanz-Montero, M.E.; Cabestrero, Ó.; Sánchez-Román, M. Microbial Mg-rich Carbonates in an Extreme Alkaline Lake (Las Eras, Central Spain). Front. Microbiol. 2019, 10, 148. [Google Scholar] [CrossRef]
- del Buey, P.; Sanz-Montero, M.E. Biomineralization of ordered dolomite and magnesian calcite by the green alga Spirogyra. Sedimentology 2022, 70, 685–704. [Google Scholar] [CrossRef]
- Vasconcelos, C.; McKenzie, J.A.; Bernasconi, S.; Crujic, D.; Tiens, A.J. Microbial mediation as a possible mechanism for natural dolomite formation at low temperatures. Nature 1995, 377, 220–222. [Google Scholar] [CrossRef]
- Corzo, A.; Luzon, A.; Mayayo, M.J.; van Bergeijk, S.A.; Mata, P.; García Lomas, J. Carbonate mineralogyalong a biogeochemical gradient in recent lacustrine sediments of Gallocanta lake (Spain). Geomigrobiol. J. 2005, 22, 283–298. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; Vasconcelos, C.; Warthmann, R.; Rivadeneira, M.A.; McKenzie, J.A. Microbial dolomite precipitation under aerobic conditions: Results from Brejo do Espinho Lagoon (Brazil) and culture experiments. In Perspectives in Carbonate Geology: A Tribute to the Career of Robert Nathan Ginsburg; Swart, P.K., Eberli, G.P., McKenzie, J.A., Jarvis, I., Stevens, T., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 167–178. [Google Scholar]
- Samylina, O.S.; Zaytseva, L.V. Characterization of modern dolomite stromatolites from hypersaline Petukhavskoe Soda Lake, Russia. Lethaia 2019, 52, 1–13. [Google Scholar] [CrossRef]
- Cabestrero, Ó.; Sanz-Montero, M.E. Brine evolution in two inland evaporative environments: Influence of microbial mats in mineral precipitation. J. Paleolimnol. 2018, 59, 139–157. [Google Scholar] [CrossRef]
- Areias, C.; Fernandez-Barbosa, C.; Soarez-Cruz, A.P.; Ariztegui, D.; Eglinton, T.; Haghipour, N.; Vasconcelos, C.; Sánchez-Román, M. Organic matter diagenesis and precipitation of Mg-rich carbonate and dolomite in modern hypersaline lagoons linked to climatic changes. Geochim. Cosmochim. Acta. 2022, 337, 14–32. [Google Scholar] [CrossRef]
- Wright, D.T. The role of sulfate-reducing bacteria and cyanobacteria in dolomite formation in distal ephemeral lakes of the Coorong region, South Australia. Sediment. Geol. 1999, 126, 147–157. [Google Scholar] [CrossRef]
- Bontognali, T.R.R.; Vasconcelos, C.; Bernasconi, S.M.; Wharthmann, R.J.; Dupraz, C.; Bernasconi, S.M.; McKenzie, J.A. Microbes produce nanobacteria-like structures, avoidilng cell entombment. Geology 2008, 36, 663–666. [Google Scholar] [CrossRef]
- Liu, D.; Fan, Q.; Papineau, D.; Yu, N.; Chu, Y.; Wang, H.; Qiu, X.; Wang, X. Precipitation of protodolomite facilitated by sulfate-reducing bacteria: The role of capsule extracellular polymeric substances. Chem. Geol. 2020, 533, 119415. [Google Scholar] [CrossRef]
- Moreira, N.F.; Walter, L.M.; Vasconcelos, C.; McKenzie, J.A.; McCall, P. Role of sulfide oxidation in dolomitization: Sediment and pore-water geochemistry of a modern hypersaline lagoon system. Geology 2004, 32, 701–704. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; Vasconcelos, C.; Schmid, T.; Dittrich, M.; McKenzie, J.A. Aerobic microbial dolomite at the nanometer scale: Implications for the geologic record. Geology 2008, 36, 879–882. [Google Scholar] [CrossRef]
- Roberts, J.A.; Benett, P.C.; González, L.A.; Macpherson, G.L.; Milliken, K.L. Micorbial precipitation of dolomite in methanogenic groundwater. Geology 2004, 32, 277–280. [Google Scholar] [CrossRef]
- Kenward, P.A.; Fowle, D.A.; Goldstein, R.H.; Ueshima, M.; González, L.A.; Roberts, J.A. Ordered low-temperature dolomite mediated by carboxylic-group density of microbial cell walls. AAPG Bulletin. 2013, 97, 2113–2125. [Google Scholar] [CrossRef]
- Moore, T.S.; Murray, R.W.; Kurtz, A.G.; Schrag, D.P. Anaerobic methane oxidation and the formation of dolomite. Earth Planet. Sci. Lett. 2004, 229, 141–154. [Google Scholar] [CrossRef]
- DiLoreto, Z.A.; Garg, S.; Bontognali, T.R.R.; Dittrich, M. Modern dolomite formation is caused by the seasonal cycling of oxygenic phototrophs and anoxygenic phototrophs in a hypersaline shabka. Sci. Rep. 2021, 11, 1–13. [Google Scholar]
- del Buey, P.; Sanz-Montero, M.E.; Sánchez-Román, M. Bioinduced precipitation of smectites and carbonates in photosynthetic diatom-rich microbial mats: Effect of culture medium. Appl. Clay. Sci. 2023, 238, 106932. [Google Scholar] [CrossRef]
- Bontognali, T.R.R. Anoxygenic phototrophs and the forgotten art of making dolomite. Geology 2019, 47, 591–592. [Google Scholar] [CrossRef]
- Visscher, P.T.; Stolz, J.F. Microbial mats as bioreactors: Populations, processes and products. Paleogeogr. Paleoclimatol. Paleoecol. 2005, 219, 87–100. [Google Scholar] [CrossRef]
- Mavromatis, V.; Power, I.M.; Harrison, A.L.; Beinlich, A.; Dipple, G.M.; Bénézeth, P. Mechanisms controlling the Mg isotope composition of hydromagnesite-magnesite playas near Atlin, British Columbia, Canada. Chem. Geol. 2021, 579, 120325. [Google Scholar] [CrossRef]
- Arenas, C.; Casanova, J.; Pardo, G. Stable-isotope characterization of the Miocene lacustrine systems of Los Monegros (Ebro Basin, Spain): Paleogeographic and paleoclimatic implications. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1997, 124, 133–135. [Google Scholar] [CrossRef]
- Anadon, P.; Utrilla, R.; Vázquez, A. Use of charophyte carbonates as proxy indicators of subtle hydrological and chemical changes in marl lakes: Example from the Miocene Bicrob Basin, eastern Spain. Sediment. Geol. 2000, 133, 325–347. [Google Scholar] [CrossRef]
- Horton, T.W.; Defliese, W.F.; Tripati, A.K.; Oze, C. Evaporation induced 18O and 13C enrichment in lake systems: A global perspective on hydrologic balance effects. Quat. Sci. Rev. 2016, 131, 364–379. [Google Scholar] [CrossRef]
- Popall, R.M.; Bolhuis, H.; Muyzer, G.; Sánchez-Román, M. Stromatolites as biosignatures of atmospheric oxygenation: Carbonate biomineralization and UV-C resilience in a Geitlerinema sp.-dominated culture. Front. Microbiol. 2020, 11, 948. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Montero, M.E.; Arroyo, X.; Cabestrero, Ó.; Calvo, J.P.; Fernández, E.; Fidalgo, C.; García del Cura, M.Á.; García-Avilés, J.; González, J.A.; Rodríguez-Aranda, J.P.; et al. Procesos de sedimentación y biomineralización en la laguna alcalina de Las Eras (Humedal Coca-Olmedo). Geogaceta 2013, 53, 97–100. [Google Scholar]
- Cabestrero, Ó.; Sanz-Montero, M.E.; Arregui, L.; Serrano, S.; Visscher, P.T. Seasonal variability of mineral formation in microbial mats subjected to drying and wetting cycles in alkaline and hypersaline sedimentary environments. Aquat. Geochem. 2018, 24, 79–105. [Google Scholar] [CrossRef]
- Finlay, K.; Vogt, R.J.; Bogard, M.J.; Wissel, B.; Tutolo, B.M.; Simpson, G.L.; Leavitt, P.R. Decrease in CO2 efflux from northern hardwater lakes with increasing atmospheric warming. Nature 2015, 519, 215–218. [Google Scholar] [CrossRef]
- Sanz-Montero, M.E.; Rodríguez-Aranda, J.P.; del Buey, P. Influencia del sustrato cenozoico en el origen y sedimentación de la laguna hiperalcalina de Caballo Alba (Segovia). Geogaceta 2021, 70, 31–34. [Google Scholar]
- Benito, M.I. Estudio Comparativo de la Evolución Sedimentaria y Diagenética de los Litosomas Carbonatados Arrecifales (Pre-Rifting) de la Cuenca de Cameros. Kimmeridgiense. La Rioja-Soria. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2001. [Google Scholar]
- Breitenbach, S.F.M.; Bernasconi, S.M. Carbon and oxygen isotope analyses of small carbonate samples (20 to 100 µg) with GasBench II preparation device. Rapid Commun. Mass Spectrom. 2011, 25, 1910–1914. [Google Scholar] [CrossRef]
- Stockhecke, M.; Sturm, M.; Brunner, I.; Schmincke, H.U.; Sumita, M.; Kipfer, R.; Cukur, D.; Kwecien, O.; Anselmetti, F.S. Sedimentary evolution and environmental history of Lake Van (Turkey) over the past 600.000 years. Sedimentology 2014, 61, 1830–1861. [Google Scholar] [CrossRef]
- McCormack, J.; Nehrke, G.; Jóns, N.; Immenhauser, A.; Kwiecien, O. Refining the interpretation of lacustrine carbonate isotope records: Implications of a mineralogy-specific Lake Van case study. Chem. Geol. 2019, 513, 167–187. [Google Scholar] [CrossRef]
- Kim, S.T.; Coplen, T.B.; Horita, J. Normalization of stable isotope data for carbonate minerals: Implementation of IUPAC guidelines. Geochim. Cosmochim. Acta. 2015, 158, 276–289. [Google Scholar] [CrossRef]
- Decho, A.W.; Kawaguchi, T.; Allison, M.A.; Louchard, E.M.; Reid, R.P.; Stephens, F.C.; Voss, K.J.; Wheatcroft, R.A.; Taylor, B.B. Sediment properties influencing upwelling spectral reflectance signatures: The “Biofilm gel effect”. Limnol. Oceanogr. 2003, 48, 431–433. [Google Scholar] [CrossRef]
- Braissant, O.; Decho, A.W.; Przekop, K.M.; Gallagher, K.L.; Glunk, C.; Dupraz, C.; Visscher, P.T. Characteristics and turnover of exopolymeric substances in a hypersaline microbial mat. FEMS Microbiol. Ecol. 2009, 67, 293–307. [Google Scholar] [CrossRef]
- Merz, M.U.E. The biology of carbonate precipitation by Cyanobacteria. Facies 1992, 26, 81–102. [Google Scholar] [CrossRef]
- Brady, A.L.; Slater, G.F.; Omelon, C.R.; Southam, G.; Druschel, G.; Andersen, D.T.; Hawes, I.; Laval, B.; Lim, D.S.S. Photosynthetic isotope biosignatures in laminated micro-stromatolitic and non-laminated nodules associated with modern, freshwater microbialites in Pavilion lake, B.C. Chem. Geol. 2010, 274, 56–67. [Google Scholar] [CrossRef]
- Vystavna, Y.; Harjung, A.; Monteiro, L.R.; Matiatos, I.; Wassenaar, L.I. Stable isotopes in global lakes integrate catchment and climatic controls on evaporation. Nat. Commun. 2021, 12, 7224. [Google Scholar] [CrossRef] [PubMed]
- Talbot, M.R. A review of paleohydrological interpretation of carbon and oxygen isotopic ratios in primary lacustrine carbonates. Chem. Geol. 1990, 80, 261–279. [Google Scholar]
- Anderson, T.F.; Arthur, M.A. Stable isotopes of oxygen and carbon and their application to sedimentologic and palaeoenvironment problems. In Stable Isotopes in Sedimentary Geology; Arthur, M.A., Anderson, T.F., Veizer, J., Land, L.S., Eds.; SEPM (Society for Sedimentary Geology): Tulsa, Oklahoma, 1983; Short Course 10; pp. 1–151. [Google Scholar]
- McKee, L.S.; La Rosa, S.L.; Westereng, B.; Eijsink, V.G.; Pope, P.B.; Larsbrink, J. Polysaccharide degradation by Bacteroidetes; mechanisms and nomenclature. Environ. Microbiol. Rep. 2021, 13, 559–581. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.W. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef]
- Gautier, Q.; Bénézeth, P.; Mavromatis, V.; Schott, J. Hydromagenesite solubility product and growth kinetics in aqueous solution from 25 to 75 °C. Geochim. Cosmochim. Acta. 2014, 138, 1–20. [Google Scholar] [CrossRef]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Li, H.C.; Ku, T.L. δ 13C—δ 18O covariance as a paleohydrological indicator for closed-basin lakes. Paleogeogr. Palaeoclimatol. Palaeoecol. 1997, 133, 69–80. [Google Scholar] [CrossRef]
- Tucker, M.E.; Wright, V.P. Carbonate Sedimentology; Blackwell Scientific Publications: Oxford, UK, 1990; p. 482. [Google Scholar]
- Jiménez López, C.; Romanek, C.S.; Caballero, E. Carbon isotope fractionation in synthetic magnesian calcite. Geochim. Cosmochim. Acta. 2006, 70, 1163–1171. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; Romanek, C.S.; Fernández-Remolar, D.C.; Sánchez-Navas, A.; McKenzie, J.A.; Amils-Pibernat, R.; Vasconcelos, C. Aerobic biomineralization of Mg-rich carbonates: Implications for natural environments. Chem. Geol. 2011, 281, 143–150. [Google Scholar] [CrossRef]
- Braithwaite, C.J.R.; Zedef, V. Hydromagnesite stromatolites and sediments in an alkaline lake, Salda Golu, Turkey. J. Sediment. Res. 1996, 66, 991–1002. [Google Scholar]
- Power, I.M.; Wilson, S.A.; Thom, J.M.; Dipple, G.M.; Southam, G. Biologically induced mineralization of dypingite by cyanobacteria from an alkaline wetland near Atlin, British Columbia, Canada. Geochem. Trans. 2007, 8, 13. [Google Scholar] [CrossRef]
- Power, I.M.; Wilson, S.A.; Harrison, A.L.; Dipple, G.; McCutcheon, J.; Southam, G.; Kenward, P.A. A depositional model for hydromagnesite-magnesite playas near Atlin, British Columbia, Canada. Sedimentology 2014, 61, 1701–1733. [Google Scholar] [CrossRef]
- Vasconcelos, C.; McKenzie, J.A. Microbial mediation of modern dolomite precipitation and diagenesis under anoxic conditions (Lagoa Vermelha, Rio de Janeiro, Brazil). J. Sediment. Res. 1997, 67, 378–390. [Google Scholar]
- Bahniuk, A.; McKenzie, J.A.; Perri, E.; Bontognali, T.R.R.; Vögeli, N.; Rezende, C.E.; Rangel, T.P.; Vasconcelos, C. Characterization of environmental conditions furing microbial Mg-carbonate precipitation and early diagenetic dolomite crust formation: Brejo do Espinho, Rio de Janeiro, Brazil. Geol. Soc. Spec. Pub. 2015, 418, 243–259. [Google Scholar] [CrossRef]
- Sanz-Montero, M.E.; Rodríguez-Aranda, J.P.; García del Cura, M.Á. Dolomite-silica stromatolites in Miocene lacustrine deposits from the Duero Basin, Spain: The role of organotemplates in the precipitation of dolomite. Sedimentology 2008, 55, 729–750. [Google Scholar] [CrossRef]
- Sanz-Montero, M.E.; Rodríguez-Aranda, J.P.; García del Cura, M.Á. Bioinduced precipitation of barite and celestite in dolomite microbialites examples from Miocene lacustrine sequences in the Madrid and Duero Basins, Spain. Sediment. Geol. 2009, 222, 138–148. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).