Calibrating a Handheld LIBS for Li Exploration in the Barroso–Alvão Aplite-Pegmatite Field, Northern Portugal: Textural Precautions and Procedures When Analyzing Spodumene and Petalite
Abstract
1. Introduction
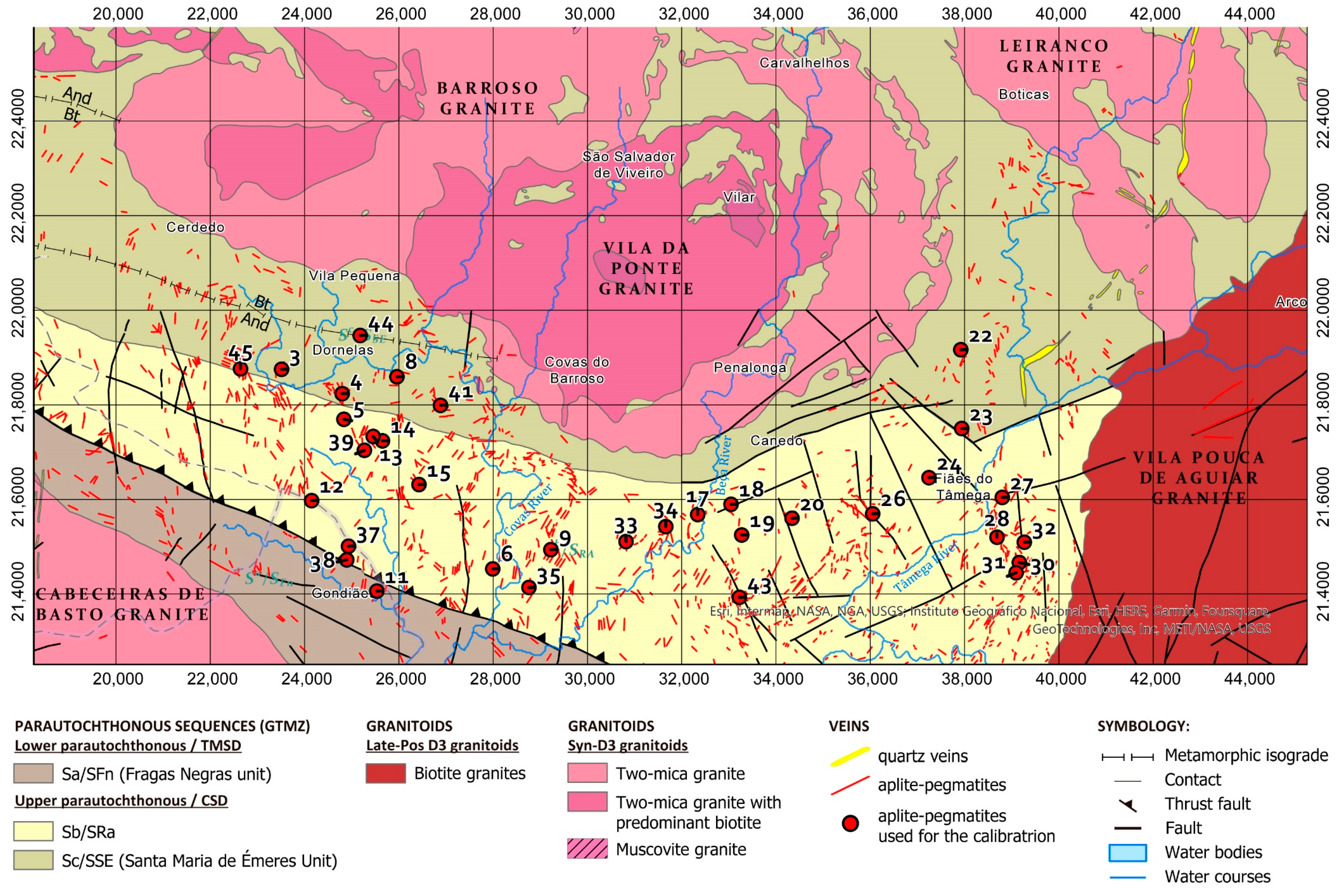
2. Geological Setting
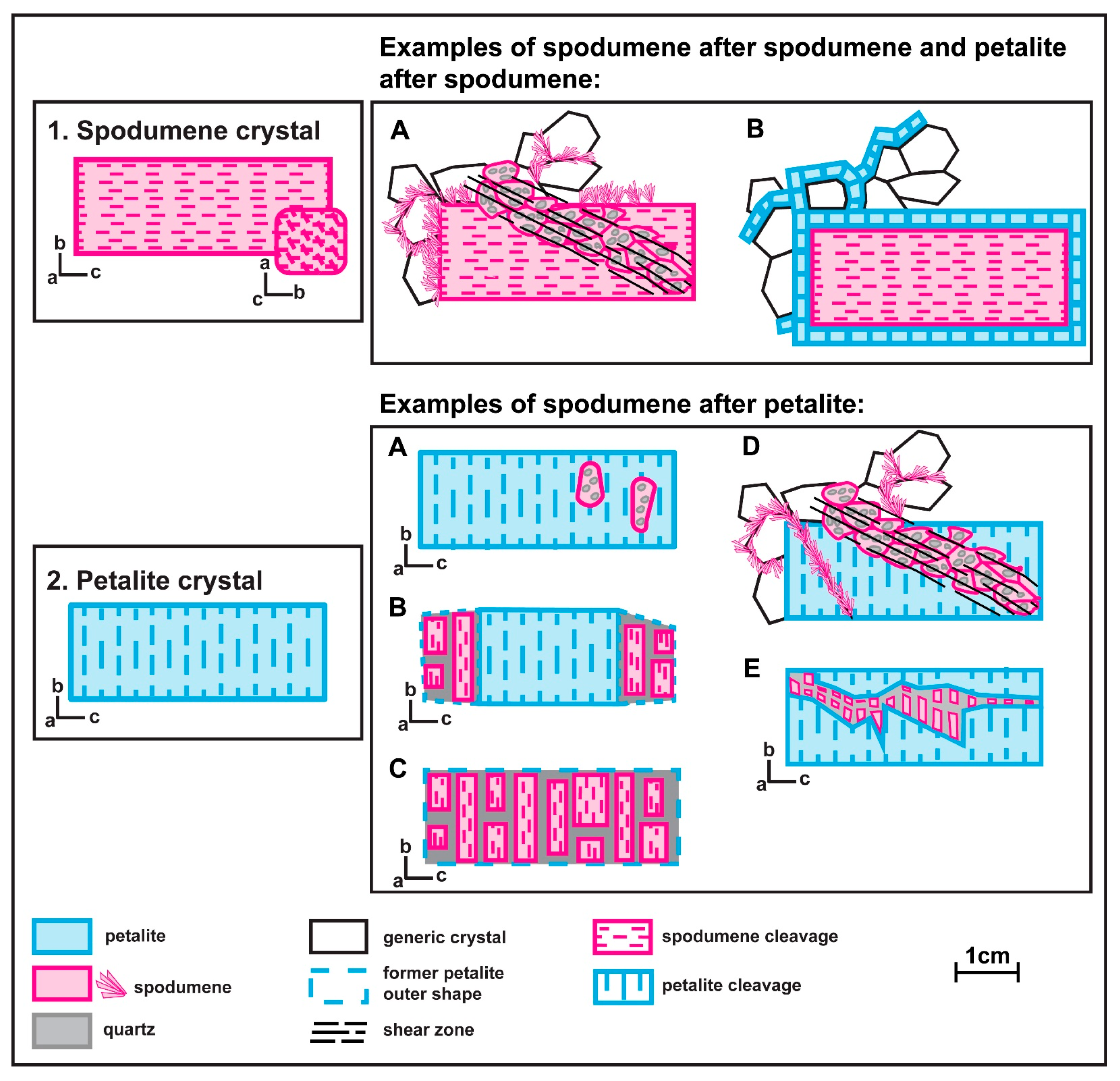
3. Spodumene and Petalite Petrography
4. Materials and Methods
Construction of Calibration Lines for the Handheld LIBS
5. Results
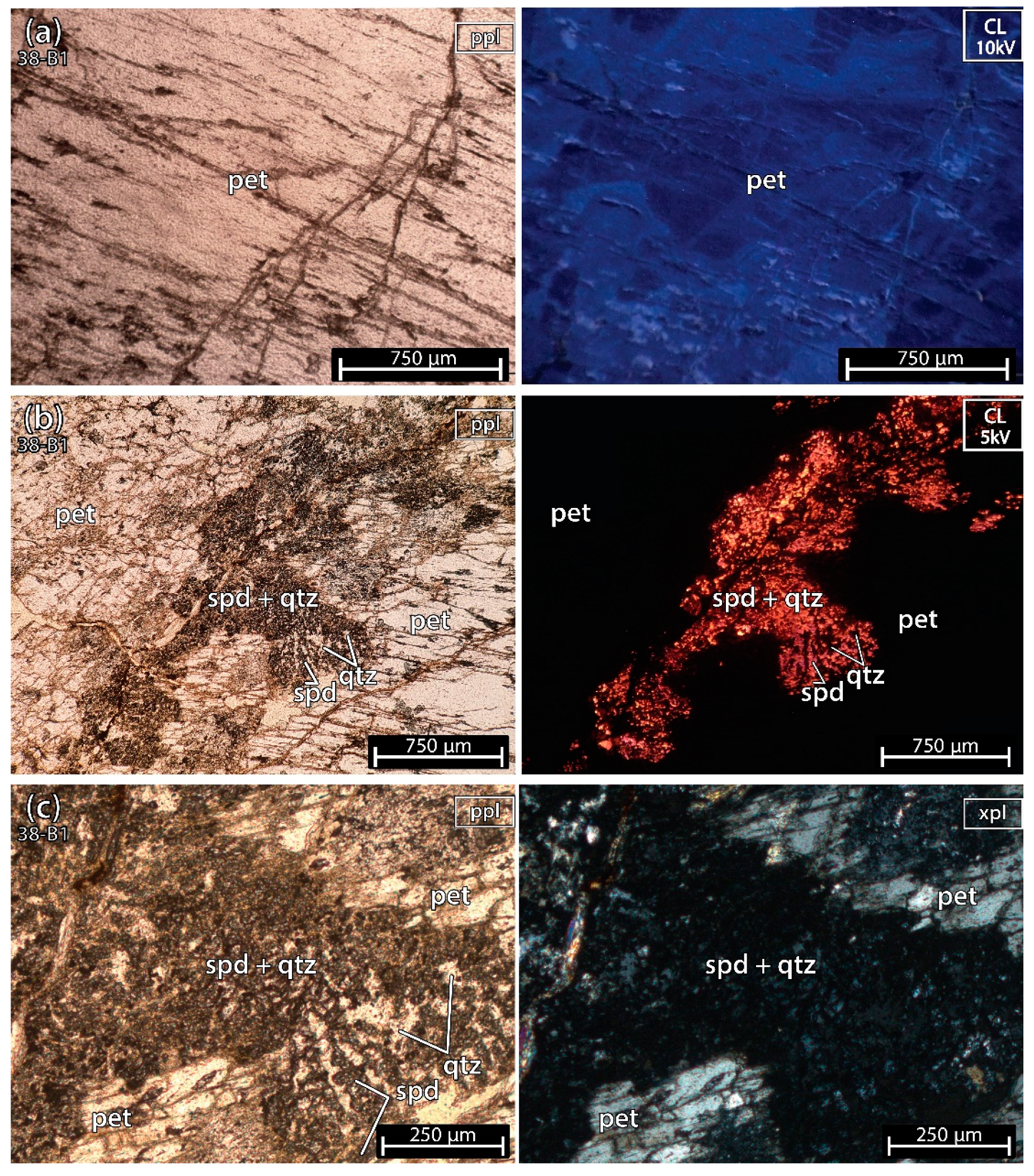
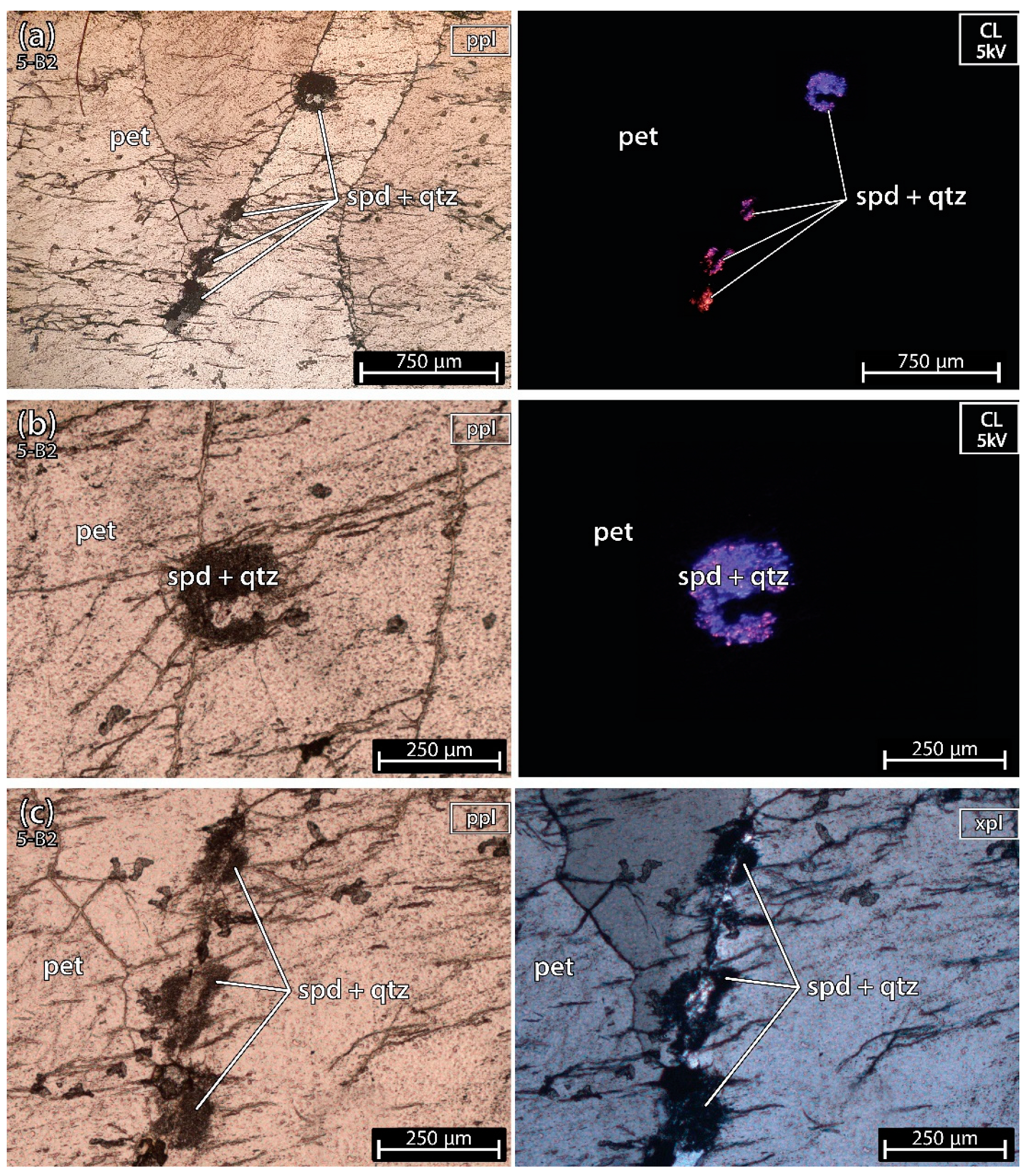
5.1. Petrography of the Thin sections
5.1.1. Petalite Crystal 1 of the Aplite-Pegmatite 38 (Also Known as Gondiães 91)
5.1.2. Petalite Crystal 2 of the Aplite-Pegmatite 5 (Also Known as 59-AL-023)
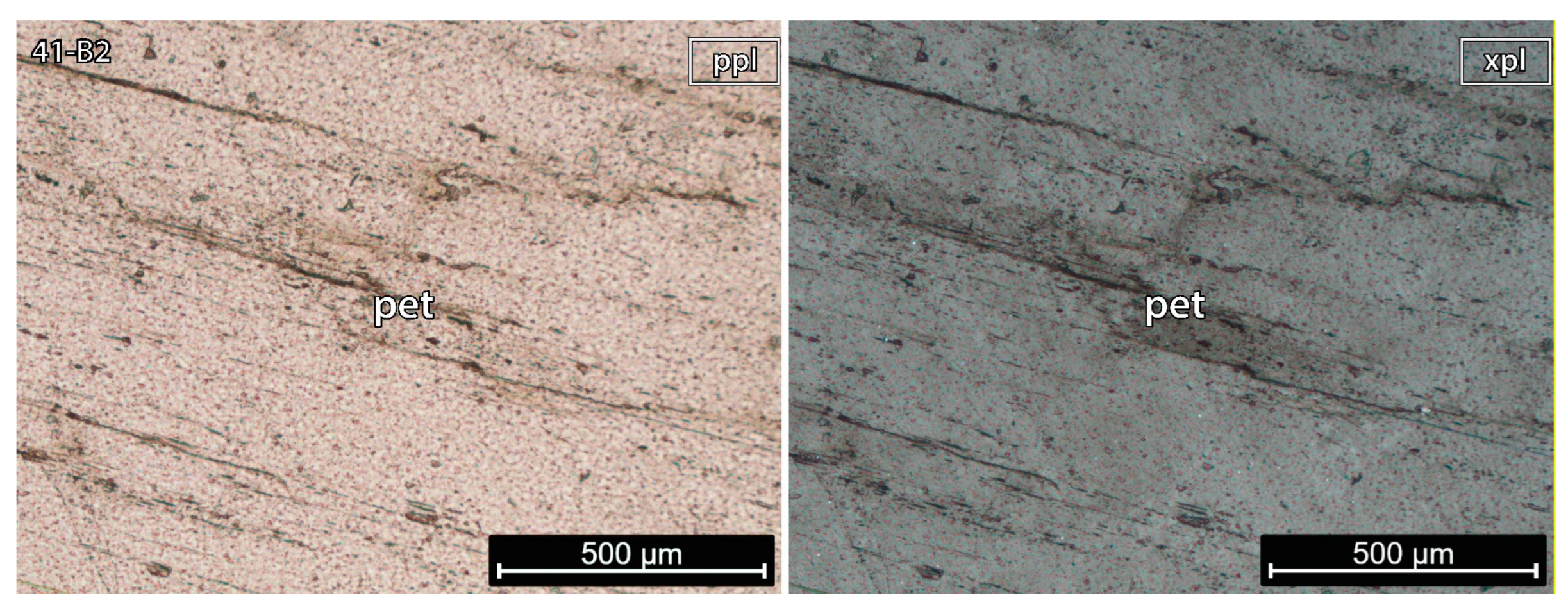
5.1.3. Petalite Crystal 2 of the Aplite-Pegmatite 41 (Also Known as NOA)
5.1.4. Petalite Crystal 2 of the Aplite-Pegmatite 44 (Also Known as Antigo)
5.1.5. Mixture of Spodumene and Quartz 2 of the Aplite-Pegmatite 8 (Also Known as 59-AL-52)
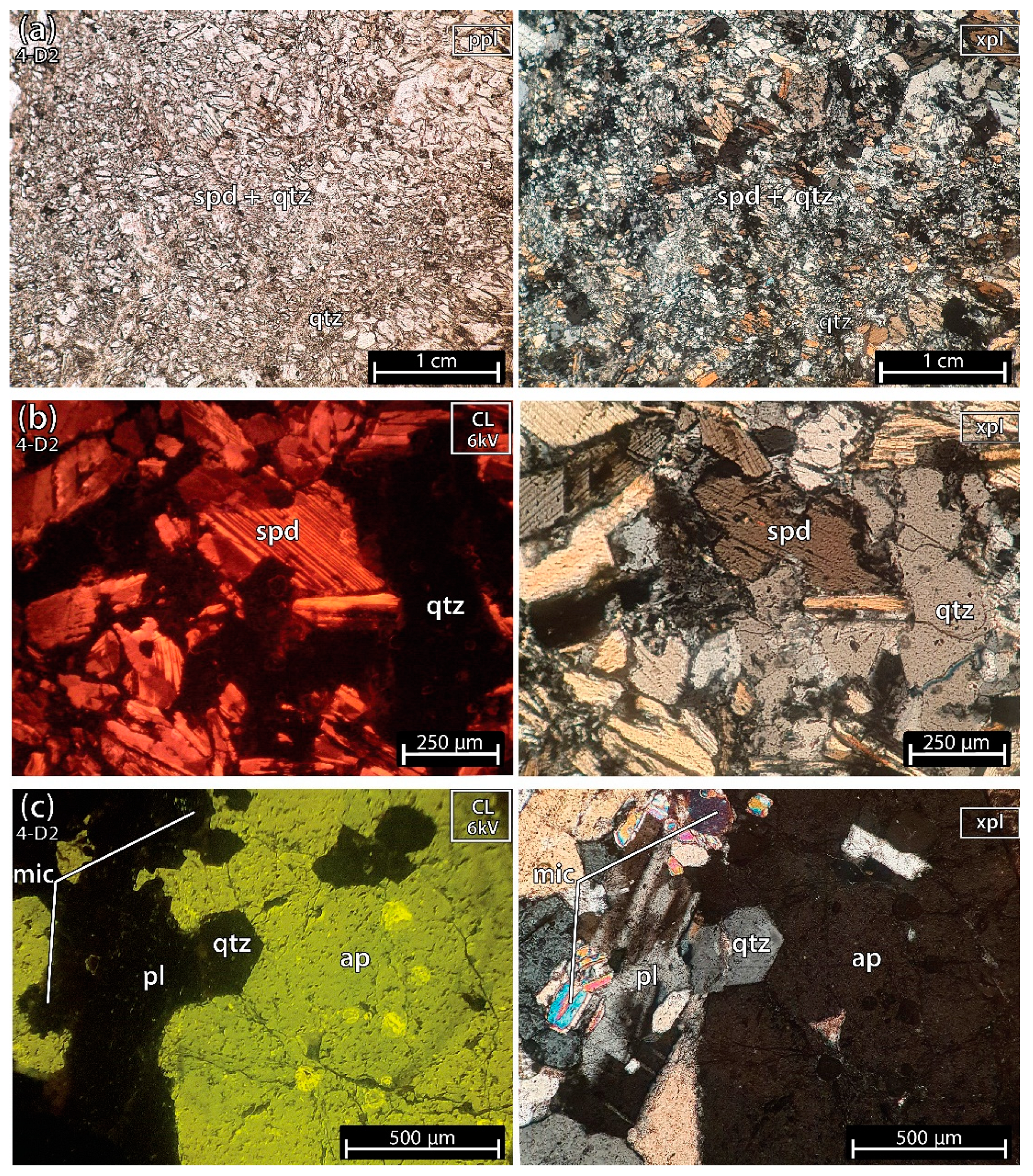
5.1.6. Mixture of Spodumene and Quartz 2 of the Aplite-Pegmatite 4 (Also Known as 59-AL-22)
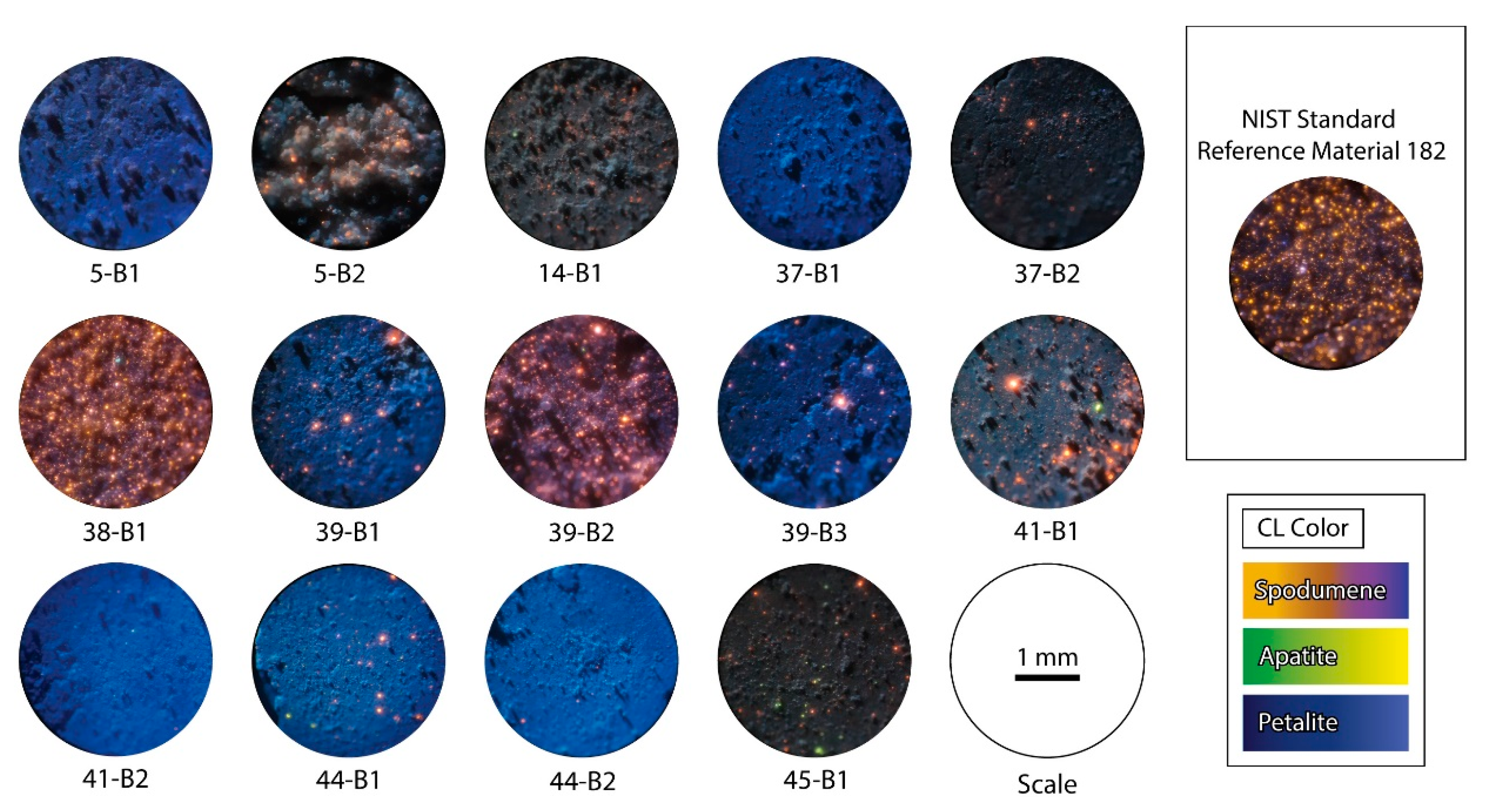
5.2. Petalite Pellets Analyzed with Cold-Cathodoluminescence Microscopy
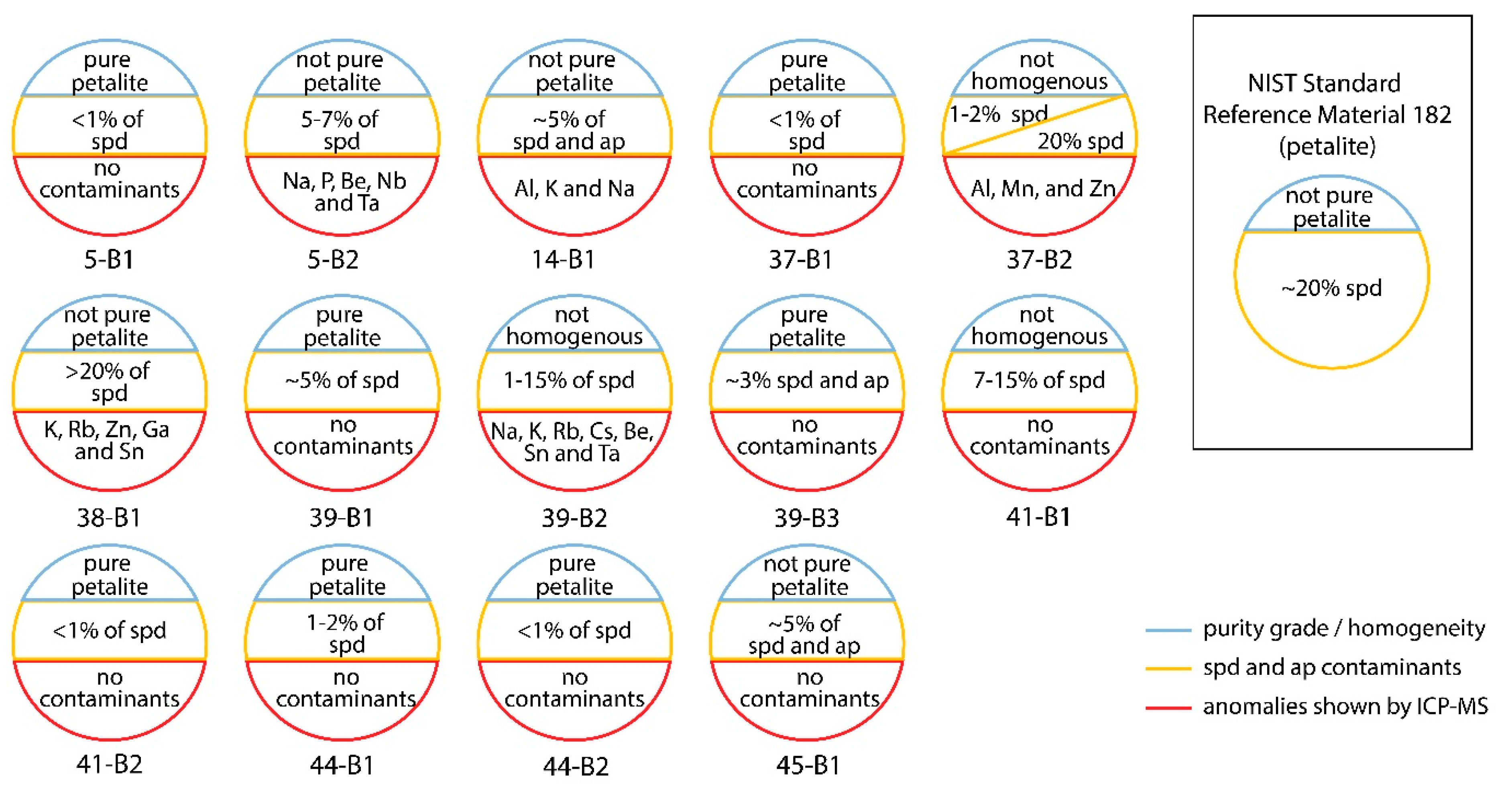
- sample 5-B1 was essentially pure petalite with less than 1% of possible spodumene and other bright minerals;
- sample 5-B2 had a destroyed surface after the LIBS ablation. However, it was possible to see approximately 5%–7% of bright orange minerals, probably spodumene. The rest of the material was very dark. This could be the result of the pellet being destroyed and the shadows formed by the irregular surface hiding the luminescence of the petalite. Alternatively, the sample could contain several non-luminescent or poorly luminescent minerals. However, since the ICP-MS results for this sample indicated positive anomalies in Na, P, Be, Nb and Ta, the last option is the most likely (Table 2);
- sample 14-B1 had around 5% of bright luminescent minerals, some green and some orange in CL, probably spodumene and apatite. The blue luminescence of the rest of the material was weaker than some of the other petalite pellets, possibly indicating the presence of other non-luminescent or poorly luminescent minerals. Since the ICP-MS results indicated positive anomalies in Al, K and Na, this could indicate the presence of feldspars and micas mixed with the petalite (Table 2).
- sample 37-B1 was essentially pure petalite with less than 1% of bright orange minerals, probably spodumene;
- sample 38-B1 seemed to have more than 20% of spodumene, since it had a high amount of bright orange minerals. Besides, the ICP-MS results indicated the possible presence of other minerals, such as feldspar and micas, since this sample contained positive anomalies in K, Rb, Zn, Ga and Sn;
- sample 39-B1 was essentially petalite with about 5% of spodumene;
- sample 39-B2 had some zones richer and other zones poorer in strong luminescent minerals, thus varying from 1 to 15% of what seems to be spodumene grains. In the ICP-MS results, this sample also had a positive anomaly in Na, K, Rb, Cs, Be, Sn and Ta (Table 2);
- sample 39-B3 was pure petalite with approximately 3% of other bright minerals, including probable spodumene;
- sample 41-B1 had some zones richer and other zones poorer in strong luminescent minerals, thus varying from 7 to 15% of what seemed spodumene grains;
- sample 41-B2 was essentially pure petalite with less than 1% of bright orange minerals;
- sample 44-B1 was almost pure petalite with 1%–2% of bright orange minerals;
- sample 44-B2 was essentially pure petalite with less than 1% of bright orange minerals;
- sample 45-B1 had around 5% of strong luminescent minerals, some bright orange, others bright green. The CL luminescence of this pellet was darker than the other petalites, but the ICP-MS results from this sample did not have any significant anomalies.
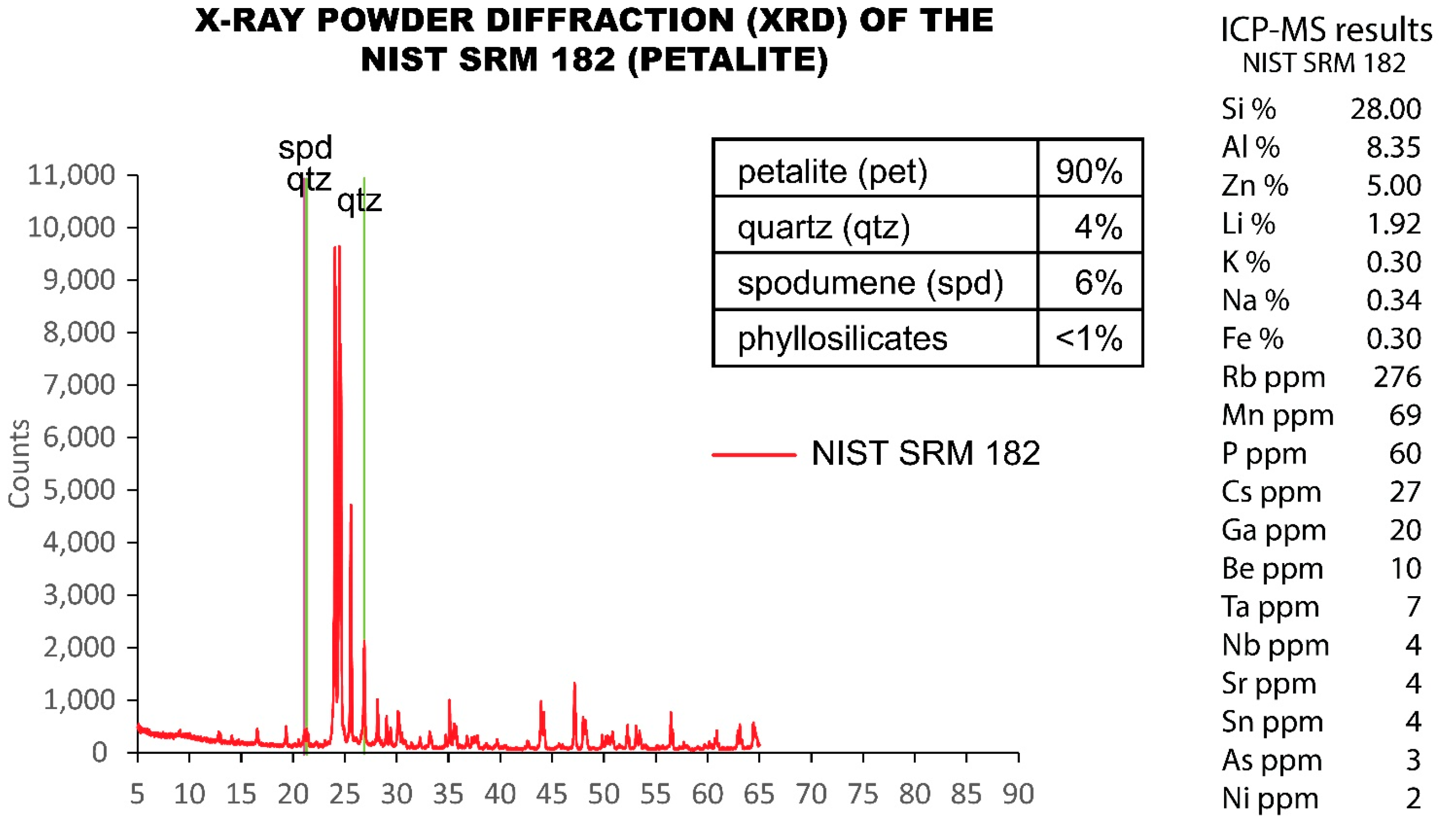
5.3. NIST SRM 182 (Petalite)
- spodumene: 16% of error for Si, 0% of error for Al, 1% of error for Li and 11% of error for Fe;
- spodumene and quartz mixture: 18% of error for Si, 1% of error for Al, 3% of error for Li and 0% of error for Fe;
- NIST SRM 182 (petalite): 5% for Li.
5.4. ICP-MS Errors from the Duplicates Made by Actlabs
6. Discussion
6.1. Implications of the Petalite Pellets Analysis with Cathodoluminescence
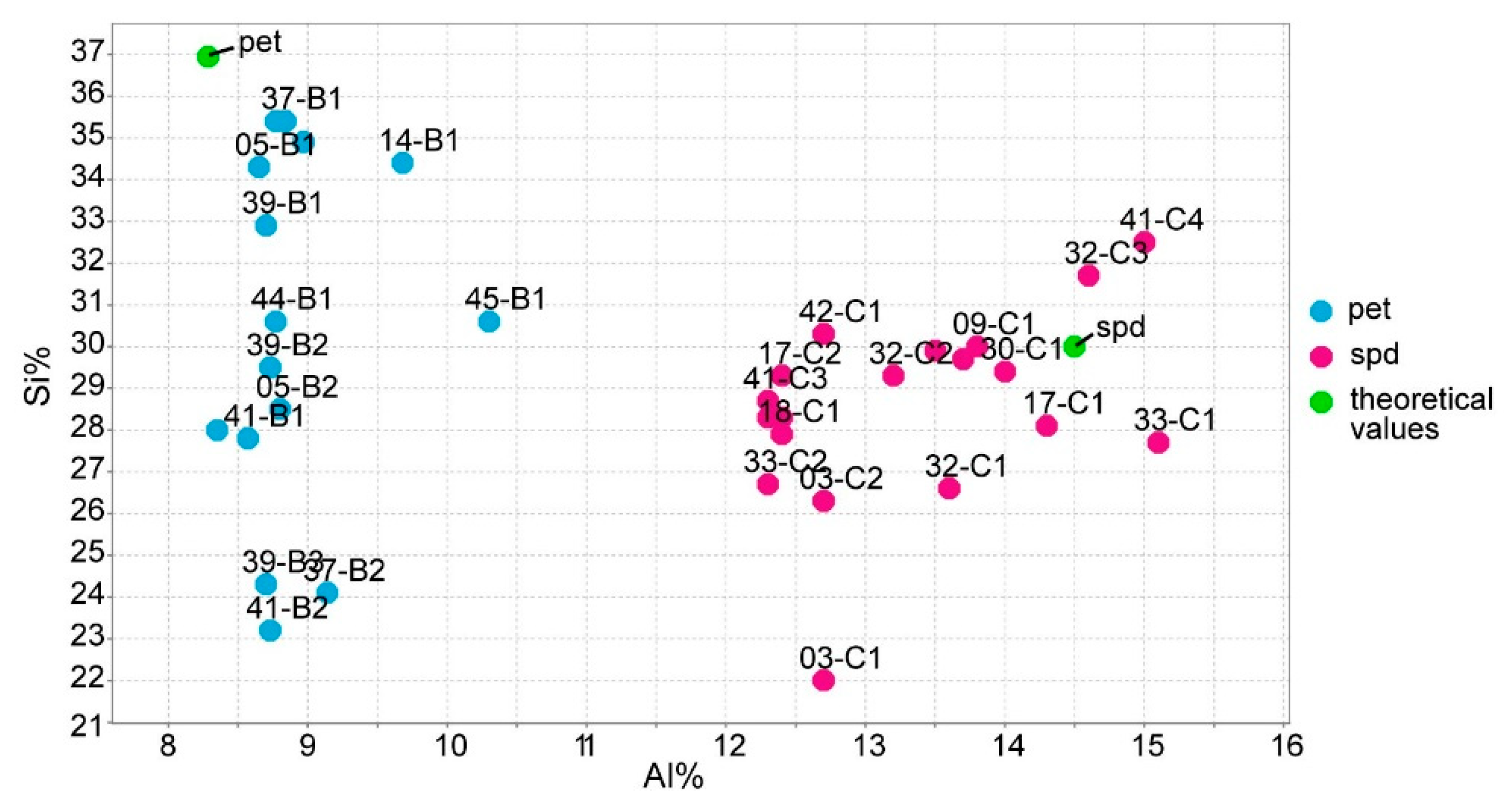
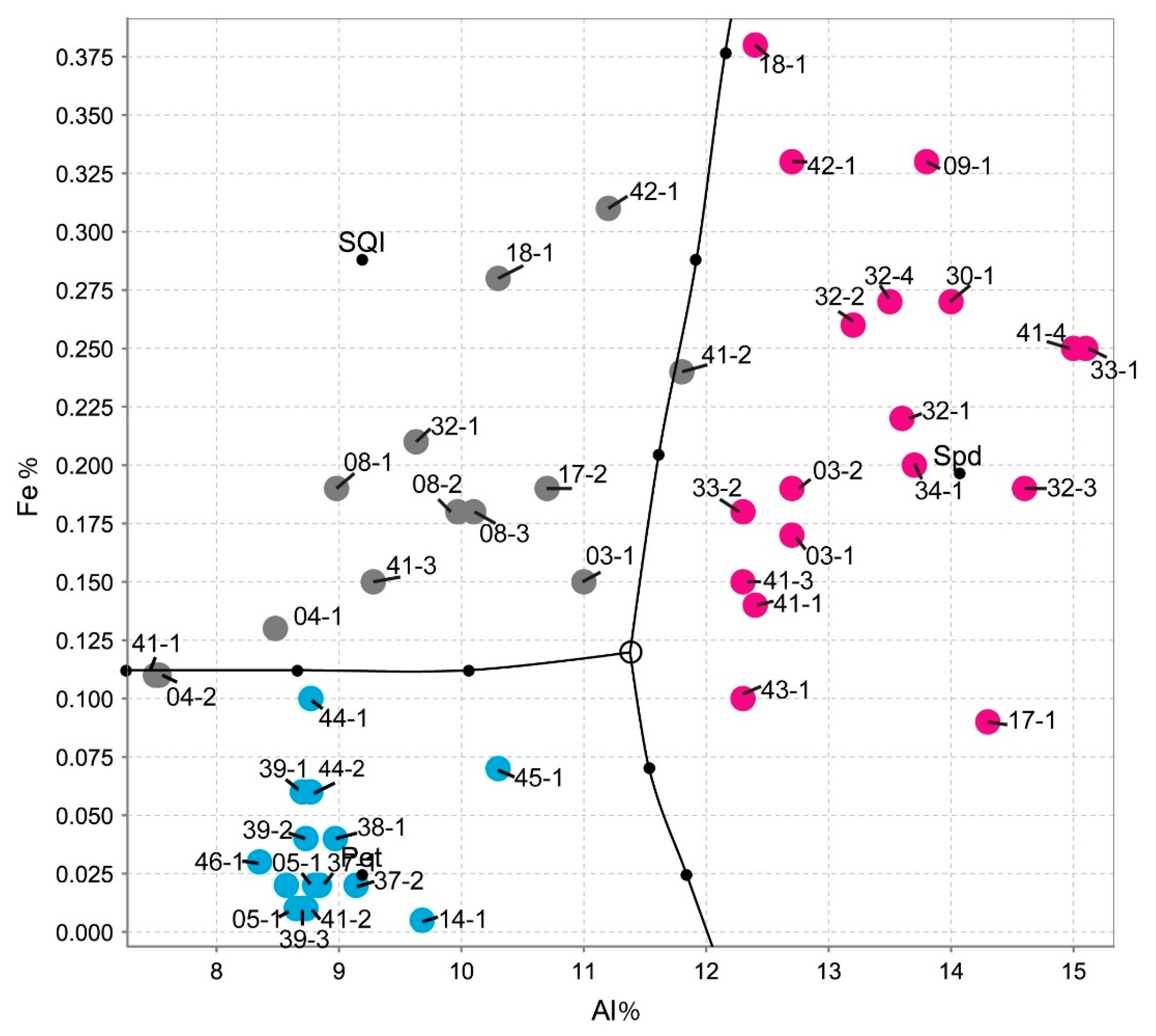
6.2. Identifying Spodumene, Petalite and Mixtures of Spodumene and Quartz Based on Their Chemical Composition
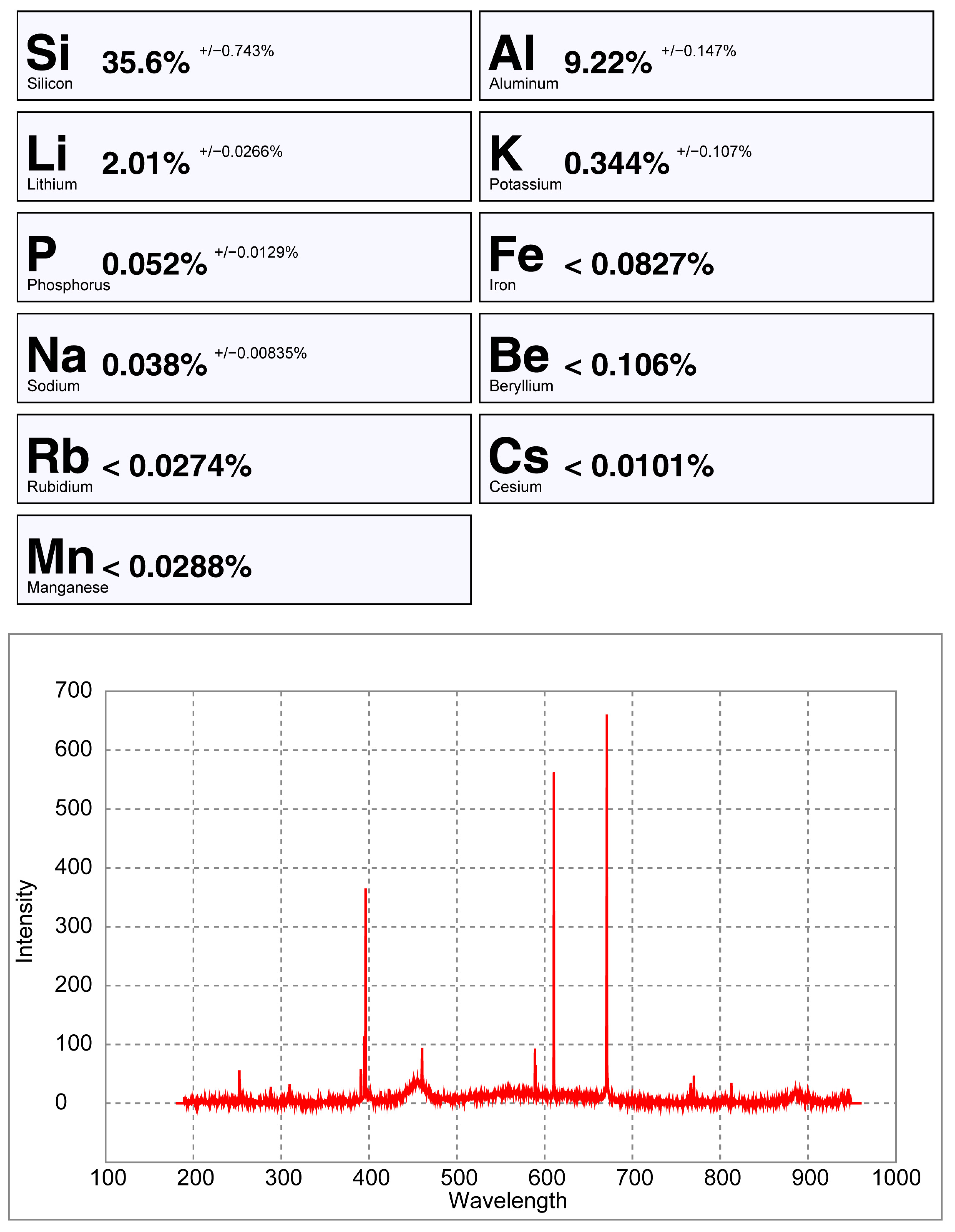
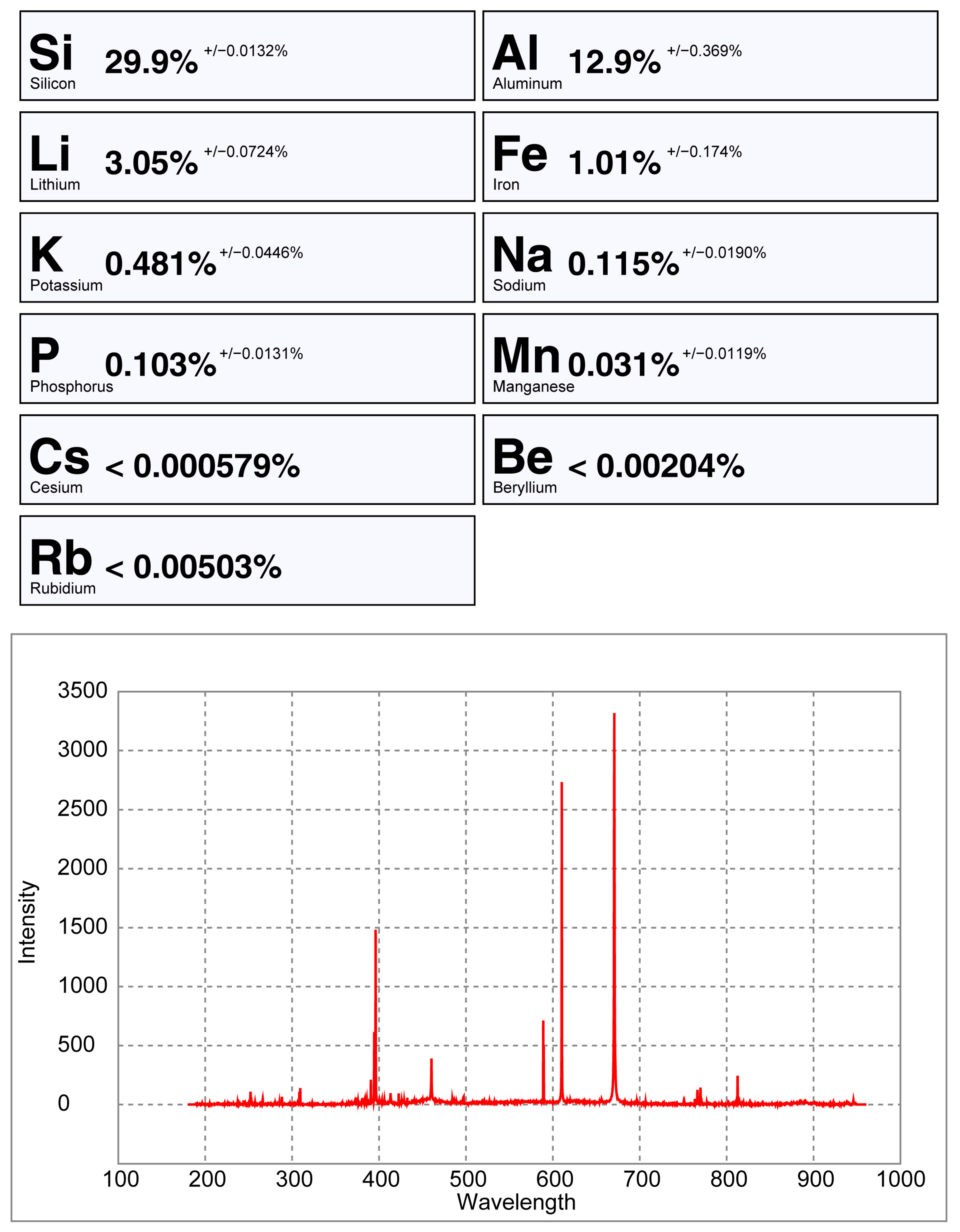
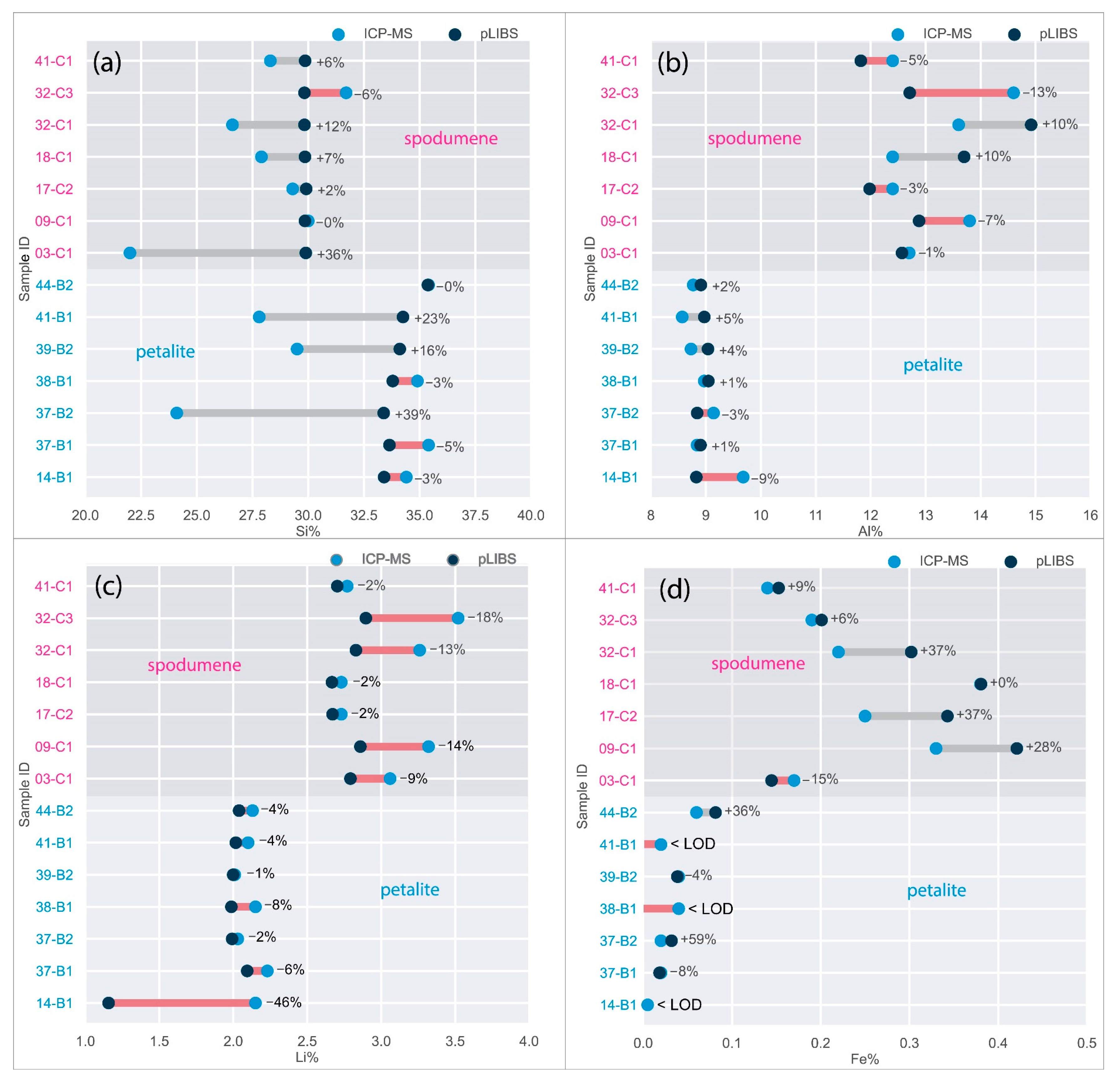
6.3. LIBS Results
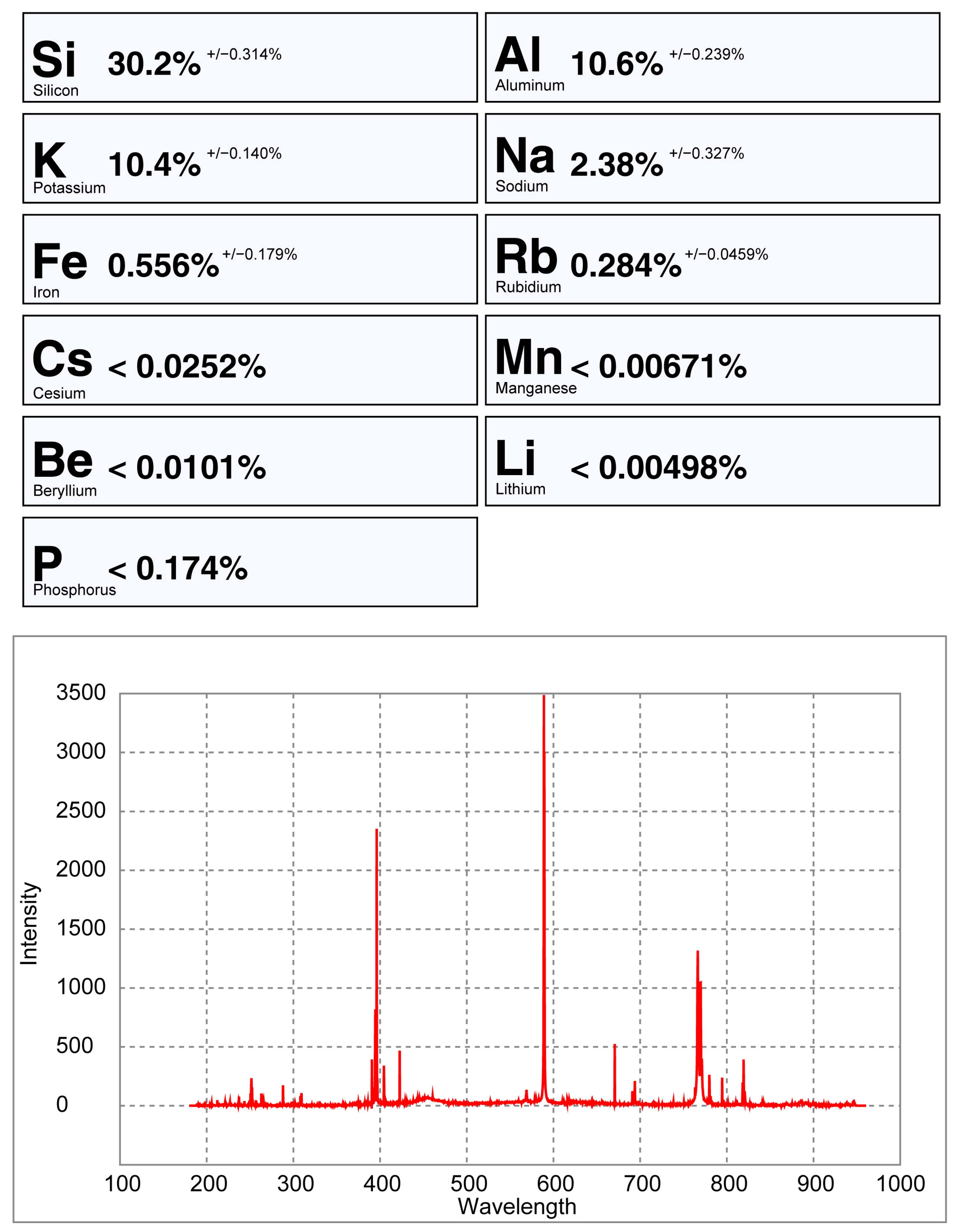
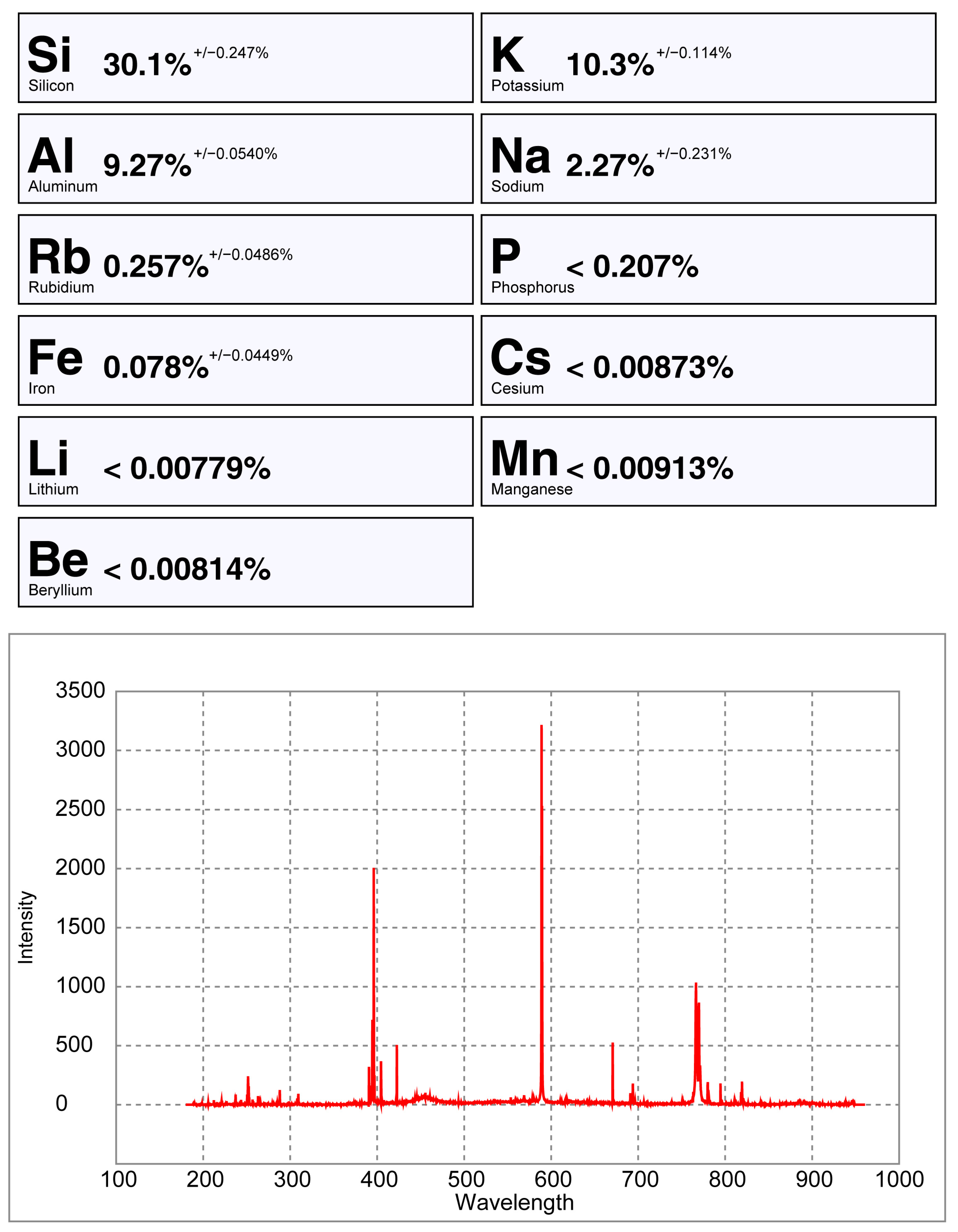
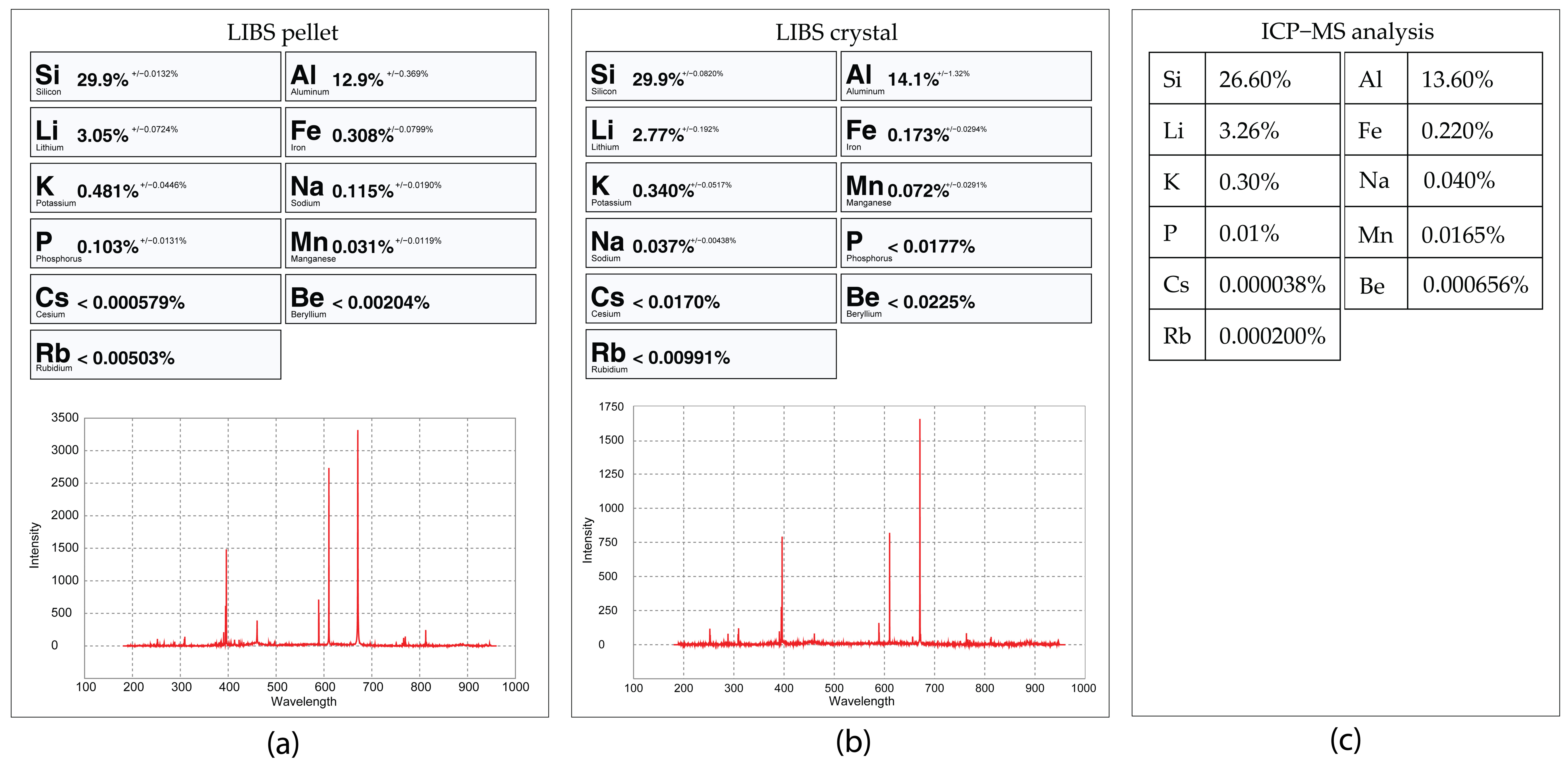
6.4. Comparing the Handheld LIBS Spectra of a Crystal Versus a Pellet of Spodumene
7. Conclusions
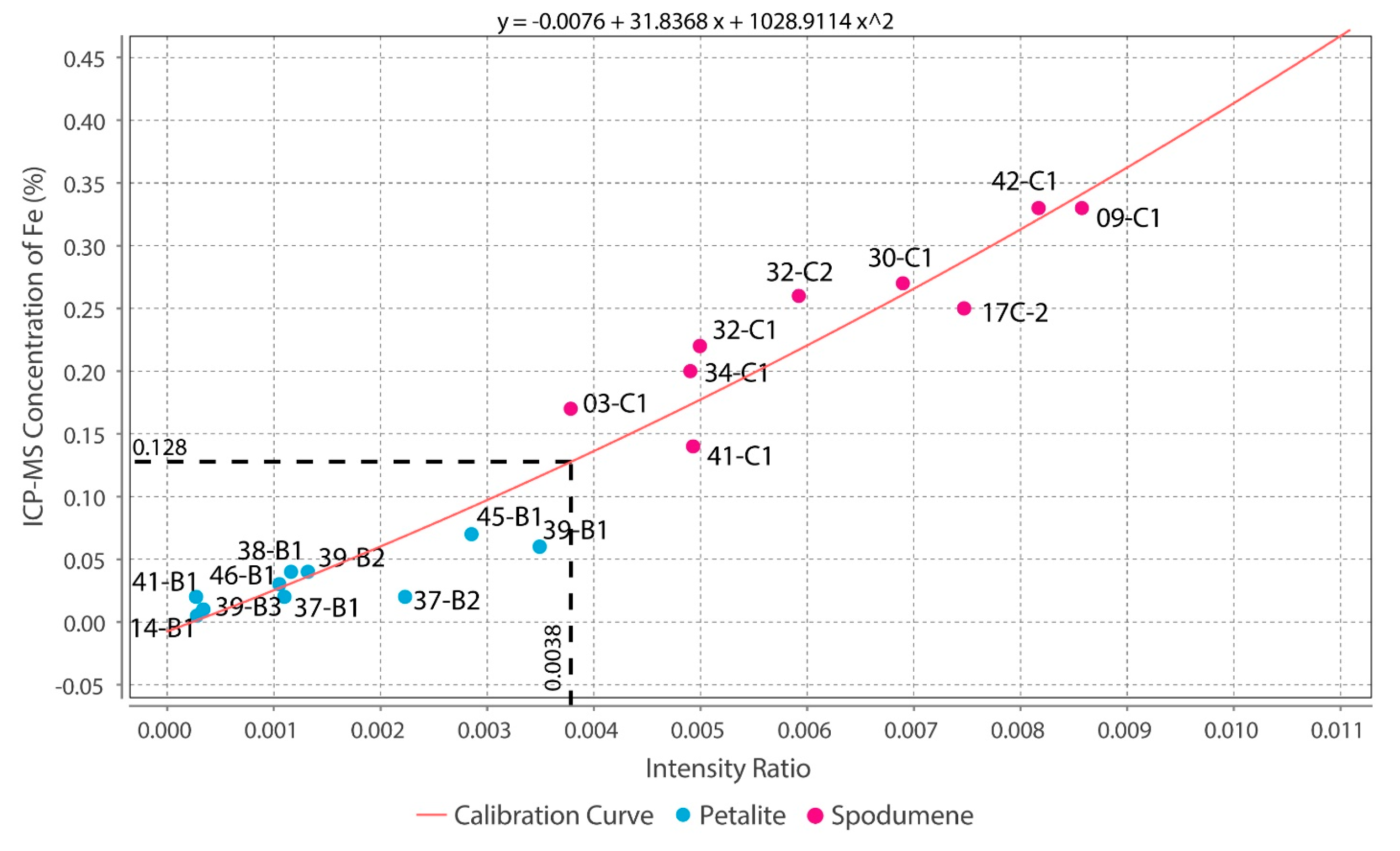
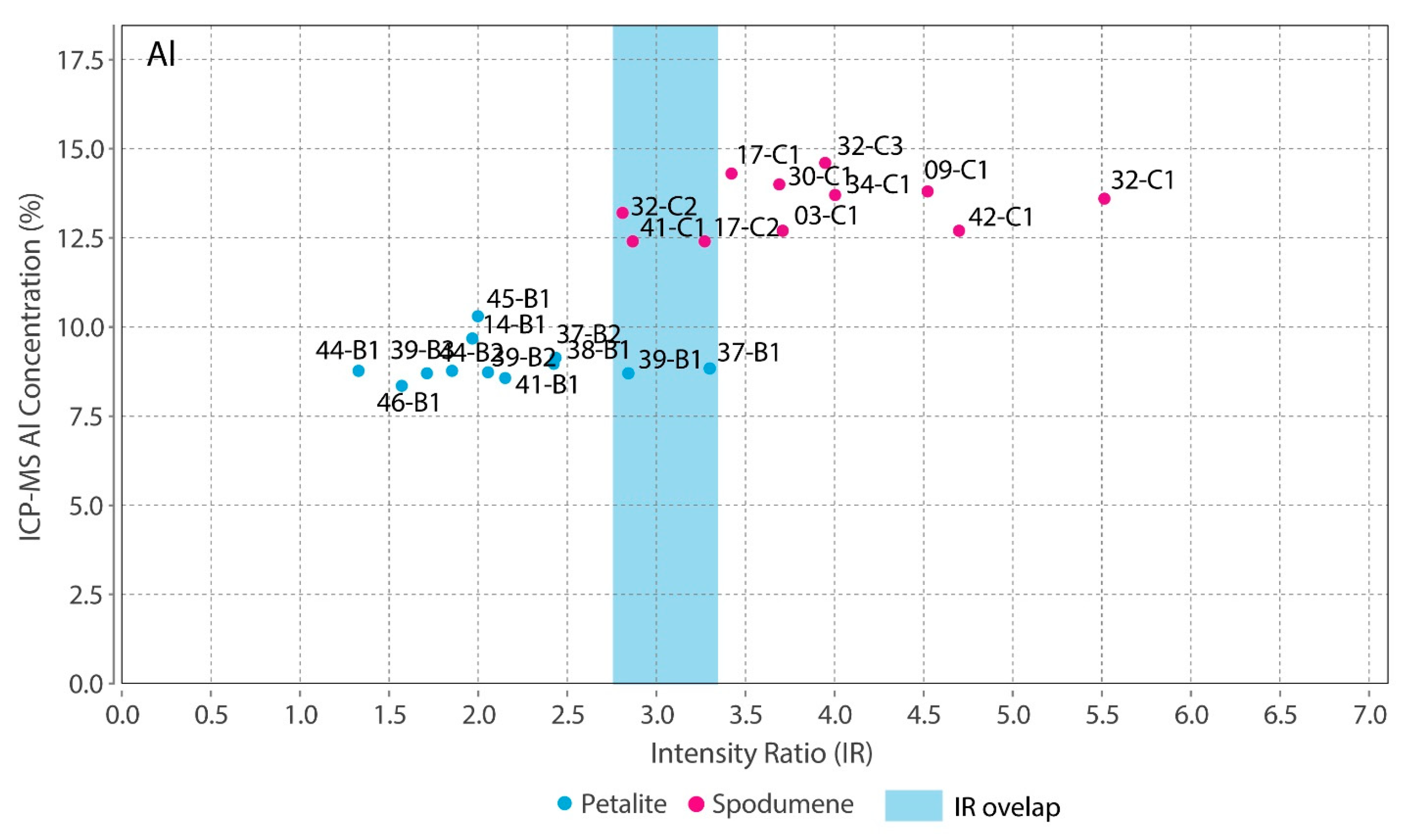
- The intensity ratios (IR) determined by dividing the total area of the peaks for one element by the total area of all the peaks in the spectra resulted in identical IRs for spodumene and petalite. This forced the creation of two calibration lines for each mineral for all the chemical elements, except for Fe, which was the only element with distinct IRs for both minerals. Thus, it was only possible to successfully distinguish and analyze spodumene and petalite because petalite had always more Fe than spodumene and IRs greater than 0.0038 (Figure 17);
- To distinguish between spodumene and petalite, the calibration Fe_Barroso must be used. For a more complete chemical analysis, the Spd_Barroso calibration must be used if Fe > 0.1%, and the Pet_Barroso calibration if Fe < 0.1%;
- The Fe calibration cannot be used carelessly in other pegmatite fields without first learning the chemical signature of the trace elements of spodumene and petalite for that field because there are petalites that can have higher Fe content than 0.1% (e.g., 0.99% of Fe in a Fe-bearing petalite from a pegmatite with spodumene and quartz from the eastern Transbaikal region, Russia [27]);
- The distinction of fine mixtures of spodumene and quartz from single crystals of spodumene and petalite was possible with the Fe and Al results from ICP-MS. However, the pLIBS could not distinguish the spd + qtz mixtures from spodumene due to the overlapped IR for Al (Figure 18);
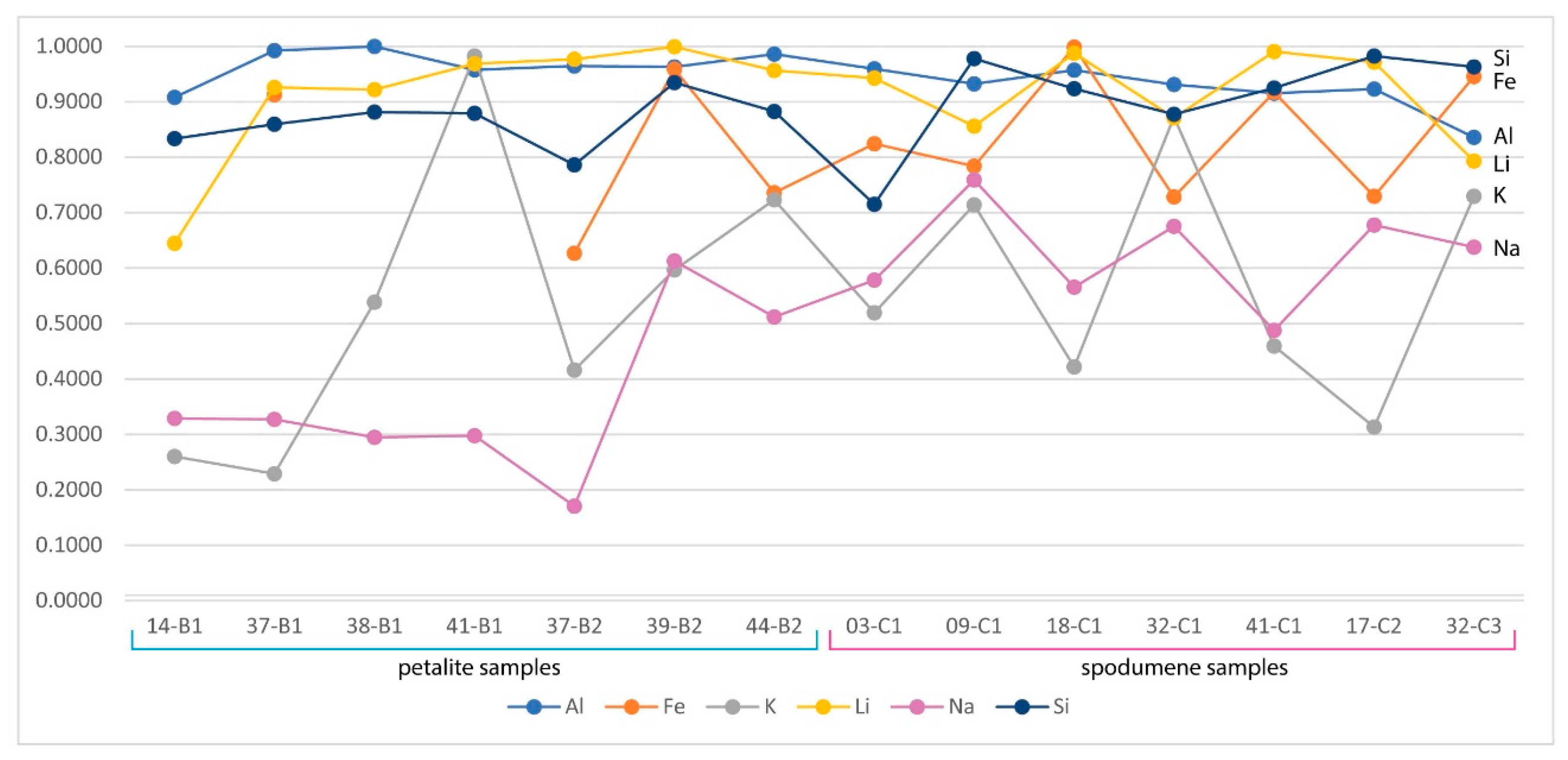
- Although LIBS is easily affected by changes in the crystal structure, the presence of spodumene in the petalite pellets did not seem to affect the results, as shown by samples 38-B1 and 39-B2, where Li and Al had errors of less than 30% when compared to ICP-MS (Figure 23);
- Analyzing pellets and crystals with a pLIBS has different results (Figure 27 and Figure 28). In the pellets, the trace elements are averaged because of the homogenized material, while the trace elements may vary along a crystal. The ICP-MS results will be more similar to a pellet than to a spot analysis in a crystal. Therefore, when calibrating a LIBS with ICP-MS analysis, it is better to use pellets instead of crystals;
- The crystal surface reflectiveness (flat or transparent) can be a problem for the pLIBS, since the equipment may not detect the sample or produce low intensity spectra with strong background signal, masking the smaller peaks and producing inaccurate results for the trace elements. Milling the samples and pressing them into pellets helps with this issue;
- The presence of quartz also seems to affect the spectra intensity even if the minerals are milled, as was observed with the spectra of the pellets of the mixtures of spodumene and quartz (Figure A1);
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- IEA. The Role of Critical Minerals in Clean Energy Transitions; IEA: Paris, France, 2021. [Google Scholar]
- Charoy, B.; Noronha, F. Rare-element (Li-rich) granitic and pegmatitic plutons: A primary or superimposed signature? Rev. Bras. De Geociências 1999, 29, 3–8. [Google Scholar] [CrossRef][Green Version]
- Ribeiro, M.A. Estudo Litogeoquímico das Formações Metassedimentares Encaixantes de Mineralizações em Trás-os-Montes Ocidental. Implicações Metalogénicas; Faculdade de Ciências da Universidade do Porto: Porto, Portugal, 1998. [Google Scholar]
- Rodríguez Fernández, L.R.; López Olmedo, F.; Oliveira, J.T.; Medialdea, T.; Terrinha, P.; Matas, J.; Martín-Serrano, A.; Martín Parra, L.M.; Rubio, F.; Marín, C.; et al. Mapa Geológico de Espanha e Portugal. Escala 1:1 000 000. IGME 2015. © Instituto Geológico y Minero de España (IGME); © Laboratório Nacional de Energia e Geologia (LNEG). Available online: https://info.igme.es/cartografiadigital/geologica/ (accessed on 29 January 2021).
- Agência Portuguesa do Ambiente. Massas de Água Superficiais Rios de Portugal Continental: Conjunto de Dados Geográfico SNIAmb. Available online: https://sniamb.apambiente.pt/content/geo-visualizador (accessed on 6 July 2019).
- Agência Portuguesa do Ambiente. Massas de Água Superficiais Rios Albufeiras de Portugal Continental Conjunto de Dados Geográfico SNIAmb. Available online: https://sniamb.apambiente.pt/content/geo-visualizador (accessed on 19 July 2022).
- Sant’Ovaia, H.; Ribeiro, M.A.; Martins, H.C.B.; Noronha, F. Notícia explicativa da Carta geológica de Portugal na escala 1:50000—Folha 6D—Vila Pouca de Aguiar. 2011. Available online: https://geoportal.lneg.pt/pt/dados_abertos/cartografia_geologica/cgp50k/ (accessed on 30 September 2019).
- Ribeiro, M.A.; Martins, H.C.B.; Almeida, A.; Noronha, F. Notícia Explicativa da Folha 6-C- Cabeceiras de Basto. Carta Geológica de Portugal na Escala 1/50 000; Instituto Geológico e Mineiro: Lisboa, Portugal, 2000; p. 48. [Google Scholar]
- Noronha, F.; Ribeiro, M.A.; Almeida, A.; Dória, A.; Guedes, A.; Lima, A.; Martins, H.C.; Sant’Ovaia, H.; Nogueira, P.; Martins, T.; et al. Jazigos Filonianos Hidrotermais e Aplitopegmatíticos Espacialmente Associados a Granitos (Norte de Portugal). In Geologia de Portugal; Dias, R., Araújo, A., Terrinha, P., Kullberg, J.C., Eds.; Escolar Editora; Volume Volume I—Geologia Pré-mesozóica de Portugal: Lisboa, Portugal, 2013; pp. 403–438. [Google Scholar]
- Charoy, B.; Lhote, F.; Dusausoy, Y.; Noronha, F. The Crystal Chemistry of Spodumene in Some Granitic Aplite-Pegmatite of Northern Portugal. Can. Mineral. 1992, 30, 639–651. [Google Scholar]
- Lima, A. Estrutura, Mineralogia e Génese dos Filões Aplitopegmatíticos com Espodumena da Região do Barroso-Alvão; University of Porto: Porto, Portugal, 2000. [Google Scholar]
- Martins, T. Multidisciplinary Study of Pegmatites and Associated Li and Sn-Nb-Ta Mineralisation from the Barroso-Alvão Region; Faculty of Sciences of University of Porto: Porto, Portugal, 2009. [Google Scholar]
- Dias, F. Lithium Mineralizations of Barroso-Alvão Aplite-Pegmatite Field. Master’s Thesis, Faculty of Sciences of the University of Porto, Porto, Portugal; p. 2016.
- Lima, A.; Dias, F. Spodumene and Quartz Intergrowth—Textural and Genesis Point of View. In Proceedings of the EGU2019, Vienna, Austria, 7–12 April 2019; 21, p. 13404. [Google Scholar]
- Martins, T.C.; Lima, A.M.C.; Vieira, R.C. Barroso-Alvão Pegmatite-Aplite Field: A Case Study in Northern Portugal. Crystallization Processes in Granitic Pegmatites. In Proceedings of the International Meeting: Crystallization Processes in Granitic Pegmatite, Elba, Italy, 23–29 May 2005; 2005; pp. 21–22. [Google Scholar]
- Charoy, B.; Noronha, F.; Lima, A. Spodumene-petalite-eucryptite; mutual relationships and pattern of alteration in Li-rich aplite-pegmatite dykes from northern Portugal. Can. Mineral. 2001, 39, 729–746. [Google Scholar] [CrossRef]
- Cremers, D.A.; Radziemski, L.J. Handbook of Laser-Induced Breakdown Spectroscopy; John Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar]
- Ribeiro, R.; Capela, D.; Ferreira, M.; Martins, R.; Jorge, P.; Guimarães, D.; Lima, A. X-ray Fluorescence and Laser-Induced Breakdown Spectroscopy Analysis of Li-Rich Minerals in Veins from Argemela Tin Mine, Central Portugal. Minerals 2021, 11, 1169. [Google Scholar] [CrossRef]
- Dias, C. Abordagem Multidisciplinar às Mineralizações de Lítio no Campo Aplito-Pegmatítico do Barroso-Alvão, Norte de Portugal. Master’s Thesis, Faculty of Sciences of the University of Porto, Porto, Portugal, 2016. [Google Scholar]
- Tucker Vasques, J. Lithogeochemistry and Prospection of Lithium-Bearing Pegmatites and Their Host-Rocks. Master’s Thesis, Faculty of Sciences of the University of Porto, Porto, Portugal, 2021. [Google Scholar]
- Pagel, M.; Barbin, V.; Blanc, P.; Ohnenstetter, D. Cathodoluminescence in Geosciences; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2000. [Google Scholar]
- Dias, F.; Lima, A. Cathodoluminescence characteristics of spodumene and petalite from the Iberian massif pegmatites. In Proceedings of the Jornadas do ICT, Faculdade de Ciências da Universidade do Porto—Zoom Meeting, Porto, Portugal, 11–12 February 2021. [Google Scholar]
- Wise, M.A.; Brown, C.D. Cathodoluminescence (CL) Microscopy—A Technique for Understanding The Dynamics of Pegmatite Crystallization. Can. Mineral. 2019, 57, 821–823. [Google Scholar] [CrossRef]
- Černý, P.; Ferguson, R.B. The Tanco pegmatite at Bernic Lake, Manitoba; IV, Petalite and spodumene relations. Can. Mineral. 1972, 11, 660–678. [Google Scholar]
- Lepore, K.H.; Fassett, C.I.; Breves, E.A.; Byrne, S.; Giguere, S.; Boucher, T.; Rhodes, J.M.; Vollinger, M.; Anderson, C.H.; Murray, R.W.; et al. Matrix Effects in Quantitative Analysis of Laser-Induced Breakdown Spectroscopy (LIBS) of Rock Powders Doped with Cr, Mn, Ni, Zn, and Co. Appl. Spectrosc. 2017, 71, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Černý, P.; London, D. Crystal Chemistry and Stability of Petalite; Tmpm Tschermaks Mineralogische Und Petrographische Mitteilungen; Springer: New York, NY, USA; Volume 31, pp. 81–96.
- Deer, W.A.; Howie, R.A.; Zussman, J. Rock-Forming Minerals: Silica Minerals, Volume 4B; Geological Society: London, UK, 2004. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. Rock-Forming Minerals: Single-Chain Silicates. Volume 2A; Geological Society: London, UK, 1978. [Google Scholar]
- Claffy, E.W. Composition, tenebrescence and luminescence of spodumene minerals. Am. Mineral. 1953, 38, 11–12. [Google Scholar]








| 3 | 3 | 9 | 17 | 17 | 18 | 30 | 32 | 32 | 32 | 32 | 33 | 33 | 34 | 41 | 41 | 41 | 42 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| spd 1 * | spd 2 | spd 1 | spd 1 | spd 2 | spd 1 | spd 1 | spd 1 | spd 2 * | spd 3 | spd 4 * | spd 1 | spd 2 | spd 1 | spd 1 | spd 3 | spd 4 | spd 1 | |
| Si % | 22.00 | 26.30 | 30.00 | 28.10 | 29.30 | 27.90 | 29.40 | 26.60 | 29.30 | 31.70 | 29.90 | 27.70 | 26.70 | 29.70 | 28.30 | 28.70 | 32.50 | 30.30 |
| Al % | 12.70 | 12.70 | 13.80 | 14.30 | 12.40 | 12.40 | 14.00 | 13.60 | 13.20 | 14.60 | 13.50 | 15.10 | 12.30 | 13.70 | 12.40 | 12.30 | 15.00 | 12.70 |
| Li % | 3.06 | 3.08 | 3.32 | 3.08 | 2.73 | 2.73 | 3.14 | 3.26 | 2.32 | 3.52 | 2.67 | 2.38 | 2.81 | 3.23 | 2.77 | 2.79 | 3.35 | 2.93 |
| K % | 0.30 | 0.30 | 0.30 | 0.70 | 0.50 | 0.30 | 0.50 | 0.30 | 1.10 | 0.40 | 1.10 | 0.70 | 0.40 | 0.40 | 0.50 | 0.60 | 0.70 | 0.30 |
| Fe % | 0.17 | 0.19 | 0.33 | 0.09 | 0.25 | 0.38 | 0.27 | 0.22 | 0.26 | 0.19 | 0.27 | 0.25 | 0.18 | 0.20 | 0.14 | 0.15 | 0.25 | 0.33 |
| Na % | 0.10 | 0.11 | 0.08 | 0.10 | 0.12 | 0.17 | 0.09 | 0.04 | 0.51 | 0.12 | 0.45 | 0.08 | 0.14 | 0.08 | 0.20 | 0.11 | 0.27 | 0.07 |
| Mn ppm | 237 | 225 | 238 | 484 | 602 | 323 | 259 | 165 | 172 | 229 | 184 | 297 | 339 | 216 | 166 | 182 | 269 | 214 |
| Sn ppm | 209 | 194 | 57 | 8 | 19 | 21 | 7 | 4 | 12 | 5 | 40 | 147 | 43 | 13 | 11 | 7 | 13 | 6 |
| P ppm | 110 | 840 | 490 | 410 | 490 | 680 | 480 | 100 | 350 | 200 | 380 | 80 | 610 | 30 | 360 | 90 | 170 | 340 |
| Be ppm | 105 | 108 | 8 | 7 | 25 | 21 | 8 | 7 | 8 | 5 | 4 | 13 | 8 | 8 | 11 | 11 | 14 | 12 |
| Ga ppm | 11 | 12 | 14 | 14 | 15 | 12 | 11 | 9 | 15 | 12 | 16 | 20 | 17 | 15 | 17 | 17 | 19 | 14 |
| Nb ppm | 9 | 9 | 18 | 2 | 3 | 6 | 1 | 1 | 6 | 2 | 7 | 3 | 14 | 12 | 6 | 5 | 6 | 5 |
| Ta ppm | 9 | 8 | 1 | 1 | 1 | 1 | 0 | 0 | 5 | 0 | 3 | 7 | 6 | 7 | 1 | 1 | 2 | 1 |
| Zn ppm | 5 | 5 | 20 | 20 | 10 | 40 | 30 | 20 | 90 | 5 | 30 | 60 | 70 | 20 | 40 | 50 | 60 | 20 |
| Rb ppm | 4 | 5 | 7 | 71 | 60 | 13 | 36 | 2 | 131 | 19 | 181 | 54 | 50 | 21 | 67 | 92 | 88 | 7 |
| Sr ppm | 3 | 3 | 24 | 12 | 34 | 37 | 1 | 0 | 6 | 0 | 1 | 2 | 106 | 1 | 3 | 2 | 8 | 2 |
| Cs ppm | 3 | 3 | 1 | 3 | 8 | 2 | 2 | 0 | 7 | 1 | 6 | 15 | 4 | 2 | 7 | 12 | 23 | 1 |
| As ppm | 1 | 0 | 4 | 1 | 2 | 6 | 3 | 1 | 2 | 2 | 1 | 5 | 1 | 0 | 1 | 0 | 1 | 5 |
| Ni ppm | 0 | 0 | 1 | 4 | 3 | 6 | 2 | 3 | 1 | 1 | 2 | 13 | 2 | 3 | 2 | 1 | 3 | 12 |
| U ppm | 0 | 1 | 1 | 0 | 1 | 1 | 5 | 1 | 3 | 1 | 3 | 3 | 2 | 5 | 2 | 2 | 4 | 0 |
| 5 | 5 | 14 | 37 | 37 | 38 | 39 | 39 | 39 | 41 | 41 | 44 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pet 1 | pet 2 * | pet 1 * | pet 1 | pet 2 * | pet 1 * | pet 1 | pet 2 * | pet 3 | pet 1 | pet 2 | pet 2 | |
| Si % | 34.30 | 28.50 | 34.40 | 35.40 | 24.10 | 34.90 | 32.90 | 29.50 | 24.30 | 27.80 | 23.20 | 35.40 |
| Al % | 8.65 | 8.80 | 9.68 | 8.84 | 9.14 | 8.97 | 8.70 | 8.73 | 8.70 | 8.57 | 8.73 | 8.77 |
| Li % | 2.24 | 1.98 | 2.15 | 2.23 | 2.03 | 2.15 | 2.07 | 2.01 | 2.18 | 2.10 | 2.13 | 2.13 |
| K % | 0.10 | 0.20 | 0.30 | 0.10 | 0.20 | 0.30 | 0.20 | 0.30 | 0.20 | 0.20 | 0.20 | 0.20 |
| Fe % | 0.01 | 0.02 | 0.01 | 0.02 | 0.02 | 0.04 | 0.06 | 0.04 | 0.01 | 0.02 | 0.01 | 0.06 |
| Na % | 0.02 | 0.16 | 0.16 | 0.06 | 0.01 | 0.01 | 0.05 | 0.31 | 0.02 | 0.02 | 0.04 | 0.04 |
| Mn ppm | 9 | 72 | 23 | 76 | 314 | 25 | 68 | 65 | 32 | 12 | 151 | 17 |
| Sn ppm | 0 | 3 | 1 | 1 | 1 | 12 | 3 | 13 | 0 | 0 | 1 | 0 |
| P ppm | 100 | 1020 | 470 | 180 | 50 | 100 | 190 | 420 | 50 | 90 | 320 | 110 |
| Be ppm | 11 | 25 | 6 | 10 | 4 | 4 | 7 | 30 | 7 | 5 | 5 | 10 |
| Ga ppm | 11 | 12 | 14 | 10 | 19 | 25 | 10 | 16 | 9 | 7 | 7 | 8 |
| Nb ppm | 1 | 9 | 0 | 0 | 0 | 1 | 2 | 4 | 0 | 0 | 0 | 0 |
| Ta ppm | 0 | 5 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 |
| Zn ppm | 20 | 10 | 30 | 30 | 70 | 80 | 20 | 5 | 5 | 5 | 5 | 5 |
| Rb ppm | 3 | 25 | 18 | 13 | 14 | 101 | 28 | 60 | 2 | 3 | 5 | 3 |
| Sr ppm | 4 | 6 | 3 | 0 | 6 | 2 | 18 | 5 | 2 | 4 | 7 | 0 |
| Cs ppm | 1 | 3 | 1 | 2 | 3 | 9 | 5 | 67 | 1 | 1 | 4 | 0 |
| As ppm | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 2 |
| Ni ppm | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 2 | 4 | 1 | 1 |
| U ppm | 3 | 1 | 4 | 7 | 7 | 2 | 0 | 1 | 8 | 0 | 1 | 1 |
| Spodumene | Spd + Qtz | NIST SRM 182 | ||||
|---|---|---|---|---|---|---|
| 03-C1 | 03-C2 | 08-D2 | 08-D3 | NIST * | ICP-MS | |
| Si | error: 16% | error: 18% | ||||
| Si 22.00% | Si 26.30% | Si 33.70% | Si 28.50% | |||
| Al | error: 0% | error: 1% | ||||
| Al 12.70% | Al 12.70% Al | Al 9.97% | Al 10.10% | |||
| Li | error: 1% | error: 3% | error: 5% | |||
| Li 3.06% | Li 3.08% | Li 2.01% | Li 2.07% | 1.92% Li | 2.01% Li | |
| K | error: 0% | error: 14% | ||||
| K 0.30% | K 0.30% | K 0.60% | K 0.70% | |||
| Na | error: 9% | error: 0% | ||||
| Na 0.10% | Na 0.11% | Na 0.21% | Na 0.21% | |||
| P | error: 87% | error: 6% | ||||
| P 0.01% | P 0.08% | P 490 ppm | P 520 ppm | |||
| Rb | error: 21% | error: 1% | ||||
| Rb 4.1 ppm | Rb 5.2 ppm | Rb 91.7 ppm | Rb 92.3 ppm | |||
| Ca | error: - | Error: 0% | ||||
| Ca < 0.01% | Ca < 0.01% | Ca 0.02% | Ca 0.02% | |||
| Fe | error: 11% | error: 0% | ||||
| Fe 0.17% | Fe 0.19% | Fe 0.18% | Fe 0.18% | |||
| Mn | error: 5% | error: 2% | ||||
| Mn 237 ppm | Mn 225 ppm | Mn 276 ppm | Mn 271 ppm | |||
| Be | error: 3% | error: 3% | ||||
| Be 105 ppm | Be 108 ppm | Be 9.02 ppm | Be 8.79 ppm | |||
| Cs | error: 3% | error: 2% | ||||
| Cs 2.74 ppm | Cs 2.66 ppm | Cs 2.55 ppm | Cs 2.6 ppm | |||
| Tl | error:33% | error: 0% | ||||
| Tl 0.04 ppm | Tl 0.03 ppm | Tl 0.58 ppm | Tl 0.58 ppm | |||
| Sr | error: 3% | error: 3% | ||||
| Sr 3.4 ppm | Sr 3.3 ppm | Sr 21.8 ppm | Sr 21.2 ppm | |||
| Ga | error: 5% | error: 2% | ||||
| Ga 10.9 ppm | 11.5 ppm Ga | Ga 11.6 ppm | Ga 11.4 ppm | |||
| Zn | error: 0% | error: 50% | ||||
| Zn 5 ppm | Zn 5 ppm | Zn 10 ppm | Zn 20 ppm | |||
| As | error: 133% | error: 0% | ||||
| As 0.7 ppm | As 0.3 ppm | As 0.9 ppm | As 0.9 ppm | |||
| Nb | error: 2% | error: 2% | ||||
| Nb 9.4 ppm | Nb 9.2 ppm | Nb 4.4 ppm | Nb 4.3 ppm | |||
| Ni | error: 25% | error: 0% | ||||
| Ni 0.3 ppm | Ni 0.4 ppm | Ni 0.5 ppm | Ni 0.5 ppm | |||
| Sn | error: 8% | error: 1% | ||||
| Sn 209 ppm | Sn 194 ppm | Sn 23.2 ppm | Sn 22.9 ppm | |||
| Ta | error: 7% | error: 0% | ||||
| Ta 8.64 ppm | Ta 8.09 ppm | Ta 0.96 ppm | Ta 0.96 ppm | |||
| U | error: 70% | error: 13% | ||||
| U 0.3 ppm | U 1 ppm | U 0.9 ppm | U 0.8 ppm | |||
| Det. Lim. | Petalite | Spodumene | Spodumene + Quartz | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 39-B2 | 39-B2 | 09-C1 | 09-C1 | 32-C4 | 32-C4 | 32-D1 | 32-D1 | 41-D3 | 41-D3 | ||
| Orig | Dup | Orig | Dup | Orig | Dup | Orig | Dup | Orig | Dup | ||
| Si | 0.01 | error: 26% | error: 2% | error: 1% | error: 13% | error: 22% | |||||
| Si 25% | Si 34% | Si 30% | Si 30% | Si 30% | Si 30% | Si 34% | Si 30% | Si 32% | Si 26% | ||
| Al | 0.01 | error: 1% | error: 0% | error: 1% | error: 0% | error: 2% | |||||
| Al 8.8% | Al 8.7% | Al 14% | Al 14% | Al 13% | Al 14% | Al 9.6% | Al 9.6% | Al 9.4% | Al 9.2% | ||
| K | 0.1 | error: 0% | error: 0% | error: 0% | error: 0% | error: 0% | |||||
| K 0.3% | K 0.3% | K 0.3% | K 0.3% | K 1.1% | K 1.1% | K 1.6% | K 1.6% | K 0.7% | K 0.7% | ||
| Li | 0.01 | error: 5% | error: 1% | error: 2% | error: 0% | error: 1% | |||||
| Li 2.1% | Li 2% | Li 3.3% | Li 3.3% | Li 2.7% | Li 2.7% | Li 1.2% | Li 1.2% | Li 1.8% | Li 1.8% | ||
| NIST Petalite | Spodumene | Spodumene + Quartz | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Det. | SRM 182 | SRM 182 | 32-C1 | 32-C1 | 11-1D | 11-D1 | 08-D2 | 08-D2 | |
| Limit | Orig | Dup | Orig | Dup | Orig | Dup | Orig | Dup | |
| Na % | 0.01 | error: 0% | error: 0% | error: 0% | |||||
| Na 0.04 | Na 0.04 | Na 0.26 | Na 0.26 | Na 0.21 | Na 0.21 | ||||
| Fe % | 0.01 | error: 10% | error: 0% | error: 0% | |||||
| Fe 0.23 | Fe 0.21 | Fe 0.13 | Fe 0.13 | Fe 0.18 | Fe 0.18 | ||||
| Ca % | 0.01 | error: - | error: 0% | error: 0% | |||||
| Ca <0.01 | Ca <0.01 | Ca 0.02 | Ca 0.02 | Ca 0.02 | Ca 0.02 | ||||
| P ppm | 10 | error: 50% | error: 0% | error: 0% | |||||
| P 120 | P 80 | P 4210 | P 4230 | P 490 | P 490 | ||||
| Rb ppm | 0.1 | error: 2% | error: 29% | error: 5% | error: 3% | ||||
| Rb 258 | Rb 262 | Rb 2.2 | Rb 1.7 | Rb 96 | Rb 91.7 | Rb 90.5 | Rb 92.9 | ||
| Mn ppm | 5 | error: 1% | error: 0% | error: 1% | |||||
| Mn 165 | Mn 166 | Mn 161 | Mn 161 | Mn 274 | Mn 277 | ||||
| Sr ppm | 0.2 | error: 6% | error: 0% | error: 2% | error: 0% | ||||
| Sr 5.2 | Sr 4.9 | Sr 0.4 | Sr 0.4 | Sr 59.5 | Sr 60.7 | Sr 21.8 | Sr 21.8 | ||
| Cs ppm | 0.05 | error: 5% | error: 3% | error: 5% | error: 1% | ||||
| Cs 24.6 | Cs 26 | Cs 0.38 | Cs 0.37 | Cs 4.75 | Cs 4.52 | Cs 2.56 | Cs 2.54 | ||
| Pb ppm | 0.5 | error: 50% | error: 14% | error: 12% | error: - | ||||
| Pb 2.1 | 1.4 | Pb 0.8 | 0.7 | Pb 2.8 | Pb 2.5 | Pb <0.5 | Pb <0.5 | ||
| Zn ppm | 2 | error: 20% | error: 0% | error: 5% | |||||
| Zn 8 | Zn 10 | Zn 41 | Zn 41 | Zn 23 | Zn 22 | ||||
| Ga ppm | 0.05 | error: 0% | error: 9% | error: 7% | error: 2% | ||||
| Ga 17.5 | Ga 17.5 | Ga 9.48 | Ga 8.7 | Ga 7.9 | Ga 7.41 | Ga 11.7 | Ga 11.5 | ||
| Be ppm | 0.05 | error: 5% | error: 0% | error: 3% | error: 1% | ||||
| Be 10.6 | Be 11.2 | Be 6.58 | Be 6.55 | Be 3.48 | Be 3.38 | Be 9.07 | Be 8.98 | ||
| Sn ppm | 0.2 | error: 8% | error: 8% | error: 3% | error: 4% | ||||
| Sn 3.4 | 3.7 | Sn 4.2 | 3.9 | Sn 11.2 | Sn 10.9 | Sn 22.8 | Sn 23.7 | ||
| Tl ppm | 0.02 | error: 1% | error: - | error: 2% | error: 2% | ||||
| Tl 1.56 | Tl 1.58 | Tl <0.02 | Tl <0.02 | Tl 0.44 | Tl 0.43 | Tl 0.57 | Tl 0.58 | ||
| Cr ppm | 1 | error: 40% | error: 67% | error: 33% | |||||
| Cr 7 | Cr 5 | Cr 5 | Cr 3 | Cr 4 | Cr 3 | ||||
| Nb ppm | 0.1 | error: 10% | error: 25% | error: 9% | error: 7% | ||||
| Nb 3.5 | Nb 3.9 | Nb 0.5 | Nb 0.4 | Nb 3.7 | Nb 3.4 | Nb 4.3 | Nb 4.6 | ||
| As ppm | 0.2 | error: 26% | error: 150% | error: 44% | error: 20% | ||||
| As 3.1 | As 4.2 | As 1 | As 0.4 | As 1.3 | As 0.9 | As 0.8 | As 1 | ||
| Ta ppm | 0.05 | error: 13% | error: 28% | error: 15% | error: 4% | ||||
| Ta 4.74 | Ta 5.47 | Ta 0.32 | Ta 0.25 | Ta 0.68 | Ta 0.59 | Ta 0.94 | Ta 0.98 | ||
| U ppm | 0.1 | error: 25% | error: 43% | error: 0% | error: 9% | ||||
| U 0.3 | U 0.4 | U 1 | U 0.7 | U 2.7 | U 2.7 | U 1 | U 1.1 | ||
| Ni ppm | 0.2 | error: 13% | error: 16% | error: 10% | error: 0% | ||||
| Ni 0.7 | Ni 0.8 | Ni 3.6 | Ni 3.1 | Ni 1.1 | Ni 1 | Ni 0.5 | Ni 0.5 | ||
| Y ppm | 0.1 | error: 0% | error: 122% | error: 0% | error: - | ||||
| Y 0.2 | Y 0.2 | Y 4 | Y 1.8 | Y 0.1 | Y 0.1 | Y <0.1 | Y <0.1 | ||
| LiAlSi4O10 (Petalite) → | LiAlSi2O6 (Spodumene) + 2SiO2 (Quartz) | |||
|---|---|---|---|---|
| 2.09% Li | 3.73% Li | 0% Li | ||
| 8.75% Al | 14.50% Al | 0% Al | ||
| 36.72% Si | 30.18% Si | 46.74% Si | ||
| 52.43% O | 51.59% O | 53.26% O | ||
| 100 vol% | → | 56.3 vol% | + | 43.7 vol% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, F.; Ribeiro, R.; Gonçalves, F.; Lima, A.; Roda-Robles, E.; Martins, T. Calibrating a Handheld LIBS for Li Exploration in the Barroso–Alvão Aplite-Pegmatite Field, Northern Portugal: Textural Precautions and Procedures When Analyzing Spodumene and Petalite. Minerals 2023, 13, 470. https://doi.org/10.3390/min13040470
Dias F, Ribeiro R, Gonçalves F, Lima A, Roda-Robles E, Martins T. Calibrating a Handheld LIBS for Li Exploration in the Barroso–Alvão Aplite-Pegmatite Field, Northern Portugal: Textural Precautions and Procedures When Analyzing Spodumene and Petalite. Minerals. 2023; 13(4):470. https://doi.org/10.3390/min13040470
Chicago/Turabian StyleDias, Filipa, Ricardo Ribeiro, Filipe Gonçalves, Alexandre Lima, Encarnación Roda-Robles, and Tânia Martins. 2023. "Calibrating a Handheld LIBS for Li Exploration in the Barroso–Alvão Aplite-Pegmatite Field, Northern Portugal: Textural Precautions and Procedures When Analyzing Spodumene and Petalite" Minerals 13, no. 4: 470. https://doi.org/10.3390/min13040470
APA StyleDias, F., Ribeiro, R., Gonçalves, F., Lima, A., Roda-Robles, E., & Martins, T. (2023). Calibrating a Handheld LIBS for Li Exploration in the Barroso–Alvão Aplite-Pegmatite Field, Northern Portugal: Textural Precautions and Procedures When Analyzing Spodumene and Petalite. Minerals, 13(4), 470. https://doi.org/10.3390/min13040470








