Ultrasonic and Microstructural Evaluation of Sulphide-Rich Tailings Cemented Paste Backfill Properties Containing Alkali-Activated Slag: Effect of Slag Fineness
Abstract
:1. Introduction
2. Materials Characterisation and Experimental Studies
2.1. Characterisation of Tailings and Binders
2.2. Preparation of the CPBs and Strength Tests
2.3. The pH and Sulphate Monitoring
2.4. Microstructural Investigation
2.5. Ultrasonic P-wave Velocity Testing
3. Results and Discussion
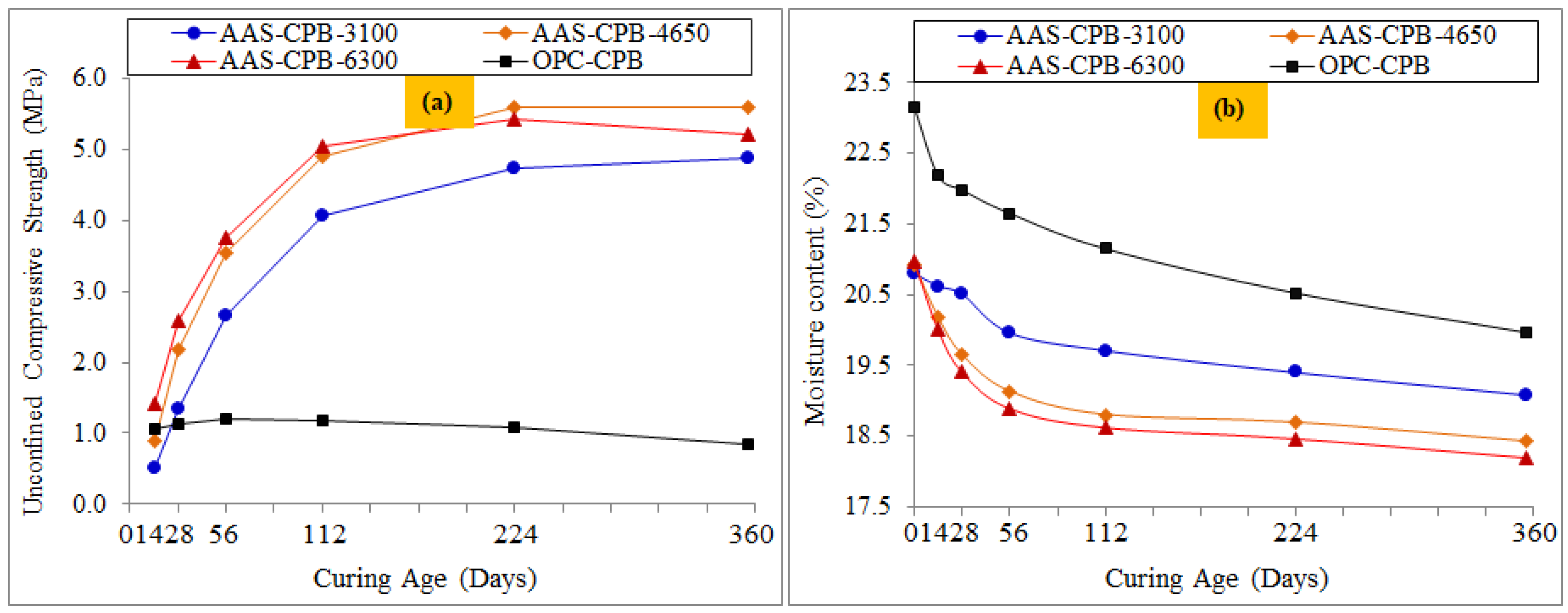
3.1. Effect of the Slag Fineness on the Strength Evolution and Durability Properties of the CPB
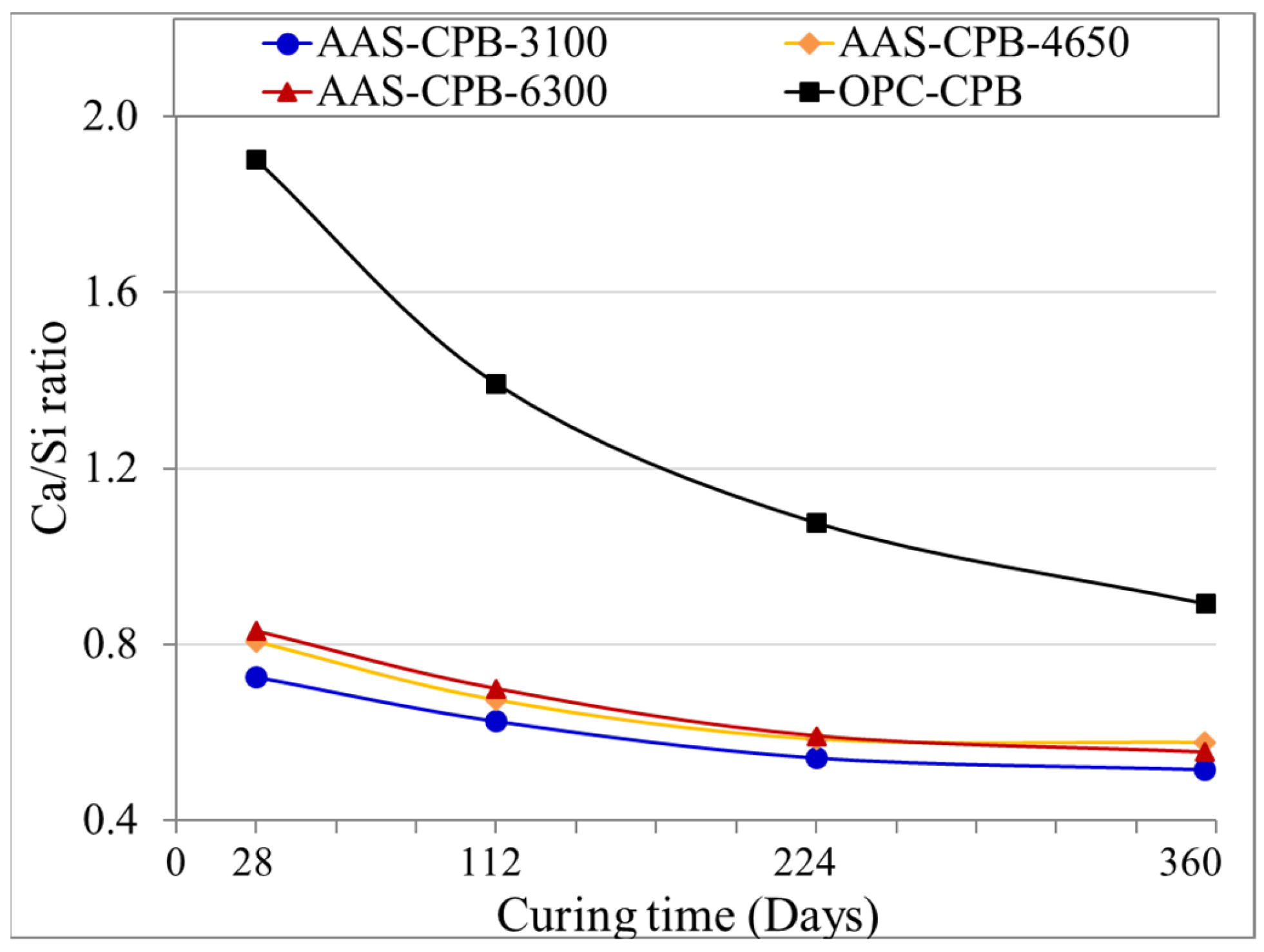
3.2. Effect of the Slag Fineness on the pH and the Sulphate Concentration Evolution of the CPBs
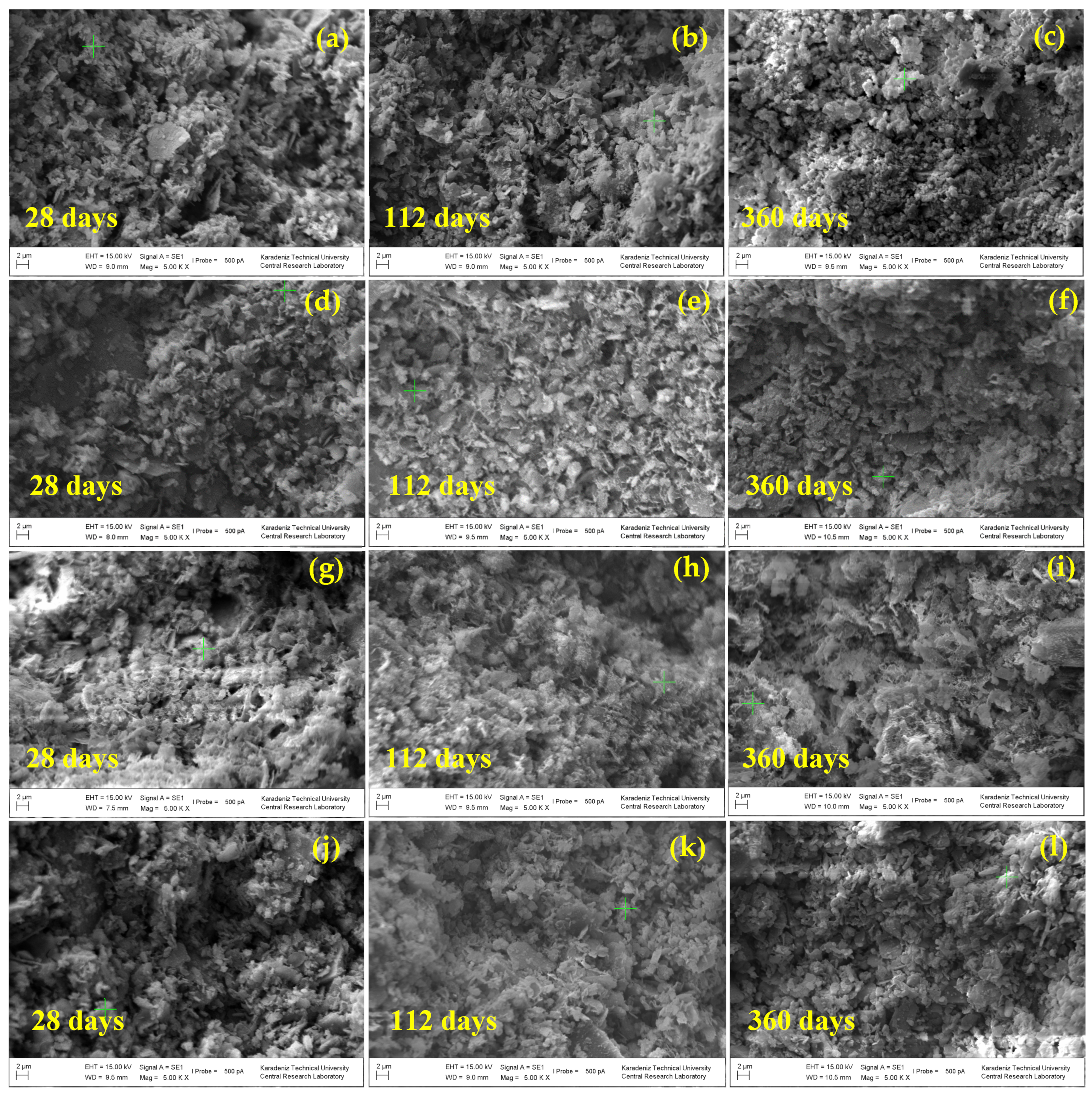
3.3. Influence of the Slag Fineness on the Microstructural Evolution of the CPBs
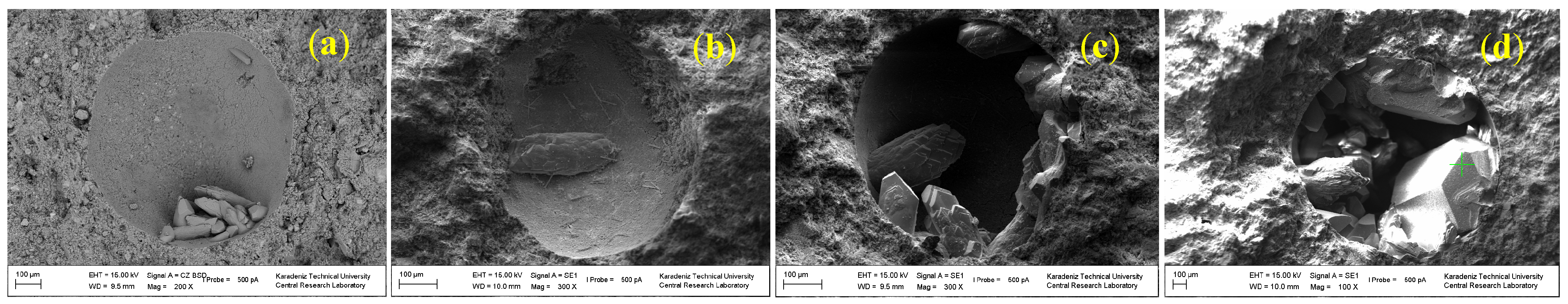
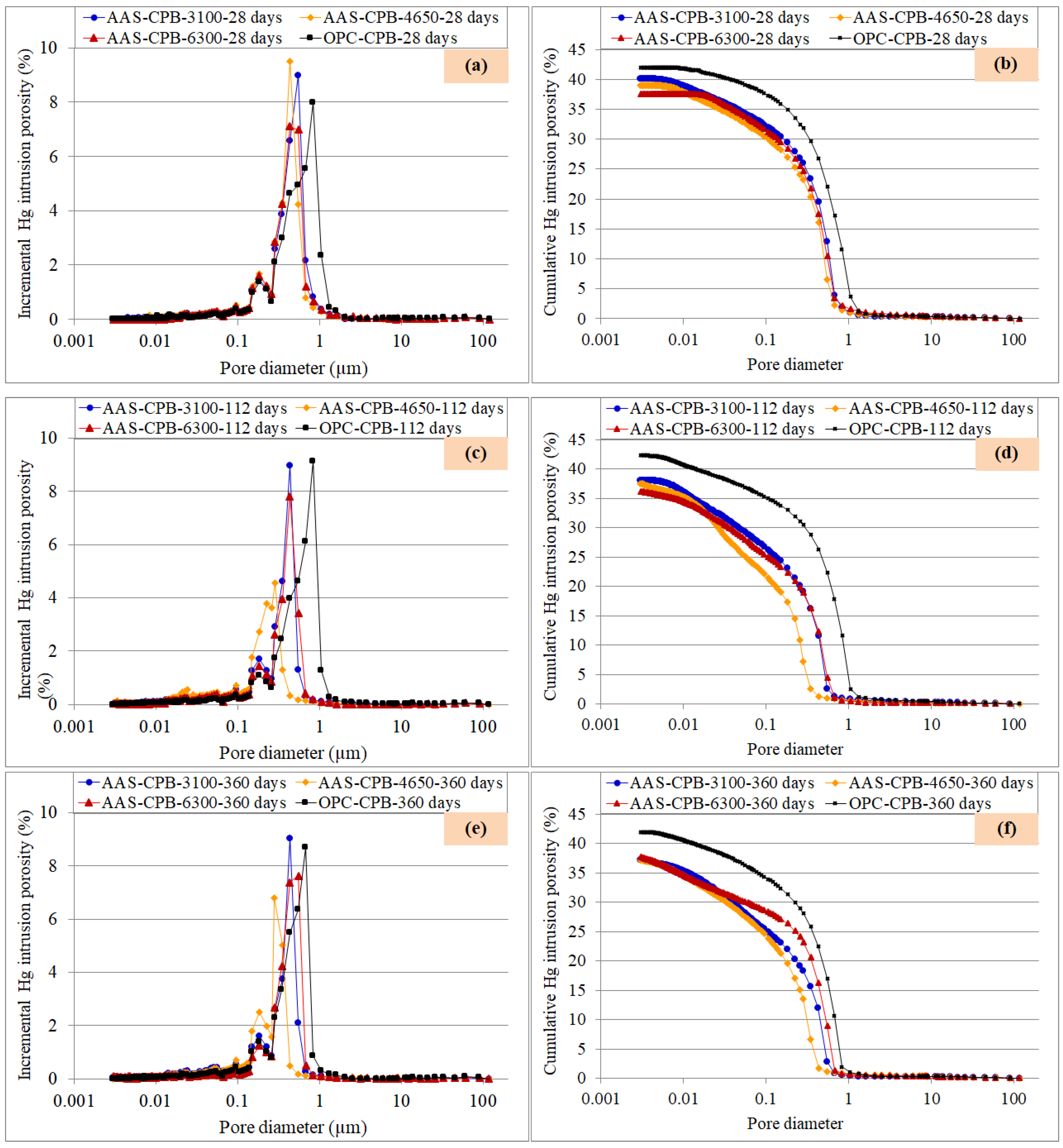
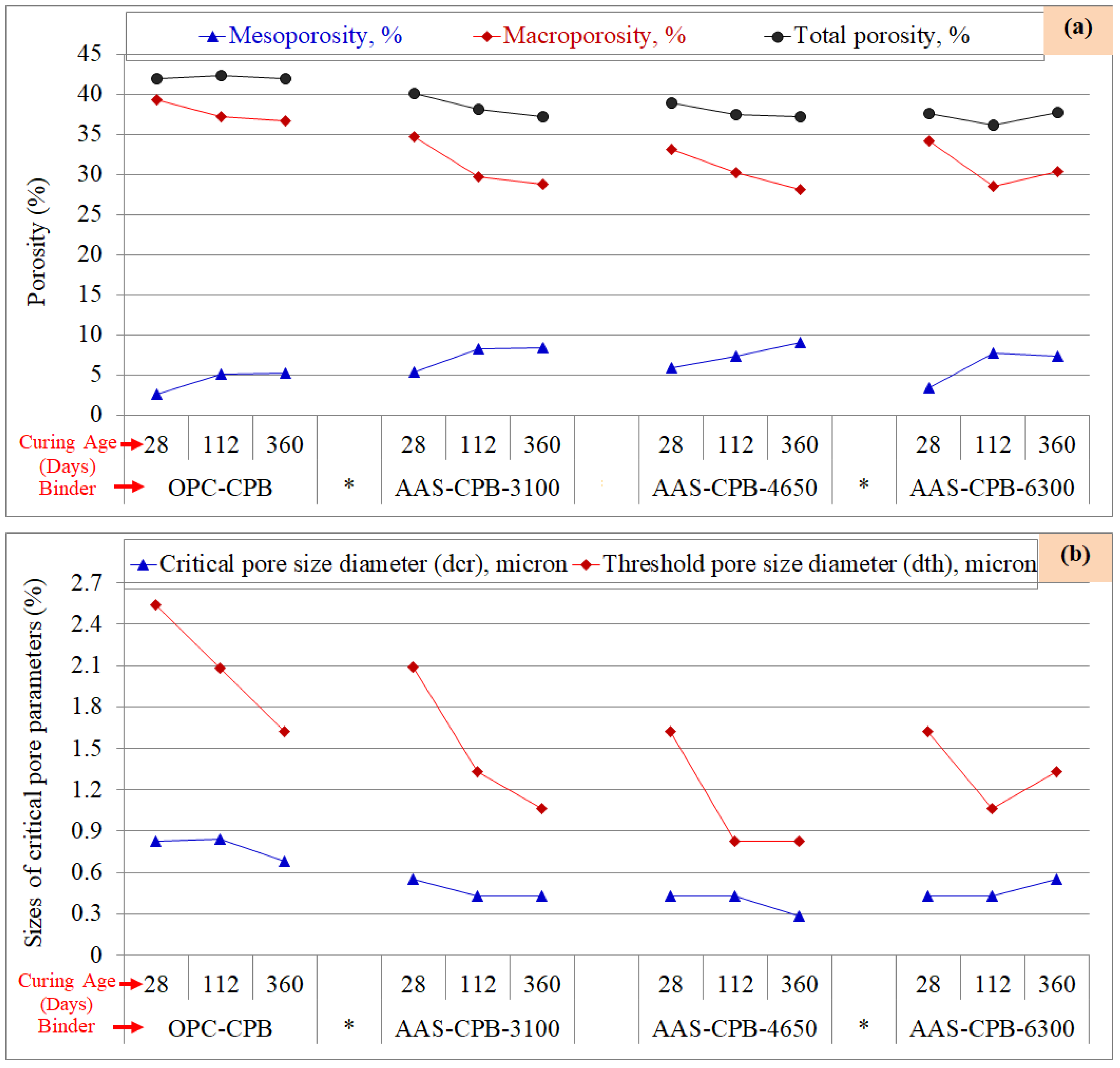
3.4. Pore Size Evolution of the CPB Samples
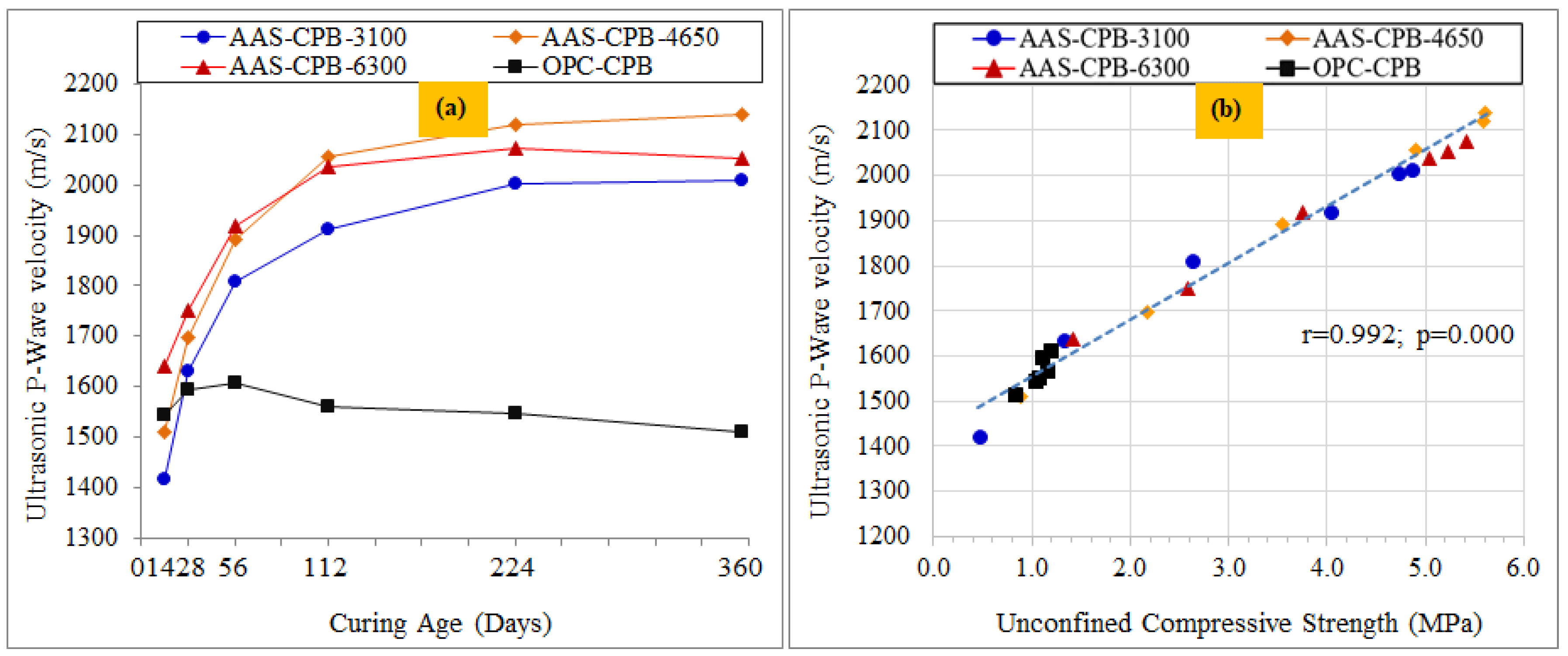
3.5. Evaluation of the CPB Strength and the Durability Properties via P-Wave Velocity (UPV)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Q.; Zhang, Q.; Qi, C.; Fourie, A.; Xiao, C. Recycling Phosphogypsum and Construction Demolition Waste for Cemented Paste Backfill and Its Environmental Impact. J. Clean. Prod. 2018, 186, 418–429. [Google Scholar] [CrossRef]
- Koc, E.; Cihangir, F.; Ercikdi, B. Chapter 3—Geochemical Evaluation of Sulfidic Tailings and Cemented Paste Backfill with Respect to Environmental Impacts. In Managing Mining and Minerals Processing Wastes Concepts, Design, and Applications; Qi, C., Benson, C.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 47–70. ISBN 978-0-323-91283-9. [Google Scholar]
- Chen, X.; Shi, X.; Zhou, J.; Du, X.; Chen, Q.; Qiu, X. Effect of Overflow Tailings Properties on Cemented Paste Backfill. J. Environ. Manag. 2019, 235, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Fall, M.; Pour, H.M. Deeper Understanding of the Strength Evolution and Deformation Characteristics of Sodium Silicate–Cemented Paste Tailing Material. Minerals 2023, 13, 1382. [Google Scholar] [CrossRef]
- Petlovanyi, M.; Mamaikin, O. Assessment of an Expediency of Binder Material Mechanical Activation in Cemented Rockfill. ARPN J. Eng. Appl. Sci. 2019, 14, 3492–3503. [Google Scholar]
- Chen, Q.; Zhou, H.; Wang, Y.; Li, X.; Zhang, Q.; Feng, Y.; Qi, C. Resistance Loss in Cemented Paste Backfill Pipelines: Effect of Inlet Velocity, Particle Mass Concentration, and Particle Size. Materials 2022, 15, 3339. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, R.; Xie, C.; Liu, S. Effect of Curing Humidity on Performance of Cemented Paste Backfill. Int. J. Miner. Metall. Mater. 2020, 27, 1046–1053. [Google Scholar] [CrossRef]
- Kasap, T.; Yilmaz, E.; Guner, N.U.; Sari, M. Recycling Dam Tailings as Cemented Mine Backfill: Mechanical and Geotechnical Properties. Adv. Mater. Sci. Eng. 2022, 2022, 6993068. [Google Scholar] [CrossRef]
- Pan, A.; Grabinsky, M. Mechanical Characterization of Cemented Paste Backfill. Eng 2023, 4, 738–747. [Google Scholar] [CrossRef]
- Kesimal, A.; Ercikdi, B.; Yilmaz, E. The Effect of Desliming by Sedimentation on Paste Backfill Performance. Miner. Eng. 2003, 16, 1009–1011. [Google Scholar] [CrossRef]
- Cihangir, F.; Ercikdi, B.; Kesimal, A.; Deveci, H.; Erdemir, F. Paste Backfill of High-Sulphide Mill Tailings Using Alkali-Activated Blast Furnace Slag: Effect of Activator Nature, Concentration and Slag Properties. Miner. Eng. 2015, 83, 117–127. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Quellet, J.; Servant, S.; Newman, P.; Verburg, R. Cementitious Backfill with High Sulfur Content Physical, Chemical, and Mineralogical Characterization. Cem. Concr. Res. 1999, 29, 719–725. [Google Scholar] [CrossRef]
- Fall, M.; Benzaazoua, M. Modeling the Effect of Sulphate on Strength Development of Paste Backfill and Binder Mixture Optimization. Cem. Concr. Res. 2005, 35, 301–314. [Google Scholar] [CrossRef]
- Liu, L.; Xin, J.; Huan, C.; Qi, C.; Zhou, W.; Song, K.I.I.L. Pore and Strength Characteristics of Cemented Paste Backfill Using Sulphide Tailings: Effect of Sulphur Content. Constr. Build. Mater. 2020, 237, 117452. [Google Scholar] [CrossRef]
- Clayton, S.; Grice, T.G.; Boger, D.V. Analysis of the Slump Test for On-Site Yield Stress Measurement of Mineral Suspensions. Int. J. Miner. Process. 2003, 70, 3–21. [Google Scholar] [CrossRef]
- Kesimal, A.; Yilmaz, E.; Ercikdi, B. Evaluation of Paste Backfill Mixtures Consisting of Sulphide-Rich Mill Tailings and Varying Cement Contents. Cem. Concr. Res. 2004, 34, 1817–1822. [Google Scholar] [CrossRef]
- Cihangir, F.; Akyol, Y. Effect of Desliming of Tailings on the Fresh and Hardened Properties of Paste Backfill Made from Alkali-Activated Slag. Adv. Mater. Sci. Eng. 2020, 2020, 4536257. [Google Scholar] [CrossRef]
- Annor, A.; Tarr, K.; Fynn, D. Mechanical Properties of a Composite Backfill Material; Core Project on Deep Mining Version: Montreal, QC, Canada, 2006; pp. 128–132. [Google Scholar]
- Cihangir, F.; Ercikdi, B.; Kesimal, A.; Ocak, S.; Akyol, Y. Effect of Sodium-Silicate Activated Slag at Different Silicate Modulus on the Strength and Microstructural Properties of Full and Coarse Sulphidic Tailings Paste Backfill. Constr. Build. Mater. 2018, 185, 555–566. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Fall, M.; Belem, T. A Contribution to Understanding the Hardening Process of Cemented Pastefill. Miner. Eng. 2004, 17, 141–152. [Google Scholar] [CrossRef]
- Cihangir, F.; Ercikdi, B.; Kesimal, A.; Turan, A.; Deveci, H. Utilisation of Alkali-Activated Blast Furnace Slag in Paste Backfill of High-Sulphide Mill Tailings: Effect of Binder Type and Dosage. Miner. Eng. 2012, 30, 33–43. [Google Scholar] [CrossRef]
- Ercikdi, B.; Baki, H.; İzki, M. Effect of Desliming of Sulphide-Rich Mill Tailings on the Long-Term Strength of Cemented Paste Backfill. J. Environ. Manag. 2013, 115, 5–13. [Google Scholar] [CrossRef]
- Pokharel, M.; Fall, M. Combined Influence of Sulphate and Temperature on the Saturated Hydraulic Conductivity of Hardened Cemented Paste Backfill. Cem. Concr. Compos. 2013, 38, 21–28. [Google Scholar] [CrossRef]
- Xue, G.; Yilmaz, E.; Song, W.; Cao, S. Compressive Strength Characteristics of Cemented Tailings Backfill with Alkali-Activated Slag. Appl. Sci. 2018, 8, 1537. [Google Scholar] [CrossRef]
- Kou, Y.; Jiang, H.; Ren, L.; Yilmaz, E.; Li, Y. Rheological Properties of Cemented Paste Backfill with Alkali-Activated Slag. Minerals 2020, 10, 288. [Google Scholar] [CrossRef]
- Jiang, H.; Han, J.; Li, Y.; Yilmaz, E.; Sun, Q.; Liu, J. Relationship between Ultrasonic Pulse Velocity and Uniaxial Compressive Strength for Cemented Paste Backfill with Alkali-Activated Slag. Nondestruct. Test. Eval. 2020, 35, 359–377. [Google Scholar] [CrossRef]
- Mohamed, O.A. A Review of Durability and Strength Characteristics of Alkali-Activated Slag Concrete. Materials 2019, 12, 1198. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-Activated Binders: A Review. Part 2. About Materials and Binders Manufacture. Constr. Build. Mater. 2008, 22, 1315–1322. [Google Scholar] [CrossRef]
- Sun, Q.; Wei, X.; Li, T.; Zhang, L. Strengthening Behavior of Cemented Paste Backfill Using Alkali-Activated Slag Binders and Bottom Ash Based on the Response Surface Method. Materials 2020, 13, 855. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhou, M.; Chen, C.; Yuan, Z.; Geng, X.; Yang, S. Solidification of Uranium Tailings Using Alkali-Activated Slag Mixed with Natural Zeolite. Nucl. Eng. Technol. 2023, 55, 523–529. [Google Scholar] [CrossRef]
- Jiang, H.; Qi, Z.; Yilmaz, E.; Han, J.; Qiu, J.; Dong, C. Effectiveness of Alkali-Activated Slag as Alternative Binder on Workability and Early Age Compressive Strength of Cemented Paste Backfills. Constr. Build. Mater. 2019, 218, 689–700. [Google Scholar] [CrossRef]
- Ji, X.; Gu, X.; Wang, Z.; Xu, S.; Jiang, H.; Yilmaz, E. Admixture Effects on the Rheological/Mechanical Behavior and Micro-Structure Evolution of Alkali-Activated Slag Backfills. Minerals 2023, 13, 30. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Alkali Activated Materials, State-of-the-Art Report, RILEM TC 224-AAM; Springer: Dordrecht, The Netherland, 2014; Volume 1, ISBN 978-94-007-7672-2. [Google Scholar]
- Kılıç, A.; Teymen, A. Determination of Mechanical Properties of Rocks Using Simple Methods. Bull. Eng. Geol. Environ. 2008, 67, 237–244. [Google Scholar] [CrossRef]
- Altindag, R. Correlation between P-Wave Velocity and Some Mechanical Properties for Sedimentary Rocks. J. S. Afr. Inst. Min. Metall. 2012, 112, 229–237. [Google Scholar]
- Karpuz, C.; Pa¸amehmetogˇlu, A.G. Field Characterisation of Weathered Ankara Andesites. Eng. Geol. 1997, 46, 1–17. [Google Scholar] [CrossRef]
- Hamdi, E.; Lafhaj, Z. Microcracking Based Rock Classification Using Ultrasonic and Porosity Parameters and Multivariate Analysis Methods. Eng. Geol. 2013, 167, 27–36. [Google Scholar] [CrossRef]
- Sousa, L.M.O.; Suárez Del Río, L.M.; Calleja, L.; Ruiz De Argandoña, V.G.; Rey, A.R. Influence of Microfractures and Porosity on the Physico-Mechanical Properties and Weathering of Ornamental Granites. Eng. Geol. 2005, 77, 153–168. [Google Scholar] [CrossRef]
- Ullemeyer, K.; Siegesmund, S.; Rasolofosaon, P.N.J.; Behrmann, J.H. Experimental and Texture-Derived P-Wave Anisotropy of Principal Rocks from the TRANSALP Traverse: An Aid for the Interpretation of Seismic Field Data. Tectonophysics 2006, 414, 97–116. [Google Scholar] [CrossRef]
- Wyering, L.D.; Villeneuve, M.C.; Wallis, I.C.; Siratovich, P.A.; Kennedy, B.M.; Gravley, D.M.; Cant, J.L. Mechanical and Physical Properties of Hydrothermally Altered Rocks, Taupo Volcanic Zone, New Zealand. J. Volcanol. Geotherm. Res. 2014, 288, 76–93. [Google Scholar] [CrossRef]
- Ullah, Z.; Qureshi, M.I.; Ahmad, A.; Khan, S.U.; Javaid, M.F. An Experimental Study on the Mechanical and Durability Properties Assessment of E-Waste Concrete. J. Build. Eng. 2021, 38, 102177. [Google Scholar] [CrossRef]
- El Jazouli, B.; Tsangouri, E. Fire-Exposed Stones in Constructions: Residual Strength, Performance Loss and Damage Mode Shift Due to Mineralogical Transformation and Micro-Cracking. Eng. Geol. 2022, 302, 106638. [Google Scholar] [CrossRef]
- Gupta, A.S.; Rao, S.K. Weathering Indices and Their Applicability for Crystalline Rocks. Bull. Eng. Geol. Environ. 2001, 60, 201–221. [Google Scholar] [CrossRef]
- Wang, P.; Xu, J.; Fang, X.; Wang, P.; Zheng, G.; Wen, M. Ultrasonic Time-Frequency Method to Evaluate the Deterioration Properties of Rock Suffered from Freeze-Thaw Weathering. Cold Reg. Sci. Technol. 2017, 143, 13–22. [Google Scholar] [CrossRef]
- Christaras, B. P-Wave Velocity and Quality of Building Materials; WSEAS Press: Cambridge, UK, 2009; pp. 295–300. [Google Scholar]
- Wu, J.; Feng, M.; Chen, Z.; Mao, X.; Han, G.; Wang, Y. Particle Size Distribution Effects on the Strength Characteristic of Cemented Paste Backfill. Minerals 2018, 8, 322. [Google Scholar] [CrossRef]
- Yan, B.; Zhu, W.; Hou, C.; Yilmaz, E.; Saadat, M. Characterization of Early Age Behavior of Cemented Paste Backfill through the Magnitude and Frequency Spectrum of Ultrasonic P-Wave. Constr. Build. Mater. 2020, 249, 118733. [Google Scholar] [CrossRef]
- Qiu, J.; Guo, Z.; Yang, L.; Jiang, H.; Zhao, Y. Effect of Tailings Fineness on Flow, Strength, Ultrasonic and Microstructure Characteristics of Cemented Paste Backfill. Constr. Build. Mater. 2020, 263, 120645. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Y.; Liu, Y. Mechanical Performance and Ultrasonic Properties of Cemented Gangue Backfill with Admixture of Fly Ash. Ultrasonics 2016, 64, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sun, X.; Yao, W.; Wu, P.; Qiu, J.; Guo, Z.; Liu, N. Strength and Ultrasonic Characteristics of Cemented Paste Backfill Incorporating Foaming Agent. Minerals 2021, 11, 681. [Google Scholar] [CrossRef]
- Ercikdi, B.; Yılmaz, T.; Külekci, G. Strength and Ultrasonic Properties of Cemented Paste Backfill. Ultrasonics 2014, 54, 195–204. [Google Scholar] [CrossRef]
- Yılmaz, T.; Ercikdi, B.; Karaman, K.; Külekçi, G. Assessment of Strength Properties of Cemented Paste Backfill by Ultrasonic Pulse Velocity Test. Ultrasonics 2014, 54, 1386–1394. [Google Scholar] [CrossRef]
- Yılmaz, T.; Ercikdi, B. Predicting the Uniaxial Compressive Strength of Cemented Paste Backfill from Ultrasonic Pulse Velocity Test. Nondestruct. Test. Eval. 2016, 31, 247–266. [Google Scholar] [CrossRef]
- Xu, S.; Suorineni, F.T.; Li, K.; Li, Y. Evaluation of the Strength and Ultrasonic Properties of Foam-Cemented Paste Backfill. Int. J. Min. Reclam. Environ. 2017, 31, 544–557. [Google Scholar] [CrossRef]
- Jiang, H.; Yi, H.; Yilmaz, E.; Liu, S.; Qiu, J. Ultrasonic Evaluation of Strength Properties of Cemented Paste Backfill: Effects of Mineral Admixture and Curing Temperature. Ultrasonics 2020, 100, 105983. [Google Scholar] [CrossRef] [PubMed]
- Sari, M.; Yilmaz, E.; Kasap, T.; Karasu, S. Exploring the Link between Ultrasonic and Strength Behavior of Cementitious Mine Backfill by Considering Pore Structure. Constr. Build. Mater. 2023, 370, 130588. [Google Scholar] [CrossRef]
- Landriault, D. Backfill in Underground Mining. In Underground Mining Methods: Engineering Fundamentals and International Case Studies; SME: Littleton, CO, USA, 2001; pp. 601–614. [Google Scholar]
- TS EN 196-6; Methods of Testing Cement—Part 6: Determination of Fineness. TSE: Ankara, Turkey, 2020.
- ISRM. The Complete Suggested Methods for Rock Characterization, Testing and Monitoring: 1974–2006; Springer: London, UK, 2007. [Google Scholar]
- Bauné, E.; Bonnet, C.; Liu, S. Reconsidering the Basicity of a FCAW Consumable—Part 1: Solidified Slag Composition of a FCAW Consumable as a Basicity Indicator. Weld. J. 2000, 79, 57–65. [Google Scholar]
- ASTM C989/C989M-22; Standard Specification for Slag Cement for Use in Concrete and Mortars. ASTM: West Conshohocken, PA, USA, 2022.
- ASTM C143/C143M-20; Standard Test Method for Slump of Hydraulic-Cement Concrete. ASTM: West Conshohocken, PA, USA, 2020.
- ASTM C39/C39M-21; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM: West Conshohocken, PA, USA, 2021.
- Komljenović, M.; Baščarević, Z.; Marjanović, N.; Nikolić, V. External Sulfate Attack on Alkali-Activated Slag. Constr. Build. Mater. 2013, 49, 31–39. [Google Scholar] [CrossRef]
- Shi, C.; Stegemann, J.A. Acid Corrosion Resistance of Different Cementing Materials. Cem. Concr. Res. 2000, 30, 803–808. [Google Scholar] [CrossRef]
- Gaitero, J.J.; Campillo, I.; Guerrero, A. Reduction of the Calcium Leaching Rate of Cement Paste by Addition of Silica Nanoparticles. Cem. Concr. Res. 2008, 38, 1112–1118. [Google Scholar] [CrossRef]
- Chen, J.J.; Thomas, J.J.; Jennings, H.M. Decalcification Shrinkage of Cement Paste. Cem. Concr. Res. 2006, 36, 801–809. [Google Scholar] [CrossRef]
- Damidot, D.; Glasser, F.P. Thermodynamic Investigation of the CaO Al2O3 CaSO4 H2O System at 25 °C and the Influence of Na2O. Cem. Concr. Res. 1993, 23, 221–238. [Google Scholar] [CrossRef]
- Cihangir, F.; Akyol, Y. Mechanical, Hydrological and Microstructural Assessment of the Durability of Cemented Paste Backfill Containing Alkali-Activated Slag. Int. J. Min. Reclam. Environ. 2018, 32, 123–143. [Google Scholar] [CrossRef]
- ASTM D4404-18; Standard Test Method for Determination of Pore Volume and Pore Volume Distribution of Soil and Rock by Mercury Intrusion Porosimetry. ASTM: West Conshohocken, PA, USA, 2018.
- Everett, D.H. I Manual of Symbols and Terminology for Physicochemical Quantities and Units, Appendix II: Definitions, Terminology and Symbols in Colloid and Surface Chemistry. Pure and Applied Chemistry. 1972, 1, 578–593. [Google Scholar] [CrossRef]
- ASTM C597-22; Standard Test Method for Pulse Velocity Through Concrete. ASTM: West Conshohocken, PA, USA, 2022.
- Shi, C.; Krivenko, P.V.; Roy, D.M. Alkali-Activated Cements and Concretes; Taylor & Francis: London, UK; New York, NY, USA, 2006; ISBN 978-0-415-70004-7. [Google Scholar]
- Wan, H.; Shui, Z.; Lin, Z. Analysis of Geometric Characteristics of GGBS Particles and Their Influences on Cement Properties. Cem. Concr. Res. 2004, 34, 133–137. [Google Scholar] [CrossRef]
- Wang, P.Z.; Trettin, R.; Rudert, V. Effect of Fineness and Particle Size Distribution of Granulated Blast-Furnace Slag on the Hydraulic Reactivity in Cement Systems. Adv. Cem. Res. 2005, 17, 161–167. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K.L.; Pratt, P.L. Factors Affecting the Strength of Alkali-Activated Slag. Cem. Concr. Res. 1994, 24, 1033–1043. [Google Scholar] [CrossRef]
- Lloyd, R.R.; Provis, J.L.; Van Deventer, J.S.J. Pore Solution Composition and Alkali Diffusion in Inorganic Polymer Cement. Cem. Concr. Res. 2010, 40, 1386–1392. [Google Scholar] [CrossRef]
- Li, Y.; Min, X.; Ke, Y.; Fei, J.; Liu, D.; Tang, C. Immobilization Potential and Immobilization Mechanism of Arsenic in Cemented Paste Backfill. Miner. Eng. 2019, 138, 101–107. [Google Scholar] [CrossRef]
- Yilmaz, E.; Belem, T.; Bussière, B.; Benzaazoua, M. Relationships between Microstructural Properties and Compressive Strength of Consolidated and Unconsolidated Cemented Paste Backfills. Cem. Concr. Compos. 2011, 33, 702–715. [Google Scholar] [CrossRef]
- Gupta, A.S.; Rao, K.S. Index Properties of Weathered Rocks: Inter-Relationships and Applicability. Bull. Eng. Geol. Environ. 1998, 57, 161–172. [Google Scholar] [CrossRef]
- Smolarkiewicz, P.P.; Nogueira, C.L.; Willam, K.J. Ultrasonic Evaluation of Damage in Heterogeneous Concrete Materials. In Proceedings of the European Congress on Computational Methods in Applied Sciences and Engineering. ECCOMAS, Barcelona, Spain, 11–14 September 2000; p. 13. [Google Scholar]
- Aydin, E.; Doven, A.G. Influence of Water Content on Ultrasonic Pulse–Echo Measurements Through High Volume Fly Ash Cement Paste—Physicomechanical Characterization. Res. Nondestruct. Eval. 2006, 17, 177–189. [Google Scholar] [CrossRef]
- Vasconcelos, G.; Lourenço, P.B.; Alves, C.A.S.; Pamplona, J. Ultrasonic Evaluation of the Physical and Mechanical Properties of Granites. Ultrasonics 2008, 48, 453–466. [Google Scholar] [CrossRef]
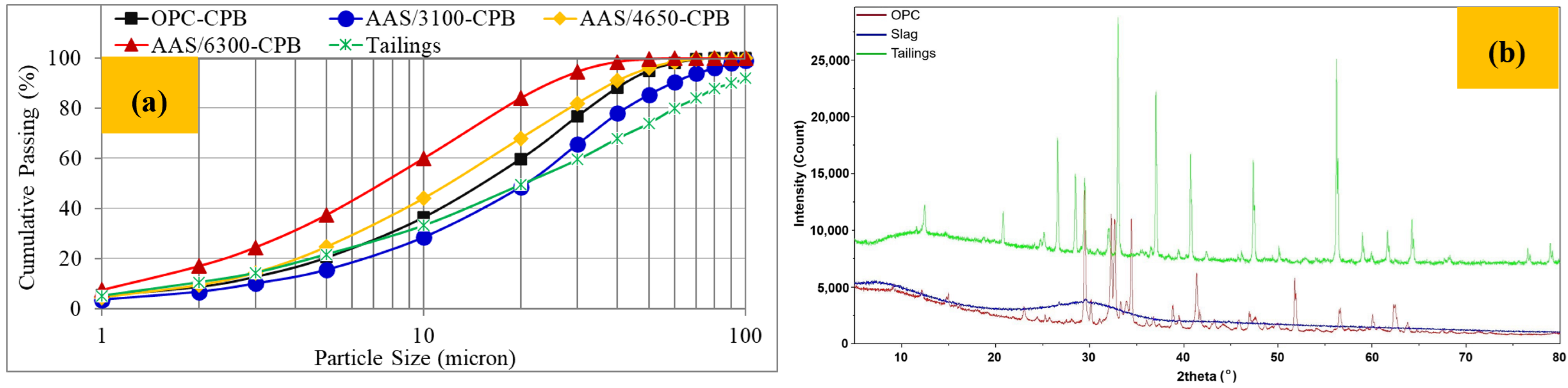


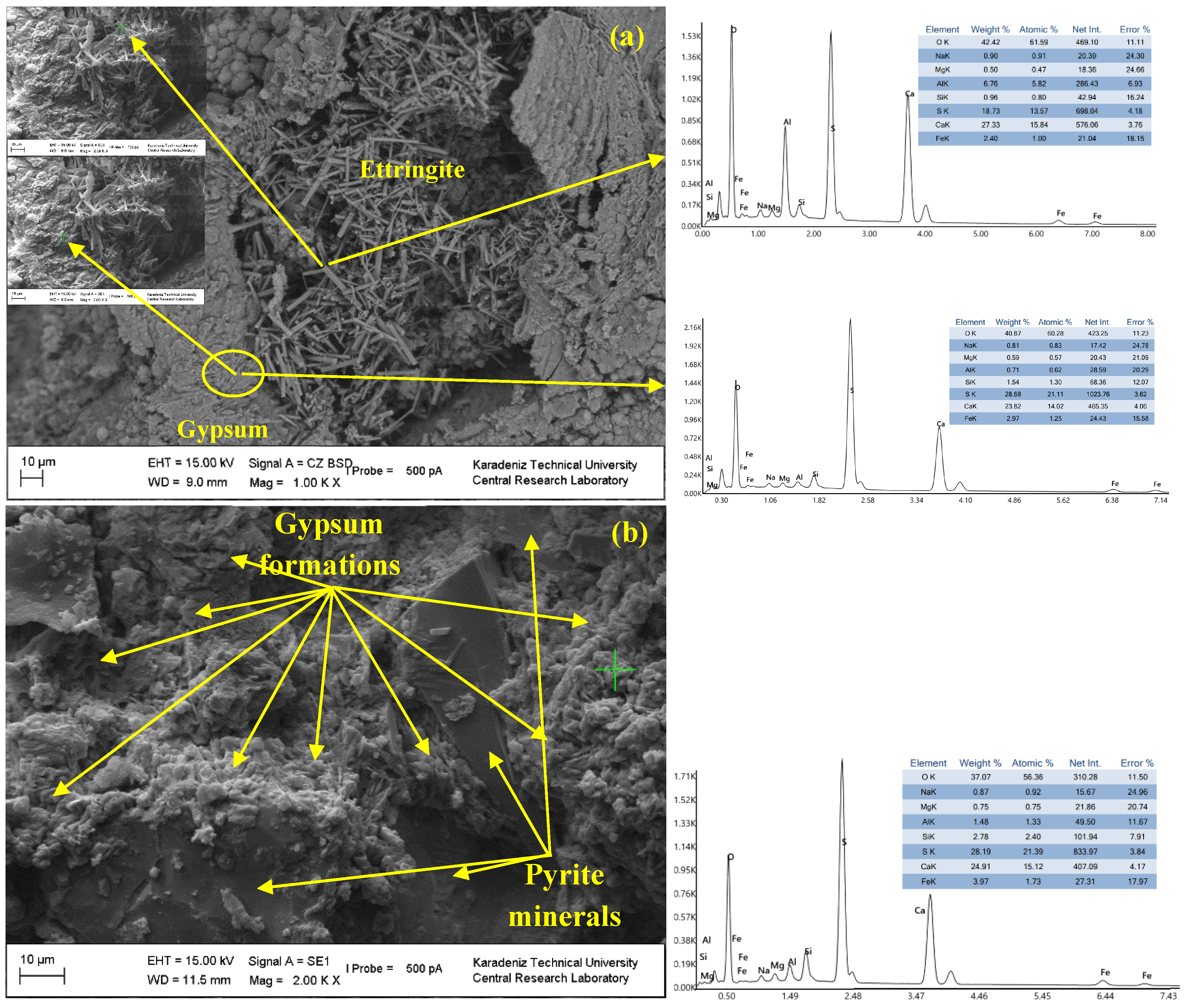
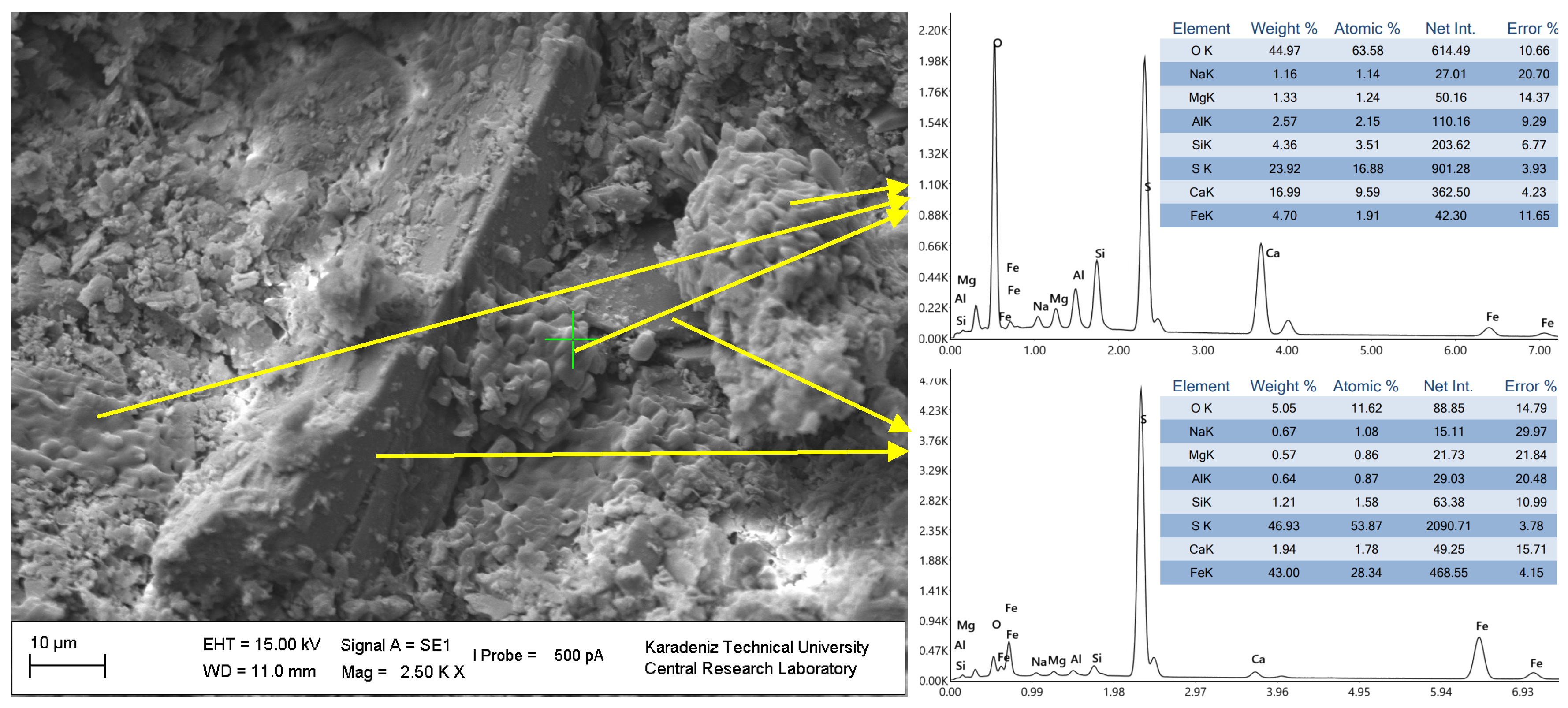


| Chemical Composition (%) | Value | Physical Properties | Value | Mineralogical Content (%) | Value |
|---|---|---|---|---|---|
| SiO2 | 15.24 | Specific gravity | 3.95 | Quartz | 15.4 |
| Al2O3 | 3.69 | Specific surface (cm2/g) | 3066 | Pyrite | 56.3 |
| Fe2O3 | 49.19 | −20 µm material content (%) | 48.85 | Clinochlore | 4.5 |
| CaO | 2.56 | D10 (µm) | 1.84 | Kaolinite | 3.1 |
| MgO | 1.64 | D30 (µm) | 8.44 | Calcite | 4.5 |
| TiO2 | 0.21 | D50 (µm) | 20.97 | Amesite | 4.1 |
| Cr2O3 | 0.03 | D60 (µm) | 30.37 | Siderite | 2.0 |
| Na2O | 0.98 | D90 (µm) | 90.37 | Dolomite | 2.4 |
| K2O | 0.22 | Cu | 16.51 | Acmite | 1.1 |
| MnO | 0.07 | Cc | 1.27 | Copiapite | 1.0 |
| P2O5 | 0.06 | Initial pH | 8.98 | Hematite | 2.7 |
| Loss on ignition | 23.30 | Initial SO42− (ppm) | 5712 | Halloysite | 0.8 |
| S2− (Sulphide) | 32.33 | Gypsum | 1.0 | ||
| Total S | 28.89 | Others | ~1.1 |
| Chemical Composition (%) | OPC (%) | Slag (%) | Physical Properties | OPC | Slag 3100 | Slag 4650 | Slag 6300 |
|---|---|---|---|---|---|---|---|
| SiO2 | 19.10 | 40.24 | Specific gravity | 3.15 | 2.90 | 2.90 | 2.90 |
| Al2O3 | 5.35 | 11.68 | Specific surface (cm2/g) | 4060 | 3100 | 4650 | 6300 |
| Fe2O3 | 3.45 | 0.66 | +90 µm sieve (%) | - | 0.8 | - | - |
| CaO | 62.29 | 36.63 | +45 µm sieve (%) | 2.64 | 12.3 | 3.85 | 0.62 |
| MgO | 0.95 | 5.90 | +32 µm sieve (%) | 7.11 | 24.6 | 8.33 | 2.78 |
| TiO2 | 0.14 | 1.00 | Mineralogical composition * | ||||
| Cr2O3 | 0.007 | 0.006 | |||||
| Na2O | 0.41 | 0.3 | C3S | 64.1 | - | - | - |
| K2O | 1.09 | 1.27 | C2S | 26.0 | - | - | - |
| MnO | 0.07 | 1.98 | C3A | 3.0 | - | - | - |
| P2O5 | 0.08 | 0.01 | C4AF | 3.7 | - | - | - |
| Loss on ignition | 1.53 | 0.9 | Basicity index | - | 0.96 | ||
| SO3 | 6.68 | 1.71 | Pozzolanic index (%; 28 days) | - | 67.6 | 84.7 | 99.3 |
| Binder Type | Binder Content | Solid Content (%) | Water Content (%) | Water/Binder Ratio | Slump (inch) |
|---|---|---|---|---|---|
| AAS-3100 | 7% | 80.31 | 19.69 | 3.50 | 8.6 |
| AAS-4650 | 7% | 80.24 | 19.76 | 3.52 | 8.4 |
| AAS-6300 | 7% | 80.16 | 19.84 | 3,54 | 8.3 |
| OPC | 7% | 77.97 | 22.03 | 4.04 | 8.4 |
| Correlation Parameters | Coefficient of Pearson Correlation (r) | Sig. (2-Tailed) |
|---|---|---|
| UCS-UPV for all CPB designs | 0.992 | 0.000 |
| UCS-UPV for AAS-3100 CPB samples | 0.982 | 0.001 |
| UCS-UPV for AAS-4650 CPB samples | 0.998 | 0.000 |
| UCS-UPV for AAS-6300 CPB samples | 0.997 | 0.000 |
| UCS-UPV for OPC-CPB samples | 0.872 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koc, E.; Cihangir, F. Ultrasonic and Microstructural Evaluation of Sulphide-Rich Tailings Cemented Paste Backfill Properties Containing Alkali-Activated Slag: Effect of Slag Fineness. Minerals 2023, 13, 1524. https://doi.org/10.3390/min13121524
Koc E, Cihangir F. Ultrasonic and Microstructural Evaluation of Sulphide-Rich Tailings Cemented Paste Backfill Properties Containing Alkali-Activated Slag: Effect of Slag Fineness. Minerals. 2023; 13(12):1524. https://doi.org/10.3390/min13121524
Chicago/Turabian StyleKoc, Ercument, and Ferdi Cihangir. 2023. "Ultrasonic and Microstructural Evaluation of Sulphide-Rich Tailings Cemented Paste Backfill Properties Containing Alkali-Activated Slag: Effect of Slag Fineness" Minerals 13, no. 12: 1524. https://doi.org/10.3390/min13121524
APA StyleKoc, E., & Cihangir, F. (2023). Ultrasonic and Microstructural Evaluation of Sulphide-Rich Tailings Cemented Paste Backfill Properties Containing Alkali-Activated Slag: Effect of Slag Fineness. Minerals, 13(12), 1524. https://doi.org/10.3390/min13121524






