Cadmium Sorption on Alumina Nanoparticles and Mixtures of Alumina and Smectite: An Experimental and Modelling Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Conditions and Materials Used
2.1.1. Sorbents
2.1.2. Adsorbate
2.2. Cadmium Sorption Experiments
2.2.1. Cadmium Sorption Quantification
2.3. Cadmium Sorption Modelling
| Parameter | A | S |
|---|---|---|
| CEC (meq/100 g) | 100 ± 4 [38] | |
| ] (µeq/m2) | 9.5∙10−3 [51] | 3.4∙10−2 [37] |
| ] (µeq/m2) | 1.1 [41] | 1.02 [52] |
| BET (m2/g) | 136 [41] | 59 [38] |
| Species and reactions | A log10 K° | S log10 K° |
| Surface sites acidity constants | ||
| 6.90 [51] | 4.8 [37] | |
| −9.7 [51] | −9.9 [37] | |
| 6.90 [41] | 5.3 [52] | |
| −9.7 [41] | −8.4 [52] | |
| Cation exchange in S | ||
| - | 0.8 § [14] | |
| - | 1.5 § [34] | |
| Cd sorption by surface complexation | ||
| 0.1 * | −1.4 [14] | |
| −2.25 * | −2.51 [14] | |
| 7.9 * | 6.10 [14] | |
| 5.4 * | 4.14 [14] | |
| −12.5 * | −11.66 [14] | |
| −13.2 * | −11.88 [14] | |
| Empirical Cd sorption at low pH | log10 Kd (mL/g) = 2.2 * | - |
| Anion sorption on A | ||
| 11.50 [41] | - | |
| 9.2 [49] | - | |
| 8.5 [41] | - |
3. Results and Discussion
3.1. Cadmium Sorption on Alumina Nanoparticles: Experiments and Modelling
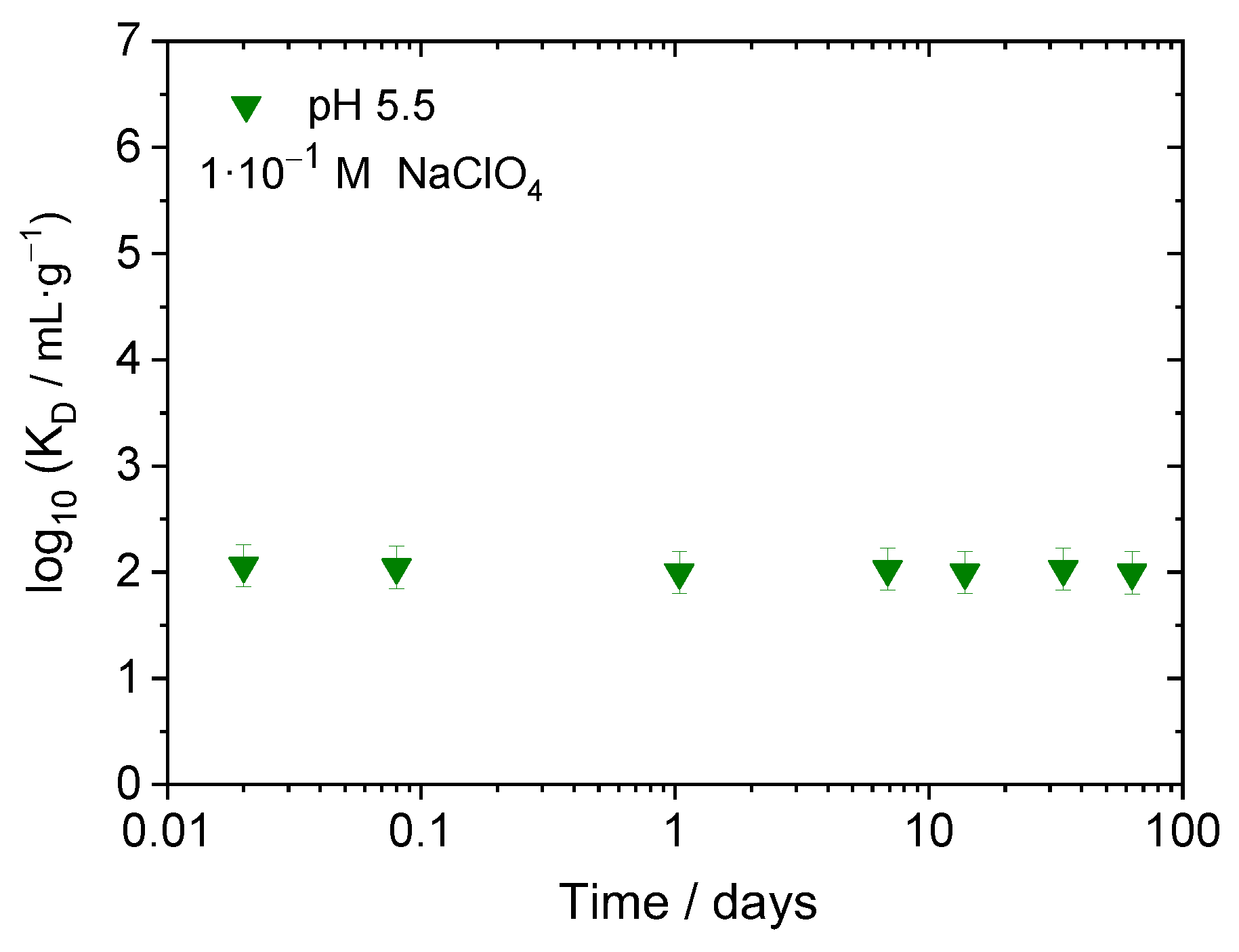
3.1.1. Effect of Time
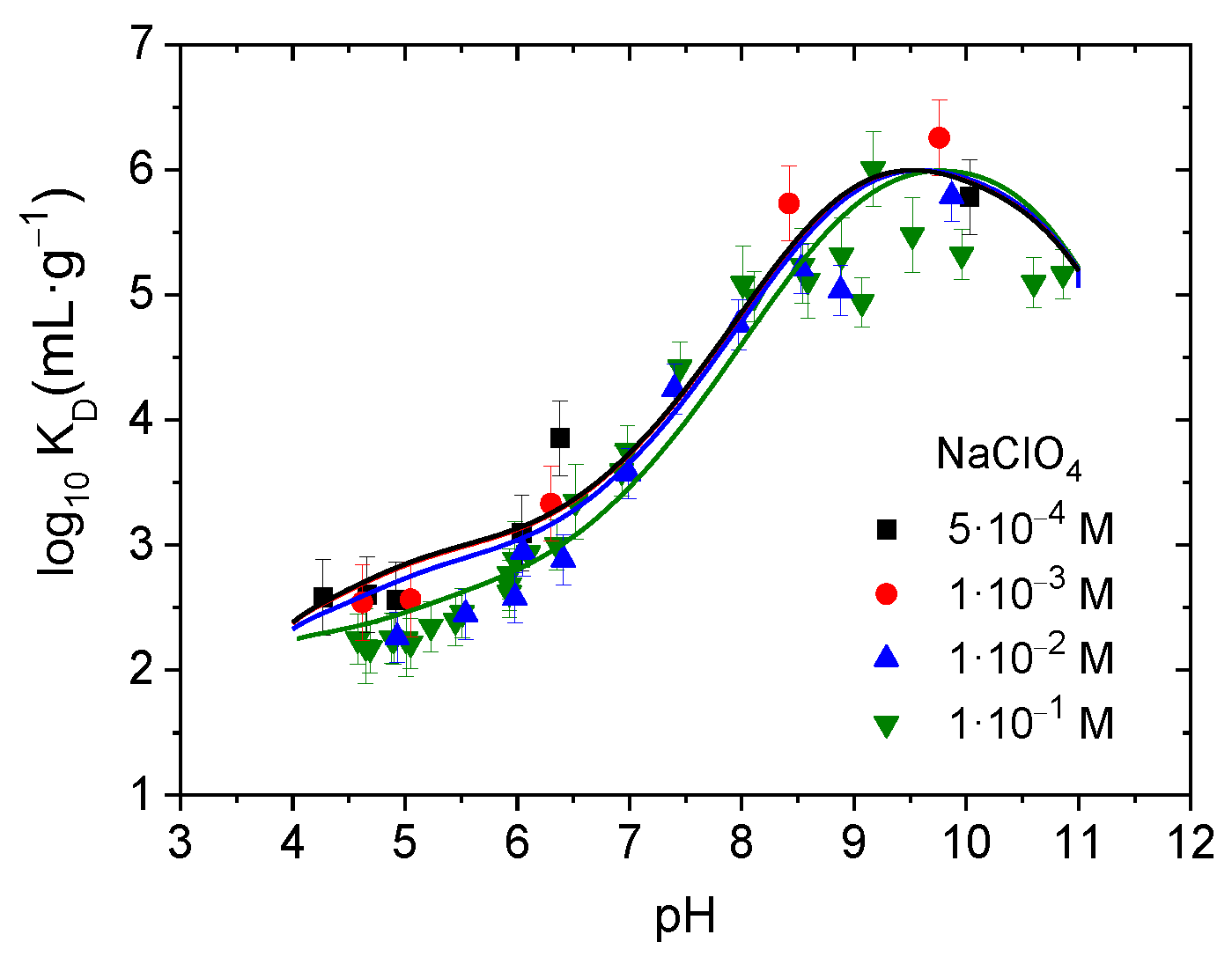
3.1.2. Effect of pH, Ionic Strength, Chloride Concentration, and Cadmium Concentration
3.1.3. Cd Sorption Model on Alumina
3.2. Cadmium Sorption on Mixtures of Alumina Nanoparticles and Smectite: Experiments and Modelling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agents Classified by the IARC Monographs Volume 1–111. Available online: http://monographs.iarc.fr/ENG/Classification/ClassificationsAlphaOrder.pdf (accessed on 10 December 2016).
- Nordberg, G.F. Historical perspectives on cadmium toxicology. Toxicol. Appl. Pharmacol. 2009, 238, 192–200. [Google Scholar] [CrossRef]
- Nordberg, G.F.; Nogawa, K.; Nordberg, M. Handbook on the Toxicology of Metals, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780444594532. [Google Scholar]
- WHO. Cadmium in Drinking-Water; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Mashhadikhan, S.; Amooghin, A.E.; Sanaeepur, H.; Shirazi, M.M.A. A critical review on cadmium recovery from wastewater towards environmental sustainability. Desalination 2022, 535, 115815. [Google Scholar] [CrossRef]
- Rao, K.S.; Mohapatra, M.; Anand, S.; Venkateswarlu, P. Review on cadmium removal from aqueous solutions. Int. J. Eng. Sci. Technol. 2010, 2, 81–103. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Naidu, R. Cadmium sorption and desorption in soils: A review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 489–533. [Google Scholar] [CrossRef]
- Padmaja, M.; Bhavani, R.; Pamila, R. Adsorption of Cadmium from Aqueous Solutions Using Low cost Materials-A Review. Int. J. Eng. Technol. 2018, 7, 26. [Google Scholar] [CrossRef]
- Mousavi, H.Z.; Asghari, A. Removal of Heavy Metal Ions in Wastewater by Semnan Natural Zeolite. Asian J. Chem. 2009, 21, 2881–2886. [Google Scholar]
- Chen, C.; Liu, H.; Chen, T.; Chen, D.; Frost, R.L. An insight into the removal of Pb(II), Cu(II), Co(II), Cd(II), Zn(II), Ag(I), Hg(I), Cr(VI) by Na(I)-montmorillonite and Ca(II)-montmorillonite. Appl. Clay Sci. 2015, 118, 239–247. [Google Scholar] [CrossRef]
- Glatstein, D.A.; Francisca, F.M. Influence of pH and ionic strength on Cd, Cu and Pb removal from water by adsorption in Na-bentonite. Appl. Clay Sci. 2015, 118, 61–67. [Google Scholar] [CrossRef]
- Franco, F.; Benítez-Guerrero, M.; Gonzalez-Triviño, I.; Pérez-Recuerda, R.; Assiego, C.; Cifuentes-Melchor, J.; Pascual-Cosp, J. Low-cost aluminum and iron oxides supported on dioctahedral and trioctahedral smectites: A comparative study of the effectiveness on the heavy metal adsorption from water. Appl. Clay Sci. 2016, 119, 321–332. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, X.; Sun, K.; Zhao, Y.; Lin, C. Sorption of tetracycline to sediments and soils: Assessing the roles of pH, the presence of cadmium and properties of sediments and soils. Front. Environ. Sci. Eng. China 2010, 4, 421–429. [Google Scholar] [CrossRef]
- Missana, T.; Alonso, U.; Mayordomo, N.; García-Gutiérrez, M. Analysis of Cadmium Retention Mechanisms by a Smectite Clay in the Presence of Carbonates. Toxics 2023, 11, 130. [Google Scholar] [CrossRef]
- Kumar, R.; Chawla, J. Removal of Cadmium Ion from Water/Wastewater by Nano-metal Oxides: A Review. Water Qual. Expo. Health 2014, 5, 215–226. [Google Scholar] [CrossRef]
- Irshad, M.A.; Nawaz, R.; Wojciechowska, E.; Mohsin, M.; Nawrot, N.; Nasim, I.; Hussain, F. Application of Nanomaterials for Cadmium Adsorption for Sustainable Treatment of Wastewater: A Review. Water Air Soil Pollut. 2023, 234, 54. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, V.; Sharma, K.; Kumar, V.; Choudhary, S.; Mankotia, P.; Kumar, B.; Mishra, H.; Moulick, A.; Ekielski, A.; et al. A review of adsorbents for heavy metal decontamination: Growing approach to wastewater treatment. Materials 2021, 14, 4702. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Siebecker, M.G.; Gou, W.; Li, L.; Li, W. A review of cadmium sorption mechanisms on soil mineral surfaces revealed from synchrotron-based X-ray absorption fine structure spectroscopy: Implications for soil remediation. Pedosphere 2021, 31, 11–27. [Google Scholar] [CrossRef]
- Tomas, R.; Visic, M.; Tominic, I.; Sokol, V. Determination of Stability Constants of Chlorocadmium Complexes in Water from Electromotive Force Measurements. Croat. Chem. Acta 2001, 74, 91–101. [Google Scholar]
- Undabeytia, T.; NIr, S.; Rytwo, G.; Morillo, E.; Maqueda, C. Modeling adsorption-desorption processes of Cd on montmorillonite. Clays Clay Miner. 1998, 46, 423–428. [Google Scholar] [CrossRef]
- Kantar, C.; Ikizoglu, G.; Koleli, N.; Kaya, O. Modeling Cd(II) adsorption to heterogeneous subsurface soils in the presence of citric acid using a semi-empirical surface complexation approach. J. Contam. Hydrol. 2009, 110, 100–109. [Google Scholar] [CrossRef]
- Di Leo, P.; O’Brien, P. Nuclear Magnetic Resonance (NMR) study of Cd2+ sorption onto montmorillonite. Clays Clay Miner. 1999, 47, 761–768. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, S.; Yang, S.; Yang, X.; Yang, S. Investigation of the sorption behavior of Cd(II) on GMZ bentonite as affected by solution chemistry. Chem. Eng. J. 2011, 166, 1010–1016. [Google Scholar] [CrossRef]
- Saeki, K.; Kunito, T. Influence of chloride ions on cadmium adsorptions by oxides, hydroxides, oxyhydroxides, and phyllosilicates. Appl. Clay Sci. 2012, 62–63, 58–62. [Google Scholar] [CrossRef]
- Taylor, P.; Lumsdon, D.G.; Evans, L.J.; Bolton, K.A. The influence of pH and chloride on the retention of cadmium, lead, mercury, and zinc by soils. Soil Sediment Contam. 1995, 4, 137–150. [Google Scholar]
- Blom, B.E. Sorption of Cadmium by Soils; National Technical Information Service: Springfield, VA, USA, 1974. [Google Scholar]
- El-Hefnawy, M.E.; Selim, E.M.; Assaad, F.F.; Ismail, A.I. The effect of chloride and sulfate ions on the adsorption of Cd2+ on clay and sandy loam egyptian soils. Sci. World J. 2014, 2014, 806252. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Fang, L.; Li, X.; Liu, T.; Wang, P.; Gao, R.; Chen, G.; Yin, H.; Yang, Y.; Huang, F.; et al. Roles of Chloride and Sulfate Ions in Controlling Cadmium Transport in a Soil-Rice System as Evidenced by the Cd Isotope Fingerprint. Environ. Sci. Technol. 2023, 57, 17920–17929. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liu, Y.; Li, T.; Zhao, H.; Alessi, D.S.; Liu, W.; Konhauser, K.O. Cadmium adsorption to clay-microbe aggregates: Implications for marine heavy metals cycling. Geochim. Cosmochim. Acta 2020, 290, 124–136. [Google Scholar] [CrossRef]
- Yang, K.; Cheng, Z.; Luo, W.; Guo, B.; Zhang, B. Adsorption performance and mechanisms of MgO-modified palygorskite/biochar composite for aqueous Cd (II): Experiments and theoretical calculation. Appl. Surf. Sci. 2023, 638, 157965. [Google Scholar] [CrossRef]
- Yu, W.; Chu, C.; Chen, B. Pyrogenic Carbon Improves Cd Retention during Microbial Transformation of Ferrihydrite under Varying Redox Conditions. Environ. Sci. Technol. 2023, 57, 7875–7885. [Google Scholar] [CrossRef] [PubMed]
- Safonov, A.; Popova, N.; Andrushenko, N.; Boldyrev, K.; Yushin, N.; Zinicovscaia, I. Investigation of materials for reactive permeable barrier in removing cadmium and chromium(VI) from aquifer near a solid domestic waste landfill. Environ. Sci. Pollut. Res. 2021, 28, 4645–4659. [Google Scholar] [CrossRef] [PubMed]
- Lützenkirchen, J.; Behra, P. A new approach for modelling potential effects in cation adsorption onto binary (hydr) oxides. J. Contam. Hydrol. 1997, 26, 257–268. [Google Scholar] [CrossRef]
- Mayordomo, N.; Alonso, U.; Missana, T. Effects of γ-alumina nanoparticles on strontium sorption in smectite: Additive model approach. Appl. Geochem. 2019, 100, 121–130. [Google Scholar] [CrossRef]
- Mayordomo, N.; Rodríguez, D.M.; Schild, D.; Molodtsov, K.; Johnstone, E.V.; Hübner, R.; Shams Aldin Azzam, S.; Brendler, V.; Müller, K. Technetium retention by gamma alumina nanoparticles and the effect of sorbed Fe2+. J. Hazard. Mater. 2020, 388, 122066. [Google Scholar] [CrossRef] [PubMed]
- Missana, T.; Garcia-Gutierrez, M.; Alonso, U. Sorption of strontium onto illite/smectite mixed clays. Phys. Chem. Earth 2008, 33, S156–S213. [Google Scholar] [CrossRef]
- Missana, T.; Garcia-Gutierrez, M. Adsorption of bivalent ions (Ca(II), Sr(II) and Co(II)) onto FEBEX bentonite. Phys. Chem. Earth 2007, 32, 55–567. [Google Scholar] [CrossRef]
- Missana, T.; Alonso, U.; García-Gutiérrez, M. Evaluation of component additive modelling approach for europium adsorption on 2:1 clays: Experimental, thermodynamic databases, and models. Chemosphere 2021, 272, 129877. [Google Scholar] [CrossRef]
- Missana, T.; Benedicto, A.; García-Gutiérrez, M.; Alonso, U. Modeling cesium retention onto Na-, K- and Ca-smectite: Effects of ionic strength, exchange and competing cations on the determination of selectivity coefficients. Geochim. Cosmochim. Acta 2014, 128, 266–277. [Google Scholar] [CrossRef]
- Missana, T.; Alonso, U.; García-Gutiérrez, M. Experimental study and modelling of selenite sorption onto illite and smectite clays. J. Colloid Interface Sci. 2009, 334, 132–138. [Google Scholar] [CrossRef]
- Missana, T.; Benedicto, A.; Mayordomo, N.; Alonso, U. Analysis of anion adsorption effects on alumina nanoparticles stability. Appl. Geochem. 2014, 49, 68–76. [Google Scholar] [CrossRef]
- Cuevas, J.; Cabrera, M.Á.; Fernández, C.; Mota-Heredia, C.; Fernández, R.; Torres, E.; Turrero, M.J.; Ruiz, A.I. Bentonite Powder XRD Quantitative Analysis Using Rietveld Refinement: Revisiting and Updating Bulk Semiquantitative Mineralogical Compositions. Minerals 2022, 12, 772. [Google Scholar] [CrossRef]
- Huertas, F.; Fuentes-Santillana, J.L.; Jullien, F.; Rivas, P.; Linares, J.; Fariña, P.; Ghoreychi, M.; Jockwer, N.; Kickmaier, W.; Martínez, M.; et al. FEBEX Project Final Report. EUR 19147; European Commission: Madrid, Spain, 2000. [Google Scholar]
- Van der Lee, J.; de Wint, L. Chess Tutorial and Cookbook; Technical Report LHM/RD/99/05; École des Mines de Paris: Fountainbleau, Paris, 1999. [Google Scholar]
- Powell, K.J.; Brown, P.L.; Byrne, R.H.; Gajda, T.; Hefter, G.; Sjöberg, S.; Wanner, H. Chemical speciation of environmentally significant metals with inorganic ligands Part 4: The Cd2+ + OH−, Cl−, CO32−, SO42− and PO43− systems. Int. Union Pure Appl. Chem. 2011, 83, 1163–1214. [Google Scholar] [CrossRef]
- Delany, J.M.; Lundeen, S.R. The LLNL Thermochemical Data Base—Revised Data and File Format for the EQ3/6 Package; Lawrence Livermore National Lab.: Livermore, CA, USA, 1991. [Google Scholar]
- Rodríguez, D.M.; Mayordomo, N.; Schild, D.; Shams Aldin Azzam, S.; Brendler, V.; Müller, K.; Stumpf, T. Reductive immobilization of 99Tc(VII) by FeS2: The effect of marcasite. Chemosphere 2021, 281, 130904. [Google Scholar] [CrossRef]
- Lagaly, G.; Imre, D. Colloid Clay Science. In Handbook of Clay Science; Bergara, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Nederlands, 2013; pp. 244–328. ISBN 978-0-08-098258-8. [Google Scholar]
- Mayordomo, N.; Alonso, U.; Missana, T. Analysis of the improvement of selenite retention in smectite by adding alumina nanoparticles. Sci. Total Environ. 2016, 572, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, M.H.; Baeyens, B. Sorption by Cation Exchange Incorporation of a Cation Exchange Model into Geochemical Computer Codes; IAEA: Vienna, Austria, 1994. [Google Scholar]
- Mayordomo, N. Experimental and Theoretical Studies of Mixed Smectite and Al2O3 Nanoparticles to Improve Pollutant Retention in Geochemical Barriers; Universidad de Alcalá: Alcalá de Henares, Spain, 2017. [Google Scholar]
- Missana, T.; Garcia-Gutierrez, M.; Fernandez, V.; Gil, P. Application of mechanistic models for the interpretation of radionuclides sorption in clays. In Part 1. CIEMAT Technical Report 2002 CIEMAT/DIAE/54610/03; CIEMAT: Madrid, Spain, 2002; p. 4. [Google Scholar]
- Sen, T.K.; Sarzali, M.V. Removal of cadmium metal ion (Cd2+) from its aqueous solution by aluminium oxide (Al2O3): A kinetic and equilibrium study. Chem. Eng. J. 2008, 142, 256–262. [Google Scholar] [CrossRef]
- Sposito, G. Chemical Equilibrium and Kinetics in Soils, 1st ed.; Oxford University Press: New York, NY, USA, 1994; ISBN 0-19-507564-1. [Google Scholar]
- Dzombak, D.A.; Morel, F.M.M. Surface Complexation Modeling. Hydrous Ferric Oxide, 1st ed.; John Wiley and Sons: Cambridge, MA, USA, 1990. [Google Scholar]
- Naiya, T.K.; Bhattacharya, A.K.; Das, S.K. Adsorption of Cd(II) and Pb(II) from aqueous solutions on activated alumina. J. Colloid Interface Sci. 2009, 333, 14–26. [Google Scholar] [CrossRef]
- Floroiu, R.M.; Davis, A.P.; Torrents, A. Cadmium adsorption on aluminum oxide in the presence of polyacrylic acid. Environ. Sci. Technol. 2001, 35, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Mayordomo, N.; Foerstendorf, H.; Lützenkirchen, J.; Heim, K.; Weiss, S.; Alonso, Ú.; Missana, T.; Schmeide, K.; Jordan, N. Selenium(IV) sorption onto γ-Al2O3: A consistent description of the surface speciation by spectroscopy and thermodynamic modeling. Environ. Sci. Technol. 2018, 52, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, E.J. Formation of layered Fe(II)-Al(III)-hydroxides during reaction of Fe(II) with aluminum oxide. Environ. Sci. Technol. 2012, 46, 4894–4901. [Google Scholar] [CrossRef]
- Forano, C.; Costantino, U.; Prévot, V.; Gueho, C.T. Layered double hydroxides (LDH). In Developments in Clay Science; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 5, pp. 745–782. ISBN 9780080982588. [Google Scholar]
- Papelis, C.; Brown, G.E.; Parks, G.A.; Leckie, J.O. X-ray Absorption Spectroscopic Studies of Cadmium and Selenite Adsorption on Aluminum Oxides. Langmuir 1995, 11, 2041–2048. [Google Scholar] [CrossRef]
- Vasconcelos, I.F.; Haack, E.A.; Maurice, P.A.; Bunker, B.A. EXAFS analysis of cadmium(II) adsorption to kaolinite. Chem. Geol. 2008, 249, 237–249. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, C.; Cui, P.; Fan, T.; Zhu, M.; Alves, M.E.; Siebecker, M.G.; Sparks, D.L.; Wu, T.; Li, W.; et al. Formation of Cd precipitates on g-Al2O3: Implications for Cd sequestration in the environment. Environ. Int. 2019, 126, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Boily, J.-F.; Fein, J.B. Experimental study of cadmium-citrate co-adsorption onto α-Al2O3. Geochim. Cosmochim. Acta 1996, 60, 2929–2938. [Google Scholar] [CrossRef]
- Warne, M.; Rayment, G.; Brent, P.; Drew, N.; Klim, E.; McLaughlin, M.; Milham, P.; Shelley, B.; Stevens, D.; Sparrow, L. Final Report of the National Cadmium Management Commitee (NCMC) (2000–2006); National Library of Australia: Sidney, Australia, 2007. [Google Scholar]
- Wilkins, B.J.; Brummel, N.; Loch, J.P.G. Influence of pH and zinc concentration on cadmium sorption in acid, sandy soils. Water. Air. Soil Pollut. 1998, 101, 349–362. [Google Scholar] [CrossRef]
- García-Miragaya, J.; Cardenas, R.; Page, A. Surface loading effect on Cd and Zn sorption by kaolinite and montmorillonite from low concentration solutions. Water Air Soil Pollut. 1986, 27, 181–190. [Google Scholar] [CrossRef]
- Karamalidis, A.K.; Dzombak, D. Surface Complexation Modeling Gibbsite, 1st ed.; Wiley: Hoboken, NJ, USA, 2010; ISBN 978-0-470-58768-3. [Google Scholar]
- Dzombak, D.; Morel, F.M. Sorption of cadmium on hydrous ferric oxide at high sorbate/sorbent ratios: Equilibrium, kinetics, and modeling. J. Colloid Interface Sci. 1986, 112, 588–598. [Google Scholar] [CrossRef]
- Schulthess, C.P.; Sparks, D.L. Two-site Model for Aluminum Oxide with Mass Balanced Competitive pH + Salt/Salt Dependent Reactions. Soil Sci. Soc. Am. J. 1987, 51, 1136–1144. [Google Scholar] [CrossRef]
- Hiemstra, T.; Van Riemsdijk, W.H.; Bolt, G.H. Multisite proton adsorption modeling at the solid/solution interface of (hydr) oxides: A new approach: II. Application to various important (hydro)oxides. J. Colloid Interface Sci. 1989, 133, 105–117. [Google Scholar] [CrossRef]
- Hiemstra, T.; Van Riemsdijk, W.H.; Bolt, G.H. Multisite proton adsorption modeling at the solid/solution interface of (hydr)oxides: A new approach. I. Model description and evaluation of intrinsic reaction constants. J. Colloid Interface Sci. 1989, 133, 91–104. [Google Scholar] [CrossRef]
- Rabung, T.; Stumpf, T.; Geckeis, H.; Klenze, R.; Kim, J.I. Sorption of Am (III) and Eu (III) onto γ-alumina: Experiment and modelling. Radiochim. Acta 2000, 716, 711–716. [Google Scholar] [CrossRef]
- Koju, N.K.; Song, X.; Wang, Q.; Hu, Z.; Colombo, C. Cadmium removal from simulated groundwater using alumina nanoparticles: Behaviors and mechanisms. Environ. Pollut. 2018, 240, 255–266. [Google Scholar] [CrossRef]
- Kosmulski, M. Adsorption of cadmium on alumina and silica: Analysis of the values of stability constants of surface complexes calculated for different parameters of triple layer model. Colloids Surf. A Physicochem. Eng. Asp. 1996, 117, 201–214. [Google Scholar] [CrossRef]
- Asencios, Y.J.O.; Sun-Kou, M.R. Synthesis of high-surface-area γ-Al2O3 from aluminum scrap and its use for the adsorption of metals: Pb(II), Cd(II) and Zn(II). Appl. Surf. Sci. 2012, 258, 10002–10011. [Google Scholar] [CrossRef]
- Kosma, C.; Balomenou, G.; Salahas, G.; Deligiannakis, Y. Electrolyte ion effects on Cd2+ binding at Al2O3 surface: Specific synergism versus bulk effects. J. Colloid Interface Sci. 2009, 331, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Plavšić, M.; Ćosović, B. Voltammetric study of the role of organic acids on the sorption of Cd and Cu ions by alumina particles. Colloids Surf. A Physicochem. Eng. Asp. 1999, 151, 189–200. [Google Scholar] [CrossRef]
- Stietiya, M.H.; Wang, J.J. Zinc and cadmium adsorption to aluminum oxide nanoparticles affected by naturally occurring ligands. J. Environ. Qual. 2014, 43, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Shiao, S.Y.; Egozy, Y.; Meyer, R.E. Adsorption of Cs(I), Sr(II), Eu(III), Co(II) and Cd(II) by Al2O3. J. Inorg. Nucl. Chem. 1981, 43, 3309–3315. [Google Scholar] [CrossRef]
- Thoenen, T.; Hummel, W.; Berner, U.; Curti, E. The PSI/Nagra Chemical Thermodynamic Database 12/07; PSI Bericht Nr. 14-04; Paul Scherrer Institute: Villigen, Switzerland, 2014. [Google Scholar]
- Barin, I. Thermochemical Data of Pure Substances, 3rd ed.; Wiley: Weinheim, Germany, 1995; ISBN 9783527619825. [Google Scholar]
- Chen, Q.; Zeng, W.; Chen, X.; Gu, S.; Yang, G.; Zhou, H.; Yin, Z. Investigation of the thermodynamic properties of γ-Al2O3. Termochimica Acta 1995, 253, 33–39. [Google Scholar] [CrossRef]
- Kersting, A.B.; Efurd, D.W.; Finnegan, D.L.; Rokop, D.J.; Smith, D.K.; Thompson, J.L. Migration of plutonium in ground water at the Nevada Test Site. Nature 1999, 397, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Novikov, A.P.; Kalmykov, S.N.; Utsunomiya, S.; Ewing, R.C.; Horreard, F.; Merkulov, A.; Clark, S.B.; Tkachev, V.V.; Myasoedov, B.F. Colloid transport of plutonium in the far-field of the Mayak Production Association, Russia. Science 2006, 314, 638–641. [Google Scholar] [CrossRef]
- Schaller, M.S.; Koretsky, C.M.; Lund, T.J.; Landry, C.J. Surface complexation modeling of Cd(II) adsorption on mixtures of hydrous ferric oxide, quartz and kaolinite. J. Colloid Interface Sci. 2009, 339, 302–309. [Google Scholar] [CrossRef]
- Alessi, D.S.; Fein, J.B. Cadmium adsorption to mixtures of soil components: Testing the component additivity approach. Chem. Geol. 2010, 270, 186–195. [Google Scholar] [CrossRef]
- Mustafa, S.; Waseem, M.; Naeem, A.; Shah, K.; Ahmad, T.; Hussain, S.Y. Selective sorption of cadmium by mixed oxides of iron and silicon. Chem. Eng. J. 2010, 157, 18–24. [Google Scholar] [CrossRef]
- Anderson, P.R.; Benjamin, M.M. Surface and Bulk Characteristics of Binary Oxide Suspensions. Environ. Sci. Technol. 1990, 24, 692–698. [Google Scholar] [CrossRef]
- Lövgren, L.; Sjöberg, S.; Schindler, P.W. Acid/base reactions and Al(III) complexation at the surface of goethite. Geochim. Cosmochim. Acta 1990, 54, 1301–1306. [Google Scholar] [CrossRef]

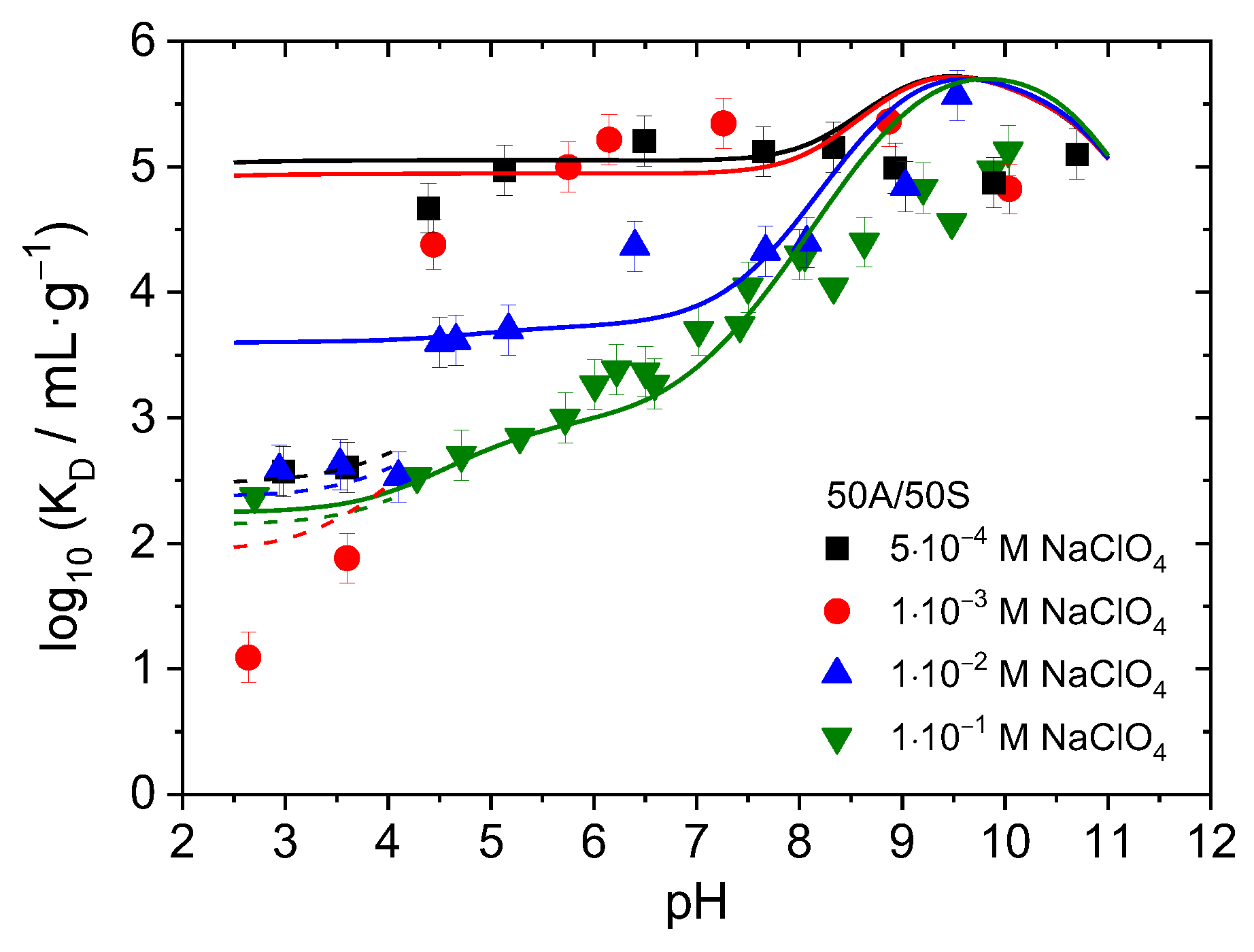
 ) 1.0∙10−2 M at pH 4.1, and (
) 1.0∙10−2 M at pH 4.1, and ( ) 1.0∙10−1 M at pH 8.0. Empty plots and colored lines represent the Cd sorption on A/S mixtures calculated using the data collected in Table 5 and including 1 mg/L of Al3+ for 10% A mixture and 2 mg/L of Al3+ for the rest of A/S mixtures. [A/S]total = 0.5 g/L, S, contact time = 7 days, and [Cd]0 = 4.8∙10−8 M.
) 1.0∙10−1 M at pH 8.0. Empty plots and colored lines represent the Cd sorption on A/S mixtures calculated using the data collected in Table 5 and including 1 mg/L of Al3+ for 10% A mixture and 2 mg/L of Al3+ for the rest of A/S mixtures. [A/S]total = 0.5 g/L, S, contact time = 7 days, and [Cd]0 = 4.8∙10−8 M.
 ) 1.0∙10−2 M at pH 4.1, and (
) 1.0∙10−2 M at pH 4.1, and ( ) 1.0∙10−1 M at pH 8.0. Empty plots and colored lines represent the Cd sorption on A/S mixtures calculated using the data collected in Table 5 and including 1 mg/L of Al3+ for 10% A mixture and 2 mg/L of Al3+ for the rest of A/S mixtures. [A/S]total = 0.5 g/L, S, contact time = 7 days, and [Cd]0 = 4.8∙10−8 M.
) 1.0∙10−1 M at pH 8.0. Empty plots and colored lines represent the Cd sorption on A/S mixtures calculated using the data collected in Table 5 and including 1 mg/L of Al3+ for 10% A mixture and 2 mg/L of Al3+ for the rest of A/S mixtures. [A/S]total = 0.5 g/L, S, contact time = 7 days, and [Cd]0 = 4.8∙10−8 M.
| Experiment | Kinetics | pH Effect | Isotherm |
|---|---|---|---|
| Solid to liquid ratio of A | 0.5 g/L | 0.5 g/L | 0.5 g/L |
| [Cd2+]0 | 2·10−9 M | 4.6·10−8 M, 1·10−5 M | 1·10−10 M–1·10−3 M |
| pH | 5.5 | 3.0–11.0 | 9.8, 8.9, 6.1/7.2 |
| [Cl−] | 4·10−9 M | 0 M, 10−8 M, 10−3 M | 1·10−3 M |
| Contact time | 30 min–64 days | 7 days | 7 days |
| Ionic strength NaClO4 | 1·10−1 M | 5·10−4 M–10−1 M | 1·10−1 M/1·10−3 M |
| Experiment | Ionic Strength Effect |
|---|---|
| Solid to liquid ratio of S | 0.5 g/L |
| [Cd2+]0 | 4.6·10−8 M |
| pH | 4.5 |
| [Cl−] | 1·10−3 M |
| Ionic strength NaClO4 | 5·10−4 M–10−1 M |
| Experiment | pH Dependency | Mixture Composition | |
|---|---|---|---|
| A/S mixtures (wt.%) | 80A/20S | 50A/50S | (100A → 100S) |
| Total solid to liquid ratio | 0.5 g/L | 0.5 g/L | 0.5 g/L |
| [Cd2+]0 | 4.8·10−8 M | 4.8·10−8 M | 4.8·10−8 M |
| pH | 3.0–11.0 | 3.0–12.0 | 4.3/8.0 |
| Ionic strength NaClO4 | 1·10−1 M | 5·10−4 M–1·10−1 M | 1·10−2 M/1·10−1 M |
| [Cl−] | 1·10−3 M | 1·10−3 M | 1·10−3 M |
| Species | Code Definition | log10 K° |
|---|---|---|
| Aqueous | ||
| Cd2+ | Basis species | |
| CdCl+ | 1 Cd[2+], 1 Cl[−] | 1.98 |
| CdCl2 (aq) | 1 Cd[2+], 2 Cl[−] | 2.64 |
| CdCl3− | 1 Cd[2+], 3 Cl[−] | 2.30 |
| CdCl(OH) (aq) | 1 Cd[2+], 1 Cl[−], 1 H2O, −1 H[+] | −7.4328 * |
| CdHCO3+ | 1 Cd[2+], 1 HCO3− | 1.50 * |
| CdCO3 (aq) | 1 Cd[2+], 1 HCO3−, −1 H[+] | −5.9288 |
| Cd(CO3)22− | 1 Cd[2+], 2 HCO3−, −2 H[+] | −14.4576 |
| CdOH+ | 1 Cd[2+], 1 H2O, −1 H[+] | −9.91 |
| Cd(OH)2 (aq) | 1 Cd[2+], 2 H2O, −2 H[+] | −20.19 |
| Cd(OH)3− | 1 Cd[2+], 3 H2O, −3 H[+] | −33.50 |
| Cd(OH)42− | 1 Cd[2+], 4 H2O, −4 H[+] | −47.28 |
| Cd2OH3+ | 2 Cd[2+], 1 H2O, −1 H[+] | −8.73 |
| Cd4(OH)44+ | 4 Cd[2+], 4 H2O, −4 H[+] | −31.8 |
| Solid | ||
| Cd (s) | 1 Cd[2+], 1 H2O, −2 H[+], −0.5 O2(aq) | −56.6 |
| CdCl2 (s) | 1 Cd[2+], 2 Cl[−] | 0.674 * |
| CdCl2:H2O (s) | 1 Cd[2+], 2 Cl[−], 1 H2O | 1.6747 * |
| CdCl(OH) (s) | 1 Cd[2+], 1 Cl[−], 1 H2O, −1 H[+] | −3.543 |
| Otavite (CdCO3 (s)) | 1 Cd[2+], 1 HCO3−, −1 H[+] | −0.1 ** |
| Cd(OH)2 (s) | 1 Cd[2+], 2 H2O, −2 H[+] | −13.72 * |
| Monteponite (CdO (s)) | 1 Cd[2+], 1 H2O, −2 H[+] | −15.097 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayordomo, N.; Missana, T.; Alonso, U. Cadmium Sorption on Alumina Nanoparticles and Mixtures of Alumina and Smectite: An Experimental and Modelling Study. Minerals 2023, 13, 1534. https://doi.org/10.3390/min13121534
Mayordomo N, Missana T, Alonso U. Cadmium Sorption on Alumina Nanoparticles and Mixtures of Alumina and Smectite: An Experimental and Modelling Study. Minerals. 2023; 13(12):1534. https://doi.org/10.3390/min13121534
Chicago/Turabian StyleMayordomo, Natalia, Tiziana Missana, and Ursula Alonso. 2023. "Cadmium Sorption on Alumina Nanoparticles and Mixtures of Alumina and Smectite: An Experimental and Modelling Study" Minerals 13, no. 12: 1534. https://doi.org/10.3390/min13121534
APA StyleMayordomo, N., Missana, T., & Alonso, U. (2023). Cadmium Sorption on Alumina Nanoparticles and Mixtures of Alumina and Smectite: An Experimental and Modelling Study. Minerals, 13(12), 1534. https://doi.org/10.3390/min13121534