Application of Shewanella xiamenensis Placed on Zeolite in Treatment of Silver-Containing Effluents
Abstract
1. Introduction
2. Materials and Methods
2.1. Effluents
2.2. Preparation of Biosorbent
2.3. Experiment Design
2.4. Applied Techniques
3. Results
3.1. Effect of pH on Metal Biosorption on Bio-Zeolite
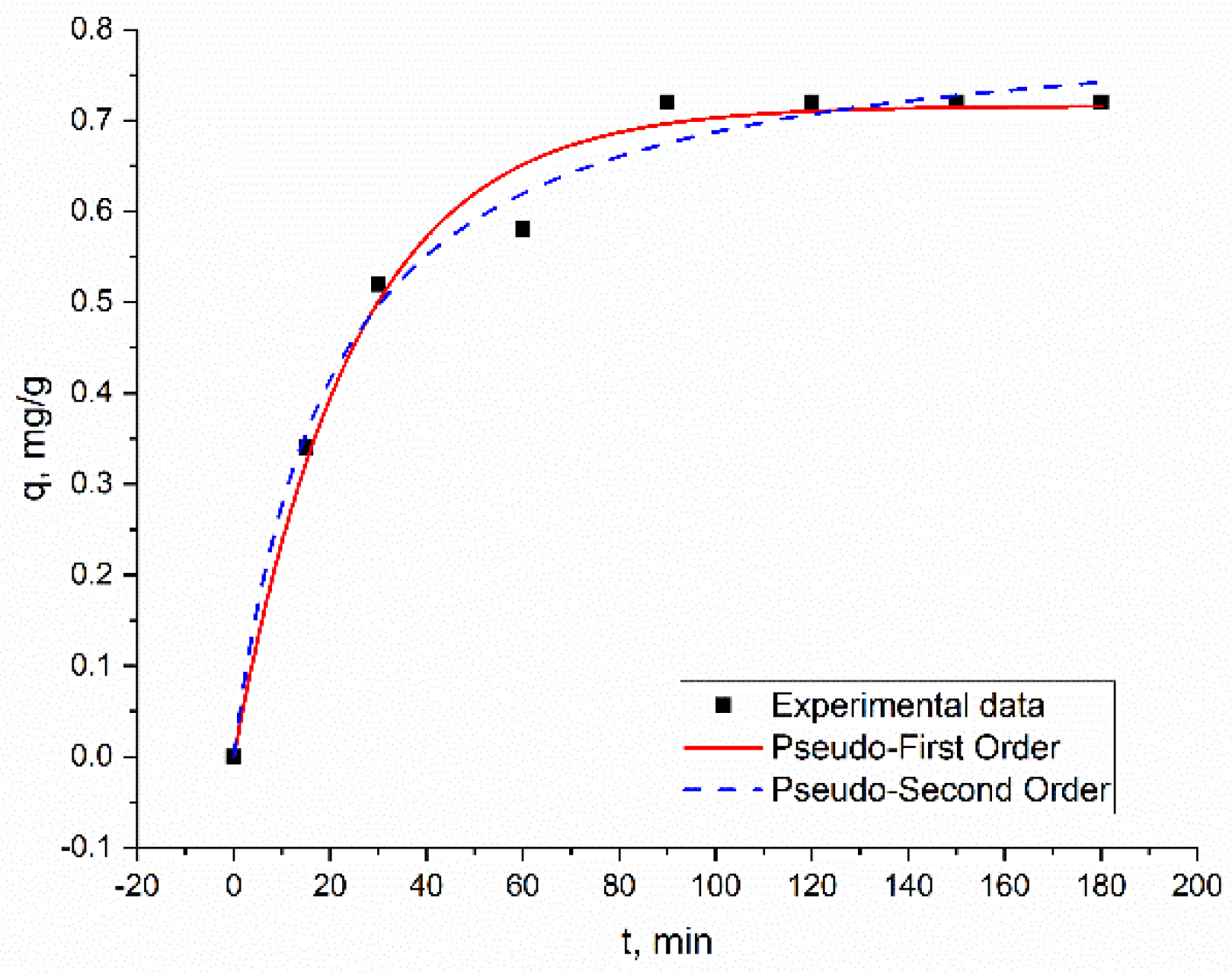
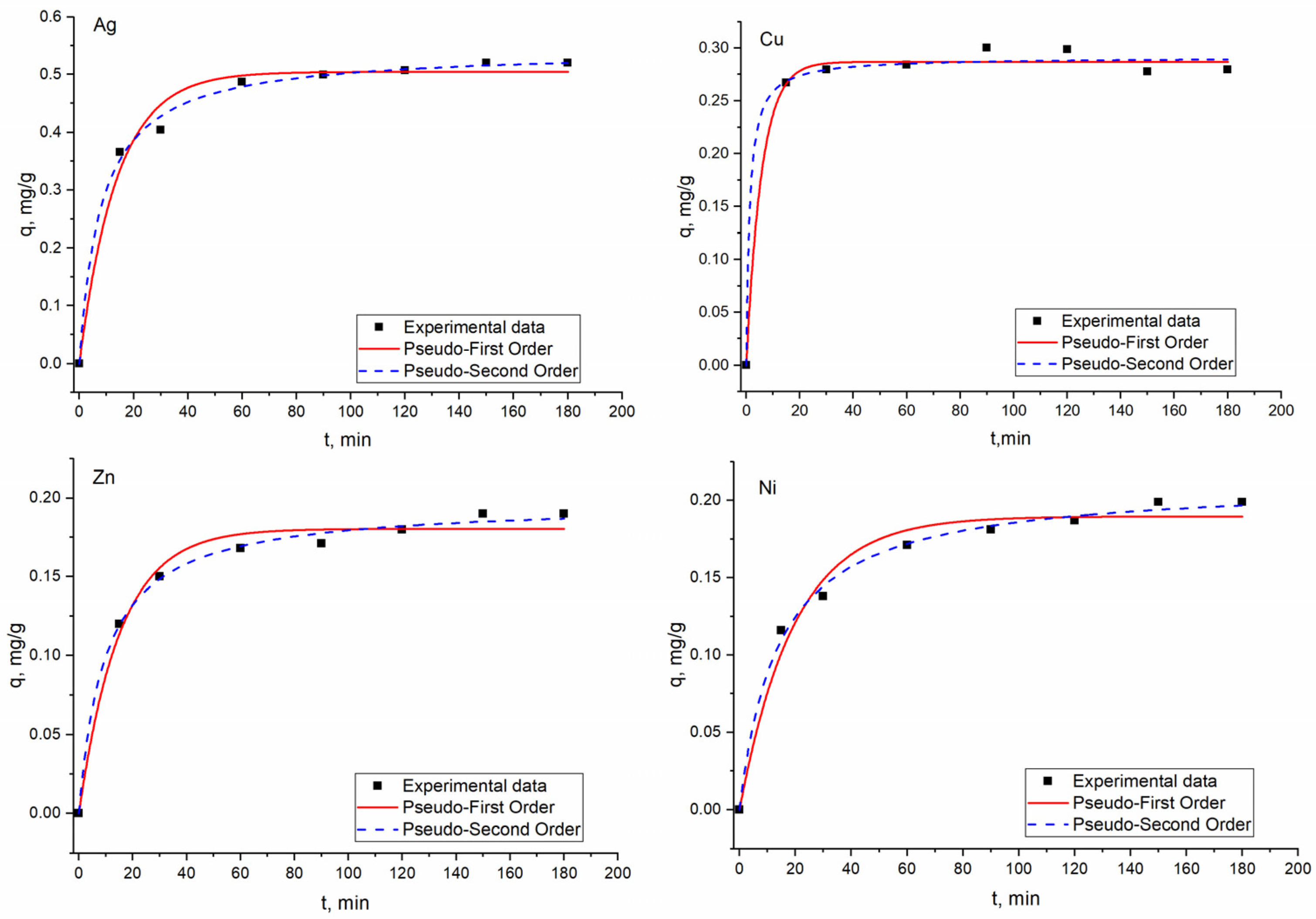
3.2. Effect of Time on Metal Biosorption on Bio-Zeolite and Kinetic Studies
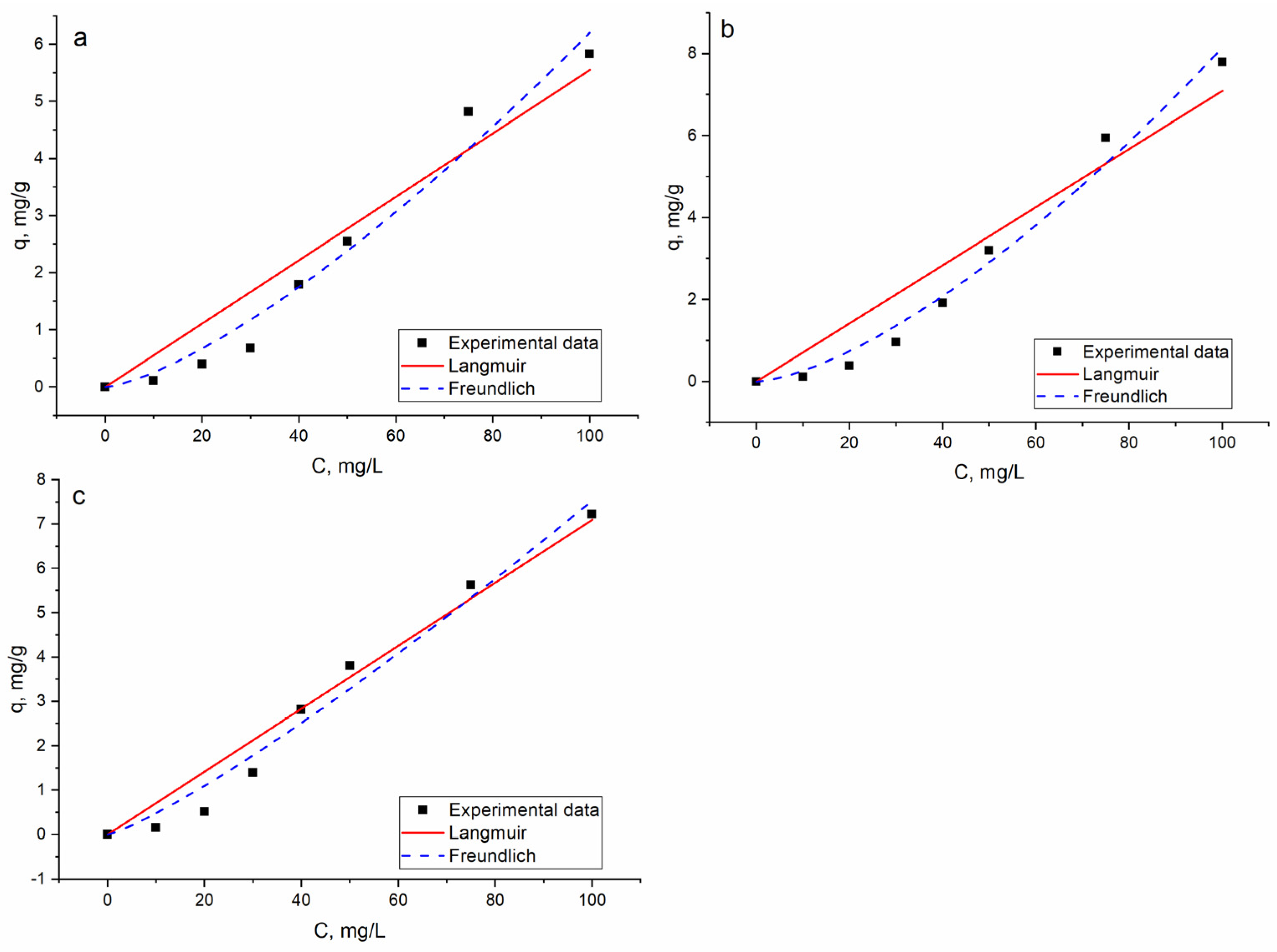
3.3. Effect of Silver Concentration on Its Biosorption on Bio-Zeolite and Equlibrium Studies
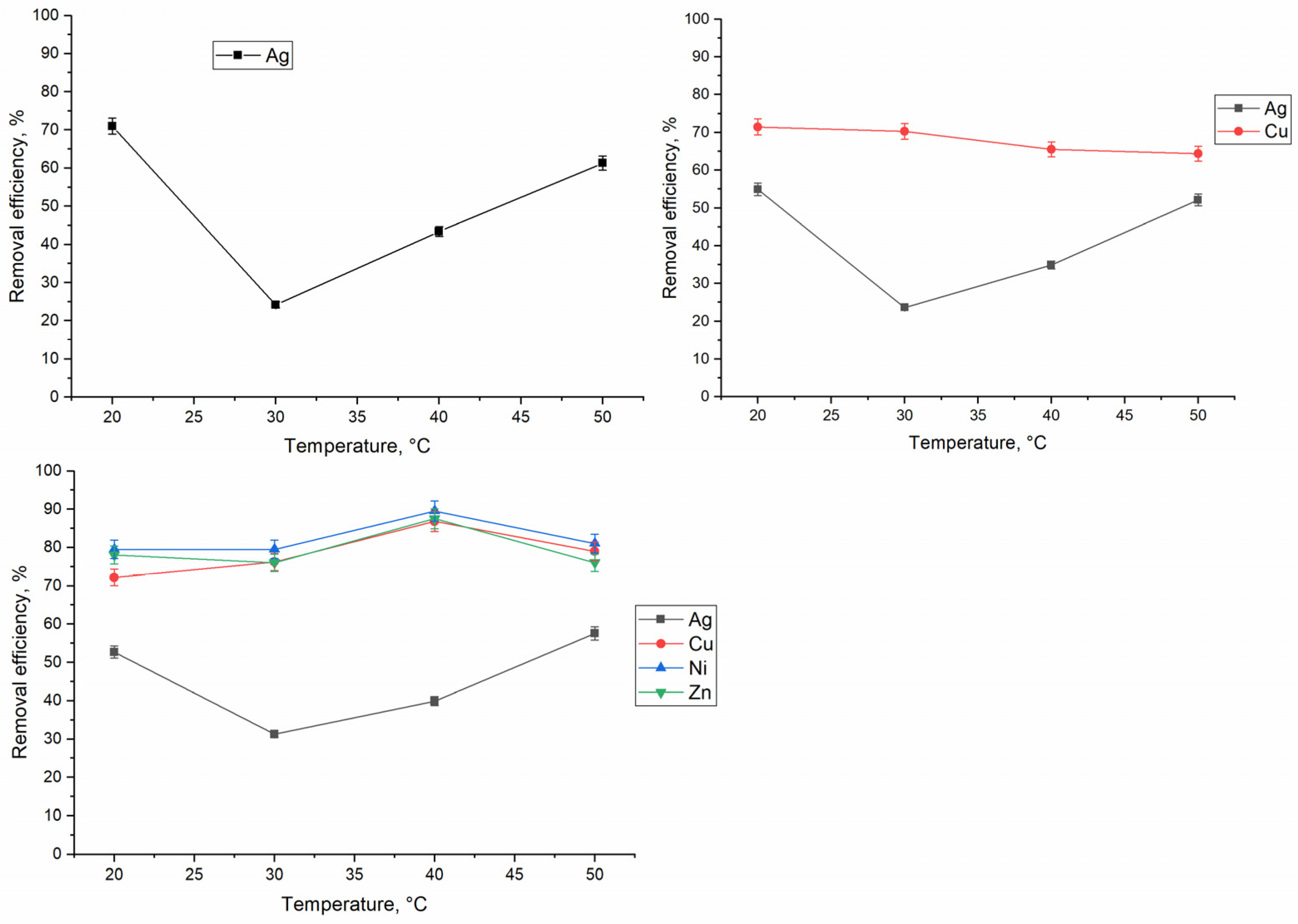
3.4. Effect of Temperature on Silver Biosorption on Bio-Zeolite and Thermodinamic Studies
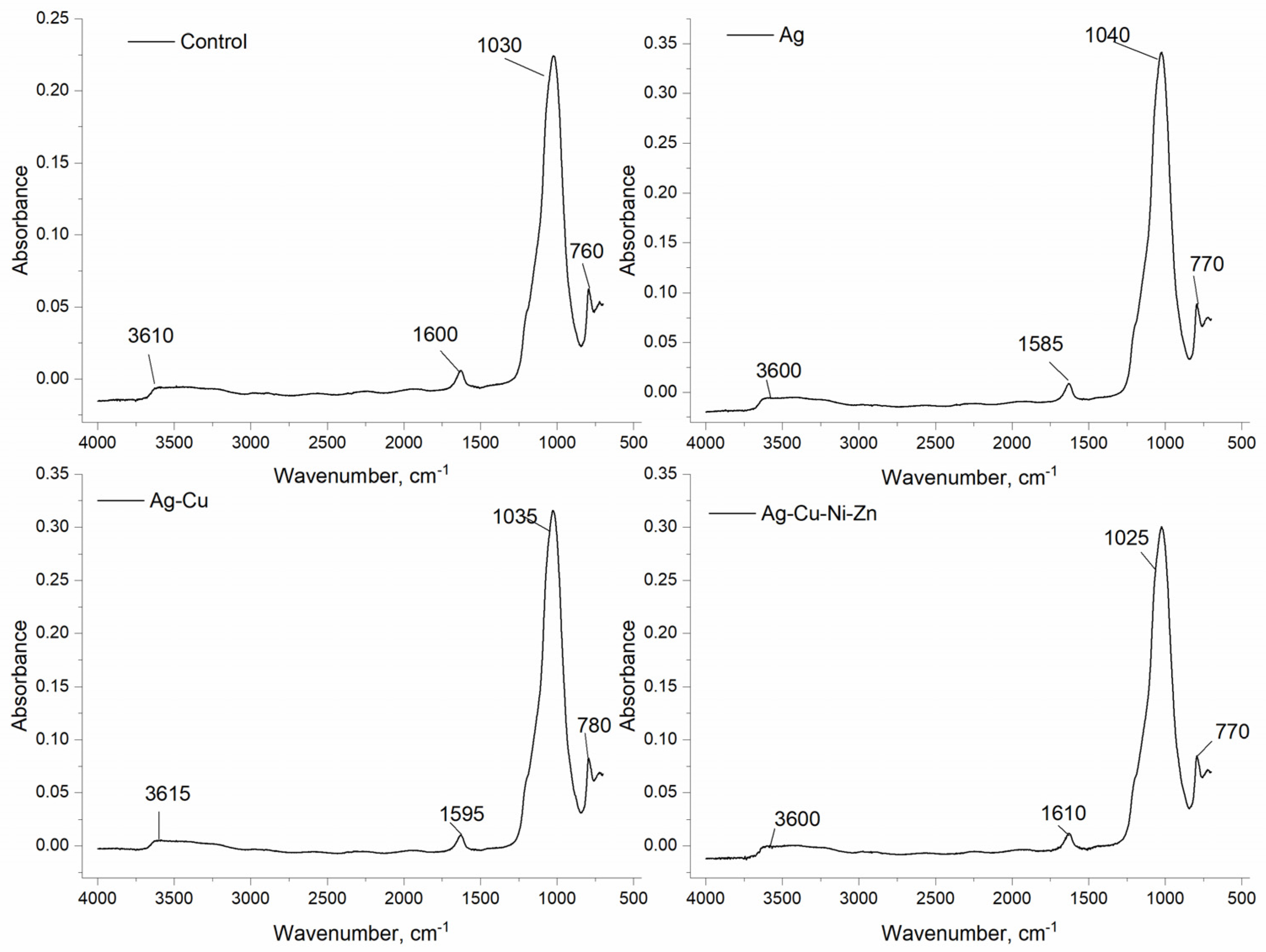
3.5. FTIR Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- de Freitas, G.R.; Vieira, M.G.A.; da Silva, M.G.C. Characterization and biosorption of silver by biomass waste from the alginate industry. J. Clean. Prod. 2020, 271, 122588. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, D.; Xie, H.; Won, S.W.; Cui, L.; Wu, G. Adsorption of Ag (I) from aqueous solution by waste yeast: Kinetic, equilibrium and mechanism studies. Bioprocess Biosyst. Eng. 2014, 38, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Ratte, H.T. Bioaccumulation and toxicity of silver compounds: A review. Environ. Toxicol. Chem. 1999, 18, 89–108. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Drake, P.L.; Hazelwood, K.J. Exposure-Related Health Effects of Silver and Silver Compounds: A Review. Ann. Occup. Hyg. 2005, 49, 575–585. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, F.; Yalamarty, S.S.K.; Filipczak, N.; Jin, Y.; Li, X. Nano Silver-Induced Toxicity and Associated Mechanisms. Int. J. Nanomed. 2022, 17, 1851–1864. [Google Scholar] [CrossRef]
- De Matteis, V. Exposure to Inorganic Nanoparticles: Routes of Entry, Immune Response, Biodistribution and In Vitro/In Vivo Toxicity Evaluation. Toxics 2017, 5, 29. [Google Scholar] [CrossRef]
- Bakand, S.; Hayes, A. Toxicological Considerations, Toxicity Assessment, and Risk Management of Inhaled Nanoparticles. Int. J. Mol. Sci. 2016, 17, 929. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, Y.; Wang, H.; Lin, W.; Wang, Y.; Sun, D.; Hong, J.; Li, Q. Simultaneous Silver Recovery and Cyanide Removal from Electroplating Wastewater by Pulse Current Electrolysis Using Static Cylinder Electrodes. Ind. Eng. Chem. Res. 2013, 52, 5871–5879. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Al-Bogami, A.S.; Guibal, E. 2-Mercaptobenzimidazole derivative of chitosan for silver sorption—Contribution of magnetite incorporation and sonication effects on enhanced metal recovery. Chem. Eng. J. 2020, 403, 126265. [Google Scholar] [CrossRef]
- Yin, X.; Long, J.; Xi, Y.; Luo, X. Recovery of Silver from Wastewater Using a New Magnetic Photocatalytic Ion-Imprinted Polymer. ACS Sustain. Chem. Eng. 2017, 5, 2090–2097. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, F.; Peng, Z. Adsorption mechanism of Cr(VI) onto GO/PAMAMs composites. Sci. Rep. 2019, 9, 3663. [Google Scholar] [CrossRef] [PubMed]
- Demey, H.; Vincent, T.; Guibal, E. A novel algal-based sorbent for heavy metal removal. Chem. Eng. J. 2018, 332, 582–595. [Google Scholar] [CrossRef]
- Tighadouini, S.; Radi, S.; Roby, O.; Hammoudan, I.; Saddik, R.; Garcia, Y.; Almarhoon, Z.M.; Mabkhot, Y.N. Kinetics, thermodynamics, equilibrium, surface modelling, and atomic absorption analysis of selective Cu(ii) removal from aqueous solutions and rivers water using silica-2-(pyridin-2-ylmethoxy)ethan-1-ol hybrid material. RSC Adv. 2021, 12, 611–625. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Yushin, N.; Grozdov, D.; Vergel, K.; Nekhoroshkov, P.; Rodlovskaya, E. Treatment of Rhenium-Containing Effluents Using Environmentally Friendly Sorbent, Saccharomyces cerevisiae Biomass. Materials 2021, 14, 4763. [Google Scholar] [CrossRef] [PubMed]
- Zinicovscaia, I.; Yushin, N.; Abdusamadzoda, D.; Grozdov, D.; Shvetsova, M. Efficient Removal of Metals from Synthetic and Real Galvanic Zinc–Containing Effluents by Brewer’s Yeast Saccharomyces cerevisiae. Materials 2020, 13, 3624. [Google Scholar] [CrossRef]
- Fathollahi, A.; Khasteganan, N.; Coupe, S.J.; Newman, A.P. A meta-analysis of metal biosorption by suspended bacteria from three phyla. Chemosphere 2021, 268, 129290. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Kim, J.; Chang, S.; Chung, W. Bacterial Biosorbents, an Efficient Heavy Metals Green Clean-Up Strategy: Prospects, Challenges, and Opportunities. Microorganisms 2022, 10, 610. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Moghaddam, S.A.E.; Harun, R.; Mokhtar, M.N.; Zakaria, R. Potential of Zeolite and Algae in Biomass Immobilization. BioMed Res. Int. 2018, 2018, 6563196. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.-S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef]
- Yuna, Z. Review of the Natural, Modified, and Synthetic Zeolites for Heavy Metals Removal from Wastewater. Environ. Eng. Sci. 2016, 33, 443–454. [Google Scholar] [CrossRef]
- Elboughdiri, N. The use of natural zeolite to remove heavy metals Cu (II), Pb (II) and Cd (II), from industrial wastewater. Cogent Eng. 2020, 7, 1782623. [Google Scholar] [CrossRef]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef]
- Senila, L.; Hoaghia, A.; Moldovan, A.; Török, I.A.; Kovacs, D.; Simedru, D.; Tomoiag, C.H.; Senila, M. The Potential Application of Natural Clinoptilolite-Rich Zeolite as Support for Bacterial Community Formation for Wastewater Treatment. Materials 2022, 15, 3685. [Google Scholar] [CrossRef] [PubMed]
- Popova, N.; Artemiev, G.; Zinicovscaia, I.; Yushin, N.; Demina, L.; Boldyrev, K.; Sobolev, D.; Safonov, A. Biogeochemical Permeable Barrier Based on Zeolite and Expanded Clay for Immobilization of Metals in Groundwater. Hydrology 2022, 10, 4. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Yushin, N.; Grozdov, D.; Abdusamadzoda, D.; Safonov, A.; Rodlovskaya, E. Zinc-Containing Effluent Treatment Using Shewanella xiamenensis Biofilm Formed on Zeolite. Materials 2021, 14, 1760. [Google Scholar] [CrossRef]
- Khosravi, A.; Javdan, M.; Yazdanpanah, G.; Malakootian, M. Removal of heavy metals by Escherichia coli (E. coli) biofilm placed on zeolite from aqueous solutions (case study: The wastewater of Kerman Bahonar Copper Complex). Appl. Water Sci. 2020, 10, 167. [Google Scholar] [CrossRef]
- Silva, B.; Pimentel, C.Z.; Machado, B.; Costa, F.; Tavares, T. Performance of a Combined Bacteria/Zeolite Permeable Barrier on the Rehabilitation of Wastewater Containing Atrazine and Heavy Metals. Processes 2023, 11, 246. [Google Scholar] [CrossRef]
- Monge-Amaya, O.; Valenzuela-García, J.L.; Félix, E.A.; Certucha-Barragan, M.T.; Leal-Cruz, A.L.; Almendariz-Tapia, F.J. Biosorptive Behavior of Aerobic Biomass Biofilm Supported on Clinoptilolite Zeolite for the Removal of Copper. Miner. Process. Extr. Met. Rev. 2013, 34, 422–428. [Google Scholar] [CrossRef]
- Quintelas, C.; Rocha, Z.; Silva, B.; Fonseca, B.; Figueiredo, H.; Tavares, T. Biosorptive performance of an Escherichia coli biofilm supported on zeolite NaY for the removal of Cr(VI), Cd(II), Fe(III) and Ni(II). Chem. Eng. J. 2009, 152, 110–115. [Google Scholar] [CrossRef]
- Gu, J.-N.; Liang, J.; Chen, C.; Li, K.; Zhou, W.; Jia, J.; Sun, T. Treatment of real deplating wastewater through an environmental friendly precipitation-electrodeposition-oxidation process: Recovery of silver and copper and reuse of wastewater. Sep. Purif. Technol. 2020, 248, 117082. [Google Scholar] [CrossRef]
- Grouzdev, D.S.; Safonov, A.V.; Babich, T.L.; Tourova, T.P.; Krutkina, M.S.; Nazina, T.N. Draft Genome Sequence of a Dissimilatory U(VI)-Reducing Bacterium, Shewanella xiamenensis Strain DCB2-1, Isolated from Nitrate- and Radionuclide-Contaminated Groundwater in Russia. Genome Announc. 2018, 6, e00555-18. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yuan, F.; Zeng, G.; Li, X.; Gu, Y.; Shi, L.; Liu, W.; Shi, Y. Influence of pH on heavy metal speciation and removal from wastewater using micellar-enhanced ultrafiltration. Chemosphere 2017, 173, 199–206. [Google Scholar] [CrossRef]
- Wei, W.; Wang, Q.; Li, A.; Yang, J.; Ma, F.; Pi, S.; Wu, D. Biosorption of Pb (II) from aqueous solution by extracellular polymeric substances extracted from Klebsiella sp. J1: Adsorption behavior and mechanism assessment. Sci. Rep. 2016, 6, 31575. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Mishra, R.; Kushwaha, P.; Saha, P. Removal of safranin from aqueous solutions by NaOH-treated rice husk: Thermodynamics, kinetics and isosteric heat of adsorption. Asia-Pac. J. Chem. Eng. 2010, 7, 236–249. [Google Scholar] [CrossRef]
- Omri, A.; Benzina, M. Adsorption characteristics of silver ions onto activated carbon prepared from almond shell. Desalination Water Treat. 2013, 51, 2317–2326. [Google Scholar] [CrossRef]
- Merroun, M.L.; Ben Omar, N.; Alonso, E.; Arias, J.M.; González-Muñoz, M.T. Silver sorption to Myxococcus xanthus biomass. Geomicrobiol. J. 2001, 18, 183–192. [Google Scholar] [CrossRef]
- Wajima, T. Removal of Ag(I) from Aqueous Solution by Japanese Natural Clinoptilolite. Adv. Chem. Eng. Sci. 2016, 6, 470–487. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Cepoi, L.; Chiriac, T.; Mitina, T.; Grozdov, D.; Yushin, N.; Culicov, O. Application of Arthrospira (Spirulina) platensis biomass for silver removal from aqueous solutions. Int. J. Phytoremediat. 2017, 19, 1053–1058. [Google Scholar] [CrossRef]
- Patel, H. Batch and continuous fixed bed adsorption of heavy metals removal using activated charcoal from neem (Azadirachta indica) leaf powder. Sci. Rep. 2020, 10, 16895. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.C.; da Costa, A.C.A.; Henriques, C.A.; Luna, A.S. Kinetic modeling and equilibrium studies during cadmium biosorption by dead Sargassum sp. biomass. Bioresour. Technol. 2003, 91, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Lameiras, S.; Quintelas, C.; Tavares, T. Biosorption of Cr (VI) using a bacterial biofilm supported on granular activated carbon and on zeolite. Bioresour. Technol. 2008, 99, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Chou, W.-L.; Wang, C.-T.; Chang, W.-C.; Chang, S.-Y. Adsorption treatment of oxide chemical mechanical polishing wastewater from a semiconductor manufacturing plant by electrocoagulation. J. Hazard. Mater. 2010, 180, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-G.; Ma, X.-L.; Sun, J.; Huang, M.-R. Powerful Reactive Sorption of Silver(I) and Mercury(II) onto Poly(o-phenylenediamine) Microparticles. Langmuir 2009, 25, 1675–1684. [Google Scholar] [CrossRef]
- Gupta, H.; Gogate, P.R. Intensified removal of copper from waste water using activated watermelon based biosorbent in the presence of ultrasound. Ultrason. Sonochem. 2016, 30, 113–122. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, P.; Kumari, S. Equilibrium studies for copper removal from aqueous solution using nanoadsorbent synthesized from rice husk. SN Appl. Sci. 2019, 1, 988. [Google Scholar] [CrossRef]
- Treto-Suárez, M.A.; Prieto-García, J.O.; Mollineda-Trujillo, Á.; Lamazares, E.; Hidalgo-Rosa, Y.; Mena-Ulecia, K. Kinetic study of removal heavy metal from aqueous solution using the synthetic aluminum silicate. Sci. Rep. 2020, 10, 10836. [Google Scholar] [CrossRef]
- Baldermann, A.; Grießbacher, A.C.; Baldermann, C.; Purgstaller, B.; Letofsky-Papst, I.; Kaufhold, S.; Dietzel, M. Removal of Barium, Cobalt, Strontium, and Zinc from Solution by Natural and Synthetic Allophane Adsorbents. Geosciences 2018, 8, 309. [Google Scholar] [CrossRef]



| Concentration, mg/L | ||||
|---|---|---|---|---|
| System | Ag | Cu | Ni | Zn |
| Ag | 10 ± 0.4 | - | - | - |
| Ag-Cu | 10 ± 0.3 | 5 ± 0.06 | - | - |
| Ag-Cu-Ni-Zn | 10 ± 0.3 | 5 ± 0.06 | 2 ± 0.01 | 2 ± 0.02 |
| Ag | Ag-Cu | Ag-Cu-Ni-Zn | ||||||
|---|---|---|---|---|---|---|---|---|
| Metal | Ag | Ag | Cu | Ag | Cu | Ni | Zn | |
| PFO | qexp, mg/g | 0.72 ± 0.02 | 0.50 ± 0.003 | 0.25 ± 0.03 | 0.52 ± 0.01 | 0.28 ± 0.003 | 0.20 ± 0.004 | 0.19 ± 0.003 |
| qe, mg/g | 0.71 ± 0.01 | 0.50 ± 0.006 | 0.25 ± 0.01 | 0.50 ± 0.01 | 0.28 ± 0.003 | 0.19 ± 0.005 | 0.18 ± 0.004 | |
| k1, min−1 | 0.04 ± 0.004 | 0.08 ± 0.007 | 0.13 ± 0.07 | 0.07 ± 0.008 | 0.17 ± 0.03 | 0.05 ± 0.006 | 0.07 ± 0.007 | |
| R2 | 0.98 | 0.99 | 0.99 | 0.98 | 0.99 | 0.97 | 0.97 | |
| Radj2 | 0.99 | 0.99 | 0.98 | 0.96 | 0.97 | 0.95 | 0.96 | |
| SSE, % | 0.09 | 0.08 | 0.39 | 0.08 | 0.02 | 0.90 | 0.2 | |
| PSO | qe, mg/g | 0.82 ± 0.02 | 0.53 ± 0.01 | 0.25 ± 0.01 | 0.54 ± 0.008 | 0.28 ± 0.005 | 0.21 ± 0.004 | 0.20 ± 0.003 |
| k2, g/mg·min | 0.06 ± 0.001 | 0.03 ± 0.009 | 1.48 ± 0.009 | 0.3 ± 0.002 | 2.7 ± 0.01 | 0.3 ± 0.03 | 0.05 ± 0.05 | |
| R2 | 0.99 | 0.98 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | |
| Radj2 | 0.99 | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 | 0.99 | |
| SSE | 0.16 | 0.18 | 0.51 | 0.10 | 0.01 | 1.0 | 0.2 | |
| Ag | Ag-Cu | Ag-Cu-Ni-Zn | ||
|---|---|---|---|---|
| Langmuir | qm, mg/g | 14.8 ± 0.08 | 32.5 ± 0.5 | 12.8 ± 0.05 |
| b, L/mg | 0.38 ± 0.003 | 0.22 ± 0.003 | 0.50 ± 0.002 | |
| RL | 0.02 | 0.04 | 0.02 | |
| R2 | 0.92 | 0.91 | 0.96 | |
| Radj2 | 0.90 | 0.90 | 0.95 | |
| Freundlich | KF, mg/g | 0.01 ± 0.007 | 0.008 ± 0.0006 | 0.03 ± 0.001 |
| 1/n | 1.39 ± 0.08 | 1.49 ± 0.06 | 1.2 ± 0.01 | |
| R2 | 0.97 | 0.98 | 0.97 | |
| Radj2 | 0.96 | 0.97 | 0.96 |
| Sorbent | qmax, mg/g | Concentrations Range, mg/L | pH | Reference |
|---|---|---|---|---|
| Bio-zeolite | 12.8–32.5 | 10–100 | 6.0 | Present study |
| Fe3O4@SiO2@TiO2 -IIP | 30.55 | 10–300 | 6.0 | [11] |
| Fe3O4@SiO2@TiO2 -NIP | 17.21 | 10–300 | 6.0 | [11] |
| Acidified biosorbent | 2.92 mmol/ g | 10–200 | 5.0 | [1] |
| Waste yeast | 18.9–41.8 | 0–750 | 3.0 | [2] |
| Arthrospira platensis | 31.6 | 5–30 | 3.0 | [40] |
| Poly(o-phenylenediamine) Microparticles | 533 | 1–10 mM | 5.0 | [46] |
| Japanese Natural Clinoptilolite | 0.64 mmol/g | 50 | 4.0 | [39] |
| System | Metal | ∆G°, kJ/mol | ∆H°, kJ/mol | ∆S°, J/mol·K | R2 | |||
|---|---|---|---|---|---|---|---|---|
| 293 K | 303 K | 313 K | 323 K | |||||
| Ag | Ag | −9.3 | −9.6 | −10.0 | −10.3 | 0.4 | 33 | 0.79 |
| Ag-Cu | Ag | −8.8 | −9.1 | −9.5 | −9.8 | 1.2 | 34 | 0.88 |
| Cu | −10.5 | −10.7 | −11.0 | −11.3 | −2.4 | 27.4 | ||
| Ag-Cu-Ni-Zn | Ag | −9.3 | −9.6 | −9.8 | −10.1 | −1.8 | 24.4 | 0.98 |
| Cu | −10.6 | −11.0 | −11.5 | −11.9 | 2.3 | 44.0 | 0.99 | |
| Ni | −9.2 | −9.6 | −10.0 | −10.4 | 2.2 | 30.0 | 0.99 | |
| Zn | −11.8 | −12.1 | −12.5 | −12.9 | −0.5 | 38.2 | 0.98 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zinicovscaia, I.; Yushin, N.; Grozdov, D.; Safonov, A. Application of Shewanella xiamenensis Placed on Zeolite in Treatment of Silver-Containing Effluents. Minerals 2023, 13, 179. https://doi.org/10.3390/min13020179
Zinicovscaia I, Yushin N, Grozdov D, Safonov A. Application of Shewanella xiamenensis Placed on Zeolite in Treatment of Silver-Containing Effluents. Minerals. 2023; 13(2):179. https://doi.org/10.3390/min13020179
Chicago/Turabian StyleZinicovscaia, Inga, Nikita Yushin, Dmitrii Grozdov, and Alexey Safonov. 2023. "Application of Shewanella xiamenensis Placed on Zeolite in Treatment of Silver-Containing Effluents" Minerals 13, no. 2: 179. https://doi.org/10.3390/min13020179
APA StyleZinicovscaia, I., Yushin, N., Grozdov, D., & Safonov, A. (2023). Application of Shewanella xiamenensis Placed on Zeolite in Treatment of Silver-Containing Effluents. Minerals, 13(2), 179. https://doi.org/10.3390/min13020179







