Abstract
In world practice, the need for high-strength concrete with an intensive gain of early strength is due to an increase in requirements for characteristics of concrete and the desire to shorten the construction period. Alkali-activated cement, based on soluble sodium silicates (SSS), can demonstrate high strength and rapid gain due to the nano-modifying effect of amorphous silica present in SSS. However, the problem with the effective use of such cement compositions is unsatisfactory short setting times. This work investigates the effect of modifying admixtures on the structure formation of alkali-activated slag cement (AASC), its physical and mechanical properties depending on characteristics of SSS and the basicity of the aluminosilicate component (precursor), which was changed by the ratio of the ordinary Portland cement (OPC) clinker and granulated blast furnace slag (GBFS). A positive synergistic effect was noticed from glycerol and trisodium phosphate, as the components of a complex admixture, to control the setting of AASC. This resulted in extending the initial setting time from 1 to 5 min to the values of 21–72 min. The compressive strength of 21–26.3 MPa by 3 h, 36.5–43.4 MPa by 1 day, and 84.7–117.1 MPa by 28 days was obtained. Proper shrinkage deformations were equal to 0.47–0.6 mm/m. It was shown that with an increase in the basicity of the aluminosilicate component, the properties of AASC increased both in the early and late stages of hardening.
1. Introduction
In global construction practice, the need for high-strength concrete with intensive early strength gain is a priority. This is due to the constant increase in requirements for the performance characteristics of concrete and the desire to shorten the construction period.
This is possible primarily due to the use of rapid-hardening high-strength cement, the most famous of which are high alumina cement, sulfoaluminate, high sulfate cement, and other ones, characterized by a strength of ≥60 MPa (Table 1) [1,2]. However, cement and concrete based on them are very expensive, and most required aluminate (aluminous) cement is not produced in Ukraine and is characterized by insufficient strength (60 MPa) with falls, especially at temperatures above 25 °C.

Table 1.
Comparative performance properties of cement.
Well-known technologies under the general term of High-Performance Concrete (HPC) are developed today [3,4,5,6]. In a general case, HPC can be characterized by high compressive strength (200 MPa and more) but requires expensive admixtures and modifiers, as well as a high culture of the technological process and care before exploitation starts.
The most promising ones in this direction are alkali-activated cement and concrete [7,8,9]. This is due to a complex of special properties, though their potential has not yet been fully determined. A series of articles are devoted to opening questions on the peculiarities of alkali-activated materials, such as the regulation of drying shrinkage [10], freeze–thaw resistance [11], crack resistance [12], transport properties [13,14], etc. Controlling the above setting times while ensuring high strength is a priority.
However, the cement made using (soluble sodium silicates) SSS, as an alkaline component, deserves special attention due to its ability to demonstrate rapid strength gain and high final strength while maintaining properties during exploitation [1,15], which is due to the nano modifying action of the amorphous and highly active silica of SSS. Of course, the effect is possible when the composition and technological process parameters are correct.
However, the problem with the effective use of cement activated by soluble high-modulus sodium silicates and the implementation of their special properties is determined, firstly, using very short settings.
Regarding the interaction of calcium silicates with SSS, this is characterized by active coagulation [16,17], and there is a necessity to modify admixtures with retarding and stabilizing effects, resistant to the action of a highly alkaline medium. The authors [18,19] proposed an admixture of potassium fluoride KF as a retarder. The effectiveness of the admixture increased when the silicate modulus of SSS was raised, and its density was reduced. However, the widespread application of the KF admixture is hindered by its high price and toxicity. There are known attempts to regulate the setting time of systems based on an OPC clinker, slags, and high-modulus SSS by adding Na3PO4·12H2O [20]. It is known that the ions of traditional anionic surfactants, formed due to dissociation, have a valency ≤ II, resulting in their limited adsorption on the surface of mineral particles. The competition of these anions with SiO32− ions causes the mentioned phenomenon. The higher valency of PO43− ions results in satisfactory adsorption and ensures the effectiveness of phosphates for the regulation of the setting time [21]. However, due to the high chemical activity of alkali-activated cement and low solubility of Na3PO4·12H2O, the amount of the last one that is dissolved is not always sufficient to ensure technologically acceptable setting times.
Triatomic polyol (glycerol) has been used as a so-called cross-linking admixture for sodium silicate hydrate xerogel structures to increase strength and water resistance [22]. The efficiency of glycerol (triol), as a modifier of alkali-activated cement, can be explained by the stability of its molecular structure [23]. It is known that glycerol accelerates the hydration of slag in the presence of SSS with the formation of additional structural joints in cement stone. This effect determines the increased strength of the cement matrix under bending loads. The use of glycerol as a modifier made it possible to propose the system “slag—SSS—glycerol” for mortars and concretes intended for rapid restoration. However, the authors did not consider the influence of the basicity of the cement due to the presence of an OPC clinker and the influence of the modifier on the structure formation of alkali-activated cement, only stating the high-performance properties of mortar and concrete. The basicity of cement is determined by the basicity modulus (Mb) of the aluminosilicate component, i.e., by the ratio of the mass concentrations of oxides (CaO + MgO)/(SiO2 + Al2O3) via an analogy with the estimation of GBFS basicity [21,24].
Other traditional retarder admixtures are partly or completely ineffective for alkali-activated cements due to the high alkalinity of their medium during hydration [25,26,27]. The formation of protective adsorption films and shells on the surface of cement particles under such conditions is impossible. The decrease in the effectiveness of admixtures is also caused by the anion of the alkaline component. An increase in the silicate modulus of SSS causes a higher concentration of SiO32− groups in the solution, determining a rise in the concentration of H+ protons [28]. This fact results in significant changes to the structure of admixtures. Thus, the option to use high-modulus SSS as an alkaline component is mostly problematic in terms of the setting time. Therefore, the development of effective methods for regulating the setting time of alkali-activated cement based on high-modulus SSS remains an urgent task.
The main task of this study was to increase the early strength of cement due to the application of SSS while solving problems with structure formation and providing effective control concerning the setting time.
The purpose of this paper is to study the effect of admixtures, the silicate modulus of SSS, and the basicity of the aluminosilicate component on the rate of structure formation and the characteristics of alkali-activated slag cement (AASC) to ensure their effective control under setting times and proper shrinkage deformations and to obtain high strength.
2. Materials and Methods
The OPC clinker and GBFS were used as aluminosilicate components of AASC. The characteristics of GBFS are presented in Table 2. The mineralogical composition of the OPC clinker: C3S—62% by mass, β-C2S—15.5% by mass, C3A—7.5, C4AF—12% by mass. The specific surface area of GBFS = 4420 cm2/g, and that of OPC clinker = 4120 cm2/g by Blaine.

Table 2.
Characteristics of GBFS.
The basicity of the binder system was corrected by changing the ratio of the GBFS and OPC clinker.
High-modulus SSS (Ms = 1.8, Ms = 2.4, and Ms = 3.0; density p = 1.30, 1.35, 1.40, and 1.45 g/mL) were used as the alkaline component.
The optimization of the compositions was carried out using the plans of the full factorial experiment of type 23.
According to [28,29,30], the salt Na3PO4·12H2O (trisodium phosphate—hereinafter referred to as TSP) was used as a modifier to increase the coagulation resistance of SSS when interacting with GBFS and, thus, to slow down the setting. The salt content was determined experimentally until an acceptable setting start time of at least 15–20 min was reached for AASC with high early strength [31].
According to the results of previous studies, glycerol was taken as a modifying agent to enhance the effect of TSP on setting times and influence the structure formation of AASC. On the other hand, glycerol is a polyfunctional organic compound that can bind the surface groups of silicon–oxygen oligomers using hydrogen bonds by cross-linking silicic acid molecules. Therefore, glycerol is also able to improve the bending strength and elasticity of hardened concrete [20]. The addition of glycerol was introduced into the solution of SSS containing TSP and was mixed with it. The resulting mixture was used to mix the cement.
The preparation of cement pastes and cement–sand mortars was carried out in a Hobart-type mixer. As a fine aggregate for mortars, standard monofractional quartz sand fr. 0.9 mm was used. The strength of the cement was determined in accordance with the national standard of Ukraine, DSTU B V.2.7-181:2009, using samples with a composition of 1:3 (AASC:sand) and 4 × 4 × 16 cm3 in size. The ratio between the volume of modified SSS and the mass of cement (hereinafter S/C) for preparing mortars was selected experimentally to ensure the diameter of the spread, measured on a standard flow table, as 106–115 mm.
Conditions for curing samples to determine the strength were as follows: after manufacturing and after up to 2 days—t = 20 ± 2 °C, relative humidity RH = 95–100%. After 3 days and until the moment of testing, the samples were kept in water.
The proper shrinkage deformations were measured according to the method described in [32]. Samples of mortar were hardened into molds for 48 h and then for another 5 days in water. The baseline (zero) measurement was recorded on the 8th day. After that, the samples were stored in a desiccator until the moment of testing over a supersaturated solution of potash, providing RH = 65% at t = 20 ± 2 °C.
To determine water resistance, two sets of samples aged 28 d were tested. The first set was hardened under normal conditions (t = 20 ± 2 °C, RH = 95% ± 5%), and then the compressive strength was determined. The second set, after curing, was pre-saturated with water. The coefficient of water resistance was determined as the ratio of the average strength of water-saturated samples to samples of normal hardening.
3. Results
3.1. Hardening Periods of Alkali-Activated Slag Cements
At the first stage, the influence of modifying admixtures and the silicate modulus of SSS on setting times of AASC were investigated, containing 5%, 50%, and 95% by mass of the OPC clinker in accordance with the plan of the full factorial experiment 23 [33]. The content of the aluminosilicate component was according to [34].
The modifying admixture Na3PO4∙12H2O was preliminarily dissolved in a solution of SSS at an amount of 6% and 12% by mass (in accordance with the plan of the factorial experiment), followed by obtaining a homogeneous solution with a density of 1.35 g/mL.
The initial data and the results of the experiment are presented in Table 3 and Table 4 and Figure 1.

Table 3.
Initial data.

Table 4.
Plan and results of the experiment.
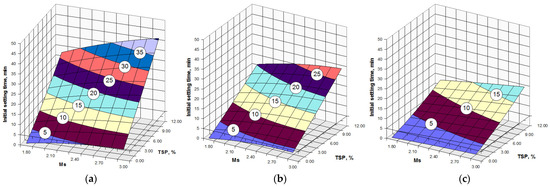
Figure 1.
Initial setting times vs. silicate modulus of SSS, the content of trisodium phosphate and content of the OPC clinker in AASC: (a) OPC clinker content 5%; (b) OPC clinker content 50%; (c) OPC clinker content 95%.
The approximation of data in Table 4 made it possible to obtain regression equations to determine the initial setting time, which were the mathematical models of the experiment:
τ(5%) = 18.77 + 15.17·X1 + 4.5·X2 + 0.83·X11 − 1.17·X22 + 2.5·X1·X2
τ(50%) = 14.11 + 11.0·X1 + 2.83·X2 + 0.33·X11 − 1.17·X22 + 1.25·X1·X2
τ(95%) = 8.11 + 6.83·X1 + 2.17·X2 + 0.83·X11 − 0.17·X22 + 1.25·X1·X2
An analysis of the results given in Table 4 and Figure 1 shows that, in the absence of Na3PO4∙12H2O, all compositions of AASC had extremely short initial setting times within 1–5 min and were of no practical interest because of technological inconvenience.
The lengthening of the setting times within the factor space of the experiment was observed as the silicate modulus, as well as the content of TSP, increased and also as the amount of the OPC clinker in AASC decreased (Figure 1).
The introduction of the Na3PO4∙12H2O admixture in a dosage ≥ 10 wt.% by volume of SSS caused the significant prolongation of the initial setting time to provide the necessary consistency retention of AASC mortar. In this case, silicate modulus could be changed within 2.4–3.0, causing an initial setting time of 20–30 min, which may be of practical interest.
To select the SSS with optimal density, its influence on the setting times of AASC was studied. The content of Na3PO4∙12H2O in the solution was 12% by weight of SSS. The resulting dependencies are shown in Figure 2.
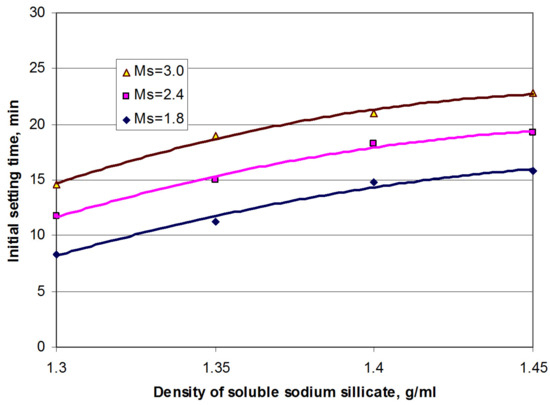
Figure 2.
Initial setting times of alkali-activated slag cement vs. density and silicate module of SSS. The content of TSP in the solution was 12% by the mass of SSS, and the content of OPC clinker in AASC was 95%.
As can be seen from Figure 2, the initial setting time could be prolonged due to the higher density of SSS and the increase in its silicate modulus.
Thus, it is possible to control the setting times of AASC based on SSS and optimize them according to this criterion, as has been shown.
Next, the effect of the complex admixture “TSP + glycerol” on the setting times of AASC containing 95% of the OPC clinker (which, to a certain extent, is an analog to CEM I 42.5R) was tested using SSS (Ms = 2.4). In this case, the initial setting time of 1–18 min, even with the addition of Na3PO4∙12H2O (Table 4), was obtained. This complex action shows that only glycerol, even in a fairly significant amount, had little effect on setting times (Figure 3). Somewhat preferable are the results with a solution of SSS containing the addition of Na3PO4∙12H2O. Additionally, only the complex admixture “glycerol + TSP” acted effectively due to the synergistic effect. In this case, solutions of SSS with a density of 1.35 g/mL and Ms = 2.4, containing 6% and 12% of Na3PO4∙12H2O, had glycerol added to them at different ratios to TSP.
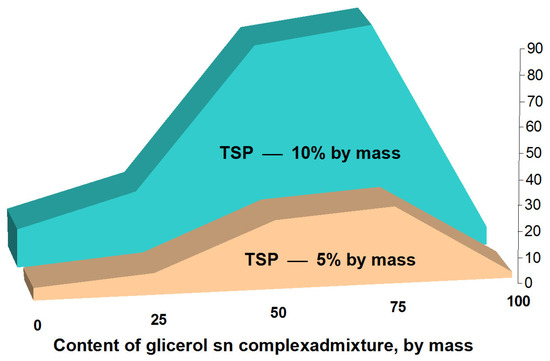
Figure 3.
Effect of the complex admixture “TSP + glycerol” on initial setting time of AASC based on SSS (p = 1.35 g/mL, Ms = 2.4).
Thus, the insurance of an initial setting time within 25–30 min is possible in the presence of glycerol in the complex admixture at an amount of 25%, that is, the ratio “glycerol: TSP” as 1:3 with a content of 12% TSP in SSS (Figure 3). A further increase in the content of glycerol allowed the initial setting time to be prolonged up to 60–90 min.
3.2. Characteristics of Alkali-Activated Slag Cements
To ensure the specified setting times in the composition, in addition to Na3PO4∙12H2O, an admixture of glycerol was introduced: the optimal amount of 2%–2.5% by AASC. Therefore, further studies to determine the main technological and physicomechanical characteristics of AASC with variable compositions (the content of the OPC clinker ranged from 5% to 95%) were carried out using high-modulus SSS, containing Na3PO4∙12H2O (12%) and the optimal amount of glycerol. In this case, the influence of silicate modulus on the strength of AASC was determined (Table 5).

Table 5.
The properties of AASC based on high modulus SSS.
The effect of silicate modulus on the strength of compositions is not directly related. The maximum early strength (up to 3 days) was fixed when SSS with Ms = 2.4 was used. Changing the value of silicate modulus in some direction resulted in less strength (Figure 4).
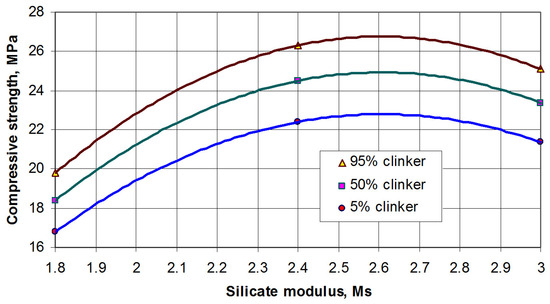
Figure 4.
Strength of AASC after 3 h of hardening vs. silicate modulus of SSS. The density of the solution was 1.35 g/mL, and the content of TSP in the solution was 12% by mass.
An increase in the content of the OPC clinker significantly intensified the early strength of AASC. For example, in the case of the 5% OPC clinker, the 3 h strength was 22.1 MPa, then, with a content of 50% and 95% for the OPC clinker—24.5 MPa and 26.3 MPa, respectively (Table 5, ## 6–8). The optimal amount of glycerol in the complex admixture made it possible to extend the initial setting time from 16 min to 21–30 min (Table 5, ## 5–8).
The introduction of an increased amount of glycerol up to 5.25% (Table 5, # 13) allowed the prolongation of the initial setting time from 23 min up to 72 min but led to a significant decrease in the strength gain at early stages (Table 5, # 12 and # 13). However, by the 28th day, the strength of such compositions equalized and slightly exceeded the strength of compositions with the optimal amount of glycerol. Perhaps this was due to some plasticizing effect on the last one. Thus, increasing the glycerol dosage from 2.5% to 5.25% reduces standard consistency from 31% to 29% and S/C from 0.43 to 0.39. The coefficient of water resistance for all compositions was equal to 0.89–0.95, i.e., they are waterproof. Higher ratios between flexural strength and compressive strength when glycerol was used compared to compositions without this admixture indicate the higher crack resistance of the modified AASC (Table 5).
Based on Figure 4, it can be noticed that the range of Ms = 2.5–2.7 was optimal for AASC from the point of early strength.
Figure 5 shows how the introduction of glycerol allowed compositions to be obtained with proper shrinkage deformations within the range of 0.47–0.6 mm/m. This is 1.2–1.53 times lower compared to the composition without the addition of glycerol. In a general case, increasing the content of the OPC clinker causes less shrinkage.
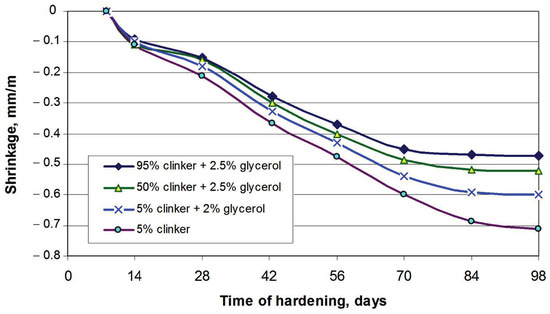
Figure 5.
Proper shrinkage deformations of AASC fine−grained concrete: SSS (Ms = 2.4, p = 1.35 g/mL); the content of TSP in the solution was 12% by mass for the compositions seen in Table 5 (## 5–8).
Table 6 shows the comparative characteristics of obtained super-rapid-hardening AASC and well-known cement with high early strength gain, i.e., alumina cement [35], rapid-hardening cement [36], super-rapid-hardening cement [37], and low water demand cement [38].

Table 6.
Comparative characteristics of cement.
4. Conclusions
It has been shown that there is a possibility of obtaining super-rapid-hardening high-strength alkali-activated slag cement with an OPC clinker content of 5%–95%, the initial setting times—21–34 min, compressive strength after 3 h—21.0–26.3 MPa, after 1 day—36.5–43.4 MPa, after 28 days—84.7–117.1 MPa, and water resistance coefficient—0.89–0.95.
The efficiency of co-using glycerol and TSP has been shown. The admixture of TSP, due to the adsorption of PO43− ions on the surface of aluminosilicate components, serves as a substrate for glycerol. The synergistic effect from the use of glycerol in complex with TSP has been established to prolong the initial setting time by 1.3–2 times or more and to enhance the properties of cement stone manifested in the plasticizing effect of glycerol (a reduction in S/C by 2.7%–5.3%), which is higher than 23.5%–35.8% of the compressive strength of artificial stone with a reduction in 1.2–1.53 times of proper shrinkage deformations to values of 0.47–0.6 mm/m, and an increase in crack resistance.
Optimal and technologically acceptable setting times and early strength can be obtained by mixing AASC with SSS (Ms = 2.3–2.5, p = 1.35 g/mL).
An increase in the basicity of the aluminosilicate component of AASC causes higher early strength, reduces shrinkage, and shortens setting times. Reducing the setting time is offset by the complex admixture “TSP + glycerol”.
In terms of early strength gain, super-rapid-hardening AASC with a content of 95% of the OPC clinker based on SSS (Ms = 2.4, p = 1.35 g/mL) is significantly superior to alumina cement, rapid-hardening cement, super-rapid-hardening cement, as well as cement with a low water demand.
Author Contributions
Project administration, funding acquisition, conceptualization, supervision, validation and writing—review and editing, P.K. and I.R.; methodology, resources, O.K. (Oleksandr Kovalchuk); data curation, software, investigation, formal analysis, visualization and writing—original draft preparation, O.G. and O.K. (Oleksandr Konstantynovskyi). All authors have read and agreed to the published version of the manuscript.
Funding
This research work was funded by the Ministry of Education and Science of Ukraine for the financial support of projects (registration No. 0123U101832 and No. 0123U101831), which are carried out at the expense of budget funding in 2023–2025.
Data Availability Statement
The data are not publicly available due to works are carried out within the framework of the mentioned project, its results will be available on request after registration of report in State scientific institution Ukrainian Institute of Scientific and Technical Expertise and Information (http://www.uintei.kiev.ua/en, accessed on 21 March 2023) using the system https://nddkr.ukrintei.ua/, accessed on 21 March 2023.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Coppola, L.; Bellezze, T.; Belli, A.; Bignozzi, M.C.; Bolzoni, F.; Brenna, A.; Cabrini, M.; Candamano, S.; Cappai, M.; Caputo, D.; et al. Binders alternative to Portland cement and waste management for sustainable construction—Part 1. J. Appl. Biomater. Funct. Mater. 2018, 16, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Sanytsky, M.; Kropyvnytska, T.; Heviuk, I.; Sikora, P.; Braichenko, S. Development of Rapid-Hardening Ultra-High Strength Cementitious Composites using Superzeolite and N-C-S-H-PCE Alkaline Nanomodifier. East.-Eur. J. Enterp. Technol. 2021, 5, 62–72. [Google Scholar] [CrossRef]
- Xue, J.; Briseghella, B.; Huang, F.; Nuti, C.; Tabatabai, H.; Chen, B. Review of ultra-high performance concrete and its application in bridge engineering. Constr. Build. Mater. 2020, 260, 119844. [Google Scholar] [CrossRef]
- Afroughsabet, V.; Ozbakkaloglu, T. Mechanical and durability properties of high-strength concrete containing steel and polypropylene fibers. Constr. Build. Mater. 2015, 94, 73–82. [Google Scholar] [CrossRef]
- Liu, S.G.; Xiang, Z.; Huang, R.H.; Wang, D.H.; Ju, Y.Z. The durability of reactive powder concrete: A review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 474, 012047. [Google Scholar] [CrossRef]
- Hohol, M.; Sanytsky, M.; Kropyvnytska, T.; Barylyak, A.; Bobitski, Y. The effect of sulfur- and carbon-codoped TiO2 nanocomposite on the photocatalytic and mechanical properties of cement mortars. East.-Eur. J. Enterp. Technol. 2020, 4, 6–14. [Google Scholar] [CrossRef]
- Ponomar, V.; Luukkonen, T.; Yliniemi, J. Revisiting alkali-activated and sodium silicate-based materials in the early works of Glukhovsky. Constr. Build. Mater. 2023, 398, 132474. [Google Scholar] [CrossRef]
- Elzeadani, M.; Bompa, D.V.; Elghazouli, A.Y. One part alkali activated materials: A state-of-the-art review. J. Build. Eng. 2022, 57, 104871. [Google Scholar] [CrossRef]
- Rossi, L.; Miranda de Lima, L.; Sun, Y.; Dehn, F.; Provis, J.; Ye, G.; De Schutter, G. Future Perspectives for Alkali-Activated Materials: From Existing Standards to Structural Applications. RILEM Tech. Lett. 2023, 7, 159–177. [Google Scholar] [CrossRef]
- Krivenko, P.; Gots, V.; Petropavlovskyi, O.; Rudenko, I.; Konstantynovskyi, O. Complex shrinkage-reducing additives for alkali activated slag cement fine concrete. Solid State Phenom. 2021, 321, 165–170. [Google Scholar] [CrossRef]
- Krivenko, P.; Rudenko, I.; Konstantynovskyi, O. Effect of technological factors on freeze-thaw resistance of alkali-activated slag cement concrete in NaCl solution. AIP Conf. Proc. 2023, 2684, 040011. [Google Scholar] [CrossRef]
- Krivenko, P.; Rudenko, I.; Konstantynovskyi, O. Comparison of influence of surfactants on the thermokinetic characteristics of alkali-activated slag cement. East.-Eur. J. Enterp. Technol. 2021, 6, 24–32. [Google Scholar] [CrossRef]
- Krivenko, P.; Rudenko, I.; Konstantynovskyi, O.; Boiko, O. Prevention of steel reinforcement corrosion in alkali-activated slag cement concrete mixed with seawater. E3S Web Conf. 2021, 280, 07004. [Google Scholar] [CrossRef]
- Krivenko, P.; Rudenko, I.; Konstantynovsky, O.; Vaičiukynienė, D. Feasibility of incorporating SO42−-ions in zeolite-like matrices based on alkaline aluminosilicate binders. Con. Build. Mat. 2023, 391, 131878. [Google Scholar] [CrossRef]
- Marushchak, U.; Sanytsky, M.; Sydor, N.; Braichenko, S. Research of Nanomodified Engineered Cementitious Composites. In Proceedings of the IEEE 8th International Conference Nanomaterials: Application & Properties (NAP), Zatoka, Ukraine, 9–14 September 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Vafaei, M.; Allahverdi, A. High strength geopolymer binder based on waste-glass powder. Adv. Powder Technol. 2017, 29, 215–222. [Google Scholar] [CrossRef]
- Li, M.; Huang, G.; Cui, Y.; Wang, B.; Chang, B.; Yin, Q.; Zhang, S.; Wang, Q.; Feng, J.; Ge, M. Coagulation mechanism and compressive strength characteristics analysis of high-strength alkali-activated slag grouting material. Polymers 2022, 14, 3980. [Google Scholar] [CrossRef]
- Ajler, R. Himija Kremnezema; Chast’ 1. Mir: Moskow, Russia, 1982; pp. 184–186. [Google Scholar]
- Blazhis, A.R.; Rostovskaya, G.S.; Glukhovskiy, V.D. Binder. Patent of USSR 1708787, 7 January 1992. [Google Scholar]
- Krivenko, P.; Petropavlovskyi, O.; Kovalchuk, O.; Rudenko, I.; Konstantynovskyi, O. Enhancement of alkali-activated slag cement concretes crack resistance for mitigation of steel reinforcement corrosion. E3S Web Conf. 2020, 166, 06001. [Google Scholar] [CrossRef]
- Krivenko, P.V.; Runova, R.F.; Rudenko, I.I. Plasticized Concrete and Mortar Based on Cements System Na2O–CaO–Al2O3–SiO2–H2O: Monography; Lira-K: Kyiv, Ukraine, 2022; 392p. [Google Scholar]
- Collepardi, M.; Monosi, S.; Moricomi, G.; Pauli, M. Influence of Gluconate, Lignosulfonate and Glucose on the C3A Hudration in the Presence of Gupsum with on without Lime. Cem. Concr. Res. 1984, 14, 105–212. [Google Scholar] [CrossRef]
- Skoryk, V.V. Backfill Mortars Based on Alkaline Cement for Variable Cure Conditions in the Well. Ph.D. Thesis, Kyiv National University of Construction and Architecture, Kyiv, Ukraine, 2018. [Google Scholar]
- DSTU B V.2.7-302:2014; Building Materials. Granulated Blast Furnace Slag for Cements, Concretes and Mortars. Technical Requirements and Conformity Assessment. EN 15167-1:2006, NEQ. Minregionbud Ukrani: Kyiv, Ukraine, 2014.
- Palacios, M.; Puertas, F. Effect of superplasticizer and shrinkage-reducing admixtures on alkali-activated slag pastes and mortars. Cem. Concr. Res. 2005, 35, 1358–1367. [Google Scholar] [CrossRef]
- Partschefeld, S.; Tutal, A.; Halmanseder, T.; Schneider, J.; Osburg, A. Investigations on stability of polycarboxylate superplasticizers in alkaline activators for geopolymer binders. Materials 2023, 16, 5369. [Google Scholar] [CrossRef]
- Alrefaei, Y.; Wang, Y.-S.; Dai, J.-G. The effectiveness of different superplasticizers in ambient cured one-part alkali activated pastes. Cem. Concr. Compos. 2019, 97, 166–174. [Google Scholar] [CrossRef]
- Chang, J.J. A study on the setting characteristics of sodium silicate-activated slag pastes. Cem. Concr. Res. 2003, 33, 1005–1011. [Google Scholar] [CrossRef]
- Jiang, D.; Shi, C.; Zhang, Z. Recent progress in understanding setting and hardening of alkali-activated slag (AAS) materials. Cem. Concr. Compos. 2022, 134, 104795. [Google Scholar] [CrossRef]
- Haha, M.B.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Krizan, D.; Zivanovic, B. Effects of dosage and modulus of water glass on early hydration of alkali-slag cements. Cem. Concr. Res. 2002, 32, 1181–1188. [Google Scholar] [CrossRef]
- Kudina, E.F.; Pechersky, G.G.; Ermolovich, O.A. Study on the process of gel formation in the “water glass-acrylamide” systems. Plast. Massy 2012, 1, 27–29. [Google Scholar]
- Wu, C.J.; Hamada, M.S. Experiments: Planning, Analysis, and Optimization; John Wiley & Son: Hoboken, NJ, USA, 2021; p. 736. [Google Scholar]
- DSTU B V.2.7-181:2011; Building Materials. Alkaline Cements. Specifications. Mіnregіonbud Ukrani: Kyiv, Ukraine, 2011.
- Zapata, J.F.; Azevedo, A.; Fontes, C.; Monteiro, S.N.; Colorado, H.A. Environmental Impact and Sustainability of Calcium Aluminate Cements. Sustainability 2022, 14, 2751. [Google Scholar] [CrossRef]
- Kropyvnytska, T.; Sanytsky, M.; Rucińska, T.; Rykhlitska, O. Development of nanomodified rapid hardening clinker-efficient concretes based on composite Portland cements. East.-Eur. J. Enterp. Technol. 2019, 6, 38–48. [Google Scholar] [CrossRef]
- Bertola, F.; Bassani, M.; Canonico, F.; Bianchi, M. Use of Rapid-Hardening Cement for Controlled Low-Strength Materials for Pavement Applications. Transp. Res. Rec. 2013, 2363, 77–87. [Google Scholar] [CrossRef]
- Khozin, V.; Khokhryakov, O.; Nizamov, R. A «carbon footprint» of low water demand cements and cement-based concrete. IOP Conf. Ser. Mater. Sci. Eng. 2020, 890, 012105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).