Rheological and Strength Properties of Steel-Slag Cemented Paste Backfill: Link to Gypsum Type and Dosage
Abstract
1. Introduction
2. Experimental Programs
2.1. Materials
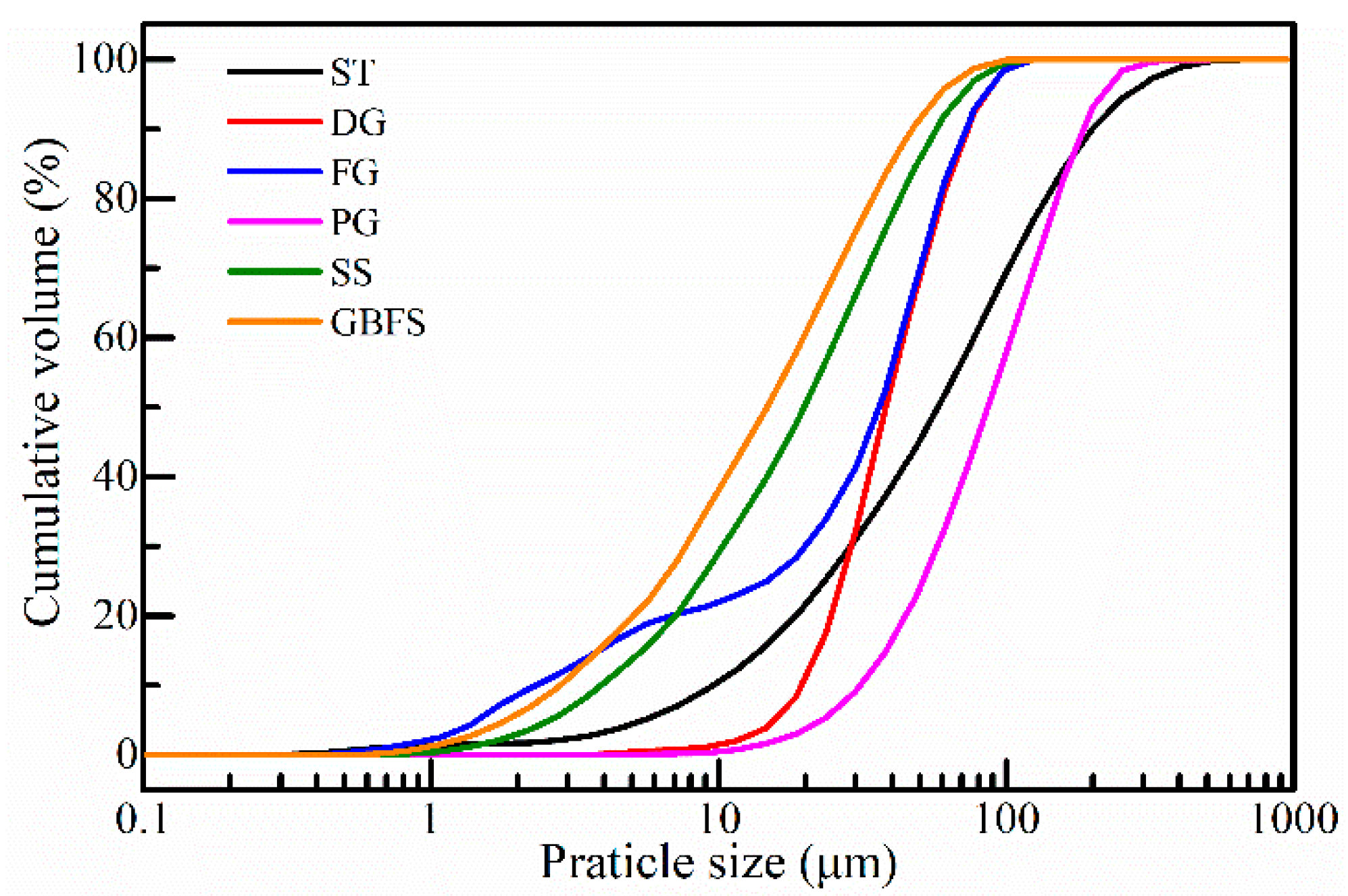
2.1.1. Aggregate
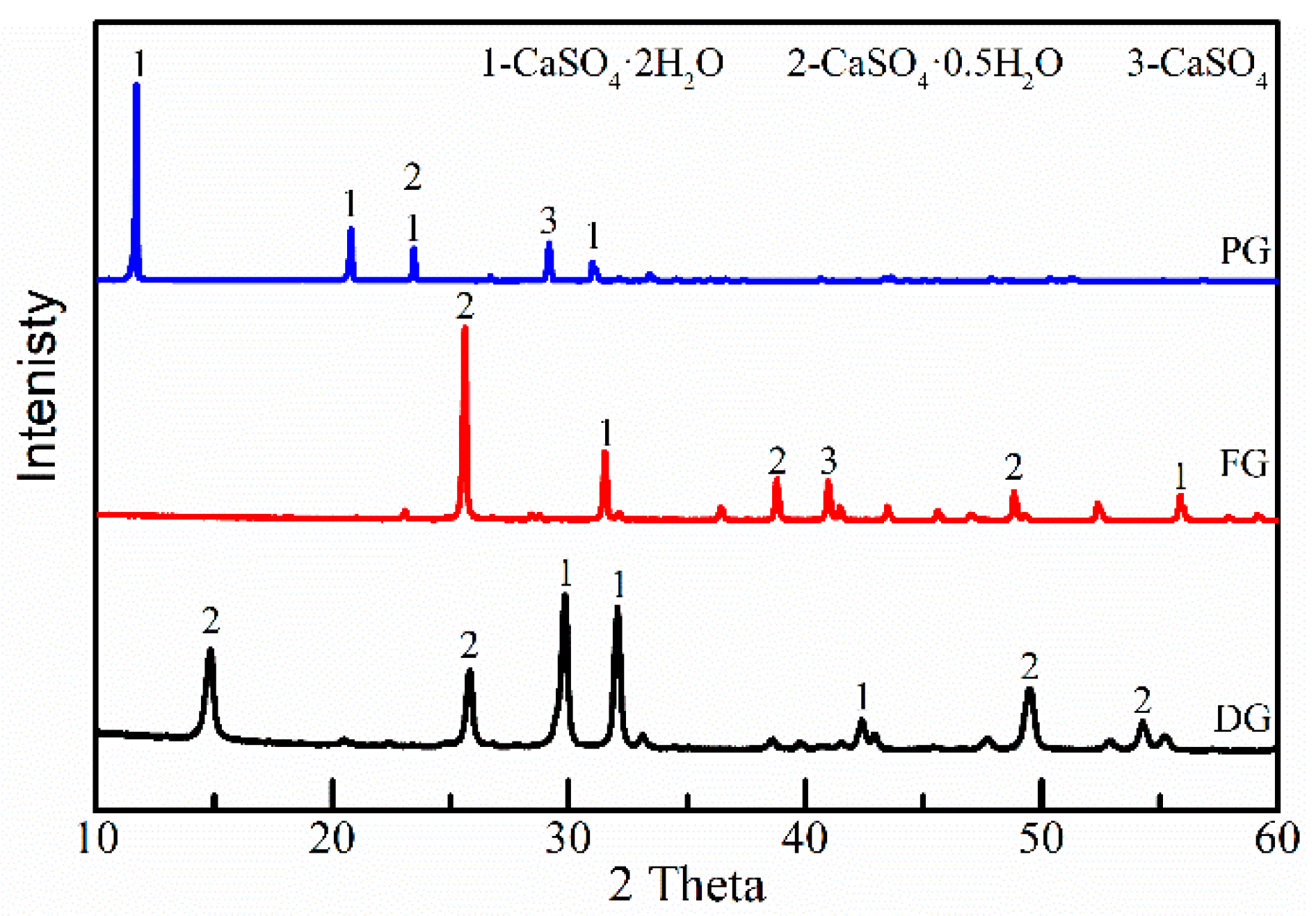
2.1.2. Binder
2.1.3. Water
2.2. Sample Preparation
2.3. Testing of Samples
2.3.1. Rheology Tests
2.3.2. Uniaxial Compressive Strength Tests
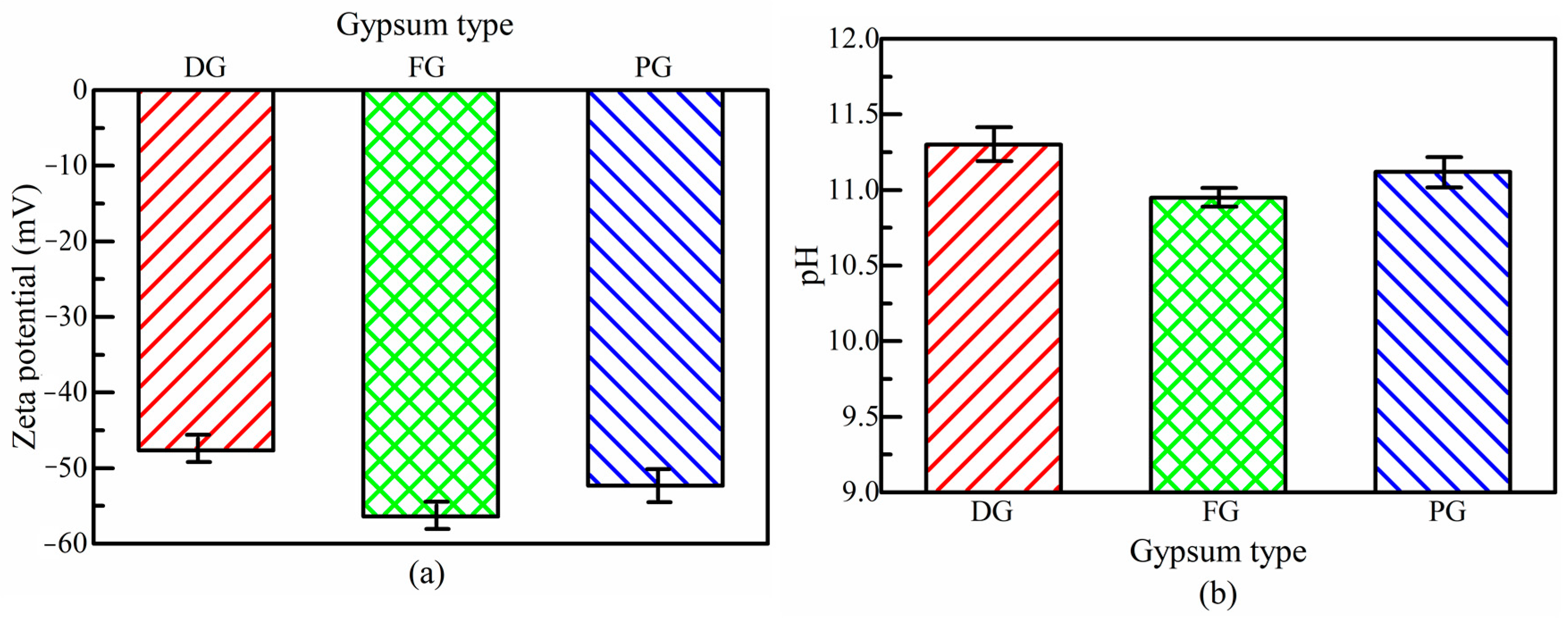
2.3.3. Zeta Potential and pH Measurements
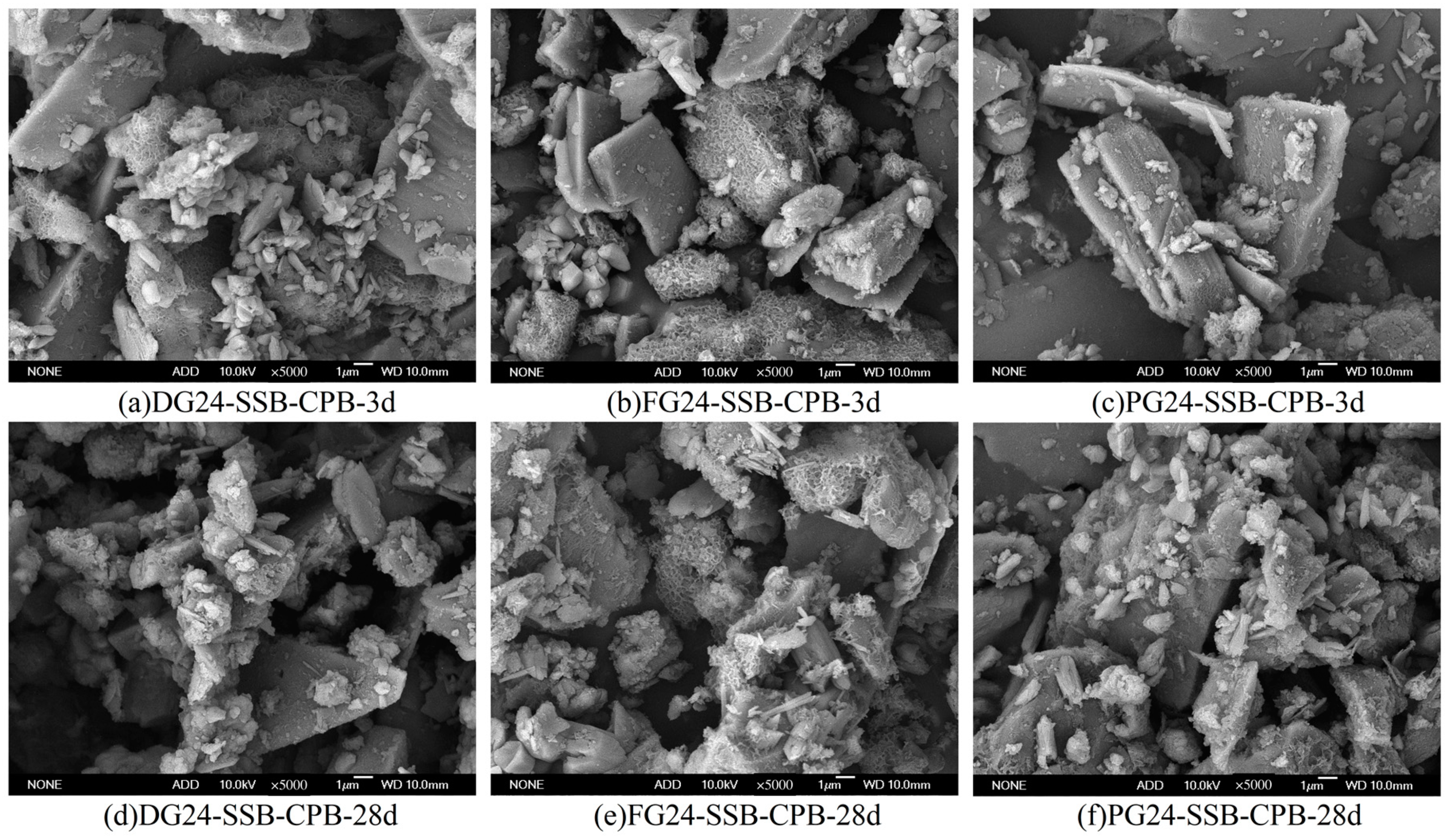
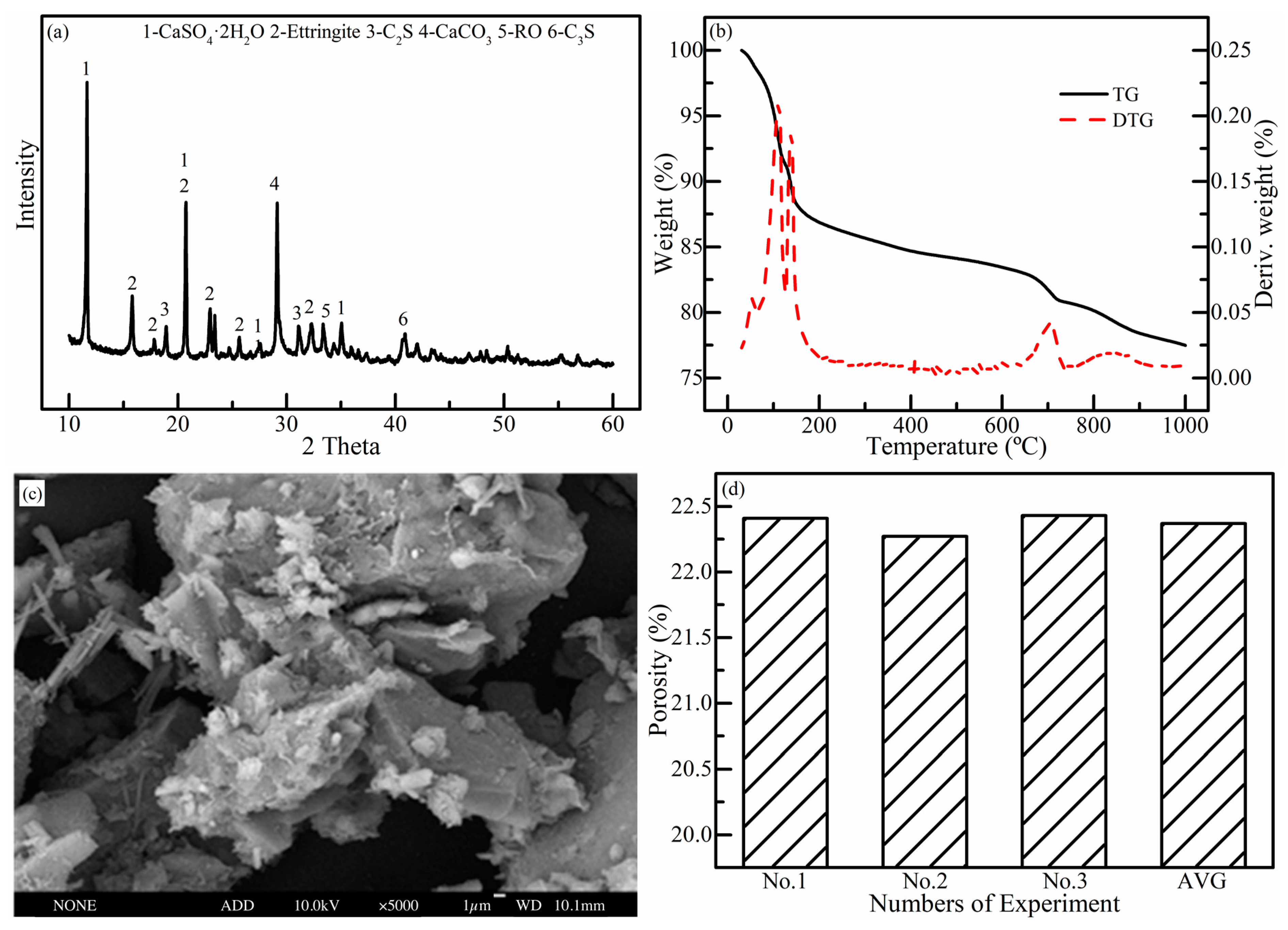
2.3.4. Microstructural Analysis
3. Results and Discussion
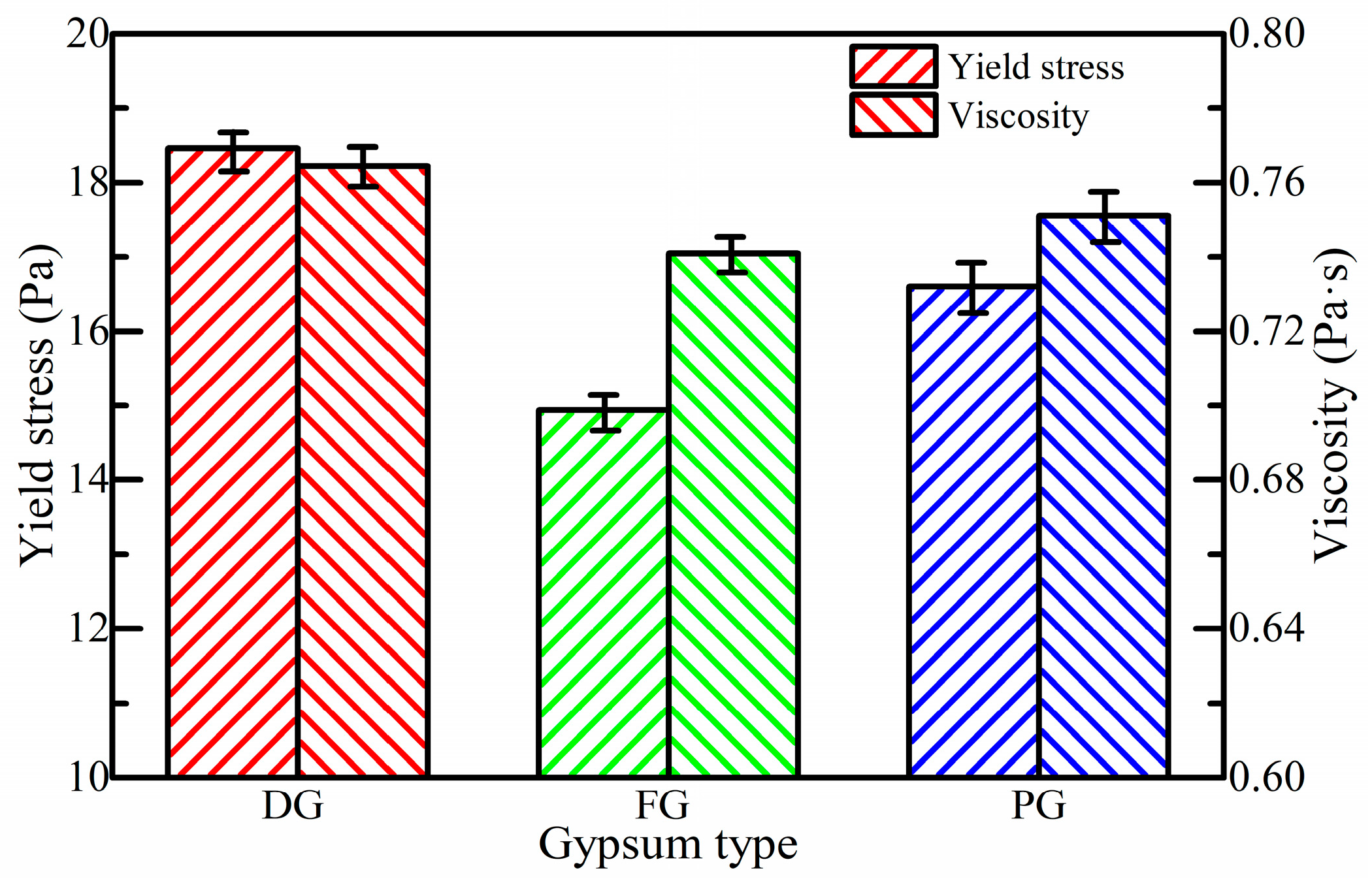
3.1. Effect of Gypsum Type on Rheological Properties of SSB-CPB
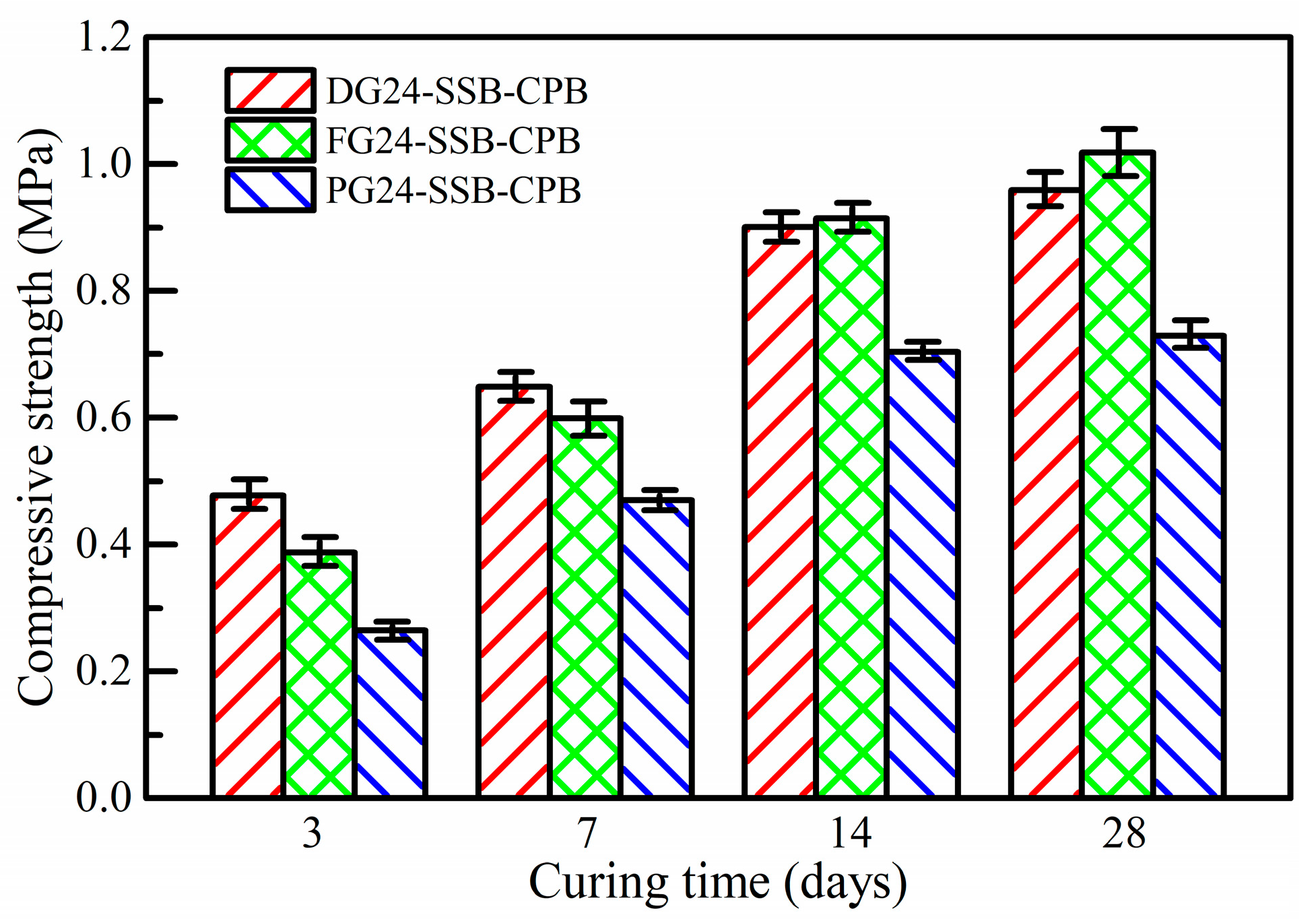
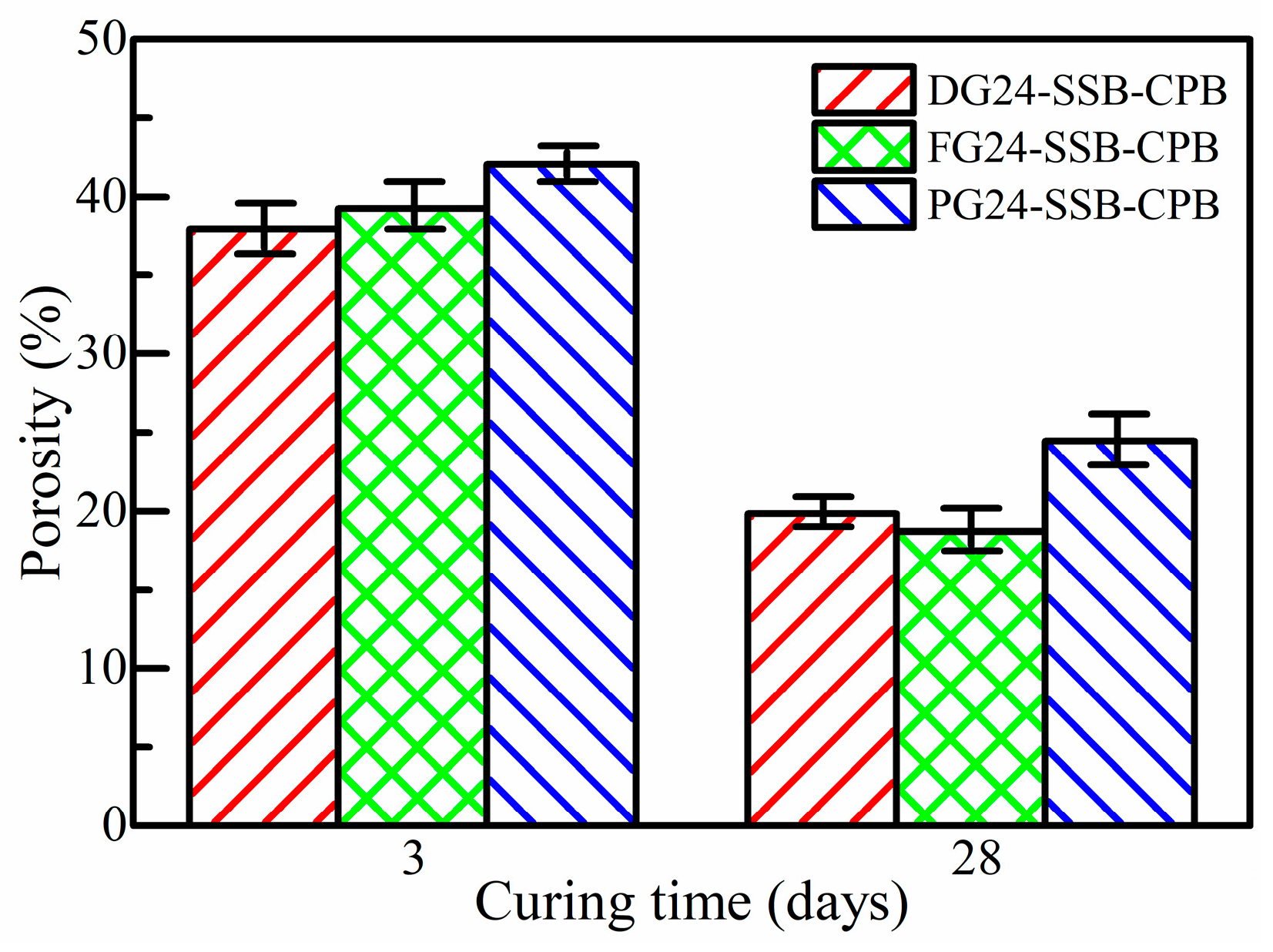
3.2. Effect of Gypsum Type on Strength Property of SSB-CPB
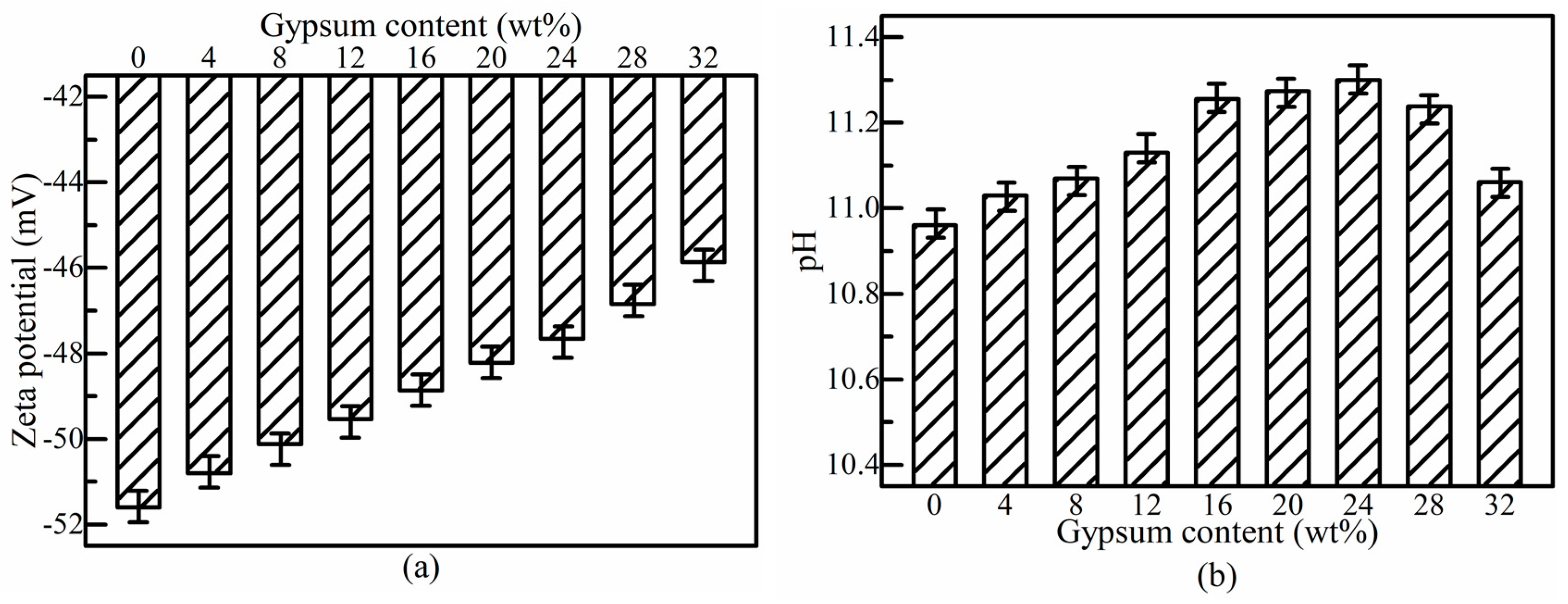
3.3. Effect of Gypsum Dosage on Rheological Properties of SSB-CPB
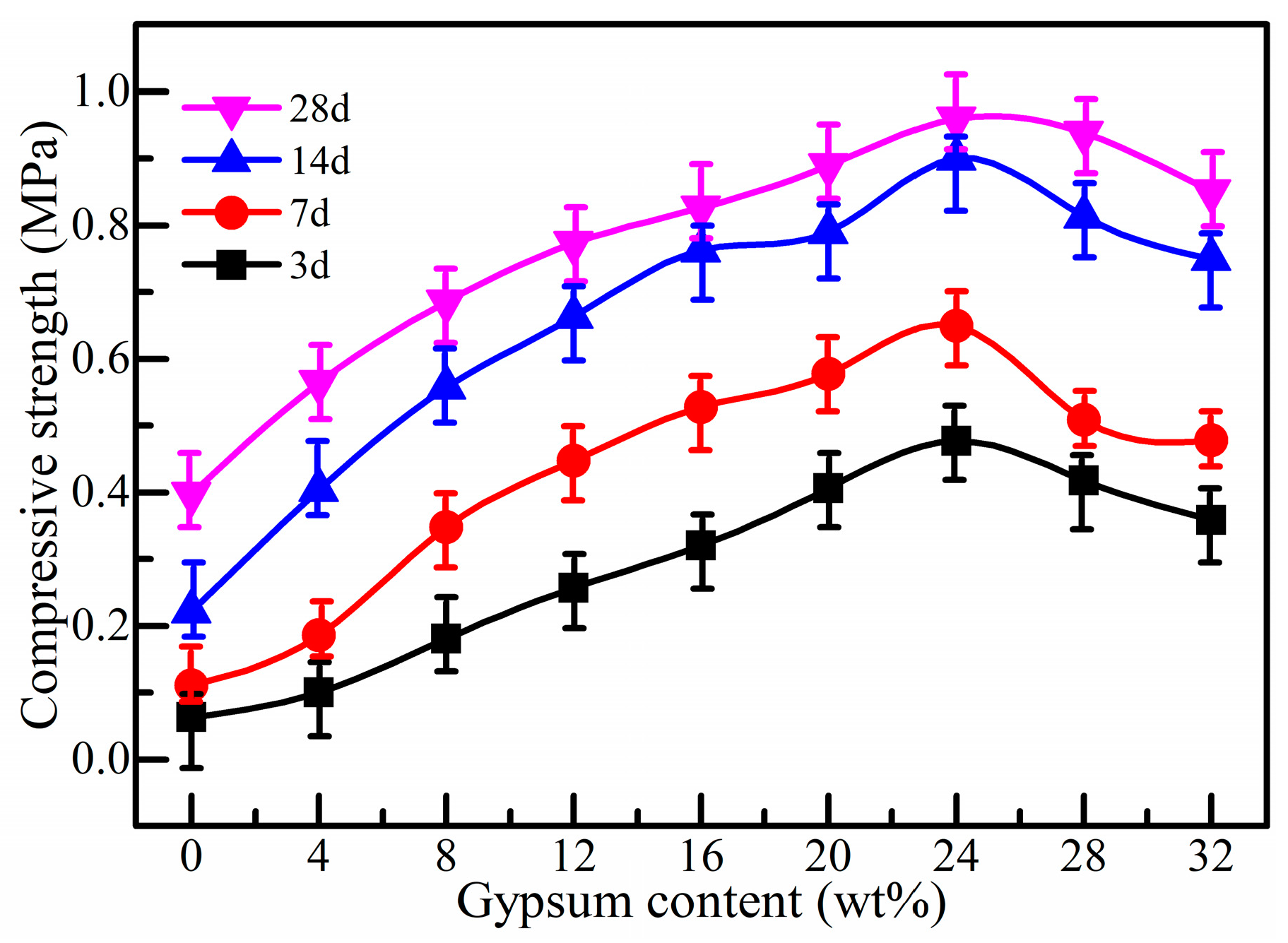
3.4. Effect of Gypsum Dosage on Strength Property of SSB-CPB
4. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roshani, A.; Fall, M. Flow ability of cemented pastefill material that contains nano-silica particles. Powder Technol. 2020, 373, 289–300. [Google Scholar] [CrossRef]
- Qiu, J.; Guo, Z.; Yang, L.; Jiang, H.; Zhao, Y. Effects of packing density and water film thickness on the fluidity behaviour of cemented paste backfill. Powder Technol. 2020, 359, 27–35. [Google Scholar] [CrossRef]
- Wang, Y.; Fall, M.; Wu, A. Initial temperature-dependence of strength development and self-desiccation in cemented paste backfill that contains sodium silicate. Cem. Concr. Compos. 2016, 67, 101–110. [Google Scholar] [CrossRef]
- Cui, L.; Fall, M. An evolutive elasto-plastic model for cemented paste backfill. Comput. Geotech. 2016, 71, 19–29. [Google Scholar] [CrossRef]
- Ghirian, A.; Fall, M. Coupled thermo-hydro-mechanical–chemical behaviour of cemented paste backfill in column experiments. Part I: Physical, hydraulic and thermal processes and characteristics. Eng. Geol. 2013, 164, 195–207. [Google Scholar] [CrossRef]
- Cui, L.; Fall, M. Mechanical and thermal properties of cemented tailings materials at early ages: Influence of initial temperature, curing stress and drainage conditions. Constr. Build. Mater. 2016, 125, 553–563. [Google Scholar] [CrossRef]
- Yang, X.; Xiaobing, Y.; Gao, Q.; He, J. Determining the pressure drop of cemented Gobi sand and tailings paste backfill in a pipe flow. Constr. Build. Mater. 2020, 255, 119371. [Google Scholar] [CrossRef]
- Tikou, B.; Mostafa, B. Design and Application of Underground Mine Paste Backfill Technology. Geotech. Geol. Eng. 2008, 26, 147–174. [Google Scholar] [CrossRef]
- Yilmaz, E.; Benzaazoua, M.; Belem, T.; Bussière, B. Effect of curing under pressure on compressive strength development of cemented paste backfill. Miner. Eng. 2009, 22, 772–785. [Google Scholar] [CrossRef]
- Yang, X.; Xiao, B.; Gao, Q. Validating the Use of Slag Binder with 91 Percent Blast Furnace Slag for Mine Backfilling. Adv. Mater. Sci. Eng. 2020, 2020, 2525831. [Google Scholar] [CrossRef]
- Fall, M.; Célestin, J.; Pokharel, M.; Touré, M. A contribution to understanding the effects of curing temperature on the mechanical properties of mine cemented tailings backfill. Eng. Geol. 2010, 114, 397–413. [Google Scholar] [CrossRef]
- Behera, S.; Ghosh, C.; Mishra, D.; Singh, P.; Mishra, K.; Buragohain, J.; Mandal, P.K. Strength development and microstructural investigation of lead-zinc mill tailings based paste backfill with fly ash as alternative binder. Cem. Concr. Compos. 2020, 109, 103553. [Google Scholar] [CrossRef]
- Wu, A.; Wang, Y.; Wang, H.; Yin, S.; Miao, X. Coupled effects of cement type and water quality on the properties of cemented paste backfill. Int. J. Miner. Process. 2015, 143, 65–71. [Google Scholar] [CrossRef]
- Shafigh, P.; Nomeli, M.A.; Alengaram, U.J.; Bin Mahmud, H.; Jumaat, M.Z. Engineering properties of lightweight aggregate concrete containing limestone powder and high volume fly ash. J. Clean. Prod. 2016, 135, 148–157. [Google Scholar] [CrossRef]
- Xu, W.; Li, Q.; Haruna, S. The Effect of Calcium Formate, Sodium Sulfate, and Cement Clinker on Engineering Properties of Fly Ash-Based Cemented Tailings Backfill. Adv. Mater. Sci. Eng. 2019, 2019, 5370360. [Google Scholar] [CrossRef]
- Zhao, Y.; Taheri, A.; Karakus, M.; Chen, Z.; Deng, A. Effects of water content, water type and temperature on the rheological behaviour of slag-cement and fly ash-cement paste backfill. Int. J. Min. Sci. Technol. 2020, 30, 271–278. [Google Scholar] [CrossRef]
- Yilmaz, E.; Belem, T.; Bussière, B.; Mbonimpa, M.; Benzaazoua, M. Curing time effect on consolidation behaviour of cemented paste backfill containing different cement types and contents. Constr. Build. Mater. 2015, 75, 99–111. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, Y.; Tian, Y.; Wu, P.; Guo, Z.; Qiu, J.; Xing, J.; Xiaowei, G. Modification of high-volume fly ash cement with metakaolin for its utilization in cemented paste backfill: The effects of metakaolin content and particle size. Powder Technol. 2021, 393, 539–549. [Google Scholar] [CrossRef]
- Cihangir, F.; Ercikdi, B.; Kesimal, A.; Turan, A.; Deveci, H. Utilisation of alkali-activated blast furnace slag in paste backfill of high-sulphide mill tailings: Effect of binder type and dosage. Miner. Eng. 2012, 30, 33–43. [Google Scholar] [CrossRef]
- Jiang, H.; Qi, Z.; Yilmaz, E.; Han, J.; Qiu, J.; Dong, C. Effectiveness of alkali-activated slag as alternative binder on workability and early age compressive strength of cemented paste backfills. Constr. Build. Mater. 2019, 218, 689–700. [Google Scholar] [CrossRef]
- Xue, G.; Yilmaz, E.; Song, W.; Cao, S. Compressive Strength Characteristics of Cemented Tailings Backfill with Alkali-Activated Slag. Appl. Sci. 2018, 8, 1537. [Google Scholar] [CrossRef]
- Li, L.; Gao, Q.; Chen, D.; Wu, F. Effect of gypsum-clinker mass ratios on properties of slag filling cementitious material and its application. J. Cent. South Univ. (Sci. Technol.) 2020, 51, 489–498. [Google Scholar] [CrossRef]
- Han, F.; Zhang, Z.; Wang, D.; Yan, P. Hydration heat evolution and kinetics of blended cement containing steel slag at different temperatures. Thermochim. Acta 2015, 605, 43–51. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P.; Yang, J.; Zhang, B. Influence of steel slag on mechanical properties and durability of concrete. Constr. Build. Mater. 2013, 47, 1414–1420. [Google Scholar] [CrossRef]
- Kourounis, S.; Tsivilis, S.; Tsakiridis, P.; Papadimitriou, G.; Tsibouki, Z. Properties and hydration of blended cements with steelmaking slag. Cem. Concr. Res. 2007, 37, 815–822. [Google Scholar] [CrossRef]
- Brand, A.S.; Roesler, J.R. Steel furnace slag aggregate expansion and hardened concrete properties. Cem. Concr. Compos. 2015, 60, 1–9. [Google Scholar] [CrossRef]
- Shi, C. Steel slag—its production, processing, characteristics, and cementitious properties. J. Mater. Civ. Eng. 2004, 16, 230–236. [Google Scholar] [CrossRef]
- Wang, T.; Wu, K.; Wu, M. Development of green binder systems based on flue gas desulfurization gypsum and fly ash incorporating slag or steel slag powders. Constr. Build. Mater. 2020, 265, 120275. [Google Scholar] [CrossRef]
- R., S.L.R.; Domínguez, O.; A., J.H.D.; García, C.; Tapia, F. Synergistic effects of rubber-tire-powder and fluorogypsum in cement-based composite. Case Stud. Constr. Mater. 2020, 14, e00471. [Google Scholar] [CrossRef]
- Kuryatnyk, T.; da Luz, C.A.; Ambroise, J.; Pera, J. Valorization of phosphogypsum as hydraulic binder. J. Hazard. Mater. 2008, 160, 681–687. [Google Scholar] [CrossRef]
- Li, W.; Fall, M. Strength and self-desiccation of slag-cemented paste backfill at early ages: Link to initial sulphate concentration. Cem. Concr. Compos. 2018, 89, 160–168. [Google Scholar] [CrossRef]
- Xiao, B.; Fall, M.; Roshani, A. Towards Understanding the Rheological Properties of Slag-Cemented Paste Backfill. Int. J. Min. Reclam. Environ. 2020, 35, 268–290. [Google Scholar] [CrossRef]
- All-China Environment Federation Professional Committee for the Treatment of Solid and Endangered Wastes. Development Report on Comprehensive Utilization of Large Industrial Solid Waste in China in 2019; Industrial Solid Waste Network: Beijing, China, 2020. [Google Scholar]
- Ke, X.; Hou, H.; Zhou, M.; Wang, Y.; Zhou, X. Effect of particle gradation on properties of fresh and hardened cemented paste backfill. Constr. Build. Mater. 2015, 96, 378–382. [Google Scholar] [CrossRef]
- Wu, D.; Cai, S.-J.; Huang, G. Coupled effect of cement hydration and temperature on rheological properties of fresh cemented tailings backfill slurry. Trans. Nonferrous Met. Soc. China 2014, 24, 2954–2963. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, A.; Zhang, L.; Jin, F.; Liu, X. Investigating the Effect of Solid Components on Yield Stress for Cemented Paste Back-fill via Uniform Design. Adv. Mater. Sci. Eng. 2018, 2018, 3839174. [Google Scholar] [CrossRef]
- Xu, W.; Chen, W.; Tian, M.; Guo, L. Effect of temperature on time-dependent rheological and compressive strength of fresh cemented paste backfill containing flocculants. Constr. Build. Mater. 2021, 267, 121038. [Google Scholar] [CrossRef]
- Xiao, B.; Wen, Z.; Wu, F.; Li, L.; Yang, Z.; Gao, Q. A simple L-shape pipe flow test for practical rheological properties of backfill slurry: A case study. Powder Technol. 2019, 356, 1008–1015. [Google Scholar] [CrossRef]
- Bharathan, B.; McGuinness, M.; Kuhar, S.; Kermani, M.; Hassani, F.P.; Sasmito, A.P. Pressure loss and friction factor in non-Newtonian mine paste backfill: Modelling, loop test and mine field data. Powder Technol. 2019, 344, 443–453. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P.; Feng, J. A discussion on improving hydration activity of steel slag by altering its mineral compositions. J. Hazard. Mater. 2011, 186, 1070–1075. [Google Scholar] [CrossRef]
- Xu, C.; Ni, W.; Li, K.; Zhang, S.; Li, Y.; Xu, D. Hydration mechanism and orthogonal optimisation of mix proportion for steel slag–slag-based clinker-free prefabricated concrete. Constr. Build. Mater. 2019, 228, 117036. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P. Hydration properties of basic oxygen furnace steel slag. Constr. Build. Mater. 2010, 24, 1134–1140. [Google Scholar] [CrossRef]
- Wu, F.; Yang, F.; Xiao, B.; Yang, Z.; Gao, Q. Influence of Steel Slag Dosage on Early Age Strength and Rheological Properties of Paste. Mater. Rev. 2021, 35, 3021–3025. [Google Scholar] [CrossRef]
- Xiao, B.L.; Miao, S.J.; Gao, Q.; Chen, B.Y.; Li, S.H. Hydration Mechanism of Sustainable Clinker-Free Steel Slag Binder and Its Application in Mine Backfill. JOM 2021, 73, 1053–1061. [Google Scholar] [CrossRef]
- Cihangir, F.; Ercikdi, B.; Kesimal, A.; Ocak, S.; Akyol, Y. Effect of sodium-silicate activated slag at different silicate modulus on the strength and microstructural properties of full and coarse sulphidic tailings paste backfill. Constr. Build. Mater. 2018, 185, 555–566. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, W.; Liu, Q.; Sheng, L. Studying with small sample for leaching behavior of heavy metals based on the fuzzy theo-ry. World J. Eng. 2015, 12, 271–282. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Guo, Z.; Ding, H. Stabilization/solidification of hexavalent chromium containing tailings using low-carbon binders for cemented paste backfill. J. Environ. Chem. Eng. 2021, 9, 104738. [Google Scholar] [CrossRef]
- Pokharel, M.; Fall, M. Combined influence of sulphate and temperature on the saturated hydraulic conductivity of hardened cemented paste backfill. Cem. Concr. Compos. 2013, 38, 21–28. [Google Scholar] [CrossRef]
- Shang, J.; Zhang, K.; Zhao, S.; Wu, G.; Li, X. Development on Evaluation Methodology of Cementitious Activity of Steel Slag. Mater. Rev. 2012, 26, 128–130,140. [Google Scholar] [CrossRef]
- Haruna, S.; Fall, M. Strength development of cemented tailings materials containing polycarboxylate ether-based superplasticizer: Experimental results on the effect of time and temperature. Can. J. Civ. Eng. 2021, 48, 429–442. [Google Scholar] [CrossRef]
- Liu, X.; Wu, A.; Wang, H.; Wang, Y. Influence mechanism and calculation model of CPB rheological parameters. J. Univ. Sci. Technol. Beijing 2017, 39, 190–195. [Google Scholar] [CrossRef]
- Xiapeng, P.; Fall, M.; Haruna, S. Sulphate induced changes of rheological properties of cemented paste backfill. Miner. Eng. 2019, 141, 105849. [Google Scholar] [CrossRef]
- Aldhafeeri, Z.; Fall, M. Sulphate induced changes in the reactivity of cemented tailings backfill. Int. J. Miner. Process. 2017, 166, 13–23. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Cui, L.; Si, Z.; Wang, H. Effect of external sulfate attack on the mechanical behavior of cemented paste backfill. Constr. Build. Mater. 2020, 263, 120968. [Google Scholar] [CrossRef]
- Chin, C.-J.; Yiacoumi, S.; Tsouris, C. Probing DLVO Forces Using Interparticle Magnetic Forces: Transition from Secondary-Minimum to Primary-Minimum Aggregation. Langmuir 2001, 17, 6065–6071. [Google Scholar] [CrossRef]
- Kuznar, Z.A.; Elimelech, M. Direct microscopic observation of particle deposition in porous media: Role of the secondary energy minimum. Colloids Surf. A Physicochem. Eng. Asp. 2007, 294, 156–162. [Google Scholar] [CrossRef]
- Hot, J.; Bessaies-Bey, H.; Brumaud, C.; Duc, M.; Castella, C.; Roussel, N. Adsorbing polymers and viscosity of cement pastes. Cem. Concr. Res. 2014, 63, 12–19. [Google Scholar] [CrossRef]
- Gao, S.; Ni, W.; Zhu, L.; Wang, Z.; Wang, J. Effect of gypsum on strength performance of cemented backfilling materials of red mud-slag system. J. Cent. South Univ. (Sci. Technol.) 2013, 44, 2259–2266. [Google Scholar]
- Xiao, B.; Wen, Z.; Miao, S.; Gao, Q. Utilization of steel slag for cemented tailings backfill: Hydration, strength, pore structure, and cost analysis. Case Stud. Constr. Mater. 2021, 15, e00621. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Li, S.; Gao, Y.; Liu, C.; Qi, Y. Effect of different gypsums on the workability and mechanical properties of red mud-slag based grouting materials. J. Clean. Prod. 2020, 245, 118759. [Google Scholar] [CrossRef]
- Sun, G.; Tang, Q.; Zhang, L.; Wang, C. Early activation effect and mechanism of high-volume fly ash. J. Harbin Eng. Univ. 2019, 40, 540–547. [Google Scholar] [CrossRef]
- Xiao, B.; Miao, S.; Gao, Q.; Wu, F. Effect of metallurgical slag cementitious material on solidification characteristics of ultra-fine tailings backfill. Chin. J. Nonferrous Met. 2022, 32, 1153–1163. [Google Scholar] [CrossRef]
- Wang, X.; Ni, W.; Li, J.; Zhang, S.; Hitch, M.; Pascual, R. Carbonation of steel slag and gypsum for building materials and associated reaction mechanisms. Cem. Concr. Res. 2019, 125, 105893. [Google Scholar] [CrossRef]
- Chang, J.; Xiong, C.; Zhang, Y.; Wang, D. Foaming characteristics and microstructure of aerated steel slag block prepared by accelerated carbonation. Constr. Build. Mater. 2019, 209, 222–233. [Google Scholar] [CrossRef]
- Cui, X.; Ni, W.; Ren, C. Hydration Mechanism of All Solid Waste Cementitious Materials Based on Steel Slag and Blast Furnace Slag. Chin. J. Mater. Res. 2017, 31, 687–694. [Google Scholar] [CrossRef]
- Dambrauskas, T.; Baltakys, K.; Škamat, J.; Kudžma, A. Hydration peculiarities of high basicity calcium silicate hydrate samples. J. Therm. Anal. Calorim. 2017, 131, 491–499. [Google Scholar] [CrossRef]
- Petlovanyi, M.; Mamaikin, O. Assessment of an expediency of binder material mechanical activation in cemented rockfill. ARPN J. Eng. Appl. Sci. 2019, 14, 3492–3503. [Google Scholar]
- Petlovanyi, M.V.; Zubko, S.A.; Popovych, V.V.; Sai, K.S. Physicochemical mechanism of structure formation and strength-ening in the backfill massif when filling underground cavities. Vopr. Khimii I Khimicheskoi Tekhnologii 2019, 6, 142–150. [Google Scholar]




| Sample | Silica Tailings | Desulfurization Gypsum | Fluorogypsum | Phosphogypsum | Steel Slag | Slag |
|---|---|---|---|---|---|---|
| CaO | 0.01 | 47.14 | 47.12 | 45.45 | 46.22 | 43.51 |
| SiO2 | 99.80 | 1.83 | 1.39 | 2.29 | 18.99 | 30.68 |
| Al2O3 | 0.05 | 0.44 | 0.32 | 0.16 | 4.45 | 14.03 |
| MgO | <0.01 | 0.00 | 0.00 | 1.19 | 4.59 | 7.35 |
| SO3 | 0.00 | 50.20 | 49.68 | 48.02 | 0.32 | 1.32 |
| Fe2O3 | 0.00 | 0.22 | 0.51 | 0.33 | 16.64 | 0.72 |
| TiO2 | 0.02 | 0.02 | 0.03 | 0.05 | 0.87 | 0.68 |
| MnO | 0.00 | 0.01 | 0.02 | 0.03 | 4.76 | 0.57 |
| K2O | 0.02 | 0.04 | 0.06 | 0.04 | 0.12 | 0.54 |
| Na2O | <0.01 | 0.00 | 0.00 | 0.04 | 0.00 | 0.33 |
| SrO | 0.00 | 0.05 | 0.01 | 0.10 | 0.04 | 0.08 |
| P2O5 | 0.00 | 0.00 | 0.00 | 1.41 | 2.25 | 0.06 |
| F | 0.00 | 0.00 | 0.80 | 0.83 | 0.00 | 0.00 |
| Sample Name | SSB | Curing Time (Days) | |||
|---|---|---|---|---|---|
| Gypsum Type | Gypsum Dosage (%) | SS (%) | Slag (%) | ||
| Effect of gypsum type | |||||
| DG24-SSB-CPB | DG | 24 | 35 | 41 | 3, 7, 14, 28 |
| FG24-SSB-CPB | FG | 24 | 35 | 41 | 3, 7, 14, 28 |
| PG24-SSB-CPB | PG | 24 | 35 | 41 | 3, 7, 14, 28 |
| Effect of gypsum dosage | |||||
| DG0-SSB-CPB | DG | 0 | 35 | 65 | 3, 7, 14, 28 |
| DG4-SSB-CPB | DG | 4 | 35 | 61 | 3, 7, 14, 28 |
| DG8-SSB-CPB | DG | 8 | 35 | 57 | 3, 7, 14, 28 |
| DG12-SSB-CPB | DG | 12 | 35 | 53 | 3, 7, 14, 28 |
| DG16-SSB-CPB | DG | 16 | 35 | 49 | 3, 7, 14, 28 |
| DG20-SSB-CPB | DG | 20 | 35 | 45 | 3, 7, 14, 28 |
| DG24-SSB-CPB | DG | 24 | 35 | 41 | 3, 7, 14, 28 |
| DG28-SSB-CPB | DG | 28 | 35 | 37 | 3, 7, 14, 28 |
| DG32-SSB-CPB | DG | 32 | 35 | 33 | 3, 7, 14, 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.; Xiao, B.; Yang, F. Rheological and Strength Properties of Steel-Slag Cemented Paste Backfill: Link to Gypsum Type and Dosage. Minerals 2023, 13, 421. https://doi.org/10.3390/min13030421
Wu F, Xiao B, Yang F. Rheological and Strength Properties of Steel-Slag Cemented Paste Backfill: Link to Gypsum Type and Dosage. Minerals. 2023; 13(3):421. https://doi.org/10.3390/min13030421
Chicago/Turabian StyleWu, Fan, Bolin Xiao, and Faguang Yang. 2023. "Rheological and Strength Properties of Steel-Slag Cemented Paste Backfill: Link to Gypsum Type and Dosage" Minerals 13, no. 3: 421. https://doi.org/10.3390/min13030421
APA StyleWu, F., Xiao, B., & Yang, F. (2023). Rheological and Strength Properties of Steel-Slag Cemented Paste Backfill: Link to Gypsum Type and Dosage. Minerals, 13(3), 421. https://doi.org/10.3390/min13030421










