Electrically Conductive Silicate Composite for Protection against Electrocorrosion
Abstract
1. Introduction
2. Theoretical Justifications for the Development of the Composite of the Electrically Conductive Silicate Composite
=CaF2 + Na2O·Al2O3·2SiO2·2H2O + 3CaO·Al2O3·6H2O.
= −4463.13 + (5017.46) + (−1113.44) − 2·(−3808.31) − (−542.57) − 8·(−237.34)
= −536.14 kJ/mol
3. Materials and Methods
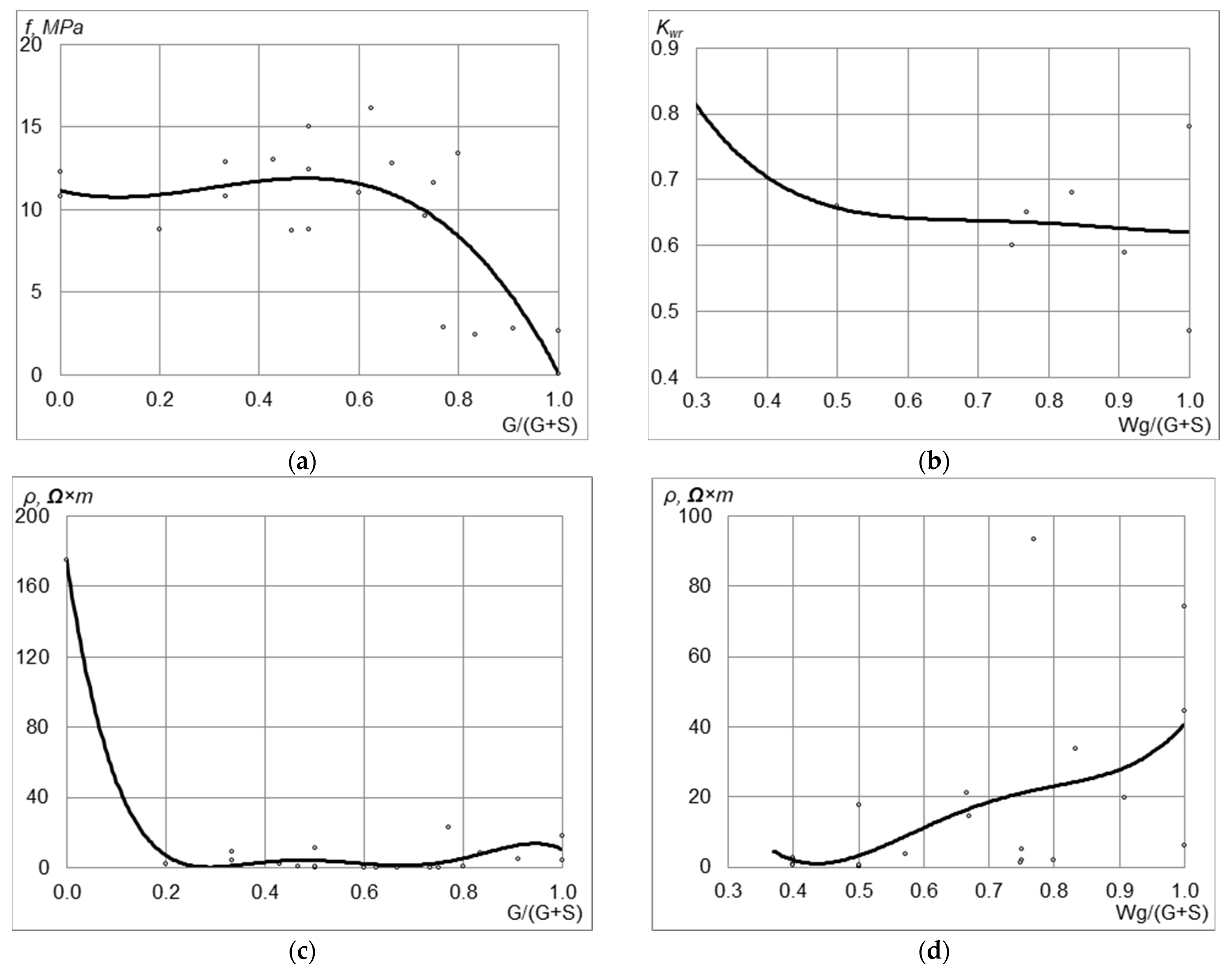
4. Results
5. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Plugin, D.; Kasyanov, V.; Konev, V.; Nesterenko, S.; Afanasiev, A. Research into the effectiveness of grounded screens of electroconductive silicate compositions for electrocorrosion protection. MATEC Web Conf. 2017, 116, 1012. [Google Scholar] [CrossRef]
- Plugin, A.A.; Pluhin, O.A.; Kasyanov, V.V.; Dyomina, O.I.; Bondarenko, D.O. Portland cement-based penetrating electrically conductive composition for protection against electrocorrosion. Funct. Mater. 2021, 28, 121–130. [Google Scholar] [CrossRef]
- Koniev, V. Electrically Conductive Silicate Composition for Protection against Electrocorrosion of Railway Structures. Ph.D. Thesis, Ukrainian State University of Railway Transport, Kharkiv, Ukraine, 2021. [Google Scholar]
- Ding, Y.; Chen, Z.; Han, Z.; Zhang, Y.; Pacheco-Torgal, F. Nano-carbon black and carbon fiber as conductive materials for the diagnosing of the damage of concrete beam. Constr. Build. Mater. 2013, 43, 233–241. [Google Scholar] [CrossRef]
- Baeza, J.; Galao, O.; Zornoza, E.; Garcés, P. Multifunctional Cement Composites Strain and Damage Sensors Applied on Reinforced Concrete (RC) Structural Elements Francisco. Materials 2013, 6, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Dinesha, A.; Sujib, D.; Pichumani, M. Electro-mechanical investigations of steel fiber reinforced self-sensing cement composite and their implications for real-time structural health monitoring. J. Build. Eng. 2022, 511, 104343. [Google Scholar] [CrossRef]
- Yehia, S.; Tuan, C.Y.; Ferdon, D.; Chen, B. Conductive Concrete Overlay for Bridge Deck Deicing: Mixture Proportioning, Optimization, and Properties. Mater. J. 2000, 97, 172–181. [Google Scholar] [CrossRef]
- Sassani, A.; Ceylan, H.; Kim, S.; Gopalakrishnan, K.; Arabzadeh, A.; Taylor, C.P. Factorial Study on Electrically Conductive Concrete Mix Design for Heated Pavement Systems. In Proceedings of the Transportation Research Board 96th Annual Meeting, Washington, DC, USA, 8–12 January 2017; Available online: https://dr.lib.iastate.edu/handle/20.500.12876/13676 (accessed on 1 January 2017).
- Khalid, T.; Albasha, L.; Qaddoumi, N.; Yehia, S. Feasibility Study of Using Electrically Conductive Concrete for Electromagnetic Shielding Applications as a Substitute for Carbon-Laced Polyurethane Absorbers in Anechoic Chambers. IEEE Trans. Antennas Propag. 2017, 65, 2428–2435. [Google Scholar] [CrossRef]
- Carmona, J.; Garcés, P.; Climent, M.A. Efficiency of a conductive cement-based anodic system for the application of cathodic protection, cathodic prevention and electrochemical chloride extraction to control corrosion in reinforced concrete structures. Corros. Sci. 2015, 96, 102–111. [Google Scholar] [CrossRef]
- Anwar, M.S.; Sujitha, B.; Vedalakshmi, R. Light-weight cementitious conductive anode for impressed current cathodic protection of steel reinforced concrete application. Constr. Build. Mater. 2014, 71, 167–180. [Google Scholar] [CrossRef]
- Sassani, A.; Ceylan, H.; Kim, S.; Gopalakrishnan, K.; Arabzadeh, A.; Taylor, P.C. Influence of mix design variables on engineering properties of carbon fiber-modified electrically conductive concrete. Constr. Build. Mater. 2017, 152, 168–181. [Google Scholar] [CrossRef]
- García-Macías, E.; D’Alessandro, A.; Castro-Triguero, R.; Pérez-Mira, D.; Ubertini, F. Micromechanics modeling of the electrical conductivity of carbon nanotube cement-matrix composites. Compos. Part B Eng. 2017, 108, 451–469. [Google Scholar] [CrossRef]
- Jang, D.; Yoon, H.N.; Yang, B.; Seo, J.; Farooq, S.Z.; Lee, H.K. Synergistic effects of CNT and CB inclusion on the piezoresistive sensing behaviors of cementitious composites blended with fly ash. Smart Struct. Syst. 2022, 29, 351–359. [Google Scholar] [CrossRef]
- Fiala, L.; Petříková, M.; Černý, R. Electrical properties and self-heating ability of alkaliactivated slag with graphite powder. Green Build. Technol. Mater. 2020, 20, 359–366. [Google Scholar] [CrossRef]
- Fiala, L.; Petříková, M.; Lin, W.-T.; Podolka, L.; Černý, R. Self-Heating Ability of Geopolymers Enhanced by Carbon Black Admixtures at Different Voltage Loads. Energies 2019, 12, 4121. [Google Scholar] [CrossRef]
- Černý, Z.; Jakubec, I.; Bezdička, P.; Šulc, L.; Macháček, J.; Bludská, J.; Roubíček, P. Preparation of electrically conductive materials based on geopolymers with graphite. Ceram. Eng. Sci. Proc. 2010, 31, 91–99. [Google Scholar]
- Wang, D.; Wang, Q.; Huang, Z. Investigation on the poor fluidity of electrically conductive cement-graphite paste: Experiment and simulation. Mater. Des. 2019, 169, 107679. [Google Scholar] [CrossRef]
- Łach, M.; Hebdowska-Krupa, M.; Mierzwiński, D.; Korniejenko, K. Mechanical properties of geopolymers reinforced with carbon and aramid long fibers. IOP Conf. Ser. Mater. Sci. Eng. 2019, 706, 012011. [Google Scholar] [CrossRef]
- Zmeskal, O.; Trhlikova, L.; Pospisil, J.; Fiala, L.; Florian, P. Investigation of electric and thermal properties of alkali-activated aluminosilicates with a cnt admixture. Ceram. Silikáty 2020, 64, 180–189. [Google Scholar] [CrossRef]
- Babushkin, V.I.; Plugin, A.A.; Zelensky, D.Y. Thermodynamic and physico-chemical studies of acid-resistant binders based on water glass and sodium silicon fluoride. Collect. Sci. Work. Ukr. State Univ. Railw. Transp. 2000, 37, 55–62. [Google Scholar]
- Palomo, A.; Krivenko, P.; Garcia-Lodeiro, I.; Kavalerova, E.; Maltseva, O.; Fernández-Jiménez, A. A review on alkaline activation: New analytical perspectives. Mater. Constr. 2014, 64, e022. [Google Scholar] [CrossRef]
- Krivenko, P. Why alkaline activation—60 years of the theory and practice of alkali-activated materials. J. Ceram. Sci. Technol. 2017, 8, 323–333. [Google Scholar] [CrossRef]
- Babushkin, V.I.; Matveyev, G.M.; Mchedlov-Petrossyan, O.P. Thermodynamics of Silicates; Original Russian edition published by Stroissdat, Moskow; Edycja Softcover Reprint of the Original, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1985; 475p. [Google Scholar]
- Ovcharenko, F.D.; Arkhipov, V.V.; Biryukov, A.I.; Plugin, A.N. Electrosurface phenomena and the evaluation of processes in the ardening of mineral binders and concretes prepared from these binders. Colloid J. USSR 1981, 43, 713–717. Available online: https://www.webofscience.com/wos/woscc/full-record/WOS:A1981NR37500007 (accessed on 1 January 1981).
- Plugin, A.N.; Vdovenko, N.V.; Biriukov, A.I.; Ovcharenko, F.D. On the mechanism of advent of the electrosurface potential of different substances in aqueous-solutions. Dokl. Akad. Nauk SSSR 1988, 298, 656–661. Available online: https://www.webofscience.com/wos/woscc/full-record/WOS:A1988M134500036 (accessed on 1 January 1988).
- Plugin, A.N.; Plugin, A.A.; Trykoz, L.V. Fundamentals of the Theory of Hardening, Strength, Destruction and Durability of Portland Cement, Concrete and Structures Made of Them. 1 Colloidal Chemistry and Physical-Chemical Mechanics of Cement Concrete; Naukova Dumka: Kyiv, Ukraine, 2011; 331p. [Google Scholar]
- Bergna, H.E. Chapter 1. Advances in Chemistry. In The Colloid Chemistry of Silica; Bergna, H.E., Ed.; American Chemical Society: Washington, DC, USA, 1994; Volume 234, pp. 1–47. [Google Scholar] [CrossRef]
- Ren, Z.; Sun, J.; Tang, W.; Zeng, X.; Zeng, H.; Wang, Y.; Wang, X. Mechanical and electrical properties investigation for electrically conductive cementitious composite containing nano-graphite activated magnetite. J. Build. Eng. 2022, 57, 104847. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.; Ceylan, H.; Kim, S.; Yang, S.; Abdualla, H. Electrically Conductive Mortar Characterization for Self-Heating Airfield Concrete Pavement Mix Design. Int. J. Pavement Res. Technol. 2015, 8, 315–324. [Google Scholar] [CrossRef]
- Pluhin, O.; Plugin, A.; Plugin, D.; Borziak, O.; Dudin, O. The effect of structural characteristics on electrical and physical properties of electrically conductive compositions based on mineral binders. MATEC Web Conf. 2017, 116, 01013. [Google Scholar] [CrossRef]
- Ahmed, S.; Kamal, I. Green Conductive Construction Materials toward Sustainable Infrastructures. ECS Trans. 2022, 107, 2139. [Google Scholar] [CrossRef]
- Frąc, M.; Szudek, W.; Szołdra, P.; Pichór, W. The applicability of shungite as an electrically conductive additive in cement composites. J. Build. Eng. 2022, 45, 103469. [Google Scholar] [CrossRef]
- Majerová, J.; Drochytka, R. Possibilities of utilization of waste materials in production of electrically conductive composites on silicate basis. Solid State Phenom. 2021, 321, 171–176. [Google Scholar] [CrossRef]
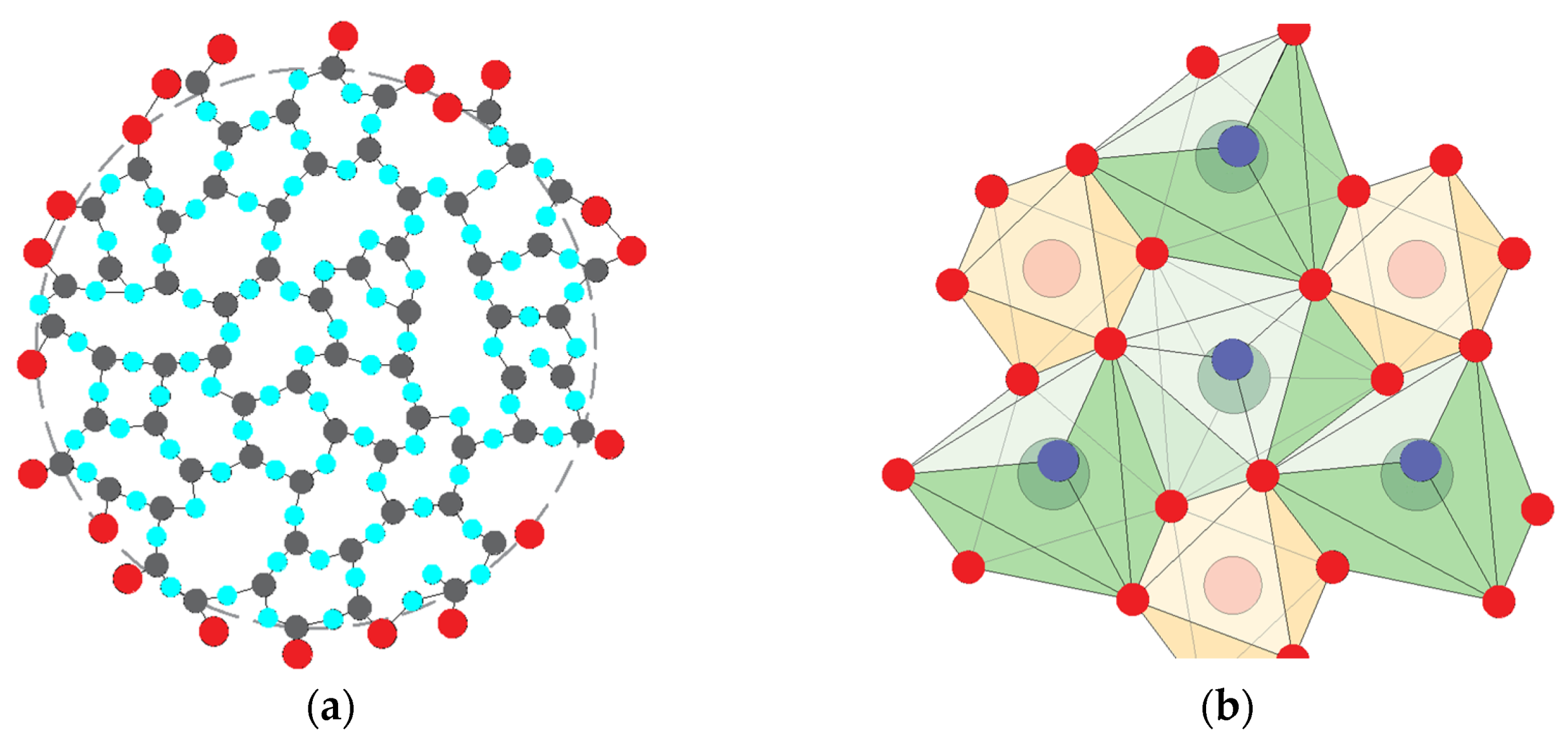




| Alkaline Component | Gibbs Free Energy ΔG, kcal/mol, of Reactions of the Interaction of Main and Additional Products | |||
|---|---|---|---|---|
| Hydronepheline NAS2H2 | Analcime NAS4H2 | |||
| ΔG | Additional Products | ΔG | Additional Products | |
| NaO·2SiO2 | −33.172 | C2S3H2.5; Ca(OH)2 | −14.792 | C2AH8; Ca(OH)2 |
| NaO·3SiO2 | −949.120 | C6S6H | 27.331 | Ca(OH)2 |
| Na2SiF6 | −208.490 | C3AH6; CaSiF6 | −29.383 | C2AH8; CaSiF6; Ca(OH)2 |
| NaF | −268.07 | C3AH6; CaF2 | 17.019 | C2AH8; CaF2; Ca(OH)2 |
| Element | Ca | Na | Al | Si | H | O | F |
|---|---|---|---|---|---|---|---|
| ψ0, B | −4.20 | −4.04 | −2.99 | −1.23 | −1.2 | +1.44 | +4.89 |
| Compound | Electrical Surface Potentials, V | ||
|---|---|---|---|
| Name, Designation | Formula | Absolute | Equilibrium |
| orthosilicic acid | Si(OH)4 | +0.03 | −0.383 |
| sodium fluoride | NaF | −0.425 | −0.838 |
| hydronepheline NAS2H2 | Na2O·Al2O3·2SiO2·2H2O | +0.346 | −0.067 |
| calcium hydroaluminate C3AH6 | 3CaO·Al2O3·6H2O | +0.541 | +0.128 |
| calcium fluoride | CaF2 | +1.86 | +1.447 |
| Oxide Compound | MgO | Al2O3 | SiO2 | SO3 | CaO | MnO | FeO | TiO2 |
|---|---|---|---|---|---|---|---|---|
| Content (%wt.) | 14.36 | 7.51 | 31.23 | 2.2 | 31.75 | 2.2 | 1.5 | 0.34 |
| Properties | Value |
|---|---|
| Ash content | 0.5% |
| Mass fraction of the copper | 0.5% |
| Mass fraction of volatile substances | 0.5% |
| Mass fraction of photo-reagents | 0.5% |
| pH value | 6.0–8.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plugin, A.; Rucińska, T.; Borziak, O.; Pluhin, O.; Zhuravel, V. Electrically Conductive Silicate Composite for Protection against Electrocorrosion. Minerals 2023, 13, 610. https://doi.org/10.3390/min13050610
Plugin A, Rucińska T, Borziak O, Pluhin O, Zhuravel V. Electrically Conductive Silicate Composite for Protection against Electrocorrosion. Minerals. 2023; 13(5):610. https://doi.org/10.3390/min13050610
Chicago/Turabian StylePlugin, Andrii, Teresa Rucińska, Olga Borziak, Oleksii Pluhin, and Vitalii Zhuravel. 2023. "Electrically Conductive Silicate Composite for Protection against Electrocorrosion" Minerals 13, no. 5: 610. https://doi.org/10.3390/min13050610
APA StylePlugin, A., Rucińska, T., Borziak, O., Pluhin, O., & Zhuravel, V. (2023). Electrically Conductive Silicate Composite for Protection against Electrocorrosion. Minerals, 13(5), 610. https://doi.org/10.3390/min13050610








